Abstract
This study was conducted to monitor the physiological and molecular responses of Catharanthus roseus (rose periwinkle) to multi-walled carbon nanotube (MWCNT) incorporation into the culture medium. The seeds were grown on hormone-free MS medium supplemented with 0, 50, 100, and 150 mgL-1of MWCNT. The supplementations of culture medium with MWCNTs led to significant increases in plant growth indexes such as leaf width, leaf area, leaf fresh weight, root length, and total plant biomass). Slight increases were also observed in chlorophyll a (Chla), Chlb, and carotenoid contents (mean = 18.6%) in MWCNT-treated seedlings. Protein concentrations increased by an average of 34% relative to the control. The application of MWCNT resulted in twofold increases in the catalase and peroxidase activities. A similar trend was also observed in the phenylalanine ammonia lyase activities (by an average of 36.5%), soluble phenols (by 23%), and alkaloids (by 1.7-fold). Moreover, upregulations (mean = 37-fold) in the transcriptions of the DAT gene resulted from the MWCNT supplementations. Exposure to MWCNT improved cell sizes and xylem conducting tissue in treated seedlings. The applications of MWCNTs also stimulated the callus initiation and performance, implying their effects on proliferation and possible differentiation. This study has provided evidence of role MWCNT play in improving plant performance and production of pharmaceutical secondary metabolites.
Keywords: Carbon nanotube, In vitro, Nanoparticle, Secondary metabolites
Introduction
The sterile and controlled environment of plant cell, tissue, organ culture has been widely used to study changes to plant anatomy, physiology, biochemistry, and genetics when the culture media is supplemented with various compounds (Kim et al. 2017; Safari et al. 2017; Asgari-Targhi et al. 2018; Moghanloo et al. 2019a, b). Attempts have been made to optimize culture media by changing the formulations and/or inclusion of highly potent elicitors through which a cellular proliferation, differentiation phenomena, and productions of valuable pharmaceutical secondary metabolites may be modified (Asgari-Targhi et al. 2018; Moghanloo et al. 2019a, b). Besides, it has been shown that nano-based products, owing to their unique physiochemical traits, may interact with a multitude of biological systems and function as an epigenetic factor (Yan et al. 2013; Asgari-Targhi et al. 2018). Taking callus, plant regeneration, and in vitro early seedling performance into account, the differential behaviors of diverse plant species in response to various nano-products have been reported in different plant species, including nano zinc oxide in rice (Salah et al. 2015; Sheteiwy et al. 2016, 2017) and pepper (Iranbakhsh et al. 2018), nano-Ag in Solanum nigrum (Ewais et al. 2015), nano chitosan in pepper (Asgari-Targhi et al. 2018), and nano silicon in Astragalus fridae (Moghanloo et al. 2019a, b). It has been reported that the chitosan nanoparticle displayed the hormone-like activity through which the micropropagations of nodal explants of pepper were accelerated (Asgari-Targhi et al. 2018). Moreover, the nanoparticles may reprogram cellular transcription (Safari et al. 2018). Considering the worldwide high production and numerous industrial functions of carbon nanotubes (CNTs), these nano-products have gained a lot of attention (De Volder et al. 2013). Furthermore, chemical modification and functionalization of CNTs with the carboxylic acid, amine, or other groups are considered as one of the most critical factors contributed to their solubility, dispensability, reactivity, bio-system reactions, and environmental remediation (Manzetti and Gabriel 2019; Seddighinia et al. 2019). Recent work of Joshi et al. (2018) and Seddighinia et al. (2019) strongly confirmed the MWCNT uptake and translocation. The utilization of CNTs as a plant growth regulator to influence diverse biological functions has been demonstrated in seed germination and early seedling performance (Joshi et al. 2018; Seddighinia et al. 2019), cell cycle (Khodakovskaya et al. 2012), growth (Oloumi et al. 2018; Joshi et al. 2018), anatomy (Joshi et al. 2018; Seddighinia et al. 2019), flowering (Seddighinia et al. 2019), nutrition (Joshi et al. 2018; Seddighinia et al. 2019), and gene expression (Yan et al. 2013). Furthermore, CNTs also showed ameliorating roles in various plant species challenged with stress conditions, including salinity in broccoli (Martínez-Ballesta et al. 2016) and Cd in Helianthus annus (Oloumi et al. 2018). On the other hand, exposure to MWCNT was found to provoke toxicity in T87 suspension cells of Arabidopsis as indicated by the reductions in cell viabilities, chlorophyll, and dry mass (Lin et al. 2009). Likewise, in rice, the presence of MWCNTs in the suspension cell cultures caused a reduction in cell viability and the accumulation of reactive oxygen species (Tan et al. 2009). Martínez-Ballesta et al. (2016) provided evidence that MWCNTs influenced plasma membrane characteristics of broccoli cells. Moreover, Yan et al. (2013) provided the molecular evidence of the CNT-associated epigenetic modification in maize seedlings. Additionally, CNTs in Satureja khuzestanica accelerate callus formation and biosynthesis of secondary metabolites (Ghorbanpour and Hadian 2015). Hence, CNTs may be a good candidate as highly potent elicitors used in plant tissue culture, thereby modifying plant growth, primary and secondary metabolism, and protecting plants when exposed to stressful conditions. However, there is a gap of knowledge of the possible roles of CNT applications as a supplement into the rooting culture medium, which needs to be further explored.
