Abstract
Stress is the exacerbating factor of itch across patients with chronic itch due to different origins. However, the precise mechanisms behind stress-induced exacerbation of itch remain unknown. Chronic stress induces hyperexcitability of the amygdala, the center of emotional processing. Recent findings on the itch neuronal pathways support a pivotal role of the amygdala for itch processing. We hypothesized that itch is enhanced by stress through hyperexcitation of the amygdala. Modulation of amygdala activity, therefore, may have therapeutic potential in the treatment of chronic itch.
Keywords: chronic itch, amygdala, scratching, chronic stress, anxiety
Background
Stress can be categorized into two different types: acute and chronic stress. Joshua Smyth and colleagues mentioned that short duration and the return to homeostasis as two essential features of acute stressors. In contrast, they defined chronic stress as (1) repeated activations, (2) low or slow adaptation, and (3) delayed or failure to return to homeostasis 1. Studies examining the effect of acute stress on itch have produced inconsistent findings. Viewing a standardized series of stressful images increased itch severity in patients with prurigo nodularis and lichen simplex chronicus2. Healthy subjects also reported higher itch from histamine iontophoresis when negative emotions were induced with violent film fragments compared to when positive emotions were induced with comedic film fragments3. In contrast, patients with atopic dermatitis reported lower itch scores after the Trier Social Stress Test, an established method to produce acute stress4. In another study, the Trier Social Stress Test did not change itch intensity and desire to scratch in patients with chronic itch5. In a rodent study, when rats were subjected to acute forced swim stress, they displayed a reduced scratching response to serotonin6. Divergent effects may depend on the type of stress and individual differences of its sensitivity. Certain types of acute stress may be capable of activating descending itch inhibitory pathways (e.g., the analog of stress-induced analgesia).
It is difficult to test the effects of chronic stress on itch in humans due to ethical reasons. However, significant correlations between itch and perceived stress were reported in the patients with atopic dermatitis 7,8. In rodent studies, it has been consistently reported that chronic stress enhances itch. When subjected to four weeks of water avoidance stress, NC/Nga mice in specific pathogen-free housing developed intense scratching and dermatitis9. In allergic contact dermatitis model mice, chronic social isolation stress led to an increase in scratching behavior and idiopathic dermatitis, appearing in areas distinct from the contact dermatitis site10. Mice subjected to 10 days of water avoidance stress displayed increased scratching after injection of compound 48/80, a mast cell degranulator11. A 9-day heterotypic chronic intermittent stress protocol, which included cold-restraint stress, water avoidance stress, and forced swim stress, led to increased scratching (but not pain-related behavior) after serotonin injection in rats12. Another animal study found that a four-week chronic unpredicted mild stress (CUMS) protocol, which induced depression-like behavior in mice, led to increased scratching after injection of histamine or chloroquine, and an increase in spontaneous scratching in allergic contact dermatitis model mice13. Interestingly, CUMS itself led to a slight increase in spontaneous scratching without any itch induction. In spite of those findings supporting the crucial role of stress in itch, the central mechanisms underlying interactions between stress and itch remain unclear.
Premises
While many chronic itch patients report that psychological stress is a factor that aggravates their itch, the mechanisms underlying stress-induced exacerbation of itch are largely unknown. Although stressor is known to increase cortisol level, there is no correlation between itch intensity and cortisol level in the patient with atopic dermatitis 7. There is likely cortisol-independent mechanism behind stress-induced exacerbation of itch. Stress has been reported as an aggravation factor of itch in a wide variety of pruritic conditions including dermatological or systemic diseases14, suggesting that the final common pathway for itch processing (brain) is involved in stress-induced exacerbation of itch.
