Abstract
Background/Objectives:
To assess whether gait speed under complex conditions predict long-term risk for mobility disability as well as or better than usual-pace gait speed.
Design:
Longitudinal cohort study
Setting/Participants:
Subsample of Health Aging and Body Composition study with follow-up from 2002–2003 to 2010–2011, including 337 community-dwelling adults (mean age=78.5, 50.7% female, 26.1% black)
Measurements:
Associations of gait speed measured under usual-pace, fast-pace, dual-task and narrow-path conditions with mobility disability, defined by any self-reported difficulty walking ¼ mile assessed annually, were tested by Cox proportional hazard models adjusted for demographic and health characteristics. Models were fitted for each walking condition and R2 statistics were used to compare predictive value across models. Models were repeated for persistent mobility disability, defined as at least two consecutive years of mobility disability.
Results:
Mobility disability occurred in 204 (60.5%) participants over the 8-year follow-up. There was a lower hazard of developing mobility disability with faster gait speed under all conditions. Hazard ratios, confidence intervals, and r2 of gait speed predicting mobility disability were very similar across all four walking conditions (r2 range 0.22–0.27), but were strongest for dual-task gait speed (HR (95% CI), r2 of fully adjusted models: 0.81 (0.75, 0.88), 0.27). Results were comparable for persistent mobility disability (r2 range 0.26–0.28).
Conclusion:
Slower gait speed under both usual-pace and complex conditions may be clinical indicators of future risk of mobility disability. These results support the call for increased use of gait speed measures in routine geriatric care.
Keywords: Gait Speed, Fast Pace, Dual Task, Mobility Disability
Introduction
The ability to walk moderate distances outside of the home is necessary for completion of many essential activities, including shopping and accessing healthcare1–3. Community mobility promotes physical function, social engagement, independent living, and higher quality of life4–8. Several indicators for future risk of mobility disability have been proposed, but few can be easily measured in the clinic.
A minimum gait speed is necessary for successful community mobility2, 3 and gait speed as measured in the clinic under simple, usual-pace conditions is a strong and independent predictor of mobility disability. However, usual-pace gait speed tests may not be the best indicator for changes in community mobility. Community mobility occurs in the context of complex community environments and therefore, requires cognitive resources and behavioral flexibility to address environmental challenges such as terrain characteristics, attentional demands, and postural transitions1. Usual-pace gait speed tests do not appear to capture these complexities, but physical (e.g. fast-pace, narrow-path) or cognitive (e.g. dual-task) challenges can be added to increase the ecological validity of walking tasks and to unmask early, subtle changes in walking. Few studies have examined the value of complex locomotor tasks in predicting disability. A previous study found that failure on complex locomotor tasks (e.g. inability to generate responses to the cognitive component of a dual-task or inability to speed up during fast pace conditions) was associated with incident mobility disability over 3 years9 but did not assess gait speed under complex conditions as a predictor. However, a dichotomous indicator of success or failure on these tasks may be more proximal to disability onset whereas slower gait speed during the tasks could be an earlier indicator of subtle changes leading to longer-term risk of mobility disability.
In this study, we assessed whether performance on complex walking tasks involving both physical (fast-pace and narrow-path) and cognitive (dual-task) challenges predicted greater risk for incident mobility disability (self-reported inability to walk ¼ mile) compared with usual-pace gait speed. We hypothesized that these complex walking tasks would be more strongly related to risk for mobility disability compared to usual-pace walking.
Methods
Sample
We utilized data from the Health Aging and Body Composition (Health ABC) study which enrolled community-dwelling black and white adults in Pittsburgh and Memphis from 1997–1998. Participants were 70–79 years old upon entry to the study and free of self-reported difficulties in performing activities of daily living, walking a quarter mile, or climbing 10 steps without resting. Men and black participants were oversampled. Dual-task walking was completed as part of an ancillary study (n=426) at the Pittsburgh site during the 2002–2003 study visit10 which serves as the baseline for these analyses.
Of the 426 participants in the dual-task ancillary study, 337 participants were included in our analytic sample. Participants were excluded if they were unable to complete any walking task (n=89), had mobility disability at baseline (n=47), or did not have at least two follow-up measures for mobility disability between baseline and the final visit in 2010–2011 (n=35; categories not mutually exclusive). Participants who were included in these analyses did not significantly differ on demographic or comorbid characteristics from those not included.
All participants provided written informed consent and all protocols were approved by the local institutional review boards.
