Abstract
Wnt/β-catenin signaling plays an important role in melanocyte biology, especially in the early stages of melanocyte transformation and melanomagenesis. β-catenin, encoded by the gene CTNNB1, is an intracellular signal transducer of Wnt signaling and activates transcription of genes important for cell proliferation and survival. Wnt/β-catenin signaling is frequently activated in melanoma through oncogenic mutations of β-catenin and elevated β-catenin levels are positively correlated with melanoma aggressiveness. Molecular mechanisms that regulate β-catenin expression in melanoma are not fully understood. MicroRNA-214 is known to function as a tumor suppressor by targeting β-catenin in several types of cancer cells. Here, we investigated regulation of β-catenin by miR-214 and its role in melanoma. We show that β-catenin mRNA levels are negatively correlated with miR-214 in melanoma. However, overexpression of miR-214 paradoxically increased β-catenin protein levels and promoted malignant properties of melanoma cells including resistance to mitogen-activated protein kinase inhibitors (MAPKi). RNA-seq analysis revealed that melanoma cells predominantly express a β-catenin mRNA isoform lacking miR-214 target site. Using matched miRNA and mRNA-seq and bioinformatics analysis, we identified novel miR-214 targets, ankyrin repeat domain 6 (ANKRD6) and C-terminal binding protein 1 (CTBP1), that are involved in negative regulation of Wnt signaling. Overexpression of miR-214 or knockdown of the novel miR-214 targets, ANKRD6 or CTBP1, increased melanoma cell proliferation, migration and decreased sensitivity to MAPKi. Our data suggest that in melanoma cells β-catenin is not regulated by miR-214 and the functions of miR-214 in melanoma are mediated partly by regulating proteins involved in attenuation of Wnt/β-catenin signaling.
Keywords: Wnt/β-catenin signaling pathway, microRNA-214, CTNNB1, metastatic melanoma, chemo-resistance, tumor growth
INTRODUCTION
Wnt-β-catenin signaling pathway is an essential and highly conserved pathway involved in regulation of a wide ranging cellular functions such as cell proliferation, cell migration, cell polarity and cell fate specifications during embryogenesis and tissue regeneration1–3. Perturbations in Wnt-β-catenin signaling are associated with malignant phenotype4. In melanocytes, the canonical Wnt/β-catenin signaling pathway plays a critical role by activating microphthalmia-associated transcription factor (MITF), the melanocyte master regulator that regulates melanocyte survival, proliferation and differentiation5. In melanoma, genetic alterations including amplification and missense mutations in CTNNB1 have been reported6. β-catenin expression is also upregulated and stabilization and accumulation of β-catenin in the nucleus increases transcriptional activity of the TCF/Lef-responsive target genes7–8. In metastatic melanoma, mechanisms responsible for dysregulation of β-catenin include mutations that produce stable β-catenin mRNA transcripts, deletion of the regulatory NH2-terminal and loss of adenomatous polyposis coli (APC), a regulator of β-catenin degradation9–11. Aberrant expression of β-catenin facilitates melanoma metastasis by activating the transcription of oncogenes like Myc and CyclinD15. In Braf/Pten−/− mouse melanoma model, β-catenin signaling was shown to control metastasis12. These findings highlight the critical role β-catenin plays in melanoma progression and the need for a better understanding of the molecular mechanisms that regulate β-catenin signaling.
In the absence of Wnt stimulation, the cellular levels of β-catenin protein are regulated post-translationally by ubiquitination and proteasomal degradation13. Phosphorylation of β-catenin at serine residues 33, 37 and 45 and threonine residue 41 by the serine-threonine kinase, GSK3, destabilizes the protein and marks it for ubiquitination and facilitates its interaction with the E3 ubiquitin ligase, β-TrCP2. Activation of Wnt signaling inhibits ubiquitination-dependent proteolysis of β-catenin and promotes transcription of oncogenes14–15.
In addition to regulation by degradation, cellular protein expression can also be regulated by small non-coding RNAs including microRNA (miRNA). Typically, microRNA bind to the 3’untranslated region (3’UTR) of target mRNA resulting in inhibition of translation or stability of mRNA16. Regulation of gene expression by miRNAs is complex because mRNAs have binding sites for multiple miRNAs and each miRNA, in turn, can bind to multiple mRNA targets17. By targeting oncogenes or tumor suppressors, miRNAs themselves can act as tumor suppressors and oncogenes, respectively18. For example, miR-214, which has been reported to target several important mRNAs including that of β-catenin, fibroblast growth factor receptor 1 (FGFR-1) and hepatoma-derived growth factor (HDGF), has been shown to act as a tumor suppressor in hepatic cancers19–23. In non-small cell lung cancer, miR-214 was reported to target the master regulator c-MYC, and reduce cancer stem-like traits and reverse resistance to the chemotherapeutic drug, cisplatin24. In melanoma, miR-214 was shown to modulate expression of the transcription factor AP-2 (Activating Protein 2, TFAP2) and enhance melanoma tumor growth in vivo25. The role of miR-214 as a critical regulator of cancer networks was elegantly reviewed by Penna et al26. Although β-catenin is a validated target of miR-214, the relationship of miR-214-mediated regulation of β-catenin to the pro-oncogenic functions of miR-214 in melanoma is not fully understood.
In this study, we investigated the relationship between miR-214 and β-catenin and show that in melanoma cells, β-catenin is protected from downregulation by miR-214 by the preferential expression of mRNA isoforms that lack the miR-214 target site. However, we found that overexpression of miR-214 increased melanoma cell proliferation and migration. Using RNA-seq and bioinformatic analysis, we identified and validated ANKRD6 and CTBP1 as novel targets of miR-214. Accordingly, overexpression of miR-214 increased malignant properties of melanoma, including potential resistance to MAP kinase inhibitors, partly due to downregulation of ANKRD6 and CTBP1,which are known to be negative regulators Wnt signaling. Our data suggest that miR-214 expression can serve as a predictor of melanoma aggressiveness including resistance to MAPK targeted therapy.
