Abstract
Physical plasmas generate unique mixes of reactive oxygen and nitrogen species (RONS or ROS). Only a bit more than a decade ago, these plasmas, operating at body temperature, started to be considered for medical therapy with considerably little mechanistic redox chemistry or biomedical research existing on that topic at that time. Today, a vast body of evidence is available on physical plasma-derived ROS, from their spatiotemporal resolution in the plasma gas phase to sophisticated chemical and biochemical analysis of these species once dissolved in liquids. Data from in silico analysis dissected potential reaction pathways of plasma-derived reactive species with biological membranes, and in vitro and in vivo experiments in cell and animal disease models identified molecular mechanisms and potential therapeutic benefits of physical plasmas. In 2013, the first medical plasma systems entered the European market as class IIa devices and have proven to be a valuable resource in dermatology, especially for supporting the healing of chronic wounds. The first results in cancer patients treated with plasma are promising, too. Due to the many potentials of this blooming new field ahead, there is a need to highlight the main concepts distilled from plasma research in chemistry and biology that serve as a mechanistic link between plasma physics (how and which plasma-derived ROS are produced) and therapy (what is the medical benefit). This inevitably puts cellular membranes in focus, as these are the natural interphase between ROS produced by plasmas and translation of their chemical reactivity into distinct biological responses.
1. Introduction to Cold Physical Plasma
The advancement in medicine could not have been possible without the introduction of innovative technologies from the field of physics to improve the diagnosis and treatment of patients. From radiation therapy to magnetic resonance imaging, these technologies have revolutionised medicine, which allow clinicians to use advanced imaging methods and sophisticated therapies to treat patients. In the last decades, another technology from the physics disciplines has gained visibility: physical plasma. Commonly referred to as the fourth state of matter [1], plasma brings multiple opportunities for patient care that range from cosmetic procedures to clinically relevant pathologies (being the focus of this review) such as wound healing and cancer treatment.
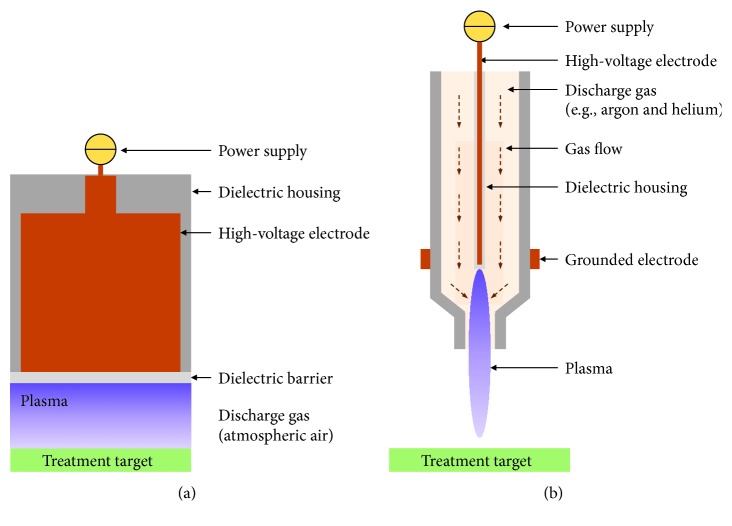
Cold physical plasma, from here on referred to as plasma, is generated by supplying energy to a gas to induce partial ionization. For medical purposes, there are two main principles, despite some sources not falling into the following categories: (i) dielectric barrier discharges (DBD) that are directly operated in ambient air and (ii) plasma jets that ionize a stream of noble or inert gas that subsequently interacts with oxygen and nitrogen of ambient air. DBDs generate plasma in atmospheric air directly onto the treatment target (Figure 1(a)). A high-voltage pulse is applied to an electrode covered with an insulating barrier and brought near the target, which acts as the second electrode. The barrier reduces the current that is passed to the tissue, making the plasma generated in the gap between the electrodes, thermally and electrically safe [2]. The electrodes used for DBD systems could be fabricated for different sizes, making them ideal for large surface treatments. While several plasma jet configurations are available, they operate on the principle that the bulk of the plasma is generated within the plasma device (Figure 1(b)), which is filled with a constant flow of discharge gas or gas mixture (e.g., argon, helium, and nitrogen) [3]. The generated plasma protrudes from the aperture of the device and is brought in contact with the biological target for treatment. The cross-section of this “plasma plume” is on the order of micrometers, which allows for high-precision treatment.
Figure 1.
Schematic of two categories of commonly used plasma devices for medical application: dielectric barrier discharges and plasma jets. In dielectric barrier discharges, plasma is generated in atmospheric air directly onto the biological target (a), while in plasma jets, plasma is generated inside the device and delivered to the target via a flow of gas (b).
Common to both principles is the presence of free electrons and ions, free radicals, and neutral molecules in constant interaction [4]. Plasmas operated at ambient pressure and body temperature are of particular interest in biomedicine. The major biologically active component of plasma is the variety of reactive oxygen and nitrogen species formed upon reaction with molecules (oxygen, nitrogen, and water) present in the ambient air [5–7]. Plasma-derived reactive species can be divided into reactive oxygen species, such as ozone (O3), superoxide (O2•-), singlet delta oxygen (1O2), atomic oxygen (O), hydroxyl radical (•OH), and hydrogen peroxide (H2O2) on the one hand, and reactive nitrogen species, such as nitrogen dioxide radical (•NO2), peroxynitrite (ONOO−), and nitric oxide (•NO) on the other [8–10]. Since all the biologically relevant RNS also contain oxygen, we will use the term ROS in this review to refer to both ROS and RNS.
ROS have been acknowledged as the main active agents responsible for the biological effects of direct and indirect plasma treatments (the latter refers to treating a liquid with plasma that is subsequently transferred to cells or tissues) [6, 11, 12]. Other physical components produced by plasma (UV photons and electromagnetic fields) seem to have a negligible cellular impact on their own [13–15] at the intensities generated with plasmas. However, their ability to exert biological effects in cells during direct plasma treatments should not be overlooked. There is evidence that exposure of cells to low electromagnetic field frequencies can induce transient changes in protein [16] and mRNA levels [17], decrease cell proliferation [18], and increase free radical levels [19]. Further studies on the effect of the physical components of plasma other than ROS are needed to elucidate their specific roles.
An advantage of plasma technology is the ability to exert different biological responses based firstly on the type of ROS delivered and secondly by their quantity. ROS have a crucial role in physiological functions, and they can induce different effects on cells depending on their nature, levels, and localization [20]. In medicine, the potential of ROS is being exploited in therapies in, e.g., dermatology, oncology, and dentistry. Direct plasma treatments benefit from the presence of highly active, short-lived ROS produced during ionization, which present a unique chemical opportunity to modulate the responses in target cells. The success of these therapies will depend on the ability of plasma to induce the desired effect in the target tissue, for which it is necessary to understand the underlying mechanisms of action.
To set the stage for a discussion of the future of plasma in the medical field, we outline the theories proposed to account for the effects of plasma-generated ROS and the corresponding signalling pathways at the cellular level. To understand the mechanistic link between plasma and its therapeutic effect, we will focus on the interactions occurring at the membrane microenvironment and the translation of such events into biological responses. The ultimate goal in plasma medicine should be to identify specific types and quantities of plasma-derived ROS (based on either different plasma sources or different operational settings for one plasma source) for the treatment of a specific pathological condition.
2. Plasma-Derived ROS in Medical Therapy
The spatiotemporal distribution of the ROS output of some plasma sources like the kINPen is exceptionally well characterized [21]. Naturally, more investigations are needed for this and other types of plasma sources, but there is a certain degree of consent on what ROS plasma sources typically generate and how this can be tuned by changing the feed and ambient gas composition. The medical effects of plasma treatment in patients are promising in dermatology and cancer, as briefly outlined below. For a comprehensive overview of other areas of medical application, the reader is referred to a recent text book covering all aspects of plasma medicine [22].
2.1. Dermatology and Skin-Based Infections
Nonhealing wounds are a devastating problem for patients and healthcare systems alike [23]. The increasing incidence of diabetes mellitus as a major ailment for diabetic foot ulcers, as well as the increase in human life expectancy, is likely to magnify this issue [24]. More than a decade ago, it was hypothesized that wound healing is subject to redox control [25–27]. As plasmas emit ROS, it was natural to test their potential effect on nonhealing wounds. Several clinical observations and studies found not only an antimicrobial activity but also a wound healing promoting activity of plasma treatment in acute as well as chronic wounds [28–35] and driveline infections [36]. Using hyperspectral imaging, an increase in wound oxygenation and blood flow was found immediately after plasma treatment [37]. Yet, the efficacy of plasma therapy varies between patients. In general, the evidence level of the majority of clinically relevant wound therapies is low [38]. Part of this problem is a lack of standardization of wound location, size, microbial colonization, and etiology as well as varying treatment procedures prior to hospitalization. Hence, a limited number of randomized clinical trials (RCTs) as well as clinical trials without randomization is reported. Due to the nature of cold physical plasma, blinding the investigators (or patients) is hardly achievable. For the medical product PlasmaDerm (NCT01415622), improved wound healing was reported [39]. For the medical product MicroPlaSter, three nonregistered RCTs showed a reduction in bacterial load and a modest improvement in wound healing [40–43], while no improvement in patients with pruritus was observed [44]. For the same device, one trial on biofilm removal in diabetic ulcers is ongoing (ISRCTN17491903). For pressure ulcers, another unregistered trial reported a reduction in microbial burden and improved wound healing using an argon DBD-based source called P-Jet [45]. To the best of our knowledge, this source has not been accredited as a medical device. For a novel, CE-marked, hand-held, and battery-driven plasma device called PlasmaCare, there is one recruiting interventional trial (ISRCTN98384076) with the primary outcome measure of a reduction of bacterial load as a basis for its prospective accreditation for wound healing. At the VU Medical Center Amsterdam, a phase I study (primary outcome: safety; secondary outcome: antimicrobial activity) using the plasma device for wound healing was recently completed (NCT03007264). A clinical trial on plasma-assisted wound healing after surgical removal of hemorrhoids (NCT03907306) is currently ongoing in the Russian Federation. Two trials to evaluate the efficacy and safety of the RenewalNail device (USA) targeting onychomycosis (fungal nail) were recently concluded (NCT03072550, NCT03216200). Another US-based device, the floating-electrode barrier discharge initially designed at Philadelphia-based Drexel University, is currently being tested by The Skin Center Dermatology Group in New York (NCT02759900) in patients with various skin disorders (actinic keratosis, acne, verruca plana (warts), and tinea corporis (superficial fungal infection)) up to the year 2023. The US-based Apyx Medical (formerly Bovie Medical Corp.) has completed a trial on their plasma device (J-plasma) for safety and effectiveness against facial wrinkles (NCT03286283).
Some of these niche applications are partially supported by clinical observations, for example, the decrease of the severity of atopic [46] and superinfected dermatitis [47] in patients. Future applications may concern treatment or pruritic disorders, leishmaniosis, erythema, fungal infections (especially onychomycosis), impetigo contagiosa, and folliculitis [48–50]. This is supported by numerous preclinical studies suggesting a microbicidal and antifungal action of plasmas, partially tested also on human skin [51–60]. Among the multiple applications of cold physical plasmas is their use in dentistry, where so far only one trial on dental restoration and caries prevention using the miniature atmospheric cold plasma brush (m-ACPB) has been completed (NCT01529606). Altogether, evidence for plasma-assisted wound decontamination and plasma-assisted wound healing based on (R)CTs is improving, although structured reviews are still missing. For other applications in dermatology, including the treatment of (pre)malignancies, RCTs are urgently warranted to increase the evidence level in plasma medical applications. The different plasma devices used across different countries will remain a drawback, each likely similar and dissimilar in several aspects at the same time. Here, basic and applied researches from physics to biology need to address the challenge of categorizing plasma sources and parameters under a unifying umbrella.
2.2. Oncology
Cancer is one of the biggest challenges in the medical field. Solely in 2018, it was responsible for almost 10 million deaths globally [61]. These striking numbers reveal the limitations of current therapy resources to improve overall survival and often also the patient's quality of life. For example, a challenge in the palliation of end-stage head-and-neck cancer patients is the extensive microbial growth on tumors, which produces a hostile odor and hampers social interaction. As these soft tumors are difficult to disinfect chemically, plasma was chosen for this purpose. While the decontamination procedure worked in all patients, tumor regression with plasma treatment was observed in some patients [62–65]. Another benefit was the healing of tumor wounds together with their decontamination with no or negligible side effects [62] and a decrease in the need for pain medication [63, 64]. These clinical results are important because they set the start point for future medical interventions with plasma, not only for palliation, but also for the treatment of less advanced cancers. However, treatment of metastatic lesions of malignant melanoma in end-stage patients with the plasma of the kINPen MED was so far of limited success [66]. Currently, one nonrandomized clinical trial (NCT03218436) in Tübingen, Germany is recruiting patients for the treatment of cervical intraepithelial neoplasia (ovarian cancer) with cold physical plasma.
A recent innovation in plasma oncology is the treatment of carcinoma in situ, e.g., actinic keratosis [67–69]. These dry, crusty, superficial lesions of the skin have a very high prevalence, and a significant percentage of lesions can develop into invasive squamous cell carcinoma over time. Patients with intraoral, precancerous leukoplakia or oral lichen planus lesions face a similar fate. Repetitive plasma treatment over several months successfully reduced and partially even removed these lesions [70]. Hence, plasma treatment may play a future role in the prevention of advanced cancer.
2.3. From Bench to Bedside to Bench
Despite the clinical success of plasma treatment with some diseases, challenges remain. First, how can the rate of nonresponders seen in wound healing and cancer be decreased based on biological mechanisms yet to be identified? Second, how can new applications based on promising in vitro and in vivo research, e.g., treatment of metastatic melanoma, be implemented? Third, which are the promising therapeutic avenues in combining plasma treatment with existing therapies, e.g., immunotherapy in cancers, to maximize clinical outcome? These questions can be addressed in multiple ways, e.g., via tuning the chemistry of existing plasma sources, construction of novel plasma sources, finding the optimal dose and frequency of plasma treatment for each clinical application, and investigating promising combination therapies with plasma that seamlessly merge into existing clinical protocols. Thus, a number of iterations need to be tested in basic research on plasma redox chemistry and biomedicine to motivate and stratify therapeutic strategies in plasma medicine. Yet, while the physics of plasma is reasonably well explored, sufficient understanding in the chemistry and biology of plasma treatment is one current bottleneck in pinpointing best-practice plasma ROS patterns for the most efficient clinical response (Figure 2). Especially cell membranes, the key interface between plasma-derived ROS and cells, have been investigated only poorly so far. With plasma medicine being a field of unparalleled multidisciplinarity from physics and engineering, over chemistry and biology to medicine, the following sections provide the current working hypothesis in the field together with key knowledge gaps that need to be addressed to accelerate progress in this field.
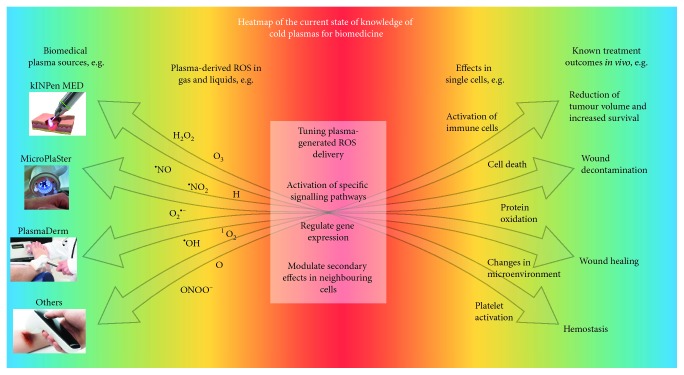
Figure 2.
Heat map of the current state of knowledge of cold plasmas for biomedicine. Blue: known and well-characterized commercial plasma sources (left) and reported effects of plasma therapies in in vivo models and human patients (right). Yellow: many biologically relevant plasma-generated ROS in air or in liquids have been described (left); however, it is still a challenge to tune the setups to deliver specific ROS mixes for different biomedical applications. In the same way, multiple effects of plasma in cells have been reported, yet the mechanisms of action of plasma-generated ROS in cells has not been fully unraveled (right). Red: the current bottleneck in the field is the little information available on how to use plasma to activate specific signalling pathways and evoke a desired effect in cells to design better and more effective therapies.
3. Biological Mechanisms in Cells Exposed to Cold Physical Plasma
A macroscopic view of plasmas in biomedicine reveals multiple positive outcomes in patients treated with this technology. However, a microscopic view of the processes evoked by plasma in cells indicates that multiple mechanisms of action at the cellular and macromolecular levels are involved in exerting such effect, most of them being underexplored. In this section, we will discuss the collection of events that lead to the biological outcome previously described, considering the current state of the field with regard to challenges (Box 1) and opportunities (Box 2). Before discussing observations in plasma medical research, a brief summary of concepts in redox biology is given as a basis for plasma medicine.
Box 1.

Current challenges in the field of plasma medicine.
Box 2.
Current opportunities in the field of plasma medicine.
3.1. Current Concepts in Redox Biology
Oxygen is a chemically aggressive molecule able to cause oxidative modifications in all biomolecules. At the same time, it is needed to preserve life in aerobic species. In order to prevent oxidative damage and maintain homeostasis, cells have developed efficient antioxidant mechanisms to cope with ROS produced by biological processes (i.e., mitochondrial respiration) and external insults (radiation, ionization). The misbalance between the levels of prooxidants and antioxidants in the cell results in oxidative stress, with the consequent accumulation of ROS and oxidative damage to the biomolecules that make up the cell. To prevent detrimental effects, cells are equipped with ROS detoxification mechanisms that can be enzymatic (catalases, peroxidases, and superoxide dismutases) and nonenzymatic (vitamin E, vitamin C, reduced glutathione, β-carotene, etc.). The outcome in redox biology will unequivocally depend on the type of ROS produced over a certain period of time at a specific location [71], as this is directly linked to the location and availability of the detoxification mechanisms to deal with the insult. The amount of ROS is also important, as low concentrations have different effects compared to higher concentrations, a phenomena coined as hormesis.
