Abstract
Neuroinflammation is a condition characterized by the elaboration of proinflammatory mediators within the central nervous system. Neuroinflammation has emerged as a dominant theme in contemporary neuroscience due to its association with neurodegenerative disease states such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease. While neuroinflammation often is associated with damage to the CNS, it also can occur in the absence of neurodegeneration, e.g., in association with systemic infection. The “acute phase” inflammatory response to tissue injury or infections instigates neuroinflammation‐driven “sickness behavior,” i.e. a constellation of symptoms characterized by loss of appetite, fever, muscle pain, fatigue and cognitive problems. Typically, sickness behavior accompanies an inflammatory response that resolves quickly and serves to restore the body to homeostasis. However, recurring and sometimes chronic sickness behavior disorders can occur in the absence of an underlying cause or attendant neuropathology. Here, we review myalgic enchepalomyelitis/chronic fatigue syndrome (ME/CFS), Gulf War Illness (GWI), and chemobrain as examples of such disorders and propose that they can be exacerbated and perhaps initiated by a variety of environmental stressors. Diverse environmental stressors may disrupt the HPA axis and contribute to the degree and duration of a variety of neuroinflammation‐driven diseases.
Keywords: ME/CFS, GWI, chemobrain “sickness behavior”, stressors, neuroimmune, neuroinflammation
Introduction
Recent research in psychoneuroimmunology shows that neuroimmune dysregulation has profound effects on neuronal function and behavior. A broad spectrum of symptoms such as lethargy, anorexia, attention deficits, and sleep disruption, constitute the basis for transient sickness behaviors [1]. Chronic mental health issues, such as major depressive disorder or cognitive dysfunction, also can involve abberations in neuroimmune signaling [2,3]. At the molecular and cellular levels, these symptoms are a product of altered peripheral and brain immune cell function leading to upregulation of proinflammatory mediators. Accordingly, behavioral responses are dictated by the magnitude and duration of the expression of brain proinflammatory mediators, commonly referred to as neuroinflammation [3]. Typically, physiological neuroinflammation (e.g. as a result of systemic infection) resolves over time with restoration of homeostasis; however, persistent dysregulation is associated with chronic or recurring behavioral effects that underlie varying neurological diseases.
The continuum of neuroinflammatory responses: from acute phase injury to neurodegeneration:
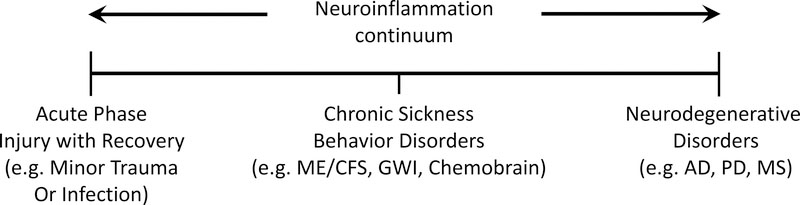
Transient inflammation in the periphery (often referred to as the acute phase response) (Fig. 1), due to mild trauma/infection at a wound site, leads to neuroinflammation manifested as a sickness behavior [1]. This response is a positive aspect of neuroinflammation because it allows for tissue remodeling at the site of injury and a slowing of overall behavior, permitting energy conservation and recovery. At the other end of the neuroinflammation continuum (Fig. 1) is a link of neuroinflammatory responses to neurodegeneration. Accordingly, the scientific and popular literature is replete with examples of an association of neuroinflammation with neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s Disease and multiple sclerosis [3]. There are other neuroinflammatory disorders, however, that can occur in the absence of evidence of underlying neurodegeneration. For example, chronic sickness behavior disorders appear to lie in the middle of the neuroinflammatory continuum (Fig. 1), where neuroinflammation persists, but without an attendant neuropathological underpinning. As we note below, such sickness behavior disorders have received relatively little attention compared to those associated with neuropathological diseases. Our focus here will be to highlight examples of neuroinflammatory conditions manifested as sickness behavior and suggest that environmental stressors may contribute to the etiology of these disorders.
Figure 1:
Neuroinflammation continuum. Neuroinflammation occurs in a continuum. It can result from acute phase injury where recovery to baseline occurs quickly after minor traumatic injury or infections, but when neuroinflammation is sustained, it has widely been associated with neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and multiple sclerosis (MS). In the middle of this neuroinflammation continuum are chronic sickness behavior disorders with no apparent underlying neurodegeneration (ME/CFS, GWI and chemobrain represent examples of distinct diseases in this non‐neurodegenerative category).
