Abstract
INTRODUCTION:
The low mild cognitive impairment (MCI)-to-cognitively normal (CN) reversion rate in the Alzheimer’s Disease Neuroimaging Initiative (2–3%) suggests the need to examine reversion by other means. We applied comprehensive neuropsychological criteria (NP Criteria) to determine the resulting MCI-to-CN reversion rate.
METHODS:
CN (n=641) or MCI (n=569) participants were classified at baseline and Year 1 using NP Criteria. Demographic, neuropsychological, and Alzheimer’s disease (AD) biomarker variables as well as progression-to-dementia were examined across Stable CN, Reversion, and Stable MCI groups.
RESULTS:
NP Criteria produced a one-year reversion rate of 15.8%. Reverters had demographics, AD biomarkers, and risk-of-progression most similar to the Stable CN group, and showed the most improvement on neuropsychological measures from baseline-to-Year 1.
DISCUSSION:
NP Criteria produced a reversion rate that is consistent with, albeit modestly improved from, reversion rates in meta-analyses. Reverters’ biomarker profiles and progression rates suggest that NP Criteria accurately tracked with underlying pathophysiologic status.
Keywords: mild cognitive impairment, reversion, diagnostic criteria, stability, neuropsychology, Alzheimer’s disease
1. Introduction
Mild cognitive impairment (MCI) is thought to represent a transitional state between normal cognition and dementia [1,2]. However, the cognitively normal (CN)-to-MCI-to-Alzheimer’s disease (AD) trajectory is not always unidirectional [3] and a diagnosis of MCI does not irreparably foreshadow progression to dementia. A large portion of individuals diagnosed with MCI revert to CN status when reevaluated after one year or more (up to 30–50%) [4–6]. Meta-analyses report reversion rates of 18% (26% when only “better quality” studies were included) [7] and 24% [8] across all (clinic- and community-based) studies, and approximately 25% to 30% when limited to community-based studies [7,8]. The MCI criteria of studies included in these meta-analyses varied widely. One included studies that defined MCI based on the International Working Group [9] or Petersen/Mayo Clinic criteria [10], clinical consensus, use of cognitive screening measures, or combination of neuropsychological and functional measures; both amnestic and non-amnestic MCI were included [7]. Another meta-analysis focused on only amnestic MCI based on traditional Mayo/Petersen criteria, requiring at least one objective memory test be at least 1.5 SDs below the normative mean; of the 25 studies included, 10 used a consensus diagnosis, 2 used an algorithmic diagnosis, 9 did not specify whether algorithmic or consensus diagnosis was used, and 4 used a clinical diagnosis from medical records [8].
Higher MCI-to-CN reversion rates are associated with younger age [11,12], better neuropsychological test performance and functional abilities [5,12–14], absence of an apolipoprotein E (APOE) ε4 allele [12,14], and a “normal” AD biomarker profile [14]. Individuals with non-amnestic and single-domain MCI revert more often than those with amnestic [5,13] and multi-domain MCI [4,5,13], respectively. Finally, diagnostic criteria for MCI that rely on only cognitive screens, rating scales, or a single impaired score on an objective memory test lead to higher rates of reversion than criteria that require poor performance on more than one neuropsychological test [6,15].
The MCI diagnostic criteria used in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [16] are similar to the conventional Petersen/Winblad criteria [9,10] and require a subjective cognitive complaint, normal global cognitive screening, minimal/mild changes on a self- and study-partner-informed interview of global functioning (Clinical Dementia Rating [CDR]), and impaired performance on a single objective memory test (delayed recall of one story from Logical Memory [LM]) [16]. ADNI’s one-year reversion rate from MCI to CN was initially reported to be a surprisingly low 2.2% [16], and similarly was noted to be 3% in a more recent inspection of the larger ADNI dataset [17]. When performance on all components of the ADNI MCI criteria at baseline and one year later (Year 1) were examined, about one-third of those who met all criteria at baseline failed to meet all criteria, particularly an impaired score on the LM test, at Year 1. Instead, it appears that the diagnosis of MCI was either carried forward from baseline to Year 1, or was primarily based on the CDR [17] without application of the LM score criterion. If the LM cutoff had been consistently applied at Year 1, the rate of reversion would have been at least 22% [17], a value more consistent with rates reported in other studies [7,8].
Lack of guidance on how to weight various components of the diagnostic criteria used in ADNI and heavy reliance on subjective cognitive complaints may also reduce diagnostic clarity [18,19]. These limitations can be overcome by using comprehensive neuropsychological criteria (NP Criteria) for MCI that are primarily based on objective neuropsychological test scores [15,20]. The NP Criteria classify someone as MCI if they do not have dementia, but performed >1 SD below a demographically-adjusted mean on two neuropsychological measures within the same cognitive domain, or >1 SD below the demographically-adjusted mean on at least one measure across three sampled cognitive domains; participants who were rated by a study partner to have a functional difficulty across at least two areas of functioning were also considered MCI [15,20]. Prior work has demonstrated that these comprehensive NP Criteria offer the optimal balance of sensitivity (i.e., >1 SD threshold for impairment compared to a 1.5 or 2 SD cut-off) with reliability (i.e., two impaired scores within a domain instead of one impaired score across the battery) [15].
When NP Criteria was applied to the ADNI cohort, we found that the standard ADNI MCI criteria had resulted in high rates of “false positive” [20,21] and “false negative” MCI diagnoses [22]. Strikingly, approximately 34% of ADNI-diagnosed MCI participants were classified as CN by actuarial NP Criteria and demonstrated normal imaging, CSF biomarker profiles, and functional trajectories [20,21,23–26]. The disagreement between NP Criteria and ADNI Criteria classifications skewed toward ADNI over-diagnosing MCI at baseline (“false-positives”); however, there is also evidence that the ADNI Criteria also missed a small portion of participants who were classified CN by ADNI but met NP Criteria for MCI (“false-negatives”) [22]. These potentially “missed” MCI participants had neuropsychological scores, cerebrospinal fluid markers, and progression rates that suggested an MCI diagnosis was warranted.
