Abstract
Background:
Early life anesthesia exposure results in long term cognitive deficits in rats. Environmental enrichment consisting of social housing, a stimulating environment and voluntary exercise can rescue this deficit. We hypothesized that exercise alone is sufficient to rescue the cognitive deficit associated with perinatal anesthesia.
Methods:
Post-natal day 7 male rats (P7) underwent isoflurane or sham exposure and were subsequently weaned at P21. They were then singly housed in a cage with a running wheel or a fixed wheel. After three weeks of exercise, animals underwent behavioral testing for spatial and recognition memory assessments. Animals were sacrificed at various time points to accomplish either bromodeoxyuridine (BrdU) labelling or qRT-PCR to quantify brain derived neurotrophic factor (BDNF) mRNA levels.
Results:
Post-weaning voluntary exercise rescued the long-term spatial memory deficit associated with perinatal isoflurane exposure. Iso-sedentary animals did not discriminate the goal quadrant, spending no more time than chance during the Barnes maze probe trial (one sample T-test p=0.524) while all other groups did (one sample T-test p Iso-exercise =0.033; p Con-sedentary = 0.004). We did not find a deficit in recognition memory tasks after isoflurane exposure as we observed previously. BrdU incorporation in the adult hippocampus of Iso-sedentary animals was decreased compared to sedentary controls (Tukey p=0.005). Exercise prevented this decrease, with Iso-exercise animals having more proliferation than Iso-sedentary (Tukey p<0.001). There was no effect of exercise or isoflurane on BDNF mRNA in either the cortex or hippocampus (Cortex: F Exercise(1,32) =0.236 p=0.631; F Iso(1,32) =0.038 p=0.847; F Interaction(1,32) =1.543 p=0.223; Hippocampus: F Exercise (1,33) =1.186 p=0.284; F Iso(1,33) =1.46 p=0.236; F Interaction (1,33) =1.78, p=0.191).
Conclusions:
Exercise restores BrdU incorporation and rescues a spatial memory deficit after early life anesthesia exposure. This demonstrates sufficiency of exercise alone in the context of environmental enrichment to recover a behavioral phenotype after a perinatal insult.
Introduction
Early life exposure to anesthesia in humans continues to be a concern given the mounting evidence of a cognitive deficit in animal models including those of non-human primates1,2. This deficit is lifelong and highly reproducible across anesthetic agents and several species3,4. The results from clinical studies on the other hand have been mixed, complicated by the nature of studying children and the inherent confounding variables of subjects who require surgery/anesthesia.
In a rodent model of early life anesthesia exposure, we have previously identified environmental enrichment (EE) as an important modulator of this phenotype5. Others have subsequently shown voluntary exercise is a key component of environmental enrichment6,7 and it can improve cognitive ability in both human and animal models. In rodents, this beneficial effect of exercise is thought to result from increasing adult hippocampal neurogenesis, the process of a neuro-progenitor cell differentiating into a fully integrated hippocampal neuron8 (although in humans, the dogma of adult neurogenesis has recently been called into question9,10). To understand this process in our animal model we hypothesize that voluntary exercise alone is sufficient to rescue this anesthetic-induced phenotype and we investigated the role of exercise on several different memory tasks after early life isoflurane exposure in rats.
Methods
This manuscript adheres to the relevant ARRIVE guidelines for reporting animal research.
Isoflurane Exposure
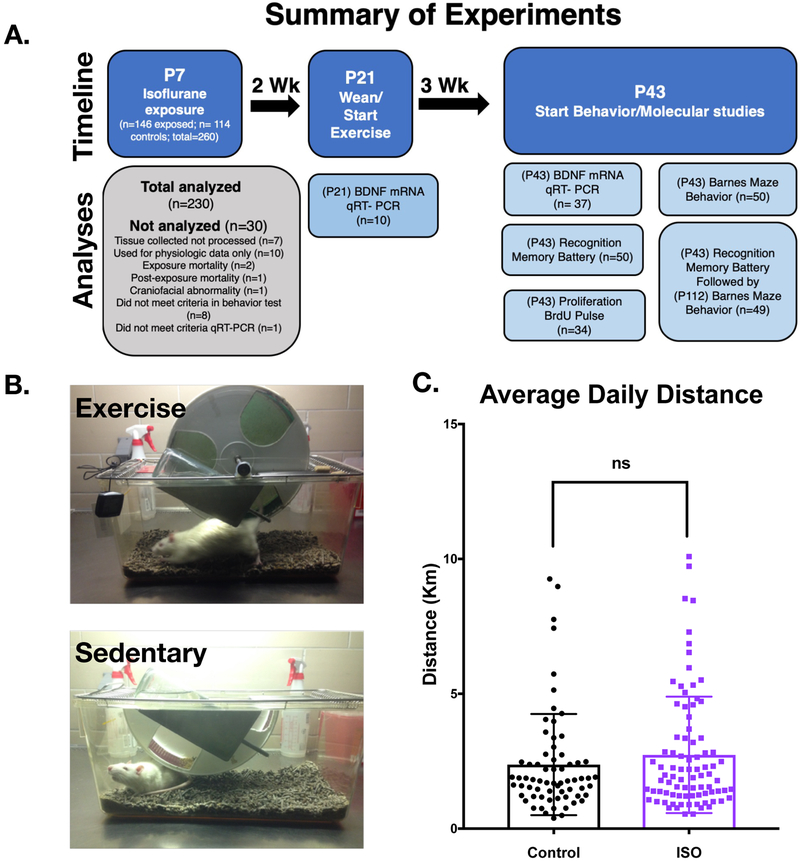
