Abstract
Single nucleotide polymorphisms (SNPs) in genes involved in mycophenolic acid (MPA) metabolism have been shown to contribute to variable MPA exposure, but their clinical effects are unclear. We aimed to determine if SNPs in key genes in MPA metabolism affect outcomes after lung transplantation. We performed a retrospective cohort study of 275 lung transplant recipients, 228 receiving mycophenolic acid and a control group of 47 receiving azathioprine. Six SNPs known to regulate MPA exposure from the SLCO, UGT and MRP2 families were genotyped. Primary outcome was one-year survival. Secondary outcomes were 3-year survival, nonminimal (≥A2 or B2) acute rejection, and chronic lung allograft dysfunction (CLAD). Statistical analyses included time-to-event Kaplan Meier with log-rank test and Cox regression modeling. We found that SLCO1B3 SNPs rs4149117 and rs7311358 were associated with decreased one-year survival [rs7311358 HR 7.76 (1.37–44.04), p=0.021; rs4149117 HR 7.28 (1.27–41.78), p=0.026], increased risk for nonminimal acute rejection [rs4149117 TT334/T334G: OR 2.01(1.06–3.81), p=0.031; rs7311358 GG699/G699A: OR 2.18(1.13–4.21) p=0.019] and lower survival through three years for MPA patients but not for azathioprine patients. MPA carriers of either SLCO1B3 SNP had shorter survival after CLAD diagnosis (rs4149117 p=0.048, rs7311358 p=0.023). For the MPA patients, Cox regression modelling demonstrated that both SNPs remained independent risk factors for death. We conclude that hypofunctional SNPs in the SLCO1B3 gene are associated with an increased risk for acute rejection and allograft failure in lung transplant recipients treated with MPA.
INTRODUCTION
Lung transplantation has become the definitive treatment for select patients with a variety of end-stage lung diseases. However, compared to other solid organ transplant recipients, lung transplantation portends a shorter life expectancy with a median survival between 5.5–6 years1. Despite advances in surgical technique and medical management, this overall survival has only slightly improved in the past 10 years. The limiting factor in survival continues to be the development of chronic lung allograft dysfunction (CLAD), which include bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS). The current immunosuppression strategy is a three-drug regimen consisting of a calcineurin inhibitor (CNI), an anti-proliferative agent and glucocorticoids. Mycophenolic acid (MPA) has largely replaced azathioprine (AZA) as the anti-proliferative drug of choice with two-thirds of lung recipients receiving as part of maintenance immunosuppression following transplant1. This occurred primarily due to studies demonstrating a decreased incidence of acute rejection and bronchiolitis obliterans as well as a potential survival benefit2, 3. MPA inhibits inosine monophosphate dehydrogenases (IMPDH1 and IMPDH2), which controls the rate limiting step for guanine monophosphate synthesis in the de novo pathway of purine synthesis used to drive lymphocyte proliferation4. It is given in one of two forms: the prodrug mycophenolate mofetil and mycophenolate sodium. Currently, patients receive a predetermined starting dose of mycophenolate mofetil that varies by institution, though usually between 2 to 3 grams per day. However, it is unknown whether variations in mycophenolate metabolism affect outcomes of lung transplant recipients.
Mycophenolic acid dose adjustments are primarily made in reaction to changes in clinical status such as leukopenia or infection with no therapeutic drug monitoring. Although MPA therapeutic drug monitoring is not currently routine in the clinical setting, several studies have evaluated MPA exposure by determining the area under the curve from 0–12 hours (AUC0–12) for non-lung solid organ transplant recipients5, 6. These studies and others have demonstrated that MPA levels vary widely between individuals7. The importance of this variability is underscored by studies in renal and cardiac transplantation that demonstrate an association between lower MPA levels and rejection, indicating a deleterious effect of suboptimal dosing on post-transplant outcomes8–10.
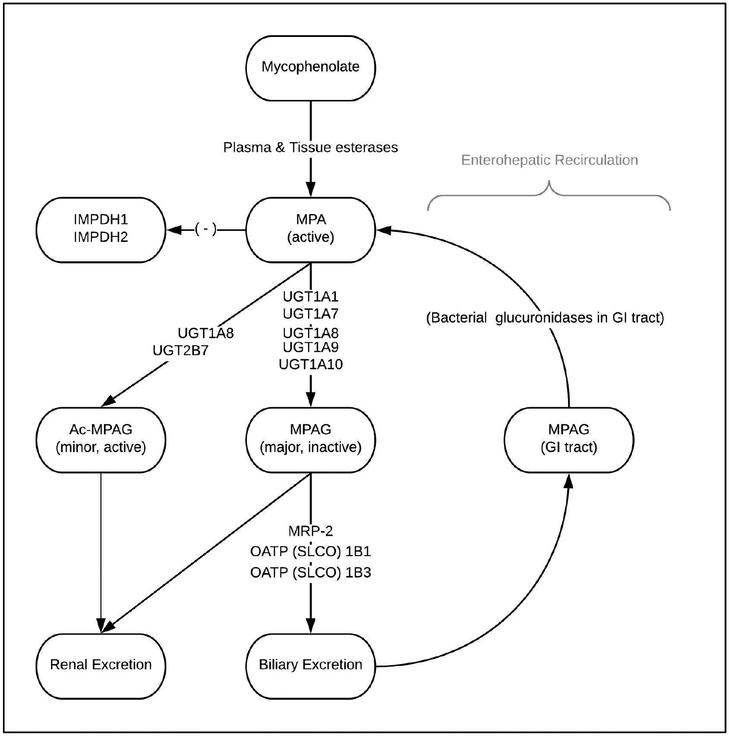
MPA exposure is regulated by two main pathways (Figure 1). Gut esterases initialize metabolism of the prodrug mycophenolate mofetil or mycophenolate sodium into MPA, accounting for approximately 60% of the total MPA accumulation in the peripheral blood. The remaining amount of MPA is converted by the Uridine 5’-diphospho-glucuronosyltransferase (UGT) family of proteins (UGT1A8, 1A9, 1A10, 2B7) into an inactive metabolite, mycophenolic acid glucuronide (MPAG), and a minor metabolite, acyl-mycophenolic acid glucuronide (Ac-MPAG). Ac-MPAG is biologically active11, but is produced in such small quantities that it has a minor impact on overall MPA effect, mainly in relation to adverse drug effects12–14. MPAG is the substrate for the remaining bulk of MPA exposure. This is regulated by MPAG uptake into the liver by the family of solute carrier organic anion (SLCO) transporters, where it is incorporated into the bile, excreted back into the intestines via the multidrug resistance associated protein 2 (MRP2, formerly ATP Binding Cassette Subfamily C Member or ABCC2) and converted back to MPA through metabolism by gut bacteria15. This second-pass enterohepatic recirculation is responsible for up to 40% of overall MPA exposure16–18.
