Abstract
Background and Purpose:
Pain is common among older adults with dementia. There are nonpharmacological options for managing pain in this population. However, the effects of physical therapist-delivered interventions have not been summarized. The purpose of this systematic review was to summarize the literature on physical therapist-delivered interventions in randomized trials for reducing pain among older adults with dementia.
Methods:
A systematic search of MEDLINE/PubMed, CINAHL, PsycINFO, and Web of Science was conducted for randomized trials of pain management in individuals aged 60 years or older with medically diagnosed dementia of any severity. Included studies addressed the effects of nonpharmacological physical therapist-delivered interventions on pain outcomes. Pain outcomes included patient or caregiver self-report, observational or interactive measures. Independent reviewers extracted relevant data and assessed methodological quality using the PEDro scale.
Results and Discussion:
Three studies (total N = 222 participants; mean age range = 82.2–84.0 years; 178 [80.2%] females) met inclusion criteria. PEDro scores ranged from 4–8/10. Interventions included passive movement and massage. Pain outcomes included the observational measures Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC), Pain in Advanced Dementia (PAINAD), and Doloplus-2 scale. Passive movement did not show better results when compared to no treatment, while massage showed pain-reducing effects in one study compared to no treatment.
Conclusions:
The evidence supporting pain-reducing physical therapy interventions for patients with dementia is limited. There is a clear gap in knowledge related to evidence-based physical therapy for managing pain in this population. Future studies should examine active physical therapist-delivered interventions and utilize interactive pain measures.
Keywords: cognition disorders, dementia, exercise, pain, massage, physical therapy modalities
INTRODUCTION
Dementia is a chronic debilitating disorder that affects an estimated 46.8 million people worldwide.1 The number of individuals with dementia is expected to rise to over 130 million people by the year 2050.1 In the United States, approximately 5 million individuals are diagnosed with dementia.2,3 Most individuals are community-dwelling, with nearly 20% of people with dementia living in either a residential care or nursing home setting.4 Dementia is associated with a range of deleterious effects including declines in neurocognitive abilities, behavioral changes, memory loss, and progression to functional dependency. Common behavioral symptoms of individuals with dementia include aggression, agitation, depression, and anxiety.5
Pain is also a predominant comorbid symptom in dementia. Prevalence estimates for pain among individuals with dementia vary considerably. Among community-dwelling individuals with dementia, approximately 23% to 57% report pain during an average day.6,7 Estimates suggest between 50% and 80% of nursing home residents with dementia or dementia-associated disorders report pain or exhibit pain behaviors.8–11 Moreover, moderate-to-severe pain has been reported by 21% of nursing home residents with dementia.12 Distinguishing type of pain can be difficult and may not be clearly differentiated (i.e., mixed pain type).12,13 However, the determination of predominant type of pain has implications for pain management, especially in directing the choice of analgesic medication.14 Van Kooten et al.12 have reported that the predominant type of pain in a sample of nursing home residents was “nociceptive pain,” with very few patients experiencing “neuropathic pain.” Additionally, the pain experienced in these patients is often associated musculoskeletal conditions like arthritis, osteoporosis, or fractures.15
Managing pain in the context of dementia is challenging, partly due to difficulty in recognizing pain amidst a complex clinical presentation.16–19 Pain in dementia can negatively influence mood, engagement with physical or recreational activity, and sleep.19 Shega et al.20 have highlighted consequences that can occur in the instance when pain is unrelieved in persons with dementia. These impacts include physical consequences such as mobility disability and increased levels of agitation and physical combativeness.20 Psychosocial consequences can include mood disturbances (i.e., depression), social isolation, and a loss of enjoyment in life.20 These consequences overlap with common behavioral symptoms of dementia and may contribute to pain that is under-detected and undertreated.
Due to the high prevalence and high-impact of pain among patients with dementia, the impetus for researchers is to identify effective nonpharmacological treatment approaches for physical therapists who treat this population. Nonpharmacological treatments can be delivered in combination with pharmacological management within a multidisciplinary approach or serve as an alternative modality. Polypharmacy and the overuse of certain medications that may have severe adverse events are two reasons for the need to identify alternative nonpharmacological options.21 Additionally, physicians may not prescribe pain medication due to concerns for side effects and limited evidence on pharmacodynamics of analgesics in patients with dementia.22 Outside of physical therapist-delivered interventions, examples of nonpharmacological interventions identified in systematic reviews of pain management in dementia include reflexology, Reiki, and rocking chair therapy.23,24 Reflexology is effective for reducing pain in one study, while Reiki and rocking chair therapy yielded small or limited pain improvement. Therapeutic touch also has potential for pain-reducing effects.24 No systematic reviews have examined studies with physical therapist-delivered interventions for pain in older adults with dementia.
Though not investigated among patients with dementia, physical therapist-delivered interventions are purported to modulate pain among older adults.25–27 For older adults with back pain, Rundell et al.28 found that active forms of physical therapy, were associated with greater improvements in pain intensity than passive intervention modes. Effect of physical therapist-delivered interventions on pain modulation in individuals with dementia has not been established. Thus, the purpose of this systematic review was to summarize the literature on physical therapist-delivered interventions in randomized trials for reducing pain among older adults with dementia. Results of this review could inform clinicians on effective treatments to embed within an individualized plan of care and guide future research by identifying knowledge gaps.
METHODS
This systematic review was performed following guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).29,30 Ethical approval was not needed for conducting this review.
