Abstract
Increased consumption of fruits may decrease the risk of chronic inflammatory diseases including inflammatory bowel disease (IBD). Gut microbiota dysbiosis plays an important etiological role in IBD. However, the mechanisms of action underlying the anti-inflammatory effects of dietary cranberry (Vaccinium macrocarpon) in the colon and its role on gut microbiota were unclear. In this study, we determined the anti-inflammatory efficacy of whole cranberry in a mouse model of dextran sodium sulfate (DSS)-induced colitis, as well as its effects on the structure of gut microbiota. The results showed that dietary cranberry significantly decreased the severity of colitis in DSS-treated mice, evidenced by increased colon length, and decreased disease activity and histologic score of colitis in DSS-treated mice compared to the positive control group (p < 0.05). Moreover, the colonic levels of pro-inflammatory cytokine (IL-1β, IL-6 and TNF-α) were significantly reduced by cranberry supplementation (p < 0.05). Analysis of the relative abundance of fecal microbiota in phylum and genus levels revealed that DSS treatment significantly altered the microbial structure of fecal microbiota in mice. α diversity was significantly decreased in the DSS group, compared to the healthy control group. But, cranberry treatment significantly improved DSS-induced decline in α-diversity. Moreover, cranberry treatment partially reversed the change of gut microbiota in colitic mice by increasing the abundance of potential beneficial bacteria, for example, Lactobacillus and Bifidobacterium, and decreasing the abundance of potential harmful bacteria, such as Sutterella and Bilophila. Overall, our results for the first time demonstrated that modification of gut microbiota by dietary whole cranberry might contribute to its inhibitory effects against the development of colitis in DSS-treated mice.
Keywords: Cranberry, Colonic inflammation, dextran sodium sulfate, Gut microbiota, inflammatory bowel disease
1. Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, causes epithelial dysfunction and increases mucosal permeability, and it is highly prevalent in Europe and North America [1, 2]. It is estimated that 1.4 million patients are suffering from IBD while $6.3 billion is used for the treatment of IBD annually [1]. Environmental factors like western diet and smoking are suspected for the major causes for the IBD, leading to impaired gut microbial structure and function of IBD patients [3, 4]. Microbiota and the immune system in the intestine form a homeostasis to maintain the health status of the intestine. Disruption of homeostasis will bring significant influences on both the host and the gut microbiota [5]. Emerging evidence has indicated that composition and functions of gut microbiota play a significant role in maintaining the physiological functions of colon and regulating the immune system [6]. It is known that some probiotics could protect the colon from inflammatory process, while the increasing amount of pathogen in colon is associated with the risks for IBD [6, 7].
Using their unique enzymes, gut microbiota can metabolize a large amount of substrates, including dietary fiber which can provide various health benefits [8]. Whole cranberry fruit has large amount of polyphenols and non-digestible dietary fiber, which cannot get absorbed and digested in the body [9]. They can reach colon nearly intact, thus gut microbiota may play a significant role in their depolymerization and metabolism [10–12]. On the other hand, the structure and function of the gut microbiota are expected to be improved by cranberry. It has been reported that dietary polysaccharides could alter the gut microbiota composition in mice and increase short chain fatty acids production, resulting in the attenuation of intestinal colitis [13].
Recently, we have reported the protective activity of whole cranberry against colon tumorigenesis [14]. We found that 1.5 wt% whole cranberry powder diminished colonic tumorigenesis in mice, and suppressed the COX-2 pro-inflammatory pathway [14]. And the anti-colitis effects of some cranberry components, such as fiber and phenolics have determined in animal models [15, 16]. Xiao et al. showed that cranberry phenolics and fiber reduced pro-inflammatory responses in dextran sodium sulfate (DSS)-treated mice, and cranberry fiber was more effective than phenolics on preventing colitis [16]. Popov et al. demonstrated that a pectic polysaccharide of cranberry inhibited acetic acid-induced by improving vascular permeability and attenuating the adhesion of peritoneal neutrophils and macrophages [15].
Nonetheless, many components of whole cranberry are indigestible and/or unabsorbed in the upper gastrointestinal tract, therefore could reach the colon intact after oral administration of cranberry. To date, the mode of interaction between whole cranberry and gut microbiota, and its role in the prevention of colonic diseases has been unknown. The present study is an innovative work that sought to determine the beneficial effects of cranberry on gut microbiota in a mouse model of colitis. Further, the molecular mechanisms underlying the anti-inflammatory effect of whole cranberry was investigated to provide a better understanding of the association between the host and gut microbiota.
2. Materials and Methods
2.1. Animals, diets and experimental procedure
The protocol for the animal experiment was approved by Institutional Animal Care and Use Committee of University of Massachusetts Amherst and all animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of University of Massachusetts Amherst. Forty male CD-1 mice (6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA, USA) and kept in a temperature-controlled animal room of 22 ± 2 °C and 50±10% humidity in a 12-h light-dark cycle with free access to water and a basal diet. The mice were then randomly divided into 4 groups (n=10 per each group) to receive either standard diet (AIN-93G) or cranberry diet. Cranberry was harvested at State Bog, Wareham, MA, and freeze-dried whole cranberry powder is deep red in color and free-flowing. Cranberry diet is made of AIN-93G diet containing 1.5 % (w/w) freeze-dried whole cranberry powder. The dietary dose of cranberry used in this study is equivalent to approximately a oral dose of 7.5 g of whole cranberry powder or 65 g fresh cranberry fruits per day for human dietary consumption in a 60 kg adult [17]. This dietary level of cranberry is readily achievable in humans, and it has been shown that 20% (w/w) freeze-dried whole cranberry reduced small intestine tumorigenesis in mice and the dose was well-tolerated [18].