All animals were housed and treated according to institutional policy with a protocol approved by the UCSF institutional animal care use committee. Post-natal day 2 (P2) male Sprague Dawley rats were purchased (Charles River) and cross fostered with dams until P7, the day of isoflurane exposure. Animals from each litter were chosen at random to be exposed to anesthesia or sham (controls) and returned to their respective dam post-exposure. Isoflurane was administered in a step-down protocol over six hours (2% Iso hrs. 0–2; 1.4% Iso hrs. 2–4, 0.8% Iso hrs. 4–6) using a custom-built anesthesia exposure box and monitored with a Datex-Ohmeda (Capnomac Ultima) gas analyzer (Supplementary Fig 1). Unlike previous experiments in which tail clamping was used in conjunction with measured volatile anesthetic concentration to determine the minimum alveolar concentration (MAC) of anesthesia in P7 male rats, we instead used the measured isoflurane concentration to guide the exposure as we did not find a difference between tail clamping and non-tail clamping in past volatile anesthetic exposures5. In addition, the step-down protocol here was developed to decrease the mortality sometimes observed when using a MAC based protocol. Skin temperature was measured by infrared thermometer every 15 minutes and controlled with a heating pad. Fresh gas was approximately 1Liter/minute, with an FiO2 of 0.4–0.6. CO2 absorbent pellets, Litholyme (Allied Healthcare), were placed in the chamber and CO2 levels were continuously monitored. On completion of isoflurane exposure, the chamber was flushed with atmospheric air and animals were allowed to emerge from anesthesia. Controls were separated from dams for 30 minutes and reunited with their isoflurane exposed littermates upon emergence from anesthesia after return of righting reflex. Out of the 146 pups exposed to anesthesia, 2 died during exposure. 114 controls were utilized for the experiments (Fig1a).
Figure 1. Exercise treatment after perinatal isoflurane exposure.
A. Outline of experiments undertaken with animal numbers. B. Images of two housing paradigms. Exercising animals had access to a running wheel with an attached odometer to measure daily exercise. Sedentary animals were singly housed with a fixed half-wheel in which they could explore but not exercise. C. Treatment animals exercised on average 2.37 Km/day for control animals (n=67) and 2.73 Km/day for Isoflurane exposed animals (n=85). There was no statistical difference between groups (Mann-Whitney p=0.428). Error bars represent standard deviation.
Exercise Treatment
Animals were weaned at P21 and placed in a solitary cage either with a running wheel and associated odometer or a cage with a fixed half wheel with which the animal could explore but could not exercise (Fig1b). Animals were given food and water ad libitum and were subjected to a 12hr. reverse light/dark cycle. Daily exercise distance was recorded.
Barnes Maze
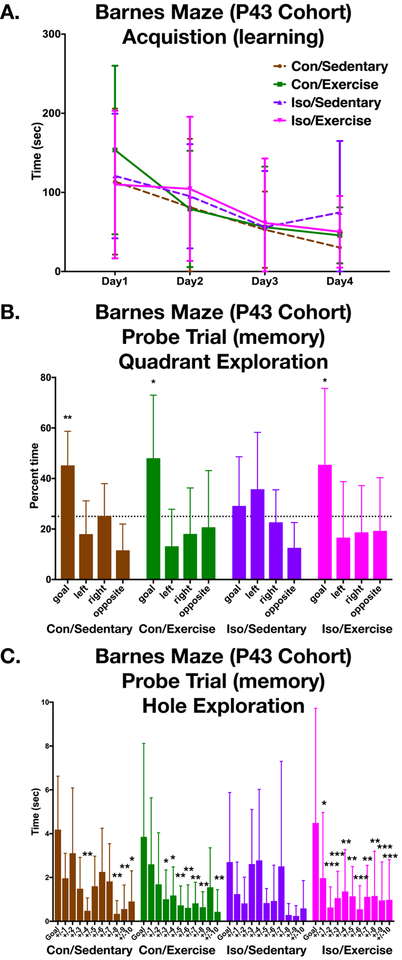
Barnes maze was conducted as previously described11. Briefly, animals were exposed to the standard rat maze with 20 holes around the perimeter. The testing arena included external spatial cues on each wall, a fan in a fixed position, bright lights in fixed positions, in an otherwise darkened room. The experimenter was hidden from view. Acquisition trials of up to 4 minutes were performed daily for 4 consecutive days, while movements were tracked using a camera (Basler aca1280) and tracking software (Ethovision XT 11.5). Probe trials were conducted 1 week after fourth acquisition day and lasted 90 seconds. Cumulative time spent at each hole and time in each quadrant of the maze were recorded. A cohort of young adults was studied after three weeks of exercise, beginning training on P43. A different cohort, which had first undergone recognition memory testing, started Barnes Maze training on P112. Animals which never found the goal hole during the learning phase were not included in the probe trial analysis (nP43 =5; n P112 =1) as well as animals which did not investigate any hole during the probe (n P43 =2; n P112 =0).