MATERIALS & METHODS
Cell culture
The MRA-series of metastatic melanoma cell lines were established at University of Wisconsin-Madison (by Dr. Mark Albertini, Department of Medicine) and maintained and passaged in DMEM, high glucose, pyruvate medium supplemented with 10% FBS and 1% penicillin-streptomycin. BRAF and NRAS mutations were confirmed by genomic DNA PCR and western blotting for expression of BRAF(V600E) protein27. The cell cultures were maintained in a CO2 (5%) incubator at 37°C. Normal human melanocytes were obtained from human neonatal foreskin tissue processed in the Cell Culture Core of the UW Skin Disease Research Center. Melanocytes were cultured in Ham’s F10 medium supplemented with 5% FBS, 85nM phorbol 12-myrisate 13-acetate, 2.5nM cholera toxin, 0.1mM 3-isobutyl-1-methylxanthine and 1% penicillin/streptomycin.
Transfection
MicroRNA-214 overexpression and inhibition was achieved by transfecting cell lines with miRVana mimic (#MC12124, Thermo Fisher Scientific, Waltham, MA) or inhibitor (#MH12124, Thermo Fisher), respectively. All experiments included transfections with a negative control oligonucleotide (#4464060, Thermo Fisher) and were performed using Lipofectamine2000™ (#11668019, Thermo Fisher). The transfection mixture was prepared in antibiotic- and serum-free DMEM and 2×105 cells plated in a 6-well plate were transfected. Next day, the media were changed to normal DMEM with 10% FBS and 1% antibiotics and the cells were collected at 24, 48 and 72 h after transfection for qPCR and western blot analysis. We found maximum transfection efficiency, measured by qPCR, when 6nM of miR-214 mimic was used to transfect 2×105 cells/ml using Lipofectamine2000™ in a 6-well plate.
TaqMan qRT-PCR
Total RNA was extracted using the miRNeasy Mini Prep Kit. RT-PCR was performed using (BioRad CFX96 thermal cycler) random primers (for cDNA of messenger RNA) or miRNA specific primers (for cDNA of the respective miRNA) (Assay name: hsa-miR-214 Cat# 002306 and Assay name: RNU48 Cat# 001006, Thermo Fisher). The RNA samples were stored at −80οC. RT-PCR was carried out in the BioRad C1000 thermal cycler (BioRad, Hercules, CA) using the cDNA obtained as templates for quantitative PCR. The TaqMan gene expression for hsa-miR-214 (AssayID#002306, Thermo Fisher), RNU48 (AssayID#001006, Thermo Fisher) and CTNNB1 (AssayID#Hs00355049_m1, Thermo Fisher) was calculated using the ΔCT or 2−ΔΔCT methods as appropriate. Reactions with no template and RNA without reverse transcriptase controls served as controls for all samples. GAPDH (AssayID#4326317E, Thermo Fisher) was used for normalizing gene expression in all experiments.
Cell viability and growth assay
Cell viability was assessed using MTT assay. Transfected cells were seeded in six replicate wells into 96-well plates at a density of 2500 cells per well. At 72 hours, 20 μl of the MTT reagent (5μg/ml) was added into each well and the plates were incubated for 1 h at 37°C and absorbance at 540nm was measured using BioTek Synergy H1 multi-mode microplate reader (BioTek, Winooski, VT).
Migration assay
Cell migration assay was performed using Ibidi (#80209, Madison, WI) cell culture inserts. Transfected cells were seeded into the chambers of the culture insert at 80% confluency. Twenty-four hours after incubation, the insert was removed carefully to obtain a well-defined cell-free gap between the two chambers. Cells were imaged at 0, 6, 12 and 24 hours. The gap between the edges of cells at each time point was normalized with the distance measured at 0 h.
Clonogenic assay
Twenty-five hundred cells were seeded in each well of a 6-well plate. After 24 h of transfection, the transfection media were changed to complete DMEM. The plates were incubated at 37°C for two weeks. Colonies were visualized using crystal violet staining method (0.05% crystal violet in 70% ethanol). The wells were photographed after drying.
Transwell assay
Transfected cells were seeded in the top chamber of a Transwell (#3464, Corning, Corning, NY) placed in 24-well plate with medium devoid of FBS. Complete DMEM (with 10% FBS and 1% penicillin/streptomycin) was placed in the bottom well. After incubation for 24 h, the migrated cells at the bottom of the Transwell were stained with crystal violet and the number of cells were counted for each treatment and plotted.
RNA-sequencing
Total RNA was isolated using miRNeasy kit from Qiagen (#217004). RNA quality was verified using a 1% TBE gel as well as a Bioanalyzer. RNA concentration was estimated using the Bioanalyzer. Total RNA was processed as per manufacturer’s instructions using the Illumina TruSeq Stranded RNA kit and sequenced using Illumina’s HiSeq 2500platform. Raw reads were analyzed for quality using FASTQC and mapped to known transcripts in human genome version hg19 using Salmon. The Salmon index for mapping was built using a k value of 25 and the UCSC genome browser CDS data hg19.
Short hairpin RNA knockdown
Scrambled shRNA (#SHC002, Sigma-Aldrich, St. Louis, MO) and shRNA specific to the open reading frame or untranslated regions of ANKRD6 (#TRCN0000162328 & #TRCN0000163365, Sigma-Aldrich) and CTBP1 (#TRCN0000285086 & #TRCN0000013738, Sigma-Aldrich) were used to transfect melanoma cells using Lipofectamine2000 (S1 Table).