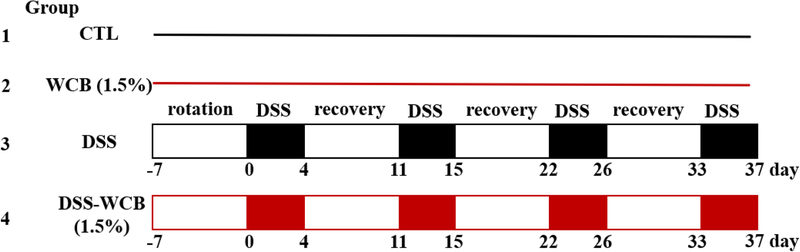
After one-week diet acclimation with standard diet, the four groups were distributed as follows: Group 1 (CTL), the healthy/non-colitic control group, which was fed with standard diet and regular tap water; Group 2 (WCB) the cranberry-supplemented non-colitic group, which received the cranberry diet and regular tap water; Group 3 (DSS) the DSS-treated colitic group, which received the standard diet and 1.5 % DSS in drinking water (wt/v, DSS salt, average molecular weight 36,000–50,000) (International Lab, Chicago, IL, USA); Group 4 (DSS-WCB) the cranberry-supplemented and DSS-treated group, which received the cranberry diet and 1.5 % DSS in drinking water (wt/v). The two DSS groups were treated with DSS in drinking water for 4 cycles (4 days/cycle, with a 7-day recovery after each of the first 3 DSS cycles) to induce colitis (Fig. 1).
Figure 1.
Experimental design.
The weight of the mice was recorded every other day during the whole experiment. At the end of the fourth cycle of DSS treatment, all mice were sacrificed by CO2 asphyxiation. The entire colon was removed and rinsed with chilled PBS, and the weight and length of colon then were measured and recorded. After measurement, the whole colon was opened and cut into two parts longitudinally for further analyses. One part of the colon was stored at −80°C for ELISA analysis while the other part was first fixed in 10% formalin for further histopathologic and immunohistochemical analysis. The fecal pellets were collected from the colon and stored at −80°C for further sequencing analysis. Both the liver and spleen were dissected weighted for evaluation of disease progression.
2.2. Disease activity index (DAI) and histological evaluation of the colon
Disease activity index was determined by the scores of body weight loss, stool consistency and rectal bleeding, which was recorded every other day (Table 1a) [19–21]. For histological evaluation, the fixed colon segments were first dehydrated by using ethanol and isopropanol, then embedded in paraffin. The specimens were then sectioned into slides, which get stained by hematoxylin and eosin (H&E). Histological grading was evaluated basing on the inflammatory criteria (Table 1b) [20–22].
Table 1A.
Scores of disease activity index (DAI)
| Score | Body weight loss (%) | Stool consistency | Fecal blood |
|---|---|---|---|
| 0 | None | Normal | None |
| 1 | 1~5 | Soft but form | Hemoccult+ |
| 2 | 5~10 | Soft | Blood |
| 3 | 10~20 | Diarrhea | Gross bleeding |
| 4 | >20 |
Table 1B.
Histological scores
| Score | Severity of inflammation | Extent of inflammation | Crypt damage |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Mild | Mucosal | Basal 1/3 |
| 2 | Moderate | Mucosal and submucosal | Basal 2/3 |
| 3 | Severe | Transmural | Crypts lost but surface epithelium present |
| 4 | Crypts and surface epithelium lost |
2.3. Measurement of inflammatory cytokines in the colonic mucosa and serum
Intestinal mucosa was collected and homogenized in phosphate buffer (PBS) solution containing 0.4 M NaCl, 0.05% Tween-20, 0.5% BSA, 0.1mM benzethonium, and protease inhibitor cocktail (Boston Bioproducts, Ashland, MA, USA). After homogenization, the samples were centrifuged at 10,000 g for 30 min at 0°C to collect the supernatant. The colonic mucosa and serum samples were loaded in U-PLEX ELISA kit (Meso Scale Discovery, Rockville, MD, USA) to determine the concentrations of IL-4, IL-13, IL-1β, IL-6, and TNF-α, according to the manufacturer’s instructions.
2.4. 16S rRNA Gut Microbiota Analysis
Fecal bacterial DNA was isolated using the QIAamp DNA stool kit (Qiagen, Inc., Shanghai, China). DNA quantity was measured by NanoDrop Spectrophotometer (Thermo Scientific, Waltham, MA, US). PCR was performed to amplify the 16S rRNA gene marker using universal primers tailed with Illumina barcoded adapters. The index PCR was performed to attach dual indices and Illumina sequencing adapters using the Nextera XT Index Kit (Illumina, San Diego, CA, US). PCR product was purified by using AMPure XP beads (Beckman Coulter, Danvers MA, US). After purification, the quantity of PCR products was determined by Qubit dsDNA BR Assay kit (Life technology, Carlsbad, CA, US) and the quality was estimated by ScreenTape Assay on Tape Station 2200 (Agilent Technologies, Santa Clara, CA). The amplicon library was loaded into the 600-cycle MiSeq Reagent kit v3 cartridge and sequenced on an Illumina MiSeq platform (Illumina Inc, San Diego, CA, US).
Quantitative insights into microbial ecology (QIIME) software pipeline v1.9.1 was used to analyze the raw data files obtained from Illumina MiSeq [23]. Operational Taxonomic Units (OTUs) were clustered into represent groups and assigned to taxonomy. OTU table and phylogenetic tree were constructed basing on the Greengenes bacterial 16S rRNA database (13.8 release) with 97% similarity threshold. Alpha-diversity and beta-diversity were analyzed to identify the species diversity. Distance matrix was visualized with principal coordinate analysis (PCoA) [24]. The functional capacity of the gut microbiota was predicted using PICRUSt with Greengenes database [25]. STAMP software was used to calculate the difference among each group [26].
2.5. Statistical analysis
A comparison of two or more groups was analyzed by either one-way parametric analysis of variance (ANOVA) followed by Tukey’s post hoc test or one-way non-parametric ANOVA (Kruskal–Wallis test) followed by Dunn’s post hoc test using SPSS 24.0 (Chicago, IL, USA). For all analysis, values of P <0.05 were considered statistically significant.
3. Results
3.1. Dietary cranberry decreased severity of DSS-induced colitis in mice
In this study, a DSS-induced experimental colitis mouse model was used to evaluate the anti-inflammatory effect of cranberry. Body weight was monitored twice a week. No difference was found between healthy control mice (Group 1) and cranberry-fed healthy mice (Group 2) throughout the entire experimental period (data not shown). There was no difference in the weight of liver and spleen between these two groups, suggesting no noticeable adverse effects caused by dietary administration of WCB to the mice.
At day 32, DSS induced severe illness in mice which was evidenced by a dramatically higher DAI score compared to the healthy control groups (including significant weight loss, evident rectal bleeding and diarrhea, Table 1a). Cranberry administration significantly decreased the DSS-elevated DAI scores compared to the DSS group (DSS-WCB vs. DSS). It is generally accepted that colon length is inversely associated with the severity of DSS-induced colitis [27]. To determine whether cranberry had a preventive effect on DSS-induced colon shortening, we compared the colon length of mice in all experimental groups. A significant shortening of colon length was observed in mice treated with DSS compared with healthy control mice (p<0.05). However, oral administration of cranberry significantly restored colon shortening effect. The spleen weight increased significantly in DSS group compared to the control group, while cranberry administration reversed the weight augment (p<0.05). Taken together, the results showed that dietary cranberry effectively diminished DSS-induced colitis in mice.