Catharanthus roseus (a member of the Apocynaceae family) commonly known as rose periwinkle has attracted a lot of concerns as a potentially important medicinal plant due to possessing unique secondary metabolites with exceptional medicinal characteristics, in particular, anticancer and antioxidant. Hence, it has been widely exploited in pharmaceutical industries to treat a multitude of important human diseases, especially cancer and diabetes (Senbagalakshmi et al. 2017). Moreover, the most efficient anticancer drugs, including vincristine and vinblastine are derived from this plant species. Figure 1 shows the schematic pathway of these terpenoid indole alkaloids in C. roseus. These compounds are originated from catharanthine and vindoline monomers, the mono-terpenoid indole alkaloids present in C. roseus (Wang et al. 2010). Furthermore, the ultimate phase of vindoline biosynthesis is mediated through the catalytic function of deacetylvindoline4-O-acetyltransferase (DAT) (Magnotta et al. 2007).
Fig. 1.
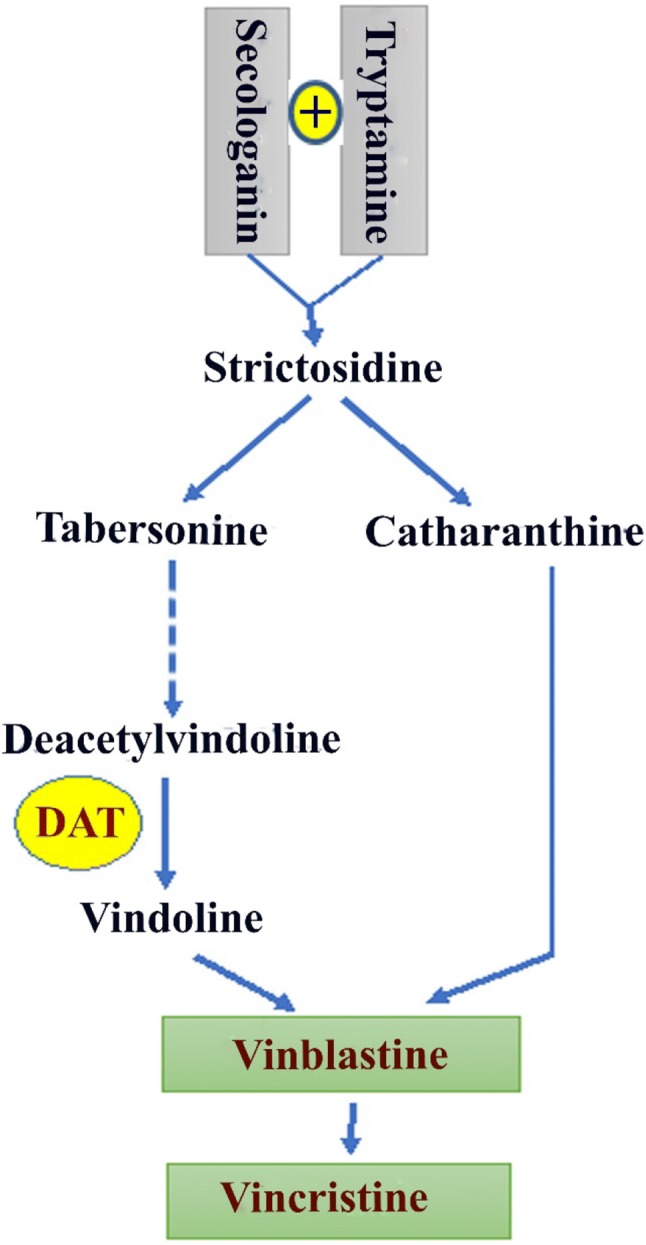
Biosynthesis route of vinblastine and vincristine (the pharmaceutically valuable terpenoid indole alkaloids) in Catharantus roseus. DAT deacetylvindoline-4-O-acetyltransferase
We hypothesize that the application of MWCNTs induces anatomical, physiological and molecular changes, thereby stimulating growth, morphogenesis, metabolism, gene transcription, and callogenesis in the plants cultured in sterile in vitro condition. This study was conducted to monitor responses of C. roseus to MWCNT incorporation into the culture medium. The key objectives of the current research were to learn the MWCNT roles in a dose-dependent manner on (I) growth, morphology, and anatomy traits; (II) photosynthetic pigments; (III) enzymatic antioxidants; (IV) secondary metabolism indexes; (V) transcription of DAT gene, and (VI) callus performance.
Materials and methods
Seeds of C. roseus were purchased from Seed and Plant Improvement Institute in Karaj, Iran. The MWCNT compound was supplied by US Research Nano Materials, Inc (3302 Twig Leaf Lane Houston, TX 77084, USA) with the characteristics, including young’s modulus (GPa): 1200, tensile strength (GPa): 150, density (g/cm3): 2.6, thermal conductivity (W/m k):3000, and electron conductivity (S/m): 105–107.
Seeds of C. roseus were soaked in water for 24 h and were then successively surface-disinfected in several steps using 1.5% sodium hypochlorite for 7 min, 0.4% Benomyl for 3 min, and 70% ethanol for 50-s. The seeds were then thoroughly rinsed three times with sterilized water after each disinfection step. Then, seeds were grown in Petri dishes containing different doses of MWCNT (0, 50, 100, and 150 mg L−1) for 3 days. Thereafter, the germinating seeds were cultured in the hormone-free MS medium (Murashige and Skoog 1962) supplemented with 0, 50, 100, and 150 mg L−1 of MWCNT. The activated charcoal was also applied in the culture medium to reduce the production of the phenolic compounds which can be toxic to plants resulting in their browning. All cultures were incubated under a controlled environment (temperature of 25 °C; photoperiod 16 h and light intensity 40 μ mol photon m−2 s−1). Finally, 60 days-old seedlings were harvested and subjected to the required analysis.
