Abstract
The accumulation of Copper in organisms can lead to altered functions of various pathways and become cytotoxic through the generation of reactive oxygen species. In yeast, cytotoxic metals such as Hg+, Cd2+, and Cu2+ are transported into the lumen of the vacuole through various pumps. Copper ions are initially transported into the cell by the copper transporter Ctr1 at the plasma membrane and sequestered by chaperones and other factors to prevent cellular damage by free cations. Excess copper ions can subsequently be transported into the vacuole lumen by an unknown mechanism. Transport across membranes requires the reduction of Cu2+ to Cu+. Labile copper ions can interact with membranes to alter fluidity, lateral phase separation and fusion. Here we found that CuCl2 potently inhibited vacuole fusion by blocking SNARE pairing. This was accompanied by the inhibition of V-ATPase H+ pumping. Deletion of the vacuolar reductase Fre6 had no effect on the inhibition of fusion by copper. This suggests that that Cu2+ is responsible for the inhibition of vacuole fusion and V-ATPase function. This notion is supported by the differential effects of chelators. The Cu2+-specific chelator TETA rescued fusion, whereas the Cu+-specific chelator BCS had no effect on the inhibited fusion.
Keywords: Membrane fusion, SNARE, V-ATPase, Sec17, Vam7, Vam3, Nyv1, Fre6
1. INTRODUCTION
Divalent cations play numerous roles in cellular maintenance and membrane trafficking. Among these cations, calcium is well known for its role in signaling via calmodulin and calcineurin, and regulating synaptic vesicle trafficking, while divalent metals such as zinc and copper are less understood. Copper is an essential trace metal that functions in aerobic respiration, superoxide dismutase activity and iron acquisition.1 Yet, excess free copper leads to toxicity and is associate with Wilson’s disease and liver failure.2 Many of the deleterious effects of elevated labile copper is the generation of reactive oxygen species (ROS) that can damage membranes, proteins and DNA, and exacerbate neurodegenerative diseases such Alzheimer’s and Huntingtin’s.3,4 Aside from the generation of ROS, copper can interact with membranes to affect lateral phase separation, membrane fluidity, and can block exocytosis in PC-12 cells.5–9 With respect to lysosomes, brief copper exposure leads to the transport of the copper pump ATP7B from the plasma membrane to lysosomes and can stimulate the exocytosis of degradative enzymes.10
In yeast, labile copper ions are transported into the vacuole lumen to reduce toxicity. Copper can subsequently be transported out of the vacuole upon its reduction from Cu2+ to Cu+ by Fre6.11 Cu+ is exported from the vacuole through the Ctr2 transporter, and its deletion results in the hyper-accumulation of vacuolar copper.12 Although the mechanism for copper export from the vacuole is known, the importer of the metal remains unclear. It is possible that copper import is carried out by a broad-spectrum transporter and not a high affinity pump.
Due to the effects of elevated copper on membranes in mammalian cells, we hypothesized that yeast vacuole dynamics could also be affected by this cation. In this study we found that addition of exogenous Cu2+ strongly inhibited vacuolar fusion. An examination of the distinct stages of membrane fusion indicated that Cu2+ inhibited trans-SNARE complex formation and H+ uptake by the V-ATPase. The effects of copper were likely due to the Cu2+ form as specific chelators rescued fusion, while Cu+ chelators had no effect.
2. RESULTS AND DISCUSSION
2.1. Copper inhibits vacuole fusion
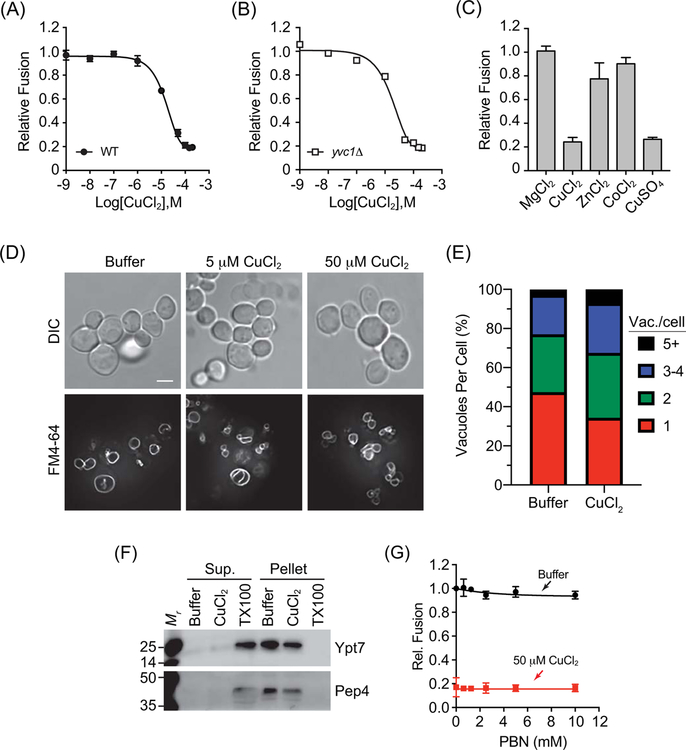
Copper toxicity leads to many effects including the redirection of membrane trafficking. For instance, toxic levels of copper block the membrane fusion of chromaffin granules in PC-12 cells.6 In HeLa cells elevated copper stimulates lysosome exocytosis in an effort to expel the excess metal.10 Here, we asked if toxic copper levels affect isolated yeast vacuoles through altering in vitro vacuole homotypic fusion. To start, we added a concentration curve of CuCl2 to vacuole fusion reactions at the start of the incubation period. We found that vacuole fusion was blocked in a dose-dependent manner with an IC50 of 15 μM (Fig. 1A). This concentration of copper might seem too high to be relevant, as micromolar levels of free copper in the cytoplasm are unlikely reached in vivo due to sequestration by chaperones upon entry13,14. Due to the cellular capacity to process and detoxify high levels of metals such as copper, toxic effects are only observed when cells are overwhelmed with concentrations that saturate the binding capacity and storage limit of the system. Only then, can the remaining free copper ions lead to toxic effects. This notion is consistent with the need for micromolar levels of copper to see effects in experimental systems.