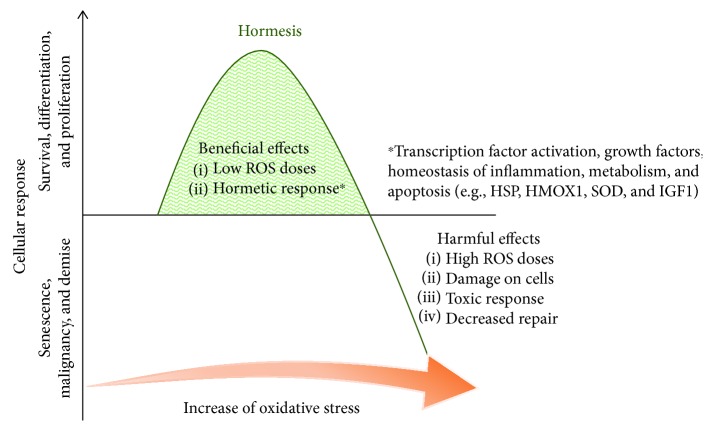
Hormesis describes the biphasic dose response to an agent whereby a stimulatory or beneficial effect is obtained with a low dose and an inhibitory or toxic effect is achieved with a high dose. As an integral process of the normal function of cells, hormesis participates in multiple physiological processes that involve ion channels, enzymes, and transcription factors [72] (Figure 3). Hormesis then could be described as an adaptive response to environmental challenges in order to preserve homeostasis [73]. The biphasic dose response can be caused by multiple stimuli such as toxins, radiation, neurotransmitters, and ROS [74]. In wound healing and cancer, low concentrations of ROS have proproliferative effects, while high concentrations are deleterious [75–77]. Importantly, in both situations, signalling in response to ROS is key in subsequent biological effects.
Figure 3.
Scheme of hormetic responses. In the concept of hormesis, small concentrations of a given substance or molecules (including ROS) can have opposing effects between small and large concentrations.
ROS are constantly and purposefully made in the human body to exert a variety of responses. On the cellular level, ROS are produced to allow the development of oocytes after fertilization [78] and to attract neutrophils to the site of injury to clear pathogens and elicit inflammation [79]. On the molecular level, responses to ROS are related to both redox and phosphorylation signalling with proteins [80]. In the former, oxidases and reductases control disulfide bond formation of thiols, while in the latter, kinases and phosphatases control phosphor residues on target proteins. The binary states activate or inactivate the (binding) activity of proteins, and often both systems act in concert to achieve distinct biological responses. For instance, growth factor binding activates Src family members to phosphorylate peroxiredoxin 1 to render this antioxidant inactive. At the same time, NAPDH oxidase (NOX) is activated to produce superoxide in the extracellular space, which then dismutates to hydrogen peroxide, enters the cell through aquaporins, and reversibly oxidizes target molecules such as protein phosphatases [81]. At the same time, redox proteins also act as sensors of ROS. For example, upon ROS exposure, thioredoxin reversibly releases the apoptosis signal-regulated kinases (ASK1) to induce subsequent pathways for cell death [82].
With the exception of supraphysiological concentrations of ROS leading to immediate necrosis, ROS-mediated cell death is a form of regulated cell death as per consensus guideline [83]. This also delineates a link between ROS and a plethora of cell death pathways, including intrinsic apoptosis, ferroptosis, NETosis, lysosome-dependent cell death, mitochondrial pore transition-driven necrosis, parthanatos, necroptosis, and autophagy, largely because of the ROS' intrinsic and pleiotropic roles in metabolism, mitochondrial homeostasis, inflammation, and immunity. Importantly, not all types of cells can undergo all types of cell death. For instance, several tumor cell types are incapable of undergoing necroptosis [84], NETosis is primarily observed in myeloid cells [85], and oxycytosis is performed by red blood cells [86]. Attributing ROS- (and hence, plasma-) induced cell death to a certain modality is made complicated not only by the heterogeneous and cell-type-specific cell death responses but also by the fact that exogenous ROS exposure can also lead to quick endogenous ROS generation, making it difficult to distinguish primary from secondary ROS responses. Pinpointing the specific type of cell death is not only an academic question, as the type of cell death has important implications for the functional outcome in diseases. For instance, in wound healing, further excessive damage (e.g., necroptosis) may be discouraged for appropriate healing response, while in the treatment of tumors, a proinflammatory type of cell death would be encouraged to unleash the power of antitumor immunity.
3.2. Functional Consequences in Plasma-Treated Cells and Tissues
Hormesis accurately describes why plasmas are useful in both wound healing and cancer therapies: while the exposure to low levels of ROS can promote cell proliferation to support tissue regeneration, platelet activation, and blood coagulation [87–89], higher doses can induce cell death [90–92], endogenous ROS generation, and DNA damage, and lipid peroxidation [93]. This has been described in HaCaT cells exposed to plasma, where a low amount of ROS delivered over a minute of treatment was better tolerated than the fast delivery of the same amount of ROS over a few seconds [94]. Similarly, a study performed in ocular cells exposed to plasma for decontamination showed stimulatory effects at low doses and toxic effects at high doses [95]. It must be noted that the mechanisms involved in the hormetic response to ROS are differently activated (regarding type and strength) among tissues and cells, and therefore this should be considered in the analysis of the adaptive protective processes evoked by plasma [96].
One favorable advantage of cold plasma is the adjustable generation of biologically active factors, such as single or complex reactive species, at the site of interest by the admixture of water, oxygen, and/or nitrogen to argon gas [97–99]. As one consequence, cold plasma induces physical or chemical changes in fluids, cells, and tissues. The relatively short lifetime and the quick reaction between plasma-generated ROS and biomolecules, such as proteins, lipids, and nucleic acids, especially the short-lived species, lead to the formation of ROS intermediates. Such intermediates can directly function as signalling or redox-reactive molecules (e.g., NO and H2O2) in secondary reactions in biological environments [8, 100, 101]. Their high reactivity, diffusion, and delivery via pores, channels, and receptors influences the cellular availability and activates downstream signalling.
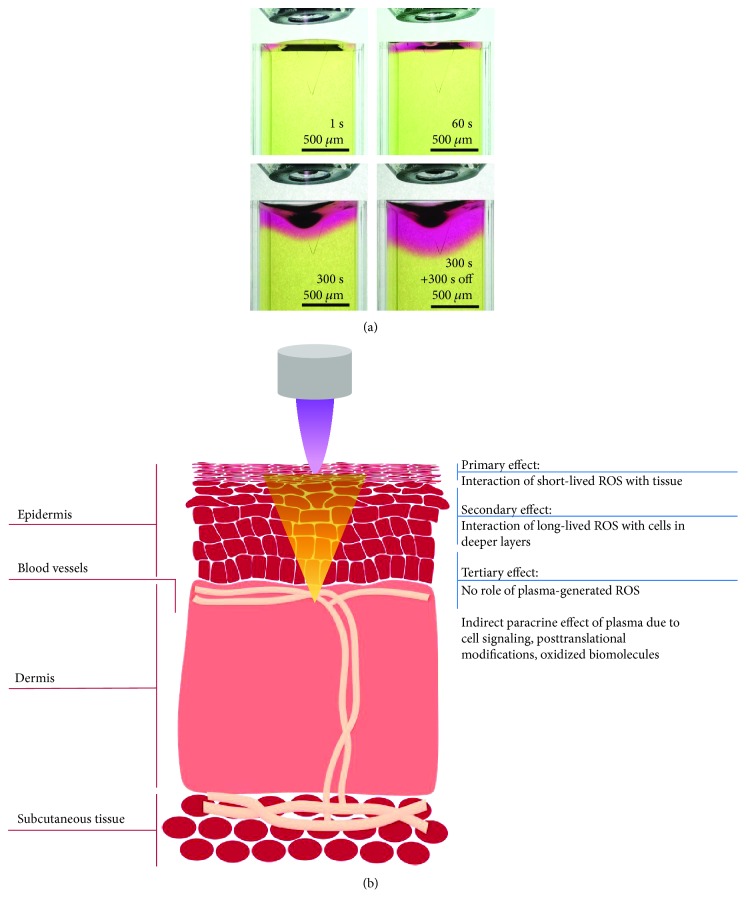
The oxidizing properties of ROS have an important impact on membrane integrity [102, 103]. Reactive species oxidize hydrophilic head groups and lipophilic tails of the phospholipid bilayer, leading to an initial membrane rigidity and an increase in fluidity [104]. Although the penetration depth of plasma in tissues ranges from 5 to 40 μm for O3 to a few millimeters for H2O2 and molecular oxygen (O2) [105, 106] (Table 1), the oxidizing nature of plasma by the oxidation of redox-sensitive cysteine and thiols in proteins [107–109] evokes paracrine effects [110, 111] and thereby changes of the microenvironment in deeper layers (Figure 4). Consequently, distant cells may benefit from cell-cell communication via paracrine mechanisms. One must also consider the presence of cells of the immune system, which are able to move across tissues and evoke a response at distant sites. Such is the case of immunogenic cell death (ICD), a mechanism proposed to mediate the effect of plasma in cancer and further discussed in this review. ICD-inducing therapies promote the expression of cell surface antigens and the release of damage-associated molecular patterns to activate cytotoxic T cells that kill the tumor cells and can stimulate antitumor immunity [112]. This mechanism is currently being studied in the field of plasma medicine [113], as it could extend the reach of plasma therapies from localized to systemic targets.
Table 1.
Overview of reported studies on penetration depths of plasma-derived ROS in original and artificial tissue models.
| Penetration depth | Plasma treatment | Tissue or biosurface studied | References |
|---|---|---|---|
| In vivo models | |||
| 10 μm | kINPen09 | Human skin | [255] |
| 36.8 ± 14.2 μm | kINPen09 | In ovo tumour of pancreatic adenocarcinoma cells | [106] |
| ~65 μm∗ | MicroPlaSter β plasma torch system | Skin wounds in 129 Sv/Ev female mice | [110] |
| 2.8 mm | Helium plasma jet | Bladder carcinoma tumors in BALB/c nu/nu male mice | [256] |
| ~50 μm∗ | Atmospheric-pressure helium plasma jet | Skin of BALB/c female mice | [257] |
| ~300–400 μm | kINPen09 | Hair follicles | [60] |
|
| |||
| In vitro surrogate models for real tissues | |||
| 1 mm | Helium plasma jet | ROS delivery through pig skin into liquid | [256] |
| 500–1500 μm | Helium+0.5% O2 plasma jet | ROS delivery through pig muscle into various liquids | [258] |
| 100–470 μm | Helium+0.5% O2 plasma jet | KI starch-containing gelatin films | [259] |
| 150 μm | Helium plasma jet | 2,7-Dichlorodihydrofluorescein/gelatin model | [260] |
| 150 μm | Helium plasma jet | ROS sensor-containing phospholipid vesicles in gelatin | [261] |
| 1 mm | Helium linear- and cross-field plasma jets | ROS delivery through gelatin or gelatin+NaNO2 films into distilled water | [262] |
| 1 mm | Helium plasma jet | ROS delivery through gelatin, gelatin+BSA, or poly(vinyl alcohol) targets into various liquids | [263, 264] |
| 6 mm (6 min) 8 mm (36 min) 11 mm (66 min) |
Argon plasma jet | KI starch gel | [265] |
| 2 mm (36 min) 4 mm (66 min) |
2% agarose | ||
| 1.5–5.8 mm | Low-temperature plasma jet | ROS delivery through agarose films into liquid | [256, 266–269] |
| 1–2 mm | Helium plasma jet | Agarose films | [270, 271] |
| 2 mm | Helium plasma jet | DNA damage in HEPES solution, phospholipid vesicles, or DNA embedded in gelatin | [272] |
|
| |||
| In silico models | |||
| Plasma ROS: 10–20 μm H2O2, O2−: 1–1.2 mm HO2: 20–250 μm O3: 5–40 μm |
Low-power He-O2 plasma | Highly hydrated biofilms and plasma-tissue interaction models | [273] |
∗Retrospectively measured with software from published images.
Figure 4.
Models for the study of the penetration of plasma-generated ROS into tissue. (a) In vitro approach for the analysis of ROS penetration using 0.02% methyl red as a reporter of ROS in 0.5% agarose gel. The treatment applied with Ar/O2 (1%) kINPen MED at 4 mm distance demonstrates that the penetration depth is directly proportional to the treatment time (unpublished/original data). (b) Proposed mechanisms of action of plasma ROS and concomitant effects in tissues. The primary effect is exerted in the first layers of cells that directly interact with the short-lived ROS. At this level, oxidative damage is induced in the extracellular matrix, cell membranes, and intracellular components of cells located in the outermost region of the tissue. The long-lived ROS able to penetrate into deeper regions of the tissue elicit a secondary oxidative effect in cells. However, the effect of plasma extends to more profound regions of the tissue due to the oxidation of redox-sensitive cysteine and thiols in proteins with paracrine effects and via cell-to-cell communication.
The maintenance of a physiological level of ROS is important for redox signalling [114–117]. An imbalance between the production and detoxification of reactive ROS intermediates affects the cellular stress level, e.g., cell cycle [118]. Cold plasma modulates numerous cellular processes related to redox signalling, and therefore, may be useful for targeting a plethora of specific, wound healing-related pathways.
3.3. Signalling Events in Wound Healing
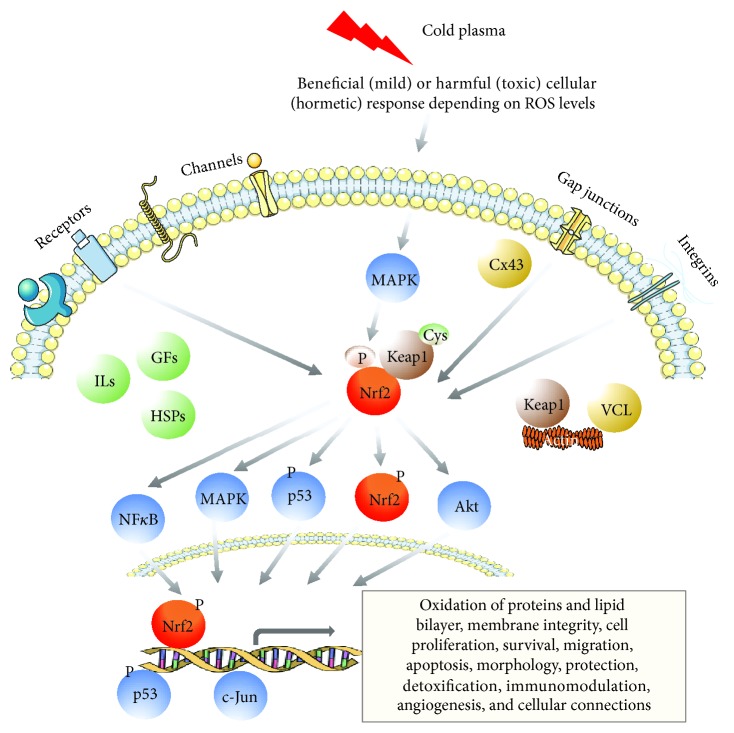
Changes in ROS levels trigger a coordinated action of redox-sensitive transcription factors (Figure 5) as part of cellular signalling (Table 2). Cold plasma significantly alters the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, as shown in global -omics analyses by microarrays, as well as by liquid chromatography and mass spectrometry, and in cytokine profiling [119–121]. In an immunocompetent murine wound model, gene and protein expression pattern revealed a strong regulation of specific targets of the Nrf2 pathway after a daily or three times per week treatment over 14 consecutive days [122, 123]. Nrf2 signalling, since its downstream targets act as sensors and/or effectors for increased oxidative stress, was ranked among the most active regulatory networks and canonical pathways after plasma treatment. Nrf2, itself, activates cellular rescue pathways against oxidative injury, inflammation, or apoptosis and functions in cellular defense against imbalances in redox homeostasis [124, 125]. The primary event in downstream signalling of Nrf2 is the recognition of plasma-generated ROS by specific oxidative stress sensors such as the actin-binding protein Kelch-like ECH-associated protein 1 (Keap1) [126]. Under basal conditions, Nrf2 is associated with Keap1. This vital factor in Nrf2 signalling cascade retains Nrf2 in the cytoplasm where Nrf2 is targeted for ubiquitin-mediated degradation [127, 128]. After the release of Nrf2 from Keap1 by oxidation events at cysteine, Nrf2 translocates to the nucleus, binds to antioxidant responsive elements (AREs) that are located in the promoters of its target genes, and activates their transcription [120, 123]. To scavenge ROS and inhibit oxidative damages, cells activate Nrf2 and its downstream genes, which encode ROS-detoxifying enzymes and antioxidant proteins. Among the most robustly increased proteins, heme oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (Nqo1), carbonyl reductase 1 (Crb1), γ-glutamylcysteine ligase catalytic (GCLC) and modifier subunit (GCLM), superoxide dismutases 1-3 (Sod1-3), thioredoxin (TRx), catalase (Cat), glutathione peroxidase (GPx), cytochrome P450, and nonenzymatic antioxidants like glutathione were found. Proteins involved in thiol group reduction or coupling (glutathione-S-transferases, e.g., GstK1, GstO1, and GstP1) showed an increased abundance (ca. 70 molecules), demonstrating that the glutathione metabolism is affected, which is a marker for an Nrf2-related signalling event. The strongly increased abundance of heat shock proteins (Hsp90 and Hsp40 derivatives) also indicates cellular response to plasma in terms of thermal or chemical stress [121].
Figure 5.
Overview of cold plasma-mediated signalling pathways, including oxidative stress (Nrf2), mitogen-activated protein (MAP) kinase, p53, Wnt/β-catenin, cytoskeletal, cell adhesion or growth factor (GF) signalling, and differentiation.
Table 2.