Figure 1:
Metabolic pathway of MPA. The salt mycophenolate mofetil is rapidly hydrolyzed by plasma and tissue esterases into the active form MPA. The UGT superfamily then converts MPA to either AcMPAG, an active minor metabolite, or MPAG, an inactive major metabolite. SLCO1B1/1B3 family of transporters help incorporate MPAG into bile where it is excreted into the intestines and gut bacteria hydrolyze it back to MPA for the enterohepatic circulation. A more detailed explanation of each protein is contained in the text. MPA: mycophenolic acid, UGT: uridine glucuronidasyl transferases, OATP: organic anion transporter protein, SLCO: soluble organic anion transporter, MRP-2: membrane resistance protein-2, IMPDH: inosine monophosphate dehydrogenase.
Several SNPs have been linked to variations in the transport and metabolism of MPA19, 20. In renal transplantation, SNPs in the UGT family and the SLCO family of membrane transporters have been shown to correlate with MPA exposure21–23. In lung transplantation, one study demonstrated an association between hypofunctional SNPs and a decrease in the measured MPA AUC but clinical outcomes were not examined24. A different group found an association between hypofunctional SNPs in the MRP2 gene and persistent acute rejection but did not correlate this with MPA levels, nor with long-term outcomes25. Here, we evaluated how the presence of SNPs leading to functional variants of the UGT, SLCO and ABCC2 proteins impacted clinical outcomes post-lung transplantation.
PATIENTS AND METHODS
Study Design, Patient Selection and Data Collection
We conducted a single-center retrospective cohort study of adult primary lung transplant recipients between 2008 and 2013 who had been previously enrolled in our recipient genetic database. Patients were excluded based on death within 30 days of lung transplantation, multi-organ recipients, age <18years at time of transplantation, transplantation at a different center and subsequent transfer of care, or if they were unable to provide a DNA sample. The cohort was divided into two groups based on the anti-metabolite agent they received for immunosuppression during the first post-transplant year, ‘Mycophenolate’ or ‘Azathioprine’. The primary outcome was survival at one-year post-transplant. Secondary outcomes were development of nonminimal (≥A2 or B2) acute cellular rejection (ACR) or lymphocytic bronchiolitis (LB), development of CLAD and 3-year survival. CLAD was defined, as previously described, as a drop of 20% or more in either forced expiratory volume in one second (FEV1) or Forced Vital Capacity (FVC) measured on two separate occasions at least three weeks apart in the absence of acute rejection or infection26.
Data collected in the retrospective genetic database included recipient and donor demographic variables, transplant surgery details, including ischemic time and use of cardiopulmonary bypass, immunosuppression through the first post-transplant year, episodes of acute rejection and date and cause of death. Data were collected through December 2016 and maintained in a secure REDCap database. Informed consent was obtained from each patient prior to enrollment in the study. The study protocol was approved with waiver of informed consent by the Institutional Review Board of Washington University in Saint Louis (IRB #201105421).
Immunosuppression Regimen
During the study period, all patients were maintained on a 3-drug immunosuppression regimen consisting of a calcineurin inhibitor, an anti-proliferative and a corticosteroid. Induction immunosuppression consisted of a single dose of 20mg of intravenous basiliximab on days 0 and 4 post-transplant and 1 gram of intravenous methylprednisolone given intraoperatively. The calcineurin inhibitor of choice was tacrolimus. Patients were maintained on tacrolimus with a trough goal of 7–10mcg/ml in the initial year following transplantation and 4–7mcg/ml subsequently. Patients with renal dysfunction had a trough goal of 3–5mcg/ml. Patients were started on their anti-proliferative agent on post-operative day 0, with it being administered intravenously until the patient was capable of taking oral medications. The study period spans a time during which the antiproliferative agent of choice transitioned from azathioprine to mycophenolate owing to studies indicating potentially improved outcomes with mycophenolate compared to azathioprine in the late 2000s2, 3. The standard starting dose of mycophenolate mofetil, the anti-proliferative agent of choice at our institution during most of the study period, was 1 gram twice daily, with adjustments made at the discretion of the treating transplant pulmonologist. Corticosteroid dosing was determined according to protocol which consisted of initial dose of 0.5mg/kg of methylprednisolone twice daily x6 doses after which patients were switched to prednisone 0.5mg/kg daily (maximum of 40mg) with a preset taper down to 5mg by six months post-transplant. Immunosuppression changes due to changes in clinical status or adverse drug effects were made at the discretion of the treating transplant pulmonologist.
DNA Collection and Identification of SNPs
Saliva samples from each lung transplant recipient were collected using the OGR-500 collection kit (DNA Genotek, Ottawa, ON, Canada). The median time to DNA collection was 144 days (range 9–1417 days) after transplantation. Genotyping was accomplished using a TaqMan® single tube genotyping assay using allelic specific primers (Life Technologies, Foster City, CA) for indicated SNPs. Allelic PCR results were analyzed using Taqman Genotyper v1.0.1 (Life Technologies) reported by DNA Genotek and entered into the REDCap database at Washington University.
Statistical Analysis
Baseline demographic and clinical variables are expressed as means with standard deviations or medians with interquartile for normal and nonnormally distributed continuous variables, respectively, and as percentages for categorical variables. Comparison of continuous demographic and clinical variables were made using the student’s t test or Wilcoxon test, depending on the results of tests of normality. Categorical variables were analyzed using the Pearson chi-square test. Survival analysis was conducted using the Kaplan-Meier curve with the log-rank test for equality of survivors. Multivariable survival modelling was performed using a backwards stepwise Cox regression analysis with inclusion of all baseline clinical and demographic variables with p<0.25 or those deemed clinically significant based on prior knowledge. Diagnostics were performed to ensure proportional hazards assumptions were met. Results were considered significant with a two-sided p-value <0.05. All statistical analyses were performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Macintosh, Version 24.0. Armonk, NY.).