Given prior evidence that use of NP Criteria improves the accuracy of the baseline MCI diagnosis in ADNI relative to their standard methods [20–22], coupled with the observation that ADNI’s diagnostic tracking produces an artificially low MCI-to-CN reversion rate [17], we aimed to determine whether the NP Criteria produces a more defensible reversion rate in ADNI. We hypothesized that application of these criteria at baseline and Year 1 would provide a more accurate and reliable characterization of those MCI participants who revert to CN status that is more in-line with reversion rates from recent meta-analyses [7,8] than the unrealistically low reversion rates using the ADNI MCI diagnoses [17]. If true, these results would provide further support for the use of the NP Criteria as a flexible method for operationally-defining MCI that may be adapted across large aging datasets to yield more accurate diagnostic and tracking information. Also, findings would be expected to improve understanding of predictors of reversion when NP Criteria are implemented. Specifically, we hypothesize that participants who revert will be more likely to have lower proportions of AD genetic susceptibility, higher levels of cerebrospinal fluid (CSF) β-amyloid (Aβ), lower levels of CSF total tau (t-tau) and hyperphosphorylated-tau (p-tau), and be more likely to have a non-amnestic MCI profile than those participants that remain MCI according to NP Criteria at both baseline and Year 1 occasions.
2. Methods
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2003 as a public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information on ADNI, see www.adni-info.org. This study was approved by the Institutional Review Boards at each of the participating institutions, and written informed consent was obtained from all participants or authorized representatives at each site.
2.1. Participants and procedure
The specific enrollment inclusion/exclusion criteria for ADNI have been described elsewhere [16]. Briefly, participants from ADNI, ADNI-GO, and ADNI-2 cohorts were diagnosed at baseline by ADNI as CN, MCI (including early- and late-MCI for ADNI-GO and ADNI-2), or AD. All non-demented ADNI participants who completed a baseline and Year 1 neuropsychological assessment were considered for the current analyses; there were also 6 ADNI participants who were classified as AD at baseline, but were considered CN based on NP Criteria who were included in the analyses (total N=1,210).
Participants were re-classified separately at baseline and Year 1 as either CN or as having MCI using the following Jak/Bondi comprehensive NP Criteria [15,20]: (1) performance >1 SD below the age/education/sex-adjusted mean on two neuropsychological measures within the same cognitive domain, or (2) performance >1 SD below the demographically-adjusted mean on at least one measure across all three sampled cognitive domains, or (3) were rated by a study partner to have a Functional Activities Questionnaire (FAQ) score >5, suggesting difficulties across at least two areas of functioning.
Six neuropsychological total test scores were used to determine MCI status via NP Criteria [21] and included two memory measures: Rey Auditory Verbal Learning Test (AVLT) delayed free recall and AVLT recognition (hits minus false positives); two language measures: 30-item Boston Naming Test (BNT), Animal Fluency; and two attention/executive function measures: Trail Making Test (TMT), Part A and Part B. The neuropsychological age/education/sex-adjusted z-scores were based on regression coefficients derived from a sample of ADNI’s CN participants who did not progress to MCI for the duration of their study participation (i.e., “robust” controls; N=385) [21,25,26]. Regressions to determine demographic adjustment were completed at each occasion. Non-demented participants that did not meet NP Criteria for MCI were considered CN.
Once all participants were re-classified as CN or MCI at baseline and CN, MCI, or AD at Year 1, diagnostic stability, reversion, and conversion were examined. We then examined several biomarker and clinical variables to determine their association with reversion from MCI to normal. A subset of participants underwent a lumbar puncture (baseline CN n=363, MCI n=337). Cerebrospinal fluid (CSF) biomarkers of AD (Aβ, t-tau and p-tau) were measured using Elecsys ® immunoassays. APOE ε4 status, based on the presence of at least one ε4 allele, was also examined. The Mini Mental State Exam (MMSE) measured global cognition; the LM delayed recall subscale was an independent measure of memory performance that was not included in the NP Criteria diagnosis; the Geriatric Depression Scale (GDS) measured depressive symptoms; the modified Hachinski Ischemia Scale measured ischemic risk; and the CDR and FAQ measured everyday functioning. Type 2 diabetes mellitus (T2DM) status was determined via baseline medical history [27] or if they were on glucose-lowering medications [28].
ADNI’s AD criteria was used in progression-to-dementia analyses in order to be independent from the components of the NP Criteria. These criteria included: (1) subjective memory complaint by the subject, study partner, or clinician; (2) abnormal memory function defined as scoring below the education-adjusted cutoffs on the LM delayed recall subscale from the Wechsler Memory Scale–Revised (≤8 for 16+ years of education, ≤4 for 8–15 years of education, and ≤2 for 0–7 years of education); (3) MMSE score <27; (4) CDR=0.5 or 1.0; and (5) met NINCDS/ADRDA criteria for probable AD [29].
2.2. Statistical Analyses
Baseline demographic and clinical characteristics for each group (CN and MCI) were compared using independent t-tests, Mann-Whitney U tests, or chi-squared tests. Proportions of the sample who remained diagnostically stable, reverted, or progressed from baseline-to-Year 1 were examined. Group (Stable CN, Reversion, Stable MCI) differences in baseline demographic, clinical, functional, AD biomarker, and neuropsychological variables were examined using one-way analyses of variance (ANOVAs; with independent t-test post-hocs), Kruskal-Wallis tests (with Mann-Whitney post-hocs), or chi-squared tests for categorical variables. All post-hoc tests of group differences were adjusted for the 3 group pairwise comparisons, so the alpha was set to .017 (.05/3 groups).
A binary logistic regression was used to examine baseline predictors of Stable MCI versus Reversion status. Because raw data were used for this analysis, demographic variables (age, education, sex) were first entered in Block 1. Then, GDS, FAQ, Hachinski score, T2DM status, APOE ε4 status, Aβ, t-tau, p-tau, MMSE, AVLT Delayed Recall and Recognition, Animal Fluency, BNT, TMT Parts A and B, and LM Delayed Recall were included in the Block 2 and a forward stepwise procedure was used to determine which of these variables were included in the model so that only predictors that add value were included.
Change in raw neuropsychological performance from baseline-to-Year 1 by group (Stable CN, Reversion, Stable MCI) was evaluated using a repeated measures ANCOVA, controlling for age, education, and sex with post-hoc analyses that adjusted for multiple group comparisons (i.e., alpha=.017). Although some of the neuropsychological measures violate the assumption of normality (e.g., BNT and TMT are skewed), the analyses were run after transforming the data using a Blom-transformation and compared to the use of the raw scores. The results were qualitatively and statistically similar, and there were no differences in the pattern of findings for the Reversion group. Thus, the raw scores were used for analysis in order to facilitate clinical translation and interpretation of the results.