Figure 1. CuCl2 inhibits vacuole homotypic fusion.
Vacuoles isolated from wild type (BJ3505 and DKY6281) (A) or yvc1Δ (RFY74–75) (B) fusion reporter strains were incubated with buffer alone or a concentration curve of CuCl2. (C) Wild type vacuoles were incubated with 100 μM MgCl2, ZnCl2, CoCl2, CuCl2, or CuSO4. Fusion reactions were incubated for 90 min at 27°C. After incubation membranes were solubilized and incubated with p-nitrophenyl phosphate to measure Pho8 activity. p-nitrophenolate was measured at OD400. Fusion values were normalized to the untreated control set to 1. (D) BJ3505 cells were grown to log phase and treated with CuCl2. Vacuoles were visualized by staining with FM4–64 and cells were visualized using DIC. (E) Quantitation of vacuole fragmentation of cells treated with 0 μM or 50 μM CuCl2 as in panel D. n=392, untreated cells, n=616, 50 μM CuCl2 treated cells. (F) DKY6281 vacuoles were incubated with 100 μM CuCl2, 0.1% TX-100 or buffer for 90 min at 27°C. After incubation, the soluble and membrane fractions were separated by centrifugation (16,000 x g, 10 min, 4°C), mixed with SDS-loading buffer and resolved by SDS-PAGE. The soluble luminal protease Pep4 and the membrane anchored Ypt7 were probed for by immunoblotting. (G) Wild type vacuoles were treated with or without CuCl2 in the presence of a concentration curve of PBN to eliminate oxygen radicals. Error bars are S.E.M. (n=3). Scale bar = 4 μm.
The disparity in the amount of copper added to what causes toxicity is inferred by many studies where high levels of copper are used to trigger a response. For example, in HeLa cells, 10–100 μM Cu2+ is needed to activate the PI3 Kinase/AKT signaling pathway15. Others have grown Candida albicans on 10–500 μM CuSO4 to find mutants that affect resistance to copper16. To show copper toxicity in growing Saccharomyces cerevisiae, Sowada et al., used millimolar concentrations of CuCl217. Finally, a recent a proteomic and genetic analysis of the response to soluble copper by yeast used ≥200 μM CuSO4 to screen their collection of strains18. Granted, these are examples of whole cells, which can process greater levels of copper in comparison to purified vacuoles. That said, we must consider that the vacuole serves as a storage compartment for multiple cations including calcium, zinc, iron and copper19,20. The vacuole also serves a storage vessel for copper and releases it when needed by the cell for numerous functions, including supplying the mitochondria with copper for respiration, or loading Cu, Zn superoxide dismutase. The large capacity of vacuole copper storage was demonstrated by Rees et al. where wild type and ctr2Δ yeast were fed 20 μM copper for 1h prior to isolating vacuoles. They found that purified wild type vacuoles stored ~20 pg of copper per μg of protein12. This was increased in the absence of the copper exporter Ctr2 to ~80 pg/μg of protein. The trend continued when cells were incubated with 100 μM copper, which would correspond to copper at 100–400 pg/μg of vacuole protein. We found that Cu2+ inhibited in vitro vacuole fusion with an IC50 of 15 μM, which corresponded to a concentration of 4.75 ng of available copper per μg of vacuole protein in the total reaction. While this was an order of magnitude higher than the reported vacuole content above, it is important to acknowledge that toxic levels of copper for S. cerevisiae are reached when the extracellular concentrations are at or near millimolar levels.
Copper has also been shown to interact with the vacuolar TRP family Ca2+ channel Yvc1.21 To test whether the inhibitory effect of Cu2+ on vacuole fusion was associated to this channel, we used vacuoles from yvc1Δ yeast. As seen in Figure 1B, the inhibitory effect of copper on yvc1Δ vacuoles was nearly indistinguishable from its effect on wild type vacuoles. The lack of a difference indicates that the effect of Cu2+ was independent of Yvc1. To verify whether the effect of CuCl2 on vacuole fusion was specific we tested other divalent cations as well as a second copper salt. As shown in Figure 1C, vacuole fusion was resistant to 100 μM ZnCl2, MgCl2, CoCl2, while vacuoles were equally sensitive to both CuCl2 and CuSO4 (Fig. 1C). We have also tested Ca2+ at 100 μM and see no effect on fusion (not shown). These data indicate the effects of copper on vacuole fusion were specific to the metal and not due to a charge effect. While Ca2+ can inhibit vacuole fusion, it only does so at millimolar concentrations.22 This makes the effect of copper at micromolar concentrations of particular interest.
The inhibition of in vitro vacuole fusion is often accompanied by in vivo vacuole fragmentation. To test the effects acute copper treatment, we incubated log-phase yeast with 0, 5 or 50 μM CuCl2. The vacuoles were stained with the vital dye FM4–64 and vacuole morphology was examined by fluorescence microscopy and whole cells were visualized with DIC. In Figure 1D–E we show that acute CuCl2 treatment led to mild vacuole fragmentation. This is in accord with our previous results, further indicating that CuCl2 inhibits vacuole fusion at low micromolar levels.
2.2. Copper does not inhibit vacuole fusion through reactive oxygen species generation
Copper ions can lead to the production of oxygen radicals that damage membranes through lipid peroxidation culminating in vesicle lysis. To test whether the effect of CuCl2 on vacuole fusion was due to membrane lysis we looked for the release of the soluble luminal protease Pep4. Fusion reactions were incubated with reaction buffer alone, 100 μM CuCl2, or 0.5% Triton X-100 (TX100). After incubation, vacuoles were pelleted through centrifugation and separated from the supernatant. Pellets were resuspended with a starting volume of reaction buffer after which both pellet and supernatant fractions were mixed with equal volumes of SDS-loading buffer. Proteins were resolved by SDS-PAGE and probed by Western blotting for Pep4 and the membrane anchored protein Rab GTPase Ypt7. We found that copper did not result in the release of Pep4 from the vacuole lumen. Rather, Pep4 remained in the pellet with Ypt7 (Fig. 1F). As expected, Triton X-100 solubilized both proteins that only appeared in the supernatant fraction. This indicates that copper did not cause vesicle lysis and that its effect on fusion was due to its effect on the fusion machinery itself.