3.2. Dietary cranberry suppressed DSS-induced tissue damages and overproduction of pro-inflammatory cytokines in colitic mice
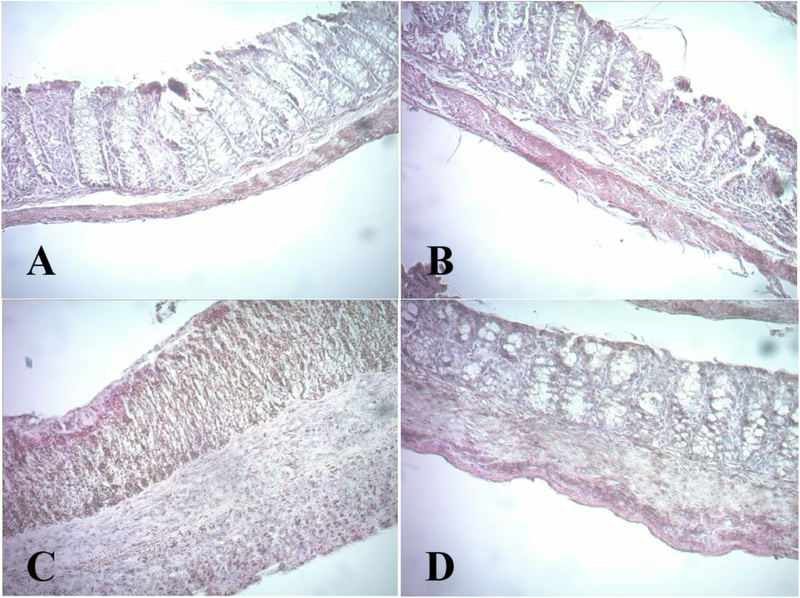
The effects of dietary cranberry on histological damages in the colons were examined following the histological grading system as described in Materials and Methods. Colonic inflammation is characterized by severe lesions in the colon mucosa, alteration of epithelial structure, increase in neutrophil population and lymphocyte infiltration into the mucosal and submucosal layers, and loss of normal crypts [27]. The colon specimens from the healthy control group exhibited normal features of typical glands and abundant goblet cells in epithelium (Figure 2, Table 2). In contrast, the colonic specimens obtained from the DSS group showed serious erosive lesions with extensive loss of glands, thickening of the mucosa, cellular infiltration of immune cells into the submucosa and lamina propria and crypt shortening, collectively resulting in a higher histological score. However, in the WCB group, dietary cranberry remarkably decreased the total histological score compared with the DSS group. The colonic specimens obtained from DSS-WCB group had no noticeable erosion with just mild inflammatory cell infiltration. In both non-colitic groups, no aberrant tissue was observed.
Figure 2.
Histological characterization of the colon mucosa. Representative images (150×) of H&E stained colon: (A) CTL, (B) WCB, (C) DSS, (D) DSS-WCB.
Table 2.
DAI, colon length, histological score and spleen weight. Data are presented as means ± SD. Different letters (a, b, c) indicate statistically significant differences between groups (p < 0.05).
| Groups | DAI | Colon length (mm) | Histological score | Spleen weight (mg) |
|---|---|---|---|---|
| CTL | 0 a | 87.39 ± 3.85 a | 0 a | 125.35 ± 7.15 a |
| WCB | 0 a | 94.19 ± 5.10 a | 0 a | 100.62 ± 4.21 a |
| DSS | 3.62 ± 0.42 b | 68.98 ± 2.01b | 7.11 ± 0.30 b | 159.25 ± 18.97 b |
| DSS-WCB | 1.56 ± 0.25 c | 80.34 ± 7.42 a | 4.55 ± 0.21c | 126.29 ± 8.91 a |
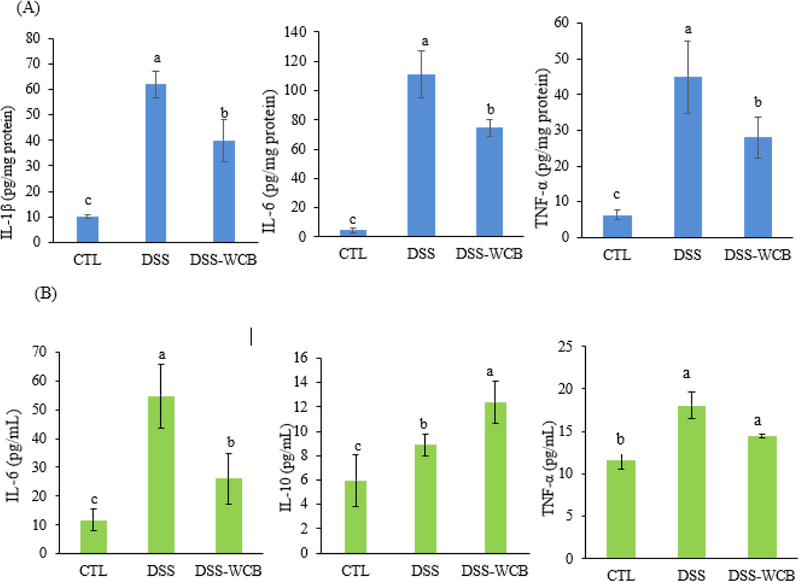
Improper elevation of circulating and colonic level of pro-inflammatory cytokines is a common hallmark of IBD [21, 27]. Figure 3A showed the concentration of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in the colonic mucosa. Compared to the healthy control group, pro-inflammatory cytokines were overexpressed in both DSS and DSS-WCB groups. However, the expression levels of pro-inflammatory cytokines in the DSS-WCB group were significantly lower than those in DSS group. The level of IL-1β was increased by 80% in the DSS group, while cranberry diet significantly reduced that by 40% (p<0.05). The concentration of IL-6 was elevated by 95% in the DSS group, and cranberry treatment significantly suppressed that by 33% (p<0.05). Similarly, the expression of TNF-α was enhanced by DSS treatment by 86% while the cranberry diet significantly alleviated that by 37% (p<0.05).
Figure 3.
