Abstract
Background:
Prior studies examining the sleep of adolescents with and without attention-deficit/hyperactivity disorder (ADHD) have relied on mean values such as average sleep duration, which masks intraindividual variability. The objective was to investigate whether adolescents with ADHD have greater intraindividual variability of sleep/wake patterns than adolescents without ADHD using actigraphy and daily sleep diaries.
Method:
Adolescents (ages 13.17±0.40 years; 45% female) with (n=162) and without (n=140) ADHD were recruited from middle schools at two sites. Participants wore actigraphs and completed sleep diaries for an average of two weeks.
Results:
Multilevel models were conducted with sex, sleep medication use, ADHD medication use, number of days with data, and social jetlag controlled for in analyses. For actigraphy, adolescents with ADHD had greater variability for time in bed, sleep onset and offset, and wake after sleep onset than adolescents without ADHD. For sleep diary data, adolescents with ADHD had greater variability in bedtime, wake time, sleep duration, sleep onset latency, sleep quality, and night wakings than adolescents without ADHD. Social jetlag was a significant predictor of variability in sleep measures based on both actigraph and daily diaries; however, ADHD status was not associated with social jetlag.
Conclusions:
This is the first study to show that adolescents with ADHD have more variable sleep/wake patterns than their peers using both objective and subjective sleep measures. Intraindividual variability of sleep/wake patterns may be important for clinicians to assess and monitor as part of treatment. Research is needed to understand the mechanisms underlying increased intraindividual variability of sleep/wake patterns in adolescents with ADHD and potential consequences for daytime functioning.
Keywords: Actigraphy, adolescence, attention-deficit/hyperactivity disorder, day-to-day, interdaily, night-to-night, jet lag
Introduction
There is clearly a link between attention-deficit/hyperactivity disorder (ADHD) and sleep/wake problems (Cortese et al., 2009; Lunsford-Avery, Krystal, & Kollins, 2016). However, the vast majority of studies have compared the sleep/wake patterns of individuals with and without ADHD using mean, summary variables, such as average sleep duration or bedtime. Although important, findings using this approach masks the intraindividual variability (IIV) of sleep/wake patterns that may be especially prevalent in adolescents with ADHD. For example, an individual who consistently goes to sleep at 10:00pm and wakes at 6:00am will have the same mean sleep duration as an individual who consistently wakes at 6:00am but alternates between going to sleep at either 8:00pm or 12:00am. ADHD is also often described as a disorder characterized by variability due to deficits in temporal processing (Castellanos & Tannock, 2002). Given both daytime and nighttime difficulties typically present in ADHD, it has also been called a “24-hour disorder” (Konofal, Lecendreux, & Cortese, 2010). The natural implication of these conceptualizations of ADHD is to examine not only daytime variability, but also sleep/wake variability.
Intraindividual variability in adults and youth with ADHD
For adults with ADHD, it is well established that intraindividual variability (IIV) exists in their circadian rhythms when compared to adults without ADHD (Baird et al., 2011; Van Veen et al., 2010). For example, Baird and colleagues used actigraphy and cortisol salivary samples to determine that adults with ADHD had significant diurnal and nocturnal hyperactivity, with significant phase delays of cortisol rhythms. Similarly, Van Veen et al. (2010) found that adults with ADHD showed higher variability and lower amplitude of their circadian rhythms than healthy controls. These studies suggest that having ADHD in adulthood is accompanied with significant changes in the circadian system, possibly negatively affecting consistency in sleep duration and sleep quality.
In contrast, fewer than 10 studies have examined the IIV of sleep/wake patterns in youth with ADHD, with mixed findings reported (for a review, see Becker et al., 2017). There is some indication, from research with school-aged children (Gruber & Sadeh, 2004; Gruber, Sadeh, & Raviv, 2000) that children with ADHD have greater IIV in their sleep (duration, true sleep time, sleep onset latency) compared to children without ADHD (Becker et al., 2017). A seminal study conducted with 38 boys with ADHD and 64 comparison boys without ADHD did not find ADHD to be associated with greater sleep/wake IIV using daily diary ratings but did find ADHD to be associated with greater IIV in sleep onset latency and duration using actigraphy (Gruber, Sadeh, & Raviv, 2000). Similarly, in a study of youth ages 6–13 years, Moreau et al. (2014) found that youth with ADHD exhibited greater variability in sleep onset latency and movement. In contrast, Owens et al. (2009) did not find significant differences in sleep/wake IIV in a sample with a similar age range (ages 6–14). Recently, Poirier and Corkum (2018) found that children with and without ADHD had substantial sleep duration IIV, yet variability was similar between the groups. A systematic review of this literature indicated that although there is some evidence for greater IIV in sleep/wake patterns among youth with ADHD compared to their peers, additional studies are needed that include larger samples and consider developmental factors (Becker et al., 2017).
Sleep/wake homeostasis and circadian timing during in adolescence
In adolescence, nightly sleep duration of 9–9.35 hours is required for optimal cognitive functioning (Crowley, Wolfson, Tarokh, & Carskadon, 2018; Short, Weber, Reynolds, Coussens, & Carskadon, 2018). However, a confluence of psychosocial and biological factors contribute to changes in sleep regulation and circadian timing. Homeostatic sleep pressure builds at a slower rate among more mature adolescents compared to less mature adolescents, giving biological ‘permission’ of later bedtimes (Jenni et al., 2005; Taylor et al., 2005). The circadian timing system also changes during adolescence, with a shift in phase preference toward eveningness possibly due to a lengthening of the intrinsic period of the circadian clock (Crowley et al., 2018). These developmental changes in homeostatic sleep pressure and a circadian phase delay contribute to differences in sleep timing and duration between school nights and weekend nights (National Sleep Foundation, 2006). This discrepancy, referred to as social jetlag, is associated with mood, academic and cognitive performance, (e.g., Diaz-Morales & Escribano, 2015), and substance use (Pasch et al, 2010 O’Brien & Mindell, 2005).
Importantly for the present study, there is a growing body of literature indicating that individuals with ADHD may have more circadian rhythm abnormalities compared to their neurotypical peers. For instance, children (Gruber et al., 2012) and adults (Kooij & Bijlenga, 2013) with ADHD may have a later circadian rhythm and/or preference than their peers. Further, there is some evidence that social jetlag is associated with more ADHD symptomatology in young adults (McGowan et al., 2016). However, we are unaware of any studies that have examined social jetlag in adolescents with and without ADHD. It is important to explore whether adolescents with and without ADHD differ in social jetlag and whether social jetlag is associated with increased sleep/wake IIV.
