Abstract
Ataxia telangiectasia and Rad3-related (ATR) is a serine/threonine-specific kinase that plays an important role in the maintenance of genomic integrity. In this study, we investigated the role of ATR in cell cycle arrest by withaferin A (WA), a cancer preventative steroidal lactone derived from Withania somnifera plant abundant in India and surrounding countries. The WA treatment decreased viability of MCF-7, MDA-MB-231, and SUM159 cells. Exposure of breast cancer cells to WA also resulted in suppression of protein level as well as phosphorylation of ATR and its downstream effector kinase (checkpoint kinase 1; CHK1). Both transcriptional and post-transcriptional mechanisms were involved in WA-mediated downregulation of ATR protein. Downregulation of ATR protein expression resulting from WA exposure was not attenuated by overexpression of manganese superoxide dismutase. On the other hand, overexpression of CHK1 attenuated WA-mediated G2/M arrest and augmented S10 phosphorylation of histone H3, a marker of mitotic arrest. The protein level of ATR was lower by about 50% in breast tumors of WA-treated mouse mammary tumor virus-neu mice when compared to vehicle-treated controls but the difference was not significant due to small sample size. WA treatment sensitized MDA-MB-231 and SUM159 cells to growth inhibition and apoptosis induction by cisplatin. Cisplatin treatment resulted in increased autophosphorylation of ATR (T1989) and CHK1 (S345) phosphorylation that was markedly suppressed in the presence of WA. These results indicate that WA is an inhibitor of ATR in human breast cancer cells.
Keywords: withaferin A, ATR, cisplatin, breast cancer
1. INTRODUCTION
Withaferin A (WA) is a promising small molecule whose anticancer activity was first reported in Ehrlich tumor cells.1 Since then, the anticancer effect of WA has been reported against a variety of solid tumor cell lines, including breast cancer cells in vitro and/or in vivo.2 Our laboratory was the first to demonstrate growth inhibitory effect of WA in human breast cancer cells.3 The in vitro survival of MCF-7 and MDA-MB-231 cells was dose-dependently and significantly inhibited after 24-h treatment with WA.3 In a therapeutic setting, the in vivo growth of MDA-MB-231 cells implanted in female nude mice was also suppressed after intraperitoneal injections of WA (4 mg/kg body weight, five times/week).3 Breast cancer is a molecularly heterogeneous disease broadly grouped into different subtypes including luminal-type, human epidermal growth factor receptor 2 (HER-2)-amplified, basal-type and normal-like.4 Studies have shown in vivo chemopreventative efficacy of WA against two different subtypes of breast cancer in rodent models.5,6 In one such study, we showed that macroscopic mammary tumor size and microscopic mammary tumor area as well as the incidence of pulmonary metastasis were decreased significantly after 28 weeks of WA administration (0.1 mg/mouse, three times per week) to female mouse mammary tumor virus-neu (MMTV-neu) transgenic mice,5 an animal model for HER-2 amplified breast cancer. Likewise, WA administration significantly decreased incidence, multiplicity, and burden of N-methyl-N-nitrosourea-induced luminal-type breast cancer in rats.6
The mechanisms underlying tumor cell growth inhibition by WA have been studied rather extensively using cellular models of breast cancer as well as other solid tumor types.2 The G2/M phase and mitotic arrest, apoptosis induction, and inhibition of cancer stem-like cells, cell migration, epithelial-mesenchymal transition, and angiogenesis are the primary anticancer responses to WA in breast cancer.2,3,7–15 At the molecular level, covalent modification of cysteine-303 residue in β-tubulin was implicated in mitotic arrest resulting from WA exposure.13 Because WA is an electrophile,13 covalent modification of other proteins has also been documented including vimentin and peptidyl-prolyl cis/trans isomerase Pin1.16–18 The WA-induced apoptotic cell death in human breast cancer cell lines was associated with production of reactive oxygen species and accompanied by induction/activation of Bax and Bak proteins.9 Induction of Bak protein expression was also observed in vivo in chemically-induced mammary tumors from WA-treated rats in comparison with controls.6 Interestingly, normal mammary epithelial cells (MCF-10A or HMEC) were shown to be markedly more resistant to cell cycle arrest and apoptosis induction by WA in comparison with breast cancer cells.9,13 Epigenetic modification was also shown for this phytochemical in breast cancer cells.19
