Abstract
Background
Obstructive lung disease (OLD) is associated with increased multimorbidity but few studies have examined its impact on mortality particularly in the older adult population. This study aims to investigate the impact of respiratory symptoms in current and former smokers with and without OLD on all-cause mortality.
Methods
This is a secondary analysis in a prospective cohort (the Health, Aging and Body Composition Study) in community-dwelling current and former smokers (N=596) aged 70-79 years. Participants were categorized into 4 mutually exclusive groups based on the modified Medical Research Council Dyspnea Scale ≥1 and forced expiratory volume in the 1st second to forced vital capacity ratio <0.70. The groups were Less Dyspnea-No OLD (N=196), More Dyspnea-No OLD (N=104), Less Dyspnea-With OLD (N=162), and More Dyspnea-With OLD (N=134).
Results
53% in Less Dyspnea-No OLD, 63% in More Dyspnea-No OLD, 67% in Less Dyspnea-With OLD, and 84% in More Dyspnea-With OLD died within the 13- year follow up period (log-rank chi-square=44.4, p<.0001). The hazard ratio was highest for participants with OLD, both with (HR=1.91, 95% CI 1.44 – 2.54; p<.0001) and without dyspnea (HR=1.52, (95% CI 1.15 – 2.02; p=.004). Participants without OLD but with dyspnea had a similar risk of death to subjects who had OLD but fewer symptoms.
Conclusions
OLD is associated with high risk of death with different risk profiles based on symptom group. Patients with symptoms of shortness of breath without OLD should be considered an at-risk group given their similar mortality to those with OLD with minimal symptoms.
Keywords: Aging, Obstructive Lung Disease, Survival, FEV1, Smoking
INTRODUCTION
There is a growing societal impact of obstructive lung disease (OLD) on quality of life, leading to economic consequences, and mortality. The age-adjusted prevalence of chronic obstructive pulmonary disease (COPD) in adults aged 40 years and over has increased by 44% over the past 3 decades.1, 2 COPD is the third leading cause of mortality worldwide with mortality rates increasing with age.3, 4 Further, smoking is associated with more than 50% the attributable risk of chronic OLD, especially COPD, and contributes to a large proportion of mortality in both high and low-income countries.5, 6 However, some smokers do not develop airflow obstruction (i.e. have preserved lung function) defined as a low forced expiratory volume in 1st second to the forced vital capacity ratio (FEV1/FVC) <0.70 based on the modified Global Initiative for Chronic Lung Disease (GOLD)7 but nevertheless have symptoms including shortness of breath, cough, and wheeze.8 Despite this, there is a minimal data, particularly in older adults, on the diagnosis and health consequences of OLD.
Understanding risks associated with increased mortality in smokers with and without OLD is important because OLD is associated with increased economic burden and adverse health outcomes. Regardless of lung function, smokers with symptoms have high rates of respiratory exacerbations and activity limitation based on 6-minute walk distance.9-12 Moreover, those with exacerbations have high healthcare use, report low health-related quality of life scores, demonstrate thoracic computed tomography evidence of emphysema or gas trapping, and were more likely to use respiratory medications despite a lack of evidence-based approach.9, 12-14 A recent task-force statement by the American Thoracic Society(ATS)/European Respiratory Society (ERS) emphasized the need for additional research in smokers with symptoms without an OLD or COPD diagnosis confirmed by spirometry, as well as the impact of age on the importance of identifying airflow limitation.15
The primary aim of this study is to investigate the risk of symptoms in tobacco exposed older adults with and without spirometric evidence of OLD on all-cause mortality in the Health, Aging, and Body Composition (Health ABC) Study. We hypothesize that irrespective of lung function and tobacco exposure, older adults who are less symptomatic will have lower risk of mortality compared to those who are more symptomatic. Further, smokers with symptoms who also have OLD will have the highest mortality.
METHODS
Study Population
The Health ABC Study protocol was approved by the University of Pittsburgh and the University of Tennessee, Knoxville Institutional Review Boards (Health ABC IRB approval # 960212 at the University of Pittsburgh and IRB approval # 5531 at the University of Tennessee, Knoxville). All participants provided written informed consent.