Limitations of prior research
The vast majority of research conducted on sleep and ADHD has focused on school-aged children. A recent review found only 19 studies that focused on sleep in adolescents with ADHD, and these studies were generally limited by small or non-representative samples and the use of non-standardized sleep measures (Lunsford-Avery, Krystal, & Kollins, 2016). Relatedly, most studies of sleep in adolescents with ADHD have relied upon subjective ratings rather than on objective measures of sleep, with only a few studies using actigraphy to examine sleep in adolescents with ADHD or ADHD symptoms (Lunsford-Avery, Krystal, & Kollins, 2016). It is crucial to examine both subjective and objective sleep in adolescents with and without ADHD given the vast developmental, neurobiological, and socio-contextual changes that occur during this period that impact sleep (Colrain & Baker, 2011; Dahl, 2004; Feinberg & Campbell, 2010; Steinberg, 2010; Tarokh, Saletin, & Carskadon, 2016). These changes may be especially relevant for adolescents with ADHD given known differences in brain and pathophysiology at the group-level (Cortese et al., 2012; Hoogman et al., 2017; Konrad & Eickhoff, 2010) as well as impairments that may impact sleep. Given the distinct context of adolescence, including heightened academic demands, extracurricular activities, increased technology/media use, growing importance of peer relationships, and greater independence over sleep/wake schedules, it is particularly important to examine sleep variability in adolescents specifically. Consistent with these developmental factors, there is evidence that increasing age/maturity is associated with increased sleep IIV among youth (Becker et al., 2017). However, only one study has examined sleep/wake IIV in adolescents with ADHD, with no difference in sleep time variability found between adolescents with and without ADHD (Moore et al., 2011). However, this study relied solely upon parent-report of ADHD diagnosis rather than a comprehensive diagnostic assessment and was limited to a sample of only 16 adolescents with ADHD (Moore et al., 2011). Additional studies examining the sleep/wake IIV in adolescents with and without ADHD are needed (Becker et al., 2017).
Finally, an important methodological consideration is the statistical approach used to assess sleep/wake IIV. Sleep IIV has traditionally been measured using either individual standard deviation or coefficient of variation (Rowe et al., 2008). However, neither of these metrics are ideal given that intensive longitudinal data have repeated measures nested within participant that are non-independent. Ignoring this non-independence, as traditional ANOVAs do, increases risk for making a Type I error (Bauer et al., 2013). Multilevel modeling (MLM; Goldstein, 2012; Hox, Moerbeek, & Schoot, 2017; Raudenbush & Bryk, 2002; Snijders & Bosker, 1999) was developed to capture sources of dependence in nested data and allows researchers to estimate both between-group variability and individual differences within groups (Bauer et al., 2013). Use of MLM has been gaining popularity in the adult (McCrae et al., 2008) and adolescent (Fuligni et al., 2017) sleep fields. However, extant research specifically examining sleep IIV in individuals with ADHD has relied on ANOVAs/MANOVAs (Gruber & Sadeh, 2004; Gruber, Sadeh, & Raviv, 2000; Moreau, Rouleau, & Morin, 2014; Owens et al., 2009) Accordingly, the present study used MLM to examine sleep IIV using actigraph and daily diary data collected over a multi-week period in adolescents with and without ADHD.
Present study
To build from and extend previous research, the current study used a multi-method design including actigraphy and daily sleep diary, and MLM to examine whether adolescents with ADHD have greater IIV in sleep/wake patterns compared to adolescents without ADHD. Importantly, our analyses also included sex, ADHD medication use, and sleep medication use as covariates. Although mixed findings have been reported, there is an indication for greater sleep/wake IIV in youth with ADHD (Becker et al., 2017) and we hypothesized that we would find evidence for this in adolescents with ADHD. The present study also builds on our prior work with sample which found mean level differences in sleep for adolescents with ADHD, such that adolescents with ADHD were more likely than adolescents without ADHD to obtain insufficient sleep on schooldays (per diary) and weekends (per diary and actigraphy; Becker et al., 2019).
Methods
Participants
Participants were 302 adolescents (167 males) in eighth grade (ages 12–14 years; M ± SDage=13.17 ± 0.40 years) who were recruited from local middle schools around two sites located in the Southeastern and Midwestern United States. The purpose of the study is to evaluate differences between adolescents with and without ADHD, and so adolescents with ADHD were purposefully oversampled in order to have an approximately 1:1 ratio. Approximately half (n=162) of the participants were diagnosed with ADHD (120 predominantly inattentive presentation, 42 combined presentation) per the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). The majority of participants were White (81.8%; 5.3% Black, 4.6% Asian, 8.1% another race or multiracial). Fifty-eight percent of the ADHD participants were taking ADHD medication and 19% were taking sleep medication or melatonin; 6% of the comparison sample was taking sleep medication or melatonin. Table 1 provides a breakdown of the types of medications participants were taking by classification. See Becker et al. (2019) for more details on the sample.
Table 1.
Medication breakdown by classification
| Type | Name (# taking) of Medication | # taking |
|---|---|---|
| ADHD Medications | ||
| ADHD Non-Stimulant | Clonidine (12), Guanfacine ER (1), Intuniv (3), Strattera (3), Tenex (5) | 24 |
| Amphetamine | Adderall (13); Adderall XR (15), Dextroamphetamine (1), D-Amphetamine Salt COM (1), D-Amphetamine Salt Com XR (1), Vyvanse (18) | 49 |
| Methylphenidate | Concerta (12), Concerta ER(5), Dexmethylphenidate (1), Dexmethylphenidate ER (1), Focalin (7), Focalin XR (4), Metadate (4), Methylphenidate (7), Methylphenidate XR (4), Quillichew (2), Quillivant XR (2), Ritalin (2) | 51 |
| Sleep Medications | ||
| Melatonin | Melatonin (31) | 31 |
| Other Sleep | DriftOff (1) | 1 |
| Emotional-behavioral Disorder Medications | ||
| Anti-anxiety | Ativan (1), Hydroxizine (1) | 2 |
| Anti-depressant | Amitriptyline (1), Citalopram (1), Fluoxetine (4), Fluvoxamine (1), Lexapro (3), Paxil (1), Pristiq (1), Prozac (3), Sertaline (2), Trazadone (1), Wellbutrin (1), Zoloft (8) |
27 |
| Anti-psychotic | Abilify (1), Risperidone (1), Risperidone ER (1) | 3 |
Note. Medications are listed in alphabetical order. 183 participants were taking no medication, 76 participants were on one medication, 30 participants were on 2 medications; 12 participants were on 3 medications; 1 participant was on 4 medications.
Procedures
This study was approved by the Virginia Commonwealth University and Cincinnati Children’s Hospital Medical Center Institutional Review Boards. Written informed consent and adolescent assent were obtained from all participants. Participants and their parents were recruited across two consecutive years (2016 and 2017) for a prospective longitudinal study examining sleep in adolescents with and without ADHD. Currently only baseline data are available for both cohorts. Potential participants were recruited via distribution of a recruitment flyer and letter to all eighth grade families by e-mail, within an information packet, or at events attended by eighth grade parents. Given the study sought to include a larger proportion of participants with ADHD, two sets of recruitment materials were used, with one set specifically directed towards youth with attention difficulties and/or ADHD. A phone screen was completed with interested families (n=405) to ensure that participants were in eighth grade and in regular education classes. Families meeting phone screen criteria (n=360) were then invited to receive a comprehensive assessment. For more details and a flow diagram, see Becker et al. (2019). At this assessment, families (n=313) completed the Children’s Interview for Psychiatric Syndromes (ChIPS), a structured psychiatric diagnostic interview, and rating scales. In addition to phone screen inclusion criteria, participants needed an estimated Full Scale IQ ≥ 80 based on results of the Wechsler Abbreviated Scale of Intelligence, Second Edition. Exclusion criteria were: (1) meeting criteria for autism spectrum disorders, bipolar disorder, a dissociative disorder, or a psychotic disorder; (2) previous diagnosis per parent report of an organic sleep disorder (e.g., obstructive sleep apnea, narcolepsy, restless leg syndrome, periodic limb movement disorder), and (3) not meeting criteria for either the ADHD or comparison group.