A Cox regression adjusting for age, education, and sex was used to determine the hazard ratio (HR) and 95% confidence interval (CI) of progression to dementia. In these analyses, time-to-dementia was the number of months (12 to 60 months) from the baseline assessment to the assessment when the participant first met criteria for dementia. Participants who did not progress to dementia during their follow-up period were censored at their last visit. Kaplan-Meier curves were used to show the rate of progression to dementia by group.
3. Results
3.1. Baseline characteristics
At baseline, there were 641 CN (53.0%) and 569 MCI (47.0%) participants based on NP Criteria, compared to 418 CN (34.5%), 786 MCI (65.0%), and 6 AD (0.5%) participants based on ADNI classifications. CN and MCI groups based on NP Criteria significantly differed on all demographic, functional, neuropsychological, and biomarker variables, with the exception of age (see Table 1). Of the participants diagnosed with MCI, 507 (89%) were diagnosed based on having two impaired neuropsychological scores within the same domain, 16 (3%) were diagnosed based on having one impaired score in each of the three cognitive domains, and 46 (8%) were diagnosed based on an FAQ score >5.
Table 1.
Baseline demographic and clinical characteristics [mean (SD) or %] by group.
| CN (N=641) | MCI (N=569) | t, U, or χ2 | p | |
|---|---|---|---|---|
| Age | 73.63 (6.95) | 73.85 (7.09) | t=−0.54 | .589 |
| Education | 16.31 (2.69) | 15.89 (2.86) | t=2.66 | .008 |
| Female, % | 47.0% | 39.9% | χ2=57.71 | .017 |
| GDS | 1.16 (1.32) | 1.59 (1.42) | U=216,896.00 | <.001 |
| CDR=0/0.5,% | 54.1% / 45.7% | 12.3% / 87.5% | χ2=233.67 | <.001 |
| FAQ | 0.60 (1.16) | 3.85 (4.51) | U=271,001.00 | <.001 |
| Hachinski | 0.57 (0.66) | 0.69 (0.77) | U=169,161.50 | .015 |
| T2DM status*, % | 7.2% | 10.5% | χ2=4.01 | .045 |
| Aβ (pg/ml) | 1292.46 (621.24) | 942.92 (555.04) | t=8.71 | <.001 |
| t-tau (pg/ml) | 243.66 (98.15) | 300.85 (129.37) | t=−7.27 | <.001 |
| p-tau (pg/ml) | 22.44 (10.20) | 29.58 (14.70) | t=−8.22 | <.001 |
| APOE ε4+, % | 33.4% | 53.8% | χ2=51.15 | <.001 |
| MMSE | 28.69 (1.47) | 27.44 (1.84) | U=108,803.00 | <.001 |
| Logical Memory | 10.73 (4.27) | 5.32 (4.02) | t=21.97 | <.001 |
| AVLT Delayed Recall | 7.53 (3.75) | 2.50 (3.00) | t=25.89 | <.001 |
| AVLT Recognition | 27.30 (2.42) | 22.38 (3.90) | t=25.88 | <.001 |
| BNT | 28.15 (1.97) | 25.54 (3.97) | U=102,273.50 | <.001 |
| Animal Fluency | 20.62 (5.06) | 15.90 (4.84) | t=16.51 | <.001 |
| TMT Part A | 32.81 (9.64) | 45.47 (20.20) | U=263,723.00 | <.001 |
| TMT Part B | 82.13 (35.67) | 130.76 (71.13) | U=263,467.00 | <.001 |
CN=Cognitively Normal; MCI=Mild Cognitive Impairment; GDS=Geriatric Depression Scale; MMSE=Mini-Mental Status Exam; CDR=Clinical Dementia Rating; FAQ=Functional Activities Questionnaire; AVLT=Rey Auditory Verbal Learning Test; BNT=Boston Naming Test (30-item); TMT=Trail Making Test; Aβ=β-amyloid; t-tau=total tau; p=tau=hyperphosporylated tau; APOE=Apolipoprotein E. Sample size for cerebrospinal fluid biomarkers: CN n=363, MCI n=337.
3.2. Classification at Year 1
Examination of stability, progression, and reversion showed that among participants classified by NP Criteria as CN at baseline (N=641), 508 (79.3%) remained CN, 125 (19.5%) progressed to MCI, and 8 (1.2%) progressed to dementia at Year 1. Of those who were classified as MCI at baseline (N=569), 381 (67%) remained MCI, 90 (15.8%) reverted to CN, and 98 (17.2%) progressed to dementia at Year 1. Consistent with what has been previously described [17], ADNI classifications showed that among participants they classified as CN at baseline (N=418), 401 (95.9%) remained CN and 17 (4.1%) progressed to MCI at Year 1. Of those who were classified by ADNI as MCI at baseline (N=786), 659 (83.8%) remained MCI, 24 (3.1%) reverted to CN, and 103 (13.1%) progressed to dementia at Year 1.
3.3. Reversion group characteristics
Within the NP Criteria-defined Reversion group, baseline MCI diagnoses were made based on two impaired scores within at least one cognitive domain in 80 participants (88.9% of reverters), based on three impaired scores across three cognitive domains in 3 participants (3.3% of reverters), and based on FAQ >5 in 7 participants (7.8% of reverters). The proportions of participants classified as MCI using the 3 different NP criteria (i.e., two impaired scores in one domain, three impaired scores across three domains, or FAQ >5) did not differ between Stable MCI and Reversion groups (χ2=0.04, df=2, p=.98).
When examining baseline MCI subtype of those participants that reverted, 46 participants were initially considered amnestic MCI (51.1%): 38 single-domain amnestic MCI and 8 multidomain amnestic MCI. There were 34 participants (37.8%) who were impaired in only non-memory domains; 16 impaired in the language domain, 6 impaired in attention/executive domain, and 2 impaired in both language and attention/execution domains. As mentioned above, 3 participants (3.3%) had diffuse impairments with one impaired score in three cognitive domains and 7 participants (7.8%) were diagnosed based on FAQ. When examining baseline amnestic and non-amnestic MCI subtypes (diagnosed based on two impaired scores in one domain), the proportion of non-amnestic MCI participants was greater in the Reversion group (34 out of 80, 42.5%) than in the Stable MCI group (97 out of 337, 28.8%), χ2=5.65, df=1, p=.017.