Walking Tasks
For all walking tasks, participants began before the start line and time was measured in seconds from the first footfall over the start line to the first footfall after the finish line. Walk times were recorded and converted to gait speed (m/s).
Usual-pace gait speed was measured over a 20 m walkway. Fast-paced walking was completed on the same 20 m walkway, with participants being asked to “walk as fast as you can”.
The dual-task paradigm used a concurrent visuospatial task as previously described10, 11. Briefly, participants were given a time of day prompt and were asked to visualize the time of day as displayed by the hands on an analog clock. Participants responded ‘same’ or ‘different’ based on whether the hands of the clock would be on the same side of a line passing through the 12 and 6 on the clock face or on different sides. The dual-task was completed while participants walked along a 20-meter corridor at usual-pace. Performance on the cognitive portion of the task was recorded; accuracy was high at 93%, so was not controlled for in these analyses.
For the narrow-path walking, participants were instructed to walk within a 6 m long and 20 cm wide path marked by tape12. Participants were asked to walk at a comfortable pace and not step on or outside of the lines. Participants were given up to three attempts to complete the task without stepping outside of the lines. If they were unable to complete the task without stepping out, they were excluded from these analyses.
Mobility Disability
Participants were asked every six months through the 2010–2011 visit if they had any difficulty walking a quarter mile, about 2 or 3 blocks, due to a health or physical problem12. Mobility disability was reported annually at in-person visits. Onset of mobility disability was defined as first onset of any reported difficulty. Persistent mobility disability was defined as at least two consecutive years with mobility disability.
Covariates
Variables associated with mobility13 were considered as potential covariates. Demographic variables were recorded at study baseline and all other variables were assessed concurrently with the walking tasks. Demographic data including age, race, sex, and education were self-reported. Chronic diseases, including coronary heart disease (CHD), hypertension, and diabetes, were assessed through self-report, physician diagnoses, recorded medications and laboratory data. Isokinetic quadriceps strength was measured as average maximum torque on a dynamometer (125 AP, Kin-Com, Chattanooga, TN). Weak muscle strength was defined by gender-specific cut-offs for quadriceps strength (<97 Nm for men and <62 Nm for women)14. Poor vision was based on self-report of fair, poor, or very poor, with glasses or contact lenses if they wore them. Poor lung function was based on previously reported15 gender-specific cutoffs for forced vital capacity as measured by spirometry (<3,066 mL for men and <2,127 mL for women) and was collected one year prior to our analytic baseline. Knee pain was self-reported for either knee most days in the past 30 days. Obesity was defined by a body mass index (BMI) greater than or equal to 30 kg/m2. Global cognitive function was tested by the Modified Mini Mental Status Exam (3MS)16. Processing speed was assessed by the Digit Symbol Substitution Test (DSST)17. Cognitive testing were not completed at the 2002–2003 visit, so we took 3MS and DSST scores from one year prior to our analytic baseline. Depressive symptoms were assessed by the Center for Epidemiologic Studies Depression Scale (CES-D) short form18. Recurrent falls were defined as at least two falls reported in the past year.
Statistical Analyses
Descriptive statistics assessed differences between those who developed any mobility disability during the 8-year follow-up and those who did not by t-tests for continuous variables and chi-square tests for categorical ones.
Cox proportional hazard models were fitted separately to assess the risk of developing either mobility disability or persistent disability as a function of four predictor variables at baseline: 1) usual-pace gait speed, 2) fast-pace gait speed, 3) narrow-path gait speed, and 4) dual-task gait speed. Models were repeated with adjustment for basic demographic characteristics (age, gender, and race) and then for health characteristics that were associated with mobility disability in bivariate analyses (diabetes, CES-D scores, obesity, knee pain, low FVC, 3MS scores). Model fit and proportionality assumptions were tested. R2 values are reported from all fully adjusted models to allow comparison of predictive value across models including different gait speed predictors.
All analyses were completed in SAS 9.4.
Results
Participants were 78.5 (SD=2.9) years of age on average, 50.7% were female, and 26.1% were black. By the end of the 8 year follow-up, 204 (60.5%) of the participants developed mobility disability and 131 (38.9%) developed persistent mobility disability. Individuals with incident mobility disability were more likely to be female, have diabetes, be obese, have knee pain, and have low pulmonary function (Table 1). Those who developed mobility disability also had higher depressive symptoms (Table 1).
Table 1.