Eligibility Criteria
Criteria for inclusion in this systematic review was based on study design, population, intervention, and outcome (Table 1). Limits were placed for peer-reviewed articles published in English. Randomized trial criteria included parallel group or crossover design that allowed an examination of comparative effects. Types of studies excluded were non-randomized designs including prospective cohort and retrospective studies, and descriptive studies including case series and single case designs. The population was older adults (e.g., 60 years of age or older) with a medical diagnosis of dementia. No limit was placed on subtype of dementia or dementia severity. Studies were included if they compared the effect of a physical therapist-delivered intervention, in isolation or as part of a multimodal approach, to a comparison group that did not include the studied intervention. Physical therapist-delivered interventions include a broad range of techniques for addressing pain that include manual therapy, thermal or electrical modalities, and various forms of exercise. No restriction was placed on type of comparison group. Comparison groups could include no treatment, usual care, or another form of active intervention so long as the effect of a specific physical therapist-delivered intervention could be determined. Studies were eligible if they included an outcome measure that specifically assessed pain intensity or pain behavior. Pain measures could include patient or caregiver self-report, observational, and interactive measures. No restriction was placed on follow-up duration for measuring outcomes after intervention completion.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Study Design | |
|
|
| Population | |
|
|
| Intervention | |
|
|
| Outcome | |
|
|
| Limits | |
|
|
Data Sources and Study Search
A systematic search was conducted for peer-reviewed articles in MEDLINE/PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycInfo, and Web of Science from each database’s inception to May 2, 2018 (see Appendix for database search strategy). A search protocol using Medical Subject Heading (MeSH) or Major Concept terms and keywords was developed prior to conducting the search. Search terms were based on eligibility criteria and included key terms such as “dementia,” “cognitive disease/disorder,” “Alzheimer,” “delirium,” “Lewy Body disease,” “physical therapy,” “exercise,” “massage,” “musculoskeletal manipulations,” “manual therapy,” “electrical stimulation,” “walking,” “strengthening,” “stretching,” “pain,” and “pain perception.” Reference lists of relevant articles were also searched.
Study Selection
Articles found from each of the database searches were imported into EndNote X7 bibliographic software (Thomson Reuters, New York, NY) for removing duplicates and title and abstract screening. Title and abstracts were independently screened by a group of authors (H.E.A., J.L.A., R.G.C., V.A.E.) and excluded based on eligibility criteria. Screening authors were trained by the primary author (R.A.C.) during a single 1-hour session that included instructions and practice of a set of sample articles. Each author reviewed the title and abstract of each article. The final list of titles identified by each author was compared and discussed for consensus agreement. A final composite list of potentially relevant articles was compiled for full-text review. The full-texts of each article were examined and articles not meeting criteria were excluded. In cases where a consensus could not be met for inclusion, a final reviewer (R.A.C.) was brought in to resolve disagreement and facilitate consensus.
Methodological Quality
The Physiotherapy Evidence Database (PEDro) scale was used to determine the methodological quality of each study.31–33 The PEDro scale is an 11-item scale used for rating studies based on quality criteria such as random and concealed allocation, baseline similarity of groups, masked evaluation, and aspects of outcome and data assessment. Each item, except for item 1, is summed to obtain total score. PEDro score greater than 5 has been considered indicative of moderate to high methodological quality.34 Two groups of reviewers (H.E.A., V.A.E. and J.L.A., R.G.C.) independently scored each included article. Agreement in scores between the two groups was excellent (ICC = 0.98 [95% CI = 0.90; 1.00]. Any discrepancies in scoring were resolved by consensus and in consultation with a final arbiter (R.A.C.).
Data Extraction and Analysis
A standardized data extraction spreadsheet was used to obtain relevant article data. Two independent authors (H.E.A. and R.A.C.) extracted data. Accuracy of data extraction was confirmed through comparison of spreadsheets and discussion. Discrepancies in data extraction were resolved through consensus. No reliability assessment was performed for data extraction. Extracted data included sample characteristics, intervention details, pain outcome, time points, and statistical values. For statistical data, pain values at baseline and follow-up, and effect estimates (e.g., mean change scores) were extracted. Descriptive summary of findings was performed. Due to limited number of studies and heterogeneity, meta-analysis could not be conducted.
RESULTS
Search Results
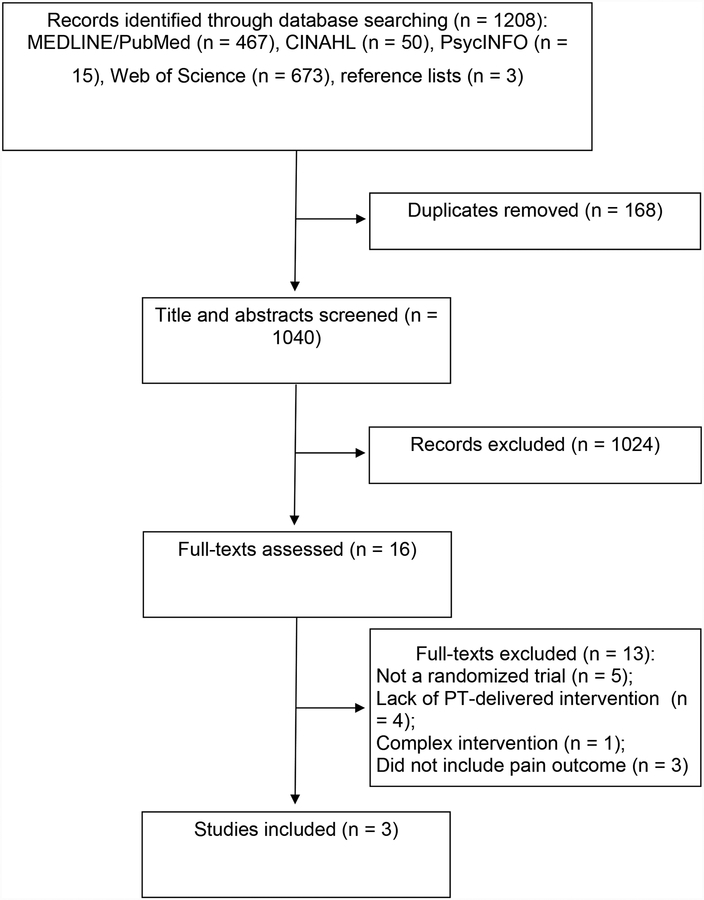
The systematic search identified a total of 1208 articles (Figure 1). After removing duplicates, the title and abstract of 1040 unique articles were screened. Of these, 1024 articles were excluded. Example reasons for exclusion at this stage were titles or abstracts unrelated to dementia, non-randomized study designs including reviews, cases and cohort studies, and interventions unrelated to physical therapy such as medication. Sixteen full-text articles were evaluated for inclusion. Thirteen articles were excluded based primarily on non-randomized design (n = 5),35–39 lack of physical therapist-delivered intervention (n = 3),40–43 complex intervention (n = 1),44 or not including a pain outcome (n = 3).45–47 Three articles met inclusion criteria.48–50
Figure 1.