3.4. Baseline group differences
3.4.1. Demographics, clinical characteristics, and vascular risk
Table 2 shows the omnibus tests for the baseline characteristics of the NP Criteria-defined Stable CN, Reversion, and Stable MCI groups. Age and education did not differ by group (p-values >.05) and only the Stable CN and Stable MCI group differed on sex (χ2=8.93, df=1, p=.003), with the Stable CN group having a larger proportion of women. All groups endorsed minimal depressive symptoms, with only the Stable CN and Stable MCI groups significantly differing from one another such that the Stable MCI group endorsed more symptoms (U=72903.50, p<.001). On measures of everyday functioning (CDR, FAQ), the Stable CN group had the least amount of functional difficulty and significantly differed from the Reversion group (CDR χ2=9.25, df=1, p=.002; FAQ U=16797.50, p<.001) and Stable MCI group (CDR χ2=242.47, df=2, p<.001; FAQ U=46752.50, p<.001). The Reversion group has less functional difficulty that the Stable MCI group (CDR χ2=65.93, df=2, p<.001; FAQ U=12123.00, p<.001). The Reversion group had higher ischemia risk (U=19019.50, p=.004) and were more likely to have T2DM (χ2=10.51, df=1, p=.001) than the Stable CN group, but did not statistically differ from the Stable MCI group (Hachinski U=15794.00, p=.197; T2DM χ2=3.81, df=1, p=.051). The Stable CN group had lower ischemia risk than the Stable MCI group (U=87674.50, p=.007), but did not significantly differ on ischemic risk or T2DM status (χ2=2.65, df=1, p=.104).
Table 2.
Baseline demographic, clinical, neuropsychological, and biomarker characteristics of Stable CN, Reversion, and Stable MCI groups.
| Stable CN (N=508) | Reversion (N=90) | Stable MCI (N=381) | F, H, χ2 | p | |
|---|---|---|---|---|---|
| Age | 73.61 (6.80) | 74.85 (6.67) | 73.73 (7.22) | F=1.22 | .295 |
| Education | 16.37 (2.68) | 15.88 (2.66) | 15.95 (2.91) | F=3.01 | .050 |
| Female, % | 47.9% | 40.0% | 38.8% | χ2=9.65 | .008 |
| GDS* | 1.10 (1.32) | 1.47 (1.55) | 1.64 (1.40) | H=42.51 | <.001 |
| CDR* = 0 / 0.5, % | 59.4% / 40.6% | 42.2% / 57.8% | 8.4% / 91.3% | χ2=242.35 | <.001 |
| FAQ* | 0.45 (0.99) | 1.58 (2.51) | 3.66 (4.42) | H=214.52 | <.001 |
| Hachinski* | 0.53 (0.64) | 0.83 (0.90) | 0.67 (0.75) | H=12.38 | .002 |
| T2DM status*, % | 6.1% | 16.1% | 9.0% | χ2=10.49 | .005 |
| Aβ (pg/ml) | 1330.63 (597.63) | 1152.50 (628.86) | 954.24 (566.27) | F=31.43 | <.001 |
| t-tau* (pg/ml) | 236.44 (93.75) | 229.51 (86.96) | 307.97 (132.27) | F=36.47 | <.001 |
| p-tau* (pg/ml) | 21.58 (9.57) | 21.63 (9.62) | 30.33 (15.08) | F=44.00 | <.001 |
| APOE ε4+*, % | 30.1% | 36.7% | 54.1% | χ2=52.49 | <.001 |
| MMSE | 28.83 (1.31) | 28.72 (1.49) | 27.32 (1.77) | H=179.54 | <.001 |
| Logical Memory | 11.37 (4.10) | 9.34 (3.35) | 5.27 (3.73) | F=268.42 | <.001 |
| AVLT Delayed Recall | 8.21 (3.63) | 4.45 (3.38) | 2.35 (2.89) | F=340.19 | <.001 |
| AVLT Recognition | 27.61 (2.16) | 24.06 (3.58) | 22.43 (3.93) | F=311.78 | <.001 |
| BNT | 28.33 (1.74) | 26.86 (2.73) | 25.52 (3.17) | H=155.17 | <.001 |
| Animal Fluency | 21.05 (5.09) | 17.17 (4.50) | 15.99 (4.95) | F=117.13 | <.001 |
| TMT Part A, total seconds* | 32.31 (9.37) | 41.98 (14.82) | 45.27 (20.50) | H=144.26 | <.001 |
| TMT Part B, total seconds* | 78.61 (31.46) | 104.79 (52.56) | 129.76 (68.84) | H=181.84 | <.001 |
Denotes variable in which lower scores are better. CN=cognitively normal; MCI= mild cognitive impairment; GDS=Geriatric Depression Scale; CDR=Clinical Dementia Rating; FAQ=Functional Activities Questionnaire; T2DM=type 2 diabetes mellitus; Aβ=β-amyloid; t-tau=total tau; p-tau=hyperphosphorylated tau; APOE ε4+ = apolipoprotein E epsilon 4 allele positivity; MMSE=Mini Mental State Exam; AVLT=Rey Auditory Verbal Learning Test; BNT=Boston Naming Test (30-item); TMT=Trail Making Test; Sample size for cerebrospinal fluid biomarkers: Stable CN n=363, Reversion n=70, stable MCI n=267.
3.4.2. AD biological markers
Examination of baseline AD biomarkers showed that Stable CN and Reversion participants did not significantly differ on baseline levels of Aβ [t(431)=2.26, p=.024], t-tau [t(431)=0.57, p=.567],or p-tau [t(431)=−0.04, p=.968] after adjustment for multiple comparisons. Stable CN and Reversion groups had higher (better) levels of Aβ [t(628)=7.99, p<.001 and t(335)=2.55, p=.011, respectively] as well as lower (better) levels of t-tau [t(453.43)=−7.55, p<.001 and t(162.58)=−5.96, p<.001, respectively] and p-tau [t(419.80)=−8.33, p<.001 and t(168.27)=−5.90, p<.001, respectively] compared to Stable MCI participants. The Stable CN and Reversion groups did not differ in proportions of participants with an APOE ε4 allele (χ2=1.53, df=1, p=.216), but both the Stable CN and Reversion groups had a smaller proportion of individuals with an ε4 allele relative to the Stable MCI group (χ2=51.87, df=1, p<.001 and χ2=8.82, df=1, p=.003, respectively).