Photosynthetic pigments
The photosynthetic pigments (Chlorophyll a (Chla), Chlorophyll b (Chlb), and carotenoid) were determined according to the equations previously represented by Lichtenthaler and Welburn (1983).
Enzyme extraction, protein concentration, and activities of catalase, and peroxidase
Enzyme extraction from the well-grounded leaves in liquid nitrogen was carried out at 4 °C in an extraction buffer (phosphate buffer (0.1 M; pH of 7.2), containing 0.5 mM ascorbic acid and 0.5 mM Na2-EDTA). The extracts were then centrifuged at 4 °C and the supernatants containing the enzyme extracts were stored at − 80 °C. The protein concentrations were measured based on the Bradford (1976) protocol. Using a spectrophotometer, the peroxidase activity was estimated in the reaction mixture containing phosphate buffer (0.05 M; pH 6.5), H2O2, and guaiacol and the quantification of the absorbance variations at 470 nm per min. The peroxidase activity was expressed in Unit E g−1 fw. Catalase activity was also spectrophotometrically assessed according to Dhindsa et al. (1981) method.
Phenylalanine ammonia lyase (PAL) activity and total soluble phenols
The PAL activity was measured according to Beaudoin-Eagan and Thorpe (1985) method. The activity of this enzyme was calculated based on the standard equation of cinnamate and expressed in μg Cin min−1 g−1 fw. In addition, the concentrations of total soluble phenols were quantified based on the Folin-Ciocalteu method from tannic acid as a standard curve (Asgari-Targhi et al. 2018).
Alkaloid determination
The concentration of total alkaloids was quantified according to the method of Harborne (1973). The homogenized leaves in 10% (v/v) acetic acid in ethanol (stand for at least 4 h) were filtered and the resulted extracts were concentrated in a water bath. The addition of concentrated ammonium hydroxide to the samples in a dropwise manner was performed until the complete precipitation appearance. The sediments were resolved in 0.1 M H2SO4. The maximum absorbance in the range between 270 and 380 nm was determined and the alkaloid level was expressed in ODg−1 fw.
Gene expression
The expression patterns of the DAT gene were monitored in leaves. The leaf samples were stored in − 80 °C. According to the manufacturer’s instructions of Kit, the RNA extractions from the well-grounded leaves in liquid nitrogen were performed. Then, using RT-PCR, the synthesis of cDNAs (complementary DNA) from the extracted RNA was carried out using the specific Kit (YT4500, YektaTajihzAzma, Iran). The forward and reverse primer sequences for DAT target gene and 18S rRNA as a housekeeping gene are displayed in Table 1. The expression of the DAT gene in each treatment group was assessed using the real-time quantitative RT-PCR approach and specific Kit (qPCRBIOSyGreen Mix Lo-ROX). The relative expression rate of the target gene (DAT) was calculated according to the convenient common ΔΔCt method (2−∆∆Ct) and expressed as fold differences (Babajani et al. 2019).
Table 1.
Forward and reverse primer sequences for deacetylvindoline O-acetyltransferase (DAT) and 18S rRNA as housekeeping gene
| Primer name | Sequence (5–3) | Tm |
|---|---|---|
| DAT-F | TCCAACCCCTCAATCCCTCA | 59.35 |
| DAT-R | CAACGGATACGCACGTTTGG | 59.35 |
| 18-F | GGACGTATATTGGCCTCCCG | 61.4 |
| 18-R | GGTCGTTCGGACACCTAGAC | 61.4 |
Histological procedure
To evaluate the anatomical differences, the cross-sections of the basal stem [1 cm above transition zone of root to shoot; fixed in ethanol/glycerol (70:30 v/v)] were made (thickness of 10 µm). The sections were then exposed to sodium hypochlorite for 15 min and acetic acid for 3 min. Finally, these samples were stained with carmine and methylene blue, observed and photographed monitored using a light microscope (Moghanloo et al. 2019a, b; Seddighinia et al. 2019).
Complementary callus experiment
For callus induction, the leaves originated from the 2nd node (from stem base) of 8-week-old sterile seedlings were subjected for preparation of explants. The explants were taken from the middle of the leaf (1 cm) containing midrib. The leaf explants were cultured on the MS medium containing 0.5 mg l−1 BAP and 1 mg l−1 2,4-d hormones. After 45 days later, the calli were harvested, photographed and subjected to the further analyses.
Statistical analysis and experimental design
The experimental design was completely randomized. There were four different treatment groups of MWCNT-treated seedlings with three replicates. The statistical analyses of data (one-way ANOVA) were conducted using SPSS software. All data were represented as mean ± standard error (SE) values of three independent replicates. The significant mean differences among the groups were assessed according to Duncan’s multiple range test at the P ≤ 0.05 level of significance.