To further verify that CuCl2 was not leading to the production of oxygen radicals, we used the free radical spin trap N-tert-butyl-α-phenylnitrone (PBN), which has been shown to reduce free radicals produced in the presence of copper23. We added a concentration curve of PBN to reactions containing 50 μM CuCl2 or buffer alone. This showed that PBN, even at 10 mM, was unable to restore fusion in reactions treated with copper (Fig. 1G). This suggests that copper does not inhibit vacuole fusion through creating oxygen radicals.
2.3. Transport of Copper ions into the vacuole lumen
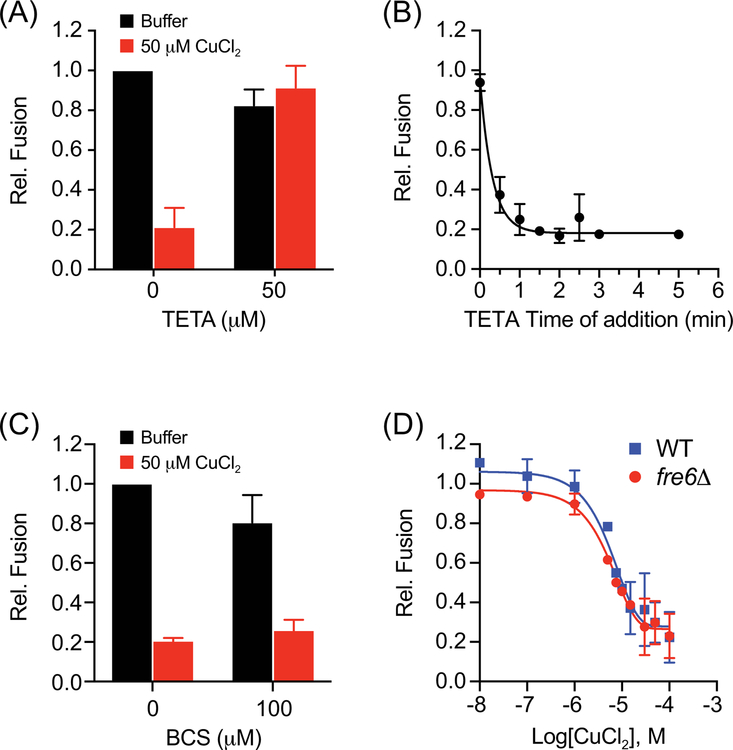
We next asked whether copper ions were transported into the vacuole lumen. One way to test this is to track resistance to an external copper chelator. The Cu2+ selective chelator Triethylenetetramine (TETA) was chosen for this experiment.24 TETA also chelates Zn2+, however, this was not relevant as Zn2+ had no effect on vacuole fusion. In Figure 2A we show that adding equimolar TETA and CuCl2 to vacuoles fully restored fusion, indicating that Cu2+ was directly responsible for the effect on fusion. Next, we added TETA at different time points after the addition of CuCl2 to vacuoles. TETA quickly lost its ability to block the effect of CuCl2 on fusion, suggesting that Cu2+ was no longer available to bind the chelator (Fig. 2B). A likely reason would be that copper ions were transported into the vacuole lumen.
Figure 2. Cu2+ and not Cu+ inhibits vacuole fusion.
(A) Fusion reactions were incubated with or without CuCl2 and treated with TETA to chelate cupric (Cu2+) ions. (B) Fusion reactions containing 50 μM CuCl2 were supplemented with TETA at the indicated times. Fusion reactions were incubated for a total of 90 min at 27°C before developing. (C) Fusion reactions were incubated with or without CuCl2 and treated with BCS to chelate cuprous (Cu+) ions. (D) Wild type and fre6Δ vacuoles were incubated with a concentration curve of CuCl2 and tested for fusion after 90 min of incubation at 27°C. Error bars are S.E.M. (n=3).
Although much is known about the transport of copper from the vacuole lumen to the cytosolic side of the vacuole through Ctr2, the identity of a specific copper importer remains unknown. Copper transport by Ctr2 at the vacuole or Ctr1 at the plasma membrane requires that copper is reduced to Cu+. At the vacuole copper is reduced by Fre6.11 Because of the relationship of cation reduction and transport, we tested whether copper was being reduced as part of its effect on fusion. To test if Cu+ was generated as part of its inhibition of fusion, we used the Cu+ chelator Bathocuproine disulfonate (BCS).25 Two BCS molecules are needed to chelate one Cu+, thus 100 μM BCS was used to chelate 50 μM CuCl2. Unlike the effect of TETA in restoring vacuole fusion, we found the BCS was unable to rescue fusion in the presence of 50 μM CuCl2 (Fig. 2C). This suggested that copper was not being reduced outside the lumen as part of its inhibitory effect on vacuole fusion. We also examined whether Cu2+ reduction was necessary to inhibit fusion by deleting Fre6. In Figure 2D, we show that fre6Δ vacuole fusion was equally sensitive to CuCl2 as compared to the wild type parent strains. These data suggest that the effects of copper on vacuole fusion are carried out by the Cu2+ cation.