Effects of DSS treatment and dietary cranberry on cytokine levels in the colonic mucosa (A) and serum (B) in mice. Data are shown as the mean ± SD. Different letters (a, b, c) indicate statistically significant differences between groups (p < 0.05, n = 3).
We then determined the circulating level of cytokines between the three groups. In addition to colonic inflammation, DSS treatment strongly enhanced the degree of systemic inflammation as evidenced by greater concentrations of IL-6 and TNF-α, compared to the healthy control group (Figure 3B). We found that cranberry treatment significantly diminished serum IL-6 (p<0.05), and showed a tendency to reduce serum TNF-α, although it did not achieve a statistical significance. IL-10 is cytokine with anti-inflammatory property, the level of IL-10 in DSS-treated colitic mice was higher than that of healthy control mice. This phenomenon may due to the self-defeating response [28, 29], indicating more severe colitis in DSS-treated mice. Despite the moderate elevation of IL-10 in DSS-treated mice, cranberry diet further enhanced the expression of IL-10 by 40% compared to DSS group. Altogether, dietary cranberry significantly ameliorated histological changes and elevated pro-inflammatory cytokines accompanied by the DSS-induced colitis.
3.3. Dietary cranberry increased gut microbiota diversity in colitic mice.
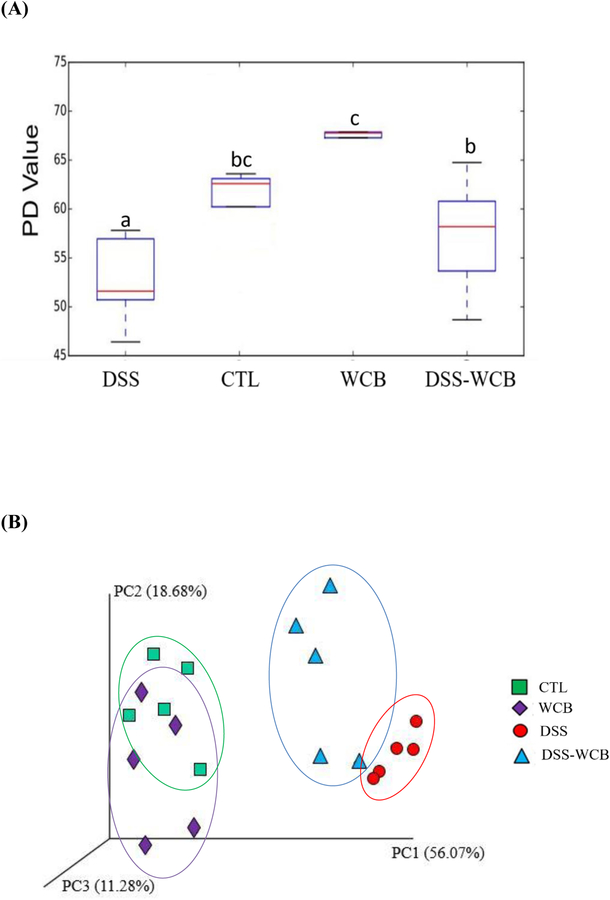
Next, we sought to determine whether cranberry modulated the structure and composition of the gut microbiota in mice by 16S rRNA sequence analysis. Bacterial α-diversity, as assayed by phylogenetic diversity (PD) whole tree, of the WCB group was significantly higher than the other three groups (Figure 4A). The α diversity was significantly decreased in the DSS group, compared to healthy control group. On the other hand, cranberry treatment significantly improved DSS-induced decline in α-diversity. Moreover, there was no significant difference between the healthy control and DSS-WCB groups.
Figure 4.
(A) Effects of DSS treatment and dietary cranberry on α diversity of gut microbiota in mice, assessed by PD whole tree analysis. Different letters (a, b, c) indicate statistically significant differences (p<0.05). (B) Effects of DSS treatment and dietary cranberry on β-diversity of gut microbiota. Weighted UniFrac distances PCoA graph was used to evaluate diversities between samples.
β diversity of the microbiota was assessed by principal coordinate analysis of the weighted UniFrac Principal Component Analysis (PCoA). The results indicated that DSS profoundly shifted the structure of the microbial community (DSS vs. CTL or WCB), while cranberry partially reversed the alteration (Figure 4B). ANOSIM with 999 permutations was used to confirm significant separation of 4 groups with distance matrices of weighted UniFrac. As expected, DSS significantly altered the microbial structure of the gut microbiota, compared with the healthy control group (DSS vs. CTL, p< 0.05, R=1.0). Moreover, cranberry diet significantly improved the gut microbial community of mice receiving DSS water (DSS-WCB vs. DSS, p< 0.05, R=0.75). Although the structure of gut microbiota in the DSS-WCB group was also significantly different from healthy control group (DSS-WCB vs. CTL, p< 0.05, R=0.73). Oral administration of cranberry to healthy mice did not further change the β diversity compared to healthy control mice (WCB vs. CTL, p> 0.05, R=0.175). Altogether, for the first time, we found that alleviation of DSS-altered diversity and structure of gut microbiota may contribute to the beneficial effects of whole cranberry on colitis.
3.4. Dietary cranberry ameliorated DSS-induced microbiota dysbiosis in colitic mice.
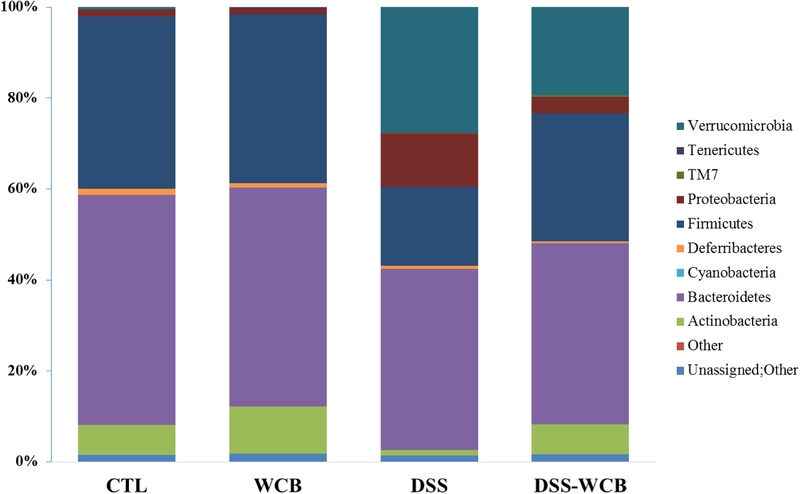
In this study, significant variations of bacteria composition were observed among the four groups. Firmicutes, Bacteroidetes, Actinobacteria, Verrucomicrobia and Actinobacteria were five major phyla present in the colonic microbiota (Figure 5). Linear discriminant analysis (LDA) effect size (LEfSe) was applied to determine differentially relative abundant bacterial phyla between each two groups. Compared to the healthy non-colitic group, Verrucomicrobia, TM7 and Proteobacteria were differentially higher expressed in the DSS group, while Firmicutes and Actinobacteria were lower (α=0.01, LDA score >3.0). DSS-treated mice with dietary cranberry (DSS-WCB group) had higher relative abundance of Verrucoicrobia and TM7, compared to the two non-colitic groups. Moreover, dietary cranberry increased the relative abundance of Verrucoicrobia, Proteobacteria and decreased that of Actinobacteria in mice received DSS (DSS-WCB vs. DSS). Taken together, dietary cranberry partially reversed the alteration of gut microbiota in DSS-induced mice.