Overview of cold plasma-mediated signalling pathways, including oxidative stress (Nrf2), mitogen-activated protein (MAP) kinase, p53, Wnt/β-catenin, cytoskeletal, cell adhesion, or growth factor signalling and differentiation. He-GIW: helium-guided ionization wave; SMD: surface microdischarge.
| Signalling | Cell type(s) | Plasma source | References |
|---|---|---|---|
| Nrf2 | Keratinocytes (HaCaT) | He-GIW | [140] |
| THP-1 monocytes (human) | kINPen | [274, 275] | |
| Breast, pancreatic, colon cancer, and melanoma | kINPen | [276] | |
| Osteosarcoma cells | kINPen | [277] | |
| Periodontal ligament (PDL) cells | Plasma one dental | [278] | |
| Rat skin cells | Single-jet system | [279] | |
| Murine skin cells | kINPen | [123] | |
| Keratinocytes (HaCaT) | kINPen | [120, 121, 134, 148] | |
| T-lymphoblastoid leukemia cells | DBD | [236, 280] | |
|
| |||
| NFκB, MAPK | Monocytes, THP-1, and Jurkat | kINPen | [119, 154] |
| Cancer cells | DBD | [281] | |
| HNC cells | Spray-type jet | [282] | |
| Cancer cells (G631) | APPJ | [283] | |
| Cancer cells (ES2) | NEAPP | [202] | |
| Keratinocytes (HaCaT) | kINPen | [284] | |
| Cancer cells (A375, 875) | Surface BD | [285] | |
|
| |||
| p53 | Melanoma cells | SMD | [286] |
| Keratinocytes (HaCaT) | DBD | [287] | |
| Cancer cells | Different | [166] | |
| Cancer cells (HSC3) | DBD oxygen | [288] | |
| Cancer cells | DBD | [281] | |
| T98G, A549, HEK293, and MRC5 | Soft plasma jet | [289] | |
| Periodontal ligament (PDL) cells | Plasma one | [278] | |
| Melanocyte cancer cells | APPJ | [283] | |
| Keratinocytes (HaCaT) | kINPen | [164] | |
| Murine skin cells | kINPen | [123] | |
| Cancer cells (Huh7, Alexander, and HepG2) | Air based | [290] | |
| Keratinocytes (HaCaT) | DBD | [291] | |
| T-lymphoblastoid leukemia cells | DBD | [236] | |
|
| |||
| Wnt/β-catenin, cell adhesion | Melanoma cells (SK-Mel-28) | kINPen | [292] |
| Keratinocytes (HaCaT) | DBD | [293, 294] | |
| Keratinocytes (HaCaT) | DBD | [131, 132, 295, 296] | |
| MNC | DBD | [297] | |
| Normal and cancer cells | Jet | [133] | |
|
| |||
| Cytoskeletal | Skin cells | DBD, kINPen | [110, 153, 298] |
| Keratinocytes (HaCaT) | DBD | [287] | |
| Cancer cells (BHP10, TPC1) | Spray-type jet | [299] | |
| Human dermal fibroblasts | Jet like | [129, 130] | |
| Skin cells (HaCaT, MRC5), melanoma cells | kINPen | [122, 292] | |
|
| |||
| Differentiation growth factors | Neuroblastoma 2a (N2a) | DBD | [300] |
| Keratinocytes (HaCaT) | kINPen | [149] | |
| Human 3D skin model | Single jet (MEF) | [301] | |
Morphological changes such as cell size [122], the reorganization of cytoskeleton, and altered cytoskeletal [129, 130] and adhesion molecule expression [131–133] are indispensable for skin repair in wounds and in the metastatic behavior of cancer cells. Plasma-generated ROS alter the barrier function and intercellular communication such as gap junctional protein expression by a transient blocking of connexin 43 (Cx43) [122] and a modulation of tight junctional zona-occludens protein 1 (ZO-1) in skin cells [134, 135]. The formation and maintenance of the skin barrier function largely depends on the regulation of these cellular connections (e.g., adherence and tight and/or gap junctions), expression of junctional proteins, surface markers, and growth factor receptors [136]. Also, wound healing requires a well-balanced expression of extracellular matrix (ECM) and matrix metalloproteinases (MMPs) [137, 138]. In this regard, chemical modifications of ECM and MMPs were shown, affecting cells and tissues by cold plasma-generated ROS [139, 140]. However, transepidermal water loss (TEWL) was only transiently reduced after plasma treatment but not further affected in the course of time [141].
Beyond the regulation of antioxidant gene expression, Nrf2 also contributes to the anti-inflammatory process by orchestrating cytokine secretion of pro- and anti-inflammatory factors, and an early infiltration and recruitment of inflammatory cells such as macrophages [142]. The regulation of most of such events, including inflammation and immune cell infiltration [123, 143, 144], depolarization of macrophages [145, 146], mitochondrial function and content [147], angiogenesis (e.g., Akt) [110, 123, 148], growth factor signalling [123, 149], and cellular viability [134, 150] are further responses after plasma treatment. Studies combining electrical fields with plasma treatment demonstrated a synergistic metabolic activation of mammalian cells [151] besides the antibacterial effect [152]. Moreover, plasma-induced activation of Nrf2 accelerates wound healing and provides a faster wound closure by a concomitant increase in basal proliferation and cellular migration [122, 153]. A rapid and transient activation of the proliferative-acting extracellular signal-related kinase ERK1/2, and a slower but sustained activation of stress-activated p38 and c-Jun N-terminal kinases was detected in skin cells [119, 154].
Beside this proliferative effect, apoptotic events include the removal of inflammatory cells and inhibition of scar formation of granulation tissue at later stages of wound healing. The lower frequency of TUNEL-positive apoptotic cells on early time points in plasma-treated wounds, either due to enhanced macrophage numbers and activity or a redox-mediated suppression caused by plasma-derived ROS intermediates, and the increasing number of TUNEL-positive apoptotic cells at later time points is an essential prerequisite in skin wound healing [123]. Redox-sensitive transcription factors, such as the tumor suppressor protein p53, are susceptible to ROS-dependent modifications, which could impact their biological functions and activities [155]. Moreover, p53 can mediate a two-phase Nrf2 response: when p53 expression is relatively low, p53 enhances the protein level of Nrf2 and its target genes to promote cellular protection and survival at basal levels in a p21-dependent manner [156]. Contrary, the Nrf2-mediated survival response is inhibited and senescence/apoptosis at higher ROS levels is supported in the repression phase [157]. This cross-talk between oxidative stress (Nrf2 signalling) and DNA damage (p53 activation) defines the critical point where cell injury may switch from an adaptation to an injury state [158]. Additionally, the phosphorylation status and therefore the activity of p53 depends on wound stages and is timely regulated [159, 160]. A transient inhibition of p53 supports the early cell proliferation required [157]. Later apoptotic events are induced via caspase activation [119, 154], cell-cycle disruption [161], and other multiple pathways [162, 163]. Cold plasma transiently enhances total p53 protein expression, induces nuclear translocation of p53, and alters the phosphorylation level of p53 in a treatment and incubation time-dependent manner [164]. Findings further suggested plasma-induced cell reactions of stress sensing, along with metabolic alterations [143, 165]. The interaction with the signal transduction pathway of p53 and related processes fosters the understanding of plasma-induced cell protection against DNA damage or DNA strand breaks.
3.4. Effects on Cancer Cells
Plasma therapies for cancer have shown promising results in multiple cancer types using a variety of plasma sources [166]. Most studies report a decrease in cell viability and elevated cytotoxicity upon plasma treatments [167–177]. Part of the damage is induced to the cell membrane, the first barrier to deal with the oxidative stress induced by plasma. The first effect observed in plasma-treated cancer cells is lipid peroxidation, a process where lipids with carbon-carbon double bounds such as glycolipids, phospholipids, and cholesterol are oxidized [178]. The extensive peroxidation of lipids upon plasma treatment, if present, may increase the entropy in the plasma membrane and alter the assembly, dynamics, and structure of lipids, facilitating pore formation [104, 179, 180]. In fact, the highly porous, disorganized plasma membrane serves as the entry door of multiple extracellular ROS, a process observed in necrotic cells [181]. Interestingly, lipid peroxidation is characteristic of ferroptosis, a Fe(II)-dependent cell death mechanism driven by oxidative stress and consecutive lipid peroxidation [182]. One report suggests that plasma treatment could promote ferroptosis in cancer cells via the reduction of Fe(III) to Fe(II) stored in ferritin [183]. In this case, the increase in Fe(II) available within the cancer cell could contribute to the Fenton reaction and the consequent formation of the highly reactive •OH radical, able to react with any biomolecule present at close proximity [184].
Cancer cells are more sensitive than normal cells to oxidative stress due to the increased steady-state ROS levels produced. The high glucose uptake and transformation to lactate, even in the presence of oxygen (also known as the Warburg effect), is responsible for the accumulation of intracellular ROS in cancer cells [185]. It has been suggested that increasing the oxidative stress by exogenous ROS (such as plasma treatments) to a threshold incompatible with cell viability could selectively eliminate cancer cells without damaging the healthy ones [186, 187]. In the plasma field, it has been suggested that an increase in aquaporins [188] or a decrease of cholesterol in the plasma membrane of cancer cells [179, 189] facilitates the transport and permeation of ROS to the intracellular compartment, supporting a selective effect of plasma on cancer over normal cells. The latter may also be mediated by cell-cycle arrest [190]. It is possible that the combination of these factors favors the selective elimination of cancer cells by plasma.
Plasma therapies for cancer have shown positive results both for localized and metastatic cancers in animal models, especially in melanoma [191]. Plasma can also induce immunogenic cell death (ICD), a regulated cell death mechanism that involves the release of damage-associated molecular patterns by cancer cells and the recruitment of immune cells to eliminate the tumor [83]. Direct plasma treatment of glioblastoma xenografts has been shown to increase the survival rate and reduce tumor volume [192], as well as to induce apoptosis and cell-cycle arrest [193]. This in turn may increase their sensitivity to common chemotherapeutic drugs such as gemcitabine [194, 195], doxorubicin [196], and novel mitochondrial complex IV [197], as well as HSP90 inhibitors [198] as well as to traditional radiotherapy [199]. Interestingly, plasma treatments could suppress the growth of irradiated and nonirradiated remote melanoma tumors in mice (known as abscopal effect), suggesting the participation of the innate immunity in the response to treatment [200]. The antiproliferative effect observed in plasma-treated tumors equally affects chemoresistant and chemosensitive cancer cells [201]. Plasma-treated solutions have proved to be effective against metastatic cancers in murine models. Intraperitoneal injections of plasma-treated medium were able to inhibit dissemination of ovarian cancer [202], and plasma-treated medium and saline solutions reduced the tumor burden, promoted the infiltration of macrophages, and increased T cell activation as well as immunogenic cancer cell death in vivo [203–205]. With direct plasma treatment, ICD can be induced in localized colorectal tumors [206] and melanoma tumors in mice by the short-lived species produced by plasma [207]. Whether plasma-induced ER stress [208] links to plasma as a type I or type II ICD inducer [209] is the subject of current investigations. To date, there is no report of resistance to plasma treatment, suggesting that plasma could be a promising therapy for cancer.
4. Cellular Membranes as a Link between Plasma Chemistry and Biology
One way for plasma treatments to be effective is that plasma-derived ROS cross or interfere with the cell membrane to affect its stability and permeability, ultimately altering the intracellular circuitry [210]. The field of redox biology has extensively addressed the effect of ROS in cell membranes; for that reason, this section will put the effects of plasma treatments on cell membrane components in context with the current knowledge in redox biology (Figure 6). Several studies have already provided evidence that skin lipids from human volunteers undergo oxidative changes upon plasma treatment, although the functional consequences remain elusive [211–214].
Figure 6.
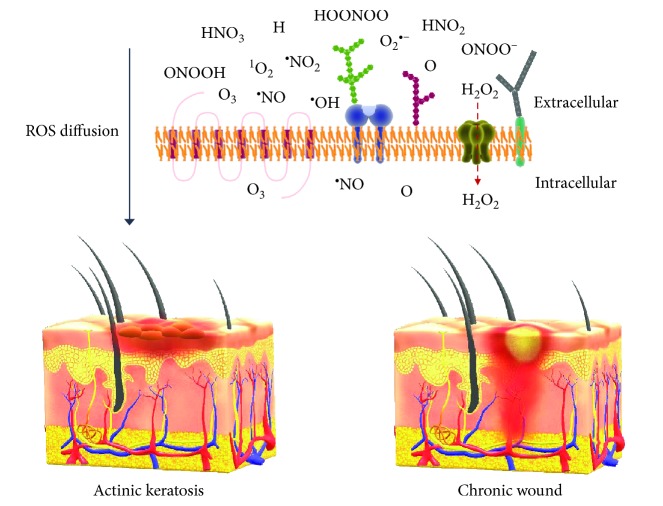
The cell membrane is the key compartment that plasma-derived ROS need to penetrate or interact with to elicit biological responses. While some ROS are able to penetrate cellular membranes (e.g., ozone, nitric oxide and atomic oxygen), other more polar ROS cannot (e.g., singlet delta oxygen, nitrite, hydroxyl radical, superoxide anion, hydrogen, and peroxynitrite). Hydrogen peroxide is actively transported into the cells via transporters such as aquaporins.
4.1. Cellular Membranes as a Target, ROS Source, and Transporter of Plasma-Derived ROS
Those ROS and RNS produced by plasma in the gas phase that are able to penetrate the liquid or soft interphase characteristic of biological substrates may directly or after transformation into additional ROS, react with cellular molecules and the extracellular matrix. The exterior of mammalian cells is composed of a complex lipid bilayer with a highly variable and dynamic chemical composition, additionally diversified by intercalated proteins (compiled in [215]). Due to their projected position and chemical nature, lipids represent “ideal” targets for oxidative modifications by plasma-derived ROS. Lipids comprise a chemically heterogeneous group of compounds that often combine hydrophilic and lipophilic substructures in the molecule [216]. In phospholipids, long-chain fatty acids are connected via a polyalcohol bridge (e.g., glycerol) to a polar head group consisting of an orthophosphate residue and an amine (choline, ethanolamine), creating a zwitterion. Various numbers of isolated double bonds are frequently found in the fatty acid tails, increasing sensitivity towards oxidative events. Attacking the weak sp1 carbon-hydrogen bond at the allyl position easily yields hydroperoxyls, hydroxylations, and radical intermediates. Subsequent reactions, like the Hock rearrangement may lead to chain breakage [217]. The resulting short-chained fatty aldehydes like 4-hydroxynonenal are relevant second messengers (see Section 4.2), and the residual aldehyde fatty acids are more polar, decreasing the order and crystallinity of the membrane [178]. Further addition or substitution reactions can occur at the double bond(s), yielding nitro- or chlorohydroxy fatty acids, depending on the attacking species [218, 219]. Accordingly, lipids are common targets of oxidative modifications by plasma-derived ROS and/or RCS (reactive chlorine species) that occur in specific conditions. Maheux et al. investigated the impact of a helium/nitrogen-driven DBD jet onto liposomes made of 1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (DOPC) [220]. Significant changes to the physical properties of the lipid particles, including size and zeta potential, were accompanied by the detection of dioxidized DOPC and chlorohydrins. Yusupov et al. revealed the impact of plasma-derived species, especially the •OH radical, on lipids and lipid complexes, e.g., bilayer models, using atomic scale simulations. Taking lipid bilayer geometry, radical species half-life, and reactivity into account, the predominant target was identified as the lipid's head group. In contrast, a strong impact on the fatty acid chain yielding cleavages was observed experimentally. A number of not fully resolved structures connected to the investigated lipid but showing cyclisation in the head group suggested that a direct interaction of short-lived species, especially •OH radicals, with the head groups cannot be excluded and may have contributed to the side-chain oxidation. Ultimately, the sum of oxidations yielded a decreased membrane stiffness of the model liposomes [104].
Plasma treatments have been shown to increase the cell membrane permeability [221, 222]. Further, ROS delivered by plasma such as O2, HOCl, O3, 1O2, •NO, and ONOO− can trigger radical chain reactions, resulting in propagated lipid oxidation [223, 224]. The superoxide anion radical O2•-, produced either by plasma and/or as a cellular product from a single-electron transfer reaction, is relatively nonreactive by itself. However, its reaction with NO yields the strong oxidant peroxynitrite, which in turn contributes to lipid oxidation. Extracellular O2•- and NO can be produced from physical plasma as well as certain types of cells as a basis for peroxynitrite generation [225]. The accumulation of oxidized lipids in the bilayer upon plasma treatment reduces the electric field threshold required for pore formation and decreases the mechanical strength, thereby increasing the permeability and fluidity of the membrane [179, 180, 226]. Similarly, lipid oxidations have been proposed to occur during the electroporation of cells to facilitate membrane permeability [180]. This suggests a concomitance of both processes and emphasizes that lipid oxidation and/or chain cleavage are key factors determining membrane fluidity and polarity and ultimately membrane penetration. Of note, the membrane lipid composition of normal and cancer cells differ in the reflection of their metabolic state, contributing to a certain graduation of the impact of plasma or other prooxidant physical treatment regimens [227, 228].
4.2. Secondary Messengers Deriving from ROS or Plasma-Derived ROS
When looking into singular lipid structures and related functional consequences in biological systems, a vast list could be compiled. Many lipid oxidation products act as second messengers having almost unrestricted access due to their ambipolarity. Well-known examples are the fatty acid oxidation derived eicosanoids with extensive impact in inflammation regulation that are also targeted by mass-market and high-selling drugs [229]. The first step, the enzymatic release of arachidic acid from a phospholipid can be achieved by plasma as well, thereby increasing the pool for the cyclooxygenases performing the following two-stage oxidation leading to the intermediate prostaglandin H2. It contains an endoperoxide, a structure that can be derived from a singlet oxygen, a common species in plasma. Downstream, this endoperoxide is replaced by oxo- and hydroxyl groups. Although these structures are complex, many steps can be performed by the plasma, opening an avenue to modulate a range of pathways, including inflammation, cardiovascular effects, or pain perception. Interestingly, a decrease of pain was repeatedly reported by patients undergoing plasma treatment of chronic wounds (see the results reported in [39]).
Some lipid oxidation products are cytotoxic and can induce apoptosis, such as 7α,β-hydroxy-, 7-oxo-, and 5,6-epoxycholesterol produced from oxidized cholesterol [230]. The reaction of •OH with cholesterol can lead to the formation hydroperoxyl radicals (HO2•) and the corresponding superoxide anion radicals (•O2−), important due to their multiple effects in cells. Excess HO2•/•O2− disproportionate spontaneously or is enzymatically reduced forming H2O2, ultimately yielding again •OH radicals through Fenton or Haber-Weiss reactions, potentially leading to the initiation of the chain oxidation of (poly-) unsaturated phospholipids [178]. In the skin, plasma-derived H2O2 and O2 have been named the main ROS responsible for cholesterol oxidation [231]. It is possible that the propagation of the reaction continues within the plasma membrane, as O2 concentrates close to the lipid tails inside the lipid bilayer where it can oxidize other lipids [189, 231]. Interestingly, 1O2 can also oxidize cholesterol to produce 5α-OOH, the most damaging hydroperoxide product due to its ability to accumulate and to migrate from the production point to more sensitive sites where iron-mediated cytotoxicity can be induced [224]. However, the participation of 5α-OOH in the response to plasma treatments is so far unknown. Other ROS-derived lipid peroxidation products such as 4-hydroxynonenal (HNE) can form DNA adducts [232]. HNE in particular is an important second messenger molecule that participates in the activation of Nrf2, a regulator of cellular resistance to oxidants [126].