Results
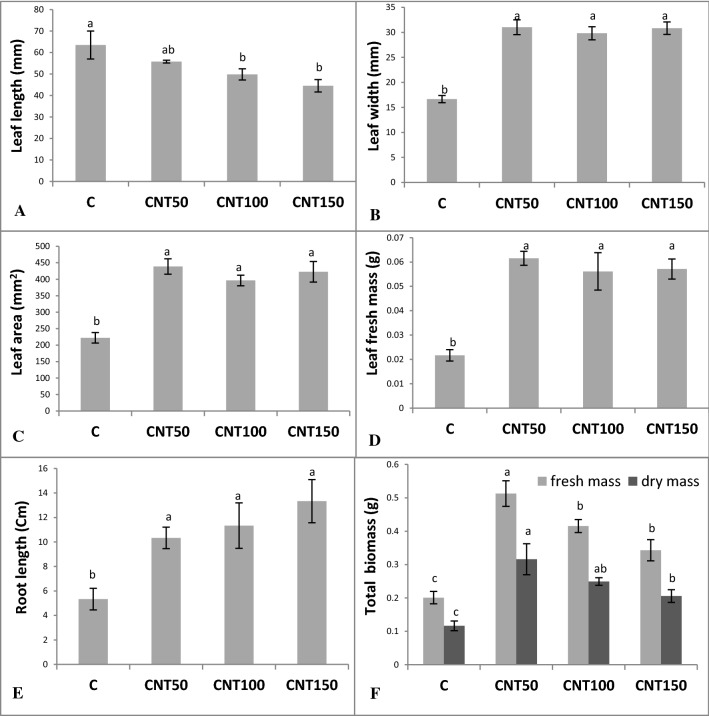
The applications of different concentrations of MWCNT improved seedling early growth and performance (Fig. 2). Leaf characteristics, including leaf length, width, area, and fresh mass were found to be altered in response to the MWCNT treatments (Fig. 3). In comparison to the untreated control, the MWCNT treatments significantly improved the leaf width, area, and fresh mass. While the slight decreases in leaf length were recorded in the MWCNT-treated seedlings (Fig. 3a–d). The supplementations of culture medium with different doses of CNT led to the significant (P ≤ 0.05) increases in root length by approximately twofolds relative to the untreated control (Fig. 3e). The CNT-treated seedlings exhibited significantly higher amounts of total fresh mass by mean twofolds when compared to the control group (Fig. 3f). Similarly, these plants significantly (P ≤ 0.05) possessed higher dry mass amounts (Fig. 3f).
Fig. 2.
The MWCNT-mediated differential growth and morphology in the 60-day old. Catharanthus roseus seedlings grown in the MS medium supplemented with different doses of MWCNTs. a Control; b CNT50; c CNT100; d CNT150
Fig. 3.
The MWCNT-associated changes in growth and biomass accumulations in 60-day old Catharanthus roseus seedlings cultured in MS medium supplemented with different concentrations of MWCNTs
The CNT50 treatment caused a slight increase in Chla content, whereas the two other CNT treatments did not find to make a significant (P ≤ 0.05) change in this trait relative to the control group (Fig. 4a). Except for CNT50, the CNT treatments of 100 and 150 resulted in the augmentations in the Chlb levels by 9.7% and 27.5% respectively (Fig. 4b). The increases in carotenoid contents were also observed in the CNT-supplemented seedlings among which the change made by the CNT50 was not found to be a significant (Fig. 4c).