Flow Diagram of Systematic Review Process.
Study Characteristics
Participants
A total of 222 participants were included in the three trials (range of sample size = 10 to 111) (Table 2). The mean age of participants in two studies ranged from 82.2 to 84.0 years,48,49 while the third study did not report a mean or median age for the sample (range of 67 to 91 years).50 Majority of participants were female (n = 178 (80.2%)). All three studies required participants to have with a diagnosis of dementia, with two studies specifying Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria.48,50 Participants in the studies were living in a nursing or residential care facility. Two studies specified eligibility criteria for pain (e.g., history of chronic pain or minimum pain score)49,50 and one study focused on paratonia.48
Table 2.
Characteristics of Included Articles (N = 3).
| Characteristic | Hobbelen et al.48 | Kapoor and Orr49 | Rodríguez-Mansilla et al.50 |
|---|---|---|---|
| Eligibility criteria |
Inclusion criteria 1. Met DSM-IV-TR criteria for dementia 2. Diagnosed with paratonia 3. Moderate-to-severe paratonia defined as a MAS score of at least 2 in at least one limb Exclusion criteria 1. Prescribed antipsychotic medication 2. Received passive movement therapy within 4 weeks prior to enrollment 3. Has an unstable health problem or disease 4. Has signs of challenging behavior toward therapist or intervention |
Inclusion criteria 1. Age 60 years or older 2. Diagnosed with dementia or advanced dementia 3. History of chronic pain 4. Living in aged care facility for more than 3 months 5. Consent provided by an Enduring Power of Attorney to participate in research Exclusion criteria 1. Diagnosis of schizophrenia or other confounding mental illness 2. Possibility of facility transfer |
Inclusion criteria 1. Age 65 years or older 2. Institutionalized in a residential home 3. Diagnosed with dementia (DSM-VI criteria) for at least one year 4. Moderate-to-severe dementia based on MMSE score 5. Doloplus2 score (pain) over 5 6. Campbell Scale score (anxiety) over 1 7. Cornell Scale (depression) score over 7 Exclusion criteria 1. Medical contraindications to massage or ear acupressure |
| Sample | N = 101 (83 female) Mean (range) age = 84 (67–98) years Setting: Nursing home |
N = 10 (9 female) Mean (SD) age = 82.2 (7.1) years Setting: Residential aged care facility |
N = 111 (86 female) Mean (range) age = NR (67–91) years Setting: Residential home |
| Intervention Group | Passive movement therapy (PMT) (n = 47): 20 minutes of standardized PMT delivered by a trained physical therapist to upper and lower extremities. The PMT intervention was delivered 3 times per week for 4 weeks. |
Massage and standard PT (n = 5): 10 minutes of massage delivered by a physical therapist to a body part associated with chronic pain, in addition to routine pain treatment including range of motion, stretching, positioning, and other pharmacological or nonpharmacological interventions. Techniques could include effleurage, kneading, or trigger point therapy. The massage intervention was delivered 4 times per week for 4 weeks. | Massage (n = 35): 20 minutes of relaxation massage delivered by a physical therapist to the back and lower extremities. Techniques included superficial effleurage and deep kneading. The massage intervention was delivered 5 days per week for a 3-month period. |
| Comparison Group(s) | No treatment (n = 54) | Routine pain treatment (n = 5): Participants received routine pain treatment including range of motion, stretching, positioning, and other pharmacological or nonpharmacological interventions. The control intervention was delivered 4 times per week for 4 weeks. | Ear acupressure (n = 40): Ear acupressure was delivered by a qualified acupuncturist with the use of herbal seeds of vaccaria. Once placed, seeds were checked daily, and replaced every 15 days over the 3 months period. No treatment (n = 36) |
| Pain Outcome | PACSLAC-D |
PAINAD | Doloplus2 Scale |
| Other Outcomes | CGI (stiffness); MAS (paratonia); PSC (daily care) | None | Campbell Scale (anxiety); Cornell Scale (depression) |
| Time Points | Baseline and 2 and 4 weeks after start of intervention | Baseline and 4 weeks after start of intervention | Baseline and monthly up to 5 months after start of intervention |
Abbreviations: CGI = Clinical Global Impressions Scale; MAS = Modified Ashworth Scale; MMSE = Mini-Mental State Examination; NR = not reported; PACSLAC-D = Pain Assessment Checklist for Seniors with Limited Ability to Communicate; PAINAD = Pain in Advanced Dementia; PSC = Patient Specific Complaint list
Physical therapist-delivered interventions
The physical therapist-delivered interventions included passive movement (one study)48 and massage (two studies).49,50 For passive movement, physical therapists moved the participant’s extremity in a slow manner to reduce muscle resistance, guided by pain response. For example, if participants expressed painful behavior, the therapist reduced the amount of passive tension. All extremities were moved passively, beginning with the left arm and ending with right leg. Sessions were 20 minutes in duration, occurring three times per week for four weeks. Physical therapists were trained to perform the passive movement over two 2-hour training sessions. In two studies, massage therapy was provided for relaxation50 or to address a specific source of chronic pain.49 In the study by Rodriguez-Mansilla et al.,50 relaxation massage was directed to the participant’s back and lower extremities with superficial effleurage and deep kneading. No specifications were provided on how depth or rhythm of massage was determined or adjusted during sessions. For this form of massage, the physical therapist provided 20-minute sessions, five days per week for a 3-month period. In the study by Kapoor and Orr,49 massage was directed to a source of chronic pain and included effleurage, kneading, and trigger point therapy. The optimal delivery of massage involved slow and regular rate and rhythm, and depth guided by tissue characteristics and pain response. The physical therapist provided 10-minute sessions, four times per week for four weeks. Neither study indicated whether therapists were trained in the massage intervention.
