Abstract
Objectives:
This study tested whether early socioeconomic status moderated links between objective and subjective sleep and weight indicators during middle childhood.
Design:
The study design was cross-sectional but included data from earlier assessment points in the study.
Setting:
Data were collected from families across the state of Arizona.
Participants:
Participants were 382 children recruited from birth records (49.5% female; Mage = 8.47 years; 56.5% European American; 25.1% Latino; 25% living at or below the poverty line).
Measurements:
Assessments included socioeconomic status at 12 months of age, and sleep and weight indicators at eight years.
Results:
Longer sleep durations predicted lower body mass index and decreased odds of being overweight/obese across all children, regardless of socioeconomic background. For children from low socioeconomic backgrounds, longer sleep duration predicted lower percent body fat, greater efficiency predicted lower percent body fat and body mass index and smaller waist circumference, and more sleep problems predicted larger waist circumference. For children from low socioeconomic backgrounds, greater sleep duration and efficiency also predicted the lowest odds of being overweight/obese, and more sleep problems predicted the greatest odds of being overweight/obese.
Conclusions:
Early life may be a sensitive period that sets the stage for stronger links between sleep and weight indicators in middle childhood. Findings offer important information regarding the protective role of sleep in the promotion of health, as well as the negative consequences that may be stronger for children who experienced low early-life socioeconomic status.
Keywords: sleep problems, weight, obesity, socioeconomic status, children
Approximately 69% of children experience at least one sleep problem (i.e., non-clinical sleep difficulties) at night one or more times per week, such as restricted sleep, poor sleep quality, and parasomnias.1,2 Importantly, early childhood sleep problems have been associated with a number of negative outcomes in childhood, including reduced cognitive functioning and greater internalizing and externalizing symptoms.2 Sleep problems have also been increasingly associated with poor physical health, such that restricted sleep and sleep problems have been linked with increased risk for obesity, higher body mass index scores (BMI), and greater adiposity.3,4 A recent report also shows that about 32% of children and adolescents (2–19 years) are classified as overweight or obese and over 100,000 deaths per year can be attributed to adiposity-related health problems.5 These statistics suggest that the “obesity epidemic” is ongoing the United States, and adiposity may be associated with a number of health-related factors such as home environment, food and activity choices, and sleep in both children and adults.6 Thus, understanding links between sleep problems and weight indicators is critical for identifying potential points of intervention to reduce both childhood sleep problems and obesity, as well as elucidating whether these associations are stronger for particular groups of children to ensure that prevention and intervention efforts are targeted for at-risk populations.
In addition to identifying relations between sleep and weight and points of intervention, other gaps in the literature for sleep-weight relationships exist. Specifically, many previous studies only examined associations between sleep duration and BMI or risk for being classified as overweight/obese (via BMI scores).4,7 However, waist circumference (WC), which estimates visceral or abdominal adipose fat tissue8 and percent body fat, which estimates the proportion of body fat to an individual’s total body weight,9 may provide valuable additional information about child body fatness that are not captured with BMI or weight status categories or be differentially related to sleep indicators. For example, numerous cross-sectional and longitudinal studies have shown that shorter sleep duration and sleep timing predict greater BMI and risk for being overweight/obese,10,11 but only a few cross-sectional studies have shown that short sleep duration is associated with WC or percent body fat.12,13 It is also possible that links between other sleep parameters and weight-related indicators or weight status may be stronger or weaker compared to established associations between sleep duration and BMI or risk for obesity. Indeed, a few studies suggest that both shorter parent-reported and actigraphy-based sleep duration are associated greater WC10 and higher percent body fat12,13 after adjusting for multiple demographic and lifestyle factors. Thus, the current study collected multiple objective and subjective sleep parameters and objective weight-related indicators to capture a more complete picture of links between sleep problems and weight in childhood, and tested whether sleep-weight relations were stronger for particular children based on their level of early-life socioeconomic status (SES) while controlling for concurrent SES.
Theoretical Mechanisms
Previous theory implicates metabolic processes as a key link between sleep and weight across the lifespan. Specifically, sleep problems may prompt changes in hormones levels (i.e., leptin, ghrelin, and insulin) and problems with glucose uptake and metabolism, which may lead to increased adiposity.14,15 Generally, research supports theory that metabolic processes underlie links between sleep problems and obesity in childhood and adolescence.16 Prior literature has shown that non-obese children with obstructive sleep apnea (OSA) do not demonstrate changes in metabolism and insulin resistance, while obese children with OSA show marked changed in various metabolic indicators, including increased insulin resistance.17 Similarly, researchers have found that metabolic indicators (i.e., IL-6, CRP) explain significant proportions of the relations between visceral body fat and OSA in a community sample of adolescents.18 One empirical study has also shown that greater sleep duration variability and shorter sleep duration were associated with greater changes in insulin levels and health risk during middle childhood.19
Combining theory about biological processes and early SES, some research suggests that early-life adversity (e.g., low SES, poverty, few resources) may lead to biological vulnerability and particular patterns of physiology and immune functioning that have long-term, negative effects on health and well-being.20,21 Indeed, previous findings with the current sample found that children who experienced higher early-life SES demonstrated longer sleep durations, less time to fall asleep, and less variable sleep in middle childhood, suggesting that SES early in life is an important predictor of later sleep behavior.22 The present study is an extension of these prior findings and aims to determine whether early-life SES is also important for associations between sleep and weight indicators in middle childhood over and above the effects of concurrent SES.
Prior Literature Linking Sleep and Weight
Recent reviews and meta-analyses generally support that shorter sleep duration is associated with higher BMI and increased risk for obesity in childhood both concurrently and longitudinally.3,4 Additionally, longer objective sleep duration has been linked to earlier bedtimes, later wake times, and lower standardized BMI (zBMI) scores in middle childhood when accounting for demographic and lifestyle factors,23 suggesting that longer sleep may also promote better quality health. Regarding other aspects of sleep, one meta-analysis has shown shorter sleep duration and poorer sleep quality are independently associated with greater risk for being overweight or obese in children.24 Lower sleep efficiency and shorter sleep duration (using actigraphy) during middle childhood have also been associated with higher BMI and obesity status after accounting for demographic factors.25 Longitudinal research also suggests links between child sleep and adiposity, such that children who show greater sleep duration on average (via parent-report and actigraphy) demonstrate earlier bedtimes,26 later wake times,26 lower BMI scores,26 slower growth in BMI scores,27 and lower risk of being overweight or obese in adolescence,26 after covarying for earlier BMI, weight, and multiple demographic factors like SES.