Baseline demographic, health, and gait characteristics of those who have incident mobility disability and those who remain free from mobility disability for 8 years (n=337).
| 8-Year Incident Mobility Disability | ||||
|---|---|---|---|---|
| Total Sample n=337 | Absent n=133 | Present n=204 | ||
| Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | p-value | |
| Age | 78.5 (2.9) | 78.2 (2.8) | 78.8 (2.9) | 0.07 |
| Female sex | 171 (50.7%) | 58 (43.6%) | 113 (55.4%) | 0.03 |
| Black race | 88 (26.1%) | 28 (21.1%) | 60 (29.4%) | 0.09 |
| ≤High school education | 175 (51.9%) | 62 (46.6%) | 113 (55.4%) | 0.1 |
| CHD | 78 (23.2%) | 26 (19.6%) | 52 (25.5%) | 0.2 |
| Diabetes | 79 (23.4%) | 19 (14.3%) | 60 (29.4%) | 0.001 |
| Hypertension | 300 (89.0%) | 115 (86.5%) | 185 (90.7%) | 0.2 |
| Recurrent falls in past year | 95 (28.4%) | 39 (29.8%) | 56 (27.6%) | 0.7 |
| CES-D score | 6.4 (6.5) | 5.5 (5.9) | 7.0 (6.8) | 0.03 |
| 3MS score | 94.1 (5.0) | 94.7 (5.1) | 93.7 (4.9) | 0.07 |
| DSST score | 40.5 (11.2) | 41.0 (10.6) | 40.1 (11.5) | 0.5 |
| Obesity | 91 (27.1%) | 15 (11.3%) | 76 (37.4%) | <0.001 |
| Low quadriceps strength | 82 (26.5%) | 32 (25.4%) | 50 (27.3%) | 0.7 |
| Knee pain | 59 (17.5%) | 10 (7.5%) | 49 (24.0%) | <0.001 |
| Low pulmonary function | 114 (37.4%) | 33 (27.3%) | 81 (44.0%) | 0.003 |
| Poor vision | 96 (28.7%) | 35 (26.5%) | 61 (30.1%) | 0.5 |
| Years of follow-up | 9.7 (2.1) | 9.7 (2.4) | 9.7 (2.0) | 0.9 |
| Gait Measures | ||||
| Usual-pace gait speed (m/s) | 1.14 (0.27) | 1.22 (0.20) | 1.08 (0.30) | <0.001 |
| Fast-pace gait speed (m/s) | 1.52 (0.30) | 1.63 (0.28) | 1.44 (0.29) | <0.001 |
| Dual-task gait speed (m/s) | 1.18 (0.23) | 1.28 (0.21) | 1.11 (0.22) | <0.001 |
| Narrow-path gait speed (m/s) | 1.11 (0.25) | 1.16 (0.24) | 1.06 (0.25) | <0.001 |
SD=standard deviation, CHD=coronary heart disease, CES-D=Center for Epidemiologic Studies Depression Scale, 3MS=Modified Mini Mental Status Exam, DSST=Digit Symbol Substitution Test, Nm=Newton meter, FVC=forced vital capacity, m/s=meters/second
Gait speed under all four conditions were correlated with one another, but not perfectly so (range of Spearman r: 0.53–0.80; all p<0.0001). The strongest correlation was between fast-pace and dual-task gait speeds.
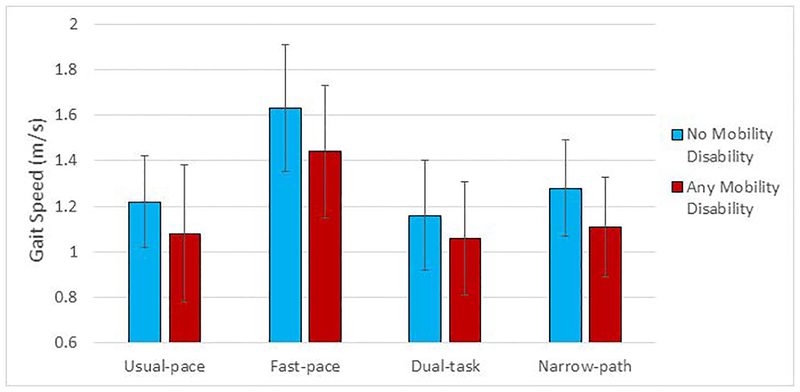
In bivariate analyses, individuals who developed incident mobility disability had 9–13% slower gait speed under all conditions compared to those who did not (Figure 1). Cox proportional hazard models indicated a lower hazard of developing mobility disability with faster gait speed under all conditions (Table 2). These associations were robust to adjustment for demographic and health characteristics. Adjusted hazard ratios and confidence intervals were very similar across all four walking conditions, but were slightly stronger for dual-task gait speed compared to usual pace (HR (95% CI) of fully adjusted models: usual-pace = 0.84 (0.78, 0.90) and dual-task = 0.81 (0.75, 0.88)). Very similar results were obtained for persistent mobility disability (Table 2). The proportion of the variance of the outcome explained was similar across gait speed measures with marginal differences between all gait conditions for both outcomes (r2 range for any mobility disability=0.22–0.27; r2 range for persistent mobility disability=0.26–0.28).