Oxidized phospholipids (OxPL) can also serve as ligands in damaged or stressed cells that are recognized by receptors in cells of the innate immune system [233]. The scavenger receptors CD36, SRA, and SRB1 (present in anti-inflammatory M2 macrophages) bind to OxPL in apoptotic cells to trigger their clearance by the immune system [234]. Plasma has been shown to effectively induce apoptosis in cancer cells [235–238], and it is possible that OxPL was formed in their plasma membranes. Interestingly, it has been shown that plasma favors monocyte differentiation towards a M2-like macrophage profile accompanied by an increased CD36 expression [145]. It is conceivable to think that plasma treatments could participate in both the induction of apoptosis in cancer cells and their clearance by macrophages. Nitrogen dioxide (NO2) generated from peroxynitrite can originate nitrofatty acids (NO2-FAs) [239] that can inhibit the propagation of lipid peroxidation and protein nitration and therefore counteract the proinflammatory and cytotoxic effects [240]. NO2-FAs can release •NO into the cell, inhibit the activation of the transcription factor NFκB, and alter the activity of proteins involved in antioxidant responses [188]. It has been shown that a plasma-treated medium attenuated the NFκB pathway in the MDAMB231 human breast adenocarcinoma cell line [241], and the direct plasma treatment combined with cetuximab modulated the NFκB and p53 signalling pathways in head-and-neck cancer cells [242]. In the same way, plasma decreased the antioxidant activity of glioblastoma, thyroid carcinoma, oral carcinoma, and nonmalignant embryonic cells [243], which suggests a possible participation of NO2 and NO2-FAs in the responses observed. Further studies of these intermediates and signalling pathways involved in the response in the context of plasma therapies should be done.
4.3. Impact of Plasma-Derived ROS on Membrane-Associated Proteins
Beside lipids as the dominant compounds in a cell membrane, numerous proteins are integrated into it. As discussed, ROS can be actively transported into the intracellular compartment (aquaporins) or neutralized by enzymes such as catalase or superoxide dismutase, thereby modulating the impact of plasma. The expression of these proteins in the membrane determine the susceptibly of cells towards plasma. These proteins are also susceptible to oxidation by exogenous ROS. Their main targets are amino acids with aromatic side chains [244] and those containing sulfhydryl groups [245]. The reaction of plasma-derived •NO and O2•- yields the strong oxidant peroxynitrite/peroxynitrous acid (ONOO−/ONOOH) which reacts with lipid hydroperoxides to form 1O2 and induce protein oxidation [178]. ROS can induce functional and structural changes in cell membrane proteins that result in their activation, change in gene expression levels, or degradation, as observed in cells treated with plasma (Table 3).
Table 3.
Overview of the main components of the cell membrane and their role in the response to plasma treatment.
| Molecule | Key physiological role(s) | Reported role in response to plasma | Redox-mediated downstream effects |
|---|---|---|---|
| Transporters | |||
| AQP1 | Water, H2O2 [302], CO2, NO, and ammonia | Favored H2O2 permeation into intracellular compartment [251] | Signalling via the Keap1/Nrf2 system [303] |
| AQP3 | Water, urea, H2O2 [304], glycerol, and ammonia. Involved in cell proliferation, invasion, and angiogenesis [305] | Unknown | Activation of the Nox-2 and PI3K/Akt or MAPK pathway [306] |
| AQP5 | Water and H2O2 [307]. Involved in tumor formation, cell proliferation, and migration [308] | Unknown | Role in tumor formation related to its phosphorylation status [309] |
| AQP8 | Water, H2O2 [310], and ammonia | Required for anticancer effect of plasma-treated medium (PTM) on glioblastoma cells [311] | EGF induces AQP8 expression via EGF/EGFR-ERK1/2 pathway [312]. H2O2 transport is controlled by redox-mediated modifications [313] |
| AQP9 | Water, H2O2 [314], urea, glycerol, lactate, and pyruvate [309] AQP9 knockdown reduced H2O2-induced cytotoxicity [314] |
Its absence does not impair H2O2 transport upon treatment with PTM in glioblastoma cells [311] | Target of protein kinase A [307]. Possible interaction with ERK1/2 and MMP9 to enhance invasion and migration of prostate cancer cells [308] |
|
| |||
| Cell membrane receptors | |||
| Epidermal growth factor receptor (EGFR) | Receptor tyrosine kinase involved in signal transduction to stimulate proliferation and cellular growth and block apoptosis | EGFR was degraded and dysfunctional in EGFR-overexpressing oral squamous carcinoma after plasma treatment [315, 316] | Moderate exogenous H2O2 induces the redox activation of EGRF and increases protein kinase activity [317]. |
| Transient receptor proteins (TRP) | Calcium-permeable and voltage-independent cation channels which act as multimodal sensors of external stimuli | Unknown | In response to oxidative stress, TRPC3 and TRPC4 increase the intracellular Ca2+ concentration that leads to cell death [318] |
| Integrins | Responsible for cell-to-matrix and cell-to-cell adhesion. Integrins transduce the external signals to the cytoskeleton | DBD/air plasma enhanced expression of α2-integrin/CD49b and β1-integrin/CD29 in HaCaT cells [295] Marginal decrease in α5- and β1-integrins in primary fibroblasts and PAM cells [319] Plasma activates β1-integrins on the cell surface of WTDF3 mouse fibroblasts [320] kINPen plasma jet treatment downregulates integrin expression in MRC5 cells [122] and increases β1-integrin in HaCaT cells [132] |
Integrin-linked kinase (ILK) signalling via PKB/Akt can suppress apoptosis and anoikis [321]. ILK is required to maintain redox balance [322] NRF2-mediated oxidative stress response |
| E-cadherin | Calcium-dependent cell-to-cell adhesion receptor | kINPen plasma jet treatment decreases E-cadherin expression in HaCaT cells [122, 132] Argon plasma modulates E-cadherin function and induces its internalization in HaCaT cells in vitro and decreases the amount of E-cadherin in mice epidermis [323]. Others report an increase in E-cadherin expression in the wounds of rats [324] |
Oxidative stress causes the selective disruption of E-cadherin and beta-catenin cell adhesion complexes [325] In response to oxidative stress, E-cadherin binds to Nrf2 to restrain Nrf2 nuclear localization and activity [326] Assembly of E-cadherin activates several small GTPases and, in turn, the activated small GTPases control the effects of E-cadherin-mediated adhesions on epithelial biogenesis [327] Involvement of ROS in the regulation of cell adhesion and signal transduction functions of integrins and cadherins, pointing to ROS as emerging strong candidates for modulating the molecular cross-talk between cell-matrix and cell-cell adhesion receptors [328] Redox-regulation of EMT [329] |
| Focal adhesions | Adhesive contact that anchors the cell to the extracellular matrix that mediates mechanical and biochemical signalling | Plasma increased the amount of vinculin and the focal adhesion size in WTDF3 mouse fibroblasts [320] | Oxidative stress activates focal adhesion kinase by Src kinase- and PI3 kinase-dependent mechanisms, which accelerates cell migration [330] |
|
| |||
| Lipids | |||
| Cholesterol | Provides rigidity to the cell membrane and controls membrane fluidity [331] | When present at low concentrations in the cell membrane, plasma oxidation facilitates pore formation and passing of ROS [179]. Unknown effect of toxic by-product 5α-OOH after plasma treatment |
Oxidation by-products such as HO•2 can generate intracellular H2O2 and •OH, and propagate lipid oxidation [178]. Induction of apoptosis by 7α,β-hydroxy-, 7-oxo-, and 5,6-epoxycholesterol [230] and formation of 5α-OOH [224] |
| Phospholipids | Main component of biological membranes | Plasma oxidizes phospholipids and affects lipid mobility [104, 332] Plasma induces apoptosis and flipping of phosphatidylserine from the inner to the outer layer of the cell membrane [140, 236, 238, 333–335] Plasma-treated cells present disrupted cell membranes [336–338] |
Apoptotic cells presenting OxPLs in the cell membrane are eliminated by M2 macrophages [234] |
| Fatty acids | Form the hydrophobic hydrocarbon tails of phospholipids | Oxidation product NO2-FAs inhibit activation of NFκB [188] | NO2-FAs stop the lipid oxidation propagation and protein nitration [240]. Peroxidation increases the rigidity of the cell membrane [339] |
| Lipid rafts | Modulate distribution of receptors and signalling molecules in the cell membrane [340] Important in oxidative stress-induced cell death [341] |
In combination with hyperthermia, plasma activates the FA receptor (abundant in lipid rafts) and causes FA-induced apoptosis [342] | Activation and aggregation of death receptors such as FAs and TNFR1 located in lipid rafts and enhanced activation of kinases recruited at the raft site [341]. Ceramides produced from the oxidation of glycosphingolipids induce apoptosis via activation of the JNK pathway and regulation of Bax [343] and bind to cathepsin D to mediate TNF-induced cell death signalling [344]. In response to H2O2, JNK activates to induce the TRAF2/RIP-dependent pathway for oxidative cell death [341]. Lipid peroxidation affects the coupling of receptors with effector systems and decreases receptor density [339] |
|
| |||
| Catalytic enzymes | |||
| NADPH oxidase (Nox) | Transmembrane enzyme that catalyzes the reduction of extracellular oxygen to O2•− | Inhibition with DPI attenuates the intracellular presence of ROS after plasma treatment, indicating a stimulation of endogenous ROS production with plasma [14] | Contributes to the elimination of malignant cells via HOCl and the NO/ONOO− signalling pathways |
| Catalase | Membrane-bound enzyme that decomposes H2O2 into water and oxygen. When membrane-bound, it provides increased resistance to exogenous H2O2 and favors tumor progression | Plasma-generated ROS supposedly induce the formation of singlet oxygen that inactivates membrane-bound catalase to favor apoptosis [345] | In malignant cells, catalase interferes with HOCl signalling by decomposing H2O2 and interferes with NO/ONOO− signalling through oxidation of NO and decomposition of ONOO− to favor tumor progression |
Although ROS can exert negative effects in cells, H2O2 is normally produced extracellularly in low concentrations to serve in both autocrine and paracrine fashion [246]. NOX present in the cell membrane generates O2•- into the outer cell environment, which is later dismutated into H2O2 [247]. The main role of H2O2 as a signalling molecule is to oxidize proteins on specific sites to modulate their function and therefore regulate gene transcription, proliferation, metabolism, and migration [248]. Because the diffusion of H2O2 across membranes is limited, aquaporins (AQPs) transport H2O2 into the intracellular space to meet the physiological demands [249]. It has been reported that cancer cells overexpress aquaporins in their cell membrane compared to normal cells, which could favor H2O2 transport into the cytosol [250, 251]. This particular feature of cancer cells could explain the selective effect of plasma in cancer cells, as described before [188]. The contribution of aquaporins to the response to plasma-generated ROS is currently under study, as only the role of AQP1, AQP8, and AQP9 in H2O2 transport upon plasma treatment has been reported (Table 3). Cancer cells that are resistant to ROS-induced apoptosis can overcome the cytotoxic activity of exogenous H2O2 by presenting catalase in the outer layer of the cell membrane [252]. Membrane-bound catalase decomposes H2O2 and ONOO− and oxidizes NO present outside the cells. Catalase therefore interferes with the ROS signalling through the HOCl and the NO/ONOO− pathway [247, 253]. Interestingly, 1O2 produced during the exposure of cancer cells to the plasma-treated medium has been shown to inactivate the enzymatic activity of membrane-bound catalase, restoring the activation of the apoptotic pathway [254].
5. Conclusion
Treatment with cold physical plasma-derived ROS provides new therapeutic avenues in the therapy of a number of diseases. While the composition of ROS in the plasma gas phase, as well as the functional consequences in cells, is reasonably well explored, much more effort is needed to explore in greater detail the interphase reactions between the ROS cocktail and cell membranes and tissues. To accelerate such research, novel tools for studying the effects of different kinds of ROS, as well as consensus guidelines of the plasma medicine community, will be of great benefit.
Acknowledgments
KW and SB acknowledge funding by the German Federal Ministry of Education and Research (grant numbers 03Z22DN11 and 03Z22DN12). The work of SB is further supported by the European Social Fund (grant number ESF/14-BM-A55-0006). APM and AB acknowledge funding by the Methusalem Project. AL acknowledges funding from the Research Foundation Flanders (grant number 12S9218N). APM thanks Yury Gorbanev for his assistance with the preparation of this review.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Burm K. T. A. L. Plasma: the fourth state of matter. Plasma Chemistry and Plasma Processing. 2012;32(2):401–407. doi: 10.1007/s11090-012-9356-1. [DOI] [Google Scholar]
- 2.Fridman A., Chirokov A., Gutsol A. Non-thermal atmospheric pressure discharges. Journal of Physics D: Applied Physics. 2005;38(2):R1–R24. doi: 10.1088/0022-3727/38/2/R01. [DOI] [Google Scholar]
- 3.Lu X., Naidis G. V., Laroussi M., Reuter S., Graves D. B., Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Physics Reports. 2016;630:1–84. doi: 10.1016/j.physrep.2016.03.003. [DOI] [Google Scholar]
- 4.Graves D. B. Reactive species from cold atmospheric plasma: implications for cancer therapy. Plasma Processes and Polymers. 2014;11(12):1120–1127. doi: 10.1002/ppap.201400068. [DOI] [Google Scholar]
- 5.Moisan M., Barbeau J., Crevier M. C., Pelletier J., Philip N., Saoudi B. Plasma sterilization. Methods and mechanisms. Pure and Applied Chemistry. 2002;74(3):349–358. doi: 10.1351/pac200274030349. [DOI] [Google Scholar]
- 6.Graves D. B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D: Applied Physics. 2012;45(26):p. 263001. doi: 10.1088/0022-3727/45/26/263001. [DOI] [Google Scholar]
- 7.Gorbanev Y., Verlackt C. C. W., Tinck S., et al. Combining experimental and modelling approaches to study the sources of reactive species induced in water by the COST RF plasma jet. Physical Chemistry Chemical Physics. 2018;20(4):2797–2808. doi: 10.1039/c7cp07616a. [DOI] [PubMed] [Google Scholar]
- 8.Wende K., von Woedtke T., Weltmann K. D., Bekeschus S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biological Chemistry. 2018;400(1):19–38. doi: 10.1515/hsz-2018-0242. [DOI] [PubMed] [Google Scholar]
- 9.Girard F., Peret M., Dumont N., et al. Correlations between gaseous and liquid phase chemistries induced by cold atmospheric plasmas in a physiological buffer. Physical Chemistry Chemical Physics. 2018;20(14):9198–9210. doi: 10.1039/C8CP00264A. [DOI] [PubMed] [Google Scholar]
- 10.Gorbanev Y., O'Connell D., Chechik V. Non-thermal plasma in contact with water: the origin of species. Chemistry - A European Journal. 2016;22(10):3496–3505. doi: 10.1002/chem.201503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbanev Y., Privat-Maldonado A., Bogaerts A. Analysis of short-lived reactive species in plasma–air–water systems: the dos and the do nots. Analytical Chemistry. 2018;90(22):13151–13158. doi: 10.1021/acs.analchem.8b03336. [DOI] [PubMed] [Google Scholar]
- 12.Weltmann K. D., von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Physics and Controlled Fusion. 2017;59(1, article 014031) doi: 10.1088/0741-3335/59/1/014031. [DOI] [Google Scholar]
- 13.Bekeschus S., Masur K., Kolata J., et al. Human mononuclear cell survival and proliferation is modulated by cold atmospheric plasma jet. Plasma Processes and Polymers. 2013;10(8):706–713. doi: 10.1002/ppap.201300008. [DOI] [Google Scholar]
- 14.Lin A., Truong B., Patel S., et al. Nanosecond-pulsed DBD plasma-generated reactive oxygen species trigger immunogenic cell death in a549 lung carcinoma cells through intracellular oxidative stress. International Journal of Molecular Sciences. 2017;18(5):p. 966. doi: 10.3390/ijms18050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehmigen K., Hahnel M., Brandenburg R., Wilke C., Weltmann K. D., von Woedtke T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Journal of Physics D: Applied Physics. 2010;7(3-4):250–257. doi: 10.1002/ppap.200900077. [DOI] [Google Scholar]
- 16.Goodman E. M., Greenebaum B., Marron M. T. Effects of electromagnetic fields on molecules and cells. International Review of Cytology. 1995;158:279–338. doi: 10.1016/S0074-7696(08)62489-4. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoudinasab H., Sanie-Jahromi F., Saadat M. Effects of extremely low-frequency electromagnetic field on expression levels of some antioxidant genes in human MCF-7 cells. Molecular Biology Research Communications. 2016;5(2):77–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan L. Q., Wang C., Zhu K., et al. The antitumor effect of static and extremely low frequency magnetic fields against nephroblastoma and neuroblastoma. Bioelectromagnetics. 2018;39(5):375–385. doi: 10.1002/bem.22124. [DOI] [PubMed] [Google Scholar]
- 19.Simko M., Mattsson M. O. Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: possible immune cell activation. Journal of Cellular Biochemistry. 2004;93(1):83–92. doi: 10.1002/jcb.20198. [DOI] [PubMed] [Google Scholar]
- 20.Weidinger A., Kozlov A. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter S., von Woedtke T., Weltmann K. D. The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. Journal of Physics D: Applied Physics. 2018;51(23, article 233001) doi: 10.1088/1361-6463/aab3ad. [DOI] [Google Scholar]
- 22.Metelmann H.-R., von Woedtke T., Weltmann K.-D. Comprehensive Clinical Plasma Medicine. Springer; 2018. [DOI] [Google Scholar]
- 23.Sen C. K., Gordillo G. M., Roy S., et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowie C. C., Rust K. F., Ford E. S., et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen C. K. The general case for redox control of wound repair. Wound Repair and Regeneration. 2003;11(6):431–438. doi: 10.1046/j.1524-475X.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- 26.Gristina R., D'Aloia E., Senesi G. S., et al. Increasing cell adhesion on plasma deposited fluorocarbon coatings by changing the surface topography. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;88B(1):139–149. doi: 10.1002/jbm.b.31160. [DOI] [PubMed] [Google Scholar]