Role of Early SES
Theoretical and empirical evidence also point to the importance of examining SES during infancy in relation to sleep and weight indicators, as the first year of life serves as a sensitive period of development for behaviors and biological processes like sleep regulation and weight.28 Furthermore, developmental theories suggest that resources and experiences in infancy and early childhood have significant and differential influences on developmental outcomes, over and above the influence of concurrent experiences.see 29 For example, multiple studies have shown associations between low early SES and poorer health later (in adulthood), after accounting for concurrent SES.see 30,31 Relevant to the current study, SES in infancy is a potent predictor of later outcomes like obesity and sleep.22,28,32,33 However, prior literature has primarily examined socioeconomic disparities in children’s sleep and weight using concurrent SES.34–36 Thus, the current study tests early SES as a possible moderator of associations between sleep and weight indicators in middle childhood over and above the effects of concurrent SES, as well as for whom these associations may be strongest.
Some studies have shown that shorter sleep duration and more sleep problems and disorders in middle childhood may be more prevalent for low SES children.37 Regarding weight indicators, research suggests that children with low SES may show greater weight and increased risk for being overweight/obese.25,38 However, prior studies have overwhelmingly utilized SES as a covariate when assessing associations between child sleep and weight indicators.11,23 Three studies to our knowledge have directly examined whether low SES children show different relations between sleep and weight indicators compared to high SES children.25,39,40 Bagley and colleagues25 found that lower objective sleep duration and efficiency cross-sectionally predicted greater zBMI scores in a large sample of European American and African-American children during middle childhood (N = 228; Mage = 10 years), with these associations significant only for children showing at least one family risk factor (including low SES). Following this first study, Bagley and colleagues39 demonstrated that greater sleep onset variability and shorter duration predicted greater zBMI scores two years later in middle childhood, particularly for children with greater cumulative risk scores, including living in poverty, lower maternal education, high maternal stress, and living in a single-parent household. Another study by O’Dea et al.40 showed stronger links between short parent-reported sleep duration and high objective BMI, particularly for children with low SES in middle childhood compared to children with high SES. However, the first two studies25,39 only examined objective sleep measures, zBMI scores, and included SES within a cumulative risk score rather than testing it as an independent moderator or examining the role of early life SES while adjusting for concurrent SES. Similarly, O’Dea et al.40 did not explore links beyond parent-reported sleep duration and objective BMI.
Current Study
While prior literature supports relations between objective and subjective sleep and weight indicators in childhood, there are a number of gaps in the current literature. Specifically, few studies have tested 1) how facets of sleep other than sleep duration are related to weight indicators beyond BMI scores or risk for obesity and 2) whether associations between sleep and weight are moderated by early SES (prior studies have relied on concurrent SES or SES as a covariate). Given these gaps in the literature, we tested whether early SES moderated links between objective (actigraphy-based) sleep duration, sleep efficiency, and subjective (parent-reported) sleep problems and objective BMI scores, WC, percent body fat, and risk for being overweight/obese in a sample of twins during middle childhood, over and above the effect of concurrent SES. We expected low sleep quantity and quality, and more sleep problems would be associated with higher BMI scores, WC, percent body fat, overweight/obese status, and that children with lower early SES would show the strongest links between sleep and weight indicators (adjusting for concurrent SES). Although the twin sample was not large enough to provide stable estimates of genetic and environmental contributions to the covariance between sleep and weight for the current sample,41,42 it was ideal for testing the goals of this study as the sample was ascertained from state birth records, representing ethnically and socioeconomically diverse children growing up in urban and rural contexts.
Methods
Participants
Participants were a subsample of children from an ongoing, longitudinal twin study recruited from birth records in Arizona (N = 382 children in subsample; 49.5% female).22,28 Families were recruited into the study when twins were approximately 12 months old (Full sample N = 591; Mage = 12.49 months, SD = 1.16; data collected from 2009–2010) and were offered the opportunity to participate again in the study when twins were approximately eight years of age (Mage = 8.47 years, SD = .45; data collected from 2016–2017; retention = 65% for current analytic sample). The full sample at 12 months includes monozygotic (MZ) and dizygotic (DZ) twin pairs (MZ = 29.2%, same-sex DZ = 36.1%, opposite-sex DZ = 32.2%, unknown zygosity = 2.6%). The partial sample is ethnically diverse with 56.5% European American, 25.1% Latino, 5.2% Asian American, 4.2% African American, 1.0% Native American, 1.6% Native Hawaiian families, and 6.3% multiethnic. Families also demonstrated a broad range of parent education levels and SES at the 12-month and eight-year assessments, with household incomes ranging from less than $20,000 to $150,000+ annually.
Procedure
At 12 months old, primary caregivers (94.6% mothers) completed telephone interviews regarding the mother’s pregnancy, family demographics (i.e., SES), and twins’ development. When twins were eight years old, primary caregivers completed two home visits separated by a week-long study protocol (71% completed during the school year). At home visits, experimenters obtained written informed consent and assent from primary caregivers and twins, and collected biological assessments (twin height, weight, WC, and percent body fat). At the first home visit, experimenters trained primary caregivers for the week-long study protocol, including how to help collect objective sleep data from twins with actigraphy watches for seven nights. Primary caregivers were also trained to complete electronic (48.7%) or paper daily diaries (44.5%; 2.1% completed both), which were used for cross-validation when cleaning actigraphy sleep data. Families were compensated for completing study questionnaires, home visits, and procedures during the study week, and twins were given small prizes at each home visit for their participation.
Measures
Objective sleep.
Objective sleep indicators were collected from each twin using wrist-based accelerometers (actigraph watches) at eight years of age (Motion Logger Micro Watch; Ambulatory Monitoring, Inc., Ardsley, NY USA). Children wore watches on their non-dominant wrist for seven nights (Mnights = 6.89, SD = .53). Actigraphy data was scored using the Action-W2 program (version 2.7.1), which includes a validated algorithm to measure sleep.43 Research suggests that actigraphy is reliable when measuring five more nights of sleep, and actigraphy sleep measurement has been validated against concurrent polysomnography.43 From scored actigraphy data, we assessed: 1) Nighttime sleep duration (total number of hours and minutes asleep each night excluding wake bouts), and 2) sleep efficiency (percentage of time asleep each night based on the amount of time in bed).
Study staff cross-checked objective actigraph sleep periods with parent-reported bed and wake times and daily sleep diaries to identify significant outliers and equipment malfunction. Sleep data were missing or excluded from analyses for 32 children (8.4%) primarily because they did not wear the watch or due to watch malfunction. However, most of the sample was highly compliant in wearing the actigraph watch (for details see Doane et al.22).
Subjective sleep.