Figure 1.
Mean and standard deviation of gait speed (meters/second (m/s)) under usual-pace and complex conditions by those who did and did not develop mobility disability over 8 years of follow-up (n=337).
Table 2.
Hazard ratios (HR) per 0.1 m/s difference in gait speeds under different conditions with incidence of mobility disability over 8 years of follow-up (n=33).
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | r-square | |
| Any MD | ||||
| Usual-pace gait speed | 0.82 (0.77, 0.86) | 0.83 (0.78, 0.88) | 0.84 (0.78, 0.90) | 0.26 |
| Fast-pace gait speed | 0.82 (0.77, 0.86) | 0.82 (0.77, 0.87) | 0.87 (0.81, 0.93) | 0.24 |
| Dual-task gait speed | 0.76 (0.71, 0.81) | 0.77 (0.72, 0.82) | 0.81 (0.75, 0.88) | 0.27 |
| Narrow-path gait speed | 0.84 (0.79, 0.89) | 0.85 (0.80, 0.91) | 0.90 (0.84, 0.97) | 0.22 |
| Persistent MD | ||||
| Usual-pace gait speed | 0.80 (0.75, 0.86) | 0.82 (0.76, 0.88) | 0.82 (0.76, 0.89) | 0.28 |
| Fast-pace gait speed | 0.77 (0.72, 0.83) | 0.77 (0.72, 0.83) | 0.83 (0.76, 0.90) | 0.28 |
| Dual-task gait speed | 0.74 (0.68, 0.80) | 0.74 (0.68, 0.81) | 0.80 (0.73, 0.88) | 0.28 |
| Narrow-path gait speed | 0.80 (0.74, 0.86) | 0.81 (0.75, 0.88) | 0.86 (0.79, 0.95) | 0.26 |
MD=mobility disability, HR=hazard ratio
Model 1 – unadjusted
Model 2 – adjusted for age, sex, race
Model 3 – Model 2 + diabetes, CES-D, obesity, knee pain, low FVC, 3MS
r2 shown for model 3
Discussion
In a sample of community-dwelling older adults, we found that gait speed under both usual-pace and complex conditions was associated with greater risk for developing mobility disability over the next 8 years. Contrary to our hypothesis, usual-pace gait speed may provide sufficient predictive ability for future mobility disability compared to more complex walking tasks, while having the advantage of being easier to obtain in the clinic. Our results suggest that adding physical or cognitive challenges only marginally improves the predictive power of gait speed for mobility disability. Results were remarkably similar for persistent mobility disability as for any mobility disability.
A previous study indicated that failure on complex walking tasks under narrow (stepping out of the 25 cm width) and fast-pace (not accelerating by at least 0.1 m/s) conditions was predictive of 3-year incidence of self-reported mobility disability9. In contrast, failure on the dual-task condition (not generating any verbal response) was not associated. These failures may be more proximal to disability onset and may not provide an opportunity to capture the early, subtle signs of walking limitations that may be captured by measuring gait speed, as was done in our analyses. Further, failure on the tasks was not compared with the predictive value of usual-pace gait speed in those analyses9. A pooled analysis of over 27,000 participants in seven cohort studies previously demonstrated the effectiveness of usual-pace gait speed as a predictor of 3-year incidence of self-reported mobility disability19. Usual-pace gait speed is also predictive of objectively measured mobility disability over 21 months of follow-up20. We extend these previous results by showing that usual-pace gait speed can be predictive of incident mobility disability for up to 8 years and that dual-task walking may have a slightly higher predictive value. Of note, our models using gait speed along with demographic and health characteristics to predict mobility disability all had r2 values below 0.29, suggesting that there are other, unmeasured contributors to mobility disability onset in our cohort. These contributors likely include environmental, behavioral, and social factors21.