- 27.Sen C. K., Roy S. Redox signals in wound healing. Biochimica et Biophysica Acta (BBA) - General Subjects. 2008;1780(11):1348–1361. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwig S., Doll C., Voss J. O., Hertel M., Preissner S., Raguse J. D. Treatment of wound healing disorders of radial forearm free flap donor sites using cold atmospheric plasma: a proof of concept. Journal of Oral and Maxillofacial Surgery. 2017;75(2):429–435. doi: 10.1016/j.joms.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Heinlin J., Zimmermann J. L., Zeman F., et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair and Regeneration. 2013;21(6):800–807. doi: 10.1111/wrr.12078. [DOI] [PubMed] [Google Scholar]
- 30.Khrupkin V. I., Zudilin A. V., Pisarenko L. V., et al. Local application of low-energy aerial and argon plasma in the treatment of suppurative wounds and trophic ulcers. Vestnik khirurgii imeni II Grekova. 2001;160(2):39–45. [PubMed] [Google Scholar]
- 31.Miyamoto K., Ikehara S., Sakakita H., Ikehara Y. Low temperature plasma equipment applied on surgical hemostasis and wound healings. Journal of Clinical Biochemistry and Nutrition. 2017;60(1):25–28. doi: 10.3164/jcbn.16-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shekhter A. B., Pekshev A. V., Vagapov A. B., et al. Physicochemical parameters of NO-containing gas flow affect wound healing therapy. An experimental study. European Journal of Pharmaceutical Sciences. 2019;128:193–201. doi: 10.1016/j.ejps.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich C., Kluschke F., Patzelt A., et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: a pilot study. Journal of Wound Care. 2015;24(5):196–203. doi: 10.12968/jowc.2015.24.5.196. [DOI] [PubMed] [Google Scholar]
- 34.Klebes M., Ulrich C., Kluschke F., et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. Journal of Biophotonics. 2015;8(5):382–391. doi: 10.1002/jbio.201400007. [DOI] [PubMed] [Google Scholar]
- 35.Vandersee S., Richter H., Lademann J., et al. Laser scanning microscopy as a means to assess the augmentation of tissue repair by exposition of wounds to tissue tolerable plasma. Laser Physics Letters. 2014;11(11, article 115701) doi: 10.1088/1612-2011/11/11/115701. [DOI] [Google Scholar]
- 36.Hilker L., von Woedtke T., Weltmann K. D., Wollert H. G. Cold atmospheric plasma: a new tool for the treatment of superficial driveline infections. European Journal of Cardio-Thoracic Surgery. 2017;51(1):186–187. doi: 10.1093/ejcts/ezw212. [DOI] [PubMed] [Google Scholar]
- 37.Daeschlein G., Rutkowski R., Lutze S., et al. Hyperspectral imaging: innovative diagnostics to visualize hemodynamic effects of cold plasma in wound therapy. Biomedical Engineering/Biomedizinische Technik. 2018;63(5):603–608. doi: 10.1515/bmt-2017-0085. [DOI] [PubMed] [Google Scholar]
- 38.Game F. L., Apelqvist J., Attinger C., et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes/Metabolism Research and Reviews. 2016;32:75–83. doi: 10.1002/dmrr.2700. [DOI] [PubMed] [Google Scholar]
- 39.Brehmer F., Haenssle H. A., Daeschlein G., et al. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm®VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial ( NCT01415622) Journal of the European Academy of Dermatology and Venereology. 2015;29(1):148–155. doi: 10.1111/jdv.12490. [DOI] [PubMed] [Google Scholar]
- 40.Isbary G., Heinlin J., Shimizu T., et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology. 2012;167(2):404–410. doi: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isbary G., Morfill G., Schmidt H. U., et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. British Journal of Dermatology. 2010;163(1):78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 42.Isbary G., Shimizu T., Zimmermann J. L., et al. Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clinical Plasma Medicine. 2014;2(2):50–55. doi: 10.1016/j.cpme.2014.07.001. [DOI] [Google Scholar]
- 43.Isbary G., Stolz W., Shimizu T., et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: results of an open retrospective randomized controlled study in vivo. Clinical Plasma Medicine. 2013;1(2):25–30. doi: 10.1016/j.cpme.2013.06.001. [DOI] [Google Scholar]
- 44.Heinlin J., Isbary G., Stolz W., et al. A randomized two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus. Journal of the European Academy of Dermatology and Venereology. 2013;27(3):324–331. doi: 10.1111/j.1468-3083.2011.04395.x. [DOI] [PubMed] [Google Scholar]
- 45.Chuangsuwanich A., Assadamongkol T., Boonyawan D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: a randomized controlled trial. The International Journal of Lower Extremity Wounds. 2016;15(4):313–319. doi: 10.1177/1534734616665046. [DOI] [PubMed] [Google Scholar]
- 46.Mertens N., Helmke A., Goppold A., et al. Low temperature plasma treatment of human tissue. Second International Conference on Plasma Medicine; 2009; San Antonio, TX, USA. [Google Scholar]
- 47.Isbary G., Morfill G., Zimmermann J., Shimizu T., Stolz W. Cold atmospheric plasma: a successful treatment of lesions in Hailey-Hailey disease. Archives of Dermatology. 2011;147(4):388–390. doi: 10.1001/archdermatol.2011.57. [DOI] [PubMed] [Google Scholar]
- 48.Isbary G., Zimmermann J. L., Shimizu T., et al. Non-thermal plasma—more than five years of clinical experience. Clinical Plasma Medicine. 2013;1(1):19–23. doi: 10.1016/j.cpme.2012.11.001. [DOI] [Google Scholar]
- 49.Emmert S., Brehmer F., Hänßle H., et al. Atmospheric pressure plasma in dermatology: ulcus treatment and much more. Clinical Plasma Medicine. 2013;1(1):24–29. doi: 10.1016/j.cpme.2012.11.002. [DOI] [Google Scholar]
- 50.Tiede R., Hirschberg J., Daeschlein G., von Woedtke T., Vioel W., Emmert S. Plasma applications: a dermatological view. Contributions to Plasma Physics. 2014;54(2):118–130. doi: 10.1002/ctpp.201310061. [DOI] [Google Scholar]
- 51.Daeschlein G., Napp M., von Podewils S., et al. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Processes and Polymers. 2014;11(2):175–183. doi: 10.1002/ppap.201300070. [DOI] [Google Scholar]
- 52.Daeschlein G., Scholz S., Arnold A., et al. In vitro susceptibility of important skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Processes and Polymers. 2012;9(4):380–389. doi: 10.1002/ppap.201100160. [DOI] [Google Scholar]
- 53.Mai-Prochnow A., Clauson M., Hong J., Murphy A. B. Gram positive and gram negative bacteria differ in their sensitivity to cold plasma. Scientific Reports. 2016;6(1, article 38610) doi: 10.1038/srep38610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthes R., Lührman A., Holtfreter S., et al. Antibacterial activity of cold atmospheric pressure argon plasma against 78 genetically different (mecA, luk-P, agr or capsular polysaccharide type) Staphylococcus aureus strains. Skin Pharmacology and Physiology. 2016;29(2):83–91. doi: 10.1159/000443210. [DOI] [PubMed] [Google Scholar]
- 55.Napp M., Daeschlein G., von Podewils S., et al. In vitro susceptibility of methicillin-resistant and methicillin-susceptible strains of Staphylococcus aureus to two different cold atmospheric plasma sources. Infection. 2016;44(4):531–537. doi: 10.1007/s15010-016-0888-9. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor N., Cahill O., Daniels S., Galvin S., Humphreys H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? Journal of Hospital Infection. 2014;88(2):59–65. doi: 10.1016/j.jhin.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 57.Ziuzina D., Boehm D., Patil S., Cullen P. J., Bourke P. Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One. 2015;10(9, article e0138209) doi: 10.1371/journal.pone.0138209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lademann J., Richter H., Patzelt A., et al. Antisepsis of the skin by treatment with tissue-tolerable plasma (TTP): risk assessment and perspectives. In: Machala Z., Hensel K., Akishev Y., editors. Plasma for Bio-Decontamination, Medicine and Food Security. Dordrecht, Netherlands: Springer; 2012. pp. 281–291. (NATO Science for Peace and Security Series A: Chemistry and Biology). [DOI] [Google Scholar]
- 59.Lademann J., Richter H., Schanzer S., et al. Comparison of the antiseptic efficacy of tissue-tolerable plasma and an octenidine hydrochloride-based wound antiseptic on human skin. Skin Pharmacology and Physiology. 2012;25(2):100–106. doi: 10.1159/000335558. [DOI] [PubMed] [Google Scholar]
- 60.Lademann O., Kramer A., Richter H., et al. Antisepsis of the follicular reservoir by treatment with tissue-tolerable plasma (TTP) Laser Physics Letters. 2011;8(4):313–317. doi: 10.1002/lapl.201010123. [DOI] [Google Scholar]
- 61.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 62.Schuster M., Rutkowski R., Hauschild A., et al. Side effects in cold plasma treatment of advanced oral cancer—Clinical data and biological interpretation. Clinical Plasma Medicine. 2018;10:9–15. doi: 10.1016/j.cpme.2018.04.001. [DOI] [Google Scholar]
- 63.Metelmann H.-R., Seebauer C., Miller V., et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clinical Plasma Medicine. 2018;9:6–13. doi: 10.1016/j.cpme.2017.09.001. [DOI] [Google Scholar]
- 64.Metelmann H.-R., Nedrelow D. S., Seebauer C., et al. Head and neck cancer treatment and physical plasma. Clinical Plasma Medicine. 2015;3(1):17–23. doi: 10.1016/j.cpme.2015.02.001. [DOI] [Google Scholar]
- 65.Metelmann H. R., Seebauer C., Rutkowski R., Schuster M., Bekeschus S., Metelmann P. Treating cancer with cold physical plasma: on the way to evidence-based medicine. Contributions to Plasma Physics. 2018;58(5):415–419. doi: 10.1002/ctpp.201700085. [DOI] [Google Scholar]
- 66.Jansen P., Schadendorf D., Bekeschus S., Klode J., Stoffels I. Laser-assisted delivery of cold atmospheric plasma in unresectable cutaneous metastasis in melanoma patients. Clinical Plasma Medicine. 2018;9:p. 46. doi: 10.1016/j.cpme.2017.12.071. [DOI] [Google Scholar]
- 67.Daeschlein G., Arnold A., Lutze S., et al. Treatment of recalcitrant actinic keratosis (AK) of the scalp by cold atmospheric plasma. Cogent Medicine. 2017;4(1, article 1412903) doi: 10.1080/2331205x.2017.1412903. [DOI] [Google Scholar]
- 68.Friedman P. C., Miller V., Fridman G., Lin A., Fridman A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: a case series. Journal of the American Academy of Dermatology. 2017;76(2):349–350. doi: 10.1016/j.jaad.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Wirtz M., Stoffels I., Dissemond J., Schadendorf D., Roesch A. Actinic keratoses treated with cold atmospheric plasma. Journal of the European Academy of Dermatology and Venereology. 2018;32(1):e37–e39. doi: 10.1111/jdv.14465. [DOI] [PubMed] [Google Scholar]
- 70.Seebauer C., Hasse S., Segebarth M., et al. Cold Atmospheric Plasma for the treatment of Oral Lichen Planus as intraoral precancerous lesion. Clinical Plasma Medicine. 2018;9:44–45. doi: 10.1016/j.cpme.2017.12.069. [DOI] [Google Scholar]
- 71.Egea J., Fabregat I., Frapart Y. M., et al. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox Biology. 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sthijns M. M. J. P. E., Weseler A. R., Bast A., Haenen G. R. M. M. Time in redox adaptation processes: from evolution to hormesis. International Journal of Molecular Sciences. 2016;17(10):p. 1649. doi: 10.3390/ijms17101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calabrese E. J., Baldwin L. A. Defining hormesis. Human & Experimental Toxicology. 2002;21(2):91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 74.Mattson M. P. Hormesis defined. Ageing Research Reviews. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang P., He X. Q., Peng L., et al. The role of oxidative stress in hormesis induced by sodium arsenite in human embryo lung fibroblast (HELF) cellular proliferation model. Journal of Toxicology and Environmental Health, Part A. 2007;70(11):976–983. doi: 10.1080/15287390701290832. [DOI] [PubMed] [Google Scholar]
- 76.Labunskyy V. M., Gladyshev V. N. Role of reactive oxygen species-mediated signaling in aging. Antioxidants & Redox Signaling. 2013;19(12):1362–1372. doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Experimental Gerontology. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Wong J. L., Creton R., Wessel G. M. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Developmental Cell. 2004;7(6):801–814. doi: 10.1016/j.devcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 79.Niethammer P., Grabher C., Look A. T., Mitchison T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459(7249):996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanschmann E. M., Godoy J. R., Berndt C., Hudemann C., Lillig C. H. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants & Redox Signaling. 2013;19(13):1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finkel T. Signal transduction by reactive oxygen species. Journal of Cell Biology. 2011;194(1):7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saitoh M., Nishitoh H., Fujii M., et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO Journal. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galluzzi L., Vitale I., Aaronson S. A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death & Differentiation. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vandenabeele P., Galluzzi L., Vanden Berghe T., Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Reviews Molecular Cell Biology. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann J. H. O., Enk A. H. Neutrophil extracellular traps in dermatology: caught in the NET. Journal of Dermatological Science. 2016;84(1):3–10. doi: 10.1016/j.jdermsci.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Minasyan H. Phagocytosis and oxycytosis: two arms of human innate immunity. Immunologic Research. 2018;66(2):271–280. doi: 10.1007/s12026-018-8988-5. [DOI] [PubMed] [Google Scholar]
- 87.Bekeschus S., Brüggemeier J., Hackbarth C., von Woedtke T., Partecke L.-I., van der Linde J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clinical Plasma Medicine. 2017;7-8:58–65. doi: 10.1016/j.cpme.2017.10.001. [DOI] [Google Scholar]
- 88.Ahmed K. M., Eldeighdye S. M., Allam T. M., Hassanin W. F. Power density measurements to optimize ac plasma jet operation in blood coagulation. Australasian Physical & Engineering Sciences in Medicine. 2018;41(3):621–632. doi: 10.1007/s13246-018-0654-7. [DOI] [PubMed] [Google Scholar]
- 89.Bekeschus S., Bruggemeier J., Hackbarth C., et al. The feed gas composition determines the degree of physical plasma-induced platelet activation for blood coagulation. Plasma Sources Science and Technology. 2018;27(3, article 034001) doi: 10.1088/1361-6595/aaaf0e. [DOI] [Google Scholar]
- 90.Wende K., Bekeschus S., Schmidt A., et al. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2016;798-799:48–54. doi: 10.1016/j.mrgentox.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Bekeschus S., Seebauer C., Wende K., Schmidt A. Physical plasma and leukocytes – immune or reactive? Biological Chemistry. 2018;400(1):63–75. doi: 10.1515/hsz-2018-0224. [DOI] [PubMed] [Google Scholar]
- 92.Bekeschus S., Rödder K., Schmidt A., et al. Cold physical plasma selects for specific T helper cell subsets with distinct cells surface markers in a caspase-dependent and NF-κB-independent manner. Plasma Processes and Polymers. 2016;13(12):1144–1150. doi: 10.1002/ppap.201600080. [DOI] [Google Scholar]
- 93.Leutner S., Eckert A., Muller W. E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. Journal of Neural Transmission. 2001;108(8):955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- 94.Szili E. J., Harding F. J., Hong S. H., Herrmann F., Voelcker N. H., Short R. D. The hormesis effect of plasma-elevated intracellular ROS on HaCaT cells. Journal of Physics D: Applied Physics. 2015;48(49, article 495401) doi: 10.1088/0022-3727/48/49/495401. [DOI] [Google Scholar]
- 95.Brun P., Brun P., Vono M., et al. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS One. 2012;7(3, article e33245) doi: 10.1371/journal.pone.0033245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliveira M. F., Geihs M. A., Franca T. F. A., Moreira D. C., Hermes-Lima M. Is “preparation for oxidative stress” a case of physiological conditioning hormesis? Frontiers in Physiology. 2018;9:p. 945. doi: 10.3389/fphys.2018.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt-Bleker A., Winter J., Bösel A., Reuter S., Weltmann K.-D. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Science and Technology. 2016;25(1, article 015005) doi: 10.1088/0963-0252/25/1/015005. [DOI] [Google Scholar]
- 98.Winter J., Tresp H., Hammer M. U., et al. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. Journal of Physics D: Applied Physics. 2014;47(28, article 285401) doi: 10.1088/0022-3727/47/28/285401. [DOI] [Google Scholar]
- 99.Winter T., Bernhardt J., Winter J., et al. Common versus noble Bacillus subtilis differentially responds to air and argon gas plasma. Proteomics. 2013;13(17):2608–2621. doi: 10.1002/pmic.201200343. [DOI] [PubMed] [Google Scholar]
- 100.Jablonowski H., von Woedtke T. Research on plasma medicine-relevant plasma–liquid interaction: what happened in the past five years? Clinical Plasma Medicine. 2015;3(2):42–52. doi: 10.1016/j.cpme.2015.11.003. [DOI] [Google Scholar]
- 101.Heusler T., Bruno G., Bekeschus S., Lackmann J.-W., von Woedtke T., Wende K. Can the effect of cold physical plasma-derived oxidants be transported via thiol group oxidation? Clinical Plasma Medicine. 2019;14, article 100086 doi: 10.1016/j.cpme.2019.100086. [DOI] [Google Scholar]
- 102.Grune T., Shringarpure R., Sitte N., Davies K. Age-related changes in protein oxidation and proteolysis in mammalian cells. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(11):B459–B467. doi: 10.1093/gerona/56.11.B459. [DOI] [PubMed] [Google Scholar]
- 103.Cordeiro R. M. Reactive oxygen species at phospholipid bilayers: distribution, mobility and permeation. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2014;1838(1):438–444. doi: 10.1016/j.bbamem.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 104.Yusupov M., Wende K., Kupsch S., Neyts E. C., Reuter S., Bogaerts A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Scientific Reports. 2017;7(1):p. 5761. doi: 10.1038/s41598-017-06412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Szili E. J., Hong S. H., Oh J. S., Gaur N., Short R. D. Tracking the penetration of plasma reactive species in tissue models. Trends in Biotechnology. 2018;36(6):594–602. doi: 10.1016/j.tibtech.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Partecke L. I., Evert K., Haugk J., et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer. 2012;12(1):p. 473. doi: 10.1186/1471-2407-12-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruno G., Heusler T., Lackmann J.-W., von Woedtke T., Weltmann K.-D., Wende K. Cold physical plasma-induced oxidation of cysteine yields reactive sulfur species (RSS) Clinical Plasma Medicine. 2019;14, article 100083 doi: 10.1016/j.cpme.2019.100083. [DOI] [Google Scholar]