Signal transducer and activator of transcription 3 (STAT3), which is an oncogenic transcription factor frequently activated in breast cancers, is another target of WA in breast, cervical, and renal cancer cells.2,8,20 In this study, we investigated the effect of WA treatment on ataxia telangiectasia and Rad3-related (ATR) signaling, which is a key component of the DNA damage response pathway for maintaining the genomic integrity,21,22 using MCF-7, MDA-MB-231 and SUM159 human breast cancer cells. The impetus for the present study stemmed from the following data: (a) WA was shown to inhibit constitutive and interleukin-6-induced activation of STAT3,8 and STAT3 has been shown to regulate ATR expression,23,24 and (b) WA treatment was shown to produce oxidative stress, which is a known modulator of DNA damage response pathway.25 Additionally, we explored the possibility of sensitization of breast cancer cells to anticancer effect of cisplatin (CIS) because ATR was shown to mediate resistance to this chemotherapeutic agent in breast cancer cells.26 CIS is a drug often used to treat triple-negative breast cancers that do not respond to hormonal therapy or HER2-targetetting (trastuzumab) antibody therapy.27 However, clinical resistance to CIS-based chemotherapy as well as toxicity are major limitations of this drug.27
2. MATERIALS AND METHODS
2.1. Reagents
Withaferin A (WA, purity > 95%) was purchased from ChromaDex (Irvine, CA), dissolved in dimethyl sulfoxide (DMSO; 20 mM stock), and stored at −80°C. CIS and anti-β-Actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). CIS was dissolved in normal saline. Cell culture media and fetal bovine serum (FBS) were purchased from MediaTech (Manassas, VA) and Atlanta Biologicals (Norcross, GA), respectively. Other reagents needed for cell culture were purchased from Invitrogen-Life Technologies (Carlsbad, CA). Antibodies against phospho(S428)-ATR, ATR, ATR interacting protein (ATRIP), phospho(S345)-checkpoint kinase 1 (CHK1), CHK1, and phospho(S10)-histone H3 were purchased from Cell Signaling Technology (Danvers, MA). Anti-phospho(T1989)-ATR antibody was from GeneTex (Irvine, CA). Anti-manganese superoxide dismutase (MnSOD) antibody was from EMD Chemicals (Gibbstown, NJ). Annexin V/propidium iodide (PI) assay kit for apoptosis detection was purchased from BD Biosciences (San Jose, CA).
2.2. Cell culture
The MCF-7 and MDA-MB-231 human breast cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA), whereas the SUM159 cell line was purchased from Asterand Bioscience (Detroit, MI). Each cell line was last authenticated by us in March of 2017, and maintained as suggested by the supplier. The MCF-7 cells were stably transfected with pcDNA3.1 empty vector or the same vector encoding MnSOD and selected in medium supplemented with 800 µg/mL of G418.
2.3. Cell viability assay
Trypan blue dye exclusion assay was performed to determine the effect of WA treatment on cell viability as described by us previously.28 Briefly, cells were plated in triplicate in 12-well plates at 70% confluency and incubated overnight for attachment. The cells were then treated with DMSO (control) or WA and/or CIS at the indicated doses for specified time periods. At the end of the treatment, the cells were collected by trypsin treatment and resuspended in phosphate-buffered saline (PBS) containing 0.1% trypan blue. Live cells were counted using a hematocytometer.
2.4. Western blotting
Details of cell lysate/tumor supernatant preparation and immunoblotting have been described by us previously.29,30 Immunoreactive bands were visualized by enhanced chemiluminescence method. Densitometric quantitation was done using UN-SCAN-IT software version 5.1 (Silk Scientific Corporation, Orem, UT).