The Health ABC Study is a prospective cohort that enrolled 3,075 well-functioning, community dwelling older adults aged 70 to 79 years old between April 1, 1997 and July 31, 1998. Participants were identified based on a random sampling of white Medicare beneficiaries and all age-eligible black community residents within designated zip code areas surrounding Pittsburgh, Pennsylvania, and Memphis, Tennessee. Participants were excluded if they had: a) had difficulty walking one quarter mile, walking up 10 steps, performing activities of daily living or, had mobility impairment b) were individuals with known life-threatening cancers; or c) planned to leave the area within 3 years. The present analysis includes 596 smokers (defined as self-reported current or former smokers with a smoking history of at least 20 pack-years) for whom complete adjudicated 13-year follow-up data were available. We excluded participants without complete data for pulmonary function testing, and 106 participants with FEV1/FVC ≥ 0.70 and FVC below the lower limit of normal (LLN) in order to minimize the misclassification of participants with restrictive ventilatory disease as having OLD.
Spirometry, OLD, and Symptom Definitions
Spirometry procedures were performed in accordance with international standards as described by the ATS/ERS.16 A more detailed description of the procedure is available.17
For this analysis, participants were categorized as with and without OLD according to the results on spirometry. Since spirometric testing in the Health ABC Study did not include post-bronchodilator reversibility testing, it was not possible to distinguish reversible from irreversible obstruction. OLD was defined by FEV1/FVC <0.70 per modified GOLD criteria.8, 18 Symptoms were quantified using questions from the modified Medical Research Council Dyspnea Scale (MMRC), a validated scale that assesses perceived respiratory disability (see Table 1).19 Since cross-sectional studies in OLD have reported 70–80% prevalence of dyspnea using an MMRC of ≥1, an MMRC ≥1 was considered a threshold of higher symptoms.20, 21 A 12-month symptom history was obtained for daily cough, and wheeze using a standardized questionnaire.
Table 1.
Dyspnea Symptoms Assessed Using the Modified Medical Research Council Dyspnea Scale
| MMRC Grade 1 |
|---|
| “Are you troubled by shortness of breath when hurrying on a level surface or walking up a slight hill?” |
| MMRC Grade 2 |
| “Do you ever have to stop for breath when walking at your own pace on a level surface?” |
| or |
| “Do you have to walk slower than people your own age when on a level surface because of breathlessness?” |
MMRC= Modified Medical Research Council Dyspnea Scale
Based on MMRC symptom profile and FEV1/FVC ratio, participants were categorized into 4 mutually exclusive groups: MMRC <1 with FEV1/FVC ≥0.70 [Less Dyspnea-No OLD (N=196)], MMRC ≥1 with FEV1/FVC ≥0.70 [More Dyspnea-No OLD (N=104)], MMRC <1 with FEV1/FVC <0.70 [Less Dyspnea-With OLD (N=162)], and MMRC ≥1 with FEV1/FVC <0.70 [More Dyspnea-With OLD (N=134)].
Mortality
The Health ABC Diagnosis and Disease Ascertainment Committee reviewed all death certificates, hospital records, autopsy data, and used informant interviews to adjudicate the underlying and immediate cause of death. All diagnosis were verified based on hospital records, interviews, and death certificates by a panel of physicians.
Covariates
Age, gender, self-reported race (black or white), and site were obtained. Education was ascertained as the highest grade completed. Additional covariates were chosen based on their known relationship with mortality. These included pack years smoking and body mass index (BMI). Comorbidity count was based on the physician-diagnosed medical disease determined using algorithms of self-reporting, physician diagnosed medical disease, and medication use. The comorbidity count was constructed from adjudicated chronic health conditions using: cardiovascular disease, congestive heart failure, peripheral vascular disease, stroke, pulmonary disease, arthritis, diabetes mellitus, peptic ulcer disease, and cancer. Usual gait speed is a measure of functional status and is measured as time needed to walk a 20-meter straight course.22 The digit symbol substitution test (DSST) is a measure of cognitive function and requires sustained attention, response speed, set shifting, and visual spatial skills. Participants are asked to fill in a series of symbols correctly within 90 seconds, with higher scores indicating better performance.23
Statistical Analysis
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). For comparison among groups we used one-way analysis of variance, the Kruskal- Wallis test, and Chi-square test as appropriate. A Bonferroni-correction for each pairwise comparison between individual groups (with adjustments made for 6 pairwise comparisons across 4 groups) was then used to examine individual differences between groups. A survival analysis was conducted amongst less dyspneic and more dyspneic current and former smokers with and without OLD. Mortality was examined with cox proportional hazard models using the above set of covariates. The association between odds of death with cognitive function, physical function, comorbid status were also examined using multivariable logistic regression controlling for age, sex, race, education, BMI, and pack year smoking history. All p-values are two sided with p value < 0.05 considered statistically significant.