RESULTS
Patient Population and SNP Genotypes
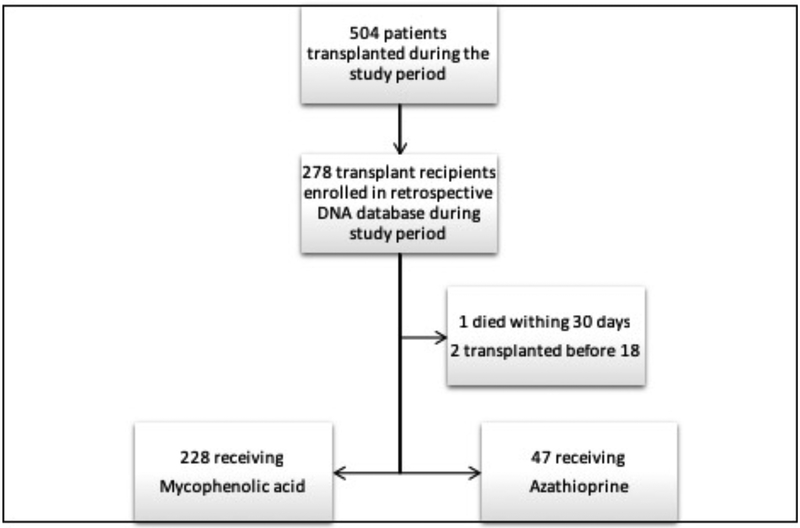
Of the 504 patients transplanted during the study period, 278 patients participated in the DNA database and were eligible for enrollment in this study (Figure 2). 228 (82.0%) of these patients were maintained on MPA through at least the first post-transplant year and 47 (16.9%) were maintained on azathioprine. SNPs in genes with known relevance to MPA metabolism were analyzed, including, UGT1A8, UGT1A9, MRP2, SLCO1B1 and SLCO1B3. The genotype frequencies of these SNPs within our cohort are outlined in Table 1. All SNPs were found to be in Hardy-Weinberg equilibrium with a p-value >0.05 for observed vs. predicted frequencies.
Figure 2:
Flowchart of Patient Selection. During the study period, a total of 504 patients were transplanted. Of those, 278 were captured in the DNA lung transplant database and eligible for enrollment in the study. 3 eligible patients were excluded for reasons indicated.
Table 1:
SNP Genotype Frequencies*
| GENE | SNP | GENOTYPE | FREQUENCIES, n (%) | |
|---|---|---|---|---|
| Mycophenolate | Azathioprine | |||
| UGT1A8 | rs1042597 | |||
| GC | 83 (37.9) | 13 (28.9) | ||
| UGT1A9 | rs2741049 | |||
| CT | 89 (41.8) | 18 (40.9) | ||
| ABCC2 (MRP2) | rs3740066 | |||
| CT | 111 (50.7) | 28 (62.2) | ||
| SLCO1B1 | rs2306283 | |||
| AG | 105 (48.8) | 25 (55.6) | ||
| SLCO1B3 | rs4149117 | |||
| GT | 50 (22.9) | 14 (31.1) | ||
| SLCO1B3 | rs7311358 | |||
| AG | 48 (22.1) | 14 (31.1) | ||
Total n varies due to DNA sample quality
SNPs in the UGT superfamily and MRP2 gene were not significantly associated with primary or secondary endpoints in either the Mycophenolate or Azathioprine group. The two SNPs in the SLCO1B3 gene, rs4149117 and rs7311358, were found to be significant among the Mycophenolate group, but not among the Azathioprine group. Tables 2 and 3 show baseline demographic and clinical variables for these SNPs among the respective groups. For both SNPs, a significantly larger number of patients with the variant allele in the Mycophenolate group were female and non-white. Additionally, for the Mycophenolate group, there was no difference in administered MPA dose between groups at 1, 6- and 12-months post-transplant. SNPs for both rs4149117 and rs7311358 were more common in the azathioprine group than in the mycophenolate group, but this did not reach statistical significance (rs 7311358: 33.3% vs. 24.5%, p=0.167; rs4149117: 33.3% vs. 24.8%, p=0.235)
Table 2:
Demographic and Clinical Factors for Mycophenolate Patient Group
| Genotype | rs4149117 | rs7311358 | |||||
|---|---|---|---|---|---|---|---|
| GG (n=164) | TT/GT (n=54) | p | AA (n=166) | GG/AG (n=51) | p | ||
| Recipient Factors | Age (years, mean ±SD) | 53.2±13.7 | 53.2±12.4 | 0.978 | 53.2±13.7 | 53.4±12.4 | 0.932 |
| Female Gender, n (%) | 61 (37.2) | 28 (51.9) | 0.057 | 62 (37.3) | 27 (52.9) | 0.048 | |
| Non-White | 6(3.7) | 7(13) | 6(3.6) | 6 (11.8) | |||
| Other | 10 (6) | 3(5.6) | 10 (6) | 3 (5.9) | |||
| LAS | 48.4±19.6 | 49.3±20.4 | 0.779 | 48.3±19.5 | 49.9±20.7 | 0.601 | |
| Donor Factors | Age, years (mean ±SD) | 37.0±14.2 | 37.1±16.1 | 0.968 | 36.9±14.2 | 37.1±16.3 | 0.931 |
| Female Gender, n (%) | 59 (36) | 20 (37) | 0.888 | 60 (36.1) | 18 (35.3) | 0.912 | |
| Non-White | 40 (24.4) | 9 (16.7) | 40 (24.1) | 9 (17.6) | |||
| Transplant Factors | |||||||
| Single | 8 (4.8) | 0 (0) | 8 (4.9) | 0 (0) | |||
| Cardiopulmonary Bypass | 81 (49.4) | 33 (61.1) | 0.135 | 81 (48.8) | 32 (62.7) | 0.081 | |
| Ischemic Time, min (mean ± SD) | 275.6±68.1 | 283.6±72.5 | 0.466 | 276.4±68 | 279.2±71.8 | 0.793 | |
| 3 | 8 (5.1) | 1 (1.9) | 8 (5.0) | 1 (2.0) | |||
| Cyclosporine | 5 (3.1) | 0 | 5 (3) | 0 | |||
| Any Acute Rejection at 1 Year, n (%) | 126 (76.8) | 36 (66.7) | 0.138 | 127 (76.5) | 35 (68.6) | 0.258 | |
| Month 12 | 1 (0–3) | 1 (0–2) | 0.345 | 1 (0–3) | 1 (0–3) | 0.549 | |
ILD: Interstitial lung disease, COPD: chronic obstructive pulmonary disease, A1AT: alpha-1 antitrypsin, LAS: lung allocation score, PGD: primary graft dysfunction
Table 3:
Demographic and Clinical Factors for Azathioprine Patient Group*
| rs4149117 | ||||
|---|---|---|---|---|
| Genotype | GG (n=30) | TT/GT (n=15) | p-value | |
| Recipient Factors | Age (years, mean ±SD) | 48.6 ±16.2 | 49.4 ± 13.9 | 0.971 |
| Female Gender, n (%) | 13 (43.3) | 8 (53.3) | 0.526 | |
| 0.041 | ||||
| Non-White | 0 (0) | 2 (13.3) | ||
| 0.701 | ||||
| Other | 4 (13.3) | 1 (6.7) | ||
| LAS, median (IQR) | 43.67 (33.17–50.13) | 38.67 (35.39–48.33) | 0.246 | |
| Donor Factors | Age, years (mean ±SD) | 35.1 ± 14.4 | 33.1 ± 13.2 | 0.782 |
| Female Gender, n (%) | 15 (50) | 5 (33.3) | 0.289 | |
| 0.612 | ||||
| Non-White | 6 (20) | 4 (26.7) | ||
| Transplant Factors | 0.153 | |||
| Single | 0 (0) | 1 (6.7) | ||
| Cardiopulmonary Bypass | 9 (30) | 5 (33.3) | 0.820 | |
| Ischemic Time, min (mean ± SD) | 280.77 ± 66.03 | 282.20 ± 62.96 | 0.754 | |
| 0.205 | ||||
| 3 | 0 (0) | 2 (13.3) | ||
| 0.041 | ||||
| Cyclosporine | 0 (0) | 2 (13.3) | ||
| Any Acute Rejection at 1 Year, n (%) | 20 (66.7) | 8 (53.3) | 0.384 | |
rs4149117 and rs7311358 demonstrated complete linkage disequilibrium in the Azathioprine group, therefore, demographics are only reported for rs4149117.