Parents reported children’s sleep difficulties at eight years of age using the Child Sleep Habits Questionnaire (CSHQ; α = .81)44 with questions regarding sleep duration problems, bedtime resistance, sleep latency, nighttime wakings, sleep anxiety, parasomnias, sleep disordered breathing, and daytime sleepiness. All items were rated on a five-point Likert scale from 1 (Always) to 5 (Never), and a mean sleep problem score was created from all items and scales for each child, such that higher CSHQ scores indicated greater sleep disturbances.
Weight indicators.
Height (to the nearest half inch) was measured once at each home visit with a tape measure. Weight (in pounds and ounces) and percent body fat (ratio or percentage of body fat to total body weight) were each measured twice at each home visit using an FDA-approved full body composition scale for children.9 WC (in inches) was collected once at each home visit using a Gulick tape measure two inches below the bottom of the lowest rib, one of the recommended methods for estimating visceral adiposity.45 The Gulick tape measure has a metal counterweight on one end that is activated when placing equal pressure around the body when assessing WC. Experimenters asked children to remove bulky clothing before assessing WC, and were trained to have the Gulick tape measure resting directly on the child’s clothing with no space between the body or clothing and the tape measure.45 One end of the Gulick tape measure is pulled around the body to meet the end of the tape with the counterweight, and the counterweight is pulled until it reaches a marker signaling equal and constant pressure against the body.
Height and weight measures were used to compute BMI scores (adjusted for age and sex) using the Centers for Disease Control child BMI formula6: weight (kg) / [height (m)]2. BMI scores were used to create weight status groups: underweight (N = 16; 4.1%), normal (N = 265; 67.8%), overweight (N = 57; 14.6%), and obese (N = 30; 7.7%). For analyses, weight status groups were collapsed such that children who were underweight or normal weight (“not overweight/obese”; 76.3%) were compared to children who were classified as overweight or obese (“overweight/obese”; 23.7%).
Socioeconomic status (SES).
SES was collected at 12 months and eight years using parent reports of primary and secondary caregiver educations (see Table 1) and total household income before taxes (used to create income to needs ratio at 12 months in 2009), and a standardized family-level composite was created to represent SES. Total household income was reported with the following categories: (1) less than $30,001; (2) $30,0001-$40,000; (3) $40,001-$50,000; (4) $50,001-$60,000; (5) $60,001-$70,000; (6) $70,001-$80,000; (7) $80,001-$90,000; (8) $90,001-$100,000; (10) $100,001-$150,000; (11) More than $150,001. A mean of the family income range was used to represent household income before taxes. Income to needs ratios at 12 months were calculated such that the household income was divided by the federal poverty threshold for each family’s household size published in 2009 (U.S. Census Bureau). Families were classified as living in poverty if they received a score of <1 (9.5% of sample), near the poverty line if scored 1–2 (20.9%), lower middle class if scored 2–3 (23.9%), and middle to upper class if scored greater than a 3 (44.8%). SES at eight years was computed in the same way at SES at 12 months but using 2016 U.S. Census Bureau information (see Table 1 for details). Parent education level was diverse at eight years of age and was similar to education levels at the 12-month assessment (r = .82, p < .001; see Table 1 for details).
Table 1.
Zero-Order Correlations and Descriptive Statistics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sleep duration (hours) | - | |||||||||
| 2. Sleep efficiency (%) | 61*** | - | ||||||||
| 3. Parent-reported sleep problemsa | −.18** | −.05 | - | |||||||
| 4. SES (12 months)b | 24*** | .12+ | −.15* | - | ||||||
| 5. Body mass index (BMI) | −.18** | −18*** | .04 | −.16** | - | |||||
| 6. Percent body fat | −.18*** | −.13* | .03 | −.15** | 84*** | - | ||||
| 7. Waist circumference (WC) | −.14* | −.13* | .04 | −.09 | 89*** | 75*** | - | |||
| 8. SES (8 years)b | 29*** | .15* | −.18** | 82*** | −.19** | −20*** | −.13+ | - | ||
| 9. Sex | .13* | .07 | .04 | −.04 | .02 | 23*** | −.04 | .02 | - | |
| 10. Race/ethnicity | .13+ | .02 | −.01 | .22** | −.04 | −.09 | −.04 | 23*** | .01 | - |
| Mean | 8.11 | 90.33 | 1.76 | .00 | 16.80 | 19.47 | 24.28 | .00 | .50 | .57 |
| Standard Deviation | .71 | 5.44 | .78 | .75 | 2.85 | 6.64 | 2.98 | .80 | - | - |
| Minimum | 5.62 | 67.18 | 1.00 | −1.67 | 13.19 | 5.00 | 16.92 | −2.06 | - | - |
| Maximum | 10.01 | 98.80 | 3.29 | 3.16 | 31.98 | 46.45 | 35.75 | 1.75 | - | - |
| Skewness | −.54 | −1.12 | .61 | −.16 | 1.90 | .92 | 1.39 | −.15 | - | - |
| Kurtosis | .30 | 1.53 | 1.00 | −.45 | 4.84 | 1.36 | 2.39 | −.55 | - | - |
Note.
Sex: 0 = male, 1 = female; Race/ethnicity: 0 = other or multiple races, 1 = non-Latino European American; Sleep duration was actigraphy-measured total number of hours and minutes slept each night on average (excluding wake periods).
Parent-reported sleep problems were reported on using the Child Sleep Habits Questionnaire (Owens et al., 2000), and a mean score was used for each participant rather than a sum score.
SES at 12 months and 8 years were standardized composites of primary caregiver education, secondary caregiver education, and income to needs ratio. Primary caregivers reported on their and their spouse/partner’s level of education with the following categories: (1) less than high school (12 months: 1.0% primary caregiver; .5% spouse/partner); (2) high school or equivalent (12 months: 6.8% primary caregiver; 13.6% spouse/partner); (3) Some college, not graduated (12 months: 22.0% primary caregiver; 19.9% spouse/partner); (4) college degree (e.g., BA, BS; 12 months: 37.2% primary caregiver; 26.7% spouse/partner); (5) Two or more years of graduate school (12 months: 3.7% primary caregiver; 2.1% spouse/partner); (6) graduate or professional degree (12 months: 20.9% primary caregiver; 15.7% spouse/partner). 8.4% of primary caregivers (12 months: 8.9% spouse/partner) declined to provide their highest education level. Income to needs ratios at 12 months: living in poverty (score <1; 9.5% of sample), near the poverty line (score of 1–2; 20.9%), lower middle class (score of 2–3; 23.9%), and middle to upper class (scores >3; 44.8%).
p ≤ .10;
p ≤ .05;
p ≤ .01;
p ≤ .001.