In multiple sensitivity analyses, we re-ran all models by redefining (a) OLD as FEV1/FVC < LLN, an alternative to the fixed ratio (FEV1/FVC <0.70) definition and (b) dyspnea as an MMRC 2 since it is considered a threshold for more severe breathlessness. A third sensitivity analysis examined mortality in participants with a restrictive ventilatory defect, given their exclusion from the main analysis.
RESULTS
Demographics
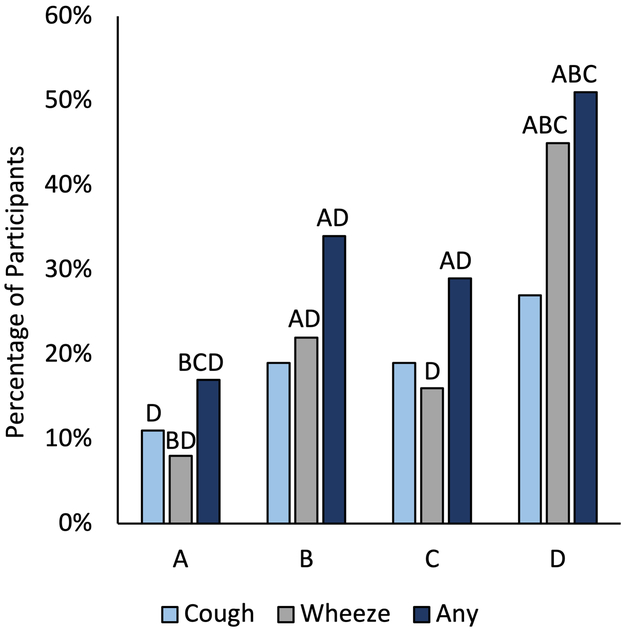
We found that irrespective of the presence of OLD, groups with More Dyspnea were similar in age, gender, race, education, pack-year smoking history, had the highest comorbidity counts, and lowest physical and cognitive function at baseline (Table 2). More Dyspnea-With OLD had the lowest FEV1% and FVC% predicted and reported more symptoms of wheeze or cough amongst all groups (Figure 1). More Dyspnea-No OLD had the highest BMI, while groups with OLD, regardless of symptoms, had the lowest BMI. More Dyspnea-With OLD were more likely to have physician diagnosed asthma, emphysema, or chronic bronchitis.
Table 2.
Baseline Characteristics of Current and Former Smokers With and Without Obstructive Lung Disease
| Without OLD | With OLD | ||||
|---|---|---|---|---|---|
| Less Dyspnea N= 196 |
More Dyspnea N= 104 |
Less Dyspnea N= 162 |
More Dyspnea N= 134 |
P value | |
| A | B | C | D | ||
| Age, years | 77.1 (2.7) | 77.2 (2.7) | 77.4 (2.7) | 77.6 (3.1) | .46 |
| Gender | .01 | ||||
| Female | 72 (37%) | 52 (50%)C | 49 (30%)B | 48 (36%) | |
| Male | 124 (63%) | 52 (50%) | 113 (70%) | 86 (64%) | |
| Race | .08 | ||||
| Black | 69 (35%) | 44 (42%) | 45 (28%) | 51 (38%) | |
| White | 127 (65%) | 60 (58%) | 117 (71%) | 83 (62%) | |
| Education | .002 | ||||
| ≤ High-school | 90 (46%)BD | 70 (67%)A | 85 (52%) | 82 (61%)A | |
| Post-secondary | 106 (54%) | 34 (33%) | 77 (48%) | 52 (39%) | |
| Current Smoking | 35 (17%) | 22 (21%) | 44 (27%) | 38 (28%) | .09 |
| Pack-years smoking | 35 (25–53)CD | 39 (26–54) | 46 (29 –60)A | 46 (31–61)A | .02 |
| *Lung Function | |||||
| FEV1, mL | 2341.0 (585.7)BCD | 2095.4 (593.2)AD | 1962.2 (598.7)AD | 1488.2 (517.9)ABC | <.0001 |
| FEV1, % | 103.4 (14.7)CD | 99.3 (14.0)CD | 81.4 (17.6)ABD | 66.3 (22.5)ABC | <.0001 |
| FVC, mL | 3067.8 (773.8)BD | 2716.9 (770.4)AC | 3125.0 (846.9)BD | 2546.4 (770.4)AC | <.0001 |
| FVC, % | 99.0 (14.0)D | 95.0 (14.0)D | 94.7 (17.1)D | 81.8 (21.0)ABC | <.0001 |
| FEV1/FVC | 0.77 (0.05)C,D | 0.77 (0.06)C,D | 0.63 (0.07)A,B,D | 0.58 (0.09)A,B,C | <.0001 |
| BMI, kg/m2 | 27.4 (4.5)BC | 29.6 (4.4)ACD | 25.1 (3.8)AB | 26.4 (5.0)B | <.0001 |
| **Comorbidity Count | |||||
| 0 | 67 (34%) | 26 (25%) | 64 (40%) | 35 (26%) | .03 |