Recognition Memory tasks
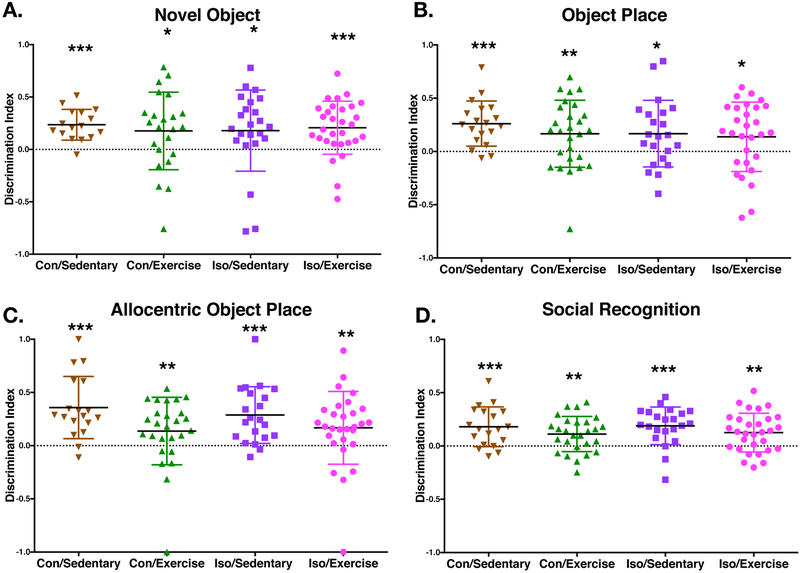
The four specific recognition memory tasks were performed on two cohorts of naïve animals, starting at P43 (following 3 weeks of exercise). Tasks were performed as previously described in detail12. Briefly, animals were placed with a set of objects in a testing box and given 4 minutes to explore. Animals were removed for two minutes and the box was cleaned and objects were moved or switched depending on the paradigm, then animals were observed for an additional 4 minutes. Movements were tracked with the aforementioned software. Social recognition memory tests, were performed as described12 using juvenile males in small cages, with a 4 minute exposure then 2hr. delay, with 4 minute test. Discrimination index was calculated by subtracting the time spent exploring the non-goal object from the goal object, divided by the total time exploring both objects.
BrdU Pulse
Animals at P43 from each of the groups studied were injected with 50mg/kg of bromodeoxyuridine (BrdU) intraperitoneally and sacrificed after four hours. Animals were briefly anesthetized with isoflurane then perfused with intracardiac injection of ice-cold phosphate buffered saline followed by 4% paraformaldehyde (PFA). Brains were dissected and fixed overnight in 4% PFA then placed in 30% sucrose until they sunk. Sixty-micron thick sections were obtained on a freezing microtome and collected serially in a 12 well plate. A single well representing 1/12 of the brain was taken for each animal and stained as floating sections using a rat anti-BrdU primary antibody (Invitrogen #MA1_82088) after exposing tissue to 30min of 2N hydrochloric acid at 38° C, then 0.5N boric acid at room temperature for 30 min. Alexa Fluor goat anti-rat 594 (Fisher A11007) was used as the secondary. Images were obtained on an upright Nikon epi-fluorescent microscope (Eclipse 80i) with a 10x objective using Stereo Investigator software (MBF biosciences). Images of each hippocampal section were acquired using consistent exposure times for each round of immunostaining. Images were exported to Image J, and the counting tool was used to count positive cells after the dentate gyrus area was defined. The person acquiring the images and analyzing the images was blinded to the groups.
qRT-PCR
Animals at P21 (weaning) and at P43 (beginning of behavior experiments) were sacrificed and brains were immediately dissected into cortex and hippocampus and homogenized with 500uL pipette in 250uL Trizol (Invitrogen). Total RNA was extracted with chloroform according to manufacturer’s instructions. Concentrations were measured by nanodrop spectrophotometer (ND1000), then samples were treated with DNAse (Invitrogen), and reverse transcriptase reaction was performed (High capacity cDNA RT Kit, Life Technologies). TaqMan brain derived neurotrophic factor (BDNF) (Rn02531967_s1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probes (Rn01775763_g1) were utilized for qPCR reaction with TaqMan Universal PCR master-mix (Applied Biosystems) on a Step-One Plus (Applied biosystems) real time PCR machine. Samples were run in duplicate with no-RT and no-sample (water) controls. CT thresholds were manually set to the same values across plates, ensuring that they were in the exponential amplification phase. Biologic replicates that did not reach the CT threshold by cycle 35 were excluded (n=1). Data was analyzed using the delta-delta CT method13 with GAPDH as the housekeeping gene and median sedentary control CT value as the refence point. Data displayed as the relative fold change on a log axis, with geometric means and 95% confidence intervals calculated.
Statistical Analysis
GraphPad Prism 7 statistical software (GraphPad Software Inc., San Diego, CA) was used for statistical analyses. Prior to intergroup testing, data was subjected to D’Agostino and Pearson normality testing. If the data was normally distributed, then parametric tests were performed, and if not, non-parametric tests were used. For all analyses, alpha was set at 0.05 using two tailed tests.
For the comparison of daily exercise, an unpaired Mann-Whitney test was performed. For the Barnes Maze three separate analyses were completed. First, learning was assessed with a two-way repeated measures ANOVA to analyze the effect of daily training vs group. Second, quadrant analysis employed one-sample T-test, asking if the time spent in the goal quadrant was significantly different from chance (0.25). Groups that met this criterion were compared with a one-way ANOVA. Third, the probe trial was analyzed by comparing the time spent at the goal hole to positions relative to the goal using Dunnett’s multiple comparison test with adjusted p values.