ADHD diagnosis.
All potential participants underwent a comprehensive ADHD diagnostic evaluation in accordance with criteria of the DSM-5. Participants met criteria for ADHD on the basis of the Children’s Interview for Psychiatric Syndromes, Parent Version (P-ChIPS. To be eligible for participation in the ADHD group, participants were required to meet all DSM-5 criteria for either the ADHD combined presentation or predominantly inattentive presentation on the P-ChIPS, including: ≥6 symptoms of inattention at clinically significant levels; presence of ADHD symptoms prior to age 12 years, presence of ADHD symptoms in two or more settings (e.g., home, school), evidence that symptoms contribute to home, academic, and/or social impairment; and symptoms of ADHD are not better explained by another mental disorder. Participants were included in the comparison group if the parent endorsed <4 symptoms of ADHD in both domains (i.e., inattention, hyperactivity/impulsivity) on the P-ChIPS. Additionally, all participants were assessed for common comorbid mental health conditions (e.g., mood and anxiety disorders, disruptive behavior disorders).
At the diagnostic assessment visit, participants were provided with an actigraph and daily diary, which they were instructed to wear and complete daily until a feedback appointment at least two weeks following the initial evaluation. Participants were instructed to wear the actigraph on their non-dominant hand at all times, except when engaging in contact sports or activities involving water (e.g., swimming, showering). However, some participants opted to only wear their actigraph after school and during sleep for a variety of reasons (e.g., wanted to wear their own watch to school, were self-conscious about wearing the actigraph to school).
Measures
Actigraphy.
Daily actigraph data were collected across a 14–30 day observation period using Actigraph GT9X Link devices. Data were downloaded using Actilife software version 6. Sixty-second epoch lengths were used to score the actigraph data. They were first validated using both the wear-time sensor built into the device and in combination with a validation algorithm (Troiano, 2007) to maximize the accuracy of when the actigraph was physically being worn by finding the times of non-wear based upon a threshold of consecutive zeros. Once data were validated, sleep scores were calculated with the Sadeh sleep scoring algorithm (Sadeh, Sharkey, & Carskadon, 1994) by individually checking and adding sleep periods to each night the device was worn for each participant, with adolescent daily diaries used alongside the actigraph scoring. Actigraph variables used in the present study are as follows: time in bed (TIB; total time from sleep onset to offset, which has been shown to be the most accurate approximation of actigraphy-derived measure of total sleep; Short et al., 2012), sleep onset time (the time the adolescent fell asleep), sleep offset time (the time the adolescent woke up), wake after sleep onset (WASO; the total number of minutes across periods of wakefulness following sleep onset), night awakenings (the number of times a participant awoke following sleep onset), and sleep efficiency (the ratio of time spent asleep to total time spent in bed).
Daily sleep diary.
Adolescent morning diary data (reporting about the previous night’s sleep) were collected during the same period that participants wore actigraphs. The following variables were collected and examined in this study: bed time (the time the adolescent reported going to bed), wake time (the time the adolescent reported waking up), sleep duration (the total number of hours the adolescent reported being asleep), sleep onset latency (SOL; the number of minutes the adolescent reported it took to fall asleep), sleep quality (the participant’s rating of overall sleep quality on a 5-point scale from 1=Very good to 5=Very bad), night awakenings (the number of times the adolescent reported waking up on a 5-point scale from1=0 wakings to 5=5 or more wakings), and WASO (adolescent-reported total number of minutes awake following sleep onset).
Daily ADHD and sleep medication use.
Each evening, participants reported on whether they took any medication for ADHD during the day; each morning, participants reported on whether they took any medications for sleep the prior evening. These time varying covariates were included in all analyses.
Social jetlag.
Social jetlag was calculated using the sleep-corrected formula for social jetlag which represents the difference between sleep onset on weekend days and schooldays for participants with longer sleep and later sleep onset on weekend days compared to schooldays, and the difference between the sleep offset on weekend days and schooldays for subjects with longer sleep and earlier sleep offset on schooldays compared to weekend days (Jankowski, 2017).
Analytic plan
First, group differences the number of days of completed actigraphy and daily diary data and in social jetlag were explored. Both of these variables were also included as predictors in main analyses given their relation with IIV. Next, to examine IIV in sleep, and potential differences in variability for participants with ADHD and comparison participants, MLM were run in SPSS Version 24 (IBM Corp., 2016) for each measure and Level 1 residuals were saved for each adolescent to reflect nightly sleep variability. This indicator of variability is a squared metric of the average variability in each measure for each adolescent (Fuligni et al., 2018). Two-level (i.e., nights nested within individuals) MLM with random intercepts to accommodate correlated errors were conducted for each outcome with the residual terms as outcome variables to examine whether group status predicted sleep variability, accounting for the effects of adolescent sex, ADHD medication status, sleep medication status, number of days of data, and social jetlag. ADHD and sleep medication status were entered as Level 1 time-varying covariates (i.e., analyses account for medication status each day of the observation period); coefficients for these covariates can be interpreted as the effect of medication status on average (1 = on medication, 0 = not on medication) on variability in sleep measures. Missing data were handled in MLM analyses using restricted maximum likelihood estimation. Our primary analyses included all days across the actigraph/sleep diary period, including both schooldays and weekends. In addition, we conducted analyses separately for weekend and school day evenings. Based on prior research suggesting that at least five schooldays of actigraph data is necessary for good reliability (Acebo et al., 1999), participants were included if they had five or more days of school day data. For weekends, participants were included if they had two or more days of weekend data. The vast majority of participants had at least five schooldays and two weekend days for both actigraph and diary. For actigraph data, only eight participants did not have at least five schooldays and 15 participants did not have at least two weekend days. For daily diary data, 12 participants did not have at least five schooldays and seven participants did not have at least two weekend days.
Power analyses for the larger study were conducted in Mplus using Monte Carlo simulations with 1,000 replications of each model. Models accounted for the nesting of adolescents within cohorts and schools. Analyses yielded effective sample sizes of 152, 122, and 102 based on intracluster correlations of .10, .15, and .20. Even with these adjustments, the power was .94 or higher for ICCs of .10, .90 or higher for ICCs of .15, and .85 or higher for ICCs of .20.