Covariate Testing and Final Covariates.
Primary caregivers reported various physical and mental health problems, developmental disabilities, learning disorders and other diagnoses for each twin that may influence sleep, weight measurement, or their associations. Within the subsample, parents reported diagnoses of hypothyroidism (N = 1), prediabetes (N = 1), immune or autoimmune disorders (N = 3), asthma or other respiratory problems (N = 85), sleep disorders (e.g., sleep walking; N = 3), ADHD (N = 18), and internalizing disorders (i.e., anxiety; N = 3; Note: the same child may have multiple diagnoses). Exploratory analyses were conducted to determine whether results were similar when controlling for the presence of child asthma or other respiratory problems (N = 85), given that this was the most prevalent health problem reported in the sample. Results indicated that there were no differences in results when controlling for the presence of asthma or other respiratory problems; thus, all children were included in the analytic sample.
Additionally, if an individual has fewer than five nights of actigraphy data, this may provide a poor estimation of regular sleep;46 thus, exploratory analyses were conducted to determine whether results were similar for children who wore the actigraphy watch for five or more nights and children who wore the watch for fewer than five nights (N = 9). Results indicated that there were no differences in results when children with four or fewer nights of sleep were excluded from analyses; thus, all children with valid actigraphy data were included in the analytic sample.
Other covariates known to be associated with sleep and weight indicators were tested, including sex (female = 1) and race/ethnicity (non-Hispanic European American = 1; Table 1), the SES composite at eight years of age, and household structure at 12 months (two-parent household = 1). Significant covariates based on correlations were included in the final analyses: sex, and race/ethnicity, and SES at eight years of age.
Results
Analytic Plan
Mixed model regression and multivariate logistic regression analyses were conducted in Mplus 7.4 using the complex command to account for twin interdependence and missing data using full information maximum likelihood with robust standard errors (MLR).47 Predictors, moderators, and covariates were centered at zero and used to create interaction terms. Unstandardized beta estimates and standard errors are reported. For significant interactions in mixed model regression analyses, simple slopes were probed at 1 SD below and above the mean of early SES using the simple slopes technique for nested data outlined by Preacher and colleagues.48 In logistic regression models, 0 = not overweight/obese, 1 = overweight/obese, and odds ratios and 95% confidence intervals are reported.
Prior to analyzing data in Mplus 7.4, we also conducted stem-and-leaf plots and analyses for univariate outliers using SPSS 24 (IBM Inc.) to determine whether any families had scores +/− 3 SDs from the mean of each of the key study variables. Any values +/− 3 SDs from the mean were windorized to 3 SD from the mean.
Preliminary Analyses
Descriptive statistics and zero-order correlations are reported in Table 1. Children spent 8.11 hours (SD = .71 or 42.6 minutes) sleeping per night on average, which is lower than the recommended hours of sleep per night for children ages 6 to 111 (recommended level: 9–12 hours of total time in bed per 24-hour period).49,50 Despite slightly lower sleep quantity than is typically recommended, children in the sample achieved a sleep efficiency of 90.33% on average (Median = 91.58%), which is adequate and suggests high sleep quality. Children had an average BMI score of 16.80, which falls into the normal/healthy range for eight-year-old males and females.6 Additionally, children demonstrated an average WC of 24.28 inches and an average percent body fat of 19.47%.
Primary Analyses
Sleep predicting BMI scores.
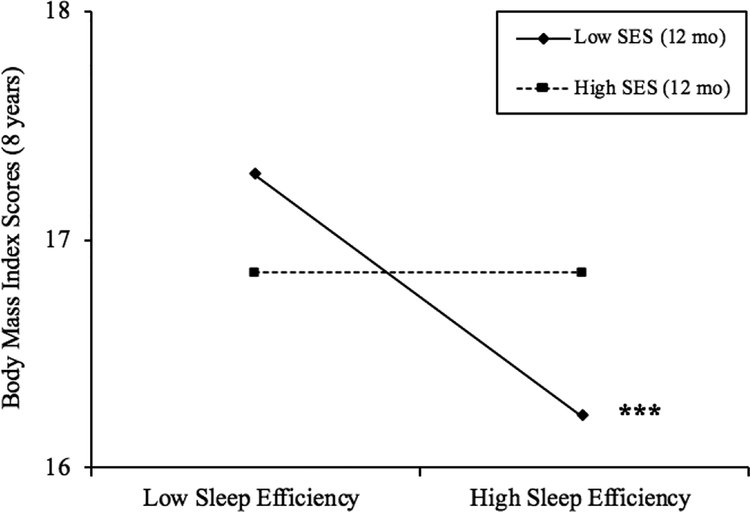
Longer sleep duration was significantly associated with lower BMI scores, and the interaction between sleep duration and early SES was not significant (Table 2). While there was no main effect of sleep efficiency on BMI, the interaction between objective sleep efficiency and early SES was significant (p = .05; Table 2), with greater sleep efficiency predicting lower BMI scores for children with low early SES (b = −.10, p < .001; about 45% of the sample; Figure 1), but not average (b = −.05, ns) or high early SES (b = .01, ns). This suggests that children with low early SES experienced .5 lower BMI scores with each 5% increase in sleep efficiency (.18 SD decrease). Neither parent-reported sleep problems nor the interaction between parent-reported sleep problems and early SES were significant in predicting BMI scores (see Table 2).
Table 2.
Objective and subjective sleep indicators and early SES predicting BMI, percent body fat, waist circumference, and risk for being classified as overweight/obese in middle childhood.