The analysis of the recognition memory task used the discrimination index to determine if animals were spending more time with the goal object than chance (theoretical mean=0). This dataset was not normally distributed, so Wilcoxon ranked test was used with a theoretical mean=0.
BrdU incorporation was analyzed using a two-way ANOVA to determine if there was an effect of exercise or isoflurane exposure. Tukey’s post-hoc analysis was used to compare between groups.
The qRT-PCR experiments were subjected to an unpaired Mann-Whitney for the P21 cohort, which had only two groups. The P43 cohort with four groups were tested with a two-way ANOVA to detect an effect of exercise or isoflurane exposure.
Given that these experiments had not been done we did not perform a power analysis a priori, but rather we based our sample size on previous experience with these assays and the literature11,12,14. We purposefully weighted the groups heavily for the isoflurane exposure because previously significant mortality would occur at the time of anesthesia. Additionally, we weighted the exercise cohort over the sedentary cohort because we were unsure how consistently the animals would exercise. In the end, we did not eliminate animals for low daily exercise. Finally, we were constrained by physical space required within our vivarium for these specially designed cages and could not accommodate larger cohorts.
Results
Isoflurane causes a spatial memory deficit that is rescued with voluntary exercise
Animals voluntarily ran in the exercise wheels with an average distance of 1.87 km and 2.22 km in the control and isoflurane exposed groups respectively (Fig1) (p=0.125). Over the course of the four-day learning acquisition phase, the animals from all groups showed a decrease in the latency to find the goal in the Barnes maze demonstrating learning is intact in all groups (Fig2a) (two-way repeated measures ANOVA, F (3,117) =10.2 p<0.001; but no effect by group or interaction). The probe trial (Fig2b), performed one week after completion of the learning phase is used to assess long-term memory of the target location and was analyzed by the time spent in the target quadrant. Iso-sedentary animals spent no more time than chance in the goal quadrant (one sample T-Test p= 0.524) while all other groups were able to discriminate the goal (pCon-sedentary =0.004; pCon-exercise =0.012; pIso-exercise=0.033). Subsequent comparison of the 3 groups that did demonstrate memory of the goal revealed no difference in time spent searching in that quadrant (ANOVA F(2,29)=0.065 p=0.937) and suggesting voluntary exercise can rescue the deficit that occurs after early exposure to isoflurane. Additional within group analysis was then performed comparing time at the goal to all other holes (Fig. 2c). The Iso-sedentary group spent no more time at the goal than any other hole while all other groups did.
Figure 2. Spatial memory acquisition and recall with the Barnes Maze (P43).
A. Animals were trained during the acquisition of the Barnes maze task to find the escape box and escape latency was measured over the four separate trials on consecutive days. Two-way repeated measures ANOVA showed a significant decrease in latency over the four days (F (3,117) =10.2 p<0.001) but there was not a significant difference among groups. (Con/Sedentary n=9, Con/Exercise n=11, Iso/Sedentary n=10, Iso/Exercise n=14). B. Quadrant analysis of probe trial, 7 days after the last training session, showed all groups except the Iso/Sedentary animals spent significantly more time at the goal quadrant than chance (two-tailed one sample T-test, chance=25%). C. No significant difference in time exploring holes for Iso/Sedentary animals between the goal hole and the non-goal holes by Dunnett’s multiple comparisons test (p values adjusted for multiple comparisons). Con/Sedentary and Con/Exercise showed significant discrimination in the holes explored with holes +/− 4,8,9,10 and +/− 3,4,5,6,7,8,10 respectively. Exercise rescued the spatial memory in the Iso/Exercise group with ten significantly different holes. Error bars represent standard deviation. *p<0.05, **p<0.01, ***p<0.001
No Recognition Memory deficit following early life anesthesia exposure.
In contrast to previous studies from our lab, we found no evidence of a deficit in recognition memory testing in the Iso-sedentary animals from two separate cohorts which are shown pooled. There was no cognitive deficit detectable in any of the groups for any of the tests (Novel Object, Object Place, Allocentric Object Place and Social recognition) which is different from previous results12 as every group had a discrimination index >0 indicating correct discrimination (Fig3). In addition, there was no significant improvement with exercise in either group when we performed an analysis based on correlation of distance and discrimination index (not shown).
Figure 3. Recognition memory preserved after early life isoflurane exposure.