What is an environmental stressor?
Classically, stressors represent factors that alter homeostasis and engender a shift of the organism to restore hemostasis. Physiologically, these stressors can take the form of heat, cold, exercise to name a few. More broadly, however, agents and conditions in one’s personal environment such as chemicals (including pharmaceuticals), infectious agents, circadian disruptions, noise, and air pollutants, as well as psychological and social stressors [2], can combine to constitute the stressor environment. Alone or combined these stressors can impact the neuroimmune/neuroinflammatory axis by actions in the periphery, a direct action on the CNS, or through interactions with the HPA axis. These effects, in turn, can manifest themselves as neuroinflammation with the attendant symptoms of lethargy, anhedonia, anorexia, depression and cognitive dysfunction, i.e. the constellation of many of the features of “sickness behavior” [1]. In addition to age and gender, stressors, both psychological and physiological, serve as environmental factors that can contribute to neurodegenerative diseases and associated neuroinflammation. Little data, however, exists to document a role of environmental stressors in neuroinflammatory disorders that do not have a neurodegenerative component. Psychological stress and associated neuroinflammation can increase susceptibility to major depressive disorder (MDD) and MDD remains one of the few recognized long‐term neurological disorders associated with proinflammatory mediators in the absence of neurodegeneration [4]. Evidence for a role of neuroinflammation and stressors, however, is emerging for other chronic neurological disorders.
The potential for chronic non‐neurodegenerative sickness behavior disorders:
A neuroimmune/neuroinflammatory response to infection (often modeled with bacteria‐derived endotoxin in both human as well as animal research) represents perhaps the most widely recognized and physiologically adaptive mechanism that engenders sickness behavior. This condition is transient and abates with the infection but various components of sickness behavior and the underlying neuroinflammation can be exacerbated and maintained by environmental stressors. Under these circumstances neuroinflammation is initiated by an inflammagen, such as endotoxin, and persists through continuing activation of the HPA axis by various stressors. Some of the disorders that may involve this dysregulated neuroimmune response include: Chronic Fatigue Syndrome, Gulf War Illness and “Chemobrain.” Data from these disorders, will be highlighted to explore and illustrate possible direct and indirect initiation of neuroinflammation and how environmental stressors “hijack” the HPA to create a chronic non‐degenerative neurological disorder.
Mimicking physiological stressors in the laboratory with the rodent stress hormone, corticosterone: exacerbation, not inhibition, of neuroinflammatory disorders:
Therapy with the classic anti‐inflammatory stress hormone, cortisol (CORT) (corticosterone in rodents), can suppress neuroinflammation and neuroinflammatory signaling caused by exposure to neurotoxins, the bacterial endotoxin, LPS [5] or the viral mimic, polyinosinic‐polycytidylic acid (PIC), a synthetic dsRNA. Paradoxically, emerging literature from experimental animals shows that prior treatment with CORT can enhance rather than suppress the neuroinflammatory response to neurotoxicants, organophosphate acetylcholinesterase inhibitors, and LPS [5,6,7]. These results suggest that glucocorticoids can either ameliorate or potentiate the response to a neuroinflammagen depending on whether the treatment occurs before or after an inflammatory exposure. Further, they suggest that environmental stressors that activate the HPA can enhance neuroinflammatory disorders. These observations already have applicability to the human condition. For example, recurring physiological stressors that elevate CORT may lead to enhanced sickness behavior due to bacterial endotoxin [8,9,10,11] or viral inflammagens [12,13]. Pesticide sprayers, or the public at large exposed to organophosphates for combating Zika by eliminating the mosquito vector, may suffer an enhanced or prolonged neuroinflammation/sickness behavior if also exposed to a prior stressor. The chronic multisymptom disorder with features of sickness behavior known as Gulf War Illness may be another example of a stressor‐enhanced proinflammatory effect of insecticide exposure, in this case, due to exposures that occurred in the 1991 Gulf War theater [14]. Finally, the ever widening recognition that cancer chemotherapy can result in cognitive deficits with sickness behavior symptoms likely represents an underlying neuroinflammatory response, one that is initiated by or at least enhanced by the immunosuppressive actions of the therapy.