Amygdala consists of the central nucleus of the amygdala (CeA) and basolateral amygdala (BLA), including the lateral (LA) and basal (BA) nuclei, and is involved in the emotional process. Recent findings suggest a role for the amygdala in the processing of itch (Fig. 1). Itch signals are transmitted from sensory neurons to projection neurons in the spinal cord and then sent to the brain through two major pathways: the spinothalamic pathway and the spinoparabrachial pathway 15–17. Recent genetic studies highlight the substantial role of latter pathway in itch 18,19. Genetic silencing of CGRP-expressing neurons or genetic depletion of Vglut2 in the parabrachial nucleus decreased pruritogen-evoked scratching. Interestingly, neuronal tracing studies reveal that the parabrachial nucleus neurons send axon projections to the CeA 20,21. It was also reported that the amygdala was activated in the subjects received pruritic stimuli 22,23 as well as rodent models of chronic and contagious itch 24,25. Moreover, we have recently reported that optogenetic activation of a subpopulation of amygdala neurons increased pruritogen-evoked scratching.26
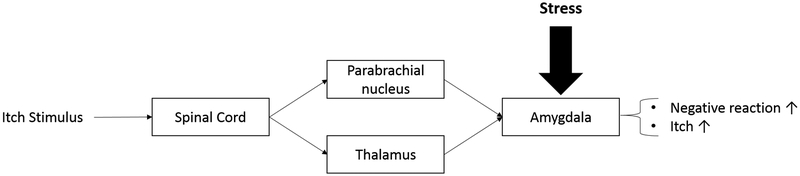
Fig. 1. Schematic diagram of a neuronal pathway that illustrates the role of the amygdala in stress-induced exacerbation of itch.
The figure shows the potential pathway through which itch signals can be projected into the amygdala. Itch signals are processed through the spinothalamic pathways or spinoparabrachial pathway before being projected to the amygdala. When there is additional stress present in the environment this also acts upon the amygdala which causes an increase in negative reaction, anxiety, and itch.
Chronic stress (social isolation stress) induces hyperexcitability of BLA principal neurons and increases anxiety-like behaviors in rats 27. Reduced expression of small-conductance Calcium-activated potassium channels accounts for hyperexcitability of BLA. Chronic restraint stress also induces hyperexcitability of LA and increases anxiety-like behaviors in rats that are sensitive to stress 28. As in rodent studies, chronic stress leads to anxiety in human 29. Human imaging studies have found amygdalar hyperactivation in subjects with high anxiety 30–33. Anxiety level was correlated with itch in the patients with atopic dermatitis 34,35. It is plausible to assume that chronic stress induces amygdala hyperactivation which contributes to the enhancement of itch.
Hypothesis
We hypothesized that stress aggravates itch through amygdala activation.
How to test the hypothesis
This hypothesis can be tested by investigating the role of the amygdala in increased spontaneous scratching by chronic stress in the chronic itch mouse model. Amygdala activities can be regulated using optogenetic, chemogenetic, or pharmacological manipulations. If the amygdala is involved in stress-evoked enhancement of itch, inhibition of amygdala activity should result in a decrease in enhanced spontaneous scratching.
Relevance and Perspectives
If the role of the amygdala in stress-induced exacerbation of itch is confirmed, this will open up avenues for understanding its molecular and cellular mechanisms to develop effective therapeutic strategies to treat chronic itch. Moreover, it would be worthwhile to identify the neuronal circuit of itch processing in the brain (e.g., Medial Prefrontal Cortex and Medial Cingulate Cortex)26 and to test how chronic stress alters this circuit. Dysregulated brain circuit may be fine-tuned by pharmacological treatments, psychological interventions, or therapeutic devices.
Acknowledgments
This project was supported by grants from the National Institutes of Health (R00AR063228 and R01AR074062) to TA.
Footnotes
Conflict of Interest
The authors have declared no competing interests.
References
- 1.Smyth J, Zawadzki M, Gerin W. Stress and Disease: A Structural and Functional Analysis. Social and Personality Psychology Compass. 2013;7(4):217–227. [Google Scholar]
- 2.Kim HJ, Park JB, Lee JH, Kim IH. How stress triggers itch: a preliminary study of the mechanism of stress-induced pruritus using fMRI. Int J Dermatol. 2016;55(4):434–442. [DOI] [PubMed] [Google Scholar]
- 3.van Laarhoven AI, Walker AL, Wilder-Smith OH, et al. Role of induced negative and positive emotions in sensitivity to itch and pain in women. Br J Dermatol. 2012;167(2):262–269. [DOI] [PubMed] [Google Scholar]
- 4.Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Schneider G, Stumpf A, Burgmer M, Broecker P, Volmering L, Stander S. Are patients with chronic pruritus more susceptible to social stress than healthy controls? An experimental case-control study. Br J Dermatol. 2018;179(5):1174–1176. [DOI] [PubMed] [Google Scholar]