ILD: Interstitial lung disease, COPD: chronic obstructive pulmonary disease, A1AT: alpha-1 antitrypsin, LAS: lung allocation score, PGD: primary graft dysfunction
Survival
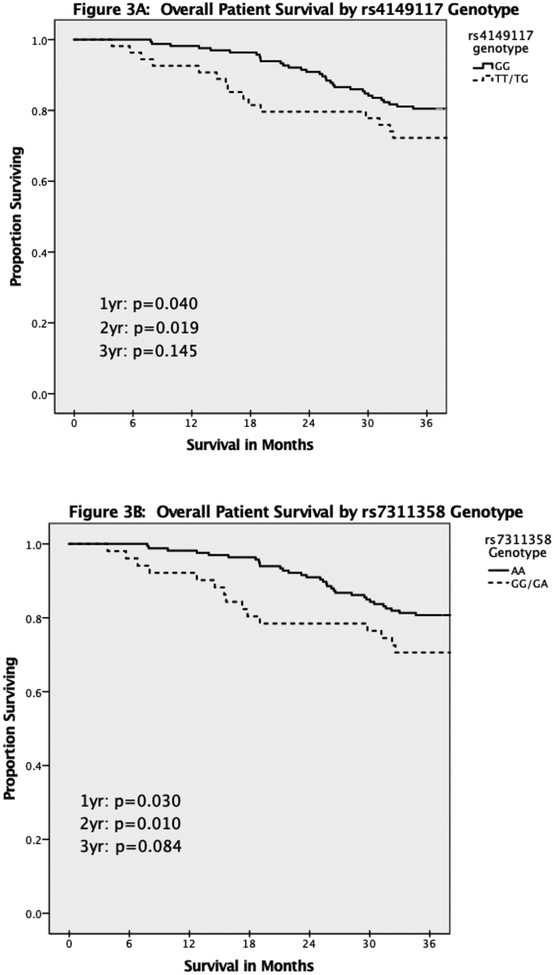
The primary outcome of one-year survival was significantly decreased in Mycophenolate patients carrying at least one copy of the SNP in both rs4149117 (334TT/TG, Figure 3A, p=0.040) and rs7311358 (699GG/GA, figure 3B, p=0.030). This difference persisted through two years, although it was no longer statistically significant at three years for either rs4149117 (p=0.14) or rs7311358 (p=0.08). This difference was not identified in Azathioprine patients (1yr: p=0.163; 2yr: p=0.235; 3yr: p=0.366, K-M curves not shown). For the Mycophenolate group, Cox regression modelling demonstrated that rs4149117 remained an independent risk factor for death through two years, while rs7311358 remained an independent risk factor through three years (Table 4). Additionally, having had no episodes of acute rejection within the first year was protective against death at one year. Finally, use of cyclosporine appeared to be associated with increased risk of death at one year when compared to tacrolimus, although limited sampling size calls for caution in interpreting this result.
Figure 3:
Kaplan Meier survival curves of overall patient survival stratified by genotype. A: Survival based on rs4149117 genotype. B: Survival based on rs7311358 genotype.
Table 4:
Cox Regression Analysis for Risk of Death by Genotype in Patients Receiving Mycophenolic Acid
| rs7311358 | |||||||
|---|---|---|---|---|---|---|---|
| Risk of Death at One Year | Risk of Death at Three Years | ||||||
| Overall Model |
Chi-square 22.082 p=0.002 |
Chi-square 11.298 p=0.080 |
|||||
| Variable | Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| Recipient Gender | 1.74 | 0.30–9.97 | 0.535 | 1.38 | 0.75–2.55 | 0.303 | |
| Recipient Race, Nonwhite | 2.74 | 0.27–27.34 | 0.391 | 2.02 | 0.71–5.75 | 0.190 | |
| Donor Race, Nonwhite | 3.72 | 0.69–20.10 | 0.127 | 1.53 | 0.80–2.90 | 0.197 | |
| Cardiopulmonary Bypass | 0.62 | 0.11–3.42 | 0583 | 0.63 | 0.35–1.14 | 0.129 | |
| No Acute Rejection at 1 year | 0.17 | 0.03–0.90 | 0.037 | -- | -- | -- | |
| rs7311358: GG or GA | 7.76 | 1.37–44.04 | 0.021 | 1.97 | 1.04–3.72 | 0.036 | |
| CNI: Cyclosporine vs. tacrolimus | 12.73 | 0.83–19.49 | 0.068 | 2.41 | 0.56–10.48 | 0.239 | |
| rs4149117 | |||||||
| Risk of Death at One Year | Risk of Death at Three Years | ||||||
| Overall Model |
Chi-square 20.993 p=0.004 |
Chi-square 8.564 p=0.128 |
|||||
| Variable | Hazard Ratio | 95% CI | p-value | Hazard Ratio | 95% CI | p-value | |
| Recipient Gender | 1.63 | 0.29–9.12 | 0.586 | 1.36 | 0.74–2.51 | 0.318 | |