- 108.Lackmann J. W., Wende K., Verlackt C., et al. Chemical fingerprints of cold physical plasmas – an experimental and computational study using cysteine as tracer compound. Scientific Reports. 2018;8(1):p. 7736. doi: 10.1038/s41598-018-25937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lackmann J. W., Bruno G., Jablonowski H., et al. Nitrosylation vs. oxidation – how to modulate cold physical plasmas for biological applications. PLoS One. 2019;14(5, article e0216606) doi: 10.1371/journal.pone.0216606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arndt S., Unger P., Berneburg M., Bosserhoff A. K., Karrer S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. Journal of Dermatological Science. 2018;89(2):181–190. doi: 10.1016/j.jdermsci.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 111.Ishaq M., Evans M. M., Ostrikov K. K. Effect of atmospheric gas plasmas on cancer cell signaling. International Journal of Cancer. 2014;134(7):1517–1528. doi: 10.1002/ijc.28323. [DOI] [PubMed] [Google Scholar]
- 112.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nature Reviews Immunology. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 113.Bekeschus S., Clemen R., Metelmann H.-R. Potentiating anti-tumor immunity with physical plasma. Clinical Plasma Medicine. 2018;12:17–22. doi: 10.1016/j.cpme.2018.10.001. [DOI] [Google Scholar]
- 114.Kuksal N., Chalker J., Mailloux R. J. Progress in understanding the molecular oxygen paradox – function of mitochondrial reactive oxygen species in cell signaling. Biological Chemistry. 2017;398(11):1209–1227. doi: 10.1515/hsz-2017-0160. [DOI] [PubMed] [Google Scholar]
- 115.Short J. D., Downs K., Tavakoli S., Asmis R. Protein thiol redox signaling in monocytes and macrophages. Antioxidants & Redox Signaling. 2016;25(15):816–835. doi: 10.1089/ars.2016.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winterbourn C. C., Hampton M. B. Redox biology: signaling via a peroxiredoxin sensor. Nature Chemical Biology. 2015;11(1):5–6. doi: 10.1038/nchembio.1722. [DOI] [PubMed] [Google Scholar]
- 117.Forman H. J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondo N., Nakamura H., Masutani H., Yodoi J. Redox regulation of human thioredoxin network. Antioxidants & Redox Signaling. 2006;8(9-10):1881–1890. doi: 10.1089/ars.2006.8.1881. [DOI] [PubMed] [Google Scholar]
- 119.Bundscherer L., Wende K., Ottmuller K., et al. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology. 2013;218(10):1248–1255. doi: 10.1016/j.imbio.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt A., Dietrich S., Steuer A., et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. Journal of Biological Chemistry. 2015;290(11):6731–6750. doi: 10.1074/jbc.M114.603555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt A., Wende K., Bekeschus S., et al. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free Radical Research. 2013;47(8):577–592. doi: 10.3109/10715762.2013.804623. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt A., Bekeschus S., Wende K., Vollmar B., von Woedtke T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Experimental Dermatology. 2017;26(2):156–162. doi: 10.1111/exd.13156. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt A., von Woedtke T., Vollmar B., Hasse S., Bekeschus S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics. 2019;9(4):1066–1084. doi: 10.7150/thno.29754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jaiswal A. K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radical Biology and Medicine. 2004;36(10):1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen T., Sherratt P. J., Nioi P., Yang C. S., Pickett C. B. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. Journal of Biological Chemistry. 2005;280(37):32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 126.Schmidt A., Bekeschus S. Redox for repair: cold physical plasmas and Nrf2 signaling promoting wound healing. Antioxidants. 2018;7(10):p. 146. doi: 10.3390/antiox7100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cullinan S. B., Gordan J. D., Jin J., Harper J. W., Diehl J. A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Molecular and Cellular Biology. 2004;24(19):8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun Z., Zhang S., Chan J. Y., Zhang D. D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Molecular and Cellular Biology. 2007;27(18):6334–6349. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gweon B., Kim D., Kim D. B., Jung H., Choe W., Shin J. H. Plasma effects on subcellular structures. Applied Physics Letters. 2010;96(10, article 101501) doi: 10.1063/1.3352316. [DOI] [Google Scholar]
- 130.Gweon B., Kim M., Kim K., et al. Role of atmospheric pressure plasma (APP) in wound healing: APP-induced antifibrotic process in human dermal fibroblasts. Experimental Dermatology. 2016;25(2):159–161. doi: 10.1111/exd.12865. [DOI] [PubMed] [Google Scholar]
- 131.Haertel B., Hahnel M., Blackert S., Wende K., von Woedtke T., Lindequist U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biology International. 2012;36(12):1217–1222. doi: 10.1042/CBI20120139. [DOI] [PubMed] [Google Scholar]
- 132.Haertel B., Wende K., von Woedtke T., Weltmann K. D., Lindequist U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Experimental Dermatology. 2011;20(3):282–284. doi: 10.1111/j.1600-0625.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 133.Kim S. J., Chung T. H. Cold atmospheric plasma jet-generated rons and their selective effects on normal and carcinoma cells. Scientific Reports. 2016;6(1, article 20332) doi: 10.1038/srep20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schmidt A., von Woedtke T., Bekeschus S. Periodic exposure of keratinocytes to cold physical plasma: an in vitro model for redox-related diseases of the skin. Oxidative Medicine and Cellular Longevity. 2016;2016:17. doi: 10.1155/2016/9816072.9816072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoentsch M., von Woedtke T., Weltmann K. D., Barbara Nebe J. Time-dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. Journal of Physics D: Applied Physics. 2012;45(2, article 025206) doi: 10.1088/0022-3727/45/2/025206. [DOI] [Google Scholar]
- 136.Brandner J. M., Houdek P., Husing B., Kaiser C., Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. Journal of Investigative Dermatology. 2004;122(5):1310–1320. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- 137.Brandner J. M., Zacheja S., Houdek P., Moll I., Lobmann R. Expression of matrix metalloproteinases, cytokines, and connexins in diabetic and nondiabetic human keratinocytes before and after transplantation into an ex vivo wound-healing model. Diabetes Care. 2008;31(1):114–120. doi: 10.2337/dc07-1304. [DOI] [PubMed] [Google Scholar]
- 138.Gill S. E., Parks W. C. Metalloproteinases and their inhibitors: regulators of wound healing. The International Journal of Biochemistry & Cell Biology. 2008;40(6-7):1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eisenhauer P., Chernets N., Song Y., et al. Chemical modification of extracellular matrix by cold atmospheric plasma-generated reactive species affects chondrogenesis and bone formation. Journal of Tissue Engineering and Regenerative Medicine. 2016;10(9):772–782. doi: 10.1002/term.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dezest M., Chavatte L., Bourdens M., et al. Mechanistic insights into the impact of cold atmospheric pressure plasma on human epithelial cell lines. Scientific Reports. 2017;7(1, article 41163) doi: 10.1038/srep41163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daeschlein G., Scholz S., Ahmed R., et al. Cold plasma is well-tolerated and does not disturb skin barrier or reduce skin moisture. Journal der Deutschen Dermatologischen Gesellschaft. 2012;10(7):509–515. doi: 10.1111/j.1610-0387.2012.07857.x. [DOI] [PubMed] [Google Scholar]
- 142.Ahmed S. M. U., Luo L., Namani A., Wang X. J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 143.Bekeschus S., Schmidt A., Weltmann K.-D., von Woedtke T. The plasma jet kINPen – a powerful tool for wound healing. Clinical Plasma Medicine. 2016;4(1):19–28. doi: 10.1016/j.cpme.2016.01.001. [DOI] [Google Scholar]
- 144.Bekeschus S., Brautigam L., Wende K., Hanschmann E. M. Oxidants and redox signaling: perspectives in cancer therapy, inflammation, and plasma medicine. Oxidative Medicine and Cellular Longevity. 2017;2017:2. doi: 10.1155/2017/4020253.4020253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Crestale L., Laurita R., Liguori A., et al. Cold atmospheric pressure plasma treatment modulates human monocytes/macrophages responsiveness. Plasma. 2018;1(2):261–276. doi: 10.3390/plasma1020023. [DOI] [Google Scholar]
- 146.Van den Bossche J., Baardman J., Otto N. A., et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Reports. 2016;17(3):684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 147.Bekeschus S., Schmidt A., Bethge L., et al. Redox stimulation of human THP-1 monocytes in response to cold physical plasma. Oxidative Medicine and Cellular Longevity. 2016;2016:11. doi: 10.1155/2016/5910695.5910695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arndt S., Schmidt A., Karrer S., von Woedtke T. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clinical Plasma Medicine. 2018;9:24–33. doi: 10.1016/j.cpme.2018.01.002. [DOI] [Google Scholar]
- 149.Barton A., Wende K., Bundscherer L., et al. Nonthermal plasma increases expression of wound healing related genes in a keratinocyte cell line. Plasma Medicine. 2013;3(1-2):125–136. doi: 10.1615/PlasmaMed.2014008540. [DOI] [Google Scholar]
- 150.Shi X., Cai J., Xu G., et al. Effect of cold plasma on cell viability and collagen synthesis in cultured murine fibroblasts. Plasma Science and Technology. 2016;18(4):353–359. doi: 10.1088/1009-0630/18/4/04. [DOI] [Google Scholar]
- 151.Steuer A., Wolff C. M., von Woedtke T., Weltmann K. D., Kolb J. F. Cell stimulation versus cell death induced by sequential treatments with pulsed electric fields and cold atmospheric pressure plasma. PLoS One. 2018;13(10, article e0204916) doi: 10.1371/journal.pone.0204916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Q., Zhuang J., von Woedtke T., et al. Synergistic antibacterial effects of treatments with low temperature plasma jet and pulsed electric fields. Applied Physics Letters. 2014;105(10, article 104103) doi: 10.1063/1.4895731. [DOI] [Google Scholar]
- 153.Arndt S., Unger P., Wacker E., et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One. 2013;8(11, article e79325) doi: 10.1371/journal.pone.0079325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bundscherer L., Nagel S., Hasse S., et al. Non-thermal plasma treatment induces MAPK signaling in human monocytes. Open Chemistry. 2014;13(1):606–613. doi: 10.1515/chem-2015-0071. [DOI] [Google Scholar]
- 155.Maillet A., Pervaiz S. Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxidants & Redox Signaling. 2012;16(11):1285–1294. doi: 10.1089/ars.2011.4434. [DOI] [PubMed] [Google Scholar]
- 156.Chen W., Jiang T., Wang H., et al. Does Nrf2 contribute to p53-mediated control of cell survival and death? Antioxidants & Redox Signaling. 2012;17(12):1670–1675. doi: 10.1089/ars.2012.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vollmar B., El-Gibaly A. M., Scheuer C., Strik M. W., Bruch H. P., Menger M. D. Acceleration of cutaneous wound healing by transient p53 inhibition. Laboratory Investigation. 2002;82(8):1063–1071. doi: 10.1097/01.LAB.0000024363.37866.45. [DOI] [PubMed] [Google Scholar]
- 158.Hiemstra S., Niemeijer M., Koedoot E., et al. Comprehensive landscape of Nrf2 and p53 pathway activation dynamics by oxidative stress and DNA damage. Chemical Research in Toxicology. 2017;30(4):923–933. doi: 10.1021/acs.chemrestox.6b00322. [DOI] [PubMed] [Google Scholar]
- 159.Greenhalgh D. G. The role of apoptosis in wound healing. The International Journal of Biochemistry & Cell Biology. 1998;30(9):1019–1030. doi: 10.1016/S1357-2725(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen P. D., Tutela J. P., Thanik V. D., et al. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair and Regeneration. 2010;18(6):553–559. doi: 10.1111/j.1524-475X.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Babington P., Rajjoub K., Canady J., Siu A., Keidar M., Sherman J. H. Use of cold atmospheric plasma in the treatment of cancer. Biointerphases. 2015;10(2, article 029403) doi: 10.1116/1.4915264. [DOI] [PubMed] [Google Scholar]
- 162.Mattia G., Puglisi R., Ascione B., Malorni W., Care A., Matarrese P. Cell death-based treatments of melanoma: conventional treatments and new therapeutic strategies. Cell Death & Disease. 2018;9(2):p. 112. doi: 10.1038/s41419-017-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan X., Zou F., Zhao S., et al. On the mechanism of plasma inducing cell apoptosis. IEEE Transactions on Plasma Science. 2010;38(9):2451–2457. doi: 10.1109/Tps.2010.2056393. [DOI] [Google Scholar]
- 164.Schmidt A., Bekeschus S., Jarick K., Hasse S., von Woedtke T., Wende K. Cold physical plasma modulates p53 and mitogen-activated protein kinase signaling in keratinocytes. Oxidative Medicine and Cellular Longevity. 2019;2019:16. doi: 10.1155/2019/7017363.7017363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.von Woedtke T., Metelmann H. R., Weltmann K. D. Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contributions to Plasma Physics. 2014;54(2):104–117. doi: 10.1002/ctpp.201310068. [DOI] [Google Scholar]
- 166.Dubuc A., Monsarrat P., Virard F., et al. Use of cold-atmospheric plasma in oncology: a concise systematic review. Therapeutic Advances in Medical Oncology. 2018;10 doi: 10.1177/1758835918786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Privat-Maldonado A., Gorbanev Y., Dewilde S., Smits E., Bogaerts A. Reduction of human glioblastoma spheroids using cold atmospheric plasma: the combined effect of short- and long-lived reactive species. Cancers. 2018;10(11):p. 394. doi: 10.3390/cancers10110394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bekeschus S., Lin A., Fridman A., Wende K., Weltmann K. D., Miller V. A comparison of floating-electrode DBD and kINPen jet: plasma parameters to achieve similar growth reduction in colon cancer cells under standardized conditions. Plasma Chemistry and Plasma Processing. 2018;38(1):1–12. doi: 10.1007/s11090-017-9845-3. [DOI] [Google Scholar]
- 169.Kwon B. S., Choi E. H., Chang B., Choi J. H., Kim K. S., Park H. K. Selective cytotoxic effect of non-thermal micro-DBD plasma. Physical Biology. 2016;13(5, article 056001) doi: 10.1088/1478-3975/13/5/056001. [DOI] [PubMed] [Google Scholar]
- 170.Bekeschus S., Kading A., Schroder T., et al. Cold physical plasma-treated buffered saline solution as effective agent against pancreatic cancer cells. Anti-Cancer Agents in Medicinal Chemistry. 2018;18(6):824–831. doi: 10.2174/1871520618666180507130243. [DOI] [PubMed] [Google Scholar]
- 171.Xu D., Cui Q., Xu Y., et al. NO2− and NO3− enhance cold atmospheric plasma induced cancer cell death by generation of ONOO−. AIP Advances. 2018;8(10) doi: 10.1063/1.5046353. [DOI] [Google Scholar]
- 172.Bekeschus S., Schmidt A., Niessner F., Gerling T., Weltmann K. D., Wende K. Basic research in plasma medicine - a throughput approach from liquids to cells. Journal of Visualized Experiments. 2017;(129, article e56331) doi: 10.3791/56331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Freund E., Liedtke K. R., Gebbe R., Heidecke A. K., Partecke L. I., Bekeschus S. In vitro anticancer efficacy of six different clinically approved types of liquids exposed to physical plasma. IEEE Transactions on Radiation and Plasma Medical Sciences. 2019;3(5):588–596. doi: 10.1109/trpms.2019.2902015. [DOI] [Google Scholar]
- 174.Bekeschus S., Wulf C., Freund E., et al. Plasma treatment of ovarian cancer cells mitigates their immuno-modulatory products active on THP-1 monocytes. Plasma. 2018;1(1):201–217. doi: 10.3390/plasma1010018. [DOI] [Google Scholar]
- 175.Hasse S., Seebauer C., Wende K., et al. Cold argon plasma as adjuvant tumour therapy on progressive head and neck cancer: a preclinical study. Applied Sciences. 2019;9(10, article 2061) doi: 10.3390/app9102061. [DOI] [Google Scholar]
- 176.Koensgen D., Besic I., Gumbel D., et al. Cold atmospheric plasma (CAP) and CAP-stimulated cell culture media suppress ovarian cancer cell growth – a putative treatment option in ovarian cancer therapy. Anticancer Research. 2017;37(12):6739–6744. doi: 10.21873/anticanres.12133. [DOI] [PubMed] [Google Scholar]
- 177.Schmidt A., Rodder K., Hasse S., et al. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Processes and Polymers. 2016;13(12):1179–1188. doi: 10.1002/ppap.201600090. [DOI] [Google Scholar]
- 178.Ayala A., Munoz M. F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:31. doi: 10.1155/2014/360438.360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Van der Paal J., Neyts E. C., Verlackt C. C. W., Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chemical Science. 2016;7(1):489–498. doi: 10.1039/c5sc02311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yusupov M., van der Paal J., Neyts E. C., Bogaerts A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochimica et Biophysica Acta (BBA) - General Subjects. 2017;1861(4):839–847. doi: 10.1016/j.bbagen.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 181.Zhang Y., Chen X., Gueydan C., Han J. Plasma membrane changes during programmed cell deaths. Cell Research. 2018;28(1):9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gaschler M. M., Stockwell B. R. Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Furuta T., Shi L., Toyokuni S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: a possible role of ferritin. Pathology International. 2018;68(7):442–443. doi: 10.1111/pin.12665. [DOI] [PubMed] [Google Scholar]
- 184.Kehrer J. P., Robertson J. D., Smith C. V. Free radicals and reactive oxygen species. In: McQueen C. A., editor. Comprehensive Toxicology. 2. Vol. 1. Oxford, UK: Elsevier; 2010. pp. 277–307. [DOI] [Google Scholar]
- 185.Cairns R. A., Harris I. S., Mak T. W. Regulation of cancer cell metabolism. Nature Reviews Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 186.Liedtke K. R., Diedrich S., Pati O., et al. Cold physical plasma selectively elicits apoptosis in murine pancreatic cancer cells in vitro and in ovo. Anticancer Research. 2018;38(10):5655–5663. doi: 10.21873/anticanres.12901. [DOI] [PubMed] [Google Scholar]
- 187.Liu J., Wang Z. Increased oxidative stress as a selective anticancer therapy. Oxidative Medicine and Cellular Longevity. 2015;2015:12. doi: 10.1155/2015/294303.294303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yan D., Talbot A., Nourmohammadi N., Sherman J. H., Cheng X., Keidar M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—a model based on aquaporins (review) Biointerphases. 2015;10(4, article 040801) doi: 10.1116/1.4938020. [DOI] [PubMed] [Google Scholar]