2.4. Cu2+ inhibits vacuole fusion after the priming stage
While we know that Cu2+ inhibits vacuole fusion, the specific stage at which it exerts its effect is unclear. Vacuole fusion traverses several stages that begins with the activation of SNAREs through a process termed priming. This stage occurs when the AAA+ protein Sec18 is recruited to inactive cis-SNARE complexes that are present on each membrane and prohibited from interacting with their cognate SNAREs on other membranes. Sec18/NSF associates with cis-SNARE complexes through its adaptor protein Sec17/α-SNAP. Sec18 hydrolyses ATP to disrupt cis-SNARE bundles resulting in the release of Sec17 from the membrane.26 Thus, priming can be measured by the loss of Sec17 from the membrane. Vacuoles were incubated with reaction buffer, 100 μM Cu2+ or 1 mM N-ethylmaleimide (NEM), an inhibitor of Sec18/NSF function.27,28 Individual reactions were incubated at increasing time increments after which they were centrifuged to separate the membrane bound (pellet) and solubilized (supernatant) fractions of Sec17. These experiments showed that Cu2+ did not negatively affect SNARE priming, as similar levels of Sec17 were released relative to the buffer control (Fig. 3A–B). By contrast, SNARE priming was fully inhibited by NEM, as demonstrated by the complete block of Sec17 release.
Figure 3. Cu2+ inhibits vacuole fusion after the docking stage.
(A) Vacuoles from BJ3505 were monitored for the release of Sec17 from the membrane upon SNARE priming. Fusion reactions containing 3 μg of vacuoles (by protein) were incubated with reaction buffer, 100 μM Cu2+ or 1 mM NEM. Vacuoles were incubated at 27°C for the indicated times after witch the organelles were pelleted by centrifugation and solubilized proteins in the supernatant were separated from the membrane bound fraction. The membrane pellets were resuspended in volumes of reaction buffer equal to the supernatant. Both fractions were mixed with SDS loading buffer and resolved by SDS-PAGE. Sec17 was detected by immunoblotting and the amount released was calculated by densitometry. (B) Normalized values were averaged and plotted over time of incubation. (C) Gain of resistance kinetic vacuole fusion assays were performed in the presence of reaction buffer or 100 μM Cu2+. Reactions were incubated at 27°C or on ice for 90 min. Reagents were added at the indicated time points. Fusion inhibition was normalized to the reactions receiving buffer alone. Data were fit with first order exponential decay. Error bars are S.E.M. (n=3). (D) Isolated vacuoles harboring GFP-Ypt7 were incubated with or without 100 μM Cu2+ for 30 min at 27°C after which reaction tubes were placed on ice and labeled with FM4–64. Vacuoles were visualized by fluorescence microscopy. Scale bar = 2 μm.
To further resolve the stage of the fusion pathway that was inhibited by Cu2+ we performed a temporal gain of resistance assay.28–30 Individual fusion reactions were treated with inhibitors at different time-points throughout the incubation period. Vacuole fusion gains resistance to an inhibitor once the target of the reagent has had completed its function. For example, an inhibitor of SNARE priming will no longer be effective to anti-Sec17 antibody as the reaction enters a later stage such as hemifusion. With this test, Cu2+ was shown to continue inhibiting the fusion reaction after docking as its resistance curve lied on the ice curve (Fig. 3C). Because this test only measures the last time an inhibitor functions, it was possible that Cu2+ could block stages between docking and full content mixing. This was in accord with the lack of an effect on vesicle docking and localization of Ypt7 at vertex sites (Fig. 3D).
2.5. Cu2+ inhibits SNARE complex formation
Next, we examined if SNARE complex formation was affected by Cu2+. For this, we used a SNARE complex formation assay using exogenous GST-Vam7 in reactions treated with anti-Sec17 IgG to block priming.31–34 Thus, only the formation of new SNARE complexes will be measured in the presence of Cu2+. After the addition of anti-Sec17 we further treated individual reactions with GDI to block tethering or Cu2+. Next, GST-Vam7 was added, and the reactions were incubated for 70 min at 27°C or on ice as indicated. After incubation, the reactions were placed on ice and processed for SNARE complex isolation as described in the Experimental Procedures. GST-Vam7 SNARE complexes were bound to reduced glutathione beads and proteins were resolved by SDS-PAGE. Specific proteins were detected by Western blotting. As previously seen, GST-Vam7 formed complexes with its cognate SNAREs Vam3 and Nyv1 when incubated at 27°C, but not when incubation on ice prevented complex formation as seen previously31,32 (Fig. 4A–B). As a negative control GDI inhibited SNARE complex formation. When 15 μM CuCl2 was added we observed nearly a 50% reduction in SNARE pairing. We were limited to using 15 μM CuCl2 due to its interference with the glutathione-GST interactions at higher concentrations. This is in accord with work showing that copper can inhibit GST function in the cytoplasm35. Nevertheless, 15 μM CuCl2, which blocks fusion by 50% was still able to substantially inhibit SNARE complex formation. Similarly, we were unable to use the trans-SNARE isolation method published by Collins and Wickner.36 This method relies on a calmodulin binding peptide (CBP) integrated between the Habc domain and the SNARE motif of the syntaxin homolog Vam3. Copper was found to interfere with the interaction between the CBP and the calmodulin resin used for protein isolation. This was not surprising as others have reported that copper can interfere with Ca2+ for calmodulin binding.37
Figure 4. Cu2+ inhibits SNARE complex formation.
(A) Large scale vacuole fusion reactions (6x) were incubated with anti-Sec17 IgG to block SNARE priming. After incubating for 15 min at 27°C, select reactions were further treated with either reaction buffer, 2 μM GDI or 15 μM Cu2+ and incubated for 5 min before adding 150 nM GST-Vam7. Reactions were then incubated for an additional 70 min. One reaction remained on ice for the duration of the assay. Reactions were then processed for glutathione pulldown of GST-Vam7 protein complexes. Isolated protein complexes were resolved by SDS-PAGE and probed for the SNAREs Vam3 and Nyv1. (B) Quantitation of SNARE complex formation in the presence or absence of CuCl2. Error bars are S.E.M. (n=3).