Comparison groups
One comparison group existed in two studies, and two comparison groups existed in one study. The comparison group included no treatment (two studies)48,50 or another active comparator (two studies)49,50 including ear acupressure and routine pain treatment. Routine pain treatment could include physical interventions such as range of motion exercise, stretching, positioning and other nonpharmacological or pharmacological interventions. In the study by Kapoor and Orr,49 routine pain treatment was provided in both the massage therapy group and as control. Thus, the effects of massage therapy are in addition to routine pain treatment.
Pain outcomes
Pain outcomes included the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC-D),48 Pain in Advanced Dementia (PAINAD),49 and Doloplus 2 Scale.50 In two studies, pain outcomes were assessed at interim periods during the intervention period.48 All studies measured pain outcomes immediately after the intervention period was completed. One study measured follow-up pain outcomes at four and eight weeks after ending the intervention.50 For this review, only post-intervention outcome data is reported.
Methodological Quality
Two of the three studies were considered moderate to high methodological quality based on PEDro scores of 5 or greater (Table 3). All studies met criteria for random allocation, groups similar at baseline, between-group statistical comparisons, and inclusion of point and variability estimates. None of the studies met criteria for blinding of subjects or therapists.
Table 3.
Methodological Quality of Articles based on PEDro Scale.
| Article | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Score* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hobbelen et al.48 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Kapoor and Orr49 | Yes | No | Yes | No | No | No | No | No | Yes | Yes | 4 |
| Rodríguez-Mansilla et al.50 | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
PEDro scoring components: 1 = subjects were randomly allocated to groups; 2 = allocation was concealed; 3 = groups were similar at baseline regarding the most important prognostic indicators; 4 = blinding of all subjects; 5 = blinding of all therapists who administered therapy; 6 = blinding of all assessors who measured at least one outcome; 7 = measures of at least one key outcome were obtained from more than 85% of subjects initially allocated to groups; 8 = all subjects for whom outcome measures were available received the treatment or control condition as allocated, or where this was not the case, data for at least one key outcome was analyzed by “intention to treat”; 9 = results of between-group statistical comparisons are reported for at least one key outcome; 10 = study provides both point measures and measures of variability for at least one key outcome.
PEDro scores of 5 or greater are considered high methodological quality.
Intervention Effects on Pain
The summary effects of physical therapist-delivered interventions on pain are described in Table 4. In the study by Hobbelen et al.,48 passive movement did not result in a greater effect on pain compared to no treatment. Rodriguez-Mansilla et al.50 found massage was more effective in reducing pain compared to no treatment, but not compared to ear acupressure. Similarly, in the study by Kapoor and Orr,49 massage did not offer an additive benefit to routine pain treatment.
Table 4.
Summary of Individual Article Effects on Pain after Intervention Completion.
| Characteristic | Hobbelen et al.48 | Kapoor and Orr49 | Rodríguez-Mansilla et al.50 |
|---|---|---|---|
| Baseline Pain |
Pre-intervention PMT: 4.0 (3.5) No treatment: 4.7 (4.1) |
Pre-intervention Massage + Exercise: 6.2 (1.9) Exercise: 6.0 (1.8) |
Pre-intervention Massage: 22.7 (6.4) Ear acupressure: 19.0 (5.1) No treatment: 21.4 (2.7) |
| Follow-up Pain* |
End of intervention at 4 weeks PMT: 3.7 (2.9) No treatment: 3.8 (3.3) |
End of intervention at 4 weeks Massage + Routine treatment: 5.2 (3.5) Routine treatment: 5.0 (3.4) |
End of intervention at 12 weeks Massage: 16.8 (4.8) Ear acupressure: 10.5 (5.5) No treatment: 25.2 (2.4) At 16 weeks (4 weeks after end of intervention) Massage: 19.4 (5.5) Ear acupressure:13.6 (4.8) No treatment: 25.9 (2.5) At 20 weeks (8 weeks after end of intervention) Massage: 21.8 (6.0) Ear acupressure: 18.0 (4.9) No treatment: 26.5 (2.3) |
| Mean Change in Pain |
End of intervention at 4 weeks PMT: −0.4 (2.4) No treatment: −0.8 (2.5) |
End of intervention at 4 weeks Massage + Routine treatment: −1.2 (1.8) Routine treatment: −0.8 (2.2) |
End of intervention at 12 weeks Massage: −5.9 (3.6) Ear acupressure: −8.6 (4.4) No treatment: 3.8 (1.4) At 16 weeks (4 weeks after end of intervention) Massage: −3.3 (3.2) Ear acupressure: −5.4 (3.6) No treatment: 4.6 (1.3) At 20 weeks (8 weeks after end of intervention) Massage: −0.9 (2.4) Ear acupressure: −1.0 (2.2) No treatment: 5.2 (1.5) |
| Summary of Results | Pain outcome: No significant difference between groups in change in pain score at the 4 week time point (p > 0.05). | Pain outcome: No significant difference between groups in change in pain score at the 4 week time point (p = 0.93). | Pain outcome: Across time points, the no treatment control group showed deterioration in pain scores, in contrast to significant pain reductions in massage and ear acupressure. Ear acupressure had significantly greater change in pain than massage at 12 (p = 0.003) and 16 weeks (p = 0.008). |
Abbreviations: PMT = passive movement therapy
All values are mean (SD) unless otherwise specified.