Figure 5.
Bacterial taxonomic profiling of the phylum level of gut microbiota from different treatment groups.
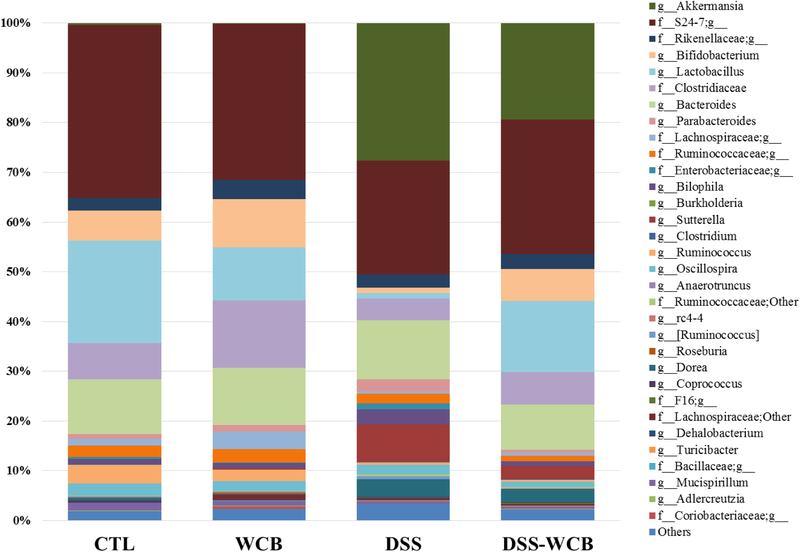
We then analyzed the relative abundance of bacteria at the genera level and found that 95 genera belonging to the 8 phyla were detected in the colonic fecal pellet (Figure 6). Among all the genera, Bifidobacterium, Bacteroides, Lactobacillus, Akkermansia, Sutterella and two unknown genera in family S24–7 and in order Clostridiales were the dominant genus accounting for more than 50% of total gut bacteria. Comparing to the healthy control group, the relative abundance of 16 genera in DSS group shifted significantly, and although no difference was detected in the phyla level between the two non-colitic groups, the relative abundance of 5 genera was significantly different.
Figure 6.
Bacterial taxonomic profiling of the genus level of gut microbiota from different treatment groups.
As shown in Table 3, Akkermansia was the most abundant genus in the DSS and DSS-WCB groups, which accounted for 28.27 and 19.32%, respectively. However, less than 1% Akkermansia was identified in two groups without DSS treatment. The aberrant dominance of Akkermansia in the two DSS-treated groups indicated that DSS treatment was in favor of the growth of Akkermansia. The cranberry diet significantly reduced the abundance of Akkermansia in DSS-WCB group (p<0.05). The abundance of Sutterella was increased significantly (p<0.05) in the DSS- group (11.01%) and DSS-WCB group (2.59%), compared to CTL group (0.01%) and WCB group (0.07%). Similarly, Bilophila proliferated significantly (p<0.05) in the DSS group (3.03%), which was three times more abundant than the other three groups. The relative abundance of genus Lactobacillus, Bifidobacterium and Ruminococcus was dramatically suppressed by the DSS treatment, compared to the two non-colitic groups (DSS vs. CTL or WCB, p<0.05). However, decline in Lactobacillus and Bifidobacterium was greatly restored by cranberry supplementation (DSS-WCB vs. DSS, p<0.05), although it did not change the abundance of Ruminococcus. Our data suggested that in addition to the improvement of overall diversity of gut microbiota, dietary cranberry also effectively modulated the composition of specific bacteria in mice.
Table 3.
Relative abundance of selected gut bacteria at the genus level. Data are presented as means ± SD. Different letters (a, b, c) indicate statistically significant differences between groups (p < 0.05).