A–C. Recognition memory tasks testing specific domains of recognition memory with increasing difficulty from Novel Object Recognition, Object Place Recognition to Allocentric Object Place Recognition (Con/Sedentary n=16, Con/Exercise n=23, Iso/Sedentary n=23, Iso/Exercise n=30). Displayed is the discrimination index for individual animals which is the time spent investigating the goal object minus the time investigating the non-goal object, divided by the total time exploring both objects. A two-way non-parametric test (Wilcoxon Signed Rank) was performed for each group which found a significant difference from chance (zero) for all groups in the three variations of recognition memory tested. D. Social recognition was tested with juvenile male rats as targets. Like the other recognition tasks, all groups had a significant difference from chance (Wilcoxon Signed Rank). Solid bar represents mean value. Error bars represent standard deviation. *p<0.05, **p<0.01, ***p<0.001
Isoflurane spatial memory deficit is not present at P112
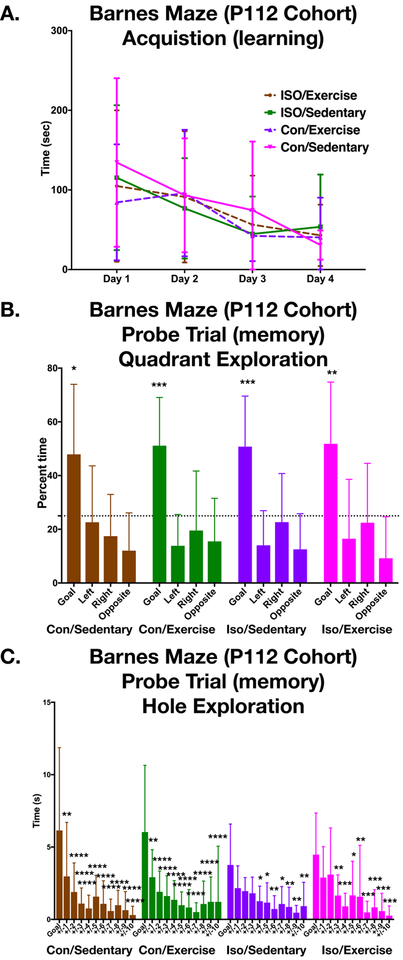
A single cohort that was exposed to the exercise/sedentary paradigm and completed the recognition memory battery, underwent Barnes maze training at P112. Like the P43 cohorts, these older animals learned the location of the goal hole by training day 4 (two-way repeated measures ANOVA: F (3,138) =12.27 p<0.001, but no effect by group or interaction) (Fig4a). In contrast to animals tested at P43 all groups in this P112 cohort successfully discriminated the correct goal quadrant from chance, including the Iso-sedentary group (Fig4b) (one sample t-test pIso-sedentary<0.001; pCon-sedentary =0.016; pCon-exercise <0.001; pIso-exercise=0.001). Subsequent within group analysis of the time spent at each hole reveals strong ability to discriminate even within the goal quadrant among this cohort of older animals (Fig4C).
Figure 4. Barnes Maze acquisition and recall in mature adult (P112 cohort) is preserved.
A. Animals that previously underwent recognition memory battery and were naïve to the Barnes Maze learned the spatial location of the escape box over the course of four consecutive days as evidenced by a decrease in latency (two-way repeated measures ANOVA F (3,138) =12.27 p<0.001) (Con/Sedentary n=11, Con/Exercise n=12, Iso/Sedentary n=12, Iso/Exercise n=13). Like the P43 cohort, there was no difference in learning between groups. B. In the Probe trial, quadrant analysis showed discrimination of the goal with significant time spent in the goal quadrant compared to chance for all groups (two-tailed one sample T-test, chance=25%). C. The Con/Sedentary and the Con/Exercise groups had significant differences between the time spent exploring the goal hole vs every other space +/− from the goal by Dunnett’s multiple comparison test (p values adjusted for multiple comparisons). Different from the P43 cohort, the Iso/Sedentary P112 group spent significant differences in the goal compared to +/−4,5,6,7,8,9,10. Like the P43 cohort, the P112 Iso/Exercise group performed slightly better that than the Iso/sedentary group with differences +/−3,4,5,6,7,8,9,10. Error bars represent standard deviation. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Proliferating cells decrease in the adult hippocampus after early life anesthesia, but increase with voluntary exercise.
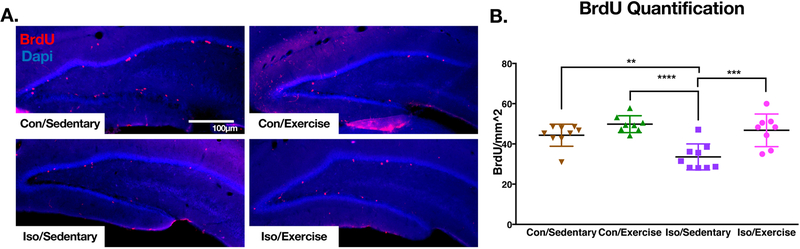
In order to explore the effect of early life isoflurane exposure on proliferating cells in the adult brain we performed a 3hr. BrdU pulse in a separate cohort of animals after 3 weeks of exercise and just prior to the start of the behavior testing protocol. Two-way ANOVA showed an effect for both isoflurane exposure and exercise, but no interaction (F ISO(1,30) =10.52, p=0.003; F Exercise(1,30) =19.25, p<0.001, F Interaction(1,30) =3.26, p=0.08). Comparing groups post-hoc, we found that isoflurane exposure on P7 led to a decrease in BrdU incorporation on P43 in the hippocampus compared to sedentary controls (Fig5a/b) (Tukey p=0.005). Exercise increased the BrdU incorporation of the Iso-exercise group relative to the Iso-sedentary group (Tukey p<0.001) and was not different from Con-Sedentary animals (Tukey p=0.850).
Figure 5. BrdU labelling at P43 in perinatally isoflurane exposed animals is decreased in sedentary animals but is increased with exercise.