| 1 | 75 (38%) | 35 (34%) | 64 (40%) | 45 (34%) | .63 |
| ≥ 2 | 54 (28%) | 43 (41%)C | 34 (20%)BD | 54 (40%)C | .0002 |
| Physician Diagnosed Pulmonary Disease | |||||
| Asthma | 3 (2%)D | 9 (8%)D | 9 (6%)D | 23 (17%)ABC | <.0001 |
| COPD | 2 (1%) | 0 (0%) | 3 (2%) | 7 (5%) | .02 |
| Emphysema | 5 (3%)D | 2 (2%)D | 9 (6%)D | 28 (21%)ABC | <.0001 |
| Chronic bronchitis | 10 (5%)D | 14 (13%) | 20 (12%)D | 35 (26%)AC | <.0001 |
| UGS, m/s | 1.15 (0.20)D | 1.09 (0.21) | 1.15 (0.20)D | 1.04 (0.20)AC | <.0001 |
| DSST (range 0-133) | 36.0 (14.5)D | 33.6 (12.8) | 33.2 (14.3) | 31.7 (13.1)A | .04 |
Note: Values are mean (SD), median (IQR), or N (%). Each superscript letter denotes categories whose column properties that differ significantly from each other at the p < .05 for each pairwise comparison (vs. the indicated group) with Bonferroni correction for multiple comparison across the 4 groups (6 comparisons).
OLD=Obstructive lung disease; BMI= Body mass index; UGS= Usual gait speed; DSST=Digit symbol substitution test
FEV1% and FVC % predicted values are derived from reference equations 37
Comorbidity count is defined from cardiovascular disease, congestive heart failure, peripheral vascular disease, stroke, pulmonary disease, arthritis, diabetes mellitus, peptic ulcer disease, and cancer
Figure 1.
Frequency of symptoms of cough and wheeze within each group of current and former smokers with or without obstructive lung disease (OLD). Each letter denotes categories whose group properties (A=Less Dyspnea-No OLD; B=More Dyspnea-No OLD; C=Less Dyspnea-With OLD; D=More Dyspnea-With OLD) differ significantly from each other at the p<.05 for each pairwise comparison (vs. the indicated group) with Bonferroni correction for multiple comparison across the 4 groups (6 comparisons).
All-Cause Mortality
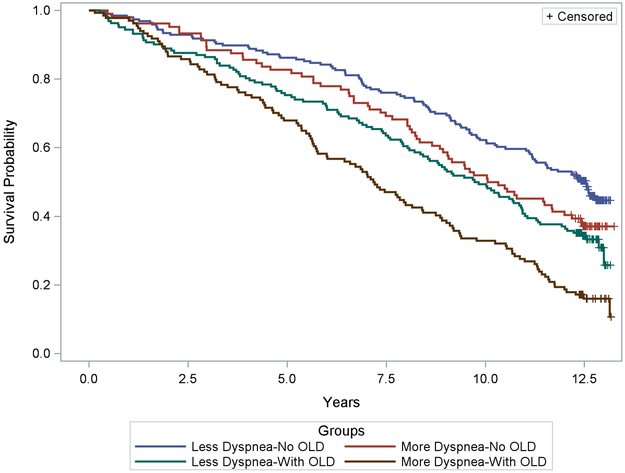
After 13-years of follow up, 103/196 (53%) in Less Dyspnea-No OLD had died as compared to 65/104 (63%) in More Dyspnea-No OLD, 109/162 (67%) in Less Dyspnea-With OLD, and 113/134 (84%) in More Dyspnea-With OLD. The corresponding 13-year Kaplan-Meier estimates for the median survival times are 12.5 yrs (95% CI 11.2 – 13.3 yrs) in Less Dyspnea-No OLD, 10.2 yrs (8.7 – 12.0 yrs) in More Dyspnea-No OLD, 9.8 yrs (8.4 – 10.9 yrs) in Less Dyspnea-With OLD, and 7.2 yrs (5.8 – 8.4 yrs) in More Dyspnea-With OLD (log-rank chi-square=44.4, p<.0001) (Figure 2). More Dyspnea-With OLD was associated with the highest mortality compared to all groups after bonferonni correction (p<.05 for all group comparisons). Comparison of survival curves between Less Dyspnea-With OLD and More Dyspnea-No OLD showed no significant difference between these two groups (p= 1.00).