Immunoblot
Cells were washed with cold HyClone Hank’s 1X Balanced Salt Solution, HBSS (GE Healthcare Life Science, Marlborough, MA) and harvested using cell scrapers. The cell suspension in HBSS was centrifuged for 5 minutes at 2500 rpm and the cells were lysed in RIPA lysis buffer (supplemented with 0.5M EDTA, protease and phosphatase inhibitor). After 20 minutes of incubation on ice, the cell lysates were sonicated (Misonix XL2000, Misonix, Farmingdale, NY) for 20 seconds and centrifuged for 30 minutes at 4°C. Protein concentration was determined using BCA Protein Assay Kit (Thermo Fisher). SDS-PAGE was performed using 15–30μg of protein, transferred to polyvinylidene difluoride (PVDF) membrane and blocked with 5% skim milk in TBS/Tween 0.1% buffer. Primary antibodies for β-catenin (#8480, Cell Signaling, Danvers, MA) Active β-catenin (#8814, Cell Signaling), GAPDH (#60004–1-Ig, Proteintech, Chicago, IL) CTBP1 (#10972–1-AP, Proteintech), ANKRD6 (#24333–1-AP, Proteintech), ARIDB (#A301–046A-T-2, Bethyl, Montgomery, TX) and FBXW11 (#13149–1-AP, Proteintech) are used at 1:1000 dilution for detecting proteins. Proteins are visualized using HRP-conjugated secondary antibodies; Anti-Rabbit IgG (#NA934VS, GE Healthcare) and Anti-Mouse IgG (#NXA93V, GE Healthcare), used at 1:5000 dilution with ECL Western Blotting Detection Reagent (Thermo Fisher). The blots were imaged using ImageQuant LAS 4000 (GE Healthcare) and ImageJ (NIH) software was used for densitometric quantification.
Statistical analysis
All statistical analysis was performed in GraphPad Prism7 software (Ver.7.03) and data were analyzed using unpaired Student’s t-test and differences with p value ≤0.05 were considered significant.
Results
Relationship between miR-214 and its target β-catenin in melanoma:
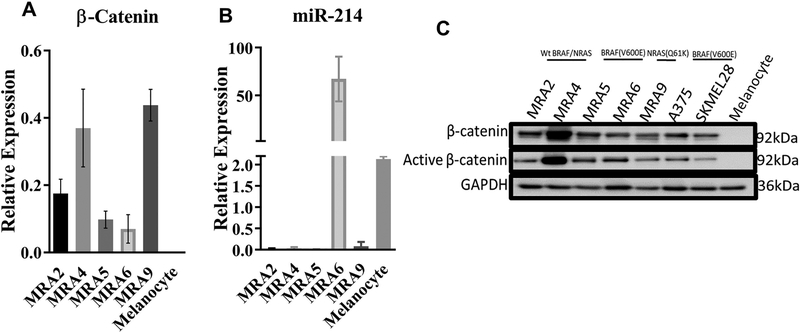
In a panel of human metastatic melanoma cell lines harboring wild type BRAF and NRAS or BRAF(V600E) or NRAS(Q61K) mutations, quantitative RT-PCR and western blot analysis showed upregulation of β-catenin mRNA and protein, both total and non-phosphorylated active β-catenin, compared to normal human melanocytes (Fig. 1A and 1C).
Fig. 1. β-catenin is upregulated in metastatic melanoma cells.
(A) TaqMan qPCR analysis of β-catenin mRNA in melanocytes and metastatic melanoma cell lines. (B) TaqMan qPCR analysis of endogenous miR-214 microRNA in melanocyte and metastatic melanoma cell lines. Data from a representative of three independent experiments are shown. (C) Western blot analysis of β-catenin and active β-catenin proteins in melanocytes and a panel of metastatic melanoma cell lines.
MicroRNA target prediction algorithms showed presence of a miR-214 binding site within the 3’UTR of β-catenin mRNA at nucleotide position 1028–1035 (Fig. S1A). To understand whether there is a relationship between miR-214 levels and β-catenin in melanoma, we queried TCGA (The Cancer Genome Atlas) dataset consisting of RNA-seq data of 342 melanoma tumors using the StarBase Pan-Cancer algorithm28. Data in S1B Fig. shows a medium to low inverse relationship between β-catenin mRNA and miR-214 expression in melanoma (Pearson’s correlation coefficient, r = −0.25 and P = 2.55381e-06).
Analysis of matched RNA- and microRNA-seq datasets of the five metastatic melanoma cell lines MRA2, −4, −5, −6 and −9 also showed an inverse relationship between expression of β-catenin and miR-214 (r = −0.392) (Fig. S1C) similar to the TCGA dataset. Consistent with the miRNA-seq data, qRT-PCR analysis also showed that miR-214 expression was lower in 4/5 of metastatic melanoma cell lines compared to melanocytes (Fig. 1B). These data raise the possibility that in melanoma cells β-catenin levels are regulated by miR-214.
Lack of miR-214-mediated regulation of β-catenin mRNA in melanoma cells:
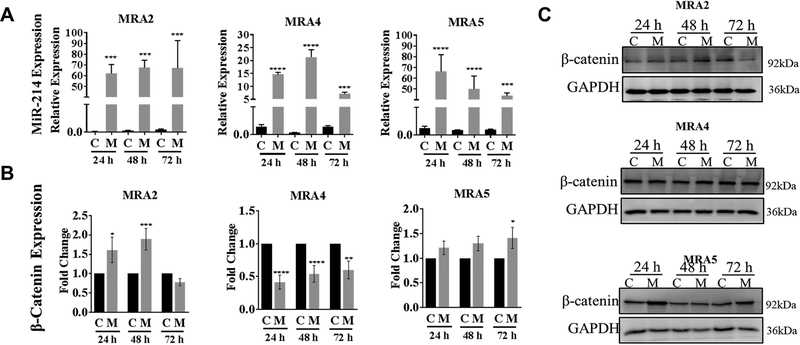
To investigate whether miR-214 regulates β-catenin mRNA and protein expression in melanoma cells, we overexpressed a miR-214 mimic in metastatic melanoma cell lines. Transfection with miR-214 mimic (M) resulted in 10–60 fold increase in miR-214 levels in different cell lines (Fig. 2A). MiR-214 overexpression caused a 50% reduction of β-catenin mRNA in MRA4 cell line (Fig. 2B) although there was no reduction in β-catenin protein levels (Fig. 2C). On the other hand, overexpression of miR-214 in MRA2 and MRA5 cell lines did not reduce β-catenin mRNA (Fig. 2B). Similarly, there was no consistently marked change in β-catenin protein levels (Fig. 2C). Thus, in melanoma cells, β-catenin protein expression did not appear to correlate with miR-214 levels.