A. Representative immunohistochemistry processed images from the hippocampus of P43 animals after a 3hr. BrdU pulse. B. Quantification reveals significant differences between Iso/Sedentary animals and every other group by Tukey’s multiple comparisons suggesting early life isoflurane exposure reduces proliferating cells in the hippocampus, but can be reversed with exercise in adulthood (p values adjusted for multiple comparisons). (Con/Sedentary n=8, Con/Exercise n=9, Iso/Sedentary n=8, Iso/Exercise n=9). Solid bars represent mean and error bars represent standard deviation. **p<0.01, ***p<0.001, ****p<0.0001
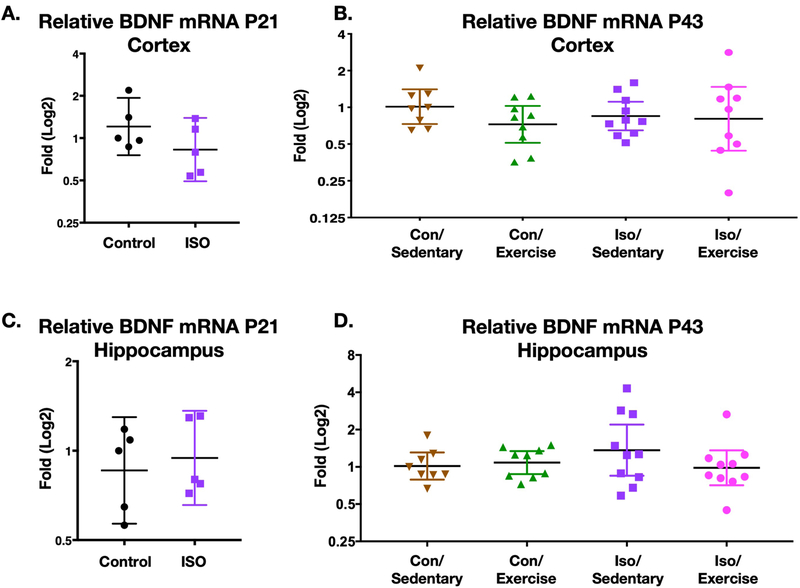
BDNF mRNA is not upregulated after exercise and is not affected by early life anesthesia in the hippocampus and cortex at P21 and P43.
The increase in neural precursor proliferation with exercise has been shown in other contexts to be linked to upregulation of BDNF15,16 which also has implications for learning and memory. To better understand the effect of exercise on spatial memory in our model we quantified mRNA levels of BDNF at P21 which was the time of weaning (Fig6a). There were no significant differences BDNF message between isoflurane exposed and non-exposed animals in either the cortex or hippocampus (Mann-Whitney pcortex =0.222 p hippocampus =0.547). Even following three weeks of exercise, there was no significant effect of exercise or isoflurane exposure on BDNF expression (two-way ANOVA; Cortex: F Exercise(1,32) =0.236 p=0.631; F Iso(1,32) =0.038 p=0.847; F Interaction(1,32) =1.543 p=0.223; Hippocampus: F Exercise (1,33) =1.186 p=0.284; F Iso(1,33) =1.46 p=0.236; F Interaction (1,33) =1.78, p=0.191) (Fig6b).
Figure 6. BDNF mRNA levels in the cortex and hippocampus of animals after perinatal isoflurane exposure.
A./C. P21 (time of weaning) relative levels of BDNF mRNA in cortex and hippocampus respectively, displayed as fold change. Neither brain region was significantly different (Cortex (n=5) p=0.222, Hippocampus (n=5) p=0.547, Unpaired two-tailed Mann-Whitney test). B./D. P43 (3 weeks of +/− exercise) relative levels of BDNF mRNA in cortex and hippocampus respectively. Unlike BrdU incorporation, there was no significant effect of exercise (Con/Sedentary n=8, Con/Exercise n=9, Iso/Sedentary n=10, Iso/Exercise n=10); ; Cortex two-way ANOVA F(1,32) =0.236 p=0.631; Hippocampus two-way ANOVA F (1,33) =1.186 p=0.284.
Geometric mean and 95% confidence intervals displayed.
Discussion
We have previously reported that environmental enrichment can rescue the deficit observed after early life exposure to volatile anesthesia. Here we tested a single component of that enrichment, voluntary exercise, to determine whether it alone can reverse the deficit we reported. The primary finding of this experiment is that in rats a spatial memory task is sensitive to early life anesthesia exposure and can be rescued by voluntary exercise beginning at the time of weaning. Additionally, we show that there is a decrease in proliferating hippocampal cells in adulthood after perinatal anesthesia, which may underlie the memory deficit. Like other reports, we found an increase in proliferating cells in the hippocampus with exercise, but unlike other reports we did not find differential BDNF mRNA levels.
This result is similar to the spatial memory deficit we reported previously in a study on environmental enrichment (EE)5. The EE protocol in the original study was composed of three components - exercise, social housing, and a complex living space. The experiments here isolate one component of that EE, exercise, which alone was able to rescue the deficit. In order to quantify the amount of exercise each animal performed they were housed individually thereby eliminating the social aspect of the original EE experiment. The environment for both exercising and non-exercising groups contains either a wheel or half-wheel and is indeed more complex than a standard cage but comparable between groups. We do not believe that group housing alone is enough to rescue the deficit because we have reported several other experiments where a deficit was seen in animals that were group housed in otherwise non-enriched cages11,17,18. One difference between our previous EE study5 and this study is the volatile anesthetic was different, sevoflurane vs isoflurane, yet the improvement of spatial memory with enrichment is similar between studies.