2.6. Copper inhibits V-ATPase activity
Copper ions have been shown to inhibit the H+-ATPase activity in aquatic fungi38, chromaffin cells39, and the yeast plasma membrane H+-ATPase Pma1.40 Because vacuole fusion is inhibited when the proton gradient is collapsed, thus implicating a role for V-ATPase function41, we proposed that Cu2+ could inhibit vacuole fusion by blocking V-ATPase activity. To test this, we used an acridine orange (AO) fluorescence quenching assay to measure V-ATPase activity.42 Transport of protons into the vacuole lumen leads to the quenching of AO fluorescence. Fluorescence quenching was reversed when the proton gradient was disrupted with FCCP. Using this method, we found that ≥50 μM CuCl2 completely inhibited AO quenching indicating that V-ATPase activity was blocked (Fig. 5A–B). Because CuCl2 had no effect on SNARE priming, which requires Mg2+-dependent ATP hydrolysis by Sec18, is unlikely that it interfered with the Mg2+ in the ATPase domains of V1.
Figure 5. V-ATPase activity is blocked by Cu2+.
(A) Acridine orange (AO) fluorescence quenching assays were used to monitor H+ pumping into the vacuole lumen. Vacuoles were incubated with dose curve of CuCl2 or buffer (± ATP) and 15 μM AO. AO fluorescence quenching was measured using a plate reader. Fluorescence was measured every 40 seconds and plotted against time. (B) Quantitation of average maximum AO fluorescence. (C) AO fluorescence quenching in the presence of PS buffer (Buf.), 100 μM MgCl2, ZnCl2 or CoCl2. Error bars are S.E.M. (n=3). (D) Log phase BJ3505 cells were incubated with 50 μM CuCl2 for 1h. Vacuoles were stained with 2 μM FM4–64 and 200 μM quinacrine. DIC (Differential Interference Contrast). Scale bar = 5 μm.
To test whether other divalent cations also blocked V-ATPase activity we next examined the effects of Mg2+, Zn2+ and Co2+on the AO assay. In Figure 5C we show that none of the cations tested blocked proton transport even at 100 μM, suggesting that the effect of Cu2+ was specific. To further verify the effects of Cu2+ on V-ATPase activity we monitored quinacrine fluorescence of yeast vacuoles. Quinacrine accumulates and fluoresces in acidic compartments.43 Quinacrine staining showed that wild type cells stained with quinacrine while those treated with CuCl2 failed to accumulate quinacrine (Fig. 5D). Thus, we have concluded that Cu2+ blocks vacuolar V-ATPase function.
Taken together, this study has demonstrated that elevated Cu2+ concentrations are deleterious to the fusion machinery and the V-ATPase. Metals such as Cu2+ are often associated with generating ROS that can lead to membrane damage through lipid peroxidation. Damaging the vacuolar membrane would lead to the loss of a membrane potential and ion gradients that are essential for vacuole homeostasis. That said, the inhibitory effect of Cu2+ on vacuole fusion was independent of ROS and membrane damage. Instead we observed an effect on vacuole acidification and SNARE complex formation. The effects of Cu2+ on the V-ATPase and SNAREs are likely due to separate direct mechanisms. The disparity can be seen in the difference in the kinetics of resistance. In this study we showed that Cu2+ was a late inhibitor with a resistance curve that overlaid the ice curve. In comparison, the inhibition of SNARE pairing has been shown many times to occur much earlier during the docking stage the pathway with a halftime of 20 min.28,44,45
While kinetically separate, the effects of Cu2+ on these functions could have a common origin such as the reduction of membrane fluidity. The mean by which Cu2+ and not Zn2+ or other metals (except for Ag2+) alter membrane fluidity is due copper-copper interactions when bound to anionic phospholipids.46. The interaction with anionic phospholipids reduces Cu2+ to Cu+ which stabilizes the complex. This occurs independent of a reductase. The formation of the copper-lipid complexes precludes chelation and is likely to be the cause for reducing membrane fluidity. Copper has been shown to reduce membrane fluidity in various cell types, organisms and artificial supported bilayers.7,46–51 Thus, Cu ions could indirectly alter the vacuolar V-ATPase, as other H+/V+-ATPases have been shown to be inhibited when membrane fluidity is reduced.49,51–54 Separately, we and others have found that SNARE function can be altered by membrane fluidity.32,33,55 Together, these notions and the data presented add another facet to pleiotropic effects of this metal to include membrane fusion.
While we show that Cu2+ inhibited V-ATPase, we cannot conclude that it was part of inhibiting vacuole fusion. For some time now the direct role of the V-ATPase in membrane fusion has been controversial. This was elegantly addressed in a commentary by Alexey Merz and thus, we will not expand on it here.56 That said, we must also remember that vacuole homeostasis is not limited to homotypic fusion. Thus, the effects of Cu2+ on V-ATPase activity could affect heterotypic fusion as well as other facets of its function, including fission.
3. MATERIALS AND METHODS
3.1. Reagents
Soluble reagents were dissolved in PIPES-Sorbitol (PS) buffer (20 mM 1,4-piperazinediethane sulfonic acid (PIPES)-KOH pH 6.8 and 200 mM sorbitol) with 125 mM KCl unless indicated otherwise. Anti-Sec17 IgG26, and Pbi2 (Protease B inhibitor)57 were prepared as described previously. Recombinant GST-Vam7 and GDI were prepared as previously described31,32,58 NEM (N-ethylmaleimide), acridine orange and FCCP were from Sigma. Quinacrine was from Cayman Biochemical.
3.2. Strains
BJ3505, DKY6281, RFY74 (BJ3505 yvc1Δ) and RFY75 (DKY6281 yvc1Δ) were previously described29,59,60 were used for fusion assays. FRE6 was deleted by homologous recombination using PCR products amplified from pAG32 with primers 5’-FRE6-KO (5’-ATCTTCTAAAGTGAAGCATGAC GACCATAGCTCGTTGAATCTGTTTAGCTTGCC TTGTCC–3’) and 3’-FRE6-KO (5’- TATAGGTG GGCGTAGGATCAGAAGGAGCCGGAGAGAAGA TGACACTGGATGGCGGCGTTA–3’). The PCR product was transformed into BJ3505 and DKY6281 yeast by standard lithium acetate methods and plated on YPD media containing hygromycin (200 μg/μl) to generate DKY6281 fre6Δ::hphMX4 (RFY93) and BJ3505 fre6Δ:: hphMX4 (RFY94).