Results
Preliminary analyses
The mean number of days in the actigraph observation period was 14 (SD=3), the mean number of schooldays was 10 (SD=2), and the mean number of weekend days was 4 (SD=1). The ADHD (M = 14.3, SD = 2.6; range = 6–20) and comparison (M = 14.4, SD = 3.0; range = 2–20) groups did not differ on number of days with actigraph data. The mean number of days in the daily diary observation period was 15 (SD=3), the mean number of schooldays was 11 (SD=3), and the mean number of weekends was 4 (SD=1). The ADHD group (M = 15, SD = 3.7; range = 2–23) had slightly fewer days of diary data than (p = .03) the comparison group (M = 15.8, SD = 2.6; range = 3–23), but the difference was less than one day. Table 2 shows the breakdown for number of days of actigraphy and daily diary data collected for participants.
Table 2.
Breakdown of number of actigraph and daily diary days collected
| Actigraphy | Daily Diaries | ||||||
|---|---|---|---|---|---|---|---|
| Number of Days | ADHD % |
Comparison % |
Total Sample % |
ADHD % |
Comparison % |
Total Sample % |
|
| <5 Days | 1.3% | 0.7% | 1.0% | 0.7% | 0.0% | 0.3% | |
| 6–10 Days | 6.7% | 9.6% | 8.0% | 7.3% | 3.7% | 5.6% | |
| 11–15 Days | 50.7% | 37.5% | 44.4% | 49.3% | 42.6% | 46.2% | |
| 16–20 Days | 38.7% | 51.5% | 44.8% | 36.0% | 48.5% | 42.0% | |
| >20 Days | 2.7% | 0.7% | 1.7% | 6.7% | 5.1% | 5.9% | |
Note. Chi-square test indicated non-significant differences in the likelihood of the ADHD group having less days of actigraphy (χ2 = 7.91, p = .10) and diary (χ2 = 6.25, p = .18) data. ADHD = attention-deficit/hyperactivity disorder.
On average the social jetlag based on actigraph data for adolescents with ADHD was 1.84 hr (SD = 2.32); the average social jetlag for adolescents without ADHD was 1.99 hr (SD = 1.07), t (278) = 0.68, p = .50. Similarly, there was a non-significant difference between groups for social jetlag based on diary data, t (290) = 0.45, p = .66, with the ADHD group experiencing an average social jetlag of 0.78 hr (SD = 1.85) and the comparison group experiencing an average social jetlag of 0.86 hr (SD = 0.99).
Actigraphy sleep/wake IIV
MLM findings for the actigraphy sleep/wake variables are summarized in Table 3. As shown, ADHD group status predicted variability in several actigraph sleep measures across the observation period and on schooldays; group status did not significantly predict variability in any actigraph sleep measure on weekends. Specifically, controlling for the effects of sex, daily ADHD medication status, daily sleep medication status, number of days of data, and social jetlag, the ADHD group had significantly more variability for TIB, sleep onset, sleep offset, and WASO across the entire observation period. When only schooldays were considered, the ADHD group had significantly more variability for TIB, sleep onset, and efficiency. Visual examples of the ADHD group showing greater variability than the comparison group on (a) TIB, (b) sleep offset, and (c) WASO across the first 14 days are presented in Figure 1. Example actograms for a randomly selected adolescent with and without ADHD are available in Figure S1 in the Supporting Information.
Table 3.
Multilevel models predicting variability in actigraph sleep outcomes
| Time in Bed Variability b (SE) |
Sleep Onset Variability b (SE) |
Sleep Offset Variability b (SE) |
Wake After Sleep Onset Variability b (SE) |
Number of Awakenings Variability b (SE) |
Efficiency Variability b (SE) |
|
|---|---|---|---|---|---|---|
| Entire Observation Period (n=285) | ||||||
| Intercept | 1791.58 (1681.53) | 0.21 (0.45) | 0.41 (0.45) | 310.00 (316.45) | 41.69 (11.18)*** | 11.21 (12.38) |
| Day | 73.80 (38.19) | 0.00 (0.01) | 0.01 (0.01) | −6.67 (6.30) | 0.43 (0.24) | 0.02 (0.35) |
| Number of Days | 44.35 (102.38) | 0.04 (0.03) | 0.02 (0.03) | 29.81 (19.29) | −0.81 (0.68) | 0.61 (0.75) |
| Social Jetlag | 779.03 (608.83)*** | 0.17 (0.04)*** | 0.36 (0.04)*** | 56.61 (29.20) | 3.83 (1.04)*** | 2.09 (1.17) |
| Sex | 986.64 (540.15) | 0.04 (0.14) | 0.27 (0.14) | 13.39 (103.08) | 0.54 (3.61) | 1.94 (3.85) |
| ADHD Med | −553.20 (644.02) | −0.06 (0.17) | −0.34 (0.17) | −119.63 (113.42) | −0.29 (4.20) | −3.21 (5.13) |
| Sleep Med | −1109.73 (826.90) | −0.48 (0.22)* | −0.49 (0.22) | −266.47 (147.97) | −10.45 (5.42) | 1.38 (6.42) |
| Group | 1742.28 (608.83)** | 0.27 (0.08)* | 0.42 (0.16)* | 303.76 (114.34)** | 5.67 (4.04) | 7.01 (4.45) |
| Schooldays (n=283) | ||||||
| Intercept | 2323.14 (1680.10) | 0.14 (0.38) | 0.45 (0.32) | 207.22 (289.11) | 33.23 (9.51)** | 12.98 (9.37) |
| Day | 115.11 (36.69)** | 0.01 (0.01) | 0.01 (0.01) | 1.01 (6.48) | 0.57 (0.23)* | 0.04 (0.25) |
| Number of Days | −54.51 (103.11) | 0.02 (0.02) | 0.00 (0.02) | 32.32 (17.74) | −0.56 (0.58) | 0.61 (0.57) |
| Social Jetlag | 448.10 (147.31)** | 0.07 (0.03)* | 0.09 (0.03)** | −50.77 (93.01) | 2.41 (3.47) | 0.46 (0.82) |
| Sex | 762.31 (541.65) | 0.20 (0.12) | 0.19 (0.10) | −50.77 (93.01) | −2.06 (3.04) | −0.20 (2.97) |
| ADHD Med | 104.16 (635.10) | 0.03 (0.15) | −0.02 (0.11) | −73.86 (110.32) | 5.11 (3.72) | −2.53 (3.81) |
| Sleep Med | −955.89 (803.17) | −0.24 (0.19) | −0.26 (0.15) | −182.25 (139.47) | −3.85 (4.68) | −3.14 (4.75) |
| Group | 1379.06 (612.41)* | 0.31 (0.14)* | 0.19 (0.12) | 205.25 (105.40) | 2.41 (3.47) | 9.11 (3.43)** |
| Weekends (n=274) | ||||||
| Intercept | −3859.09 (3273.77) | −0.69 (1.02) | −3.00 (0.98)** | 130.17 (676.72) | 44.53 (24.53) | −22.68 (37.54) |
| Day | 57.72 (93.19) | −0.00 (0.03) | 0.05 (0.03) | −19.31 (14.66) | 0.64 (0.63) | 0.14 (1.08) |
| Number of Days | 225.44 (188.67) | 0.06 (0.06) | 0.03 (0.06) | 25.85 (39.14) | −1.86 (1.42) | 1.12 (2.17) |
| Social Jetlag | 4168.41 (465.23)*** | 1.14 (0.14)*** | 2.79 (0.14)*** | 333.27 (97.93)** | 20.44 (3.50)*** | 17.16 (5.29)** |
| Sex | 1130.02 (948.55) | −0.46 (0.30) | 0.24 (0.28) | 105.34 (202.83) | 4.66 (7.22) | 7.13 (10.87) |
| ADHD Med | −38.22 (1325.69) | 0.07 (0.41) | −0.26 (0.39) | 60.23 (250.92) | −6.56 (9.67) | −2.34 (15.27) |
| Sleep Med | 1503.34 (1697.21) | −0.65 (0.53) | 0.24 (0.51) | −172.96 (337.96) | −7.06 (12.64) | 18.55 (19.52) |
| Group | 1125.40 (1088.18) | −0.13 (0.34) | 0.30 (0.33) | 383.58 (226.48) | 6.23 (8.20) | −1.76 (12.47) |
Note. Group coded comparison=0, ADHD=1. Sex coded male=0, female=1. Med= Medication, 0=did not take ADHD/sleep medication that day, 1= took ADHD/sleep medication that day.