- 6.Spradley JM, Davoodi A, Carstens MI, Carstens E. Effects of acute stressors on itch- and pain-related behaviors in rats. Pain. 2012;153(9):1890–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schut C, Weik U, Tews N, Gieler U, Deinzer R, Kupfer J. Coping as mediator of the relationship between stress and itch in patients with atopic dermatitis: a regression and mediation analysis. Exp Dermatol. 2015;24(2):148–150. [DOI] [PubMed] [Google Scholar]
- 8.Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27(2):e239–242. [DOI] [PubMed] [Google Scholar]
- 9.Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33(3):566–573. [DOI] [PubMed] [Google Scholar]
- 10.Kitagaki H, Hiyama H, Kitazawa T, Shiohara T. Psychological stress with long-standing allergic dermatitis causes psychodermatological conditions in mice. J Invest Dermatol. 2014;134(6):1561–1569. [DOI] [PubMed] [Google Scholar]
- 11.Zhao P, Hiramoto T, Asano Y, Kubo C, Sudo N. Chronic psychological stress exaggerates the compound 48/80-induced scratching behavior of mice. Pharmacol Biochem Behav. 2013;105:173–176. [DOI] [PubMed] [Google Scholar]
- 12.Peng XY, Huang Y, Wang XL, et al. Adrenergic beta2-receptor mediates itch hypersensitivity following heterotypic chronic stress in rats. Neuroreport. 2015;26(17):1003–1010. [DOI] [PubMed] [Google Scholar]
- 13.Wang X-D, Yang G, Bai Y, Feng Y-P, Li H. The behavioral study on the interactive aggravation between pruritus and depression. Brain and behavior. 2018;8(6):e00964–e00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. 2018;87:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson S, Zhang X, Khasabov SG, et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108(6):1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama T, Curtis E, Nguyen T, Carstens MI, Carstens E. Anatomical evidence of pruriceptive trigeminothalamic and trigeminoparabrachial projection neurons in mice. J Comp Neurol. 2016;524(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen NA, Giesler GJ Jr. Response characteristics of pruriceptive and nociceptive trigeminoparabrachial tract neurons in the rat. J Neurophysiol. 2015;113(1):58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature. 2018;555(7698):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu D, Deng J, Liu KF, et al. A central neural circuit for itch sensation. Science. 2017;357(6352):695–699. [DOI] [PubMed] [Google Scholar]
- 20.Sarhan M, Freund-Mercier MJ, Veinante P. Branching patterns of parabrachial neurons projecting to the central extended amgydala: single axonal reconstructions. J Comp Neurol. 2005;491(4):418–442. [DOI] [PubMed] [Google Scholar]
- 21.Lu YC, Chen YZ, Wei YY, et al. Neurochemical properties of the synapses between the parabrachial nucleus-derived CGRP-positive axonal terminals and the GABAergic neurons in the lateral capsular division of central nucleus of amygdala. Mol Neurobiol. 2015;51(1):105–118. [DOI] [PubMed] [Google Scholar]
- 22.Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. NeuroImage. 2012;59(4):3611–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vierow V, Forster C, Vogelgsang R, Dorfler A, Handwerker HO. Cerebral Networks Linked to Itch-related Sensations Induced by Histamine and Capsaicin. Acta Derm Venereol. 2015;95(6):645–652. [DOI] [PubMed] [Google Scholar]
- 24.Yu YQ, Barry DM, Hao Y, Liu XT, Chen ZF. Molecular and neural basis of contagious itch behavior in mice. Science. 2017;355(6329):1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong KY, Kang JH. Investigation of the pruritus-induced functional activity in the rat brain using manganese-enhanced MRI. J Magn Reson Imaging. 2015;42(3):709–716. [DOI] [PubMed] [Google Scholar]
- 26.Sanders KM, Sakai K, Henry TD, Hashimoto T, Akiyama T. A Subpopulation of Amygdala Neurons Mediates the Affective Component of Itch. J Neurosci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. J Neurosci. 2015;35(26):9730–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetzel A, Rosenkranz JA. Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology. 2014;39(9):2114–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S, Khan A. Chronic Stress Leads to Anxiety and Depression. Ann Psychiatry Ment Health. 2017;5(1)(1091):1–4. [Google Scholar]
- 30.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. [DOI] [PubMed] [Google Scholar]
- 31.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American journal of psychiatry. 2007;164(10):1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25(6):496–505. [DOI] [PubMed] [Google Scholar]
- 33.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasul A, El-Nour H, Lonne-Rahm SB, et al. Serotonergic Markers in Atopic Dermatitis. Acta Derm Venereol. 2016;96(6):732–736. [DOI] [PubMed] [Google Scholar]
- 35.Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90(6):582–588. [DOI] [PubMed] [Google Scholar]