| Body Mass Index (BMI) | Body Fat (%) | Waist Circumference (WC) | Odds of Overweight/Obese Status | |
|---|---|---|---|---|
| Model Predictors | Est(SE) | Est(SE) | Est(SE) | OR (95% CIs) |
| Intercept | 16.81(.18)*** | 19.36(.41)*** | 23.01(.20)*** | 1.28(.98–1.57)*** |
| Sex | .32(.34) | 3.27(.78)*** | −.18(37) | 1.48(84–2.61) |
| Ethnicity | .03(.39) | −.44(.88) | −.01(.43) | 1.16(.65–2.08) |
| SES (8 yrs.) | −.63(.53) | −1.93(1.05)† | −.69(.60) | .68(.38–1.20) |
| SES (12 mo.) | .08(.50) | .80(1.00) | .38(.61) | 1.19(.64–2.20) |
| Sleep Duration (hrs; 8 yrs.) | −.50(.23)* | −1.29(.53) | −.37(.24) | .52(.34-.78)** |
| Sleep Duration x SES | .21(.28) | 1.33(.65)* | .28(.33) | 1.76(.98–3.24)† |
| Intercept | 16.81(.18)*** | 19.42(.41)*** | 23.01(.19)*** | 1.24(.96–1.52)*** |
| Sex | .31(.35) | 3.30(.79)*** | −.14(37) | 1.40(.81–2.43) |
| Ethnicity | −.01(38) | −.51(.87) | −.01(.43) | 1.13(.64–2.0) |
| SES (8 yrs.) | −.69(.54) | −2.01(1.07)† | −.69(.62) | .60(.34–1.07)† |
| SES (12 mo.) | .07(.50) | .74(1.03) | .35(.61) | 1.16(.62–2.15) |
| Sleep Efficiency (%; 8 yrs.) | −.05(.03) | −. 13(.07)† | −.06(.03)* | .95(.91–1.0)* |
| Sleep Efficiency x SES | .06(.03)* | .22(.08)** | .09(.03)** | 1.10(1.02–1.19)** |
| Intercept | 16.81(.18)*** | 19.49(.42)*** | 23.00(.19)*** | 1.29(1.0–1.54)*** |
| Sex | .22(.34) | 3.11(.79)*** | −.25(.37) | 1.16(.67–2.02) |
| Ethnicity | −.03(.38) | −.62(.88) | −.08(.43) | 1.20(.67–2.15) |
| SES (8 yrs.) | −.74(.52) | −2.16(1.03)* | −.76(.59) | .55(.42–.98)* |
| SES (12 mo.) | .09(.50) | .77(1.02) | .41(.61) | 1.12(.60–2.08) |
| Sleep Problems (8 yrs.) | −.04(.43) | −.31(1.03) | .21(46) | .58(.24–1.39) |
| Sleep Problems x SES | −.83(.57) | −1.52(1.27) | −1.54(.61)** | .25(.09-.74)** |
Note. All models run independently. Covariates, predictors, and moderator were grand mean centered. Sex (1 = female), ethnicity (1 = non-Hispanic European American), and objective and subjective sleep were collected at 8 years of age. Interaction terms were computed after centering the predictors and moderator. Est. = unstandardized partial regression coefficient estimate. SE = robust standard error. OR = logistic estimated odds ratio. CIs = 95% confidence intervals for estimated odds ratios. Odds ratio significance based on unstandardized partial regression coefficient estimates for odds ratios.
p ≤ .10;
p ≤ .05;
p ≤ .01;
p ≤ .001.
Figure 1.
Simple slopes plots for BMI scores by levels of average sleep efficiency (%) for high (+1 SD) and low (−1 SD) levels of SES. Simple slope associations between sleep efficiency and BMI scores were significant for children with low SES (b = −.10, p < .001). Region of significance analyses indicate that simple slopes were significant for families with SES scores less than −.11 or about 44.8% of the sample. *p ≤ .05; ** p ≤ .01; *** p ≤ .001.
Sleep predicting WC.
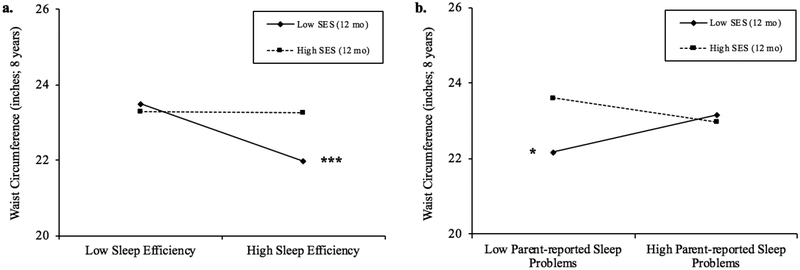
Greater sleep efficiency was associated with marginally smaller WC (p = .06). There was also a significant interaction between sleep efficiency and early SES when predicting WC (p < .01), such that greater sleep efficiency predicted smaller concurrent WC for children with low (b = −.13, p < .001) and average early SES (b = −.06, p = .05; about 57% of the sample; Figure 2a), but not high early SES (b = .001, ns). This suggests that children with low early SES showed .65 lower WC for each 5% increase in sleep efficiency (.22 SD lower) and children from average early SES backgrounds showed a .30 lower WC with each 5% increase in sleep efficiency (.10 SD lower). Similarly, the interaction between parent-reported sleep problems and early SES when predicting WC was significant (p < .01), with fewer sleep problems predicting smaller WC for children with low early SES (b = 1.38, p = .04; about 19% of the sample; Figure 2b), but not average (b = .21, ns) or high early SES (b = −.96, ns). This also indicates that children with low early SES experienced 1.38 greater WC (.46 SD greater) with each one-point increase in parent-reported sleep problem scores. There were no associations between sleep duration and early SES with WC.
Figure 2.
a. Simple slope plots for waist circumference by levels of average sleep efficiency (%) for high (+1SD) and low (−1SD) levels of SES. Simple slope associations between sleep efficiency and waist circumference were significant for children with low SES (b = −.13, p < .001) and average SES (b = −.06, p = .05). Region of significance analyses indicate that simple slopes were significant for families with SES scores less than .011 or about 57.4% of the sample. b. Simple slope plots for waist circumference by levels of parent-reported total sleep problem scores for high (+1SD) and low (−1SD) levels of SES. Simple slope associations between parent-reported sleep problems and waist circumference were significant for children with low SES (b = 1.38, p = .04). Region of significance analyses indicate that simple slopes were significant for families with SES less than −.64 or about 19.2% of the sample. *p ≤ .05; ** p ≤ .01; *** p ≤ .001.
Sleep predicting percent body fat.
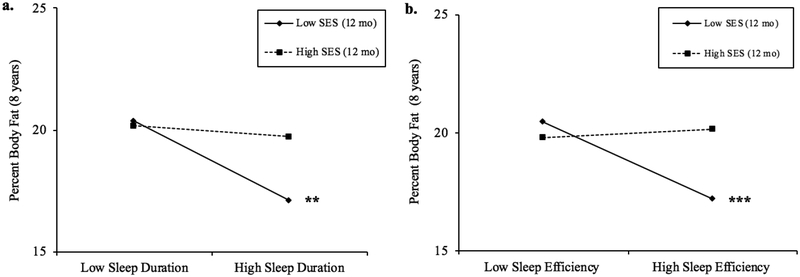
There was a significant interaction between objective sleep duration and early SES when predicting percent body fat (p < .05), such that greater sleep duration predicted lower percent body fat for children with low (b = −2.31, p < .01) and average early SES (b = −1.29, p = .01; about 60% of the sample; Figure 3a), but not high early SES (b = - .03, ns). This suggests that children with low early SES showed 2.31 lower percent body fat (.35 SD lower) and children from average early SES backgrounds showed a 1.29 lower percent body fat with each one-hour increase in sleep duration (.19 SD lower). Similarly, the interaction between objective sleep efficiency and early SES was significant when predicting percent body fat (p < .01), with greater sleep efficiency predicting lower percent body fat for children with low early SES (b = −.29, p < .001; about 48% of the sample; Figure 3b), but not average (b = −.13, p = .07) or high early SES (b = .04, ns). This shows that children with low early SES had 1.45 lower percent body fat with each 5% increase in sleep efficiency (.22 SD lower).