2.5. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Cells were plated at 70% confluency in 6-cm dishes in triplicate, allowed to attach by overnight incubation, and then treated with DMSO (control) or 2 µM of WA for 8 or 16 h. Total RNA was isolated using RNeasy® mini kit from Qiagen (Germantown, MD). One µg RNA was used for cDNA synthesis with the use of SuperScript III reverse transcriptase and oligo (dT)20 primer. qRT-PCR was done using 2× SYBR Green master mix (Applied Biosystems-Life Technologies). Primers for ATR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were as follows. ATR-forward: 5ʹ- TGCAGTAATGTCAATGGTTGG-3ʹ; ATR-reverse: 5’- CTGGAACTTCAAAGGTTTCTCC-3’; GAPDH-forward: 5ʹ- GGACCTGACCTGCCGTCTAGAA-3ʹ; GAPDH-reverse: 5’- GGTGTCGCTGTTGAAGTCAGAG-3’. The PCR conditions were as follows: 95°C for 10 min followed by 40 cycles of 95°C for 15 s, 60°C for 1 min and 72°C for 30 s. Relative gene expression levels were calculated using the method of Livak and Schmittgen.31
2.6. Transient transfection
MDA-MB-231 cells were transiently transfected with 6 µg of empty vector (pCMV3-C-GFPSpark) or CHK1 expressing plasmid (pCMV3-CHK1-GFPSpark) using FuGENE® HD transfection reagent (Promega, Madison, WI). Overexpression of CHK1 was confirmed by western blot analysis prior to other assays.
2.7. Determination of cell cycle distribution
MDA-MB-231 cells were plated in 6-cm dishes in triplicate at 50% confluency and transiently transfected with 6 µg of empty vector (EV) or 6 µg of CHK1-expressing plasmid for 24 h. Subsequently, the cells were treated with DMSO (control) or 2 μM of WA for 8 h or 16 h. At the end of the treatment, the cells were collected by trypsin treatment and fixed with 70% ethanol overnight at 4°C. Fixed cells were washed with PBS, stained with PI, and analyzed using a BD Accuri™ C6 flow cytometer (BD Biosciences).
2.8. Cell proliferation assay
Cell proliferation was measured using a kit from Promega (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay kit). Cells were plated in quadruple in 96-well plates at 70% confluency. Following overnight incubation, the cells were treated with DMSO (control) or WA and/or CIS for 24 h. Supplier’s instructions were followed for the assay.
2.9. Annexin V/PI assay
The Annexin V/PI assay kit was used to measure total (early and late) apoptosis. The cells were plated in triplicate in 6-well plates at 70% confluency and incubated overnight to allow cell attachment. The cells were then treated with DMSO (control) or WA and/or CIS for 24 h. At the end of the treatment, the cells were collected by trypsin treatment, resuspended in Annexin V/PI solution, incubated for 30 min at room temperature with gentle shaking in the dark, and analyzed using a BD Accuri™ C6 flow cytometer.
2.10. Statistical analysis
GraphPad Prism (version 7.00) was used to perform statistical analyses. Statistical significance of difference for two group comparisons was determined by two-tailed Student’s t-test. One-way analysis of variance (ANOVA) followed by Dunnett’s test (for dose-response comparison) or Bonferroni’s multiple comparisons test were used for multiple group comparisons.
3. RESULTS
3.1. WA treatment suppressed expression of ATR in human breast cancer cells
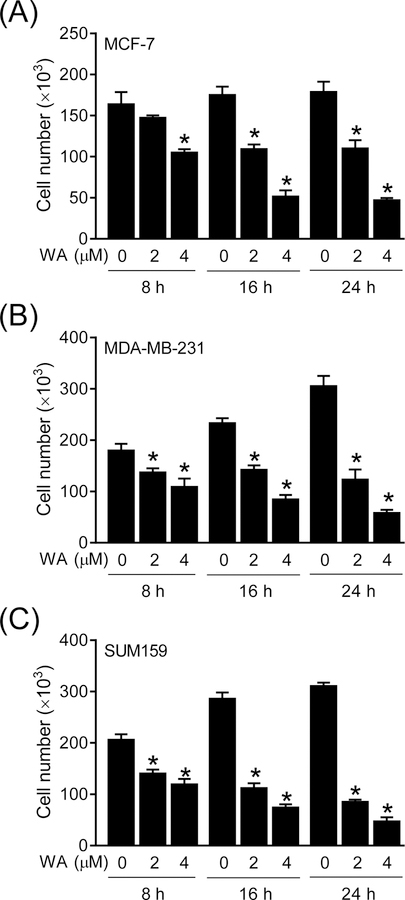
Initially, we performed a time-course study to determine the effect of WA treatment on viability of MCF-7, MDA-MB-231, and SUM159 cells. The MCF-7 is a luminal-type cell line whereas MDA-MB-231 and SUM159 are well-characterized representatives of basal-like (triple-negative) breast cancer. It is important to point out that pharmacokinetics of WA has been studied in mice after intraperitoneal administration, but its oral bioavailability is yet to be determined. The peak plasma concentration of WA after intraperitoneal administration to mice (4 mg/kg body weight) was about 1.8 µM.10 We have also shown previously that WA is bioavailable in the mammary tumors of the MMTV-neu and that of chemically-induced rats.5,6 Hence, 2 µM WA concentration was used in most in vitro studies in this study. Viability of each cell line was inhibited significantly in the presence of WA, and this effect was observed as early at 8-h post-treatment at least at the 4 µM concentration in each cell line (Figure 1A–C). Figure 2 summarizes western blotting data for the effect of WA treatment on expression of ATR-CHK1 pathway-associated proteins. Protein levels of ATR and ATRIP as well as S428 phosphorylation of ATR were suppressed following WA treatment in each tested cell line (Figure 2). The protein level and S345 phosphorylation of CHK1 were also markedly lower in WA-treated cells when compared to solvent-treated control cells. These results indicated that cell viability inhibition by WA treatment in breast cancer cell lines was associated with inhibition of expression and activity of ATR. To the best of our knowledge, inhibitory effect of WA on ATR signaling has not been documented previously.