Fig. 4.
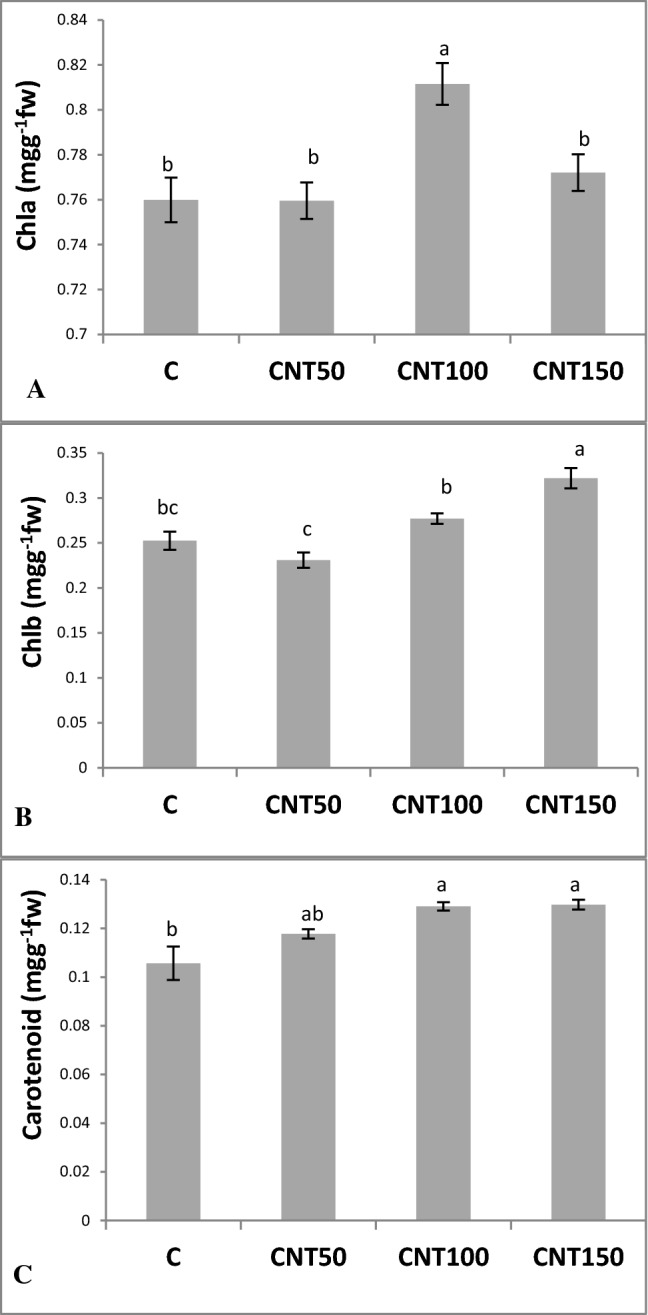
Changes in photosynthetic pigments in 60-day old Catharanthus roseus seedlings following supplementation of MS medium with different doses of MWCNTs
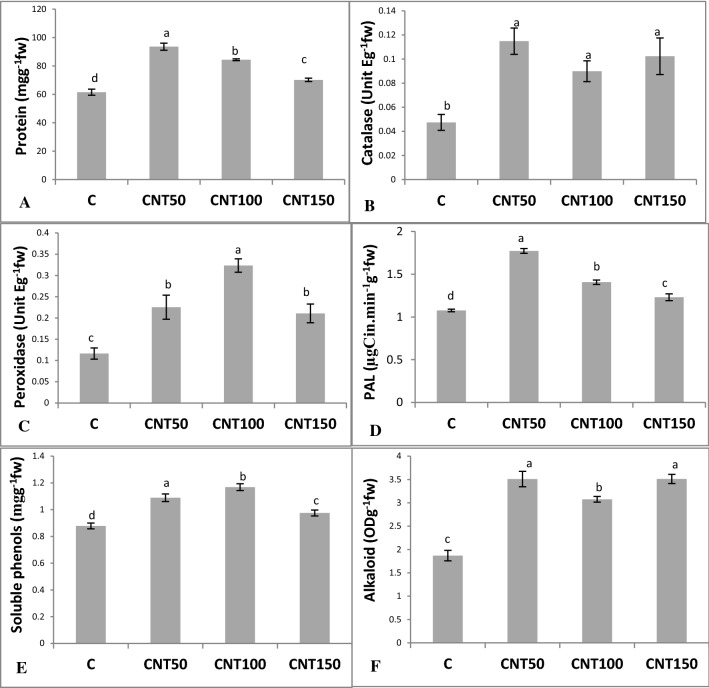
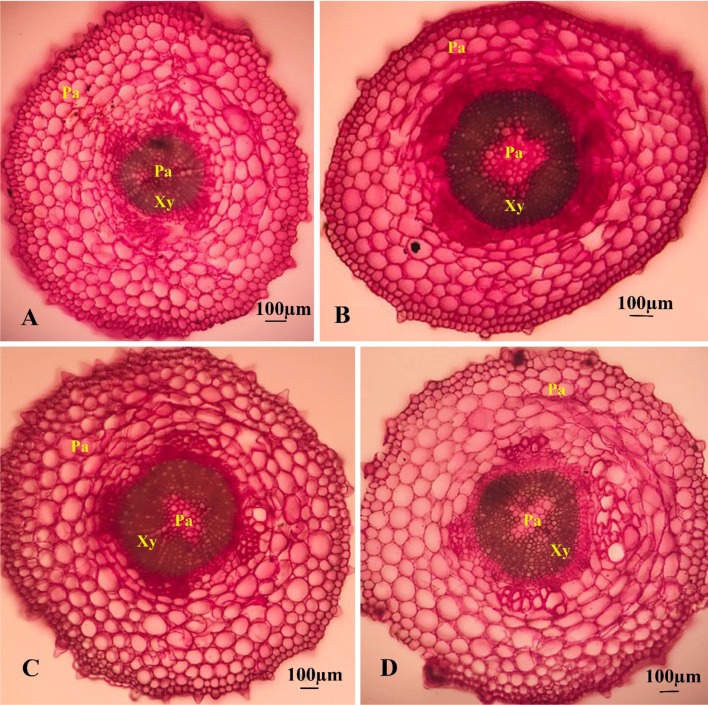
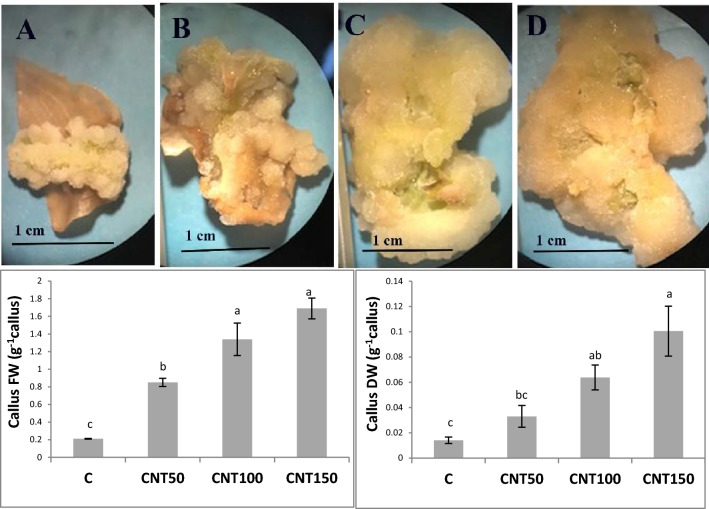
The CNT treatments significantly (P ≤ 0.05) increased concentrations of protein by a mean of 34% (Fig. 5a). These treatments also led to significant twofold induction in the leaf catalase activities relative to the untreated control (Fig. 5b). With a similar trend, the CNT-treated seedlings had higher peroxidase activity in leaves than the control (Fig. 5c). The PAL activities were significantly (P ≤ 0.05) induced by an average of 36.5% (Fig. 5d). Likewise, the utilization of CNTs significantly enhanced the total soluble phenols by a mean of 23.6% over than the control (Fig. 5e). Also, the CNT treatments significantly improved the production of alkaloids when compared to the control (Fig. 5f). Moreover, the CNT applications significantly upregulated the expression of DAT gene (Fig. 6). The cell sizes and the magnitude of xylem conducting tissue were also increased in response to the CNT treatments (Fig. 7). In the complementary experiment, the presence of CNTs in the culture medium stimulated callogenesis process (Fig. 8). The MWCNT treatments also enhanced the callus fresh and dry weights (Fig. 8).
Fig. 5.