- 189.Van der Paal J., Verheyen C., Neyts E. C., Bogaerts A. Hampering effect of cholesterol on the permeation of reactive oxygen species through phospholipids bilayer: possible explanation for plasma cancer selectivity. Scientific Reports. 2017;7(1, article 39526) doi: 10.1038/srep39526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Volotskova O., Hawley T. S., Stepp M. A., Keidar M. Targeting the cancer cell cycle by cold atmospheric plasma. Scientific Reports. 2012;2(1):p. 636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pasqual-Melo G., Gandhirajan R. K., Stoffels I., Bekeschus S. Targeting malignant melanoma with physical plasmas. Clinical Plasma Medicine. 2018;10:1–8. doi: 10.1016/j.cpme.2018.03.001. [DOI] [Google Scholar]
- 192.Vandamme M., Robert E., Dozias S., et al. Response of human glioma U87 xenografted on mice to non thermal plasma treatment. Plasma Medicine. 2011;1(1):27–43. doi: 10.1615/PlasmaMed.v1.i1.30. [DOI] [Google Scholar]
- 193.Vandamme M., Robert E., Lerondel S., et al. ROS implication in a new antitumor strategy based on non-thermal plasma. International Journal of Cancer. 2012;130(9):2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 194.Brulle L., Vandamme M., Ries D., et al. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS One. 2012;7(12, article e52653) doi: 10.1371/journal.pone.0052653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Masur K., von Behr M., Bekeschus S., et al. Synergistic inhibition of tumor cell proliferation by cold plasma and gemcitabine. Plasma Processes and Polymers. 2015;12(12):1377–1382. doi: 10.1002/ppap.201500123. [DOI] [Google Scholar]
- 196.Sagwal S. K., Pasqual-Melo G., Bodnar Y., Gandhirajan R. K., Bekeschus S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of SLC22A16. Cell Death & Disease. 2018;9(12):p. 1179. doi: 10.1038/s41419-018-1221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gandhirajan R., Griguer C., Bekeschus S. Cytochrome c oxidase inhibition and exogenous oxidants synergize in melanoma cell death induction. Free Radical Biology & Medicine. 2017;112:84–85. doi: 10.1016/j.freeradbiomed.2017.10.121. [DOI] [Google Scholar]
- 198.Bekeschus S., Lippert M., Diepold K., Chiosis G., Seufferlein T., Azoitei N. Physical plasma-triggered ROS induces tumor cell death upon cleavage of HSP90 chaperone. Scientific Reports. 2019;9(1):p. 4112. doi: 10.1038/s41598-019-38580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lin L., Wang L., Liu Y., Xu C., Tu Y., Zhou J. Non‑thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncology Reports. 2018;40:3405–3415. doi: 10.3892/or.2018.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mizuno K., Yonetamari K., Shirakawa Y., Akiyama T., Ono R. Anti-tumor immune response induced by nanosecond pulsed streamer discharge in mice. Journal of Physics D: Applied Physics. 2017;50(12, article 12LT01) doi: 10.1088/1361-6463/aa5dbb. [DOI] [Google Scholar]
- 201.Utsumi F., Kajiyama H., Nakamura K., et al. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8(12, article e81576) doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakamura K., Peng Y., Utsumi F., et al. Novel intraperitoneal treatment with non-thermal plasma-activated medium inhibits metastatic potential of ovarian cancer cells. Scientific Reports. 2017;7(1):p. 6085. doi: 10.1038/s41598-017-05620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Freund E., Liedtke K. R., van der Linde J., et al. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Scientific Reports. 2019;9(1):p. 634. doi: 10.1038/s41598-018-37169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liedtke K. R., Bekeschus S., Kaeding A., et al. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Scientific Reports. 2017;7(1):p. 8319. doi: 10.1038/s41598-017-08560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liedtke K. R., Freund E., Hackbarth C., Heidecke C.-D., Partecke L.-I., Bekeschus S. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clinical Plasma Medicine. 2018;11:10–17. doi: 10.1016/j.cpme.2018.07.001. [DOI] [Google Scholar]
- 206.Lin A. G., Xiang B., Merlino D. J., et al. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. OncoImmunology. 2018;7(9, article e1484978) doi: 10.1080/2162402X.2018.1484978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lin A., Gorbanev Y., De Backer J., et al. Non-thermal plasma as a unique delivery system of short-lived reactive oxygen and nitrogen species for immunogenic cell death in melanoma cells. Advanced Science. 2019;6(6, article 1802062) doi: 10.1002/advs.201802062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lin A., Truong B., Pappas A., et al. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Processes and Polymers. 2015;12(12):1392–1399. doi: 10.1002/ppap.201500139. [DOI] [Google Scholar]
- 209.Garg A. D., Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunological Reviews. 2017;280(1):126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 210.Stewart M. P., Sharei A., Ding X., Sahay G., Langer R., Jensen K. F. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 211.Hirschberg J., Gerhard C., Braun A., et al. Validation of the suitability of stripped lipid as a skin model in plasma medical investigations. Open Journal of Applied Sciences. 2015;5(2):40–49. doi: 10.4236/ojapps.2015.52005. [DOI] [Google Scholar]
- 212.Hirschberg J., Loewenthal L., Krupp A., Emmert S., Viöl W. Plasma induced changes in human lipid composition as revealed through XPS-analysis. Natural Science. 2016;8(3):125–137. doi: 10.4236/ns.2016.83016. [DOI] [Google Scholar]
- 213.Marschewski M., Hirschberg J., Omairi T., et al. Electron spectroscopic analysis of the human lipid skin barrier: cold atmospheric plasma-induced changes in lipid composition. Experimental Dermatology. 2012;21(12):921–925. doi: 10.1111/exd.12043. [DOI] [PubMed] [Google Scholar]
- 214.Striesow J., Lackmann J. W., Ni Z., et al. Oxidative modification of skin lipids by cold atmospheric plasma (CAP): a standardizable approach using RP-LC/MS2 and DI-ESI/MS2. Chemistry and Physics of Lipids. 2019;(article 104786) doi: 10.1016/j.chemphyslip.2019.104786. [DOI] [PubMed] [Google Scholar]
- 215.Yeagle P. L. The Structure of Biological Membranes. CRC press; 2011. [DOI] [Google Scholar]
- 216.Fahy E., Cotter D., Sud M., Subramaniam S. Lipid classification, structures and tools. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2011;1811(11):637–647. doi: 10.1016/j.bbalip.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yin H., Xu L., Porter N. A. Free radical lipid peroxidation: mechanisms and analysis. Chemical Reviews. 2011;111(10):5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 218.Kansanen E., Jyrkkanen H. K., Levonen A. L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radical Biology & Medicine. 2012;52(6):973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 219.Robaszkiewicz A., Greig F. H., Pitt A. R., Spickett C. M., Bartosz G., Soszynski M. Effect of phosphatidylcholine chlorohydrins on human erythrocytes. Chemistry and Physics of Lipids. 2010;163(7):639–647. doi: 10.1016/j.chemphyslip.2010.05.201. [DOI] [PubMed] [Google Scholar]
- 220.Maheux S., Frache G., Thomann J. S., et al. Small unilamellar liposomes as a membrane model for cell inactivation by cold atmospheric plasma treatment. Journal of Physics D: Applied Physics. 2016;49(34, article 344001) doi: 10.1088/0022-3727/49/34/344001. [DOI] [Google Scholar]
- 221.Kaneko T., Sasaki S., Hokari Y., Horiuchi S., Honda R., Kanzaki M. Improvement of cell membrane permeability using a cell-solution electrode for generating atmospheric-pressure plasma. Biointerphases. 2015;10(2, article 029521) doi: 10.1116/1.4921278. [DOI] [PubMed] [Google Scholar]
- 222.Sasaki S., Honda R., Hokari Y., Takashima K., Kanzaki M., Kaneko T. Characterization of plasma-induced cell membrane permeabilization: focus on OH radical distribution. Journal of Physics D: Applied Physics. 2016;49(33, article 334002) doi: 10.1088/0022-3727/49/33/334002. [DOI] [Google Scholar]
- 223.Smith W. L., Murphy R. C. Oxidized lipids formed non-enzymatically by reactive oxygen species. Journal of Biological Chemistry. 2008;283(23):15513–15514. doi: 10.1074/jbc.R800006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Girotti A. W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research. 1998;39(8):1529–1542. [PubMed] [Google Scholar]
- 225.Bekeschus S., Kolata J., Winterbourn C., et al. Hydrogen peroxide: a central player in physical plasma-induced oxidative stress in human blood cells. Free Radical Research. 2014;48(5):542–549. doi: 10.3109/10715762.2014.892937. [DOI] [PubMed] [Google Scholar]
- 226.Jarerattanachat V., Karttunen M., Wong-Ekkabut J. Molecular dynamics study of oxidized lipid bilayers in NaCl solution. The Journal of Physical Chemistry B. 2013;117(28):8490–8501. doi: 10.1021/jp4040612. [DOI] [PubMed] [Google Scholar]
- 227.Eggers L. F., Muller J., Marella C., et al. Lipidomes of lung cancer and tumour-free lung tissues reveal distinct molecular signatures for cancer differentiation, age, inflammation, and pulmonary emphysema. Scientific Reports. 2017;7(1, article 11087) doi: 10.1038/s41598-017-11339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fhaner C. J., Liu S., Ji H., Simpson R. J., Reid G. E. Comprehensive lipidome profiling of isogenic primary and metastatic colon adenocarcinoma cell lines. Analytical Chemistry. 2012;84(21):8917–8926. doi: 10.1021/ac302154g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Buczynski M. W., Dumlao D. S., Dennis E. A. Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. Journal of Lipid Research. 2009;50(6):1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Murphy R. C., Johnson K. M. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. Journal of Biological Chemistry. 2008;283(23):15521–15525. doi: 10.1074/jbc.R700049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yadav D. K., Kumar S., Choi E. H., Sharma P., Misra S., Kim M. H. Insight into the molecular dynamic simulation studies of reactive oxygen species in native skin membrane. Frontiers in Pharmacology. 2018;9:p. 644. doi: 10.3389/fphar.2018.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Blair I. A. DNA adducts with lipid peroxidation products. Journal of Biological Chemistry. 2008;283(23):15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hazen S. L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. Journal of Biological Chemistry. 2008;283(23):15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Freigang S. The regulation of inflammation by oxidized phospholipids. European Journal of Immunology. 2016;46(8):1818–1825. doi: 10.1002/eji.201545676. [DOI] [PubMed] [Google Scholar]
- 235.Yan D., Sherman J. H., Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8(9):15977–15995. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Turrini E., Laurita R., Stancampiano A., et al. Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in T-lymphoblastoid leukemia cells. Oxidative Medicine and Cellular Longevity. 2017;2017:13. doi: 10.1155/2017/4271065.4271065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vermeylen S., de Waele J., Vanuytsel S., et al. Cold atmospheric plasma treatment of melanoma and glioblastoma cancer cells. Plasma Processes and Polymers. 2016;13(12):1195–1205. doi: 10.1002/ppap.201600116. [DOI] [Google Scholar]
- 238.Weiss M., Gümbel D., Hanschmann E. M., et al. Cold atmospheric plasma treatment induces anti-proliferative effects in prostate cancer cells by redox and apoptotic signaling pathways. PLoS One. 2015;10(7, article e0130350) doi: 10.1371/journal.pone.0130350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Freeman B. A., Baker P. R. S., Schopfer F. J., Woodcock S. R., Napolitano A., d'Ischia M. Nitro-fatty acid formation and signaling. Journal of Biological Chemistry. 2008;283(23):15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rubbo H. Nitro-fatty acids: novel anti-inflammatory lipid mediators. Brazilian Journal of Medical and Biological Research. 2013;46(9):728–734. doi: 10.1590/1414-431X20133202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xiang L., Xu X., Zhang S., Cai D., Dai X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radical Biology & Medicine. 2018;124:205–213. doi: 10.1016/j.freeradbiomed.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 242.Chang J. W., Kang S. U., Shin Y. S., et al. Combination of NTP with cetuximab inhibited invasion/migration of cetuximab-resistant OSCC cells: involvement of NF-κB signaling. Scientific Reports. 2015;5(1, article 18208) doi: 10.1038/srep18208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kaushik N. K., Kaushik N., Park D., Choi E. H. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS One. 2014;9(7, article e103349) doi: 10.1371/journal.pone.0103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Benedikt J., Mokhtar Hefny M., Shaw A., et al. The fate of plasma-generated oxygen atoms in aqueous solutions: non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O(aq) Physical Chemistry Chemical Physics. 2018;20(17):12037–12042. doi: 10.1039/c8cp00197a. [DOI] [PubMed] [Google Scholar]
- 245.Zhang K., Perussello C. A., Milosavljevic V., Cullen P. J., Sun D. W., Tiwari B. K. Diagnostics of plasma reactive species and induced chemistry of plasma treated foods. Critical Reviews in Food Science and Nutrition. 2019;59(5):812–825. doi: 10.1080/10408398.2018.1564731. [DOI] [PubMed] [Google Scholar]
- 246.Palmer A. E., Dittmer P. J. Snap-shots of hydrogen peroxide in cells. Chemistry & Biology. 2010;17(4):318–319. doi: 10.1016/j.chembiol.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 247.Riethmuller M., Burger N., Bauer G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biology. 2015;6:157–168. doi: 10.1016/j.redox.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bedard K., Krause K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 249.Bienert G. P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840(5):1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 250.Papadopoulos M. C., Saadoun S. Key roles of aquaporins in tumor biology. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2015;1848(10):2576–2583. doi: 10.1016/j.bbamem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 251.Yusupov M., Yan D., Cordeiro R. M., Bogaerts A. Atomic scale simulation of H2O2 permeation through aquaporin: toward the understanding of plasma cancer treatment. Journal of Physics D: Applied Physics. 2018;51(12, article 125401) doi: 10.1088/1361-6463/aaae7a. [DOI] [Google Scholar]
- 252.Bauer G. Increasing the endogenous NO level causes catalase inactivation and reactivation of intercellular apoptosis signaling specifically in tumor cells. Redox Biology. 2015;6:353–371. doi: 10.1016/j.redox.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Böhm B., Heinzelmann S., Motz M., Bauer G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biological Chemistry. 2015;396(12):p. 1339. doi: 10.1515/hsz-2014-0234. [DOI] [PubMed] [Google Scholar]
- 254.Bauer G. Signal amplification by tumor cells: clue to the understanding of the antitumor effects of cold atmospheric plasma and plasma-activated medium. IEEE Transactions on Radiation and Plasma Medical Sciences. 2018;2(2):87–98. doi: 10.1109/trpms.2017.2742000. [DOI] [Google Scholar]
- 255.Fluhr J. W., Sassning S., Lademann O., et al. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Experimental Dermatology. 2012;21(2):130–134. doi: 10.1111/j.1600-0625.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 256.Szili E. J., Oh J. S., Fukuhara H., et al. Modelling the helium plasma jet delivery of reactive species into a 3D cancer tumour. Plasma Sources Science and Technology. 2017;27(1, article 014001) doi: 10.1088/1361-6595/aa9b3b. [DOI] [Google Scholar]
- 257.Kos S., Blagus T., Cemazar M., Filipic G., Sersa G., Cvelbar U. Safety aspects of atmospheric pressure helium plasma jet operation on skin: in vivo study on mouse skin. PLoS One. 2017;12(4, article e0174966) doi: 10.1371/journal.pone.0174966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nie L., Yang Y., Duan J., Sun F., Lu X., He G. Effect of tissue thickness and liquid composition on the penetration of long-lifetime reactive oxygen and nitrogen species (RONS) generated by a plasma jet. Journal of Physics D: Applied Physics. 2018;51(34, article 345204) doi: 10.1088/1361-6463/aad427. [DOI] [Google Scholar]
- 259.Liu D., He T., Liu Z., et al. Spatial-temporal distributions of ROS in model tissues treated by a He+O2 plasma jet. Plasma Processes and Polymers. 2018;15(10) doi: 10.1002/ppap.201800057. [DOI] [Google Scholar]
- 260.Szili E. J., Bradley J. W., Short R. D. A ‘tissue model’ to study the plasma delivery of reactive oxygen species. Journal of Physics D: Applied Physics. 2014;47(15, article 152002) doi: 10.1088/0022-3727/47/15/152002. [DOI] [Google Scholar]
- 261.Marshall S. E., Jenkins A. T. A., al-Bataineh S. A., et al. Studying the cytolytic activity of gas plasma with self-signalling phospholipid vesicles dispersed within a gelatin matrix. Journal of Physics D: Applied Physics. 2013;46(18, article 185401) doi: 10.1088/0022-3727/46/18/185401. [DOI] [Google Scholar]
- 262.He T., Liu D., Liu Z., et al. The mechanism of plasma-assisted penetration of NO2− in model tissues. Applied Physics Letters. 2017;111(20, article 203702) doi: 10.1063/1.4999366. [DOI] [Google Scholar]
- 263.He T., Liu D., Xu H., et al. A ‘tissue model’ to study the barrier effects of living tissues on the reactive species generated by surface air discharge. Journal of Physics D: Applied Physics. 2016;49(20, article 205204) doi: 10.1088/0022-3727/49/20/205204. [DOI] [Google Scholar]
- 264.Gaur N., Szili E. J., Oh J. S., et al. Combined effect of protein and oxygen on reactive oxygen and nitrogen species in the plasma treatment of tissue. Applied Physics Letters. 2015;107(10, article 103703) doi: 10.1063/1.4930874. [DOI] [Google Scholar]
- 265.Ghimire B., Szili E. J., Lamichhane P., et al. The role of UV photolysis and molecular transport in the generation of reactive species in a tissue model with a cold atmospheric pressure plasma jet. Applied Physics Letters. 2019;114(9, article 093701) doi: 10.1063/1.5086522. [DOI] [Google Scholar]
- 266.Oh J. S., Szili E. J., Gaur N., et al. How to assess the plasma delivery of rons into tissue fluid and tissue. Journal of Physics D: Applied Physics. 2016;49(30, article 304005) doi: 10.1088/0022-3727/49/30/304005. [DOI] [Google Scholar]
- 267.Oh J.-S., Szili E. J., Ito S., et al. Slow molecular transport of plasma-generated reactive oxygen and nitrogen species and O2 through agarose as a surrogate for tissue. Plasma Medicine. 2015;5(2-4):125–143. doi: 10.1615/PlasmaMed.2016015740. [DOI] [Google Scholar]