3.3. Vacuole Isolation and in vitro fusion
Vacuoles were isolated as described.59 In vitro fusion reactions (30 μl) contained 3 μg each of vacuoles from BJ3505 (PHO8 pep4Δ) and DKY6281 (pho8Δ PEP4) backgrounds, reaction buffer 20 mM PIPES-KOH pH 6.8, 200 mM sorbitol, 125 mM KCl, 5 mM MgCl2), ATP regenerating system (1 mM ATP, 0.1 mg/ml creatine kinase, 29 mM creatine phosphate), 10 μM CoA, and 283 nM Pbi2. Fusion was determined by the processing of pro-Pho8 (alkaline phosphatase) from BJ3505 by the Pep4 protease from DK6281. Fusion reactions were incubated at 27°C for 90 min and Pho8 activity was measured in 250 mM Tris-HCl pH 8.5, 0.4% Triton X-100, 10 mM MgCl2, and 1 mM p-nitrophenyl phosphate. Pho8 activity was inhibited after 5 min by addition of 1 M glycine pH 11 and fusion units were measured by determining the p-nitrophenolate levels by measuring absorbance at 400 nm.
3.4. GST-Vam7 SNARE complex isolation
SNARE complex isolation was performed as described previously using GST-Vam7.31,32,34,61 Briefly, 5X fusion reactions were incubated with 85 μg/ml anti-Sec17 IgG to block priming. After 15 min, 2 μM GDI or 15 μM CuCl2 was added to selected reactions and incubated for an additional 5 min before adding 150 nM GST-Vam7. After a total of 90 min, reactions were sedimented (11,000 g, 10 min, 4°C), and the supernatants were discarded before extracting vacuoles with solubilization buffer (SB: 20 mM HEPES-KOH, pH 7.4, 100 mM NaCl, 2 mM EDTA, 20% glycerol, 0.5% Triton X-100, 1 mM DTT) with protease inhibitors (1 mM PMSF, 10 μM Pefabloc-SC, 5 μM pepstatin A, and 1 μM leupeptin). Vacuole pellets were overlaid with 100 μl SB and resuspended gently. An additional 100 μl SB was added, gently mixed, and incubated on ice for 20 min. Insoluble debris was sedimented (16,000 g, 10 min, 4°C) and 176 μl of supernatants were removed and placed in chilled tubes. Next, 16 μl was removed from each reaction as 10% total samples, mixed with 8 μl of 3X SDS loading buffer and heated (95°C, 5 min). Equilibrated glutathione beads (30 μl) were incubated with the remaining extracts (15 h, 4°C, nutation). Beads were sedimented and washed 5X with 1 ml SB (735 g, 2 min, 4°C), and bound material was eluted with 40 μl 1X SDS loading buffer. Protein complexes were examined by Western blotting.
3.5. Proton Pumping
The proton pumping activity of isolated vacuoles was performed as described by others with some modifications.42 In vitro H+ transport reactions (60 μl) contained 20 μg vacuoles from BJ3505 backgrounds, fusion reaction buffer, 10 μM CoA, 283 nM Pbi2, and 15 μM of the H+ probe acridine orange. Reaction mixtures were loaded into a black, half-volume 96-well flat-bottom plate with nonbinding surface. ATP regenerating system or buffer was added and reactions were incubated at 27°C while acridine orange fluorescence was monitored. Samples were analyzed in a fluorescence plate reader with the excitation filter at 485 nm and emission filter at 520 nm. Reactions were initiated with the addition of ATP regenerating system following the initial measurement. After fluorescence quenching plateaus we added 30 μM FCCP to collapse the proton gradient and restore acridine orange fluorescence.
3.6. Vacuole docking
Docking reactions (30 μl) contained 6 μg of vacuoles from GFP-Ypt7 yeast were incubated in docking buffer (20 mM PIPES-KOH pH 6.8, 200 mM sorbitol, 100 mM KCl, 0.5 mM MgCl2), ATP regenerating system (0.3 mM ATP, 0.7 mg/ml creatine kinase, 6 mM creatine phosphate), 20 μM CoA, and 283 nM Pbi2 (Protease B inhibitor).62 Reactions were treated with 100 μM CuCl2 or buffer alone for 1 h at 27°C. After incubating, reaction tubes were placed on ice and vacuoles were stained with 1 μM MDY-64 and mixed with 50 μl of 0.6% low-melt agarose. Samples were vortexed to disrupt non-specific clustering, mounted on slides and imaged by fluorescence microscopy. Images were acquired using a Zeiss Axio Observer Z1 inverted microscope equipped with an X-Cite 120XL light source, Plan Apochromat 63X oil objective (NA 1.4), and an AxioCam CCD camera.