Figure 2.
Kaplan Meier survival probability plot of less dyspneic and more dyspneic current and former smokers with and without obstructive lung disease (OLD) as a function of time (years). Each color represents the curves of a separate group.
In unadjusted models, the risk of death for More Dyspnea-No OLD compared to Less Dyspnea-No OLD (reference) was not significant (Table 3). The risk of death for Less-Dyspnea-With OLD was 1.56 times higher (95% CI 1.19 – 2.04, p=.001) and the risk of death for More Dyspnea-With OLD was 2.45 times higher (95% CI 1.88 – 3.21, p<.0001) compared to Less Dyspnea-No OLD. In models adjusting for covariates, these trends in mortality persisted (Table 2). In the fully adjusted model there was a lower risk of death in Less Dyspnea-With OLD by 2%, HR 1.52 (95% CI 1.15 −2.02, p=.004) though this remained significant. The risk of death in More Dyspnea-With OLD was attenuated by 28%, 1.91 (95% CI 1.44 – 2.54, p<.0001). When compared to Less Dyspnea-With OLD, the risk of death for More Dyspnea-No OLD was similar (HR 0.86, 95% CI 0.62 – 1.19, p=.35) and this also remained non-significant. However, while compared to More Dyspnea-With OLD, the risk of death for More Dyspnea-No OLD was 45% lower (HR 0.55 95% CI 0.41 – 0.75, p=.02).
Table 3.
Models for All-Cause Mortality Amongst Current and Former Smokers With and Without Obstructive Lung Disease
| Less Dyspnea- No OLD |
More Dyspnea- No OLD |
P value | Less Dyspnea - With OLD |
P value | More Dyspnea- With OLD |
P value | |
|---|---|---|---|---|---|---|---|
| Model 1 | Reference | 1.34 (0.98-1.82) |
.07 | 1.56 (1.19- 2.04) |
.001 | 2.45 (1.88- 3.21) |
<.0001 |
| Model 2 | Reference | 1.31 (0.96- 1.79) |
.09 | 1.59 (1.20- 2.09) |
.001 | 2.37 (1.81- 3.11) |
<.0001 |
| Model 3 | Reference | 1.39 (1.01- 1.91) |
.04 | 1.46 (1.10- 1.94) |
.009 | 2.29 (1.74- 3.02) |
<.0001 |
| Model 4 | Reference | 1.30 (0.95- 1.79) |
.10 | 1.52 (1.15- 2.02) |
.004 | 1.91 (1.44- 2.54) |
<.0001 |
Data presented as hazard ratio (95% confidence interval).
OLD=Obstructive lung disease.
Model 1: Unadjusted.
Model 2: Adjusted for age, sex, race, study site.
Model 3: Model 2 + education, body mass index (BMI), BMI2 pack years smoking.
Model 4: Model 3 + comorbidity count, usual gait speed, digit symbol sign test score.
A multivariate analysis of the impact of comorbid status, physical function, and cognitive function on the odds of death in all smokers regardless of their lung function and symptoms was subsequently done (Supplementary Table S1). Of these, faster gait speed and higher DSST scores (better cognitive function) were associated with lower odds of death. Higher comorbidity count was associated with higher odds of death.
Sensitivity Analyses
Survival is presented in Supplementary Figure S1 using FEV1/FVC < LLN as a cutoff to define OLD. Overall, the sensitivity analysis yielded similar results in unadjusted and adjusted models (Supplementary Table S3). However, there was higher risk of death in Less Dyspnea-With OLD (HR 1.50, 95% CI 1.04 – 2.15, p=.03) compared to More Dyspnea-Without OLD in fully adjusted models. While the risk of death between both groups with More Dyspnea was similar to the main analysis.
Survival is presented in Supplementary Figure S2 using MMRC of 2 to define the threshold of dyspnea. The sensitivity analysis yielded similar results for both groups with OLD in unadjusted and adjusted models to the main analysis (Supplementary Table S4). More Dyspnea-Without OLD had higher risk of death compared to Less Dyspnea-Without OLD (Reference), in all models. Similar to the main analysis, there was no difference in the risk of death between More Dyspnea-Without OLD and Less Dyspnea-With OLD. However, More Dyspnea-Without OLD and More Dyspnea-With OLD-With OLD also had similar risk of death.