| Recipient Race, Nonwhite | 2.22 | 0.22–22.26 | 0.498 | -- | -- | -- | |
| Donor Race, Nonwhite | 4.08 | 0.75–22.17 | 0.103 | 1.51 | 0.79–2.87 | 0.205 | |
| Cardiopulmonary Bypass | 0.62 | 0.11–3.38 | 0.576 | 0.67 | 0.38–1.20 | 0.181 | |
| No Acute Rejection at 1 year | 0.18 | 0.04–0.96 | 0.045 | -- | -- | -- | |
| rs4149117: TT or TG | 7.28 | 1.27–41.78 | 0.026 | 1.86 | 0.99–3.48 | 0.054 | |
| CNI: Cyclosporine vs. tacrolimus | 12.28 | 0.83–182.69 | 0.069 | 2.23 | 0.53–9.95 | 0.265 | |
Acute Rejection
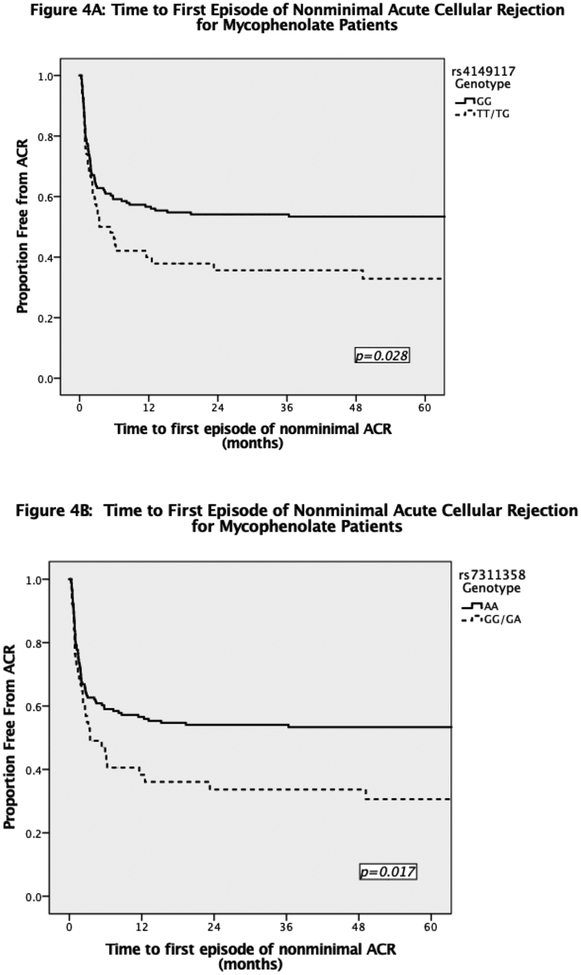
Overall, there was no significant difference in acute cellular rejection in patients in Mycophenolate vs. Azathioprine groups (62% vs. 71%, OR 0.64(0.33–1.24), p=0.183). We did find a significantly increased incidence of nonminimal acute rejection within the first post-transplant year for Mycophenolate patients with at least one copy of either SNP. Patients with the rs4149117 SNP were at a 2-fold increased risk of developing nonminimal acute rejection (OR 2.01, 1.06–3.81, p=0.031). Likewise, patients with the rs7311358 SNP were at similar increased risk (OR 2.18, 1.13–4.21, p=0.019). Azathioprine patients had no significant difference in nonminimal acute rejection for either rs4149117 or rs7311358 genotypes (p=0.429). Additionally, the time to first episode of nonminimal acute rejection was shorter for Mycophenolate group with at least one copy of either rs4149117 or rs7311358 SNPs (Figure 4). It was not significantly different for the Azathioprine group (data not shown).
Figure 4:
Time to First Episode of Nonminimal Acute Cellular Rejection for Patients Receiving Mycophenolate by A) rs4149117 genotype and B) rs7311358 genotype.
CLAD
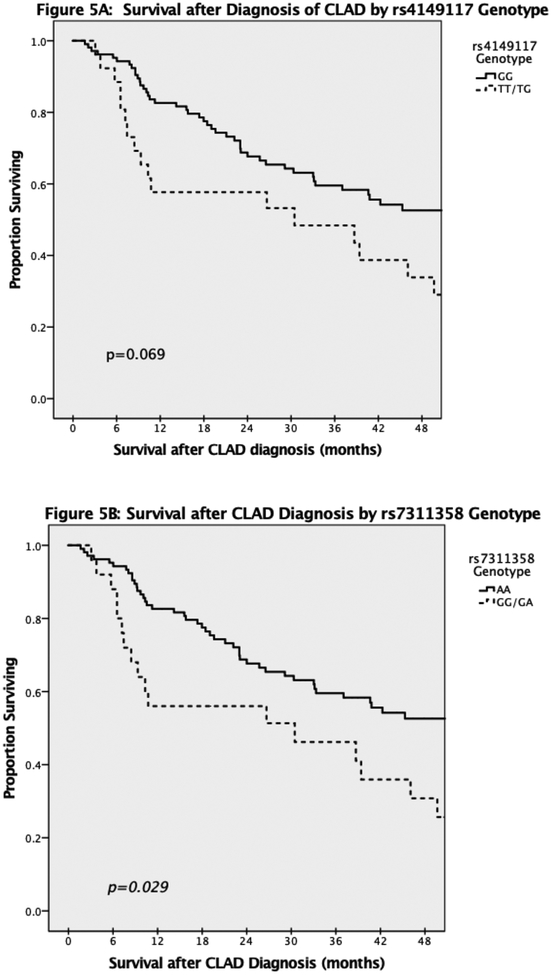
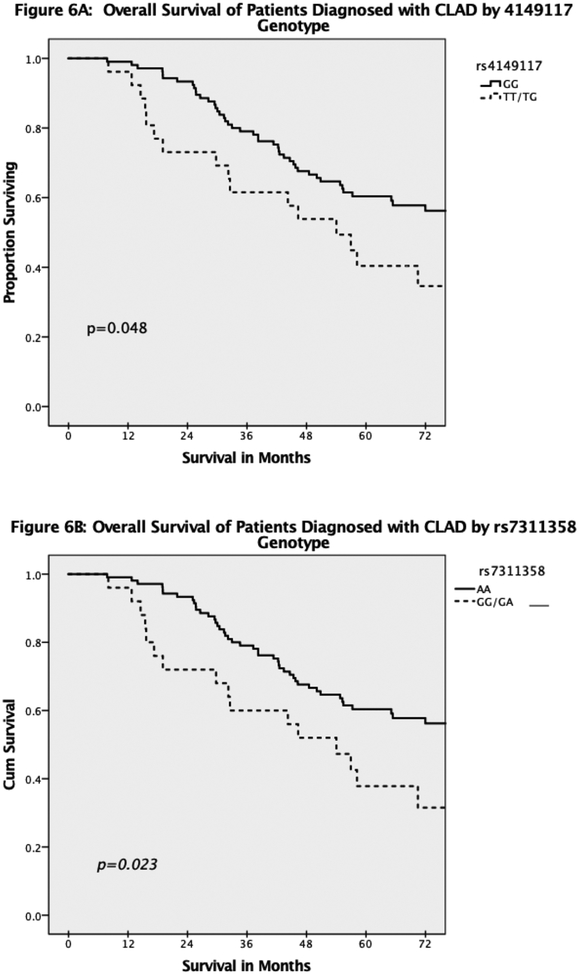
In total, 131 of 228 (57.5%) Mycophenolate patients and 23 of 47 (48.9%) Azathioprine patients developed CLAD during the follow up period. Overall, the median time to diagnosis of CLAD was 30.4 months (IQR 14.9–64.3). Interestingly, Mycophenolate patients without the SNPs had a higher rate of CLAD during the study follow up period for both rs4149117 (64% vs 48.1%, p=0.039) and rs7311358 (63.3% vs 49%, p=0.070). However, of the Mycophenolate patients diagnosed with CLAD, those with at least one copy of either SNP had a shorter survival after diagnosis of CLAD (Figure 5). In addition, of those Mycophenolate patients diagnosed with CLAD at any point, overall post-transplant survival was significantly decreased in patients who carried either of the SNPs (Figure 6). Among Azathioprine patients, there was no difference in either development of CLAD or survival after CLAD based on SNP genotype.