Synopsis:
In this study we found that Cu2+ inhibits in vitro vacuole homotypic fusion. The inhibition was not due to generating reactive oxygen radicals and membrane damage. Rather Cu2+ blocked fusion through reducing trans-SNARE complex formation and by blocking the V-ATPase function and vacuole acidification.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Institutes of Health (GM101132) and National Science Foundation (MCB 1818310) to RAF.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
References
- 1.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim Biophys Acta. 2012;1823(9):1580–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;101237–50. [DOI] [PubMed] [Google Scholar]
- 5.Christian DA, Tian A, Ellenbroek WG, Levental I, Rajagopal K, Janmey PA, Liu AJ, Baumgart T, Discher DE. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat Mater. 2009;8(10):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan C, Bica L, Crouch PJ, Caragounis A, Lidgerwood GE, Parker SJ, Meyerowitz J, Volitakis I, Liddell JR, Raghupathi R, Paterson BM, Duffield MD, Cappai R, Donnelly PS, Grubman A, Camakaris J, Keating DJ, White AR. Copper modulates the large dense core vesicle secretory pathway in PC12 cells. Metallomics 2013;5(6):700–714. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JJ, Martínez-Ballarín E, Millán-Plano S, Allué JL, Albendea C, Fuentes L, Escanero JF. Effects of trace elements on membrane fluidity. J Trace Elem Med Biol. 2005;19(1):19–22. [DOI] [PubMed] [Google Scholar]
- 8.Rock E, Gueux E, Mazur A, Motta C, Rayssiguier Y. Anemia in copper-deficient rats: role of alterations in erythrocyte membrane fluidity and oxidative damage. Am J Physiol. 1995;269(5 Pt 1):C1245–9. [DOI] [PubMed] [Google Scholar]
- 9.Suwalsky M, Ungerer B, Quevedo L, Aguilar F, Sotomayor CP. Cu2+ ions interact with cell membranes. J Inorg Biochem. 1998;70(3–4):233–238. [DOI] [PubMed] [Google Scholar]
- 10.Peña K, Coblenz J, Kiselyov K. Brief exposure to copper activates lysosomal exocytosis. Cell Calcium. 2015;57(4):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees EM, Thiele DJ. Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem. 2007;282(30):21629–21638. [DOI] [PubMed] [Google Scholar]
- 12.Rees EM, Lee J, Thiele DJ. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem. 2004;279(52):54221–54229. [DOI] [PubMed] [Google Scholar]
- 13.Cyert MS, Philpott CC. Regulation of cation balance in Saccharomyces cerevisiae. Genetics. 2013;193(3):677–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eide DJ, Clark S, Nair TM, Gehl M, Gribskov M, Guerinot ML, Harper JF. Characterization of the yeast ionome: a genome-wide analysis of nutrient mineral and trace element homeostasis in Saccharomyces cerevisiae. Genome Biol. 2005;6(9):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrakhovitch EA, Lordnejad MR, Schliess F, Sies H, Klotz LO. Copper ions strongly activate the phosphoinositide-3-kinase/Akt pathway independent of the generation of reactive oxygen species. Arch Biochem Biophys. 2002;397(2):232–239. [DOI] [PubMed] [Google Scholar]
- 16.Douglas LM, Konopka JB. Plasma membrane architecture protects Candida albicans from killing by copper. PLoS Genet. 2019;15(1):e1007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowada N, Stiller B, Kubisch C. Increased copper toxicity in Saccharomyces cerevisiae lacking VPS35, a component of the retromer and monogenic Parkinson disease gene in humans. Biochem Biophys Res Commun. 2016;476(4):528–533. [DOI] [PubMed] [Google Scholar]
- 18.Rong-Mullins X, Winans MJ, Lee JB, Lonergan ZR, Pilolli VA, Weatherly LM, Carmenzind TW, Jiang L, Cumming JR, Oporto GS, Gallagher JEG. Proteomic and genetic analysis of the response of S. cerevisiae to soluble copper leads to improvement of the antimicrobial function of cellulosic copper nanoparticles. Metallomics. 2017;91304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54(3):266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.W C, G GM. Uptake and cellular distribution of copper, cobalt and cadmium in strains of Saccharomyces cerevisiae cultured on elevated levels of these metals. FEMS Micbiology Ecology. 1986;38277–283. [Google Scholar]
- 21.Ruta LL, Popa CV, Nicolau I, Farcasanu IC. Calcium signaling and copper toxicity in Saccharomyces cerevisiae cells. Environ Sci Pollut Res Int. 2016;23(24):24514–24526. [DOI] [PubMed] [Google Scholar]
- 22.Ungermann C, Wickner W, Xu Z. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc Natl Acad Sci U S A. 1999;96(20):11194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knauert S, Knauer K. The role of Reactive Oygen Species in Copper Toxicity to Two Freshwater Green Alage. J Phycol. 2008;44(2):311–319. [DOI] [PubMed] [Google Scholar]
- 24.Nurchi VM, Crisponi G, Crespo-Alonso M, Lachowicz JI, Szewczuk Z, Cooper GJ. Complex formation equilibria of Cu(II) and Zn(II) with triethylenetetramine and its mono- and di-acetyl metabolites. Dalton Trans. 2013;42(17):6161–6170. [DOI] [PubMed] [Google Scholar]
- 25.Laggner H, Hermann M, Gmeiner BM, Kapiotis S. Cu2+ and Cu+ bathocuproine disulfonate complexes promote the oxidation of the ROS-detecting compound dichlorofluorescin (DCFH). Anal Bioanal Chem. 2006;385(5):959–961. [DOI] [PubMed] [Google Scholar]
- 26.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85(1):83–94. [DOI] [PubMed] [Google Scholar]
- 27.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164(11):5850–5857. [DOI] [PubMed] [Google Scholar]
- 28.Starr ML, Hurst LR, Fratti RA. Phosphatidic acid sequesters Sec18p from cis-SNARE complexes to inhibit priming. Traffic. 2016;17(10):1091–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miner GE, Sullivan KD, Guo A, Jones BC, Hurst LR, Ellis EC, Starr ML, Fratti RA. Phosphatidylinositol 3,5-Bisphosphate Regulates the Transition between trans-SNARE Complex Formation and Vacuole Membrane Fusion. Mol Biol Cell. 2019;30201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasser T, Qiu QS, Karunakaran S, Padolina M, Reyes A, Flood B, Smith S, Gonzales C, Fratti RA. Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion. J Biol Chem. 2012;287(3):2221–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fratti RA, Wickner W. Distinct Targeting and Fusion Functions of the PX and SNARE Domains of Yeast Vacuolar Vam7p. J Biol Chem. 2007;282(17):13133–13138. [DOI] [PubMed] [Google Scholar]