Figure 3.
a. Simple slope plots for percent body fat by levels of average sleep duration (hours) for high (+1SD) and low (−1SD) levels of SES. Simple slope associations between sleep duration and percent body fat were significant for children with low SES (b = −2.31, p < .01) and average SES (b = −1.29, p = .01). Region of significance analyses indicate that simple slopes were significant for families with SES scores less than .18 or about 60.2% of the sample. b. Simple slope plots for percent body fat by levels of average sleep efficiency (%) for high (+1SD) and low (−1SD) levels of SES. Simple slope associations between sleep efficiency and percent body fat were significant for children with low SES (b = −.29, p < .001). Region of significance analyses indicate that simple slopes were significant for families with SES scores less than - .04 or about 47.8% of the sample.
Sleep predicting weight status.
A one-hour increase in objective sleep duration at eight years was associated with 50% reduced odds of being classified as overweight/obese (p < .01). The interaction between sleep duration (eight years) and SES at 12 months was also significant (p = .05), such that a one-hour increase in sleep duration was associated with a 66% decrease in odds of being overweight/obese for children with low SES, a 48% decrease in odds at mean SES, and a 29% decrease in odds at high SES at 12 months (all ps < .01; Table 2). Similarly, a one-percent increase in objective sleep efficiency at eight years was associated with 5% reduced odds of being classified as overweight/obese (p < .05). The interaction between sleep efficiency (eight years) and SES at 12 months was also significant (p < .01), with a one-percent increase in sleep efficiency associated with a 25% decrease in odds of being classified as overweight/obese at low SES, a 5% decrease in odds at mean SES, and a 2.3% increase in odds at high SES at 12 months (all ps < .001; Table 2). Finally, the interaction between parent-reported sleep problems and SES at 12 months was significant (p < .01), with a one-point increase in sleep problem scores predicting a 65% increase in odds of being classified as overweight/obese at low SES (p = .06), and a 42% decrease in odds at mean SES at 12 months (p < .05; Table 2). Greater SES at eight year was associated with a 45% decrease in odds of being classified as overweight/obese (p < .05).
Discussion
We replicated prior empirical and meta-analytic findings showing that longer sleep duration was associated with lower BMI and reduced odds of being overweight/obese in childhood.4,11 Importantly, we identified novel associations between objective sleep quality, parent-reported sleep problems, and weight indicators in middle childhood, particularly for children who experienced low early SES environments. Greater objective sleep efficiency was associated with lower BMI and percent body fat for children from low early SES backgrounds, smaller WC for children with low and mean early SES, and significantly reduced odds of being overweight/obese at all levels of early SES. Greater objective sleep duration was also linked with healthier weight indicators (lower percent body fat and reduced odds of being overweight/obese) for children with low and mean levels of early SES. Finally, greater sleep problems were linked with larger WC for children with low early SES (with fewer problems linked to smaller WC), and increased risk of being overweight/obese for children with low and mean early SES.
Consistent with prior literature, we found a main effect of sleep duration on BMI scores (Table 2). Additionally, we found that greater objective sleep duration in middle childhood and early SES together predicted lower percent body fat for children with low and average levels of early SES. Further, findings suggest that children with low early SES experienced 2.31 lower percent body fat (.35 SD lower) and children from average early SES backgrounds showed a 1.29 lower percent body fat with each one-hour increase in sleep duration (.19 SD lower). However, we found that objective sleep efficiency (estimate of sleep quality) showed significant interactions with early SES and predicted all weight-related outcomes in the current study. These findings suggest that sleep quality, not just quantity, may be critically important when considering associations between sleep and weight, and sleep quality has implications for not just body mass, but also the amount of body fat. For example, research suggests that sleep quality may have stronger effects on emotional, behavioral and cognitive outcomes compared to sleep duration,24 and poor sleep quality (independent of sleep duration) may alter various aspects of sleep architecture and lead to changes in hormones that impact weight gain.51 Indeed, our findings demonstrate that having less fragmented sleep and fewer nighttime wakings (indicative of better sleep quality) may be particularly important, given that research shows that less fragmentation and fewer nighttime waking promote better physiological, endocrine, and immune regulation, making it less likely that hormone imbalances will increase body fat and weight.14 This is also in line with theory which suggests that sleep problems may prompt changes in hormones levels (e.g., insulin, leptin) and lead to problems with glucose uptake and metabolism, which can increase adiposity.14,15 At least one empirical study found that greater sleep duration variability and shorter sleep duration were associated with significant changes in insulin levels and greater health risk during middle childhood.19
However, we also found that parent-reported sleep problems were an important predictor of child weight indicators in middle childhood, such that fewer parent-reported sleep problems were associated with smaller WC for children with low early SES. This suggests that fewer sleep problems are associated with healthier weight and WC outcomes. Conversely, broader child sleep problems detected by parents may also be linked with greater abdominal fat. These findings highlight the need for multimethod sleep measurement, given differential associations between objective and subjective sleep and various weight outcomes like WC.51 Indeed, a number of studies demonstrate that shorter sleep duration and later sleep timing predict greater BMI and risk for being overweight/obese both concurrently and over time,10,11 which is in agreement with our findings. However, we did not find main effects of short sleep duration on WC or percent body fat as with prior studies,12,13 and our findings extend the previous literature by showing that objective sleep quality is associated with WC and percent body fat and subjective sleep quality predicts WC.