Figure 5:
Survival after Diagnosis of CLAD by genotype. Time 0 along the x-axis indicates time of diagnosis of CLAD and not time of transplantation. A: stratified by rs4149117 genotype. B: stratified by rs7311358 genotype.
Figure 6:
Overall survival of patients diagnosed with CLAD. Time 0 on the x-axis indicates time of transplantation. A: stratified by rs4149117 genotype. B: stratified by rs7311358 genotype
Influence of Calcineurin Inhibitor Use
Given that the type of calcineurin inhibitor used was associated with measured outcomes both in our cohort and that the small sample size precludes subgroup analysis based on calcineurin inhibitor type, we performed sensitivity analyses excluding those patients not maintained on tacrolimus. We found consistent survival outcomes with both SNPs as previously noted. Mycophenolate patients with the SNPs demonstrated decreased survival (Supplemental Figure S1, Supplemental Table S1) and Azathioprine patients with SNPs demonstrated no significant difference in survival when compared to patients without the SNPs. Additionally, we found that the difference in time to first episode of nonminimal ACR remained significant for Mycophenolate patients (Supplemental Figure S2) and insignificant for Azathioprine patients.
DISCUSSION
Our data demonstrate that SNPs in the SLCO1B3 gene are significantly associated with nonminimal acute rejection and graft survival in lung transplant recipients receiving mycophenolic acid, but not azathioprine for immunosuppression. Specifically, we found that patients with the wildtype (334GG and 699AA) genotypes in the SLCO1B3 gene had significantly better graft survival than patients with at least one copy of either SNP (TT334/T334G or GG699/G699A). This was most pronounced during the first post-transplant year but persisted through three years of follow up. The difference in early post-transplant survival has a potentially profound impact on overall survival post-transplant since we know from prior data that while the median survival for lung transplant patients overall is about 6 years, it is significantly longer in patients who survive the first year1.
This study is, to our knowledge, the first to provide evidence of a link between hypofunctional polymorphisms in the SLCO1B3 gene and both acute rejection and graft failure in lung transplant recipients receiving mycophenolic acid. Although it may not be surprising that patients with hypofunctional SNPs are at an increased risk of high grade acute rejection, research into which specific mutations are linked to poor outcomes remains limited25. It has previously been demonstrated that inadequate immunosuppression is independently associated with development of nonminimal acute cellular rejection27, 28. Furthermore, many prior studies have demonstrated that nonminimal acute rejection is associated both with the development of BOS and with graft survival29–33. Therefore, the increased rates of nonminimal acute rejection among patients who carry the SNPs is one plausible explanation for their decreased graft survival.
An interesting finding was that patients who did not carry the SNPs actually had an increased rate of development of CLAD, despite having better survival. One explanation for this finding is that nonminimal acute cellular rejection is usually treated with augmented immunosuppression, i.e. high-dose systemic steroids, increased maintenance immunosuppression and, possibly, antithymocyte globulin therapy. This augmentation in immunosuppression can decrease the risk of further rejection and might explain why patients with nonminimal acute rejection had lower rates of CLAD34–36. Another consideration is that MPA-induced airway damage might depend on SNP-related differences in cellular MPA metabolism. Indeed, a recent study demonstrated that MPA can be toxic and lead to loss of integrity in airway epithelial cells in a dose-dependent manner37. Patients with these hypofunctional SLCO1B3 SNPs may be protected from this MPA-induced airway injury due to lower MPA serum levels. Further investigation regarding the impact of these SNPs on epithelial biology, including recipient versus donor tissues, are needed.
The SLCO1B3 gene is part of a family of soluble organic anion transporters. Its influence on MPA pharmacokinetics has been previously examined in renal transplantation. In a pharmacokinetic analysis of 70 renal transplant recipients receiving MPA with either tacrolimus or sirolimus, Picard et al found that 334GG patients had lower exposure to MPA when compared to TT334 or T334G patients, but similar exposure to MPAG. It was concluded that the 334GG patients had decreased exposure to MPA because of decreased uptake of MPAG and subsequent decreased enterohepatic circulation38. However, MPAG serum levels were no different between the two groups, which makes their conclusions questionable. Conversely, in a study of 87 renal transplant recipients, Miura et al found that patients with the 334GG and 699AA genotype had significantly higher MPA AUC6–12 compared with TT334/GG699 and T334G/G699A patients22. Those 334GG/699AA patients also tended to have higher MPA AUC0–12, although this did not reach statistical significance. This means 334GG/699AA patients had higher measured levels of MPA over the course of 12 hours, leading to an increased exposure to MPA. They concluded that this increased late MPA exposure is indicative of increased MPAG uptake and increased enterohepatic circulation in those with 334GG/699AA. This conclusion is also supported by a study showing that the 334GG and 699AA genotypes were associated with increased SLCO1B3 activity39. Unfortunately, neither of these studies put their findings in context of clinical outcomes. The current study attempts to fill that gap in knowledge by evaluating the clinical consequences of these SNPs. Indeed, our findings that TT334/GG699 and T334G/G699A patients have decreased survival and higher rates of acute rejection are supportive of the aforementioned pharmacokinetic studies demonstrating these patients have decreased exposure to MPA given the same dose as those without the polymorphisms.
