Abstract
Background
Integrating Geriatric Assessment (GA) in the management of older adults with cancer is recommended, yet rarely practiced in routine oncologic care. Our objective was to assess the feasibility of integrating routine GA in the management of older adults with gastrointestinal (GI) malignancies and characterize impairments in this population.
Methods
Patients ≥60yo referred for consultation to the GI Oncology clinic were asked to complete the Cancer and Aging Resilience Evaluation (CARE) on their first visit. CARE was adapted from the Cancer and Aging Research Group GA with modifications to create a completely patient- reported version of the GA. Feasibility was defined as completion of CARE by ≥80% of eligible patients during the initial consultation.
Results
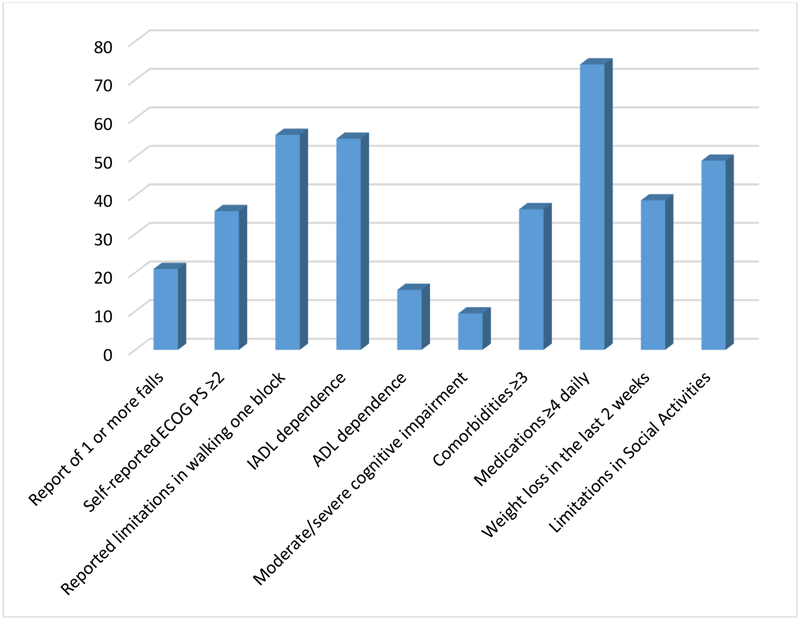
Of the eligible 354 new patients seen in the GI Oncology Clinic, 323 (91.2%) completed the CARE survey. Most patients (83.1%) felt the length of time to complete was appropriate (median time of 10 minutes [IQR 10–15.7 minutes]). GA impairments were prevalent: 54.7% reported dependence in Instrumental Activities of Daily Living, 15.5% reported dependence in Activities of Daily Living, 20.9% reported ≥1 fall, 35.9% reported a performance status ≥2, 55.7% were limited in walking one block, 74.0% reported polypharmacy (≥4 medications), and 36.4% had ≥3 comorbidities.
Conclusions
Performing a GA in the routine care of older adults with GI malignancies is feasible, and GA impairments are common amongst this population. A fully patient-reported GA such as the CARE may facilitate broader incorporation of GA in the routine clinic work flow.
Keywords: geriatric assessment, cancer, geriatric oncology, aged, gastrointestinal malignancy
Introduction:
Cancer is predominantly a disease of older adults, and the aging process results in a wide variability in health status among older adults presenting with a new diagnosis, thus complicating clinical management. Chronologic age and performance status alone are insufficient to characterize the heterogeneous aging process.1 Geriatric Assessment (GA) is a multidimensional tool developed to assess the medical, functional, and psychosocial abilities of older adults.2 A traditional GA performed by a geriatrician can take several hours to perform, and it was recognized early on in the growing field of geriatric oncology that a comprehensive yet brief measure needed to be developed. Dr. Arti Hurria led the seminal early development and validation of a GA tool designed for use in older adults with cancer.3,4 This brief GA was later adopted across several research studies and highlighted the large number of GA impairments that are often missed by routine clinical examination.1,5,6 Incorporation of a GA is recommended in the management of older adults over the age of 65 or in younger patients with age-related concerns.7,8 However, despite the growing evidence regarding the value of GA in cancer care, few oncology centers utilize GA due to perceived logistical concerns and lack of resources.