A key component of our findings is that associations between objective and subjective sleep and weight indicators in middle childhood were strongest for children who had low (or average) SES early in life, suggesting that early-life experiences and environment may have long-term effects on child health behaviors. When children demonstrated greater sleep quantity and quality, those who experienced low or average SES environments early in life showed the strongest protective effect of good quality sleep during middle childhood, thereby experiencing the greatest benefits of longer sleep duration and better sleep efficiency. Specifically, children who experienced low SES early in life demonstrated a 66% decrease in odds of being overweight/obese for each additional hour of sleep, and a 25% decrease in odds of being overweight/obese for each 1% increase in sleep efficiency. Thus, greater sleep quantity and quality may protect children who had low SES early in life from developing higher BMI scores, greater percent body fat, and larger WC, and reduce risk for being classified as overweight/obese later in childhood. Conversely, our findings demonstrate that children who experienced higher SES during infancy do not demonstrate increases in BMI scores, WC, and percent body fat in middle childhood with poor sleep; rather these high SES children show similar BMI scores, WC, and percent body under both poor and optimal sleep conditions in middle childhood, suggesting early SES may be protective for children in regards to links between sleep and weight later in childhood. These patterns of findings mirror studies that show no differences between children from high and low SES backgrounds in associations between high sleep quality and low sleep variability and cognitive functioning;52 however, when children experienced poor sleep quality and greater variability, children from high SES backgrounds showed better cognitive functioning compared to children from low SES backgrounds. Thus, our findings suggest that having high SES early in life may buffer strong, negative associations between sleep duration and percent body fat and serve as a protective factor.
Consideration of why we found significant associations between sleep and weight indicators only for children with low and average SES during infancy merits further and ongoing discussion. It is possible that lower income families tend to have less accessibility to multiple food options and/or affordable food options,53 which may account for the strength of associations between sleep and weight. Additionally, low SES families may be more likely to experience significant stress,54 as being both in a low SES family as well as experiencing recurrent and persistent early life stress has been linked to poor physiological and biological regulation later in life (e.g., increased cortisol output),54 which could include sleep and weight dysregulation.
Strengths, Limitations and Future Directions
The present study has a number of strengths including a large proportion of Latino families and significant socioeconomic diversity in the sample, multimethod assessment of sleep, and use of multiple indicators of weight. However, links between sleep and weight indicators are likely bidirectional and persist across development, with some associations between sleep and weight indictors stronger than others (e.g., sleep efficiency with percent body fat).40,55 Future studies should test longitudinal and bidirectional associations between sleep and weight indicators to determine which associations may be most important across developmental transitions, as most current analyses are cross-sectional and cannot determine direction of effects between sleep and weight. Studies should also aim to examine additional sleep variables such as sleep timing and rhythmicity, as studies have shown that children who experience shorter sleep duration, greater sleep duration variability, and later bedtimes demonstrate higher BMI scores and are more likely to consume sugar and sugary drinks, energy-dense foods, and fewer vegetables.56–58 Associations between sleep and weight also likely differ according to racial/ethnic composition, with Latino and African American children showing greater adiposity and poorer sleep in comparison to their European American counterparts.59,60 While the proportions of various racial/ethnic groups are not large enough for multigroup analyses in the present study, future studies should examine whether associations between sleep and weight are stronger or weaker for different racial/ethnic groups. Although we utilized a sample of twins which may limit generalizability of findings, estimates for traits like sleep and BMI from twin studies (including ours) are similar to studies of singletons in middle childhood.11,27,38 Finally, future studies with larger samples should estimate genetic influences on links between sleep and weight indicators, as sleep indicators are 30–70% heritable,61 and BMI and WC scores are as much as 70% heritable.62 Elucidating genetic and environmental influences on associations between sleep and weight indicators may clarify whether there is an underlying (genetic) dysregulation accounting for poor sleep and weight, or if links between sleep and weight are primarily accounted for by environmental or lifestyle factors.
Conclusion
Sleep and weight indictors were associated in middle childhood, particularly for children who experienced low early-life SES. Findings identify early SES as a potent moderator of associations between sleep and weight indicators in middle childhood and inform clinicians that maximizing and improving child sleep quantity and quality may be candidate targets for interventions looking to reduce BMI and obesity, particularly for children who experience fewer resources early in life.
Acknowledgments
The authors would like to thank all of the participants, research assistants, graduate students, and staff of the Arizona Twin Project without whom this paper would not be possible.
Sources of Funding: This research was supported by grants from the Institute for Mental Health Research and Arizona State University’s Challenged Child Project and the T. Denny Sanford School of Social and Family Dynamics awarded to K.L.C., the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079520 to L.D.D. and K.L.C. and Carlos Valiente, and a William T. Grant Foundation Scholar Award to L.D.D. Any opinion, findings, and conclusions expressed in this manuscript do not necessarily reflect views of funding agencies.
Conflict of Interest: There are no financial, institutional, or consultant conflicts of interest for any of the authors. This was not an industry-supported study, and does not include off-label or investigational use. This study is not linked to any clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Sleep Foundation. 2014 Sleep in America Poll – Sleep in the Modern Family: Summary of Findings. March 3, 2014. Available at: http://sleepfoundation.org/sleep-polls-data/2014-sleep-the-modern-family.