| CTL | WCB | DSS | DSS-WCB | |
|---|---|---|---|---|
| Lactobacillus | 25±3.2 % a | 16 ± 1.2% a | 1 ± 0.03% b | 22 ± 2.5% a |
| Akkermansia | 1 ± 0.01% a | 1 ± 0.01% a | 28 ± 2.1% b | 19 ± 1.2% c |
| Bifidobacterium | 8 ± 2.7% a | 12 ± 2.6% b | 1 ± 0.3% c | 5 ± 1.4% b |
| Sutterella | 1 ± 0.01% a | 1 ± 0.01% a | 11 + 3.4% b | 3 ± 0.5% c |
| Ruminococcus | 2.6± 0.1% a | 2.1 ± 0.1% a | 0.4± 0.01%b | 0.4± 0.01% b |
| Bilophila | 1 ± 0.01% a | 1 ± 0.01% a | 3 ± 0.9% b | 1 ± 0.02% a |
3.5. Dietary cranberry modified predicted metagenome functions of microbiota in colitic mice
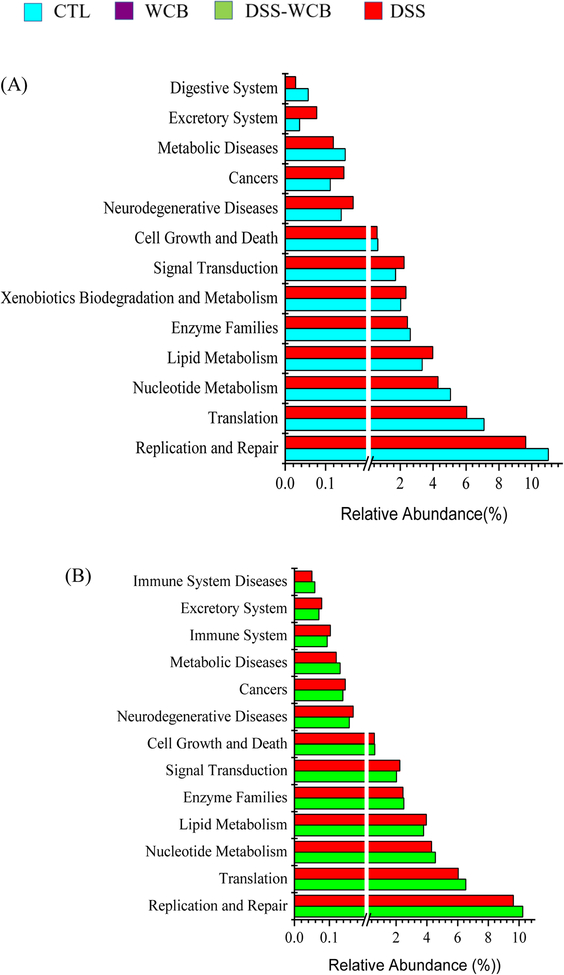
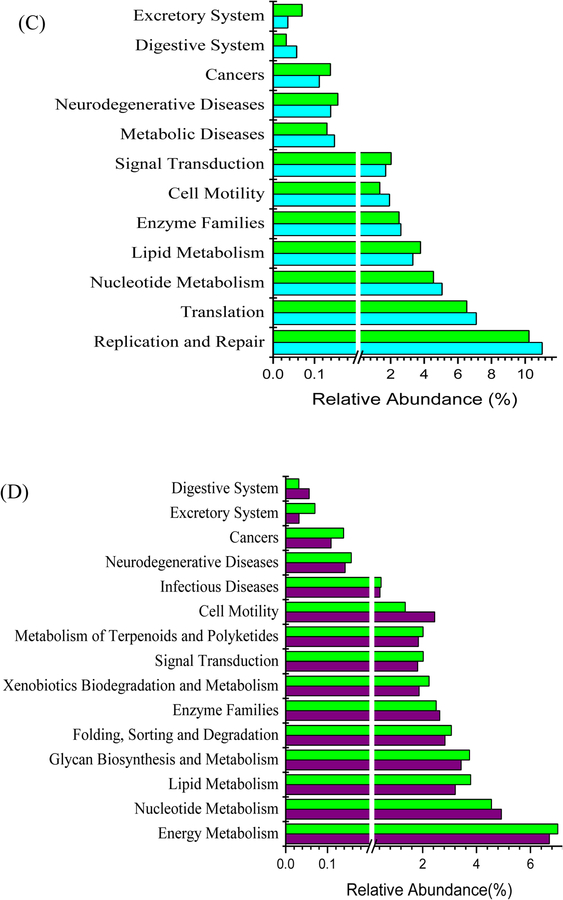
Gut bacteria functional profile was predicted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) based on 16S rRNA sequencing data (Figure 7). The functions including cancers, neurodegenerative disease and xenobiotics biodegradation were expressed higher in DSS group than the healthy control group, while the expression level of genes related to cell growth, replication and repair, digestive system was lower (Figure 7A, p<0.05). Comparing to DSS-WCB group, the relative abundance of 6 functional genes in DSS group were significantly higher including excretory system, immune system, cancers, lipid metabolism, neurodegenerative disease and signal transduction, but gut bacteria of the DSS group expressed more genes related to replication and repair, enzyme families, cell growth and death (Figure 7B, p<0.05). The bacterial functional genes associated with cell motility, nucleotide metabolism and replication and repair were more abundant in the healthy control group than in DSS-WCB group. In contrast, the DSS-WCB group had higher gene expression for excretory system, cancers, signal transduction, lipid metabolism and neurodegenerative diseases (Figure 7C, p<0.05). The DSS-WCB group mice had 15 genes expression level significantly different from the WCB group. In WCB group, the proportion of genes related to cell motility, digestive system and nucleotide metabolism were higher, while the proportion of other genes was lower such as cancers and infectious diseases (Figure 7D, p<0.05). There is no significant difference detected between two non-colitic healthy groups (CTL vs. WCB).
Figure 7.
Significant differences in relative abundance of predicted metagenome function between groups: (A) DSS group vs. CTL group. (B) DSS group vs. DSS-WCB group. (C) DSS-WCB vs. CTL group. (D) DSS-WCB group vs. WCB group. The STAMP was used to detect significant differences function.
4. Discussion
IBD can cause several severe syndromes in the patients, including abdominal pain, rectal bleeding and diarrhea [1, 2]. The high incidence rate of IBD in developed countries has been attributed to the western diet with high fat, high calorie and low consumption of vegetable and fruits [1, 2]. Current therapies for IBD mainly suppress the immune response in the colon, resulting in the several side effects such as drug toxicity and immune damage [22]. Therefore, plant-based foods and the phytochemicals presented in these foods, which have been widely studied for their various bioactivities such as anti-oxidative and anti-inflammatory effect, are thought to be a possible preventive strategy for IBD with low or no adverse effects. The purpose of this study was to investigate the chemopreventive effect of cranberry on DSS-induced colitis. The results of this study showed that DSS caused severe colonic inflammatory syndromes in mice including weight loss, diarrhea and rectal bleeding (Table 2). The shortened colon length and the increased spleen weight further confirmed the inflammatory process in DSS-treated mice. Besides, DSS induced the damage of colonic mucosal structure, leading to the crypt lesion and inflammatory cell infiltration (Figure 2). However, oral administration of cranberry in diet significantly diminished the inflammatory process and damages caused by DSS. The DAI score in DSS-WCB group was significantly lower than DSS group, indicating that cranberry diet alleviated the syndromes of colitis. No difference in the colon length and spleen weight was observed between DSS-WCB group and the two non-colitic groups, which further supported the anti-inflammatory effect of cranberry on the DSS induced colitis.