Fig. 2. Effect of miR-214 overexpression on β-catenin protein.
(A) Overexpression of miR-214 evaluated by TaqMan qPCR analysis of metastatic melanoma cells transfected with 6nM mirVana mir-214 mimic at 24 h, 48 h and 72 h time points. miR-214 expression is shown relative to RNU48 expression. (B) TaqMan qPCR analysis of β-catenin mRNA in metastatic melanoma cells transfected with 6nM mirVana miR-214 mimic (M) or negative control oligo (C). Total RNA was extracted at 24 h, 48 h and 72 h post-transfection, analyzed by qRT-PCR and fold change in β-catenin mRNA over controls is shown. Data from 3 replicates are analyzed using unpaired Student’s t test where * indicates P values: *≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. (C) Western blot analysis for β-catenin protein expression in metastatic melanoma cells transfected with miR-214 mimic (M) or negative control (C). GAPDH levels show equal protein loading. Representative data from two independent experiments are shown.
Melanoma cells express β-catenin mRNA isoform lacking miR-214 target site:
We asked whether the lack of negative regulation of β-catenin mRNA by miR-214 in MRA2 and MRA5 cells could be due to the absence of the miR-214 binding site in the β-catenin 3’UTR (as a result of either alternative splicing or mutations in the miR-214 target sequence). There are five known splice variants of β-catenin that code for the full the length 781 kDa β-catenin protein (Ensemble Genome Browser: http://useast.ensembl.org/index.html). Three of these five transcripts (ENST00000349496, ENST00000396185 and ENST00000396183) have the seed sequence CCUGCUG, for miR-214 binding while ENST00000453024 and ENST00000405570 have a truncated 3’UTR (Table S2 and Fig. S2A). Employing Salmon, which uses two-phase inference algorithm29, we analyzed the RNA-seq data and quantitated the expression of β-catenin transcript variants in melanocytes and melanoma cells (Fig. S2B). In majority of melanoma cell lines, the combined reads for β-catenin transcripts with the miR-214-binding site were lower (5–100 fold) compared to transcripts without miR-214-binding site (Fig. S2B). The splice variant that lacks the binding site for miR-214, CTNNB1–210, was the most abundant β-catenin transcript in both melanocytes and melanoma cells (36 transcripts per million and 73.53 to 555.64 transcripts per million, respectively). Interestingly, MRA4 melanoma cells, which show relatively higher abundance of β-catenin transcripts with the miR-214-binding site, also show a decrease in β-catenin mRNA upon overexpression of miR-214 (Fig. 2B).
Overexpression of miR-214 promotes malignant properties of melanoma cells:
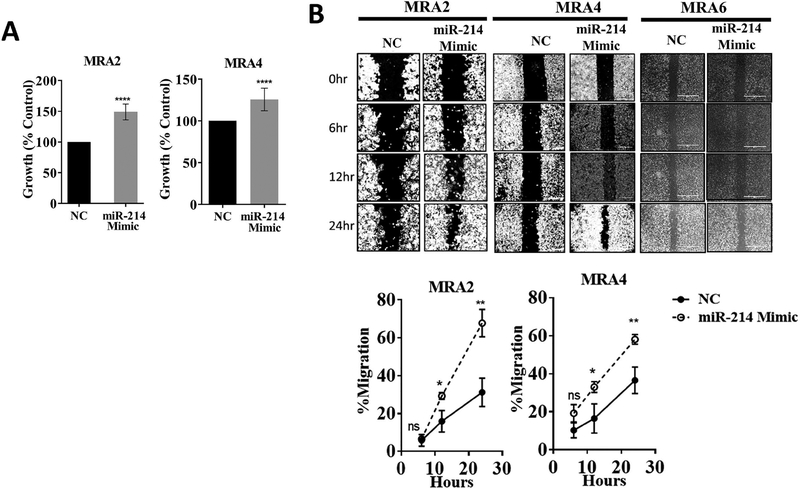
To investigate the role of miR-214 in malignant phenotype of melanoma cells, we transfected miR-214-low melanoma cells MRA2 and MRA4 with miR-214 mimic. Overexpression of miR-214 caused a significant increase in cell number (as measured by MTT assay) in both cell lines (Fig 3A). Data in Fig. 3B and Fig. S3A show that overexpression of miR-214 increased the migration of both MRA2 and MRA4 melanoma cells. Interestingly, MRA6 cells, which express highest levels of miR-214 expression, did not show markedly increased rate of migration and although overexpression of miR-214 did appear to increase their migration (Fig. 3B) suggesting that miR-214 is not the sole determinant of melanoma migration. Mir-214 also promoted clonogenic growth of metastatic melanoma cells (Fig. S3B). These results show that overexpression of miR-214 promotes the malignant properties of melanoma cells.
Fig. 3. MiR-214 promotes malignant properties of melanoma cells.
(A) miR-214 overexpression increases cell growth/survival of melanoma cells. Cells were transfected with 6nM of miR-214 mimic or negative control oligo (NC) and cell survival was assessed using MTT assay 72 h post-transfection. Data from six replicates are represented as percent growth compared to cells transfected with control oligo. (B) Left: Representative photomicrographs (obtained with 10X objective) of wound healing/migration assay. MRA2, MRA4 and MRA6 cells were transfected with 6nM of miR-214 mimic or negative control oligo (NC), trypsinized 24 h after transfection and 5×105 cells/ml were plated into the chambers of Ibidi culture inserts. Migration was monitored and photographed at 0, 6, 12 and 24 h after removing the culture inserts. Right: Quantitation of percent cells migrated. Data from 6 replicates are analyzed using unpaired Student’s t-test where * indicates P values: *≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. Data from a representative of three independent experiments are shown.