Figure 1. WA treatment inhibited viability of human breast cancer cells.
Quantification of cell viability by trypan blue dye exclusion assay in MCF-7 (A), MDA-MB-231 (B), and SUM159 (C) human breast cancer cells after treatment with DMSO or the indicated doses of WA for specified time periods. Results are presented as mean ± SD (n=3). *Significantly different (P < 0.05) compared with control as determined by one-way ANOVA followed by Dunnett’s test. Experiment was repeated two times with comparable results.
Figure 2. WA treatment suppressed ATR-CHK1 pathway in human breast cancer cells.
Western blotting for ATR-CHK1 pathway-related proteins in MCF-7, MDA-MB-231, and SUM159 human breast cancer cells after 8-h or 16-h treatment with DMSO (control) or 2 µM of WA. Numbers below bands are relative expression level of each protein compared to corresponding DMSO-treated control. Experiment was repeated at least two times with comparable results.
3.2. Mechanisms underlying WA-mediated downregulation of ATR
As shown in Figure 3A, WA treatment resulted in a significant decrease in mRNA expression of ATR in MCF-7, MDA-MB-231, and SUM159 cell lines (Figure 3A). Moreover, WA-mediated downregulation of both ATR and CHK1 proteins was partially attenuated in the presence of MG132, an inhibitor of proteasome degradation machinery (Figure 3B). These results indicated involvement of both transcriptional and post-transcriptional mechanisms in ATR downregulation resulting from WA treatment.
Figure 3. Both transcriptional and post-transcriptional mechanisms were involved in WA-mediated downregulation of ATR and CHK1 proteins.
(A) Quantification of ATR mRNA levels in human breast cancer cell after 8 h or 16 h treatment with DMSO (control) or 2 µM of WA. Results are presented as mean ± SD (n=3). *Significantly different (P < 0.05) compared with control as determined by two-tailed Student’s t-test. (B) Representative immunoblots showing levels of total and phosphorylated ATR and CHK1 proteins in MCF-7 cells treated with WA and/or MG132. The cells were pretreated with 10 µM MG132 for 1 h and then treated with DMSO (control) or 2 µM WA for 8 h or 16 h in the absence or presence of MG132. Numbers below bands are relative level of each protein compared to DMSO-treated control at each time point. Arrow signifies the correct band for p(S345)-CHK1 protein. Comparable results were observed in replicate experiments.