Studies showed that the inflammation process in the colon is modulated by the expression level of pro-inflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-12 IFN-γ and TNF-α and anti-inflammatory cytokines like IL-4 and IL-10 [30]. Our result showed that DSS indeed strongly elevated the expression of pro-inflammatory cytokines in colonic mucosa, including IL-1β, IL-6 and TNF-α, compared to the healthy control mice (Figure 3A). In contrast, the intake of cranberry diet significantly suppressed the expression of these three cytokines in colonic mice, suggesting that cranberry can prevent the colonic inflammation through reducing the overproduction of pro-inflammatory cytokines. IL-1β modulates the inflammatory process through multiple ways. IL-1β can promote the activities of dendritic cells, macrophages and neutrophils and activate T cell and CD4+TH17 cells [31]. TNF-α plays an important role on activation of various inflammatory cells and modulation of epithelial cell permeability [22]. IL-6 is involved in the inflammatory process through modulating the proliferation of T cell. Over expression of IL-6 can activate the anti-apoptotic genes Bcl-xl, leading to the expansion of T-cell, which further cause the chronic intestinal inflammation [32]. The pathological studies indicated that the excessive numbers of T cells as well as the increasing level of TNF-α in the colonic mucosa induce Crohn’s disease [33].
Besides, the result of serum cytokines showed that DSS also greatly enhanced the circulating level of IL-6 and TNF-α which was same in the colonic mucosa (Figure 3B). Interestingly, DSS also up-regulated the anti-inflammatory cytokine IL-10 in serum compared to the healthy mice. IL-10 can mediate and suppress the expression of pro-inflammatory cytokines, resulting in the protective effect against the inflammation and infections [34]. In fact, IL-10 can be stimulated by multiple pro-inflammatory agents, including 12-O-tetradecanoylphorbol 13-acetate (TPA), DSS, and trauma-hemorrhage to counter-regulate inflammatory response [35, 36]. The elevated level of IL-10 found in DSS group thus confirmed that a greater level of inflammation was presented, thus more anti-inflammatory cytokine IL-10 was produced. Cranberry diet significantly lowered the IL-6 expression and further increased the concentration of IL-10, in comparison to DSS group. Taken together, by reducing the overexpression of pro-inflammatory cytokines and promoting the secretion of anti-inflammatory cytokine, cranberry significantly ameliorated the inflammatory process in DSS-treated mice.
Accumulating evidence has showed that the development of IBD is associated with the disrupted equilibrium of gut microbiota [3]. Gut microbiota has long been known to play an important part in colonic immune system development [37], and it can modulate both pro-inflammatory and anti-inflammatory cytokines through their presence or activities [37, 38]. This study used high throughput sequencing to identify changes in the microbial community caused by DSS in mice and the beneficial effects of cranberry diet. The Firmicutes/ Bacterioidetes ratio was significantly lower in DSS group (0.43) than in CTL group (0.75), WCB (0.77) and DSS-WCB group (0.71). The lower ratio of Firmicutes/Bacterioidetes was found in IBD patients [39]. The Firmicutes/Bacterioidetes ratio in DSS-WCB group was close to two non-colitic groups, suggesting that the cranberry diet could protect the DSS-caused gut microbiota disruption.
Moreover, the relative abundance of Verrucomicrobia phylum significantly increased in DSS group (Figure 5), which could be mainly due to the excessive growth of Akkermansia genus (Table 3). Akkermansia is commonly distributed in the colon of mammals, which is located within the mucus layer and is capable of degrading mucin [40]. The aberrant number of Akkermansia in DSS group may due to the lesion of mucus layer caused by DSS, which may further lead to the over production of mucin. Dharmani et al. reported that the increasing gene expression of Muc2 and Muc 3 in DSS treated animal [41]. The increasing amount of mucin could be the food source of Akkermansia and promoted their growth. However, one study indicated that the abundance of Akkermansia was lower in the IBD patients, suggesting the potential beneficial effect of Akkermansia against IBD [42]. Derrien et al. reported that the colonization of Akkermansia in germ free mice did not induce inflammation [40]. Therefore, the excessive proliferation of Akkermansia might be the result of DSS-induced colitis rather than the cause of the inflammation. This notion was in agreement with the fact that decreased abundance of Akkermansia was observed in mice with suppressed colitis by WCB treatment.
Bifidobacterium and Lactobacillus are generally recognized as probiotic, which may reduce the risk of intestinal inflammation [43]. The reduction of these two bacterial genera in DSS group indicated that inflammatory process might inhibit the growth of them. However, the cranberry diet counteracted the reduction and the presence of cranberry may exert the anti-inflammatory effect. The results showed that DSS treatment reduced the gut microbial diversity and relative abundance of some probiotics, as well as enhanced the relative abundance of the pathogenic. In contrast, cranberry diet significantly amplified the diversity in non-colitic mice, and considerably alleviated the reduction of overall diversity and abundance of probiotics and diminished the abundance of some pathogenic bacteria, compared to the DSS group.
DSS treatment altered the functional profile of gut microbiota in a pro-inflammatory direction. For example, DSS treatment had an increased capacity on xenobiotics metabolism which link to the host adrenergic stress with bacterial virulence [44]. The metabolic function of lipid metabolism also increased in this study and a study with the FUT2 null mice, a model of Crohn’s disease, indicating the enhanced energy harvest of gut microbiota [45]. On the other hand, dietary cranberry greatly improved these changes caused by DSS. Dietary cranberry significantly decreased the bacterial functional genes associated with cancer and neurodegenerative diseases and increased that of replication and repair. The shift in the function of gut microbiota was also demonstrated by a study with IBD patients and healthy subjects, which showed that 12% of analyzed pathways changed in the IBD patients [46]. Further study will be needed to investigate whether the change of gene function is the cause or consequence of intestinal inflammation.
In conclusion, for the first time, we demonstrated the preventive effect of dietary whole cranberry against colonic inflammation in DSS-treated mice. The anti-inflammatory effect of whole cranberry was closely related to its ability to alleviate the DSS-induced alterations in composition and the function of gut microbiota. Clinical studies are needed to demonstrate the suppressive effects of cranberry on IBD and its beneficial impact on gut microbiota in humans.
Acknowledgements
This work was supported by United State Department of Agriculture (NIFA grant #2019-67017-29249), Leo and Anne Albert Charitable Trust, UMass Cranberry Health Research Center/UMass President’s S&T Initiative, and National Institutes of Health (R01AT010229). The authors declare no conflict of interest.