Last, there was no difference in mortality or risk of death in adjusted and fully adjusted models in participants with a restrictive ventilatory defect compared to those without a restrictive ventilatory defect (Supplementary Figure S3 and Supplementary Table S5).
DISCUSSION
To our knowledge, this is the first study that investigates the risk of respiratory symptoms, specifically dyspnea, in older adults with history of current and former smoking and its relationship to mortality. Our study demonstrates that current and former smokers with dyspnea, regardless of the presence of OLD are at increased risk for 13-year mortality. The mortality risk differs across dyspnea profiles with participants with OLD and prominent respiratory symptoms, including cough and wheeze, having the highest mortality. While participants with Less Dyspnea-With OLD had better lung function, better physical function, less comorbid disease, lower risk for all-cause mortality compared to More Dyspnea-With OLD, their risk of death was still 56% and 52% higher compared to those with Less Dyspnea-No OLD (reference) in unadjusted and fully adjusted models. Somewhat unexpectedly, the risk of death for More Dyspnea-No OLD was similar to the Less Dyspnea-With OLD group.
Our findings highlight the importance of evaluating not only lung function, but dyspnea, in patients at risk for OLD, which is in agreement with other studies derived from the US and Canadian populations.9, 11-13, 18 These studies demonstrated that despite normal FEV1, smokers with symptoms and preserved FEV1/FVC ratio have pathologic characteristics of chronic lung disease such as COPD however did not study mortality.9, 13 OLD has been associated with increased risk of death,18 and our study shows this association differs by dyspnea group. Smokers with dyspnea and preserved FEV1/FVC ratio (More Dyspnea-No OLD) had a similar mortality profile as smokers with less dyspnea who have an FEV1/FVC ratio <0.70 (Less Dyspnea-With OLD) and therefore should be considered an at-risk group. Further, More-Dyspnea-No OLD share similar demographic profile as the More Dyspnea-With OLD group including low physical and cognitive function at baseline.
Our results provide novel data on the impact of physical and cognitive function on mortality in smokers. Older adults who smoke, who have a faster usual gait speed and higher DSST, independent of their symptoms, have a lower mortality risk. In COPD, a reduction in gait speed has been associated with short-term mortality.24 However, improvement in gait speed in older adults has been shown to predict improved survival.25 Cognitive impairment without dementia pathology is also associated with mortality in older adults.26 Further, COPD has been shown to be a risk factor for cognitive impairment.27 As such, targeting co-occurring conditions including functional decline and cognitive impairment through early assessment and treatment may improve and impact survival.28 Our study suggest the assessment of physical and cognitive function is important in older adults with and without OLD and may present some opportunities to modify risk factors.
Our study is relevant to the under-diagnosis of OLD. We acknowledge the evidence that screening for OLD has shown that it could potentially lead to harm including increased false positive results in potentially healthy persons. However early diagnosis through screening can lead to early or modifications in pharmacologic or non-pharmacologic therapy.29 Low lung function is associated with mortality in OLD as well as risk of increased dyspnea and suggests the need for intervention.7, 18 These results suggest that these patients should be considered for early pharmacologic or non-pharmacologic therapy because of their increased risk for death. Further, our study shows that 9% in More Dyspnea-No OLD, 44% in Less Dyspnea-With OLD, and 78% in More Dyspnea-With OLD have an FEV1 % predicted <80% and therefore should be considered high risk related to multimorbidity and potential risk of death. The typical presentation of OLD, including asthma, in older adults, may lead to mis-diagnosis or under-diagnosis and therefore can lead to undertreatment due to multimorbidity patterns.30, 31 Older adults with asthma may have variable presentation and do not all present with airflow limitation.32 Further, there is no agreed upon spirometry defined cutoff for the diagnosis COPD in older adults.33 Studies have also shown some evidence of dissociation between lung function and symptoms of dyspnea, cough and wheeze suggesting that current standards need reassessment when evaluating for respiratory impairment in this group of patients.34, 35 Due to their high symptom burden and risk of death, development of diagnostic standards in older adults should be prioritized.
Our sensitivity analysis demonstrates the impact on the LLN and severity of dyspnea used to define our groups on mortality outcomes. Our sensitivity analysis for FEV1/FVC < LLN showed a lower risk of death in More Dyspnea-Without OLD compared to Less Dyspnea-With OLD. In addition, our study show that those with an MMRC of ≥1 are at an overall increased risk of death. However, More Dyspnea-Without OLD with an MMRC of 2 may be at a risk comparable to More Dyspnea-With OLD. More studies are necessary to compare different approaches to provide precision outcome measures for these groups.