p<.05,
p< 01,
p<.001.
Figure 1.
Exemplars of Intra-individual Variability for ADHD and Comparison participants for sleep onset actigraph data. Example of a randomly selected participant from the comparison and ADHD groups. Sleep onset is in extended military time (e.g., 22 = 10pm, 25 = 1am).
With regard to our predictor variables and covariates, social jetlag was associated with sleep/wake variability in all sleep outcomes (TIB, sleep onset, sleep offset, and number of awakenings across the entire observation period; TIB, sleep onset, sleep offset on schooldays; all sleep outcomes on weekends). Sex, number of days of data, taking ADHD medication, and sleep medication were not associated with variability in sleep measures, with the exception of sleep medication which was related to sleep onset variability across the entire observation period.
Daily diary sleep/wake IIV
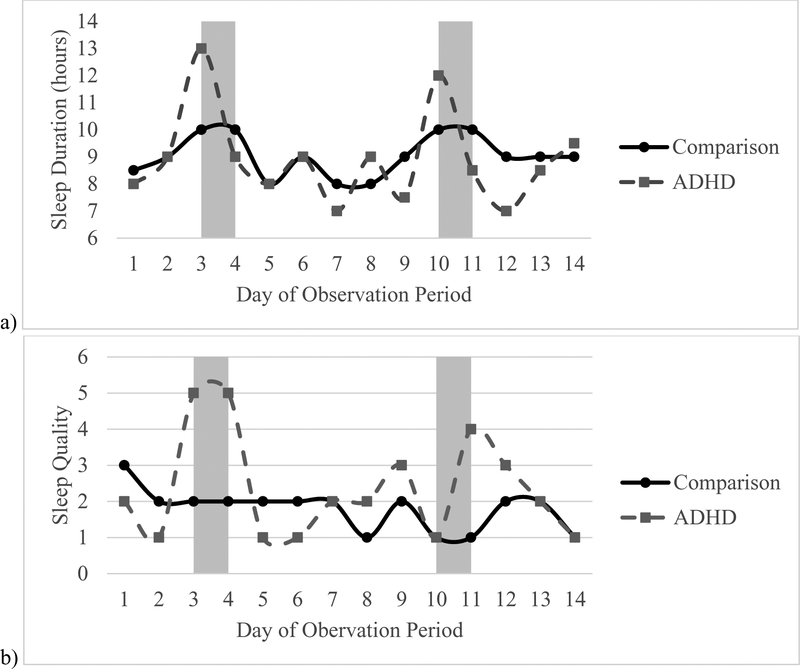
MLM findings for the daily diary sleep/wake variables are summarized in Table 4. As shown, ADHD group status predicted variability in several daily diary sleep measures across the entire observation period, on schooldays, and to a lesser extent on weekends. Specifically, controlling for the effects of sex, ADHD medication status, sleep medication status, number of days with diary data, and social jetlag, the ADHD group had significantly more variability for bedtime, wake time, duration, sleep onset latency, sleep quality, and number of awakenings across the entire observation period. When only schooldays were considered, the ADHD group had significantly more variability in wake time, duration, sleep quality, and number of awakenings; for weekends, the ADHD group had significantly more variability in sleep onset latency and number of awakenings. Visual examples of the ADHD group showing greater variability than the comparison group on (a) duration, (b) sleep quality, and (c) awakenings across the first 14 days are presented in Figure 2.
Table 4.
Multilevel models predicting variability in daily diary sleep outcomes
| Bedtime Variability b (SE) |
Wake Time Variability b (SE) |
Duration Variability b (SE) |
Sleep Onset Latency Variability b (SE) |
Quality Variability b (SE) |
Awakenings Variability b (SE) |
Wake After Sleep Onset Variability b (SE) |
|
|---|---|---|---|---|---|---|---|
| Entire Observation Period (n=286) | |||||||
| Intercept | 0.29 (0.31) | 0.67 (0.51) | 1.60 (0.47)** | 419.34 (113.31)*** | 0.51 (0.13)*** | 0.95 (0.26)*** | 142.25 (57.32)* |
| Day | −0.00 (0.01) | 0.00 (0.01) | −0.01 (0.01) | −5.60 (2.34)* | −0.00 (0.00) | −0.01 (0.00)** | −0.92 (1.17) |
| Number of Days | 0.02 (0.02) | 0.02 (0.03) | −0.01 (0.03) | −12.43 (6.61) | −0.01 (0.01) | −0.03 (0.02) | −4.69 (3.35) |
| Social Jetlag | 0.23 (0.05)*** | 0.45 (0.09)*** | 0.01 (0.08) | 1.69 (19.05) | 0.01 (0.02) | −0.07 (0.04) | −5.08 (9.44) |
| Sex | 0.10 (0.10) | 0.71 (0.17)*** | 0.38 (0.16)* | −21.32 (38.30) | 0.11 (0.04)* | 0.37 (0.09)*** | 27.04 (19.16) |
| ADHD Med | −0.24 (0.12) | −0.35 (0.19)** | −0.47 (0.18)* | 45.47 (43.60) | 0.05 (0.05) | 0.00 (0.09) | 39.78 (21.84) |
| Sleep Med | −0.36 (0.16) | −0.37 (0.26) | −0.41 (0.24) | 38.14 (58.58) | −0.06 (0.07) | −0.04 (0.12) | 30.77 (28.90) |
| Group | 0.22 (0.11)* | 0.50 (0.19)* | 0.54 (0.18)** | 84.46 (42.49)* | 0.12 (0.05)* | 0.25 (0.10)* | 8.51 (21.30) |
| Schooldays (n=286) | |||||||
| Intercept | 0.29 (0.25) | −0.24 (0.39) | 1.09 (0.44)* | 501.82 (125.31)*** | 0.48 (0.14)** | 1.02 (0.29)*** | 86.37 (66.57) |
| Day | −0.00 (0.01) | −0.00 (0.01) | −0.00 (0.01) | −3.30 (2.76) | −0.00 (0.00) | −0.01 (0.01)** | −1.00 (1.34) |
| Number of Days | 0.02 (0.01) | 0.06 (0.02) | −0.00 (0.03) | −17.90 (7.35)* | −0.00 (0.01) | −0.03 (0.02) | −1.48 (3.91) |
| Social Jetlag | 0.05 (0.04) | 0.10 (0.06) | −0.14 (0.07) | −3.22 (20.23) | −0.00 (0.02) | −0.08 (0.05) | −7.52 (10.56) |
| Sex | 0.12 (0.08) | 0.62 (0.13) | 0.30 (0.14)* | −39.25 (40.99) | 0.11 (0.05)* | 0.32 (0.10)** | 29.36 (21.71) |
| ADHD Med | −0.08 (0.09) | −0.36 (0.14) | −0.21 (0.17) | 128.19 (49.17)** | 0.09 (0.06) | −0.04 (0.10) | 30.50 (25.06) |
| Sleep Med | −0.14 (0.12) | −0.09 (0.19) | −0.46 (0.22)* | 18.65 (63.72) | −0.07 (0.07) | −0.03 (0.13) | 8.09 (32.21) |
| Group | 0.11 (0.09) | 0.30 (0.14)* | 0.41 (0.16)* | 43.36 (46.25) | 0.11 (0.05)* | 0.22 (0.10)* | 24.30 (24.35) |
| Weekends (n=285) | |||||||
| Intercept | 0.17 (0.62) | 1.67 (1.18) | 2.49 (0.92)** | 299.83 (164.03) | 0.62 (0.20)** | 1.02 (0.35)** | 247.39 (89.53)** |
| Day | 0.01 (0.02) | 0.05 (0.02) | −0.03 (0.02) | −10.93 (4.47)* | −0.00 (0.01) | −0.02 (0.01)* | −0.66 (2.25) |
| Number of Days | 0.04 (0.04) | −0.02 (0.07) | −0.03 (0.05) | −4.03 (9.49) | −0.01 (0.01) | −0.04 (0.02) | −10.84 (5.18)* |
| Social Jetlag | 0.84 (0.11)*** | 1.50 (0.22)*** | 0.40 (0.17)* | 10.36 (29.46) | 0.06 (0.04) | −0.08 (0.06) | −0.39 (15.77) |
| Sex | −0.04 (0.21) | 0.69 (0.41) | 0.53 (0.32) | 25.65 (56.24) | 0.11 (0.07) | 0.50 (0.12)*** | 28.94 (30.36) |
| ADHD Med | −0.25 (0.28) | −0.77 (0.47) | −0.67 (0.40) | −94.29 (74.17) | −0.02 (0.09) | 0.05 (0.15) | 50.46 (38.93) |
| Sleep Med | −0.58 (0.38) | −0.11 (0.69) | 0.41 (0.56) | 125.66 (101.90) | 0.09 (0.12) | 0.18 (0.21) | 124.41 (53.25)* |