Abbreviations:
- IBD
inflammatory bowel disease
- DSS
dextran sodium sulfate
- WCB
whole cranberry
- PD
phylogenetic diversity
- PCoA
Principal Coordinates Analysis
References
- 1.Maharshak N, et al. , Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes, 2013. 4(4): p. 316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SC, et al. , Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet, 2018. 390(10114): p. 2769–2778. [DOI] [PubMed] [Google Scholar]
- 3.Round JL and Mazmanian SK, The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol, 2009. 9(5): p. 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xavier RJ and Podolsky DK, Unravelling the pathogenesis of inflammatory bowel disease. Nature, 2007. 448(7152): p. 427–34. [DOI] [PubMed] [Google Scholar]
- 5.Guinane CM, and Cotter Paul D., <Role of the gut microbiota in health and chronic gastrointestinal disease- understanding a hidden metabolic organ.pdf>. Therapeutic advances in gastroenterology, 2013. 6(4): p. 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimesova K, et al. , Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm Bowel Dis, 2013. 19(6): p. 1266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gkouskou KK, et al. , The gut microbiota in mouse models of inflammatory bowel disease. Front Cell Infect Microbiol, 2014. 4: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez B, et al. , Probiotics, gut microbiota, and their influence on host health and disease. Molecular Nutrition & Food Research, 2017. 61(1). [DOI] [PubMed] [Google Scholar]
- 9.Yan X, et al. , Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J Agric Food Chem, 2002. 50(21): p. 5844–9. [DOI] [PubMed] [Google Scholar]
- 10.van Duynhoven J, et al. , Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci U S A, 2011. 108 Suppl 1: p. 4531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Kaoutari A, et al. , The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol, 2013. 11(7): p. 497–504. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg JB, et al. , Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015. Adv Nutr, 2016. 7(4): p. 759S–70S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, et al. , Dietary Non-digestible Polysaccharides Ameliorate Intestinal Epithelial Barrier Dysfunction in IL-10 Knockout Mice. J Crohns Colitis, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, et al. , Chemopreventive Effects of Whole Cranberry (Vaccinium macrocarpon) on Colitis-Associated Colon Tumorigenesis. Mol Nutr Food Res, 2018. 62(24): p. e1800942. [DOI] [PubMed] [Google Scholar]
- 15.Popov SV, et al. , Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J Gastroenterol, 2006. 12(41): p. 6646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao X, et al. , Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem, 2015. 167: p. 438–46. [DOI] [PubMed] [Google Scholar]
- 17.Freireich EJ, et al. , Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep, 1966. 50(4): p. 219–44. [PubMed] [Google Scholar]
- 18.Jin D, et al. , Dietary feeding of freeze-dried whole cranberry inhibits intestinal tumor development in Apc(min/+) mice. Oncotarget, 2017. 8(58): p. 97787–97800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper HS, et al. , Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest, 1993. 69(2): p. 238–49. [PubMed] [Google Scholar]
- 20.Hu Q, et al. , Dietary intake of Pleurotus eryngii ameliorated dextran sulfate sodium-induced colitis in mice. Mol Nutr Food Res, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Han Y, et al. , Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis. J Agric Food Chem, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Park MY, Ji GE, and Sung MK, Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci, 2012. 57(2): p. 355–63. [DOI] [PubMed] [Google Scholar]
- 23.Caporaso JG KJ, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI et al. , QIIME allows analysis of high-throughput community sequencing data. Nature methods, 2010. 7(5): p. 335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navas-Molina JA, et al. , Advancing our understanding of the human microbiome using QIIME. Methods Enzymol, 2013. 531: p. 371–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langille MG, et al. , Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol, 2013. 31(9): p. 814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks DH, et al. , STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics, 2014. 30(21): p. 3123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh SY, et al. , Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med, 2014. 33(2): p. 333–40. [DOI] [PubMed] [Google Scholar]
- 28.De Filippo K, et al. , Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood, 2013. 121(24): p. 4930–7. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CP, Schwacha MG, and Chaudry IH, The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta, 2004. 1689(1): p. 22–32. [DOI] [PubMed] [Google Scholar]
- 30.Obermeier F, et al. , Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clinical and Experimental Immunology, 1999. 116(2): p. 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coccia M, et al. , IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med, 2012. 209(9): p. 1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atreya R and Neurath MF, Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol, 2005. 28(3): p. 187–96. [DOI] [PubMed] [Google Scholar]
- 33.Adegbola SO, et al. , Anti-TNF Therapy in Crohn’s Disease. International Journal of Molecular Sciences, 2018. 19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang W, et al. , Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol, 2011. 29: p. 71–109. [DOI] [PubMed] [Google Scholar]
- 35.Monk JM, et al. , White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. Journal of Nutritional Biochemistry, 2015. 26(7): p. 752–760. [DOI] [PubMed] [Google Scholar]
- 36.Schneider CP, Schwacha MG, and Chaudry IH, The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochimica Et Biophysica Acta-Molecular Basis of Disease, 2004. 1689(1): p. 22–32. [DOI] [PubMed] [Google Scholar]
- 37.Sellon RK, et al. , Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and Immunity, 1998. 66(11): p. 5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegazy SK and El-Bedewy MM, Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol, 2010. 16(33): p. 4145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokol H, et al. , Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis, 2009. 15(8): p. 1183–9. [DOI] [PubMed] [Google Scholar]
- 40.Derrien M, et al. , Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol, 2011. 2: p. 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dharmani P, Leung P, and Chadee K, Tumor Necrosis Factor-alpha and Muc2 Mucin Play Major Roles in Disease Onset and Progression in Dextran Sodium Sulphate-Induced Colitis. Plos One, 2011. 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Png CW, et al. , Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol, 2010. 105(11): p. 2420–8. [DOI] [PubMed] [Google Scholar]
- 43.Isolauri E, Kirjavainen PV, and Salminen S, Probiotics: a role in the treatment of intestinal infection and inflammation? Gut, 2002. 50 Suppl 3: p. III54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooks MG; Veiga P; Wardwell-Scott LH; Tickle T; Segata N; Michaud M; Gallini CA; Beal C; van Hylckama-Vlieg JE; Ballal SA, Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. The ISME journal 2014, 8, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong M; McHardy I; Ruegger P; Goudarzi M; Kashyap PC; Haritunians T; Li X; Graeber TG; Schwager E; Huttenhower C, Reprograming of gut microbiome energy metabolism by the FUT2 Crohn’s disease risk polymorphism. The ISME journal 2014, 8, 2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan XC; Tickle TL; Sokol H; Gevers D; Devaney KL; Ward DV; Reyes JA; Shah SA; LeLeiko N; Snapper SB, Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome biology 2012, 13, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]