Role of miR-214 in melanoma drug resistance:
Next, we asked whether miR-214 plays a role in the sensitivity of melanoma cells to BRAF(V600E) inhibitor (BRAFi) PLX4032 (vemurafenib) and MEK inhibitor (MEKi) AZD6244 (selumetinib). We overexpressed miR-214 in BRAF(V600E) mutant MRA6 cells and assessed the sensitivity of control and miR-214 transfected cells to BRAFi and MEKi (Fig. 4A and Fig. S4A). Treatment of cells with 1μM of BRAFi and MEKi for 72 h caused 50% and 70% decrease, respectively, in survival of MRA6 cells. Overexpression of miR-214 attenuated the sensitivity of MRA6 cells to these inhibitors resulting in a 20% decrease in cell killing (Fig 4C and Figs. S4A and S5A)>. The effect of overexpression of miR-214 on the cell growth also could partially contribute the observed decrease in cell killing by these inhibitors.
Fig. 4. Role of miR-214 in melanoma drug resistance.
(A) MTT assay performed on MRA6 cells transfected with miR-214 mimic or negative control and treated with increasing concentration of BRAFi (PLX4032) for 72 h. (B) TaqMan qPCR analysis of miR-214 levels in parental cell line MRA6 and resistant cell line MRA6-MR. MEKi-sensitive MRA6 cells (C and D) and MEKi-resistant MRA-6MR cells (E) were transfected with miR-214 mimic (C) or miR-214 anti-miR (E), treated with 1μM of MEK inhibitor AZD6244 for 72 h and cell survival was assessed using MTT assay. Data are shown as percent cell survival compared to DMSO treated cells. All data are shown as percent survival after inhibitor treatment compared to DMSO control. Data from 4–6 replicates were analyzed using unpaired Student’s t test where * indicates P values: *≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. Data from a representative of three independent experiments are shown.
To test whether miR-214 contributes to acquired resistance of melanoma cells to MAPKi, we used resistant MRA6 cell line MRA6MR generated in the laboratory by culturing the MRA6 cells in the presence of AZD6244 and expanding the surviving population27 (Fig. S4B). The resistant cell line MRA6MR has significantly higher expression of endogenous miR-214 compared to the parental cell line, MRA6 (Fig. 4B). To test the role of miR-214 in acquired resistance to MAPKi, we inhibited endogenous miR-214 (using an anti-miR-214) in MRA6 and MRA6MR cells and tested their sensitivity to MEKi. We transfected the parental MRA6 and the resistant MRA6MR cells with anti-miR for 24h and then treated the cells with increasing concentrations of MEKi, AZD6244. As shown in Fig. 4D and 4E (Figs. S4C and S4D) inhibition of miR-214 increased the sensitivity of both MRA6 and MRA6MR cells to MEKi. These data show that miR-214 overexpression decreases the sensitivity of melanoma cells MEKi, suggesting that miR-214 plays a role in resistance of melanoma cells to MEK inhibitor.
Overexpression of miR-214 downregulates the negative regulators of Wnt signaling:
To determine potential targets of miR-214 in melanoma, we first identified mRNA targets of miR-214 computationally predicted by four miRNA target prediction algorithms, namely miRtar Base, miRNAMap, miRDB and Target Scan. We found 324 potential targets of miR-214 predicted by all four algorithms. Then, we queried the matched RNA- and miRNA-seq datasets of five melanoma cell lines for mRNAs that are negatively correlated to miR-214 levels. We identified 2567 mRNAs that show negative correlation (cut off value of r = −0.3) with miR-214 levels across all melanoma cell lines. Intersection of computationally predicted miR-214 targets with mRNAs negatively correlated with its expression levels in melanoma cells identified 199 candidate targets (Fig. S5A and Table S3).
Gene Ontology Enrichment analysis of the 199 candidate targets (Fig. S5B and Table S4) revealed the largest number of miR-214 target genes enriched belong to the Wnt signaling pathway (P = 0.0127). These genes include FBXW11, BTRC, CTBP1, DVL1, CELSR2, WNT11, ACVR1B, CTNNB1, ARID1B and ANKRD6 (Table S5). DVL1, CELSR2 and WNT11 are core Wnt/PCP signaling transducers that have been implicated in aberrant planar cell polarity and malignant phenotype in gastric cancer30. BTRC is involved in a negative feedback loop for β-catenin/TCF signaling31 and a single nucleotide polymorphism in BTRC gene was associated with survival of patients with cutaneous melanoma32. Regulation of ANKRD6, CTBP1, FBXW11 and ARIDB1 by miR-214 and their role in melanoma has not been investigated. The Pearson’s Correlation between expression of these targets and miR-214 levels (based on matched miRNA and mRNA-seq data) in five melanoma cell lines is shown in Fig. S5C. These targets ANKRD6 (Ankyrin Repeat Domain 6)33, CTBP1 (C-Terminal Binding Protein 1)34–35, FBXW11 (F-Box and WD Repeat Domain Containing 11)36–38 and ARID1B (AT-Rich Interaction Domain 1B)39 have a conserved miR-214 binding site in their 3’UTRs (Fig. 5A). Therefore, we tested whether overexpression of miR-214 downregulates these targets in melanoma cells. Western blot analysis of MRA2 and MRA4 melanoma cells transfected with miR-214 mimic showed reduction of ANKRD6, CTBP1 and FBXW11 (but not ARID1B) in cells overexpressing miR-214 compared to cells transfected with negative control miRNA (Fig. 5B and 5C). We selected ANKRD6 and CTBP1 for further analysis.
Fig. 5. Identification of novel targets of miR-214.
(A) Clustalω alignment of 3’UTRs of ANKRD6, ARID1B, CTBP1 and FBXW11 genes showing the conserved miR-214 binding site (B) Western blot analysis of ANKRD6, FBWX11, CTBP1 and ARID1B in MRA2, MRA4 and MRA9 cells transfected with miR-214 mimic (M) and negative control oligo (NC). (C) Densitometric quantitation of a representative western blot in (B).