| Group | 0.29 (0.24) | 0.67 (0.45) | 0.61 (0.35) | 145.01 (63.10)* | 0.14 (0.08) | 0.31 (0.14)* | −28.81 (33.94) |
Note. Group coded comparison=0, ADHD=1. Sex coded male=0, female=1. Medication=Sleep Medication, 0=not taking sleep medication, 1=taking sleep medication.
p<.05,
p< .01,
p<.001.
Figure 2.
Exemplars of Intra-individual Variability for ADHD and Comparison participants for diary data. Example of a randomly selected participant from the comparison and ADHD groups for a) sleep duration, b) sleep quality, and c) Awakenings. Observations in shaded bars are weekend nights.
Several of the predictors/covariates also significantly predicted sleep/wake IIV. Specifically, social jetlag was associated with variability in bedtime, wake time, and duration (bedtime and wake time across the entire observation period; bedtime, wake time, and duration on weekends). Females experienced more variability in wake time, duration, sleep quality, and number of awakenings across the entire observation period. Additionally, taking ADHD medication was associated with less variability in wake time, sleep duration across the entire observation period, and more variability in sleep onset latency on schooldays. Finally, taking sleep medication was associated with less variability in sleep duration during schooldays and more variability in WASO on weekends.
Discussion
This study provides the first evidence that adolescents with ADHD have greater IIV in sleep/wake patterns than adolescents without ADHD. The present study represents a significant contribution to the literature and addresses major methodological limitations of existing research by using (1) a large sample adolescents with and without ADHD, (2) both actigraphy and sleep diary measures of sleep/wake patterns, and (3) MLM that uses the available time point and accounts for the non-independence of the data while also controlling for important variables including sex and medication use. Our findings are consistent with the notion that ADHD is a 24-hour disorder and characterized by variability, including sleep/wake variability.
Although there has been long-standing interest in whether youth with ADHD have more variable sleep/wake patterns than their peers without ADHD (Becker et al. 2017) all but one study in this area has been conducted in school-aged children or samples spanning childhood and adolescence. Adolescents with ADHD may be especially prone to variable sleep/wake patterns since parents have less oversight of bedtimes (Short et al., 2011), adolescents with ADHD are likely to experience impairments such as homework problems and parent-teen conflict that can impact sleep (Lunsford-Avery, Krystal, & Kollins, 2016; Becker & Langberg, 2017) and adolescents with ADHD may be more likely than their peers without ADHD to have a greater eveningness preference/later chronotype that can exacerbate phase delay (Coogan & McGowan, 2017). The one prior study examining sleep/wake IIV in adolescents with ADHD was a preliminary study with a small sample size that relied on parent-report of a previous diagnosis of ADHD (Moore et al., 2011). In contrast to the null findings reported in that study, results of the present study are consistent with the hypothesis that adolescents with ADHD experience greater IIV in sleep/wake patterns, with evidence found for both actigraphy- and diary-measured variables.
Bolstering confidence in the results obtained, findings for several sleep domains were consistent across actigraphy and daily sleep diary analyses. Specifically, across both actigraphy and diary methods, adolescents with ADHD had greater sleep/wake IIV than their peers for sleep duration (time in bed was used for actigraphy since this variable is the most accurate actigraphy-derived approximation of total sleep in adolescence; Short et al., 2011), wake time, and night wakings frequency (diary) or duration (actigraphy). We further found evidence for adolescents with ADHD displaying greater IIV in diary-assessed bedtime, sleep onset latency, and sleep quality. These findings are consistent with some previous studies (Gruber & Sadeh, 2004; Gruber, Sadeh, & Raviv, 2000) but inconsistent with others (Owens et al., 2009; Poirier & Corkum, 2018; Moore et al., 2011). However, previous studies evaluated sleep across the entire observation period and included participants across a broad age range (i.e., childhood and early adolescence). The only prior study examining sleep variability in adolescents with ADHD evaluated whether parent-reported ADHD was a unique predictor of variability and did not evaluate group differences in IIV. In addition, some studies that did not find greater sleep/wake IIV in children with ADHD used samples that were medication-naïve and/or free of any co-occurring psychiatric conditions, which may have resulted in less severe or representative samples being recruited for participation (Becker et al., 2017). Taken together, these findings highlight the need for research focused on possible differences across developmental periods, as well as research that uses large samples and statistical methods well-suited to daily-level data and variations.