3.4.3. Neuropsychological functioning
Table 2 displays the mean baseline neuropsychological scores across NP Criteria-defined Stable CN, Reversion, and Stable MCI groups and omnibus tests. Baseline neuropsychological performance of the Reversion group was significantly worse than the Stable CN group on all measures except the MMSE (U=22553.50, p=.832), including LM [t(140.85)=5.10, p<.001], AVLT Delayed Recall [t(595)=9.10, p<.001], AVLT Recognition [t(99.52)=9.10, p<.001], BNT (U=15685.50, p<.001), Animal Fluency, TMT Part A (U=12952.50, p<.001), and TMT Part B (U=14695.00, p<.001). The Reversion group performed better than the Stable MCI group on the MMSE (U=9167.00, p<.001), LM [t(469)=9.51, p<.001], AVLT Delayed Recall [t(119.87)=5.40, p<.001], AVLT Recognition [t(467)=3.57, p<.001], BNT (U=13991.00, p=.007), and TMT Part B (U=13149.50, p=.001), but not Animal Fluency [t(469)=2.06, p=.040] or TMT Part A (U=16138.50, p=.386). On average, the Reversion group’s lowest baseline neuropsychological scores were on the AVLT Delayed Recall (mean z-score=−0.82) and AVLT Recognition (mean z-score=−1.03). The Stable CN group performed better than the Stable MCI groups across all neuropsychological tests: MMSE (U=48374.00, p<.001), LM [t(887)=22.83, p<.001], AVLT Delayed Recall [t(883.82)=26.74, p<.001], AVLT Recognition [t(548.30)=23.21, p<.001], BNT (U=50437.50, p<.001), Animal Fluency [t(887)=14.85, p<.001], TMT Part A (U=53659.50, p<.001), and TMT Part B (U=45217.50, p<.001).
3.5. Unique predictors of Reversion
Table 3 shows the results, including odds ratios, of the logistic regression that examined the predictors of Reversion versus Stable MCI group status at Year 1. Block 1 that included demographic variables did not initially improve fit relative to the null model (χ2=2.78, df=3, p=.428). For Block 2, the forward stepwise procedure resulted in the following variables included in the model: FAQ, t-tau, MMSE, AVLT delayed recall, TMT Part B, and LM. Block 2 showed significant incremental improvement in model fit over Block 1 (χ2=98.13, df=6, p<.001; Nagelkerke R2=.421) and correctly classified 81.9% of the participants (sensitivity=.37; specificity=.94; positive predictive value=.61, negative predictive value=.85).
Table 3.
Final logistic regression predicting Reversion group status (compared to Stable MCI).
| OR | 95% CI | p | |
|---|---|---|---|
| Block 1 | |||
| Age | 1.092 | 1.034–1.153 | .001 |
| Education | 0.872 | 0.762–0.998 | .046 |
| Female | 0.765 | 0.375–1.559 | .461 |
| Block 2 | |||
| FAQ* | 0.855 | 0.765–0.956 | .006 |
| t-tau* (pg/ml) | 0.996 | 0.992–0.999 | .025 |
| MMSE | 1.307 | 1.040–1.642 | .021 |
| AVLT Delayed Recall | 1.159 | 1.038–1.294 | .009 |
| TMT Part B, total seconds* | 0.988 | 0.980–0.995 | .002 |
| Logical Memory | 1.180 | 1.059–1.314 | .003 |
Denotes variable in which lower scores are better. OR=Odds Ratio; CI=Confidence Interval; FAQ=Functional Activities Questionnaire; t-tau=total tau; MMSE=Mini Mental State Exam; AVLT=Auditory Verbal Learning Test; TMT=Trail Making Test. There were 321 participants with all available data for this analysis; the Reversion group (n=67) was coded as 1 and the Stable MCI group (n=254) was coded as 0 for this logistic regression.
3.6. Baseline to Year 1 changes in neuropsychological test scores
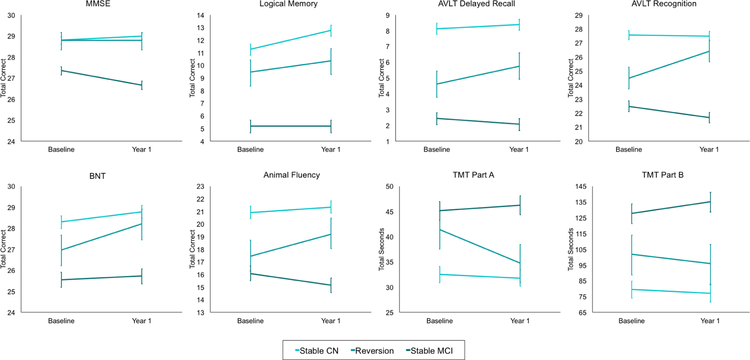
Repeated measures ANCOVAs were used to examine neuropsychological raw score change over time by group. The omnibus statistics for the group x time interactions across all neuropsychological tests are included in Supplemental Table 1. Notably, all omnibus group x time interactions were significant (ps<.001). Figure 1 shows the change in neuropsychological scores by group. Post-hoc analyses revealed that the Stable CN group showed significant improvement from baseline-to-Year 1 on the MMSE [t(501)=2.56, p=.012], LM [t(501)=9.70, p<.001], and BNT [t(500)=5.63, p<.001], but not on other neuropsychological tests (ps>.017). The Reversion group had significant improvements from baseline-to-Year 1 across most neuropsychological measures, including LM [t(84)=2.43, p=.015], AVLT Delayed Recall [t(84)=3.67, p<.001], AVLT Recognition [t(84)=6.91, p<.001], BNT [t(85)=6.02, p<.001], Animal Fluency [t(85)=4.04, p<.001], and TMT Part A [t(85)=−4.80, p<.001], but not on the MMSE or TMT Part B (ps>.150). With the exception of LM, the raw score improvements were larger for the Reversion group than for the Stable NC group. Conversely, the Stable MCI group showed significant baseline-to-Year 1 declines on the MMSE [t(376)=−7.66, p<.001], AVLT Delayed Recall [t(374)=−2.48, p=.014], AVLT Recognition [t(374)=−5.16, p<.001], Animal Fluency [t(375)=−4.43, p<.001], and TMT Part B [t(374)=3.34, p=.001] over the one-year interval, but not on other neuropsychological testis (ps>.017). At Year 1, the Stable CN and Reversion groups no longer significantly differed on the MMSE [t(591)=−2.29, p=.022], BNT [t(591)=−1.71, p=.086] and Trails A [t(590)=1.66, p=.097], but the Reversion group continued to perform worse than the Stable CN group on LM [t(464)=−4.85, p<.001], AVLT Delay Recall [t(465)=−6.86, p<.001], AVLT Recognition [t(465)=−3.11, p=.002], Animal Fluency [t(466)=−4.05, p<.001], and TMT Part B [t(466)=−3.14, p<.001].