The lack of improved predictive power from the complex walking tasks, particularly dual-task walking, may be due in part to the relatively high cognitive function of our sample (mean 3MS score=94). We found in bivariate analyses that DSST, a measure of processing speed, and 3MS, a measure of general cognitive function, were not significantly associated with incident mobility disability. The association of cognitive function with usual-pace and dual-task gait speed is well established22, 23 but the role of cognitive function in onset of mobility disability is less well studied. Two studies have indicated that a Folstein Mini-Mental State Examination (MMSE) score indicative of cognitive impairment (<24) is related to increased risk for self-reported mobility disability24, 25, though one study indicated that this association was mediated through physical function25. A third study found no association of low MMSE scores with incident mobility disability26. A recent analysis utilizing cognitive assessments across multiple domains with objectively measured onset of mobility disability also found an association of cognitive function with mobility disability that was mediated through physical function27. Our results may not apply to those with cognitive impairment as we had few individuals with 3MS scores indicative of impairment. There is a need to repeat these analyses in a sample with a sufficient range of cognitive function to determine if these findings apply to those with lower cognitive function.
Our sample was relatively high functioning at baseline for a sample in their 70s. However, our sample did include a large proportion of individuals with risk factors for mobility disability. We did not have more detailed measures of gait, such as stance time variability, which may be more strongly related to mobility disability risk than speed alone28, 29. These more detailed measures of gait require instrumentation to measure and therefore, may not be as practical to implement clinically on a large scale. Finally, we only had a dual-task paradigm utilizing a visuospatial cognitive task and results may be different with other types of dual-tasks30. Our study benefited from a large, well-characterized cohort with a long follow-up for mobility disability that was assessed annually.
Mobility disability prevalence is estimated to be 31% in the Medicare population31. Mobility disability can have serious consequences for the health and well-being of older adults and should therefore, be a public health priority32. Early identification of those most at risk could enhance the effectiveness of prevention efforts. Usual-pace gait speed has proven to be a powerful predictor of many health outcomes in older adults, including falls33, hospitalization33, 34, and mortality35. Dual-task and fast-pace gait speed may add predictive value over usual-pace gait speed for both mobility disability and cognitive decline36. These results add to the growing body of evidence that gait speed represents a summary measure of health across multiple organ systems37 and that it should be measured routinely in clinical settings as an indicator of those older adults most at risk for a number of health outcomes38, 39.
Acknowledgements
Funding: Health ABC was supported by National Institute on Aging (NIA) contracts (N01-AG-6–2101, N01-AG-6–2103, N01-AG-6–2106), NIA grant R01-AG-028050, and NINR grant R01-NR-012459. This research was supported in part by the Intramural Research Program of the NIH, National institute on Aging. These analyses were supported by the NIA (K01 AG053431–01).
Footnotes
Conflict of Interest: The authors have no conflicts.
References
- [1].Patla AE, Shumway-Cook A. Dimensions of mobility: Defining the complexity and difficulty associated with community mobility. Journal of Aging and Physical Activity. 1999;7: 7–19. [Google Scholar]
- [2].Andrews AW, Chinworth SA, Bourassa M, Garvin M, Benton D, Tanner S. Update on distance and velocity requirements for community ambulation. J Geriatr Phys Ther. 2010;33: 128–134. [PubMed] [Google Scholar]
- [3].Salbach NM, O’Brien K, Brooks D, et al. Speed and distance requirements for community ambulation: a systematic review. Archives of physical medicine and rehabilitation. 2014;95: 117–128 e111. [DOI] [PubMed] [Google Scholar]
- [4].Sheppard KD, Sawyer P, Ritchie CS, Allman RM, Brown CJ. Life-space mobility predicts nursing home admission over 6 years. Journal of aging and health. 2013;25: 907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosso AL, Taylor JA, Tabb LP, Michael YL. Mobility, disability, and social engagement in older adults. Journal of aging and health. 2013;25: 617–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bentley JP, Brown CJ, McGwin G Jr., Sawyer P, Allman RM, Roth DL. Functional status, life-space mobility, and quality of life: a longitudinal mediation analysis. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2013;22: 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women’s health and aging study. Journal of the American Geriatrics Society. 2005;53: 198–203. [DOI] [PubMed] [Google Scholar]