Changes to different biochemical traits, including protein content (a), catalase activity (b), peroxidase activity (c), PAL activity (d), total soluble phenols (e), and concentrations of alkaloids (f) in 60 day old Catharanthus roseus in response to supplementations of MS medium with different concentrations of MWCNTs
Fig. 6.
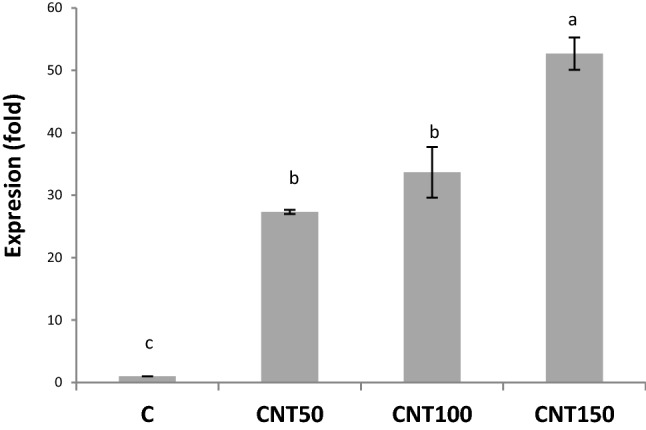
The MWCNT-mediated changes in expression of DAT gene in 60-day old Catharanthus roseus grown in the MS medium supplemented with different doses of MWCNTs
Fig. 7.
The cross sections (10 µm thickness) of basal stems (1 cm above transition zone of root to stem) of the 60-day old Catharanthus roseus seedlings cultured in the hormone-free MS medium containing different concentrations of MWCNT. a Control; b CNT50; c CNT100; d CNT150. Pa parenchyma, Xy xylem
Fig. 8.
The MWCNT-associated modifications in the callus induction, morphology, and biomass. The calli were derived from the 45 days old leaf explants of Catharanthus roseus cultured in the MS medium containing 0.5 mg l−1 BAP and 1 mg l−1 2, 4-d hormones and different doses of MWCNT. a Control; b CNT50; c CNT100; d CNT150
Discussion
The results underline the considerable potencies of MWCNTs as the elicitor/growth-promoting supplements when used under in vitro conditions. The incorporations of MWCNT into the culture medium modified growth, tissue differentiation, anatomy, physiology, biochemistry, and transcription program.
Our findings in C. roseus further substantiate the growth-promoting roles of CNTs which have recently been reported in various other plant species (Khodakovskaya et al. 2012 in tobacco; Zhai et al. 2015 in maize and sorghum; Joshi et al. 2018 in wheat; Seddighinia et al. 2019 in bitter melon). The MWCNT-associated modifications in properties of plasma membranes (Martínez-Ballesta et al. 2016), cellular elongation (Joshi et al. 2018; Seddighinia et al. 2019), cell cycle (Khodakovskaya et al. 2012), root system (Yan et al. 2013; Joshi et al. 2018; Seddighinia et al. 2019), tissue differentiation, especially in xylem and phloem conducting tissues (Seddighinia et al. 2019), nutrition, and water uptake (Lahiani et al. 2013; Martínez-Ballesta et al. 2016), photosynthesis efficiency (Lahiani et al. 2018), transcriptional regulation (Lahiani et al. 2016), epigenetic control (Yan et al. 2013), and metabolism (Hatami et al. 2017) have been illustrated as the underlying mechanisms through which CNTs may improve plant growth, development, and physiology. Moreover, the CNT-mediated up-regulation in SLR1 and RTCS (genes involved in root development) and down-regulations in RTH1 and RTH3 genes (implicated in root-hair development) have been proposed as the casual mechanisms through which CNT treatments in maize seedlings altered root phenotypes (Yan et al. 2013). Furthermore, the growth-promoting effect of MWCNT presence has been attributed to the up-regulation in transcriptions of genes contributed to several critical biological events, including the cell division (CycB), cell wall extension (NtLRX1), and aquaporin (NtPIP1) (Khodakovskaya et al. 2012). Several lines of evidence have confirmed the phytotoxicity of high doses of CNTs (Tan et al. 2009; Begum et al. 2014; Wang et al. 2014). However, in our study, the MWCNT exposure resulted in no detectable phytotoxicity. Hence, further work needs to explore this kind of interaction.