Although several previous reports have described the feasibility of performing a GA in a variety of settings, these studies have been conducted in the context of a research study with research staff facilitating the collection of information related to GA.4–6 To improve the widespread use of GA in routine practice, it is vital to develop new care models that integrate GA into routine oncologist care without additional resource allocation. The overarching goal of our study was to evaluate the implementation of incorporating a GA in the routine care of older adults with gastrointestinal (GI) malignancies referred for initial consultation. We sought to determine the proportion of eligible patients who were able to complete the GA at their initial consultation visit. Secondary outcomes included time to completion, satisfaction with the questionnaire length, whether there was any difficulty understanding questions, whether assistance was required with the GA, and the number of missing items. Lastly, we characterized GA-identified impairments in this population.
Methods
All new patients over the age of 60 with a new patient visit scheduled with the GI oncology team at the University of Alabama at Birmingham (UAB) were identified as potential participants. Performing GA is recommended as part of routine evaluation and management of older adults with cancer,2,8,9 therefore assessments were performed as part of routine clinical care. At check-in for their appointments, front desk staff provided a copy of the paper questionnaire on a clip board. During nurse triage the survey was collected and submitted to the clinical team prior to the clinical encounter. Clinical team reviewed and utilized as deemed appropriated for clinical care. After consultation with oncology providers, patients were approached for consent to have their data stored in our registry for future research. For any patients not wishing to consent, GA information was used only for clinical purposes. This study was approved by the institutional review board of UAB in September 2017 and recruitment began soon thereafter. Enrollment was performed on a rolling basis, starting with one provider and expanding to include all GI oncology providers over the first 3 months.
The assessment employed in this study was a modified version of the Cancer and Aging Research Group (CARG) GA originally developed by Arti Hurria.4 Modifications from the prior CARG version were made in order to streamline the assessment, tailor to a GI cancer population, and create an entirely patient-reported assessment that could be completed without the involvement of additional staff. The assessment included an evaluation of all essential domains of the GA including functional status, physical function, nutrition, health-related quality of life (HRQOL), social support, social activities, psychological status, cognitive function, comorbidities and polypharmacy.7 The objective cognitive assessment was replaced with the Patient- Reported Outcomes Measurement Information System (PROMIS®) cognitive function short-form 4 to reduce staff burden and promote ease of use.10 In addition, due to prior issues and uncertainty around the scoring of the mental health index (MHI) assessment of anxiety and depression, we used the PROMIS® anxiety and depression short forms. The social support questionnaire from the Medical Outcome Study was shortened to 8- items based on updated psychometric evaluations of the tool.11 As cancer cachexia and nutrition is of particular importance in GI malignancies, we added the Patient-Generated Subjective Global Assessment (PG-SGA) of nutrition that includes 4 patient-generated components of weight history, food intake, symptoms, and activities/function.12,13 Lastly, given the importance of HRQOL in older adults, we incorporated the PROMIS global health 10. The resultant assessment was termed the Cancer and Aging Resilience Evaluation (CARE) and consisted of 82 items across 6 pages (see supplemental materials).
The questionnaire was offered to all new patients seen for the first time in the outpatient GI oncology clinics at UAB and completion of the assessment was tracked. Reasons for non-completion were recorded. In order to demonstrate successful implementation of the assessment, our initial goal was to have ≥80% of all new patients seen for consultation to complete GA at their initial visit. Secondarily, we evaluated the self-reported time to completion, the participants’ satisfaction with length and understandability, whether assistance was required with the questionnaire, and the completeness of data by item and domains. Descriptive statistics were used to characterize the sample, and GA impairments were identified per literature based cut- points.1,6 Wilcoxon non-parametric tests and Pearson Chi-Square were used to compare distributions of time required to complete the CARE survey and proportions requiring assistance between those <75 and ≥75 years of age.