Figure 1. Baseline to Year 1 change in neuropsychological scores by group.
Error bars represent the 99% confidence interval. MMSE=Mini Mental State Exam; AVLT=Rey Auditory Verbal Learning Test; BNT=Boston Naming Test (30-item); TMT=Trail Making Test. For TMT Parts A and B, lower scores represent better (faster) performance. Raw scores have been adjusted for age, sex, and education.
3.7. Progression to dementia by group
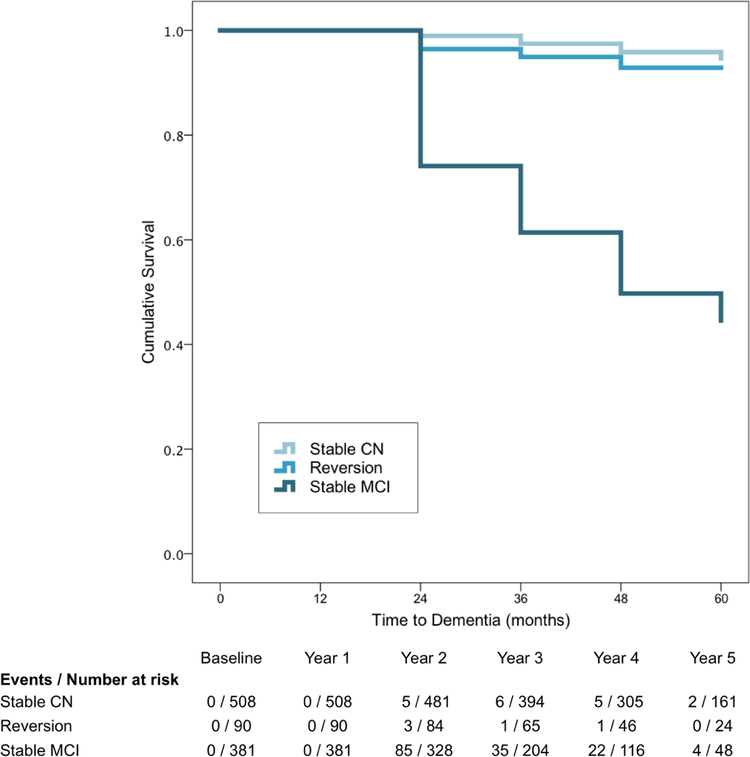
Differential rates of progression to dementia were examined between NP Criteria-defined Stable CN, Reversion, and Stable MCI groups. Cox regressions, adjusting for age, education, and sex, showed that, relative to the Stable CN group, the Reversion group did not differ in rate of progression (HR 1.67, 95% CI [0.63, 4.55], p=.301), while the Stable MCI group progressed at a faster rate than the Stable CN (HR 15.78, 95% CI [9.62, 25.86], p<.001) and Reversion groups (HR 9.35, 95% CI [3.83, 22.83], p<.001). Kaplan-Meier curves and numbers of events/persons at risk are shown in Figure 2.
Figure 2.
Kaplan-Meier curves for Stable CN, Reversion, and Stable MCI group rates of progression to dementia.
4. Discussion
Implementation of the NP Criteria at baseline and Year 1 produced an MCI-to-CN reversion rate of 15.8%. This reversion rate is largely consistent with, if not modestly lower than, the reversion rates of 18% (26% when only “better quality” studies were included) [7] and 24% [8] reported in recent meta-analyses. Moreover, it is much more realistic than the 2.2% and 3% reversion rates found in ADNI [8,16,17], which appear to be artificially low due to seemingly weighting the subjective CDR more heavily than the LM element of the ADNI MCI criteria [17].
The NP Criteria-defined Reversion group did not differ from the Stable CN or Stable MCI groups on demographics or baseline depressive symptoms. Compared to the Stable CN group, the Reversion group had more difficulty on baseline measures of everyday functioning, including the CDR (an independent measure not used in the NP Criteria), but had better functional scores than the Stable MCI group, which is consistent with previous studies [5,12]. Notably, the Reversion group did not have a greater proportion of participants classified as MCI at baseline based on the FAQ >5 element of the NP Criteria relative to the Stable MCI group, suggesting that the NP Criteria reversion rate was not driven by this element of the NP Criteria. Consistent with previous work [5,13,15], the Reversion group had a higher proportion of participants who were diagnosed with non-amnestic MCI (i.e., impaired in language or attention/executive functions), relative to the Stable MCI group.
Consistent with the greater likelihood of non-amnestic impairments in reverters, examination of vascular risk variables such as the Hachinski ischemia risk index and T2DM status showed that the Reversion group had the highest scores on the Hachinski index and the highest proportion of participants with T2DM across the groups, significantly differing from the Stable CN group. Possible vascular- or diabetes-related variability in performance at baseline with subsequent regression to the mean at Year 1 (e.g., due to transient cerebral blood flow disruptions and/or fluctuating glucose levels) is a hypothesis that needs further investigation.
When examining AD-related biomarkers, the NP Criteria-defined Reversion group did not differ from the Stable CN group across CSF measures of Aβ, t-tau, and p-tau, or proportion of APOE ε4 carriers, but the Reversion group differed from the Stable MCI group across all markers examined. These biomarker findings are consistent with the rates of progression to dementia across groups. Specifically, the Stable CN and Reversion groups had slower rates of progression to dementia over 5 years than the Stable MCI group, but did not differ from each other. The Reversion group’s HR of 1.67 when compared to the Stable CN group is lower than the rate of progression for reverters in other studies (e.g., HR=6.6 in the Mayo study [5] and HR=6.4 in the Sydney Memory and Ageing Study [4]). Thus, the participants identified as reverters via the NP Criteria do not appear to be at the same elevated risk for dementia as reverters from other studies, suggesting that the NP Criteria, with most participants being classified based on two tests within a cognitive domain, may provide improvement in prediction of progression compared to other criteria. An alternative or additional reason for these findings may be that this study used dementia criteria that were completely independent of the NP Criteria for MCI. Other studies often use similar criteria for classifying MCI and dementia, with the primary difference being a greater degree of severity to meet dementia criteria (e.g., CDR =0.5 for MCI and 1.0 for dementia).