3.3. MnSOD overexpression was dispensable for ATR downregulation by WA
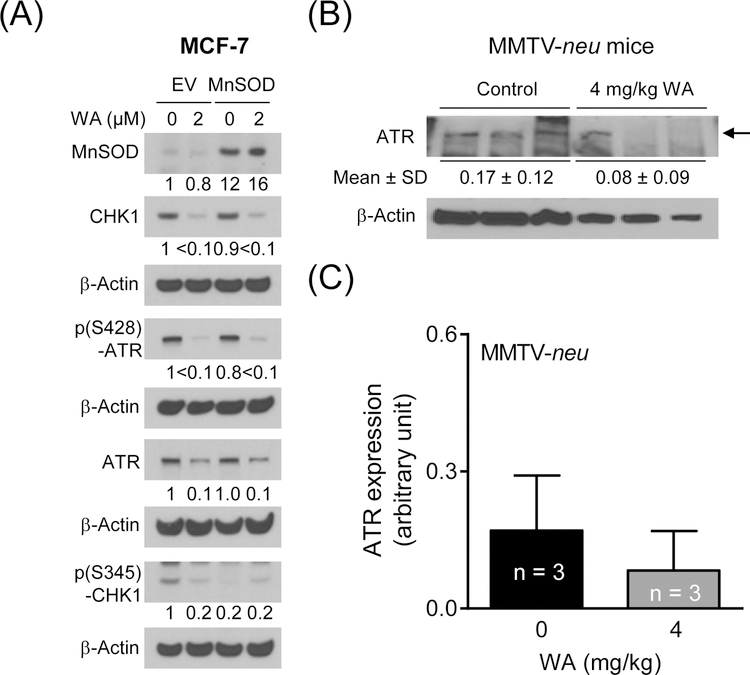
Next, we considered the possibility of whether ATR downregulation by WA was dependent on its pro-oxidant effect as WA treatment causes production of reactive oxygen species.9 Western blotting confirmed overexpression of MnSOD in stably transfected cells (Figure 4A). The level of MnSOD protein was only slightly decreased by WA treatment. However, MnSOD overexpression did not have a meaningful impact on WA-mediated downregulation of ATR and CHK1 proteins (Figure 4A). These results indicated that ATR downregulation by WA was not dependent on its pro-oxidant effect.
Figure 4. MnSOD was dispensable in WA-mediated downregulation of ATR/CHK1 proteins, and WA administration decreased the ATR protein expression in mammary tumors of MMTV-neu mice.
(A) Western blotting for MnSOD, and total and phosphorylated ATR and CHK1 proteins using lysates from empty vector (EV) transfected cells and MnSOD overexpressing MCF-7 cells treated with DMSO (control) or 2 µM WA for 8 h. Numbers below bands are relative expression level of each protein compared to DMSO-treated EV cells. (B) Western blot for ATR protein using mammary tumor supernatants from control and WA-treated MMTV-neu mice. Arrow indicates the correct band for ATR protein. (C) Quantitation of the ATR protein level from the western blot results shown in panel B. Results are shown as mean ± SD (n=3).
3.4. Effect of WA administration on ATR protein level in vivo
We used mammary tumor supernatants from control and WA-treated MMTV-neu mice5 to test in vivo significance of the cellular findings. The protein level of ATR was about 50% lower in the mammary tumors of WA-treated mice when compared to controls (Figure 4B, C) but the difference was not significant due variability and small sample size of n=3. Further studies are needed to determine if WA administration downregulates ATR protein level in vivo. In this context, we tried the mammary tumors from control and WA-treated rats6 but were unable to reproducibly detect ATR protein in these samples.
3.5. Effect of CHK1 overexpression on WA-mediated cell cycle arrest
Because of known functions of ATR/CHK1 signaling in cell cycle regulation21,22, we proceeded to determine the functional significance of ATR/CHK1 downregulation by WA. Figure 5A shows overexpression of CHK1 protein in transiently transfected cells in comparison with EV cells (Figure 5A). A small increase in S phase cells was observed in cells overexpressing CHK1 when compared to empty vector transfected control cells but this effect was abolished by WA treatment (Figure 5B). WA is known to cause G2/M phase cell cycle arrest in breast cancer cells.7 Overexpression of CHK1 also resulted in accumulation of G2/M phase cells in the absence of WA treatment (Figure 5C). However, WA-mediated G2/M phase cell cycle arrest was partly attenuated by CHK1 overexpression (Figure 5C). Because the flow cytometry cannot differentiate G2 phase and mitotic cells, we performed western blotting for S10 phosphorylated histone H3, which is a marker of mitotic cells. As described previously,13 WA treatment increased phosphorylation of S10 phosphorylated histone H3 specially at the 16-h time point and this effect was augmented by CHK1 overexpression (Figure 5D). These results indicated that ATR inhibition by WA modulated its effect on cell cycle distribution.
Figure 5. Overexpression of CHK1 affected WA-mediated cell cycle arrest.