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS):
According to the Centers for Disease Control and Prevention (http://intranet.cdc.gov/ncezid/dhcpp/branches/cvdb.html) and reports from the National Academy of Medicine [15], ME/CFS afflicts upwards of 2.5 million Americans. Due to the difficulty of diagnosing this condition, another million or more may have the disorder. ME/CFS is characterized by fatigue that persists for 6 months or more in the absence of diseases or other contributing factors that could explain the condition [16,17]. The current case definition for ME/CFS includes an expansion of the criteria of Fukuda [18] to encompass a minimum of 4 of 8 symptoms, including memory impairment, pain, headaches and sleep disturbance. The pathophysiological basis of ME/CFS remains unknown but its etiology has been attributed to a number of factors, including chemical exposures and infectious agents. As with other “sickness behavior” disorders, neuroinflammatory mediators are thought to underlie CFS [16]. Recent advances in the understanding of CFS pathogenesis has led to the elucidation of patterns of CFS biomarker expression, providing the first hints of a molecular signature of the disorder including factors associated with inflammation, immune system activation, autonomic dysfunction, neuroendocrine and altered functioning in the hypothalamic‐pituitary‐adrenal axis. Further in depth systems biology analysis has identified several cytokines, e.g., IL‐1α, IL‐6, and IL‐8 that may function as biomarkers of ME/CFS and provide an indication of the duration and severity of illness [19]. Moving forward, the use of these biomarkers will provide an opportunity to understand potential exposures/triggers that result in CFS and to develop effective treatments.
Stressors, both psychological (e.g. emotional trauma) [20] and physiological (e.g. exercise) long have been implicated as contributing to the onset, severity and duration of the symptoms of ME/CFS. More recently, stressor and ME/CFS symptoms have been linked to an increase in salivary levels of the stress hormone, cortisol, when sampled in the evening, as well as increased proinflammatory cytokines in serum [19,20]. These finding support a role for HPA axis dysfunction known to be associated with depression and heightened inflammation. While not yet causally linked, these overall associations of stressor/neuroimmune effects to the severity of ME/CFS are consistent with a chronic neuroinflammatory disorder that occurs in the absence of CNS pathology. Recent suggestions of an involvement of the hippocampus in ME/CFS [21] where an “allostatic overload” [see 22] disrupts homeostatic drives to a degree that hippocampal neurocognitive impairments result in an alternate homeostatic state [23], provide the basis for integrating many of the symptoms of ME/CFS through the hippocampus and other higher centers of the CNS.
Gulf War Illness:
Gulf War Illness (GWI) is a chronic multi‐symptom disorder characterized by persistent cognitive impairment, fatigue, depression, sleep disruption, muscle pain, and GI and dermatologic problems [24]. These constellation of symptoms are consistent with a chronic sickness behavior syndrome. While similar in symptomatology to ME/CFS, GWI is a distinct disorder with its own case definitions [24,25]. While the illness is believed to be the result of various toxic exposures during the 1991 Persian Gulf War, the pathophysiology of GWI, and factors that contribute to its persistence for over 25 years, are still under active investigation. Clearly, and in common with ME/CFS, the features of sickness behavior associated with GWI implicate an underlying neuroinflammation/neuroimmune basis for this disease [26]. Moreover, by evaluating animal models subjected to “in theater” exposure conditions, it has become apparent that toxicants such as the nerve agent, sarin, or its surrogate, diisopropyl fluorophosphate (DFP), and various organophosphate insecticides, e.g., chlorpyrifos, result in neuroinflammation across the brain [6,27,28]. Furthermore, when these exposures are coupled with a stressor or a stressor mimic, such as exogenous corticosterone, the resultant neuroinflammation, as assessed by qPCR of broad categories of cytokines and chemokine mRNA, is exacerbated in degree and duration [6,27,28]. Consistent with these animal data, exercise as a physiological stressor, results in post‐exertional malaise in veterans suffering from Gulf War Illness [29]. While exposure to a single agent or exposure regimen may not explain the pathophysiological basis of GWI, a combination of stressors and chemical toxicants in theater may have shifted the neuroinflammation threshold, priming the brain in a manner that results in an exacerbated response to subsequent inflammatory challenges occurring in the daily lives of the ill veterans for the past 25 years.