Follow-up time points represented are after intervention period was complete
DISCUSSION
This systematic review identified three randomized trials that examined the direct effect of a physical therapist-delivered intervention on pain in older adults with dementia. Interventions included passive movement and massage. Only one study showed a comparative benefit from a physical therapist-delivered intervention, where massage had greater pain-reducing effect than no treatment but was less effective than ear acupressure. To date, no randomized trials have examined active forms of physical therapist-delivered interventions such as therapeutic exercise or functional training. These results highlight an urgent need to develop evidence on the effectiveness of physical therapist-delivered interventions for addressing pain in this population.
In randomized trials, physical therapist-delivered passive interventions including passive movement and massage does not appear to result in consistent pain-reducing effects either in isolation or as an additional modality to routine pain treatment. In a non-randomized, single-group pre-post-test study, Ellis et al.51 examined the effects of a pragmatic 8-week physical therapist-delivered intervention program that could include massage and transcutaneous electrical nerve stimulation (TENS) on pain ratings in 95 participants (56% with dementia) living in a residential care facility. Reductions in pain were observed across the entire 8-week period. Specific interventions varied within sessions: massage was delivered most often in isolation, followed by TENS alone, massage with exercise, and finally massage with TENS. The combination of massage with TENS had the largest within-session effect compared to massage or TENS in isolation or in combination with exercise.51 When accounting for dementia, the effects of the interventions were not as large compared to those without dementia.51 In the current review, we did not find any randomized trials for TENS in patients with dementia, which could be an area of further study.
While the current review did not find consistent evidence from randomized trials for pain-relieving effects on massage, preliminary evidence shows that massage has positive effects on factors such as stress and agitation,24,52,53 which could be linked to pain in dementia. One moderate quality prospective, non-randomized study found massage was associated with reduced agitation levels in 52 older adults with dementia.52 Non-randomized design was a barrier to determining a causal effect. Wu et al.53 examined 11 studies, including 10 randomized trials, that included various forms of therapeutic touch procedures including massage, reflexology, and acupressure. Meta-analysis results showed a statistically significant effect following these touch procedures on symptoms including physical and verbal aggressive and non-aggressive behaviors.53 The main limitation of these studies were small sample sizes and generally low methodological quality. Pain was not an outcome in this meta-analysis.53
Few studies have examined active interventions such as exercise or functional training for potential pain-reducing effects in dementia.42,51 In older adults with dementia, physical inactivity and pain may be part of a vicious cycle,54 thus warranting strategies to promote activity and exercise in physical therapy interventions. Plooij et al.54 analyzed the link between pain and physical inactivity and showed a positive relationship in older adults with and without dementia. In a study among participants with subtle to moderate cognitive impairment with or without dementia, 20 weeks of Tai Chi delivered by a certified instructor showed greater effects on pain compared to classes providing health and cultural information.42 The level of cognitive impairment as determined by the Mini-Mental State Examination did not influence the main effects following Tai Chi.42
Active exercise can have widespread health benefits for individuals with dementia. Structured exercise and physical activity can result in not only a reduction of brain atrophy and neurodegeneration, but also improvement in cognitive functioning.55 A randomized trial on effects of exercise on apathy and agitation in individuals with dementia compared 12 weeks of physical therapist-delivered lower extremity strengthening and balance exercise to light physical activity or other pleasure activities.56,57 The exercise group showed reduced apathy and agitation, while the control group showed increased levels of apathy and agitation.56,57 Aman et al.58 showed that 15 minutes of aerobic and 15 minutes of strengthening and balance exercise can significantly decrease agitation levels among individuals with cognitive impairment. Brett et al.59 examined randomized trials that tested effects of physical exercise on various outcomes including depression, agitation, and unmet needs and found statistically significant improvements after physical exercise. Abd El-Kader and Al-Jiffri60 found 2 months of physical therapist-delivered aerobic (treadmill) and stretching exercise provided 3 times per week for patients with Alzheimer’s disease resulted in significant improvements in psychological functioning (depression, self-esteem, mood) and quality of life, and reductions in inflammatory markers. Similar effects were not seen in patients receiving a no intervention control.60 There is a clear need to examine the pain-modulating effect of exercise in patients with dementia.
Pain assessment among individuals with dementia is difficult due to communication barriers. For example, individuals with dementia may have an inability to properly communicate or experience confusion, agitation, or irritability, which may impact the reliability of pain reporting.61 Pain measures appropriate for individuals with dementia may rely on pain behavior as well as self-report. Observational signs of pain including breathing, facial expressions, body language, and physiological indicators.61–63 However, there is discrepancy in how well these measures can detect pain.64 The MOBID-2 is a responsive and reliable pain assessment scale specifically suited for individuals with advanced dementia.63,65 This tool is an interactive hybrid instrument where the first part categorizes pain behavior (e.g., noises, grimaces) during movement and activities of daily living (e.g., stretching, bedside transitioning); while the second part quantifies pain intensity for pain behavior based on the health professional’s perspective. None of the included studies in the current review examined physical therapist-delivered interventions using the MOBID-2 as an outcome measure.
Limitations
This review followed PRISMA guidelines and used a comprehensive search strategy of four databases to identify articles. Despite this approach, we are unable to confirm that pertinent peer-reviewed articles were not missed. Accuracy of data extraction and reporting was confirmed through group discussion and consensus after independent review. However, reliability of these processes was not assessed. An additional limitation of this review is that few randomized trials exist that looked specifically at physical therapist-delivered interventions for pain in individuals with dementia. None of the trials found in this review involved participants living in the community, which is surprising given the higher number of individuals with dementia living in community settings. The findings of this review are limited in generalizing to this population. Future work should examine nonpharmacological physical therapist-directed interventions across clinical settings and include community-dwelling individuals with dementia. Physical therapists may also deliver pain management interventions as a component of multi-step and/or multimodal interdisciplinary team care.38 In this context, study of physical therapy intervention in isolation may not be pragmatic. Overall, the lack of trials limits definitive clinical recommendations on the usefulness of physical therapy interventions within an evidence-based approach. None of the included studies appeared to consider type of pain (i.e., nociceptive, nociplastic, or neuropathic)66 as a potential target for the studied interventions. Mechanism-based approaches to pain management have been advocated and there may be utility in discerning the predominant pain type for matching treatment.66 However, the first step will be to determine the capacity for identifying type of pain among older adults with dementia, given the propensity for communication restrictions.