In our study, the induced activities of PAL (a key enzyme in the phenylpropanoid metabolism), higher concentrations of phenolic compounds and alkaloids, and the up-regulated expression of DAT gene (contributed to the synthesis of alkaloids) were recorded in the MWCNT-supplemented seedlings. There are significant correlations among the activities of enzymes involved in secondary metabolism (especially PAL), growth performance, and plant resistance against stress condition (Babajani et al. 2019; Sheteiwy et al. 2019). Moreover, the MWCNT treatments induced the activities of catalase and peroxidase (two main enzymatic antioxidants). Sheteiwy et al. (2019) highlighted the importance of activations in antioxidant machinery and secondary metabolism in response to seed priming. These findings can support this hypothesis that the MWCNT application may associate with the activation of defense system through which plant resistance against the unfavorable condition. These data underline the MWCNT function as a highly potent elicitor. In agreement with our observation, the MWCNT treatments ranging from 25 to 500 μg ml−1 in Satureja khuzestanica (a medicinal plant) were found to act as an efficient elicitor, thereby enhancing secondary metabolism (evaluated based on the concentrations of caffeic acid, rosmarinic acid, flavonoids, and phenolics and PAL activity) and inducing antioxidant machinery as estimated by the activity of peroxidase and polyphenol oxidase (Ghorbanpour and Hadian 2015). Furthermore, as complementary evidence, the MWCNT treatments were found to efficiently modify the callus initiation and performance, implying its effects on the proliferation and differentiation processes. Likewise, incorporating MWCNT at 25 and 50 μg ml−1 into the culture medium stimulated callus performance initiated from the leaf explants of Satureja khuzestanica, whereas the contrary results were observed at 100, 250, and 500 μg ml−1 (Ghorbanpour and Hadian 2015). It seems that MWCNT effect on proliferation and gene transcriptions may be dose-dependent. For instance, low concentrations of MWCNT displayed the growth-promoting role in tobacco cell culture, whereas dramatic declines in the cellular growth were recorded in the samples exposed to the high doses (Khodakovskaya et al. 2012). Furthermore, the SWCNT treatments in maize caused epigenetic modification via inducing the transcriptions of HDA101, HDA102, and HDA106 genes (histone acetyltransferases) and down-regulating HDA108, HD1b and HD2 genes (histone deacetylases) and consequently provoking an augmentation in the global histone acetylation in the genome (Yan et al. 2013). It appears that the incorporations of MWCNTs into the culture medium act as a highly potent elicitors/epigenetic factor influencing plant growth, differentiation, development, metabolism, and gene regulation through modulating the transcription program, epigenetic modifications, and physiological alteration. This hypothesis is supported by recent work of several researchers (for example Lin et al. 2009; Khodakovskaya et al. 2012; Yan et al. 2013; Lahiani et al. 2016; Joshi et al. 2018; Seddighinia et al. 2019).
Conclusion
The convincing evidence was provided on the potential benefits of MWCNT application as a supplement in C. roseus grown under in vitro conditions. This study emphasizes the considerable potencies of MWCNTs as a high potent elicitor or growth-promoting agents. Interestingly, the MWCNT application modified seedling growth, tissue differentiation, anatomy, physiology, antioxidants, secondary metabolism, and transcription program. Moreover, the MWCNT treatments efficiently improved the callus initiation and performance, implying its effects on the proliferation and differentiation processes. It could be concluded that MWCNT (a cost-effective nanomaterial product) can improve plant growth, performance, and production of pharmaceutically valuable secondary metabolites.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
References
- Asgari-Targhi G, Iranbakhsh A, Oraghi Ardebili Z. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol Biochem. 2018;127:393–402. doi: 10.1016/j.plaphy.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Babajani A, Iranbakhsh A, Ardebili ZO, Eslami B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ Sci Poll Res. 2019;26:24430. doi: 10.1007/s11356-019-05676-z. [DOI] [PubMed] [Google Scholar]
- Beaudoin-Eagan LD, Thorpe TA. Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol. 1985;78:438–441. doi: 10.1104/pp.78.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum P, Ikhtiari R, Fugetsu B. Potential impact of multi-walled carbon nanotubes exposure to the seedling stage of selected plant species. Nanomaterials. 2014;4:203–221. doi: 10.3390/nano4020203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. AnalBiochem. 1976;72(1–2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Ewais EA, Desouky SA, Elshazly EH. Evaluation of callus responses of Solanum nigrum L. Exposed to biologically synthesized silver nanoparticles. Nanosci Nanotechnol. 2015;5(3):45–56. [Google Scholar]
- Ghorbanpour M, Hadian J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Saturejakhuzestanica grown in vitro. Carbon. 2015;94:749–759. doi: 10.1016/j.carbon.2015.07.056. [DOI] [Google Scholar]
- Harborne JB. Phytochemical methods. London: Chapman and Hall Ltd.; 1973. pp. 49–188. [Google Scholar]
- Hatami M, Hadigan J, Ghorbanpour M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus nigerduring drought stress simulated by polyethylene glycol. J Hazard Mater. 2017;324:306–320. doi: 10.1016/j.jhazmat.2016.10.064. [DOI] [PubMed] [Google Scholar]
- Iranbakhsh A, Ardebili ZO, Ardebili NO, Ghoranneviss M, Safari N. Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology. Acta Physiol Plant. 2018;40(8):154. doi: 10.1007/s11738-018-2730-8. [DOI] [Google Scholar]
- Joshi A, Kaur S, Dharamvir K, Nayyar H, Verma G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticumaestivum L.) J Sci Food Agric. 2018;98(8):3148–3160. doi: 10.1002/jsfa.8818. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. 2012;6(3):2128–2135. doi: 10.1021/nn204643g. [DOI] [PubMed] [Google Scholar]