Results
From September 2017 through April 2019, 354 new patients over the age of 60 were seen in the UAB GI Oncology Clinic, of which 323 (91.2%) completed the CARE survey during their initial consultation and consented to be included in our registry. Of those that did not complete the survey, 17 were missed by the clinical team and 14 patients refused the assessment. Most common reasons for refusal were not interested in research (9 patients, 61.5%) or feeling overwhelmed (2 patients,15.4%), and some gave no specific reason (3 patients, 23.1%). The most frequent reason the clinical team missed the survey was due to patients’ severity of illness and requiring hospitalization or hospice. Median time to completion was 10 minutes (Interquartile Range 10–15.7 minutes). Most patients (83.1%) felt the length of time to complete was appropriate and 93.1% reported there were no difficult questions to understand. About a quarter of patients (27.2%) required assistance from a caregiver in completing the survey. All items and domains had less than 10% missing data. Patients ≥75 years required more assistance (22.8 vs. 40.7%, p=<0.01) and took longer (median of 10 vs 15 minutes, p=0.04) to complete the CARE survey.
The mean age was 70y (range 60–96) and the most common tumor types included colon (23.2%), pancreatic (22.9%), and rectal (10.5%) cancer with predominately advanced stage diseases (stage III/IV: 70.7%). GA impairments were highly prevalent with 54.7% dependent in Instrumental Activities of Daily Living (IADL), 15.5% dependent in Activities of Daily Living. Furthermore, 20.9% reported ≥1 fall, 35.9% reported a performance status ≥2, 55.7% were limited in walking one block, 74.0% reported polypharmacy (≥4 medications), 36.4% had ≥3 comorbid conditions, 9.4% with moderate/severe cognitive impairment, 38.7% reported weight loss in past 2 weeks, and 49.0% reported limitations in social activities.
Discussion
Over 90% of new patients seen for routine initial oncologic consultation completed the CARE GA during their first clinic visit without the assistance of research staff or additional resources. This study demonstrates the ease with which a GA can be integrated into routine oncology practice without significant resource allocation or burden to staff. The modified and completely patient-reported version of the GA maintained evaluation of all the core domains of the GA while shortening the time required and minimizing burden on staff. The time to complete the questionnaire was shorter in comparison to the CARG GA (10 minutes versus 27 minutes) with similar overall length satisfaction.4 As the GA is recommended for routine use in older adults with cancer, the fully patient-reported and streamlined CARE may improve the implementation of a GA in the routine cancer management.
On November 7th, 2018, Arti Hurria died tragically and the world lost an irreplaceable leader in the field of geriatric oncology, and great friend and mentor to all of us in cancer and aging research. Few researchers have been as instrumental and impactful in developing and moving a field of research forward as Dr. Hurria, and her passion for improving the care of older adults with cancer was infectious. Through her development of CARG, she fostered the creation of a vast international network of multidisciplinary researchers with the single minded determination of improving the care of the growing number of older adults with cancer. Dr. Hurria not only made considerable strides by creating the brief GA tool as highlighted in the introduction, she later went on to develop and validate a chemotherapy toxicity calculator using this tool, which can be used to facilitate treatment discussions when weighting the risks and benefits of systemic chemotherapy.14,15 Arti Hurria’s impact on the developing field of geriatric oncology is immeasurable, and she trail-blazed a path of career success as a researcher focused at the cross-section of cancer and aging that many of us strive to emulate. With our work we hope to honor Dr. Hurria’s legacy of developing the brief GA, and demonstrate a model that may help expand the number of older adults able to have a GA performed as part of their routine oncologic management in clinics across the globe.