In contrast to our own previous studies, we did not find a recognition memory deficit after early life isoflurane exposure. There are a number of possible explanations for the differences in recognition test results in our study compared to previous experiments. One possibility is the difference in isoflurane exposure. We had previously exposed animals to 4hrs. of isoflurane at 1 MAC, defined by tail clamping, which resulted in a down titration of isoflurane concentration from 4% to 1.1% over the four hrs.12,14. We experienced significant animal deaths during that anesthetic exposure. In order to counteract this, we attempted to give that same amount of isoflurane but spread out over 6 hrs., in a stepdown fashion. Although this is roughly the same area under the curve, or MAC-hours of exposure in both protocols, it is possible that this longer anesthetic is overall less potent than the previously published 4hr. anesthetic. Alternatively, the differences could also be affected by the social housing of the animals. In our previous studies, the animals were jointly housed, but because of the exercise protocol and to isolate the effect of exercise, all animals were singly housed for these experiments. In other publications social exposure has been linked to differences in behavioral outcomes19.
These studies also raise the possibility that spatial memory is more sensitive to early life anesthesia than recognition memory since we did not find a difference in recognition memory but did find deficits in the Barnes maze probe trial. The Barnes maze may be a more sensitive test compared to the recognition memory battery, or it may take a more substantial insult to induce a long-term deficit in recognition memory as we have previously reported a deficit using a different exposure paradigm12,14. Others have noted that age, training and handling can affect Barnes maze performance20 which might explain differences in performance of the spatial memory tests between the P43 and P112 cohorts. Despite a lack of difference between groups in the quadrant analysis Iso exposed animals seem less able to discriminate between nearby holes in the maze.
How the benefits of exercise are extolled to the brain is the subject of continued study, but the mechanism in rodents includes mediation by BDNF and hippocampal neurogenesis which both solidify and strengthen memories21. Recently, exogenous BDNF has been injected into the hippocampus after early anesthesia and was shown to improve spatial memory22. Exercise in humans has been specifically linked to a positive relationship between BDNF and recognition memory23. In rodents, neurogenesis is doubled following exercise which correlates with improved plasticity and memory8. While recent observational studies in humans have called into question the role of adult hippocampal neurogenesis9,10, there is an overwhelming body of evidence that support the positive effects of exercise. BDNF has been a presumed critical factor in improving learning and memory given the number of studies showing exercise induced upregulation at mRNA and protein levels24–26. Our study found no significant changes in BDNF mRNA, suggesting that there may be non-BDNF dependent pathways critical for strengthening long-term learning and memory. We did not quantify BDNF protein however, and it is possible that this could be discordant with the mRNA results although many studies report an increase in mRNA with exercise. This is an area that could be the subject of future investigation. It is significant that while perinatal anesthesia exposure has a life-long behavioral effect, voluntary exercise is sufficient to rescue this phenotype. This is similar to our previous findings that EE, instituted at the time of weaning, could rescue the deficit5 which argues for EE or exercise alone to be used as a post-exposure treatment.
Limitations of this study include the inherent constraints of the P7 rodent model which includes the immaturity of the animals, the lack of control of ventilation and the difficulty in correlating animal age with human age. However, similar methods have been used in multiple non-human primate studies with behavioral deficits observed which suggests conservation of the mechanism across species and evolution2,27. Here we used male rats exclusively as was done in the EE enrichment study since we have also described sex specific differences in susceptibility to anesthesia at P714, so the conclusions drawn are limited to males. While we have no reason to suspect that a similar beneficial effect of exercise would not be present in females, this hypothesis remains untested.
In summary, we show that early life anesthesia exposure in rodents leads to a spatial memory deficit and a decrease in proliferating cells in the hippocampus in the adult. Voluntary exercise can improve spatial memory function and rescue the loss of hippocampal proliferation. This effect cannot be attributed to BDNF mRNA levels, which in this model appeared unchanged despite daily exercise. Exercise is a simple, inexpensive, low risk intervention that can offset potential deleterious side effects of early life anesthesia exposure and improve spatial memory.
Supplementary Material
Supplementary Figure 1.Anesthetic variables measured A. Isoflurane concentration recorded over 6 hrs. of exposure of P7 rat pups. Step down approach with initial 2 hours kept at 2% Iso, then from 2–4hrs. at 1.4% Iso, and from 4–6 hrs. 0.8% Iso. B. Oxygen concentration in the chamber was kept at approximately 45%. C. Skin Temperature was recorded every fifteen minutes and regulated with a water infused warming pad. After initial temperature drop at anesthesia induction, animals were warmed and kept a constant normothermic temperature. D. Carbon dioxide was monitored within anesthesia chamber. Litholyme absorption pellets were placed in the anesthesia chamber and CO2 levels were maintained around 0.4%. For all graphs, data is from 6 separate isoflurane exposures at P7. Error bars represent standard deviation.
Key Points Summary.
Question: Is exercise sufficient to rescue the cognitive deficit associated with early life anesthesia exposure in rats?
Findings: Exercise can rescue a spatial memory deficit from perinatal isoflurane exposure and is associated with an increase in proliferating cells in the adult hippocampus.
Meaning: While previous work has shown the deficit caused by early life anesthesia can be rescued with environmental enrichment, this study specifically demonstrates that post-weaning voluntary exercise is sufficient to rescue this phenotype.