- 2.Smaldone A, Honig JC, Byrne MW. Sleepless in America: Inadequate sleep and relationships to health and well-being of our nation’s children. Pediatrics. 2007;119:S29–S37. [DOI] [PubMed] [Google Scholar]
- 3.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–298. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obes. 2008; 16:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Healthy weight. June 7, 2017. Available at: http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 7.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: A systematic review. Sleep Med Rev. 2012;16:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivier P, Tompkins C. Health consequences of obesity in children and adolescents, in Handbook of Childhood and Adolescent Obesity. Edited by Jelalian, Steele RG. New York, NY, Springer Science+Business Media, LLC, 2008, pp. 11–24. [Google Scholar]
- 9.Tanita. Understanding your measurements. June 10, 2017. Available at: http://www.tanita.com/en/understanding-your-measurements/
- 10.Chaput J, Brunet M, Tremlay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes. 2006;30:1080–1085. [DOI] [PubMed] [Google Scholar]
- 11.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep – a cross sectional study. Int J Obes. 2002;26:710–716. [DOI] [PubMed] [Google Scholar]
- 12.Nixon GM, Thompson JMD, Han DY, Becroft DM, Clark PM, Robinson E, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. Br Med J (Clin Res Ed). 2011;342: d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. [DOI] [PubMed] [Google Scholar]
- 16.Hakim F, Kheirandish-Gozal L, Gozal D. Obesity and altered sleep: a pathway to metabolic derangements in children?. Semin Pediatr Neurol. 2015;22: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaines J, Vgontzas AN, Fernandez-Mendoza J, Calhoun SL, He F, Liao D, et al. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab. 2016;311:E851–E858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatr. 2011;127:e345–e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller GE, Chen E. The biological residue of childhood poverty. Child Dev Perspect. 2013;7:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doane LD, Breitenstein RS, Beekman C, Clifford S, Smith TJ, Lemery-Chalfant L. Early life socioeconomic disparities in children’s sleep: the mediating role of the current home environment. J Youth Adolesc. 2018;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekstedt M, Nyberg G, Ingre M, Ekblom Ö, Marcus C. Sleep, physical activity and BMI in six to ten-year-old children measured by accelerometry: A cross-sectional study. Int J Behav Nutr Phys Act. 2013;10:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: A meta-analysis. Obes Rev. 2016;17:1154–1166. [DOI] [PubMed] [Google Scholar]
- 25.Bagley EJ, El-Sheikh M. Familial risk moderates the association between sleep and zBMI in children. J Pediatr Psychol. 2013;38:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78:309–323. [DOI] [PubMed] [Google Scholar]
- 27.El-Sheikh M, Bagley EJ, Keiley MK, Erath SA. Growth in body mass index from childhood into adolescence: The role of sleep duration and quality. J Early Adolesc. 2014;34:1145–1166. [Google Scholar]
- 28.Lemery-Chalfant K, Clifford S, McDonald K, O’Brien TC, Valiente C. Arizona twin project: A focus on early resilience. Twin Res Hum Genet. 2013;16:404–411. [DOI] [PubMed] [Google Scholar]
- 29.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships chance with age? Psychol Bull. 2002;128:295–329. [DOI] [PubMed] [Google Scholar]
- 30.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci. 2009;106:14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs BG, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9:135–146. [DOI] [PubMed] [Google Scholar]
- 33.Parsons TJ, Power C, Logan S, Summerbet CD. Childhood predictors of adult obesity: a systematic review. Int J Obes. 1999;23(Suppl 8):S1–S107. [PubMed] [Google Scholar]
- 34.El-Sheikh M, Bagley EJ, Keiley M, Elmore-Staton L, Chen E, Buckhalt JA. Economic adversity and children’s sleep problems: multiple indicators and moderation effects. Health Psychol. 2013;32:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrin DC, McGrath JJ, Quon EC. Objective and subjective socioeconomic gradients exist for sleep in children and adolescents. Health Psychol. 2014;33:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 2012;28:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckhalt JA. Insufficient sleep and the socioeconomic status achievement gap. Child Dev Perspect. 2011;5:59–65. [Google Scholar]
- 38.Delva J, O’Malley PM, Johnston LD. Racial/ethnic and socioeconomic status differences in overweight and health-related behaviors among American students: National trends 1986–2003. J Adolesc Health. 2006;39:536–545. [DOI] [PubMed] [Google Scholar]
- 39.Bagley EJ, Kelly RJ, El-Sheikh M. Longitudinal relations between children’s sleep and body mass index: the moderating role of socioeconomic risk. Sleep Health. 2015;1:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Dea JA, Dibley MJ, Rankin NM. Low sleep and low socioeconomic status predict high body mass index: A 1-year longitudinal study of Australian schoolchildren. Pediatr Obes. 2012;1:295–303. [DOI] [PubMed] [Google Scholar]
- 41.Neale MC, Maes HHM. Power and sample size, in Methodology for genetic studies of twins and families. Dordrecht, the Netherlands, Kluwer Academic Publishers B.V, 2004, pp. 117–123. [Google Scholar]
- 42.van der Sluis S, Dolan CV, Neale MC, Posthuma D A general test for gene-environment interaction in sib pair-based association analysis of quantitative traits. Behav Gen. 2008;38:372–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadeh A, Sharkey M, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17:201–207. [DOI] [PubMed] [Google Scholar]
- 44.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1–9. [PubMed] [Google Scholar]
- 45.Davis M. Measuring adiposity in health research, in Handbook of physiological research methods in health psychology. Edited by Luecken LJ, Gallo LG. Thousand Oaks, CA, SAGE Publications, Inc, 2008, pp. 259–288. [Google Scholar]
- 46.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. [DOI] [PubMed] [Google Scholar]
- 47.Muthén B, Kaplan D. A comparison of some of the methodologies for the factor analysis of non-normal Likert variables. Brit J Math Stat Psy. 1985;38:171–89. [Google Scholar]
- 48.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 49.American Academy of Sleep Medicine. Recharge with sleep: pediatric sleep recommendations promoting optimal health. June 13, 2016; Available at: https://aasm.org/recharge-with-sleep-pediatric-sleep-recommendations-promoting-optimal-health/
- 50.American Academy of Pediatrics. American academy of pediatrics supports childhood sleep guidelines. June 13, 2016. Available at: https://www.aap.org/en-us/about-the-aap/aap-press-room/Pages/American-Academy-of-Pediatrics-Supports-Childhood-Sleep-Guidelines.aspx
- 51.Michels N, Verbeiren A, Aherns W, De Henauw S, Sioen I. Children’s sleep quality: relation with sleep duration and adiposity. Public Health. 2014;128:488–490. [DOI] [PubMed] [Google Scholar]
- 52.Buckhalt JA, El-Sheikh M, Keller PS. Children’s sleep and cognitive functioning: race and socioeconomic status as moderators of effects. Child Dev. 2007;78:213–231. [DOI] [PubMed] [Google Scholar]
- 53.Wang MC, Kim S, Gonzalez AA, MacLeod KE, Winkleby MA. Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. J Epidemiol Community Health. 2007;61:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 55.Bub KL, Buckhalt JA, El-Sheikh M. Children’s sleep and cognitive performance: a cross-domain analysis of change over time. Dev Psychol. 2011;47:1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obes. 2011;19:1374–1381. [DOI] [PubMed] [Google Scholar]
- 57.Franckle RL, Falbe J, Gortmaker S, Ganter C, Taveras EM, Land T, et al. Insufficient sleep among elementary and middle school students is linked with elevated soda consumption and other unhealthy dietary behaviors. Prev Med. 2015;74:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golley RK, Maher CA, Matricciani L, Olds TS. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int Obes. 2013;37:546–551. [DOI] [PubMed] [Google Scholar]
- 59.Biggs SN, Lushington K, James Martin A, van den Heuvel C, Declan Kennedy J. Gender, socioeconomic, and ethnic differences in sleep patterns in school-aged children. Sleep Med. 2013;14:1304–1309. [DOI] [PubMed] [Google Scholar]
- 60.Wisniewski AB, Chernausek SD. Gender in childhood obesity: Family environment, hormones, and genes. Gend Med, 2009;6(SUPPL. 1):76–85. [DOI] [PubMed] [Google Scholar]
- 61.Gregory AM, Rijsdijk FV, Eley TC. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77:1668–1679. [DOI] [PubMed] [Google Scholar]
- 62.Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the ovesogenic environment. Am J Clin Nutr. 2008;87:398–404. [DOI] [PubMed] [Google Scholar]