Although not the focus of our study, we did not find adolescents with ADHD to experience more social jetlag compared to adolescents without ADHD. Accordingly, social jetlag does not seem to be a likely mechanism underlying our findings for greater sleep/wake IIV in adolescents with ADHD compared to their peers. However, greater social jetlag was associated with sleep/wake IIV across a number of actigraphy- and diary-assessed domains. This is not surprising since social jetlag represents the difference in sleep timing and duration between school nights and weekend nights. It is worth noting that social jetlag was also associated with sleep/wake IIV when examining school nights and weekend nights separately, suggesting that social jetlag is related to more variable sleep beyond merely the weekday-weekend difference. Adolescents who experience greater phase delay likely find it especially difficult to maintain consistent sleep/wake patterns across weekdays and weekends, consistent with the ‘perfect storm’ model whereby bioregulatory pressures interact with societal (e.g., school start time) and psychosocial (e.g., autonomy, academic pressure, screen time) pressures to contribute to insufficient and ill-timed sleep in adolescence (Crowley et al., 2018). Studies are needed to more directly test these interrelations in adolescents with ADHD specifically. Some factors of the perfect storm model are modifiable, whereas others are not (Crowley et al., 2018). Intriguingly, a recent cognitive-behavioral intervention conducted with adolescents with an evening chronotype not only improved adolescent- and parent-reported sleep, but also resulted in a decrease in evening circadian preference, an earlier endogenous circadian phase (i.e., dim light melatonin onset), and less weeknight-weekend discrepancy in total sleep time and wake time (Harvey et al., 2018). No studies have tested behavioral sleep interventions in adolescents with ADHD; testing cognitive-behavioral and other interventions for improving sleep and daytime outcomes is an important area for investigation (Becker, 2019).
Our findings indicate that sleep stability and variability are important to assess in studies aiming to understand the sleep of adolescents with ADHD. It is likely insufficient for clinicians to ask only about “typical” sleep (e.g., average sleep duration), which may mask variability. There is growing evidence that sleep IIV is associated with poorer physical and emotional health (Becker et al., 2017), though studies have yet to examine these associations in adolescents with ADHD specifically. As such, important next steps in this line of research include evaluating both predictors and consequences of sleep IIV in adolescents with ADHD. Co-occurring internalizing symptoms, homework problems, technology use, exercise, sleep environment, and parent restrictions and monitoring behaviors are all factors with potential to explain the increased sleep IIV found in adolescents with ADHD. Understanding the specific factors that lead to sleep IIV will be critical for intervention development. Given the wide-ranging impairments experienced by adolescents with ADHD, it will also be informative to examine whether sleep IIV contributes to, or exacerbates, daytime functioning and impairments. For instance, research has yet to explore if adolescents with ADHD with more variable sleep have poorer outcomes relative to adolescents with ADHD with more stable sleep. Finally, it will be important to examine possible transactional processes. Staying up to complete homework or a school project may contribute to more variable sleep patterns, which may in turn contribute to greater inattention and emotional problems during the day, resulting in a negative cascade. Models such as this have yet to be tested and would be informative for both theory and clinical intervention.
Findings of the present study should be interpreted in the context of several limitations. First, actigraph and sleep diary data are not without limitations. Sleep diaries may be subject to memory distortions or social/parental desirability (e.g., adolescents may not want their parents to know how late they actually were up). Further, actigraphy is limited in its ability to detect transitions between sleep and wake states, resulting in limited ability to accurately measure sleep onset latency. Additionally, since the larger study’s main purpose was to look at sleep, we used the Sadeh sleep scoring algorithm to code actigraphy data during periods of sleep rather than throughout the entire day and thus are unable to examine non-parametric analysis of circadian rhythms, to extract measures of interdaily stability and IIV. This is an important area for future research to explore. Second, a limited number of actigraph and sleep diary data were available for weekends, which resulted in some participants having only two weekend points despite the mean number of weekend data points being 4. Thus, the weekend results should be interpreted with caution (Acebo et al., 1999). Third, medication use was controlled for in the present analyses, but given the small number of participants taking sleep medication we were unable to examine whether our results differed between individuals on vs. not on sleep medications. Finally, although all adolescents were instructed to wear the actigraph and complete the daily diaries for a two-week period, there was variability in adherence. However, adolescents with ADHD and comparisons had equivalent actigraph data and adolescents with ADHD had less than one day fewer diary data points.
In conclusion, this study represents a significant contribution to the ADHD sleep literature by examining sleep IIV in objective and subjective measures using MLM in a large sample of adolescents with and without ADHD. Results suggest that it is important for sleep research with adolescents with ADHD to examine sleep IIV rather than averages of sleep variables. Future research should examine predictors of sleep IIV (e.g., internalizing symptoms, technology use, difficulty with homework) and whether sleep IIV predicts important outcomes within this at risk sample.
Supplementary Material
Figure S1. Exemplars of 7 days worth of actograms for a participant a) without ADHD (88.03% Efficiency) and b) with ADHD (77.01% Efficiency).
Key Points.
It is unknown whether adolescents with attention-deficit/hyperactivity disorder (ADHD) have more day-to-day sleep variability than adolescents without ADHD.
In a study that included both actigraphy and daily sleep diaries collected over a 14–30 day period, adolescents with ADHD (n=162) had significantly greater sleep variability than adolescents without ADHD (n=140). Group differences were more common on schooldays, particularly for actigraph data.
Evaluating sleep patterns and problems of adolescents with ADHD at a group/average level may mask important day-to-day variability in sleep. Day-to-day variability differences and the factors that cause them may be important targets for intervention.