(A) Western blot showing overexpression of CHK1 protein in transiently transfected MDA-MB-231 cells (lane 2) in comparison with EV cells (lane 1). Quantification of cell population in the S phase (B) or G2/M phase (C) of transiently transfected MDA-MB-231 cells following 8 h or 16 h treatment with DMSO or 2 µM WA. Combined data from two independent experiments are shown are mean ± SD (n=4). Significantly different (P < 0.05) compared with *respective control or #at the same dose level between EV and CHK1 groups by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (D) Representative immunoblot showing the level of S10 phosphorylated Histone H3 in EV transfected and CHK1 overexpressing cells treated for 8 h or 16 h with DMSO or 2 µM WA. Numbers below bands are relative level of p(S10)-Histone H3 compared to corresponding DMSO-treated EV cells. Each experiment was repeated two times with comparable results.
3.6. WA treatment sensitized basal-like breast cancer cells to CIS
Because ATR has been implicated in mediating resistance to CIS,26 which is widely used to treat triple-negative (basal-like) breast cancer,32 we asked the question of whether cells can be sensitized to anticancer effects of CIS. CIS or WA alone significantly inhibited viability of MDA-MB-231 and SUM159 basal-like breast cancer cells, but the combination was more effective than single agents alone especially at the higher dose (Figure 6A). Results of the cell proliferation assay also revealed sensitization of both cell lines to CIS in the presence of WA (Figure 6B). Breast cancer cells exposed to the CIS plus WA combination exhibited more apoptotic cell death when compared to single agent alone (Figure 6C). CIS is known to increase autophosphorylation of ATR at T1989.33 As can be seen in Fig. 6D, the autophosphorylation of ATR and, consequently phosphorylation of CHK1, was increased by CIS treatment. The autophosphorylation of ATR and CHK1 phosphorylation resulting from CIS treatment was abolished in the presence of WA. Collectively, these results indicated that WA can sensitize basal-like breast cancer cells to growth inhibition and apoptosis induction by CIS.
Figure 6. WA increased anti-tumor effect of CIS in triple-negative human breast cancer cells.
(A) Viability of MDA-MB-231 (left panel) and SUM159 (right panel) human breast cancer cells after 24 h treatment with DMSO or WA and/or CIS as determined by trypan blue dye exclusion assay. Results are presented as mean ± SD (n=3). (B) Proliferation of MDA-MB-231 (left panel) and SUM159 (right panel) human breast cancer cells after 24 h treatment with DMSO or WA and/or CIS as determined by MTS assay. Results are presented as mean ± SD (n=4). (C) Total apoptosis in MDA-MB-231 (left panel) and SUM159 (right panel) human breast cancer cells after 24 h treatment with DMSO or WA and/or CIS as determined by Annexin V/PI assay. Results are presented as mean ± SD (n=3). Statistically significant difference (panels A–C) was observed compared with DMSO-treated control (*, P < 0.05) or CIS alone (#, P < 0.05) or WA alone (ω, P < 0.05) as determined by one-way ANOVA followed by Bonferroni’s multiple comparisons test. (D) Western blots for phosphorylated ATR and CHK1 proteins using lysates from control and WA and/or CIS-treated cells. Numbers below bands represent quantitation of the immunoreactive band in comparison with DMSO-treated control cells. Each experiment was repeated two times with comparable results.
4. DISCUSSION
The ATR protein is expressed in most tissues and its mutation causes autosomal recessive disorders like ataxia telangiectasia.34 ATR is a large kinase with a molecular weight of 300 kDa and belongs to the phosphoinositide 3-kinase family, whereas ATRIP interacts with the N-terminal of ATR.21,22 The ATR/CHK1 signaling plays an important role in DNA damage response pathway.21,22 ATR is activated by replication stress during cell division or genotoxic insult and acts at the S and G2 phases of the cell cycle.21,22 Studies have shown that ATR overexpression is an independent predictor of poor outcome in breast cancer.35 High mRNA level of CHK1 was also shown to be associated with aggressive phenotype in breast cancer.35 Intense efforts are underway for clinical development of ATR and CHK1 inhibitors for treatment of cancers and for combination regimens with chemotherapy and radiation therapy.22,36 The present study shows downregulation of ATR and its activity inhibition reflected by suppression of CHK1 phosphorylation by WA in breast cancer cells. In this context, it is important to mention that studies have shown WA-mediated sensitization of cultured cancer cells to chemotherapy drugs or radiation,2 which could be mediated by ATR/CHK1 suppression.