The current study has several limitations. The first is the retrospective nature of the study. There is an inherent selection bias for patients who survived long enough to provide DNA samples, sometimes months or even years after transplant. However, this might have served to diminish the observed impact of these SNPs in patients who developed graft failure before enrollment into the study. Additionally, nuanced changes in some clinical variables, such as alterations to immunosuppression agent selection beyond the first year or initiation of azithromycin for attenuation of CLAD, were not available in the database. Although the retrospective nature of the study precluded the collection of and direct correlation with pharmacokinetic data in this cohort, we were able to demonstrate that MPA dose at several time points in the first post-transplant year were not significantly different among the groups ruling out the possibility that the differences we observed were due to actual differences in MPA dose. Finally, the pharmacodynamics of MPA must be considered given the variable activity of its target, inosine monophosphate dehydrogenase (IMPDH). Earlier reports have shown that IMPDH activity varies among transplant recipients40–43 and polymorphisms in IMPDH can alter MPA effectiveness44–48. There is also some evidence that MPA exposure itself can alter IMPDH expression49, 50. We did not account for these pharmacodynamics differences in the current study. Despite these limitations, this study provides new evidence to suggest a critical link between a gene involved in MPA pharmacokinetics and early graft failure.
Overall MPA exposure and effectiveness is likely regulated by the complex interplay of genetic, pharmacokinetic and pharmacodynamic factors. Nevertheless, the current study provides evidence that the interplay of these factors has a significant impact on clinical outcomes. Further study is needed to further elucidate the role of pharmacogenetics in MPA metabolism and translate those findings into better clinical practice, more individualized medication regimens and, hopefully, improved outcomes in lung transplant patients.
Supplementary Material
ACKNOWLEDGEMENTS
L.K. Tague is supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 and by the Washington University Division of Pulmonary and Critical Care Medicine grant T32HL007317–39 from the National Institutes of Health (NIH). H.S. Kulkarni is supported by National Center for Advancing Translational Sciences grant KL2 TR002346 from the NIH. A. E. Gelman is supported by grants from the Barnes Jewish Foundation, R01HL113436–01A1, 2RHL094601, R01HL121218–01 and P01AI116501–01. The content is solely the responsibility of the authors and does not necessarily represent the official view of Washington University, Barnes Jewish Foundation or the NIH.
Footnotes
CONFLICT OF INTEREST
The authors declare no financial conflict of interest.
Supplementary information is available at The Pharmacogenomics Journal’s website.
REFERENCES
- 1.Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016; 35(10): 1170–1184. [DOI] [PubMed] [Google Scholar]
- 2.Speich R, Schneider S, Hofer M, Irani S, Vogt P, Weder W, et al. Mycophenolate mofetil reduces alveolar inflammation, acute rejection and graft loss due to bronchiolitis obliterans syndrome after lung transplantation. Pulm Pharmacol Ther 2010; 23(5): 445–449. [DOI] [PubMed] [Google Scholar]
- 3.Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009; 87(6): 785–794. [DOI] [PubMed] [Google Scholar]
- 4.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs 1996; 51(2): 278–298. [DOI] [PubMed] [Google Scholar]
- 5.Cox VC, Ensom MH. Mycophenolate mofetil for solid organ transplantation: does the evidence support the need for clinical pharmacokinetic monitoring? Ther Drug Monit 2003; 25(2): 137–157. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LM, Nicholls A, Hale M, Armstrong VW, Oellerich M, Yatscoff R, et al. Therapeutic monitoring of mycophenolic acid. A consensus panel report. Clin Biochem 1998; 31(5): 317–322. [DOI] [PubMed] [Google Scholar]
- 7.Langers P, Press RR, Inderson A, Cremers SC, den Hartigh J, Baranski AG, et al. Limited sampling model for advanced mycophenolic acid therapeutic drug monitoring after liver transplantation. Ther Drug Monit 2014; 36(2): 141–147. [DOI] [PubMed] [Google Scholar]
- 8.Sarangi SC, Reeta KH, Agarwal SK, Kaleekal T, Guleria S, Gupta YK. A pilot study on area under curve of mycophenolic acid as a guide for its optimal use in renal transplant recipients. Indian J Med Res 2012; 135: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeNofrio D, Loh E, Kao A, Korecka M, Pickering FW, Craig KA, et al. Mycophenolic acid concentrations are associated with cardiac allograft rejection. J Heart Lung Transplant 2000; 19(11): 1071–1076. [DOI] [PubMed] [Google Scholar]
- 10.Woillard JB, Saint-Marcoux F, Monchaud C, Youdarene R, Pouche L, Marquet P. Mycophenolic mofetil optimized pharmacokinetic modelling, and exposure-effect associations in adult heart transplant recipients. Pharmacol Res 2015; 99: 308–315. [DOI] [PubMed] [Google Scholar]
- 11.Schutz E, Shipkova M, Armstrong VW, Wieland E, Oellerich M. Identification of a pharmacologically active metabolite of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Clin Chem 1999; 45(3): 419–422. [PubMed] [Google Scholar]
- 12.Bernard O, Tojcic J, Journault K, Perusse L, Guillemette C. Influence of nonsynonymous polymorphisms of UGT1A8 and UGT2B7 metabolizing enzymes on the formation of phenolic and acyl glucuronides of mycophenolic acid. Drug Metab Dispos 2006; 34(9): 1539–1545. [DOI] [PubMed] [Google Scholar]
- 13.Wieland E, Shipkova M, Schellhaas U, Schutz E, Niedmann PD, Armstrong VW, et al. Induction of cytokine release by the acyl glucuronide of mycophenolic acid: a link to side effects? Clin Biochem 2000; 33(2): 107–113. [DOI] [PubMed] [Google Scholar]
- 14.Gensburger O, Picard N, Marquet P. Effect of mycophenolate acyl-glucuronide on human recombinant type 2 inosine monophosphate dehydrogenase. Clin Chem 2009; 55(5): 986–993. [DOI] [PubMed] [Google Scholar]
- 15.Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics 2014; 24(1): 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol 2007; 2(1): 184–191. [DOI] [PubMed] [Google Scholar]
- 17.Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34(6): 429–455. [DOI] [PubMed] [Google Scholar]
- 18.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 2007; 46(1): 13–58. [DOI] [PubMed] [Google Scholar]
- 19.Barraclough KA, Lee KJ, Staatz CE. Pharmacogenetic influences on mycophenolate therapy. Pharmacogenomics 2010; 11(3): 369–390. [DOI] [PubMed] [Google Scholar]