Acknowledgements
This research was supported by the Institute of Education Science (IES), U.S. Department of Education (grant R305A160126; Drs. Langberg and Becker). S.P.B. is supported by grant K23MH108603 from the National Institute of Mental Health (NIMH). The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of this article:
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, & Carskadon MA (1999). Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep, 22(1), 95–103. [DOI] [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, & Thome J (2012). Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Molecular psychiatry, 17(10), 988. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Gottfredson NC, Dean D, & Zucker RA (2013). Analyzing repeated measures data on individuals nested within groups: Accounting for dynamic group effects. Psychological methods, 18(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP (2019). The triple threat of sleep, adolescence, and ADHD In Hiscock H & Sciberras E (Eds.), Sleep and ADHD: An evidence-based guide to assessment and treatment (pp. 257–294). San Diego, CA: Elsevier/Academic Press. doi: 10.1016/B978-0-12-814180-9.00011-9 [DOI] [Google Scholar]
- Becker SP, & Langberg JM (2017). Difficult to bed and difficult to rise: Complex interplay among ADHD, sleep, and adolescence. The ADHD Report, 25(1), 7–13. [Google Scholar]
- Becker SP, Langberg JM, Eadeh H-M, Isaacson PA, & Bourchtein E (2019). Sleep and daytime sleepiness in adolescents with and without ADHD: Differences across ratings, daily diary, and actigraphy. Journal of Child Psychology and Psychiatry. Advance online publication. doi: 10.1111/JCPP.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SP, Sidol CA, Van Dyk TR, Epstein JN, & Beebe DW (2017). Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: a systematic review. Sleep medicine reviews, 34, 94–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience, 3(8), 617. [DOI] [PubMed] [Google Scholar]
- Colrain IM, & Baker FC (2011). Changes in sleep as a function of adolescent development. Neuropsychology review, 21(1), 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, & McGowan NM (2017). A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. ADHD Attention Deficit and Hyperactivity Disorders, 9(3), 129–147. [DOI] [PubMed] [Google Scholar]
- Cortese S, Faraone SV, Konofal E, & Lecendreux M (2009). Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. Journal of the American Academy of Child & Adolescent Psychiatry, 48(9), 894–908. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, & Castellanos FX (2012). Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. American Journal of Psychiatry, 169(10), 1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Wolfson AR, Tarokh L, & Carskadon MA (2018). An update on adolescent sleep: New evidence informing the perfect storm model. Journal of adolescence, 67, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Díaz-Morales JF, & Escribano C (2015). Social jetlag, academic achievement and cognitive performance: Understanding gender/sex differences. Chronobiology international, 32(6), 822–831. [DOI] [PubMed] [Google Scholar]
- Feinberg I, & Campbell IG (2010). Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain and cognition, 72(1), 56–65. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Arruda EH, Krull JL, & Gonzales NA (2018). Adolescent sleep duration, variability, and peak levels of achievement and mental health. Child development, 89(2), e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AJ, Bai S, Krull JL, & Gonzales NA (2017). Individual differences in optimum sleep for daily mood during adolescence. Journal of Clinical Child & Adolescent Psychology, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H (2011). Multilevel statistical models (Vol. 922). John Wiley & Sons. [Google Scholar]
- Gruber R, & Sadeh A (2004). Sleep and neurobehavioral functioning in boys with attention-deficit/hyperactivity disorder and no reported breathing problems. Sleep, 27(2), 267–273. [DOI] [PubMed] [Google Scholar]
- Gruber R, Sadeh AVI, & Raviv A (2000). Instability of sleep patterns in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 39(4), 495–501. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Hein K, Dolsen MR, Dong L, Rabe-Hesketh S, Gumport NB, … & Smith RL (2018). Modifying the impact of eveningness chronotype (“night-owls”) in youth: a randomized controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry, 57(10), 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren L, & Franke B (2017). Subcortical brain volume differences of participants with ADHD across the lifespan: An ENIGMA collaboration. Lancet Psychiatry, 4(4), 310–319.28219628 [Google Scholar]
- Hox JJ, Moerbeek M, & Van de Schoot R (2017). Multilevel analysis: Techniques and applications. Routledge. [Google Scholar]
- IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jankowski KS (2017). Social jet lag: Sleep-corrected formula. Chronobiology international, 34(4), 531–535. [DOI] [PubMed] [Google Scholar]
- Jenni OG, & O’Connor BB (2005). Children’s sleep: an interplay between culture and biology. Pediatrics, 115(Supplement 1), 204–216. [DOI] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, & Cortese S (2010). Sleep and ADHD. Sleep medicine, 11(7), 652–658. [DOI] [PubMed] [Google Scholar]
- Konrad K, & Eickhoff SB (2010). Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human brain mapping, 31(6), 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JS, & Bijlenga D (2013). The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Review of Neurotherapeutics, 13(10), 1107–1116. [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery JR, Krystal AD, & Kollins SH (2016). Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clinical psychology review, 50, 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, McNamara JP, Rowe MA, Dzierzewski JM, Dirk J, Marsiske M, & Craggs JG (2008). Sleep and affect in older adults: Using multilevel modeling to examine daily associations. Journal of sleep research, 17(1), 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan NM, Voinescu BI, & Coogan AN (2016). Sleep quality, chronotype and social jetlag differentially associate with symptoms of attention deficit hyperactivity disorder in adults. Chronobiology international, 33(10), 1433–1443. [DOI] [PubMed] [Google Scholar]
- Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, & Redline S (2011). Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep medicine, 12(3), 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Rouleau N, & Morin CM (2014). Sleep of children with attention deficit hyperactivity disorder: actigraphic and parental reports. Behavioral sleep medicine, 12(1),69–83. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. (2006). 2006 Sleep in America Poll. Washington, DC: National Sleep Foundation. [Google Scholar]
- O’Brien EM, & Mindell JA (2005). Sleep and risk-taking behavior in adolescents. Behavioral sleep medicine, 3(3), 113–133. [DOI] [PubMed] [Google Scholar]
- Owens J, Sangal RB, Sutton VK, Bakken R, Allen AJ, & Kelsey D (2009). Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep medicine, 10(4), 446–456. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Laska MN, Lytle LA, & Moe SG (2010). Adolescent sleep, risk behaviors, and depressive symptoms: Are they linked? American journal of health behavior, 34(2), 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier A, & Corkum P (2018). Night-to-night variability of sleep in children with ADHD and typically developing controls. Journal of attention disorders, 22(10), 942–946. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical linear models: Applications and data analysis methods (Vol. 1). Sage. [Google Scholar]
- Rowe M, McCrae C, Campbell J, Horne C, Tiegs T, Lehman B, & Cheng J (2008). Actigraphy in older adults: comparison of means and variability of three different aggregates of measurement. Behavioral sleep medicine, 6(2), 127–145. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey M, & Carskadon MA (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep, 17(3), 201–207 [DOI] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Wright H, Lack LC, Dohnt H, & Carskadon MA (2011). Time for bed: parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep, 34(6), 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright H, & Carskadon MA (2012). The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep medicine, 13(4), 378–384. [DOI] [PubMed] [Google Scholar]
- Short MA, Weber N, Reynolds C, Coussens S, & Carskadon MA (2018). Estimating adolescent sleep need using dose-response modeling. Sleep, 41(4), zsy011. [DOI] [PubMed] [Google Scholar]
- Snijders TA, & Bosker RJ (1999). Multilevel analysis: an introduction to basic and advanced multilevel modelling.
- Steinberg L (2010). Commentary: A behavioral scientist looks at the science of adolescent brain development. Brain and cognition, 72(1), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Saletin JM, & Carskadon MA (2016). Sleep in adolescence: physiology, cognition and mental health. Neuroscience and biobehavioral reviews, 70, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, & Carskadon MA (2005). Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. Journal of sleep research, 14(3), 239–244. [DOI] [PubMed] [Google Scholar]
- Troiano RP (2007). Large-scale applications of accelerometers: new frontiers and new questions. Medicine & Science in Sports & Exercise, 39(9), 1501. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JS, Boonstra AM, Gordijn MC, & Van Someren EJ (2010). Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biological Psychiatry, 67(11), 1091–1096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Exemplars of 7 days worth of actograms for a participant a) without ADHD (88.03% Efficiency) and b) with ADHD (77.01% Efficiency).