Performing a GA not only helps assess the presence of age-related conditions and overall fitness, it has been specifically shown to predict chemotherapy toxicity and survival in older adults with cancer.2,16 This prognostic information can help inform the risk/benefit balance of many treatment decisions particularly in complex older patients, and potentially guide treatment management.8,17 As such, GA results have been demonstrated to strongly influence oncologists’ treatment decisions.18 Lastly, the GA can identify many areas of impairment, such as dependency in IADL and falls, that have known effective interventions shown to be beneficial in general older adult population; how these targeted interventions impact cancer outcomes remain less understood.19,20
The length of time required to complete the CARE survey is notably shorter than prior reports using the CARG GA (10 vs. 23–27 minutes).4,6 Besides for the complete elimination of the objective measures (specifically the Timed Up and Go and Blessed Orientation Memory and Concentration), which previously took about 6 minutes, the likely single biggest reason for the time reduction was not having patients write out all their medication list. The CARE survey only includes the number of medications taken on a daily basis and does not entail the participant writing out all their medications. This change was made as a medication review is already obtained as a routine part of clinical practice in most institutions/practices, and an updated list of medications is routinely available within the electronic medical record. We felt having participants repeat this medication review only lengthened the survey with little to no additional benefit.
Our study is not without some limitations. Although we were able to track all new patients within our clinic and evaluate whether they completed the GA, we did not assess how implementing the GA altered oncologic management. However, prior work has already demonstrated how GA-related information strongly influences oncologists’ treatment decisions, and our subsequent plans of the CARE registry is to explore how the GA impacts clinical management and develop improved methods for sharing this information with providers.18 While we acknowledge the importance of objective physical and cognitive measures traditionally included in the GA and recognize the potential drawbacks of relying entirely on patient-reported information, we chose to omit the objective measures as part of our base assessment and instead have elected to incorporate these as optional assessments for specific patients were this may be of particular importance. This decision has most impacted the domain of cognition, as the use of self-reported cognitive dysfunction is relatively new within oncology.21 Some early results suggest patient-reported cognitive dysfunction may underreport cognitive impairment while others have shown modest-high correlation with objectively assessed cognition, both of which were associated with a higher likelihood of not returning to work.22,23 We also plan to examine the concordance of objective measures with patient- reported measures to see how many additional patients this may identify with impairment and further examine associations of patient-reported cognitive dysfunction with adverse outcomes. We purposefully used a low age (60 years) for enrollment into our registry as we felt many components of the GA are relevant to younger older patients (nutrition, anxiety, depression, etc.) and in order to allow for more meaningful age-related sub-analyses in the future. Finally, our study sample consists of patients with GI malignancies from a single center in southeast US and may not be representative of all older adult with cancer.
Conclusions
In summary, we have demonstrated that a patient-reported GA can be implemented as part of routine oncologic care in the management of older adults with GI malignancies and identifies a high prevalence of GA impairments in this population. As eloquently stated by Hamaker and colleagues, it’s “time to stop saying geriatric assessment is too time consuming.”24 In the era of precision medicine, it is critical for clinicians to develop personalized treatment plans that go beyond tumor-specific markers to also include comprehensive assessments of the patient such as the GA provides.25 Further research is currently underway to determine how best GA results can be incorporated to guide management and improve clinical decision-making for older adults in oncology.
Supplementary Material
Figure 1: Geriatric Assessment Identified Impairments by Domain.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; IADL, Instrumental Activities of Daily Living; ADL, Activities of Daily Living.
Table 1.
Patient Characteristics and Implementation Results
| Total Patients | N=323 |
|---|---|
| Age, mean (SD) | 70 (6.9) |
| Sex, n (%) | |
| Male | 175 (54.2) |
| Race, n (%) | |
| White | 237 (73.4) |
| Black | 82 (25.4) |
| Other | 4 (1.2) |
| Educational Level, n (%) | |
| Less than high school | 47 (15.1) |
| High school graduate | 85 (27.2) |
| Associate/Bachelors | 135 (43.3) |
| Advanced Degree | 45 (14.4) |
| Marital Status, n (%) | |
| Single | 25 (8.0) |
| Widowed/Divorced | 85(27.1) |
| Married | 204 (65.0) |
| Cancer Type,n (%) | |
| Colon | 75 (23.2) |
| Pancreatic | 74 (22.9) |
| Rectal | 34 (10.5) |
| Esophageal-gastric | 33 (10.2) |
| Neuroendocrine | 30 (9.3) |
| Other | 77 (23.9) |
| Cancer Stage, n (%) | |
| l/ll | 94 (29.3) |
| III/IV | 227 (70.7) |
| Geriatric Assessment Implementation Results | |
| Time to Completion | |
| Median (IQR) | 10 minutes (10–15.7) |
| Length of time to complete, n (%) | |
| Too short | 2 (0.6) |
| Just right | 256 (83.1) |
| Too long | 50 (16.2) |
| Required Assistance, n (%) | |
| Yes | 88 (27.2) |
Abbreviations: SD, Standard deviation; IQR, Inter-quartile Range.