Our study has limitations. First, study participants in the Health ABC Study were selected based on voluntary participation and good functional capacity. Therefore, our study participants may not represent the general older adult population. Second, the Health ABC Study was not specifically designed to enroll participants with OLD. Post-bronchodilator spirometry was not performed on participants limiting the evaluation for chronic airflow limitation characteristic of COPD. Third, we cannot ascertain whether the reported dyspnea is due to cardiac, respiratory, or unexplained cause and cardiopulmonary exercise stress test was not done in the study. Supplementary Table S2 shows adjudicated physician diagnoses within each group. This demonstrates differences in comorbidity distribution including higher prevalence of physician diagnosed coronary heart disease in groups with More Dyspnea compared to those with Less Dyspnea though similar prevalence of heart failure, vascular disease, cancer, and ulcer. Previous studies have showed that lower comorbidity burden was associated with OLD which was not seen in our study18. Perhaps this may be related to multimorbidity of aging or comorbidity burden related to COPD, a subset of OLD.18, 36 Despite being a covariate in our models, comorbidity may be contributing factor to mortality. Lastly, the possibility of regression dilution cannot be excluded given the 13-year horizon.
Our study shows that participants with both dyspnea and OLD had significantly increased mortality when compared with less dyspneic smokers. Similar mortality was seen in participants with dyspnea but with normal lung function compared to those with less dyspnea but with OLD suggesting that the clinical relevance of normal spirometry (the first group) and less dyspnea (in the latter group) should not be underestimated. These results support the routine measurement of lung function in patients ≥65 years with ≥20 pack years smoking history. Future research should focus on the assessment of dyspnea with and without OLD in the older patient population with the goal of focusing on treatment guidelines that decrease mortality in these groups of patients.
Supplementary Material
Table S1. Multivariate models on the impact of demographics and multi-morbidity on odds of death in current and former smokers regardless of the presence of obstructive lung disease (N=596).
Table S2. Adjudicated Comorbid Conditions by Groups
Table S3. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers When the Lower Limit of Normal (LLN) Is Used to Define the Threshold of Obstructive Lung Disease
Table S4. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers When the Modified Medical Research Council Dyspnea Scale of 2 or Greater As a Threshold to Define Dyspnea
Table S5. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers With and Without Restrictive Ventilatory Defect
Figure S1. Kaplan Meier Survival Probability Plot of Less Dyspneic And More Dyspneic Current And Former Smokers With And Without Obstructive Lung Disease Using The Lower Limit Of Normal (LLN) To Define The Threshold Of Obstructive Lung Disease
Figure S2. Kaplan Meier Survival Probability Plot of Less Dyspneic and More Dyspneic Current and Former Smokers With and Without Obstructive Lung Disease Using the Modified Medical Research Council Dyspnea Scale of 2 or Greater As a Threshold to Define Dyspnea
Figure S3. Kaplan Meier Survival Probability Plot of Less Dyspneic and More Dyspneic Current and Former Smokers With and Without Restrictive Ventilatory Defect
ACKNOWLEDGEMENTS
Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6–2101; N01-AG-6–2103; N01-AG-6–2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program at the NIA. This work was also supported by the National Institutes of Health (grant R01 AG051624 to B.J.N.) and the Wake Forest CTSI Program (grant UL1TR001420).
Footnotes
Conflict of Interest: None disclosed.
Sponsor’s Role: The sponsor played no role in the data extraction, analysis, or drafting of the manuscript.