- 20.Burckart GJ, Hutchinson IV, Zeevi A. Pharmacogenomics and lung transplantation: clinical implications. Pharmacogenomics J 2006; 6(5): 301–310. [DOI] [PubMed] [Google Scholar]
- 21.Geng F, Jiao Z, Dao YJ, Qiu XY, Ding JJ, Shi XJ, et al. The association of the UGT1A8, SLCO1B3 and ABCC2/ABCG2 genetic polymorphisms with the pharmacokinetics of mycophenolic acid and its phenolic glucuronide metabolite in Chinese individuals. Clin Chim Acta 2012; 413(7–8): 683–690. [DOI] [PubMed] [Google Scholar]
- 22.Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, et al. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol 2007; 63(12): 1161–1169. [DOI] [PubMed] [Google Scholar]
- 23.van Schaik RH, van Agteren M, de Fijter JW, Hartmann A, Schmidt J, Budde K, et al. UGT1A9 −275T>A/−2152C>T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther 2009; 86(3): 319–327. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz J, Herrero MJ, Boso V, Megias JE, Hervas D, Poveda JL, et al. Impact of Single Nucleotide Polymorphisms (SNPs) on Immunosuppressive Therapy in Lung Transplantation. Int J Mol Sci 2015; 16(9): 20168–20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng HX, Zeevi A, McCurry K, Schuetz E, Webber S, Ristich J, et al. The impact of pharmacogenomic factors on acute persistent rejection in adult lung transplant patients. Transpl Immunol 2005; 14(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 26.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant 2014; 33(2): 127–133. [DOI] [PubMed] [Google Scholar]
- 27.Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg 1995; 110(1): 4–13; discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 28.Sanquer S, Amrein C, Grenet D, Guillemain R, Philippe B, Boussaud V, et al. Expression of calcineurin activity after lung transplantation: a 2-year follow-up. PLoS One 2013; 8(3): e59634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002; 21(2): 271–281. [DOI] [PubMed] [Google Scholar]
- 30.Burton CM, Iversen M, Scheike T, Carlsen J, Andersen CB. Is lymphocytic bronchiolitis a marker of acute rejection? An analysis of 2,697 transbronchial biopsies after lung transplantation. J Heart Lung Transplant 2008; 27(10): 1128–1134. [DOI] [PubMed] [Google Scholar]
- 31.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008; 177(9): 1033–1040. [DOI] [PubMed] [Google Scholar]
- 32.Shino MY, Weigt SS, Li N, Derhovanessian A, Sayah DM, Huynh RH, et al. Impact of Allograft Injury Time of Onset on the Development of Chronic Lung Allograft Dysfunction After Lung Transplantation. Am J Transplant 2017; 17(5): 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis WA, Finlen Copeland CA, Todd JL, Snyder LD, Martissa JA, Palmer SM. Spirometrically significant acute rejection increases the risk for BOS and death after lung transplantation. Am J Transplant 2012; 12(3): 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilinger RA, Paradis IL, Dauber JH, Yousem SA, Williams PA, Keenan RJ, et al. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med 1995; 152(6 Pt 1): 2037–2043. [DOI] [PubMed] [Google Scholar]
- 35.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 1999; 159(3): 829–833. [DOI] [PubMed] [Google Scholar]
- 36.Izhakian S, Wasser WG, Fox BD, Vainshelboim B, Reznik JE, Kramer MR. Effectiveness of Rabbit Antithymocyte Globulin in Chronic Lung Allograft Dysfunction. Transplant Proc 2016; 48(6): 2152–2156. [DOI] [PubMed] [Google Scholar]
- 37.Bellon H, Vandermeulen E, Verleden SE, Heigl T, Vriens H, Lammertyn E, et al. The Effect of Immunosuppression on Airway Integrity. Transplantation 2017; 101(12): 2855–2861. [DOI] [PubMed] [Google Scholar]
- 38.Picard N, Yee SW, Woillard JB, Lebranchu Y, Le Meur Y, Giacomini KM, et al. The role of organic anion-transporting polypeptides and their common genetic variants in mycophenolic acid pharmacokinetics. Clin Pharmacol Ther 2010; 87(1): 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 2000; 275(30): 23161–23168. [DOI] [PubMed] [Google Scholar]
- 40.Chiarelli LR, Molinaro M, Libetta C, Tinelli C, Cosmai L, Valentini G, et al. Inosine monophosphate dehydrogenase variability in renal transplant patients on long-term mycophenolate mofetil therapy. Br J Clin Pharmacol 2010; 69(1): 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda T, Goebel J, Thogersen H, Maseck D, Cox S, Logan B, et al. Inosine monophosphate dehydrogenase (IMPDH) activity as a pharmacodynamic biomarker of mycophenolic acid effects in pediatric kidney transplant recipients. J Clin Pharmacol 2011; 51(3): 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weimert NA, Derotte M, Alloway RR, Woodle ES, Vinks AA. Monitoring of inosine monophosphate dehydrogenase activity as a biomarker for mycophenolic acid effect: potential clinical implications. Ther Drug Monit 2007; 29(2): 141–149. [DOI] [PubMed] [Google Scholar]
- 43.Glander P, Hambach P, Braun KP, Fritsche L, Giessing M, Mai I, et al. Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 2004; 4(12): 2045–2051. [DOI] [PubMed] [Google Scholar]
- 44.Sombogaard F, van Schaik RH, Mathot RA, Budde K, van der Werf M, Vulto AG, et al. Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet Genomics 2009; 19(8): 626–634. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Zeevi A, Webber S, Girnita DM, Addonizio L, Selby R, et al. A novel variant L263F in human inosine 5’-monophosphate dehydrogenase 2 is associated with diminished enzyme activity. Pharmacogenet Genomics 2007; 17(4): 283–290. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Yang JW, Zeevi A, Webber SA, Girnita DM, Selby R, et al. IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther 2008; 83(5): 711–717. [DOI] [PubMed] [Google Scholar]
- 47.Winnicki W, Weigel G, Sunder-Plassmann G, Bajari T, Winter B, Herkner H, et al. An inosine 5’-monophosphate dehydrogenase 2 single-nucleotide polymorphism impairs the effect of mycophenolic acid. Pharmacogenomics J 2010; 10(1): 70–76. [DOI] [PubMed] [Google Scholar]
- 48.Gensburger O, Van Schaik RH, Picard N, Le Meur Y, Rousseau A, Woillard JB, et al. Polymorphisms in type I and II inosine monophosphate dehydrogenase genes and association with clinical outcome in patients on mycophenolate mofetil. Pharmacogenet Genomics 2010; 20(9): 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu TY, Fridley BL, Jenkins GD, Batzler A, Wang L, Weinshilboum RM. Mycophenolic acid response biomarkers: a cell line model system-based genome-wide screen. Int Immunopharmacol 2011; 11(8): 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Lee W, Chun S, Um TH, Min WK. Expression of IMPDH mRNA after mycophenolate administration in male volunteers. Biomed Res Int 2014; 2014: 870209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.