“Chemobrain”:
Cognitive impairment following chemotherapy, termed “chemobrain”, is a relatively common side effect, affecting approximately 25% of patients receiving a variety of cancer chemotherapeutics [30]. While many cases of chemobrain are temporary, cognitive deficits may persist for many years after the treatment [30]. While the hallmark of chemobrain is the perceived cognitive deficits [30,31] other symptoms such as fatigue, sleep disorders, and depression also are often present [30]. Thus, chemobrain, like ME/CFS and Gulf War Illness, can be considered a sickness behavior disorder. Also in common with ME/CFS and GWI, is the absence of underlying evidence for neuronal damage, therefore, the chemotherapeutics are not likely inducing a neural damage‐related cognitive dysfuntion [32]. Not unexpectedly, as with other “sickness behavior” disorders, systemic inflammation and neuroinflammation are associated with chemobrain. Both cancer and chemotherapeutics can initiate inflammatory and neuroinflammtory responses in patients and animal models [30,33]. Thus, chemotherapeutics can be considered the stressor that contributes to neuroinflammation associated with cancer. On the other hand, cancer diagnosis is associated with physiological and emotional stressors that could be considered as the ongoing stressors contributing to the neuroinflammatory effects of chemotherapeutics [30,33]. Regardless of the initiator or propagator of sickness behavior associated with chemobrain, it seems likely that neuroinflammatory homeostasis has been disrupted and reset to the extent that neuroinflammatory responses are primed and sensitized long after chemotherapy has ended and perhaps post remission and cure of the cancer. Thus, chemobrain can be considered a chronic neuroinflammatory disorder not causally related to an associated cancer.
Potential therapeutics to treat chronic sickness behavior disorders:
As can be noted from the descriptions of ME/CFS, GWI and chemobrain above, these are symptom‐based disorders. As such, the etiology and pathophysiology of these diseases remain unknown. Beyond a strong link to disrupted neuroimmune signaling, and an exacerbation by environmental stressors, little evidence exists that point to therapeutic targets/pathways. Selected immune therapies from small molecule drugs to biologicals to stem cells have been attempted but target selection remains difficult and likely too focused given the complexity of these disorders. Likely, prevention will be the best strategy and recognizing and limiting the role of environmental stressors would appear to be an effective starting point in limiting the severity and duration of these chronic diseases.
Summary:
This review highlights the possibility that brain inflammation (neuroinflammation) in the absence of brain damage can be the consequence of disparate exposures/stressors and that activated HPA axis enhances the neuroinflammation condition. These outcomes demonstrate the potential for recurrent stressors in the environment to greatly exacerbate and prolong chronic inflammatory disorders. The complexity of these disorders remain a barrier to the development of therapeutic approaches.
Acknowledgements:
The authors thank Drs. Kimberly A. Kelly and Lindsay Michalovicz Gill for informative discussions on the role of neuroinflammation in chronic sickness behavior disorders.
Publication of this article was supported by the College International de Recherche Servier (CIRS)
Footnotes
Conflicts of Interest: None
Disclaimer: “The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References:
- [1].Danzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev Neurosci 2008;9:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 2015;289: 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DiSabato DJ, Quan N, Godbout P. Neuroinflammation: the devil is in the details. J. Neurochem 2016; 139: 136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller DB and O’Callaghan JP: Depression, Cytokines and Glial Function. Metabolism 2005; 54: 33–88. [DOI] [PubMed] [Google Scholar]
- [5].Kelly KA, Michalovicz LT, Miller JV, Castranova V, Miller DB, O’Callaghan JP. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PLoS One 2018; 13(1):e0190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM. Corticosterone primes the neuroinflammatory response to DFP in mice:potential animal model of Gulf War Illness. J. Neurochem 2015; 133:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain. Behav. Immun 2010;24:19–30. [DOI] [PubMed] [Google Scholar]
- [8].Browne CA, O’Brien FE, Connor TJ, Dinan TG, Cryan JF. Differential lipopolysaccharide‐induced immune alterations in the hippocampus of two mouse strains: Effects of stress. Neuroscience. 2012;225:237–48. [DOI] [PubMed] [Google Scholar]
- [9].Espinosa‐Oliva AM, de Pablos RM, Villarán RF, Argüelles S, Venero JL, Machado A, et al. Stress is critical for LPS‐induced activation of microglia and damage in the rat hippocampus. Neurobiol. Aging 2011;32:85–102. [DOI] [PubMed] [Google Scholar]