At baseline, as expected based on their initial MCI diagnoses, the Reversion group performed worse than the Stable CN participants on almost all neuropsychological measures, but also generally performed better than the Stable MCI group, with the exception of a couple tests (animal fluency and TMT Part A). When predicting Reversion vs. Stable MCI status at Year 1, only baseline FAQ score, t-tau, MMSE, AVLT delayed recall, TMT Part B, and LM emerged as unique predictors of reversion. Notably, even with a model that considered demographics and available clinical, health, CSF, and cognitive predictors, only 37.3% of the participants who reverted at Year 1 were correctly classified using this information, suggesting that it is difficult to determine who may be more likely to revert. Future analyses should also consider the interactive effects of known predictors of reversion in order to produce models that may better identify those participants who are more likely to revert vs. remain cognitively impaired.
The Stable CN group demonstrated largely consistent neuropsychological raw score performances from baseline-to-Year 1, and showed improvements on MMSE, LM, BNT, and Animal Fluency, which likely represents an expected practice effect. The Reversion group improved on all measures except MMSE and TMT Part B, and the improvements were generally greater than those observed by the Stable CN. Thus, the improvements in the Reversion group likely go beyond the expected practice effect, in that they may also be consistent with an overall trend of regression to the mean. Conversely, the Stable MCI group declined across most neuropsychological measures. The dependent variable of the repeated measures analyses was in raw score metric, as this is most clinically relevant; however, the NP Criteria (e.g., >1 SD below mean cutoff on tests) was applied to the demographically-adjusted z-scores. The z-scores were created separately for each occasion, so the robust control participant scores at baseline were used to create the baseline z-score and the robust control participant scores at Year 1 were used to create the Year 1 z-scores. This approach allowed for the mean practice effect of the robust control participants to be accounted for in the z-scores. This method, again, supports the likelihood that practice effects alone cannot fully explain the greater improvement of the Reversion group.
The current findings, however, do highlight the potential for using measures of change, and accounting for practice effects, as a way of capturing those at risk for future progression. Previous work in a population-based sample has shown that practice effects may be reduced in individuals with incident MCI/dementia within a year of testing [30]. Practice effects are an important consideration for any longitudinal observational study, clinical trial, or clinical evaluation that involves serial testing [31], and it may be possible to derive predictive information about future cognitive outcomes by determining if a practice effect is less than or greater than expected [32,33].
Accurate characterization of reversion is critical in multiple settings. In research, clinical ‘trial ready’ cohorts are being assembled for manytypes of studies. Although it may take some effort to characterize people with MCI longitudinally, applying a consistent, objective neuropsychological criteria could help to winnow a cohort such that those most appropriate candidates for clinical trials are included [34]. Additionally, in clinical practice, patients may be labeled as having MCI for a variety of reasons, not all of which portend a high risk of AD. Re-assessment in enough detail to capture reversion would prevent people from carrying forward a label of MCI, with the negative ramifications that may result from the inaccurate label and have the potential to adversely impact their social interactions, self-perceptions, and life decisions (e.g., retirement).
For both research and clinical settings, older adults may also lack access to a knowledgeable informant; objective neuropsychological assessment would reduce this as a barrier to enrollment in a clinical trial or accurate determination of cognitive status, as it is able to serve as a stand-alone method to determine diagnosis [15,20] and reversion. The meta-analyses that examined reversion rates generally used criteria that differed from the NP Criteria; the criteria also varied within and between meta-analyses. Several of the studies included in both meta-analyses appeared to rely on a consensus diagnosis, which often includes multiple clinicians integrating information not only from a neuropsychological assessment but also from the participant and an informant about the course of cognitive symptoms and functional changes to form a diagnosis. Interestingly, the NP Criteria produced a similar or modestly better reversion rate than those reported in the meta-analyses despite only requiring the objective neuropsychological data and consistent application of the NP Criteria. This finding, in addition to the prior work showing that subjective report of cognitive difficulties may increase false positive MCI diagnostic errors [19,35], provides support for the use of a NP Criteria. Furthermore, the meta-analyses examined reversion rates over a variable number of years, often ≥2 years. Malek-Ahmadi [8] suggested that a longer follow-up period may produce lower rates of reversion; thus, the one-year follow-up interval in this study may have produced a slightly higher reversion rate than would have been be produced over a longer follow-up period.
Our study is limited in that the examination of stability and reversion was constrained to the baseline-to-Year 1 interval. Further work examining the reversion and fluctuation of diagnoses over a longer follow-up period will expand on the current findings. Our findings, however, offer an alternative and empirically-supported method of MCI classification using a comprehensive neuropsychological approach that can be applied across research and clinical settings and is adaptable to different neuropsychological batteries [15,20,36]. Notably, the NP Criteria resulted in a reversion rate that is more accurate than the extremely low reversion rate previously reported using the ADNI-based diagnoses [14,16,17], and it is also modestly lower than those reported in meta-analyses [7,8]. Furthermore, the NP Criteria appear to reliably capture true “reverters” as evidenced by the low AD biomarkers and progression-to-dementia risk in the Reversion group, both of which were consistent with those of the Stable CN group.
Supplementary Material
Highlights
Neuropsychological Criteria for MCI produced a reversion rate of 15.8%
15.8% is consistent with, or modestly improved, relative to the literature
Reverters had AD biomarkers and dementia risk similar to the Stable Normal group
Neuropsychological Criteria offer an empirically-proven MCI classification method
Results extend previous work that these MCI criteria may reduce diagnostic error
Research in Context
Systematic review:
The authors reviewed studies (using PubMed) related to MCI reversion and predictors of reversion. Reversion rates and correlates of reversion vary significantly across studies, but in general, the MCI-to-normal reversion rate reported in the Alzheimer’s Disease Neuroimaging Initiative was lower than all other studies included in a meta-analysis.
Interpretation:
Our findings demonstrate that MCI diagnosed based on objective neuropsychological performance, rather than a heavy reliance on subjective criteria, produced a realistic reversion rate that is consistent with, or slightly improved, relative to meta-analyses. These results extend evidence that MCI diagnoses based on neuropsychological performance offer an empirically-supported classification method.
Future directions:
Future work will examine the fluctuation of diagnoses over a longer follow-up period to determine whether performance variability is related to AD-related changes and progression risk. We will also continue efforts to compare objective performance and subjective report methods of detecting those at risk for future decline.