- Kim DH, Gopal J, Sivanesan I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv. 2017;7(58):36492–36505. doi: 10.1039/C7RA07025J. [DOI] [Google Scholar]
- Lahiani MH, Dervishi E, Chen J, Nima Z, Gaume A, Biris AS, Khodakovskaya MV. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl MaterInterfaces. 2013;5:7965–7973. doi: 10.1021/am402052x. [DOI] [PubMed] [Google Scholar]
- Lahiani MH, Dervishi E, Ivanov I, Chen J, Khodakovskaya M. Comparative study of plant responses to carbon-based nanomaterials with different morphologies. Nanotechnology. 2016;27:265102. doi: 10.1088/0957-4484/27/26/265102. [DOI] [PubMed] [Google Scholar]
- Lahiani MH, Nima ZA, Villagarcia H, Biris AS, Khodakovskaya MV. Assessment of effects of the long-term exposure of agricultural crops to carbon nanotubes. J Agric Food Chem. 2018;66:6654–6662. doi: 10.1021/acs.jafc.7b01863. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H, Wellburn A. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. BiochemSoc Trans. 1983;603:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- Lin C, Fugetsu B, Su Y, Watari F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J Hazard Mater. 2009;170(2–3):578–583. doi: 10.1016/j.jhazmat.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Magnotta M, Murata J, Chen J, De Luca V. Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochemistry. 2007;68(14):1922–1931. doi: 10.1016/j.phytochem.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Manzetti S, Gabriel JC. Methods for dispersing carbon nanotubes for nanotechnology applications: liquid nanocrystals, suspensions, polyelectrolytes, colloids and organization control. Int Nano Lett. 2019;9(1):31–49. doi: 10.1007/s40089-018-0260-4. [DOI] [Google Scholar]
- Martínez-Ballesta MC, Zapata L, Chalbi N, Carvajal M. Multi-walled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J Nanobiotechnol. 2016;14(1):42. doi: 10.1186/s12951-016-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghanloo M, Iranbakhsh A, Ebadi M, Ardebili ZO. Differential physiology and expression of phenylalanine ammonia lyase (PAL) and universal stress protein (USP) in the endangered species Astragalus fridae following seed priming with cold plasma and manipulation of culture medium with silica nanoparticles. 3 Biotech. 2019;9(7):288. doi: 10.1007/s13205-019-1822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghanloo M, Iranbakhsh A, Ebadi M, Satari TN, Ardebili ZO. Seed priming with cold plasma and supplementation of culture medium with silicon nanoparticle modified growth, physiology, and anatomy in Astragalusfridae as an endangered species. Acta Physiol Plant. 2019;41(4):54. doi: 10.1007/s11738-019-2846-5. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Oloumi H, Mousavi EA, Nejad RM. Multi-wall carbon nanotubes effects on plant seedlings growth and cadmium/lead uptake in vitro. Russ J Plant Physiol. 2018;65(2):260–268. doi: 10.1134/S102144371802019X. [DOI] [Google Scholar]
- Safari N, Iranbakhsh A, Ardebili ZO. Non-thermal plasma modified growth and differentiation process of Capsicum annuum PP805 Godiva in in vitro conditions. Plasma Sci Technol. 2017;19(5):055501. doi: 10.1088/2058-6272/aa57ef. [DOI] [Google Scholar]
- Safari M, Ardebili ZO, Iranbakhsh A. Selenium nano-particle induced alterations in expression patterns of heat shock factor A4A (HSFA4A), and high molecular weight glutenin subunit 1Bx (Glu-1Bx) and enhanced nitrate reductase activity in wheat (Triticum aestivum L.) Acta Physiol Plant. 2018;40(6):117. doi: 10.1007/s11738-018-2694-8. [DOI] [Google Scholar]
- Salah SM, Yajing G, Dongdong C, Jie L, Aamir N, Qijuan H, Weimin H, Mingyu N, Jin H. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L) under nano-ZnO stress. Sci Rep. 2015;5:14278. doi: 10.1038/srep14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddighinia F, Iranbakhsh A, OraghiArdebili Z, NejadSatari T, Soleimanpour S. Seed priming with cold plasma and multi-walled carbon nanotubes modified growth, tissue differentiation, anatomy, and yield in Bitter melon (Momordica charantia) J Plant Growth Regul. 2019 doi: 10.1007/s00344-019-09965-2. [DOI] [Google Scholar]
- Senbagalakshmi P., Rao M. V., Senthil Kumar T. Catharanthus roseus. Cham: Springer International Publishing; 2017. In Vitro Studies, Biosynthesis of Secondary Metabolites and Pharmacological Utility of Catharanthus roseus (L.) G. Don.: A Review; pp. 153–199. [Google Scholar]
- Sheteiwy MS, Fu Y, Hu Q, Nawaz A, Guan Y, Li Z, Huang Y, Hu J. Seed priming with polyethylene glycol induces antioxidative defense and metabolic regulation of rice under nano-ZnO stress. Environ Sci Poll Res. 2016;23(19):19989–20002. doi: 10.1007/s11356-016-7170-7. [DOI] [PubMed] [Google Scholar]
- Sheteiwy MS, Dong Q, An J, Song W, Guan Y, He F, Huang Y, Hu J. Regulation of ZnO nanoparticles-induced physiological and molecular changes by seed priming with humic acid in Oryza sativa seedlings. Plant Growth Regul. 2017;83(1):27–41. doi: 10.1007/s10725-017-0281-4. [DOI] [Google Scholar]
- Sheteiwy MS, An J, Yin M, Jia X, Guan Y, He F, Hu J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma. 2019;256:79–99. doi: 10.1007/s00709-018-1279-0. [DOI] [PubMed] [Google Scholar]
- Tan XM, Lin C, Fugetsu B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon. 2009;47(15):3479–3487. doi: 10.1016/j.carbon.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yuan F, Pan Q, Li M, Wang G, Zhao J, Tang K. Isolation and functional analysis of the Catharanthus roseus deacetylvindoline-4-O-acetyltransferase gene promoter. Plant Cell Rep. 2010;29(2):185–192. doi: 10.1007/s00299-009-0811-2. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu H, Chen J, Tian Y, Shi J, Li D, Guo C, Ma Q. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J Hazard Mater. 2014;274:404–412. doi: 10.1016/j.jhazmat.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhao L, Li H, Zhang Q, Tan J, Huang M, He S, Li L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J Hazard Mater. 2013;246:110–118. doi: 10.1016/j.jhazmat.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Zhai G, Gutowski SM, Walters KS, Yan B, Schnoor JL. Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ Sci Technol. 2015;49:7380–7390. doi: 10.1021/acs.est.5b01145. [DOI] [PubMed] [Google Scholar]