Knockdown of miR-214 targets promotes malignant phenotype of melanoma cells:
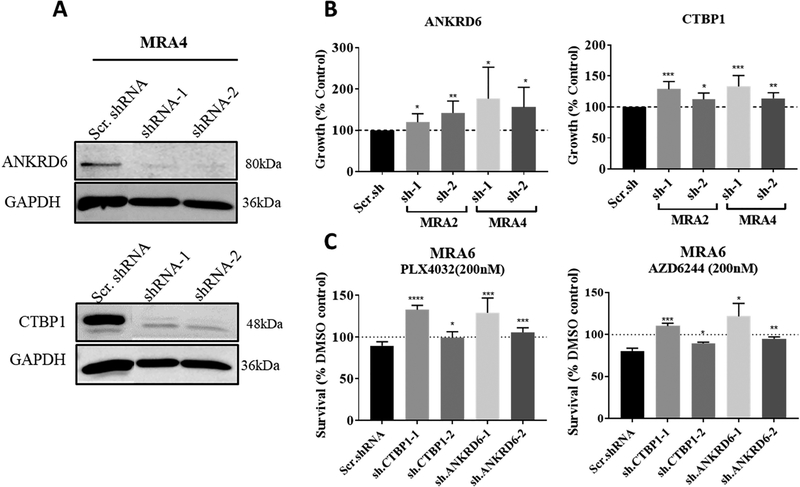
To test whether downregulation of miR-214 target genes ANKRD6 and CTBP1 results in similar phenotype as observed with miR-214 overexpression, we transfected MRA2 and MRA4 cells with ANRD6 or CTBP1 short-hairpin RNAs (shRNAs). The efficiency of knockdown by shRNAs was tested in MRA4 cell line (Fig. 6A). Knockdown of both, ANKRD6 and CTBP1, increased cell growth compared to cells transfected with scrambled shRNA (Fig. 6B). To test the role of these novel miR-214 targets in the response of melanoma cells to MEKi, we transfected the drug-sensitive MRA6 cells with ANKRD6 shRNA and CTBP1 shRNA. Data in Fig. 6C show that knockdown of miR-214 targets ANKRD6 and CTBP1 resulted in increased resistance to MAPK inhibitors, AZD6244 and PLX4032 similar to miR-214 overexpression (Figs. 4A and S4A). In summary, these data show that regulation of Wnt signaling regulators ANKRD6 and CTBP1, but not β-catenin, by miR-214 contributes to its pro-oncogenic functions in melanoma.
Fig. 6. Knockdown of miR-214 targets ANKRD6 and CTBP1 promotes malignant properties.
(A) Western blot analysis of shRNA knockdown of ANKRD6 and CTBP1 in MRA4 cell line. Data from a representative of 3 independent experiments are shown. (B) Effect of knockdown of the miR-214 targets ANKRD6 and CTBP1 on melanoma cell survival. MRA2 and MRA4 cells were transfected with ANKRD6 shRNAs or CTBP1 shRNAs for 72 h and cell survival was assessed by MTT assay. (C) Effect of knockdown of miR-214 targets ANKRD6 and CTBP1 on sensitivity of MRA6 cells to BRAF(V600E) inhibitor PLX4032 and MEK inhibitor AZD6244. Cells were transfected with two shRNAs each for ANKRD6 and CTBP1 and then treated with 1μM MEKi AZD6244 or 1μM BRAFi PLX4032 for 72 h. All data are shown as percent survival after inhibitor treatment compared to DMSO control. Data from 4–6 replicates were analyzed using unpaired Student’s t test where * indicates P values: *≤0.05; ** ≤0.01; *** ≤0.001 and **** ≤0.0001. Data from a representative of three independent experiments are shown.
DISCUSSION
In this study, we have identified novel targets of miR-214 and show that downregulation of these targets promotes malignant phenotype of melanoma cells. It is now well established that microRNAs play important roles in cancer including melanoma40–41. β-catenin, the critical component of Wnt signaling pathway, has been shown to be regulated by miR-21420–21. Wang et al reported that miR-214 is downregulated in human hepatocellular carcinoma (HCC) and overexpression of miR-214 caused G0/G1 arrest resulting in inhibition of cell proliferation and also caused downregulation of β-catenin and its transcriptional activity measured by expression of its several downstream genes20. Similarly, miR-214 was reported to act as a tumor suppressor in cervical cancer by suppressing RelA, β-catenin and STAT342. However, in breast cancer and melanoma, miR-214 was shown to inhibit tumor cell dissemination43. In gastric cancer tissue (GCT) and GCT-derived mesenchymal stem cells, from 10 patients, miR-214 expression was higher compared to non-cancerous gastric tissue44. These observations suggest a dual role for miR-214 in different tumor types presumably due to the expression of tumor-selective miR-214 targets.
β-catenin expression is upregulated in melanoma compared to melanocytes. The role of β-catenin in melanoma aggressiveness has been well documented12, 45–46. Although β-catenin has been shown to be downregulated by miR-214 in other cancers20–21, our data show that β-catenin escapes downregulation by miR-214. Alternative splicing of mRNAs in the Wnt signaling pathway and the consequent loss of regulatory binding sites on mRNA for post-transcriptional regulation have been described47. Alternate splicing can also result stabilization of the target mRNA48. For example, in prostate cancer alternate splice variant of splicing factor, hnRNPA2, binds to β-catenin mRNA thereby stabilizing the mRNA and protein which in turn increased prostate cell proliferation49. Our RNA-seq data identified an abundantly expressed isoform of β-catenin mRNA without a miR-214 binding site, which could explain the loss of miR-214-mediated regulation of β-catenin.