We have shown previously that WA treatment causes G2/M phase arrest in MCF-7 and MDA-MB-231 breast cancer cells.7 The WA-mediated enrichment of G2/M phase fraction in breast cancer cells was accompanied by a decrease in protein levels of cyclin-dependent kinase 1 and cell division cycle (Cdc)25C and/or Cdc25B proteins.7 Moreover, Cdc25C overexpression in MDA-MB-231 cells resulted in partial but statistically significant attenuation of G2/M phase cell cycle arrest resulting from WA exposure.7 Interestingly, Cdc25C is a phosphorylation target of CHK1.36 Phosphorylation of Cdc25C by CHK1 results in inhibition of cyclin-dependent kinase 1 activity and G2/M phase cell cycle arrest.36,37 The possibility that WA treatment decreases phosphorylation of Cdc25C because of ATR/CHK1 inhibition cannot be excluded. Further research is necessary to explore this possibility. It is presently unclear why WA treatment causes more robust mitotic arrest, reflected by an increase in S10 phosphorylation of Histone H3, in CHK1 overexpressing cells in comparison with empty vector transfected cells. Inhibition of ATR/CHK1 by WA was expected to facilitate mitotic entry. One possibility is that WA treatment inhibits mitotic exit in CHK1 overexpressing cells.
CIS continues to be an important drug for the treatment of triple-negative breast cancer due to lack of targeted therapy for this subtype of the disease.32 Despite clinical benefit, the limitations of CIS and other platinum-based chemotherapies include emergence of drug resistance and toxicity.28,26,38 ATR was shown to mediate CIS resistance in breast cancer cells.26 CIS treatment also promotes autophosphorylation of ATR33 that was also observed in the present study. The CIS-induced autophosphorylation of ATR is inhibited markedly in the presence of WA (present study). In addition, co-treatment with CIS and WA combination more effectively inhibits survival and proliferation of triple-negative breast cancer cells than single treatment. In agreement with these results, the CIS+WA combination more potently induces apoptotic cell death in both MDA-MB-231 and SUM159 cells when compared CIS or WA alone. These results suggest that WA may be used in combination with CIS to achieve a greater anti-tumor response as well as to reduce side effects of CIS therapy.
In conclusion, the present study demonstrates that WA is a potent inhibitor of expression and activity of ATR kinase in luminal-type (MCF-7) and basal-like (MDA-MB-231 and SUM159) breast cancer cells. It appears that WA triggers both transcriptional downregulation and post-transcriptional degradation in suppression of ATR expression. WA-mediated downregulation of ATR expression is functionally important as its downstream effector (CHK1) is also inactivated by this steroidal lactone. Finally, we also show that anti-tumor effect of CIS can be augmented in the presence of WA that is reflected by both suppression of cell viability and apoptosis induction. While these results suggest that a combination regimen with WA and CIS achieves greater therapeutic efficacy in triple-negative breast cancer, additional in vivo efficacy testing of this combination is essential prior to clinical investigations.
ACKNOWLEDGEMENT
The authors thank Heidi Schmidt for technical assistance, and Kamayani Singh for assistance in preparation of the manuscript. This study was supported by the National Cancer Institute grants RO1 CA142604 and RO1 CA219180 (to SVS). This research used the Flow Cytometry Facility supported in part by UPMC Hillman Cancer Center Support Grant from the National Cancer Institute (P30 CA047904; Dr. Robert L. Ferris, Director of the Cancer Center is the Principal Investigator on this institutional grant).
Abbreviations:
- ANOVA
analysis of variance
- ATR
ataxia telangiectasia and Rad3-related
- ATRIP
ATR interacting protein
- CHK1
checkpoint kinase 1
- Cdc25
cell division cycle 25
- Cdk1
cyclin-dependent kinase 1
- CIS
cisplatin
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HER-2
human epidermal growth factor receptor 2
- MMTV-neu
mouse mammary tumor virus-neu
- MnSOD
manganese superoxide dismutase
- PBS
phosphate-buffered saline
- PI
propidium iodide
- qRT-PCR
quantitative real-time reverse transcription polymerase chain reaction
- STAT3
signal transducer and activator of transcription 3
- WA
withaferin A
Footnotes
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists.