- 32.Fratti RA, Collins KM, Hickey CM, Wickner W. Stringent 3Q: 1R composition of the SNARE 0-layer can be bypassed for fusion by compensatory SNARE mutation or by lipid bilayer modification. J Biol Chem. 2007;282(20):14861–14867. [DOI] [PubMed] [Google Scholar]
- 33.Karunakaran S, Fratti R. The Lipid Composition and Physical Properties of the Yeast Vacuole Affect the Hemifusion-Fusion Transition. Traffic. 2013;14(6):650–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miner GE, Starr ML, Hurst LR, Sparks RP, Padolina M, Fratti RA. The Central Polybasic Region of the Soluble SNARE (Soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor) Vam7 Affects Binding to Phosphatidylinositol 3-Phosphate by the PX (Phox Homology) Domain. J Biol Chem. 2016;291(34):17651–17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letelier ME, Martínez M, González-Lira V, Faúndez M, Aracena-Parks P. Inhibition of cytosolic glutathione S-transferase activity from rat liver by copper. Chem Biol Interact. 2006;164(1–2):39–48. [DOI] [PubMed] [Google Scholar]
- 36.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci U S A. 2007;104(21):8755–8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills JS, Johnson JD. Metal ions as allosteric regulators of calmodulin. J Biol Chem. 1985;260(28):15100–15105. [PubMed] [Google Scholar]
- 38.Azevedo MM, Guimarães-Soares L, Pascoal C, Cássio F. Copper and zinc affect the activity of plasma membrane H+-ATPase and thiol content in aquatic fungi. Microbiology. 2016;162(5):740–747. [DOI] [PubMed] [Google Scholar]
- 39.Wimalasena DS, Wiese TJ, Wimalasena K. Copper ions disrupt dopamine metabolism via inhibition of V-H+-ATPase: a possible contributing factor to neurotoxicity. J Neurochem. 2007;101(2):313–326. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes AR, Sá-Correia I. Comparative effects of Saccharomyces cerevisiae cultivation under copper stress on the activity and kinetic parameters of plasma-membrane-bound H(+)-ATPases PMA1 and PMA2. Arch Microbiol. 1999;171(4):273–278. [DOI] [PubMed] [Google Scholar]
- 41.Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409(6820):581–588. [DOI] [PubMed] [Google Scholar]
- 42.Müller O, Neumann H, Bayer MJ, Mayer A. Role of the Vtc proteins in V-ATPase stability and membrane trafficking. J Cell Sci. 2003;116(Pt 6):1107–1115. [DOI] [PubMed] [Google Scholar]
- 43.Flannery AR, Graham LA, Stevens TH. Topological characterization of the c, c’, and c” subunits of the vacuolar ATPase from the yeast Saccharomyces cerevisiae. J Biol Chem. 2004;279(38):39856–39862. [DOI] [PubMed] [Google Scholar]
- 44.Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387(6629):199–202. [DOI] [PubMed] [Google Scholar]
- 45.Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396(6711):543–8. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X, Zhang J, Zhou B, Li P, Hu X, Zhu Z, Tan Y, Chang C, Lü J, Song B. Anomalous behavior of membrane fluidity caused by copper-copper bond coupled phospholipids. Sci Rep. 2018;8(1):14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Salah KM, al-Othman AA, Lei KY. Lipid composition and fluidity of the erythrocyte membrane in copper-deficient rats. Br J Nutr. 1992;68(2):435–443. [DOI] [PubMed] [Google Scholar]
- 48.Calucci L, Pinzino C, Quartacci MF, Navari-Izzo F. Copper Excess Reduces the Fluidity of Plasma Membrane Lipids of Wheat Roots: a Spin Probe EPR Study. J Phys Chem B. 2003;10712021–12028. [Google Scholar]
- 49.Myers BM, Prendergast FG, Holman R, Kuntz SM, Larusso NF. Alterations in hepatocyte lysosomes in experimental hepatic copper overload in rats. Gastroenterology. 1993;105(6):1814–1823. [DOI] [PubMed] [Google Scholar]
- 50.Quartacci MF, Pinzino C, Sgherri CLM, Dalla Vecchia F, Navari-Izzo F. Growth in excess copper induces changes in the lipid composition and fluidity of PSII‐enriched membranes in wheat. Pysiol Plant 2000;10887–93. [Google Scholar]
- 51.Yan D, Lin X, Qi Y, Liu H, Chen X, Liu L, Chen J. Crz1p Regulates pH Homeostasis in Candida glabrata by Altering Membrane Lipid Composition. Appl Environ Microbiol. 2016;82(23):6920–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexandre H, Mathieu B, Charpentier C. Alteration in membrane fluidity and lipid composition, and modulation of H(+)-ATPase activity in Saccharomyces cerevisiae caused by decanoic acid. Microbiology. 1996;142(Pt 3):469–475. [DOI] [PubMed] [Google Scholar]
- 53.Costa GA, de Souza SB, da Silva Teixeira LR, Okorokov LA, Arnholdt ACV, Okorokova-Façanha AL, Façanha AR. Tumor cell cholesterol depletion and V-ATPase inhibition as an inhibitory mechanism to prevent cell migration and invasiveness in melanoma. Biochim Biophys Acta Gen Subj. 2018;1862(3):684–691. [DOI] [PubMed] [Google Scholar]
- 54.Qi Y, Liu H, Yu J, Chen X, Liu L. Med15B Regulates Acid Stress Response and Tolerance in Candida glabrata by Altering Membrane Lipid Composition. Appl Environ Microbiol. 2017;83(18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang ST, Kreutzberger AJB, Lee J, Kiessling V, Tamm LK. The role of cholesterol in membrane fusion. Chem Phys Lipids. 2016;199136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merz AJ. What are the roles of V-ATPases in membrane fusion. Proc Natl Acad Sci U S A. 2015;112(1):8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slusarewicz P, Xu Z, Seefeld K, Haas A, Wickner WT. I2B is a small cytosolic protein that participates in vacuole fusion. Proc Natl Acad Sci U S A. 1997;94(11):5582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Starai VJ, Jun Y, Wickner W. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proc Natl Acad Sci U S A. 2007;104(34):13551–13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones EW, Zubenko GS, Parker RR. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982;102(4):665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miner GE, Starr ML, Hurst LR, Fratti RA. Deleting the DAG kinase Dgk1 augments yeast vacuole fusion through increased Ypt7 activity and altered membrane fluidity. Traffic. 2017;18(5):315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167(6):1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]