In a detailed study of miR-214 targets in melanoma by both computational prediction and experimental strategies (overexpression of miR-214), Penna et al. identified and validated integrin α3 (ITGA3) and transcription factor AP-2γ (TFAP2C) as targets of miR-214. These targets were implicated in the ability of miR-214 to coordinate the various steps of metastasis25. In our study, we also employed computational prediction strategy, but identified 199 putative targets by negative correlation of expression at physiological levels using matched mRNA- and miRNA-seq. Gene Ontology analysis in our studies identified Wnt signaling pathway as overrepresented with the largest number of genes (ten genes). Therefore, we validated four selected targets in this pathway by showing downregulation upon miR-214 overexpression. Use of different melanoma cell lines and our strategy of identifying targets based on endogenous levels of miR-214 could explain identification of different targets. Interestingly, several of these miR-214 targets are also involved in regulation of β-catenin turnover. For example, Valenta et al showed that CTBP1 indirectly represses the transcriptional activity of β-catenin by binding to the C-terminal of TCF-4, a HMG box transcription factor50. In human embryonic kidney cells, ANKRD6 was shown to downregulate β-catenin by acting upstream of GSK3β51. Vasileiou et al showed that in cell lines with aberrant Wnt/β-catenin signaling, overexpression of ARID1B inhibited while knockdown of ARID1B increased β-catenin activity39. The role of six other identified miR-214 targets in melanoma remains to be investigated.
As reported in other cancer cells, miR-214 expression increased melanoma cell survival, growth and migration. A role for miR-214 in tumor cell survival and migration has been documented in gastric cancer52. Consistent with this finding, we showed that miR-214 overexpression in the MRA series of melanoma cells increased cell growth and survival. It was previously reported that activation of Wnt/β-catenin signaling pathway is associated with chemo-resistance in several cancers53–55. Hur et al. showed that in melanoma cells, β-catenin activation indirectly contributed to resistance to MEK inhibitor by upregulating immunoglobulin transcription factor-256. Wang et al showed enhanced resistance of miR-214 expressing non-small cell lung cancers to gefitinib57. In this study, employing a MAPKi-sensitive BRAF(V600E) mutant melanoma cell line and MAPKi-resistant cell lines derived from this line, we show that overexpression of miR-214 not only decreased the sensitivity of both drug-sensitive and resistant cell lines but knockdown of miR-214 enhanced the resistance to MAPK inhibitors.
In summary, our data suggest that in melanoma miR-214 regulates Wnt signaling pathway primarily by modulating the negative regulators of this pathway, but not by the regulation of β-catenin. We propose that strategies that upregulate or activate these miR-214 target proteins could potentially sensitize melanoma to MAPK targeted therapies.
Supplementary Material
Acknowledgements
This work was supported in part by VA Merit Award 1 I01 BX002623 (VS), Department of Defense W81XWH-14-1-0332 (CA130316) (VS) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30 AR066524, Skin Diseases Research Center at the University of Wisconsin. RJP was supported by National Institutes of Health grants CA165184, NCI 5P30CA030199 and Florida Department of Health, Bankhead-Coley Cancer Research Program 5BC08. We acknowledge the technical help of Subramaniam Shyamalagovindarajan in performing RNA-seq.
The data that support the findings of this study are openly available in Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/
The reference numbers are: GSE109244 and GSE109245Accession Numbers of RNA-seq GEO
Source of Funding: This work was supported in part by VA Merit Award 1 I01 BX002623 (VS), Department of Defense W81XWH-14-1-0332 (CA130316) (VS) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30 AR066524, Skin Diseases Research Center at the University of Wisconsin. RJP was supported by National Institutes of Health grants CA165184, NCI 5P30CA030199 and Florida Department of Health, Bankhead-Coley Cancer Research Program 5BC08.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 2009:10:468–77. [DOI] [PubMed] [Google Scholar]
- 2.Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 2007:134:479–89. [DOI] [PubMed] [Google Scholar]
- 3.Acebron SP, Karaulanov E, Berger BS, Huang YL, Niehrs C. Mitotic wnt signaling promotes protein stabilization and regulates cell size. Mol Cell 2014:54:663–74. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell 2012:149:1192–205. [DOI] [PubMed] [Google Scholar]
- 5.Larue L, Delmas V. The WNT/Beta-catenin pathway in melanoma. Front Biosci 2006:11:733–42. [DOI] [PubMed] [Google Scholar]
- 6.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012:2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of beta-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer 2001:92:839–42. [DOI] [PubMed] [Google Scholar]
- 8.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 1997:275:1790–2. [DOI] [PubMed] [Google Scholar]
- 9.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A 1995:92:3046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol 1996:16:4088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worm J, Christensen C, Gronbaek K, Tulchinsky E, Guldberg P. Genetic and epigenetic alterations of the APC gene in malignant melanoma. Oncogene 2004:23:5215–26. [DOI] [PubMed] [Google Scholar]
- 12.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D, Rimm DL, McMahon M, Bosenberg M. beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011:20:741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol 1996:16:2128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002:108:837–47. [DOI] [PubMed] [Google Scholar]
- 15.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nature Reviews Cancer 2012:13:11. [DOI] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004:116:281–97. [DOI] [PubMed] [Google Scholar]
- 17.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005:433:769–73. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016:1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih TC, Tien YJ, Wen CJ, Yeh TS, Yu MC, Huang CH, Lee YS, Yen TC, Hsieh SY. MicroRNA-214 downregulation contributes to tumor angiogenesis by inducing secretion of the hepatoma-derived growth factor in human hepatoma. J Hepatol 2012:57:584–91. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z, Jiang J. MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of beta-catenin. Biochem Biophys Res Commun 2012:428:525–31. [DOI] [PubMed] [Google Scholar]