- [10].Nowacka MM, Paul‐Samojedny M, Bielecka AM, Obuchowicz E. Chronic social instability stress enhances vulnerability of BDNF response to LPS in the limbic structures of female rats: A protective role of antidepressants. Neurosci. Res. 2014;88:74–83. [DOI] [PubMed] [Google Scholar]
- [11].Audet MC, Jacobson‐Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain. Behav. Immun. 2011;25:1197–205. [DOI] [PubMed] [Google Scholar]
- [12].Chijiwa T, Oka T, Lkhagvasuren B, Yoshihara K, Sudo N. Prior chronic stress induces persistent polyI:C‐induced allodynia and depressive‐like behavior in rats: Possible involvement of glucocorticoids and microglia. Physiol. Behav 2015;147:264–73. [DOI] [PubMed] [Google Scholar]
- [13].Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain. Behav. Immun 2007;21:477–89. [DOI] [PubMed] [Google Scholar]
- [14].White RF, Steele L., O’Callaghan JP, Sullivan K, Barlow C, Binns JH, et al. Gulf War illness and other health problems in veterans of the 1990‐1991 Gulf War: Effects of exposures to neurotoxicants. Cortex 2016;74: 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clayton EW, Alegría M, Bateman L, Chu L, Cleeland CS, Davis R, et al. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Institute of Medicine . Report Brief, February 2015. [Google Scholar]
- [16].Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain Behav Immun 2012;26:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arnett SV, Korossy‐Horwood R, Clark IA. Chronic fatigue syndrome – a neuroimmunological model. Medical Hypothesis 2011;77:77–83. [DOI] [PubMed] [Google Scholar]
- [18].Fukuda K, Straus SE, Hickie I, Shapre MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome study group. Ann Intern Med 1994; 121: 953–9. [DOI] [PubMed] [Google Scholar]
- [19].Russell L, Broderick G, Taylor R, Fernandez H, Harvey J, Barnes Z, et al. Illness progression in chronic fatigue syndrome: a shifting immune baseline. BMC Immunology 2016; 17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Milrad SF, Hall DL, Jutagir DR, Lattie EG, Czaja SJ, Perdomo DM et al. Depression, evening salivary cortisol and inflammation in chronic fatigue syndrome: a psychoneuroendocrinological structural regression model. Int J Psychophysiol 2017; 17: 30162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saury J‐M. The role of the hippocampus in the pathogenesis of myalgic encephalomyelitis/chronic fatique syndrome (ME/CFS). Medical Hypothesis 2016; 86: 30–38. [DOI] [PubMed] [Google Scholar]
- [22].McEwen BS. The brain of stress: toward an integrative approach to brain, body, and behavior. Perspect Psychol Sci 23013; 8: 673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Craddock TJA, Fritsch P, Rice MA, del Rosario RM, Miller DB, Fletcher MA et al. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf war illness and chronic fatigue syndrome. PLoS One 2014; 9 (1): e84838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Steele L. Prevalence and patternsof Gulf War illness in Kansas Veterans: association of symptoms with characteristics of person, place and time of military service. Am J Epidemiol 2000; 152: 992–1002. [DOI] [PubMed] [Google Scholar]
- [25].Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 1998;280: 981–988. [DOI] [PubMed] [Google Scholar]
- [26].Coughlin SS. A Neuroimmune Model of Gulf War Illness. J Environ Health Sci. 2017; 3: 10.15436/2378-6841.17.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP. Corticosterone primes the neuroinflammatory response to Gulf War illness‐relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 2017; 142: 444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koo BB, Michalovicz LT, Calderazzo S, Kelly KA, Sullivan K, Killiany RJ, et al. Corticosterone potentiates DFP‐induced neuroinflammation and affects high‐order diffusion imaging in a rat model of Gulf War Illness. Brain Behav. Immun 2018. January; 67: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, et al. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One. 2013. June 14;8(6):e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang XM, Walitt B, Saligan L, Tiwari AFY, Cheung CW, Zhang ZJ. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 2015; 72: 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaiser j, Bledowski C, Dietrich J. Neural correlates of chemotherapy‐related cognitive impairment. Cortex 2014;54: 33–50. [DOI] [PubMed] [Google Scholar]
- [32].Button KS, Loannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–376. [DOI] [PubMed] [Google Scholar]
- [33].Lacourt TE, Heijnen CJ. Mechanisms of neurotoxic symptoms as a result of breast cancer and its treatment: Considerations on the contribution of stress, inflammation, and cellular bioenergetics. Curr Breast Cancer Rep. 2017;9:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]