Acknowledgements
We would like to thank Jason Leong (UC San Francisco, CA, USA) for technical help conducting the behavioral experiments and maintenance of the running wheel cages.
Funding
The work represented here was funded in part by NIH RO1GM112831 (JWS), NIH UCSF-CTSI Grant TL1 TR001871 (JSR) and NIH T32 GM08440 (GAC).
Glossary
- BDNF
brain derived neurotrophic factor
- BrdU
bromodeoxyuridine
- Con
control
- EE
environmental enrichment
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Iso
isoflurane
- MAC
minimum alveolar concentration
- PFA
paraformaldehyde
- P(x)
post-natal day (x)
- qRT-PCR
quantitative real-time polymerase chain reaction
Footnotes
Conflicts of Interest: NONE
References
- 1.Paule MG, Li M, Allen RR, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33(2):2202013230. doi: 10.1016/j.ntt.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123(5):1084–1092. doi: 10.1097/ALN.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stratmann G Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113(5):1170–1179. doi: 10.1213/ANE.0b013e318232066c [DOI] [PubMed] [Google Scholar]
- 4.Sinner B, Becke K, Engelhard K. General anaesthetics and the developing brain: an overview. Anaesthesia. 2014;69(9):1009–1022. doi: 10.1111/anae.12637 [DOI] [PubMed] [Google Scholar]
- 5.Shih J, May LDV, Gonzalez HE, et al. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116(3):586–602. doi: 10.1097/ALN.0b013e318247564d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobilo T, Potter MC, van Praag H. Neurogenesis and Exercise. Encycl Behav Neurosci. 2010:404–409. doi: 10.1016/B978-0-08-045396-5.00239-6 [DOI] [Google Scholar]
- 7.Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/J.NEUROSCIENCE.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldrini M, Fulmore CA, Tartt AN, et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell. 2018;22(4):589–599.e5. doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth. 2019;122(4):490–499. doi: 10.1016/j.bja.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BH, Chan JT, Hazarika O, Vutskits L, Sall JW. Early exposure to volatile anesthetics impairs long-term associative learning and recognition memory. PLoS One. 2014;9(8). doi: 10.1371/journal.pone.0105340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 14.Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology. 2014;83:9–17. doi: 10.1016/j.neuropharm.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/S0166-2236(02)02143-4 [DOI] [PubMed] [Google Scholar]
- 16.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026 [DOI] [PubMed] [Google Scholar]
- 17.Lee BH, Hazarika OD, Quitoriano GR, et al. Effect of combining anesthetics in neonates on long-term cognitive function. Int J Dev Neurosci. 2014;37:87–93. doi: 10.1016/J.IJDEVNEU.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramage TM, Chang FL, Shih J, et al. Distinct long-term neurocognitive outcomes after equipotent sevoflurane or isoflurane anaesthesia in immature rats. Br J Anaesth. 2013;110:i39–i46. doi: 10.1093/bja/aet103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendershott TR, Cronin ME, Langella S, McGuinness PS, Basu AC. Effects of environmental enrichment on anxiety-like behavior, sociability, sensory gating, and spatial learning in male and female C57BL/6J mice. Behav Brain Res. 2016;314:215–225. doi: 10.1016/j.bbr.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld CS, Ferguson SA. Barnes maze testing strategies with small and large rodent models. J Vis Exp. 2014;(84):e51194–e51194. doi: 10.3791/51194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu PZ, Nusslock R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci. 2018;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Bie B, Naguib M. Epigenetic manipulation of brain-derived neurotrophic factor improves memory deficiency induced by neonatal anesthesia in rats. Anesthesiology. 2016;124(3):624–640. doi: 10.1097/ALN.0000000000000981 [DOI] [PubMed] [Google Scholar]
- 23.Whiteman AS, Young DE, Budson AE, Stern CE, Schon K. Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morphometry study. Neuroimage. 2016;126:229–238. doi: 10.1016/j.neuroimage.2015.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x [DOI] [PubMed] [Google Scholar]
- 25.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, Antidepressant Medications, and Enhanced Brain Derived Neurotrophic Factor Expression. Neuropsychopharmacology. 1999;21:679. [DOI] [PubMed] [Google Scholar]
- 26.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266. [DOI] [PubMed] [Google Scholar]
- 27.Coleman K, Robertson ND, Dissen GA, et al. Isoflurane Anesthesia Has Long-term Consequences on Motor and Behavioral Development in Infant Rhesus Macaques. Anesthesiology. 2017;126(1):74–84. doi: 10.1097/ALN.0000000000001383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1.Anesthetic variables measured A. Isoflurane concentration recorded over 6 hrs. of exposure of P7 rat pups. Step down approach with initial 2 hours kept at 2% Iso, then from 2–4hrs. at 1.4% Iso, and from 4–6 hrs. 0.8% Iso. B. Oxygen concentration in the chamber was kept at approximately 45%. C. Skin Temperature was recorded every fifteen minutes and regulated with a water infused warming pad. After initial temperature drop at anesthesia induction, animals were warmed and kept a constant normothermic temperature. D. Carbon dioxide was monitored within anesthesia chamber. Litholyme absorption pellets were placed in the anesthesia chamber and CO2 levels were maintained around 0.4%. For all graphs, data is from 6 separate isoflurane exposures at P7. Error bars represent standard deviation.