CONCLUSIONS
Three studies have examined the direct effects of physical therapist-delivered interventions to address pain in older adults with dementia. Only passive interventions have been tested, with massage showing pain-reducing benefit in a single study compared to no treatment. The evidence supporting pain-reducing physical therapy interventions for individuals with dementia is limited. There is a clear gap in knowledge related to evidence-based physical therapy for managing pain in this population. Future studies should examine active physical therapist-delivered interventions and utilize interactive pain measures.
ACKNOWLEDGEMENTS
Dr. Coronado was affiliated with the University of Texas Medical Branch and supported by an NIH K12 Rehabilitation Research Career Development Program grant (HD055929) during the time this project was conducted.
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. At the time this manuscript was developed, Dr. Coronado was supported by an NIH K12 Rehabilitation Research Career Development Program grant (HD055929) at the University of Texas Medical Branch.
APPENDIX
Appendix Table 1.
CINAHL Search Results.
| Step | Search terms | Results |
|---|---|---|
| 1 | “MH” randomized controlled trial OR “MH” single-blind studies OR “MH” double-blind studies OR “MH” clinical trials OR randomized controlled trial OR controlled clinical trial OR clinical trials | 301450 |
| 2 | “MH” dementia OR “MH” delirium OR “MH” cognition disorders OR “MH” delirium, dementia, amnestic, cognitive disorders OR “MH” Alzheimer’s disease OR “MH” Lewy Body disease OR dementia OR cognitive disease OR cognitive disorder OR Alzheimer OR Delirium OR Lewy Body disease | 93813 |
| 3 | “MH” physical therapy OR “MH” exercise OR “MH” resistance training OR “MH” therapeutic exercise OR “MH” electric stimulation OR “MH” massage OR “MH” manual therapy OR “MH” walking OR “MH” physical activity OR “MH” muscle strengthening OR “MH” stretching OR nonpharmacological OR physical therapy OR physiotherapy OR activity OR exercise OR electrical stimulation OR massage OR manual therapy OR walking OR strengthening OR stretching | 489824 |
| 4 | “MH” pain OR pain | 241526 |
| 5 | 1 AND 2 AND 3 AND 4 | 50 |
Appendix Table 2.
PsycINFO Search Results.
| No | Search | Results |
|---|---|---|
| 1 | “randomized controlled trial” OR “controlled clinical trial” OR “clinical trial” | 41070 |
| 2 | dementia OR “cognitive disease” OR cognitive disorder OR Alzheimer OR delirium OR “Lewy Body disease” | 107947 |
| 3 | nonpharmacological OR “physical therapy” OR physiotherapy OR activity OR exercise OR “electrical stimulation” OR massage OR “manual therapy” OR walking OR strengthening OR stretching | 547939 |
| 4 | Pain | 102150 |
| 5 | 1 AND 2 AND 3 AND 4 | 15 |
Appendix Table 3.
MEDLINE/PubMed Search Results.
| No | Search | Results |
|---|---|---|
| 1 | randomized controlled trial [Mesh] OR random allocation [Mesh] OR single-blind method [Mesh] OR double-blind method [Mesh] OR randomized controlled trial OR controlled clinical trial OR clinical trial | 1174356 |
| 2 | dementia [Mesh] OR delirium [Mesh] OR cognition disorders [Mesh] OR Alzheimer disease [Mesh] OR Lewy Body disease [Mesh] OR dementia OR cognitive disease OR cognitive disorder OR Alzheimer OR delirium OR Lewy Body disease | 336624 |
| 3 | physical therapy modalities[Mesh]) OR exercise [Mesh] OR electric stimulation [Mesh] OR massage [Mesh] OR musculoskeletal manipulations [Mesh] OR walking [Mesh] OR resistance training [Mesh] OR muscle stretching exercises [Mesh] OR nonpharmacological OR physical therapy OR physiotherapy OR activity OR exercise OR electrical stimulation OR massage OR manual therapy OR walking OR strengthening OR stretching | 3231329 |
| 4 | pain perception [Mesh] OR pain [Mesh] OR pain | 729576 |
| 5 | 1 AND 2 AND 3 AND 4 | 467 |
Appendix Table 4.
Web of Science Search Results.