DATA SHARING
The data shown in this manuscript that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Shohat B, Gitter S, Lavie D. Effect of withaferin A on Ehrlich ascites tumor cells-cytological observations. Int J Cancer 1970;5:244–252. [DOI] [PubMed] [Google Scholar]
- 2.Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J 2014;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res 2008;68:7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahm ER, Lee J, Kim SH, et al. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst 2013;105:1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samanta SK, Sehrawat A, Kim SH, et al. Disease subtype-independent biomarkers of breast cancer chemoprevention by the ayurvedic medicine phytochemical withaferin A. J Natl Cancer Inst 2017;109: djw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer 2008;60(Suppl 1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 2010;31:1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahm ER, Moura MB, Kelley EE, Van Houten B, Shiva S, Singh SV. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS One 2011;6:e23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaiparambil JT, Bender L, Ganesh T, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer 2011;129:2744–2755. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Garcia A, Xu S, et al. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS One 2013;8:e75069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Singh SV. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res (Phila) 2014;7:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antony ML, Lee J, Hahm ER, et al. Growth arrest by the antitumor steroidal lactone withaferin A in human breast cancer cells is associated with down-regulation and covalent binding at cysteine 303 of β-tubulin. J Biol Chem 2014;289:1852–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Hahm ER, Marcus AI, Singh SV. Withaferin A inhibits experimental epithelial-mesenchymal transition in MCF-10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog 2015;54:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan R, Hammers HJ, Bargagna-Mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004;7:115–122. [DOI] [PubMed] [Google Scholar]
- 16.Bargagna-Mohan P, Hamza A, Kim YE, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol 2007;14:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanta SK, Lee J, Hahm ER, Singh SV. Peptidyl-prolyl cis/trans isomerase Pin1 regulates withaferin A-mediated cell cycle arrest in human breast cancer cells. Mol Carcinog 2018;57:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gambhir L, Checker R, Sharma D, et al. Thiol dependent NF-κB suppression and inhibition of T-cell mediated adaptive immune responses by a naturally occurring steroidal lactone Withaferin A. Toxicol Appl Pharmacol 2015;289:297–312. [DOI] [PubMed] [Google Scholar]
- 19.Royston KJ, Paul B, Nozell S, Rajbhandari R, Tollefsbol TO. Withaferin A and sulforaphane regulate breast cancer cell cycle progression through epigenetic mechanisms. Exp Cell Res 2018;368:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz N, Minton S, Cox C, et al. Activation of Stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated Src and Survivin expression. Clin Cancer Res 2006;12:20–28. [DOI] [PubMed] [Google Scholar]
- 21.Awasthi P, Foiani M, Kumar A. ATM and ATR signaling at a glance. J Cell Sci 2015;128:4255–4262. [DOI] [PubMed] [Google Scholar]
- 22.Fokas E, Prevo R, Hammond EM, Brunner TB, McKenna WG, Muschel RJ. Targeting ATR in DNA damage response and cancer therapeutics. Cancer Treat Rev 2014;40:109–117. [DOI] [PubMed] [Google Scholar]
- 23.Barry SP, Townsend PA, Knight RA, Scarabelli TM, Latchman DS, Stephanou A. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol 2010;91:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao XH, Zheng L, He HP, et al. STAT3 regulated ATR via microRNA-383 to control DNA damage to affect apoptosis in A431 cells. Cell Signal 2015;27:2285–2295. [DOI] [PubMed] [Google Scholar]
- 25.Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004;3:1109–1115. [DOI] [PubMed] [Google Scholar]
- 26.Gomes LR, Rocha CRR, Martins DJ, et al. ATR mediates cisplatin resistance in 3D-cultured breast cancer cells via translesion DNA synthesis modulation. Cell Death Dis 2019;10:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene 2012;31:1869–1883. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene 2004;23:5594–5606. [DOI] [PubMed] [Google Scholar]
- 29.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003;24:891–897. [DOI] [PubMed] [Google Scholar]
- 30.Powolny AA, Bommareddy A, Hahm ER, et al. Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer. J Natl Cancer Inst 2011;103:571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 32.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson CT, Miller R, Pemberton HN, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun 2016;7:13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008;9:759–769. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Fatah TM, Middleton FK, Arora A. Untangling the ATR-CHEK1 network for prognostication, prediction and therapeutic target validation in breast cancer. Mol Oncol 2015;9:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rundle S, Bradbury A, Drew Y, Curtin NJ. Targeting the ATR-CHK1 axis in cancer therapy. Cancers (Basel) 2017;9:pii E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 2008;9:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]