- 21.Xia H, Ooi LL, Hui KM. MiR-214 targets beta-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One 2012:7:e44206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Li J, Wang X, Zheng C, Ma W. Downregulation of microRNA-214 and overexpression of FGFR-1 contribute to hepatocellular carcinoma metastasis. Biochem Biophys Res Commun 2013:439:47–53. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LL, Guo YJ, Zhao CN, Gao JY. Effects and mechanism of miR-214 on hepatocellular carcinoma. Asian Pac J Trop Med 2015:8:392–8. [DOI] [PubMed] [Google Scholar]
- 24.Li QQ, Xie YK, Wu Y, Li LL, Liu Y, Miao XB, Liu QZ, Yao KT, Xiao GH. Sulforaphane inhibits cancer stem-like cell properties and cisplatin resistance through miR-214-mediated downregulation of c-MYC in non-small cell lung cancer. Oncotarget 2017:8:12067–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, Poliseno L, Haimovic A, Osella-Abate S, De Pitta C, Pinatel E, Stadler MB, Provero P, Bernengo MG, Osman I, Taverna D. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. EMBO J 2011:30:1990–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol 2015:135:960–969. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez CI, Castro-Pérez E, Prabhakar K, Block L, Longley BJ, Wisinski JA, Kimple ME, Setaluri V. EPAC-RAP1 Axis-Mediated Switch in the Response of Primary and Metastatic Melanoma to Cyclic AMP. Mol Cancer Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet 2013:45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 2017:14:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh M WNT/PCP signaling pathway and human cancer (review). Oncol Rep 2005:14:1583–8. [PubMed] [Google Scholar]
- 31.Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY. Wnt/beta-catenin signaling induces the expression and activity of betaTrCP ubiquitin ligase receptor. Mol Cell 2000:5:877–82. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q, Liu H, Han P, Li C, Wang Y, Wu W, Zhu D, Amos CI, Fang S, Lee JE, Han J, Wei Q. Genetic Variants in WNT2B and BTRC Predict Melanoma Survival. J Invest Dermatol 2017:137:1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allache R, Wang M, De Marco P, Merello E, Capra V, Kibar Z. Genetic studies of ANKRD6 as a molecular switch between Wnt signaling pathways in human neural tube defects. Birth Defects Res A Clin Mol Teratol 2015:103:20–6. [DOI] [PubMed] [Google Scholar]
- 34.Fang M, Li J, Blauwkamp T, Bhambhani C, Campbell N, Cadigan KM. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J 2006:25:2735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamada F, Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Dev Cell 2004:7:677–85. [DOI] [PubMed] [Google Scholar]
- 36.Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 1999:9:207–10. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Ji J, Song J, Li X, Han S, Lian W, Cao C, Zhang X, Li M. MicroRNA-182 promotes pancreatic cancer cell proliferation and migration by targeting beta-TrCP2. Acta Biochim Biophys Sin (Shanghai) 2016:48:1085–1093. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 2004:23:2028–36. [DOI] [PubMed] [Google Scholar]
- 39.Vasileiou G, Ekici AB, Uebe S, Zweier C, Hoyer J, Engels H, Behrens J, Reis A, Hadjihannas MV. Chromatin-Remodeling-Factor ARID1B Represses Wnt/beta-Catenin Signaling. Am J Hum Genet 2015:97:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduction And Targeted Therapy 2016:1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mione M, Bosserhoff A. MicroRNAs in melanocyte and melanoma biology. Pigment Cell Melanoma Res 2015:28:340–54. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekaran KS, Sathyanarayanan A, Karunagaran D. miR-214 activates TP53 but suppresses the expression of RELA, CTNNB1, and STAT3 in human cervical and colorectal cancer cells. Cell Biochem Funct 2017:35:464–471. [DOI] [PubMed] [Google Scholar]
- 43.Orso F, Quirico L, Virga F, Penna E, Dettori D, Cimino D, Coppo R, Grassi E, Elia AR, Brusa D, Deaglio S, Brizzi MF, Stadler MB, Provero P, Caselle M, Taverna D. miR-214 and miR-148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Cancer Res 2016:76:5151–62. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F, Cai J, Zhu Q, Zhu W, Qian H, Xu W. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer 2014:110:1199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, Garbe C, Schittek B. beta-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PLoS One 2011:6:e23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elcheva I, Tarapore RS, Bhatia N, Spiegelman VS. Overexpression of mRNA-binding protein CRD-BP in malignant melanomas. Oncogene 2008:27:5069–74. [DOI] [PubMed] [Google Scholar]
- 47.Sumithra B, Saxena U, Das AB. Alternative splicing within the Wnt signaling pathway: role in cancer development. Cell Oncol (Dordr) 2016:39:1–13. [DOI] [PubMed] [Google Scholar]
- 48.Lee HK, Choi YS, Park YA, Jeong S. Modulation of oncogenic transcription and alternative splicing by beta-catenin and an RNA aptamer in colon cancer cells. Cancer Res 2006:66:10560–6. [DOI] [PubMed] [Google Scholar]
- 49.Stockley J, Villasevil ME, Nixon C, Ahmad I, Leung HY, Rajan P. The RNA-binding protein hnRNPA2 regulates beta-catenin protein expression and is overexpressed in prostate cancer. RNA Biol 2014:11:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valenta T, Lukas J, Korinek V. HMG box transcription factor TCF-4’s interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. Nucleic Acids Res 2003:31:2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev 2002:16:2073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang TS, Yang XH, Wang XD, Wang YL, Zhou B, Song ZS. MiR-214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int 2013:13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui J, Jiang W, Wang S, Wang L, Xie K. Role of Wnt/beta-catenin signaling in drug resistance of pancreatic cancer. Curr Pharm Des 2012:18:2464-71. [DOI] [PubMed] [Google Scholar]
- 54.Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, So CW. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 2010:18:606–18. [DOI] [PubMed] [Google Scholar]
- 55.Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, Katano M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res 2010:30:2041–8. [PubMed] [Google Scholar]
- 56.Hur EH, Goo BK, Moon J, Choi Y, Hwang JJ, Kim CS, Bae KS, Choi J, Cho SY, Yang SH, Seo J, Lee G, Lee JH. Induction of immunoglobulin transcription factor 2 and resistance to MEK inhibitor in melanoma cells. Oncotarget 2017:8:41387–41400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang YS, Wang YH, Xia HP, Zhou SW, Schmid-Bindert G, Zhou CC. MicroRNA-214 regulates the acquired resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant cell lines. Asian Pac J Cancer Prev 2012:13:255–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.