| No | Search | Results |
|---|---|---|
| 1 | randomized controlled trial OR controlled clinical trial OR clinical trial | 829275 |
| 2 | dementia OR cognitive disease OR cognitive disorder OR Alzheimer OR delirium OR Lewy Body disease | 364602 |
| 3 | nonpharmacological OR physical therapy OR physiotherapy OR activity OR exercise OR electrical stimulation OR massage OR manual therapy OR walking OR strengthening OR stretching | 989046 |
| 4 | Pain | 526600 |
| 5 | 1 AND 2 AND 3 AND 4 | 673 |
REFERENCES
- 1.Prince M, Wimo A, Guerchet M, Ali GC, Wu Y, Prina M. World Alzheimer Report 2015: The global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International. 2015;https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. [Google Scholar]
- 2.Langa KM, Larson EB, Crimmins EM, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepore M, Ferrell A, Wiener JM. Living arrangements of people with Alzheimer’s Disease and related dementias: implications for services and supports An Issue Brief for the Office of the Assistant Secretary for Planning and Evaluation. Washington, DC: RTI International;2017. [Google Scholar]
- 5.Ballard C, Corbett A. Agitation and aggression in people with Alzheimer’s disease. Curr Opin Psychiatry. 2013;26(3):252–259. [DOI] [PubMed] [Google Scholar]
- 6.Mantyselka P, Hartikainen S, Louhivuori-Laako K, Sulkava R. Effects of dementia on perceived daily pain in home-dwelling elderly people: a population-based study. Age Ageing. 2004;33(5):496–499. [DOI] [PubMed] [Google Scholar]
- 7.Barry HE, Parsons C, Passmore AP, Hughes CM. Exploring the prevalence of and factors associated with pain: a cross-sectional study of community-dwelling people with dementia. Health Soc Care Community. 2016;24(3):270–282. [DOI] [PubMed] [Google Scholar]
- 8.Zwakhalen SM, Koopmans RT, Geels PJ, Berger MP, Hamers JP. The prevalence of pain in nursing home residents with dementia measured using an observational pain scale. Eur J Pain. 2009;13(1):89–93. [DOI] [PubMed] [Google Scholar]
- 9.Takai Y, Yamamoto-Mitani N, Okamoto Y, Koyama K, Honda A. Literature review of pain prevalence among older residents of nursing homes. Pain Manag Nurs. 2010;11(4):209–223. [DOI] [PubMed] [Google Scholar]
- 10.Gibson SJ. IASP global year against pain in older persons: highlighting the current status and future perspectives in geriatric pain. Expert Rev Neurother. 2007;7(6):627–635. [DOI] [PubMed] [Google Scholar]
- 11.Lin WC, Lum TY, Mehr DR, Kane RL. Measuring pain presence and intensity in nursing home residents. J Am Med Dir Assoc. 2006;7(3):147–153. [DOI] [PubMed] [Google Scholar]
- 12.van Kooten J, Smalbrugge M, van der Wouden JC, Stek ML, Hertogh C. Prevalence of Pain in Nursing Home Residents: The Role of Dementia Stage and Dementia Subtypes. J Am Med Dir Assoc. 2017;18(6):522–527. [DOI] [PubMed] [Google Scholar]
- 13.Power JD, Perruccio AV, Gandhi R, et al. Neuropathic pain in end-stage hip and knee osteoarthritis: differential associations with patient-reported pain at rest and pain on activity. Osteoarthritis Cartilage. 2018;26(3):363–369. [DOI] [PubMed] [Google Scholar]
- 14.Scherder EJ, Plooij B. Assessment and management of pain, with particular emphasis on central neuropathic pain, in moderate to severe dementia. Drugs Aging. 2012;29(9):701–706. [DOI] [PubMed] [Google Scholar]
- 15.Lin PC, Li CH, Chou PL, Chen YM, Lin LC. Prevalence of pain-related diagnoses in patients with dementia: a nationwide study. J Pain Res. 2018;11:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett A, Husebo B, Malcangio M, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. 2012;8(5):264–274. [DOI] [PubMed] [Google Scholar]
- 17.Brecher DB, West TL. Underrecognition and Undertreatment of Pain and Behavioral Symptoms in End-Stage Dementia. Am J Hosp Palliat Care. 2016;33(3):276–280. [DOI] [PubMed] [Google Scholar]
- 18.Lautenbacher S Pain assessment in special patient groups such as those with dementia: at the finishing line or just starting from scratch? Pain. 2014;155(8):1419–1420. [DOI] [PubMed] [Google Scholar]
- 19.van Dalen-Kok AH, Pieper MJ, de Waal MW, Lukas A, Husebo BS, Achterberg WP. Association between pain, neuropsychiatric symptoms, and physical function in dementia: a systematic review and meta-analysis. BMC Geriatr. 2015;15:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shega J, Emanuel L, Vargish L, et al. Pain in persons with dementia: complex, common, and challenging. J Pain. 2007;8(5):373–378. [DOI] [PubMed] [Google Scholar]
- 21.Corbett A, Husebo BS, Achterberg WP, Aarsland D, Erdal A, Flo E. The importance of pain management in older people with dementia. Br Med Bull. 2014;111(1):139–148. [DOI] [PubMed] [Google Scholar]
- 22.Parsons C Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieper MJ, van Dalen-Kok AH, Francke AL, et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev. 2013;12(4):1042–1055. [DOI] [PubMed] [Google Scholar]
- 24.Anderson AR, Deng J, Anthony RS, Atalla SA, Monroe TB. Using Complementary and Alternative Medicine to Treat Pain and Agitation in Dementia: A Review of Randomized Controlled Trials from Long-Term Care with Potential Use in Critical Care. Crit Care Nurs Clin North Am. 2017;29(4):519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tse MM, Tang SK, Wan VT, Vong SK. The effectiveness of physical exercise training in pain, mobility, and psychological well-being of older persons living in nursing homes. Pain Manag Nurs. 2014;15(4):778–788. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Hughes AK. Nonpharmacological approaches to the management of chronic pain in community-dwelling older adults: a review of empirical evidence. J Am Geriatr Soc. 2012;60(3):555–568. [DOI] [PubMed] [Google Scholar]
- 28.Rundell SD, Sherman KJ, Heagerty PJ, Mock C, Jarvik JG. Patient-reported outcomes associated with use of physical therapist services by older adults with a new visit for back pain. Phys Ther. 2015;95(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W264. [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 31.Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther. 2000;5(4):223–226. [DOI] [PubMed] [Google Scholar]
- 32.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 33.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. [DOI] [PubMed] [Google Scholar]