- [8].Rantakokko M, Portegijs E, Viljanen A, Iwarsson S, Kauppinen M, Rantanen T. Changes in life-space mobility and quality of life among community-dwelling older people: a 2-year follow-up study. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2016;25: 1189–1197. [DOI] [PubMed] [Google Scholar]
- [9].Deshpande N, Metter EJ, Guralnik J, Ferrucci L. Can failure on adaptive locomotor tasks independently predict incident mobility disability? American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2013;92: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Faulkner KA, Redfern MS, Rosano C, et al. Reciprocal influence of concurrent walking and cognitive testing on performance in older adults. Gait & posture. 2006;24: 182–189. [DOI] [PubMed] [Google Scholar]
- [11].Faulkner KA, Redfern MS, Cauley JA, et al. Multitasking: association between poorer performance and a history of recurrent falls. Journal of the American Geriatrics Society. 2007;55: 570–576. [DOI] [PubMed] [Google Scholar]
- [12].Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56: M644–649. [DOI] [PubMed] [Google Scholar]
- [13].Rosso AL, Studenski SA, Longstreth WT Jr., Brach JS, Boudreau RM, Rosano C. Contributors to Poor Mobility in Older Adults: Integrating White Matter Hyperintensities and Conditions Affecting Other Systems. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Metti AL, Rosano C, Boudreau R, et al. Catechol-O-Methyltransferase Genotype and Gait Speed Changes over 10 Years in Older Adults. Journal of the American Geriatrics Society. 2017;65: 2016–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanders JL, Boudreau RM, Penninx BW, et al. Association of a Modified Physiologic Index with mortality and incident disability: the Health, Aging, and Body Composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48: 314–318. [PubMed] [Google Scholar]
- [17].Wechsler D Manual for the Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corp, 1981. [Google Scholar]
- [18].Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). American journal of preventive medicine. 1994;10: 77–84. [PubMed] [Google Scholar]
- [19].Perera S, Patel KV, Rosano C, et al. Gait Speed Predicts Incident Disability: A Pooled Analysis. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang M, Cohen-Mansfield J, Ferrucci L, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. Journal of the American Geriatrics Society. 2004;52: 2094–2098. [DOI] [PubMed] [Google Scholar]
- [21].Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38: 1–14. [DOI] [PubMed] [Google Scholar]
- [22].Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Al-Yahya E, Mahmoud W, Meester D, Esser P, Dawes H. Neural Substrates of Cognitive Motor Interference During Walking; Peripheral and Central Mechanisms. Frontiers in human neuroscience. 2018;12: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Annals of internal medicine. 2012;156: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Siltanen S, Portegijs E, Saajanaho M, et al. The Combined Effect of Lower Extremity Function and Cognitive Performance on Perceived Walking Ability Among Older People: A Two-year Follow-up Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. [DOI] [PubMed] [Google Scholar]
- [26].Deshpande N, Metter JE, Guralnik J, Bandinelli S, Ferrucci L. Sensorimotor and psychosocial determinants of 3-year incident mobility disability in middle-aged and older adults. Age and ageing. 2014;43: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Handing EP, Chen H, Rejeski WJ, et al. Cognitive Function as a Predictor of Major Mobility Disability in Older Adults: Results From the LIFE Study. Innov Aging. 2019;3: igz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brach JS, Wert D, VanSwearingen JM, Newman AB, Studenski SA. Use of stance time variability for predicting mobility disability in community-dwelling older persons: a prospective study. J Geriatr Phys Ther. 2012;35: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Menant JC, Sturnieks DL, Brodie MA, Smith ST, Lord SR. Visuospatial tasks affect locomotor control more than nonspatial tasks in older people. PloS one. 2014;9: e109802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L. Mobility limitations in the Medicare population: prevalence and sociodemographic and clinical correlates. Journal of the American Geriatrics Society. 2005;53: 1217–1221. [DOI] [PubMed] [Google Scholar]
- [32].Satariano WA, Guralnik JM, Jackson RJ, Marottoli RA, Phelan EA, Prohaska TR. Mobility and aging: new directions for public health action. American journal of public health. 2012;102: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60: 1304–1309. [DOI] [PubMed] [Google Scholar]
- [34].Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53: 1675–1680. [DOI] [PubMed] [Google Scholar]
- [35].Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011;305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosso AL, Metti AL, Faulkner K, et al. Complex Walking Tasks and Risk for Cognitive Decline in High Functioning Older Adults. J Alzheimers Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA : the journal of the American Medical Association. 2014;311: 2061–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13: 881–889. [DOI] [PubMed] [Google Scholar]