Other Twitter Handles: @Bjnicklas (BJN); @FILES_DC (DCF); @agingup (SBK)
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Respiratory medicine. 2017;5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cory S, Ussery-Hall A, Griffin-Blake S, et al. Prevalence of selected risk behaviors and chronic diseases and conditions-steps communities, United States, 2006–2007. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2010;59: 1–37. [PubMed] [Google Scholar]
- 3.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017;390: 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burney PG, Patel J, Newson R, Minelli C, Naghavi M. Global and regional trends in COPD mortality, 1990–2010. The European respiratory journal. 2015;45: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet (London, England). 2007;370: 765–773. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. The New England journal of medicine. 2013;368: 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187: 347–365. [DOI] [PubMed] [Google Scholar]
- 8.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. The European respiratory journal. 2017;49. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med. 2016;374: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez CH, Murray S, Barr RG, et al. Respiratory Symptoms Items from the COPD Assessment Test Identify Ever-Smokers with Preserved Lung Function at Higher Risk for Poor Respiratory Outcomes. An Analysis of the Subpopulations and Intermediate Outcome Measures in COPD Study Cohort. Annals of the American Thoracic Society. 2017;14: 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez CH, Kim V, Chen Y, et al. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respiratory medicine. 2014;108: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan WC, Bourbeau J, Hernandez P, et al. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax. 2014;69: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med. 2015;175: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowler RP, Kim V, Regan E, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146: 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli BR, Decramer M, Wedzicha JA, et al. An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2015;191: e4–e27. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal. 2005;26: 319–338. [DOI] [PubMed] [Google Scholar]
- 17.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed Obstructive Lung Disease in the United States. Associated Factors and Long-term Mortality. Annals of the American Thoracic Society. 2015;12: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekstrom M, Schioler L, Gronseth R, et al. Absolute values of lung function explain the sex difference in breathlessness in the general population. The European respiratory journal. 2017;49. [DOI] [PubMed] [Google Scholar]
- 21.Mullerova H, Lu C, Li H, Tabberer M. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PloS one. 2014;9: e85540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society. 2009;57: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama. 1998;279: 585–592. [DOI] [PubMed] [Google Scholar]
- 24.Polkey MI, Spruit MA, Edwards LD, et al. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. American journal of respiratory and critical care medicine. 2013;187: 382–386. [DOI] [PubMed] [Google Scholar]
- 25.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society. 2007;55: 1727–1734. [DOI] [PubMed] [Google Scholar]
- 26.Perna L, Wahl HW, Mons U, Saum KU, Holleczek B, Brenner H. Cognitive impairment, all-cause and cause-specific mortality among non-demented older adults. Age and ageing. 2015;44: 445–451. [DOI] [PubMed] [Google Scholar]
- 27.Singh B, Mielke MM, Parsaik AK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA neurology. 2014;71: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Jama. 2006;296: 2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin K, Watkins B, Johnson T, Rodriguez JA, Barton MB. Screening for chronic obstructive pulmonary disease using spirometry: summary of the evidence for the U.S. Preventive Services Task Force. Annals of internal medicine. 2008;148: 535–543. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen QD, Wu C, Odden MC, Kim DH. Multimorbidity Patterns, Frailty, and Survival in Community-Dwelling Older Adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guisado-Clavero M, Roso-Llorach A, Lopez-Jimenez T, et al. Multimorbidity patterns in the elderly: a prospective cohort study with cluster analysis. BMC geriatrics. 2018;18: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallazzi R, Jorayeva A, Beatty BL, et al. Predicting asthma in older adults on the basis of clinical history. Respiratory medicine. 2018;142: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz Fragoso CA, Concato J, McAvay G, et al. Chronic obstructive pulmonary disease in older persons: A comparison of two spirometric definitions. Respiratory medicine. 2010;104: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus BS, McAvay G, Gill TM, Vaz Fragoso CA. Respiratory symptoms, spirometric respiratory impairment, and respiratory disease in middle-aged and older persons. Journal of the American Geriatrics Society. 2015;63: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of Spirometric Impairment in an Aging Population. American journal of respiratory and critical care medicine. 2016;193: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. International journal of chronic obstructive pulmonary disease. 2014;9: 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159: 179–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariate models on the impact of demographics and multi-morbidity on odds of death in current and former smokers regardless of the presence of obstructive lung disease (N=596).
Table S2. Adjudicated Comorbid Conditions by Groups
Table S3. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers When the Lower Limit of Normal (LLN) Is Used to Define the Threshold of Obstructive Lung Disease
Table S4. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers When the Modified Medical Research Council Dyspnea Scale of 2 or Greater As a Threshold to Define Dyspnea
Table S5. Cox Proportional Hazard Models for All-Cause Mortality Amongst Current and Former Smokers With and Without Restrictive Ventilatory Defect
Figure S1. Kaplan Meier Survival Probability Plot of Less Dyspneic And More Dyspneic Current And Former Smokers With And Without Obstructive Lung Disease Using The Lower Limit Of Normal (LLN) To Define The Threshold Of Obstructive Lung Disease
Figure S2. Kaplan Meier Survival Probability Plot of Less Dyspneic and More Dyspneic Current and Former Smokers With and Without Obstructive Lung Disease Using the Modified Medical Research Council Dyspnea Scale of 2 or Greater As a Threshold to Define Dyspnea
Figure S3. Kaplan Meier Survival Probability Plot of Less Dyspneic and More Dyspneic Current and Former Smokers With and Without Restrictive Ventilatory Defect