- 268.Oh J.-S., Szili E. J., Gaur N., et al. In-situ UV absorption spectroscopy for monitoring transport of plasma reactive species through agarose as surrogate for tissue. Journal of Photopolymer Science and Technology. 2015;28(3):439–444. doi: 10.2494/photopolymer.28.439. [DOI] [Google Scholar]
- 269.Szili E. J., Oh J. S., Hong S. H., Hatta A., Short R. D. Probing the transport of plasma-generated rons in an agarose target as surrogate for real tissue: dependency on time, distance and material composition. Journal of Physics D: Applied Physics. 2015;48(20, article 202001) doi: 10.1088/0022-3727/48/20/202001. [DOI] [Google Scholar]
- 270.Kawasaki T., Sato A., Kusumegi S., et al. Two-dimensional concentration distribution of reactive oxygen species transported through a tissue phantom by atmospheric-pressure plasma-jet irradiation. Applied Physics Express. 2016;9(7, article 076202) doi: 10.7567/Apex.9.076202. [DOI] [Google Scholar]
- 271.Kawasaki T., Mitsugi F., Koga K., Shiratani M. Local supply of reactive oxygen species into a tissue model by atmospheric-pressure plasma-jet exposure. Journal of Applied Physics. 2019;125(21, article 213303) doi: 10.1063/1.5091740. [DOI] [Google Scholar]
- 272.Szili E. J., Gaur N., Hong S.-H., et al. The assessment of cold atmospheric plasma treatment of DNA in synthetic models of tissue fluid, tissue and cells. Journal of Physics D: Applied Physics. 2017;50(27, article 274001) doi: 10.1088/1361-6463/aa7501. [DOI] [Google Scholar]
- 273.Chen C., Liu D. X., Liu Z. C., et al. A model of plasma-biofilm and plasma-tissue interactions at ambient pressure. Plasma Chemistry and Plasma Processing. 2014;34(3):403–441. doi: 10.1007/s11090-014-9545-1. [DOI] [Google Scholar]
- 274.Bekeschus S., Iseni S., Reuter S., Masur K., Weltmann K. D. Nitrogen shielding of an argon plasma jet and its effects on human immune cells. IEEE Transactions on Plasma Science. 2015;43(3):776–781. doi: 10.1109/Tps.2015.2393379. [DOI] [Google Scholar]
- 275.Bekeschus S., von Woedtke T., Kramer A., Weltmann K.-D., Masur K. Cold physical plasma treatment alters redox balance in human immune cells. Plasma Medicine. 2013;3(4):267–278. doi: 10.1615/PlasmaMed.2014011972. [DOI] [Google Scholar]
- 276.Bekeschus S., Freund E., Wende K., Gandhirajan R., Schmidt A. Hmox1 upregulation is a mutual marker in human tumor cells exposed to physical plasma-derived oxidants. Antioxidants. 2018;7(11):p. 151. doi: 10.3390/antiox7110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gümbel D., Bekeschus S., Gelbrich N., et al. Cold atmospheric plasma in the treatment of osteosarcoma. International Journal of Molecular Sciences. 2017;18(9):p. 2004. doi: 10.3390/ijms18092004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kleineidam B., Nokhbehsaim M., Deschner J., Wahl G. Effect of cold plasma on periodontal wound healing—an in vitro study. Clinical Oral Investigations. 2019;23(4):1941–1950. doi: 10.1007/s00784-018-2643-3. [DOI] [PubMed] [Google Scholar]
- 279.Kubinova S., Zaviskova K., Uherkova L., et al. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Scientific Reports. 2017;7(1, article 45183) doi: 10.1038/srep45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gherardi M., Turrini E., Laurita R., et al. Atmospheric non-equilibrium plasma promotes cell death and cell-cycle arrest in a lymphoma cell line. Plasma Processes and Polymers. 2015;12(12):1354–1363. doi: 10.1002/ppap.201500033. [DOI] [Google Scholar]
- 281.Hou J., Ma J., Yu K. N., et al. Non-thermal plasma treatment altered gene expression profiling in non-small-cell lung cancer A549 cells. BMC Genomics. 2015;16(1):p. 435. doi: 10.1186/s12864-015-1644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kang S. U., Cho J. H., Chang J. W., et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death & Disease. 2014;5(2, article e1056) doi: 10.1038/cddis.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kumar N., Attri P., Yadav D. K., Choi J., Choi E. H., Uhm H. S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Scientific Reports. 2014;4(1):p. 7589. doi: 10.1038/srep07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schmidt A., Bekeschus S., Jablonowski H., Barton A., Weltmann K. D., Wende K. Role of ambient gas composition on cold physical plasma-elicited cell signaling in keratinocytes. Biophysical Journal. 2017;112(11):2397–2407. doi: 10.1016/j.bpj.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Xia J., Zeng W., Xia Y., et al. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. Journal of Biophotonics. 2019;12(1, article e201800046) doi: 10.1002/jbio.201800046. [DOI] [PubMed] [Google Scholar]
- 286.Arndt S., Wacker E., Li Y. F., et al. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Experimental Dermatology. 2013;22(4):284–289. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- 287.Blackert S., Haertel B., Wende K., von Woedtke T., Lindequist U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (HaCaT) Journal of Dermatological Science. 2013;70(3):173–181. doi: 10.1016/j.jdermsci.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 288.Hayashi N., Miyamaru Y., Aijima R., Yamashita Y. Activation of p53-mediated apoptosis pathway in HSC3 cancer cell irradiated by atmospheric DBD oxygen plasma. IEEE Transactions on Plasma Science. 2019;47(2):1093–1099. doi: 10.1109/tps.2018.2867431. [DOI] [Google Scholar]
- 289.Kaushik N., Uddin N., Sim G. B., et al. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Scientific Reports. 2015;5(1):p. 8587. doi: 10.1038/srep08587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Smolkova B., Lunova M., Lynnyk A., et al. Non-thermal plasma, as a new physicochemical source, to induce redox imbalance and subsequent cell death in liver cancer cell lines. Cellular Physiology and Biochemistry. 2019;52(1):119–140. doi: 10.33594/000000009. [DOI] [PubMed] [Google Scholar]
- 291.Strassenburg S., Greim U., Bussiahn R., et al. Comparison of biological effects on human keratinocytes using different plasma treatment regimes. Plasma Medicine. 2013;3(1-2):57–69. doi: 10.1615/PlasmaMed.2014008219. [DOI] [Google Scholar]
- 292.Schmidt A., Bekeschus S., von Woedtke T., Hasse S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clinical Plasma Medicine. 2015;3(1):24–31. doi: 10.1016/j.cpme.2015.05.003. [DOI] [Google Scholar]
- 293.Choi J. H., Nam S. H., Song Y. S., et al. Treatment with low-temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E-cadherin-mediated cell junctions. Archives of Dermatological Research. 2014;306(7):635–643. doi: 10.1007/s00403-014-1463-9. [DOI] [PubMed] [Google Scholar]
- 294.Choi J. H., Song Y. S., Song K., Lee H. J., Hong J. W., Kim G. C. Skin renewal activity of non-thermal plasma through the activation of β-catenin in keratinocytes. Scientific Reports. 2017;7(1):p. 6146. doi: 10.1038/s41598-017-06661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Haertel B., Strassenburg S., Oehmigen K., Wende K., von Woedtke T., Lindequist U. Differential influence of components resulting from atmospheric-pressure plasma on integrin expression of human HaCaT keratinocytes. BioMed Research International. 2013;2013:9. doi: 10.1155/2013/761451.761451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Haertel B., Woedtke T. ., Weltmann K. D., Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomolecules & Therapeutics. 2014;22(6):477–490. doi: 10.4062/biomolther.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Haertel B., Volkmann F., von Woedtke T., Lindequist U. Differential sensitivity of lymphocyte subpopulations to non-thermal atmospheric-pressure plasma. Immunobiology. 2012;217(6):628–633. doi: 10.1016/j.imbio.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 298.Arndt S., Landthaler M., Zimmermann J. L., et al. Effects of cold atmospheric plasma (CAP) on β-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS One. 2015;10(3, article e0120041) doi: 10.1371/journal.pone.0120041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chang J. W., Kang S. U., Shin Y. S., et al. Non-thermal atmospheric pressure plasma inhibits thyroid papillary cancer cell invasion via cytoskeletal modulation, altered MMP-2/-9/uPA activity. PLoS One. 2014;9(3, article e92198) doi: 10.1371/journal.pone.0092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jang J. Y., Hong Y. J., Lim J., et al. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials. 2018;156:258–273. doi: 10.1016/j.biomaterials.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 301.Wiegand C., Fink S., Beier O., et al. Dose- and time-dependent cellular effects of cold atmospheric pressure plasma evaluated in 3D skin models. Skin Pharmacology and Physiology. 2016;29(5):257–265. doi: 10.1159/000450889. [DOI] [PubMed] [Google Scholar]
- 302.Almasalmeh A., Krenc D., Wu B., Beitz E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS Journal. 2014;281(3):647–656. doi: 10.1111/febs.12653. [DOI] [PubMed] [Google Scholar]
- 303.Lennicke C., Rahn J., Lichtenfels R., Wessjohann L. A., Seliger B. Hydrogen peroxide – production, fate and role in redox signaling of tumor cells. Cell Communication and Signaling. 2015;13(1):p. 39. doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Miller E. W., Dickinson B. C., Chang C. J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dajani S., Saripalli A., Sharma-Walia N. Water transport proteins–aquaporins (AQPS) in cancer biology. Oncotarget. 2018;9(91):36392–36405. doi: 10.18632/oncotarget.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Satooka H., Hara-Chikuma M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Molecular and Cellular Biology. 2016;36(7):1206–1218. doi: 10.1128/MCB.00971-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rodrigues C., Mósca A., Martins A., et al. Rat aquaporin-5 is pH-gated induced by phosphorylation and is implicated in oxidative stress. International Journal of Molecular Sciences. 2016;17(12):p. 2090. doi: 10.3390/ijms17122090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.De Ieso M. L., Yool A. J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Frontiers in Chemistry. 2018;6:p. 135. doi: 10.3389/fchem.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Li C., Wang W. Molecular biology of aquaporins. In: Yang B., editor. Aquaporins. Vol. 969. Springer; 2017. pp. 1–34. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 310.Bienert G. P., Møller A. L. B., Kristiansen K. A., et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry. 2007;282(2):1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 311.Yan D., Xiao H., Zhu W., et al. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. Journal of Physics D: Applied Physics. 2017;50(5, article 055401) doi: 10.1088/1361-6463/aa53d6. [DOI] [Google Scholar]
- 312.Chang H., Shi Y. H., Talaf T. K., Lin C. Aquaporin-8 mediates human esophageal cancer Eca-109 cell migration via the EGFR-Erk1/2 pathway. International Journal of Clinical and Experimental Pathology. 2014;7:7663–7671. [PMC free article] [PubMed] [Google Scholar]
- 313.Medrano-Fernandez I., Bestetti S., Bertolotti M., et al. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxidants & Redox Signaling. 2016;24(18):1031–1044. doi: 10.1089/ars.2016.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Watanabe S., Moniaga C. S., Nielsen S., Hara-Chikuma M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochemical and Biophysical Research Communications. 2016;471(1):191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- 315.Lee J. H., Om J. Y., Kim Y. H., Kim K. M., Choi E. H., Kim K. N. Selective killing effects of cold atmospheric pressure plasma with no induced dysfunction of epidermal growth factor receptor in oral squamous cell carcinoma. PLoS One. 2016;11(2, article e0150279) doi: 10.1371/journal.pone.0150279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Yusupov M., Lackmann J.-W., Razzokov J., Kumar S., Stapelmann K., Bogaerts A. Impact of plasma oxidation on structural features of human epidermal growth factor. Plasma Processes and Polymers. 2018;15(8) doi: 10.1002/ppap.201800022. [DOI] [Google Scholar]
- 317.Corcoran A., Cotter T. G. Redox regulation of protein kinases. FEBS Journal. 2013;280(9):1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 318.Halestrap A. P. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochemical Society Transactions. 2006;34(2):232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 319.Shashurin A., Stepp M. A., Hawley T. S., et al. Influence of cold plasma atmospheric jet on surface integrin expression of living cells. Plasma Processes and Polymers. 2010;7(3-4):294–300. doi: 10.1002/ppap.200900086. [DOI] [Google Scholar]
- 320.Volotskova O., Stepp M. A., Keidar M. Integrin activation by a cold atmospheric plasma jet. New Journal of Physics. 2012;14(5, article 053019) doi: 10.1088/1367-2630/14/5/053019. [DOI] [Google Scholar]
- 321.Wu C., Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. The Journal of Cell Biology. 2001;155(4):505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Im M., Dagnino L. Protective role of integrin-linked kinase against oxidative stress and in maintenance of genomic integrity. Oncotarget. 2018;9(17):13637–13651. doi: 10.18632/oncotarget.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lee H. Y., Choi J. H., Hong J. W., Kim G. C., Lee H. J. Comparative study of the ar and he atmospheric pressure plasmas on E-cadherin protein regulation for plasma-mediated transdermal drug delivery. Journal of Physics D: Applied Physics. 2018;51(21, article 215401) doi: 10.1088/1361-6463/aabd8c. [DOI] [Google Scholar]
- 324.Hung Y. W., Lee L. T., Peng Y. C., Chang C. T., Wong Y. K., Tung K. C. Effect of a nonthermal-atmospheric pressure plasma jet on wound healing: an animal study. Journal of the Chinese Medical Association. 2016;79(6):320–328. doi: 10.1016/j.jcma.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 325.Schmelz M. Selective disruption of cadherin/catenin complexes by oxidative stress in precision-cut mouse liver slices. Toxicological Sciences. 2001;61(2):389–394. doi: 10.1093/toxsci/61.2.389. [DOI] [PubMed] [Google Scholar]
- 326.Kim W. D., Kim Y. W., Cho I. J., Lee C. H., Kim S. G. E-cadherin inhibits nuclear accumulation of Nrf2: implications for chemoresistance of cancer cells. Journal of Cell Science. 2012;125(5):1284–1295. doi: 10.1242/jcs.095422. [DOI] [PubMed] [Google Scholar]
- 327.Watanabe T., Sato K., Kaibuchi K. Cadherin-mediated intercellular adhesion and signaling cascades involving small gtpases. Cold Spring Harbor Perspectives in Biology. 2009;1(3, article a003020) doi: 10.1101/cshperspect.a003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Goitre L., Pergolizzi B., Ferro E., Trabalzini L., Retta S. F. Molecular crosstalk between integrins and cadherins: do reactive oxygen species set the talk? Journal of Signal Transduction. 2012;2012:12. doi: 10.1155/2012/807682.807682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jiang J., Wang K., Chen Y., Chen H., Nice E. C., Huang C. Redox regulation in tumor cell epithelial–mesenchymal transition: molecular basis and therapeutic strategy. Signal Transduction and Targeted Therapy. 2017;2(1, article 17036) doi: 10.1038/sigtrans.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Basuroy S., Dunagan M., Sheth P., Seth A., Rao R. K. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;299(1):G186–G195. doi: 10.1152/ajpgi.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yeagle P. L. Cholesterol and the cell membrane. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1985;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 332.Beranova L., Cwiklik L., Jurkiewicz P., Hof M., Jungwirth P. Oxidation changes physical properties of phospholipid bilayers: fluorescence spectroscopy and molecular simulations. Langmuir. 2010;26(9):6140–6144. doi: 10.1021/la100657a. [DOI] [PubMed] [Google Scholar]
- 333.Razzokov J., Yusupov M., Vanuytsel S., Neyts E. C., Bogaerts A. Phosphatidylserine flip-flop induced by oxidation of the plasma membrane: a better insight by atomic scale modeling. Plasma Processes and Polymers. 2017;14(10, article 1700013) doi: 10.1002/ppap.201700013. [DOI] [Google Scholar]
- 334.Keidar M., Walk R., Shashurin A., et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. British Journal of Cancer. 2011;105(9):1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Karki S. B., Yildirim-Ayan E., Eisenmann K. M., Ayan H. Miniature dielectric barrier discharge nonthermal plasma induces apoptosis in lung cancer cells and inhibits cell migration. BioMed Research International. 2017;2017:12. doi: 10.1155/2017/8058307.8058307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Virard F., Cousty S., Cambus J. P., Valentin A., Kemoun P., Clement F. Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS One. 2015;10(8, article e0133120) doi: 10.1371/journal.pone.0133120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hirst A. M., Simms M. S., Mann V. M., Maitland N. J., O'Connell D., Frame F. M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. British Journal of Cancer. 2015;112(9):1536–1545. doi: 10.1038/bjc.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Gandhirajan R. K., Rodder K., Bodnar Y., et al. Cytochrome c oxidase inhibition and cold plasma-derived oxidants synergize in melanoma cell death induction. Scientific Reports. 2018;8(1, article 12734) doi: 10.1038/s41598-018-31031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Van der Vliet A., Bast A. Effect of oxidative stress on receptors and signal transmission. Chemico-Biological Interactions. 1992;85(2-3):95–116. doi: 10.1016/0009-2797(92)90055-P. [DOI] [PubMed] [Google Scholar]
- 340.Casem M. L. Membranes and membrane transport. In: Casem M. L., editor. Case Studies in Cell Biology. Boston, MA, USA: Academic Press; 2016. pp. 105–125. [DOI] [Google Scholar]
- 341.Morgan M. J., Kim Y. S., Liu Z. Lipid rafts and oxidative stress–induced cell death. Antioxidants & Redox Signaling. 2007;9(9):1471–1484. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- 342.Moniruzzaman R., Rehman M. U., Zhao Q. L., et al. Cold atmospheric helium plasma causes synergistic enhancement in cell death with hyperthermia and an additive enhancement with radiation. Scientific Reports. 2017;7(1, article 11659) doi: 10.1038/s41598-017-11877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Morales A., Lee H., Goni F. M., Kolesnick R., Fernandez-Checa J. C. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 344.Heinrich M., Wickel M., Schneider-Brachert W., et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. The EMBO Journal. 1999;18(19):5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bauer G. Targeting protective catalase of tumor cells with cold atmospheric plasma- activated medium (PAM) Anti-Cancer Agents in Medicinal Chemistry. 2018;18(6):784–804. doi: 10.2174/1871520617666170801103708. [DOI] [PubMed] [Google Scholar]