Funding:
Supported in part by the Walter B. Frommeyer Fellowship in Investigative Medicine at the University of Alabama at Birmingham and the National Cancer Institute of the National Institutes of Health (K08CA234225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previously presented as a poster presentation at the American Society of Clinical Oncology Gastrointestinal (GI) Cancers Symposium. San Francisco, CA. January 19th, 2019.
Conflict of Interest:
The authors have no conflict to disclose.
References
- 1.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. The oncologist. 2015;20(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(24):2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurria A We need a geriatric assessment for oncologists. Nature clinical practice. Oncology. 2006;3(12):642–643. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. [DOI] [PubMed] [Google Scholar]
- 5.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. Journal of geriatric oncology. 2014;5(3):245–251. [DOI] [PubMed] [Google Scholar]
- 7.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13(9):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018:JCO2018788687. [Google Scholar]
- 9.VanderWalde N, Jagsi R, Dotan E, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. Journal of the National Comprehensive Cancer Network : JNCCN. 2016;14(11):1357– 1370. [DOI] [PubMed] [Google Scholar]
- 10.Saffer BY, Lanting SC, Koehle MS, Klonsky ED, Iverson GL. Assessing cognitive impairment using PROMIS((R)) applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res. 2015;226(1):169–172. [DOI] [PubMed] [Google Scholar]
- 11.Moser A, Stuck AE, Silliman RA, Ganz PA, Clough-Gorr KM. The eight-item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. Journal of clinical epidemiology. 2012;65(10):1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott J, Teleni L, McKavanagh D, Watson J, McCarthy AL, Isenring E. Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) is a valid screening tool in chemotherapy outpatients. Support Care Cancer. 2016;24(9):3883–3887. [DOI] [PubMed] [Google Scholar]
- 13.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur 2002;56(8):779–785. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25(2):307–315. [DOI] [PubMed] [Google Scholar]
- 17.Gajra A, Loh KP, Hurria A, et al. Comprehensive Geriatric Assessment-Guided Therapy Does Improve Outcomes of Older Patients With Advanced Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(33):4047–4048. [DOI] [PubMed] [Google Scholar]
- 18.Mohile SG, Magnuson A, Pandya C, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. Journal of geriatric oncology. 2016;7(4):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerard EJ, Deal AM, Williams GR, Jolly TA, Nyrop KA, Muss HB. Falls in Older Adults With Cancer: Evaluation by Oncology Providers. Journal of oncology practice / American Society of Clinical Oncology. 2015;11(6):470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savard J, Ganz PA. Subjective or Objective Measures of Cognitive Functioning-What’s More Important JAMA Oncol. 2016;2(10):1263–1264. [DOI] [PubMed] [Google Scholar]
- 22.Sinha P, Wong AWK, Kallogjeri D, Piccirillo JF. Baseline Cognition Assessment Among Patients With Oropharyngeal Cancer Using PROMIS and NIH Toolbox. JAMA Otolaryngol Head Neck Surg. 2018;144(11):978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murdaugh D, Bosworth A, Patel S, et al. Self-Endorsed Cognitive Problems Vs. Objectively- Assessed Cognitive Impairment in Blood or Marrow Transplantation (BMT) Recipients — a Longitudinal Study. Blood. 2018;Supplement 1:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaker ME, Wildes TM, Rostoft S. Time to Stop Saying Geriatric Assessment Is Too Time Consuming. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35(25):2871–2874. [DOI] [PubMed] [Google Scholar]
- 25.Williams GR. Geriatric Assessment: Precision Medicine for Older Adults With Cancer. Journal of oncology practice / American Society of Clinical Oncology. 2018;14(2):97–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.