- 34.Maher CG. A systematic review of workplace interventions to prevent low back pain. Aust J Physiother. 2000;46(4):259–269. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Mansfield J Nonpharmacologic treatment of behavioral disorders in dementia. Curr Treat Options Neurol. 2013;15(6):765–785. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M. What are the barriers to performing nonpharmacological interventions for behavioral symptoms in the nursing home? J Am Med Dir Assoc. 2012;13(4):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Souto Barreto P, Denormandie P, Lepage B, et al. Effects of a long-term exercise programme on functional ability in people with dementia living in nursing homes: Research protocol of the LEDEN study, a cluster randomised controlled trial. Contemp Clin Trials 2016;47:289–295. [DOI] [PubMed] [Google Scholar]
- 38.Pieper MJ, Achterberg WP, Francke AL, van der Steen JT, Scherder EJ, Kovach CR. The implementation of the serial trial intervention for pain and challenging behaviour in advanced dementia patients (STA OP!): a clustered randomized controlled trial. BMC Geriatr. 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjogren K, Lindkvist M, Sandman PO, Zingmark K, Edvardsson D. Person-centredness and its association with resident well-being in dementia care units. J Adv Nurs. 2013;69(10):2196–2205. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson NA, Andersen S. The clinical efficacy of reflexology in nursing home residents with dementia. J Altern Complement Med. 2008;14(3):269–275. [DOI] [PubMed] [Google Scholar]
- 41.Kunik ME, Snow AL, Wilson N, et al. Teaching Caregivers of Persons with Dementia to Address Pain. Am J Geriatr Psychiatry. 2017;25(2):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai PF, Chang JY, Beck C, Kuo YF, Keefe FJ. A pilot cluster-randomized trial of a 20-week Tai Chi program in elders with cognitive impairment and osteoarthritic knee: effects on pain and other health outcomes. J Pain Symptom Manage. 2013;45(4):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park H, Chun Y, Gang MS. Effects of the Patient-Centered Environment Program on Behavioral and Emotional Problems in Home-Dwelling Patients With Dementia. J Gerontol Nurs. 2015;41(12):40–48. [DOI] [PubMed] [Google Scholar]
- 44.Chapman DG, Toseland RW. Effectiveness of advanced illness care teams for nursing home residents with dementia. Soc Work. 2007;52(4):321–329. [DOI] [PubMed] [Google Scholar]
- 45.de Souto Barreto P, Cesari M, Denormandie P, Armaingaud D, Vellas B, Rolland Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J Am Geriatr Soc. 2017;65(9):E123–E129. [DOI] [PubMed] [Google Scholar]
- 46.Liu JYW, Leung DYP. Pain Treatments for Nursing Home Residents with Advanced Dementia and Substantial Impaired Communication: A Cross-Sectional Analysis at Baseline of a Cluster Randomized Controlled Trial. Pain Med. 2017;18(9):1649–1657. [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Freedman L. Efficacy of nonpharmacologic interventions for agitation in advanced dementia: a randomized, placebo-controlled trial. J Clin Psychiatry. 2012;73(9):1255–1261. [DOI] [PubMed] [Google Scholar]
- 48.Hobbelen JH, Tan FE, Verhey FR, Koopmans RT, de Bie RA. Passive movement therapy in severe paratonia: a multicenter randomized clinical trial. Int Psychogeriatr. 2012;24(5):834–844. [DOI] [PubMed] [Google Scholar]
- 49.Kapoor Y, Orr R. Effect of therapeutic massage on pain in patients with dementia. Dementia (London). 2017;16(1):119–125. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Mansilla J, Gonzalez Lopez-Arza MV, Varela-Donoso E, Montanero-Fernandez J, Gonzalez Sanchez B, Garrido-Ardila EM. The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin Rehabil. 2015;29(7):683–693. [DOI] [PubMed] [Google Scholar]
- 51.Ellis JM, Wells Y, Ong JSM. Non-Pharmacological Approaches to Pain Management in Residential Aged Care: a Pre-Post-Test Study. Clin Gerontol. 2017:1–11. [DOI] [PubMed] [Google Scholar]
- 52.Moyle W, Murfield JE, O’Dwyer S, Van Wyk S. The effect of massage on agitated behaviours in older people with dementia: a literature review. J Clin Nurs. 2013;22(5–6):601–610. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Wang Y, Wang Z. The effectiveness of massage and touch on behavioural and psychological symptoms of dementia: A quantitative systematic review and meta-analysis. J Adv Nurs. 2017;73(10):2283–2295. [DOI] [PubMed] [Google Scholar]
- 54.Plooij B, Scherder EJ, Eggermont LH. Physical inactivity in aging and dementia: a review of its relationship to pain. J Clin Nurs. 2012;21(21–22):3002–3008. [DOI] [PubMed] [Google Scholar]
- 55.Gregory SM, Parker B, Thompson PD. Physical activity, cognitive function, and brain health: what is the role of exercise training in the prevention of dementia? Brain Sci. 2012;2(4):684–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Telenius EW, Engedal K, Bergland A. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: a single blinded randomized controlled trial. BMC Geriatr. 2015;15:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Telenius EW, Engedal K, Bergland A. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: an assessor blinded randomized controlled trial. PLoS One. 2015;10(5):e0126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aman E, Thomas DR. Supervised exercise to reduce agitation in severely cognitively impaired persons. J Am Med Dir Assoc. 2009;10(4):271–276. [DOI] [PubMed] [Google Scholar]
- 59.Brett L, Traynor V, Stapley P. Effects of Physical Exercise on Health and Well-Being of Individuals Living With a Dementia in Nursing Homes: A Systematic Review. J Am Med Dir Assoc. 2016;17(2):104–116. [DOI] [PubMed] [Google Scholar]
- 60.Abd El-Kader SM, Al-Jiffri OH. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr Health Sci. 2016;16(4):1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lichtner V, Dowding D, Esterhuizen P, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Husebo BS, Achterberg W, Flo E. Identifying and Managing Pain in People with Alzheimer’s Disease and Other Types of Dementia: A Systematic Review. CNS Drugs. 2016;30(6):481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Husebo BS, Ostelo R, Strand LI. The MOBID-2 pain scale: reliability and responsiveness to pain in patients with dementia. Eur J Pain. 2014;18(10):1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achterberg WP, Pieper MJ, van Dalen-Kok AH, et al. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Ljunggren AE. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) Pain Scale in a clinical setting. Scand J Caring Sci. 2010;24(2):380–391. [DOI] [PubMed] [Google Scholar]
- 66.Chimenti RL, Frey-Law LA, Sluka KA. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys Ther. 2018;98(5):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]