Acknowledgements:
This work was supported by NIH grants R01 AG049810 (M.W.B.), K24 AG026431 (M.W.B.), and P50 AG005131 (D.R.G., D.P.S, S.D.E), the Alzheimer’s Association (AARF-17–528918 to K.R.T. and AARG-17–500358 to E.C.E.), and the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2 CX001415–01A1 to E.C.E.). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Abbreviations:
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ANOVA
Analysis of Variance
- APOE
Apolipoprotein E
- AVLT
Rey Auditory Verbal Learning Test
- BNT
Boston Naming Test
- CDR
Clinical Dementia Rating
- CI
Confidence interval
- CN
Cognitively normal
- CSF
Cerebrospinal Fluid
- GDS
Geriatric Depression Scale
- FAQ
Functional Activities Questionnaire
- HR
Hazard ratio
- LM
Logical Memory
- MCI
Mild Cognitive Impairment
- MMSE
Mini Mental State Exam
- NP
Neuropsychological
- NINCDS/ADRDA
National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association
- p-tau
hyperphosphorylated tau
- T2DM
Type 2 diabetes mellitus
- t-tau
total tau
- TMT
Trail Making Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013;29:753–72. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 2011;7:270–9. doi: 10.1016/J.JALZ.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lopez OL, Becker JT, Chang Y-F, Sweet RA, DeKosky ST, Gach MH, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology 2012;79:1599–606. doi: 10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aerts L, Heffernan M, Kochan NA, Crawford JD, Draper B, Trollor JN, et al. Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology 2017;88:2225–32. doi: 10.1212/WNL.0000000000004015. [DOI] [PubMed] [Google Scholar]
- [5].Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJH, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014;82:317–25. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Loewenstein DA, Acevedo A, Small BJ, Agron J, Crocco E, Duara R. Stability of different subtypes of mild cognitive impairment among the elderly over a 2- to 3-year follow-up period. Dement Geriatr Cogn Disord 2009;27:418–23. doi: 10.1159/000211803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, et al. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. J Am Med Dir Assoc 2016;17:943–8. doi: 10.1016/J.JAMDA.2016.06.020. [DOI] [PubMed] [Google Scholar]
- [8].Malek-Ahmadi M Reversion From Mild Cognitive Impairment to Normal Cognition. Alzheimer Dis Assoc Disord 2016;30:324–30. doi: 10.1097/WAD.0000000000000145. [DOI] [PubMed] [Google Scholar]
- [9].Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–6. [DOI] [PubMed] [Google Scholar]
- [10].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild Cognitive Impairment. Arch Neurol 1999;56:303. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- [11].Gao S, Unverzagt FW, Hall KS, Lane KA, Murrell JR, Hake AM, et al. Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. Am J Geriatr Psychiatry 2014;22:670–81. doi: 10.1016/j.jagp.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of Reversion from Mild Cognitive Impairment to Normal Cognition. Dement Geriatr Cogn Disord 2017;43:204–14. doi: 10.1159/000456070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pandya SY, Clem MA, Silva LM, Woon FL. Does mild cognitive impairment always lead to dementia? A review. J Neurol Sci 2016;369:57–62. doi: 10.1016/J.JNS.2016.07.055. [DOI] [PubMed] [Google Scholar]
- [14].Park MH, Han C. Is there an MCI reversion to cognitively normal? Analysis of Alzheimer’s disease biomarkers profiles. Int Psychogeriatrics 2015;27:429–37. doi: 10.1017/S1041610214002129. [DOI] [PubMed] [Google Scholar]
- [15].Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of Five Neuropsychological Approaches to Defining Mild Cognitive Impairment. Am J Geriatr Psychiatry 2009;17:368–75. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thomas KR, Eppig JS, Weigand AJ, Edmonds EC, Wong CG, Jak AJ, et al. Artificially low mild cognitive impairment to normal reversion rate in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Dement 2019;15:561–9. doi: 10.1016/J.JALZ.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subjective Cognitive Complaints Contribute to Misdiagnosis of Mild Cognitive Impairment. J Int Neuropsychol Soc 2014;20:836–47. doi: 10.1017/S135561771400068X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Edmonds EC, Weigand AJ, Thomas KR, Eppig J, Delano-Wood L, Galasko DR, et al. Increasing Inaccuracy of Self-Reported Subjective Cognitive Complaints Over 24 Months in Empirically Derived Subtypes of Mild Cognitive Impairment. J Int Neuropsychol Soc 2018;24:842–53. doi: 10.1017/S1355617718000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates. J Alzheimer’s Dis 2014;42:275–89. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s Dement 2015;11:415–24. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Edmonds EC, Delano-Wood L, Jak AJ, Galasko DR, Salmon DP, Bondi MW. “Missed” Mild Cognitive Impairment: High False-Negative Error Rate Based on Conventional Diagnostic Criteria. J Alzheimer’s Dis 2016;52:685–91. doi: 10.3233/JAD-150986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bangen KJ, Clark AL, Werhane M, Edmonds EC, Nation DA, Evangelista N, et al. Cortical Amyloid Burden Differences Across Empirically-Derived Mild Cognitive Impairment Subtypes and Interaction with APOE ɛ4 Genotype. J Alzheimer’s Dis 2016;52:849–61. doi: 10.3233/JAD-150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, et al. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology 2016;87:2108–16. doi: 10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thomas KR, Edmonds EC, Delano-Wood L, Bondi MW. Longitudinal Trajectories of Informant-Reported Daily Functioning in Empirically Defined Subtypes of Mild Cognitive Impairment. J Int Neuropsychol Soc 2017;23:521–7. doi: 10.1017/S1355617717000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eppig JS, Edmonds EC, Campbell L, Sanderson-Cimino M, Delano-Wood L, Bondi MW, et al. Statistically Derived Subtypes and Associations with Cerebrospinal Fluid and Genetic Biomarkers in Mild Cognitive Impairment: A Latent Profile Analysis. J Int Neuropsychol Soc 2017;23:564–76. doi: 10.1017/S135561771700039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li W, Risacher SL, Huang E, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative F the ADN. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016;87:595–600. doi: 10.1212/WNL.0000000000002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moran C, Beare R, Phan TG, Bruce DG, Callisaya ML, Srikanth V, et al. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 2015;85:1123–30. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- [30].Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, et al. Practice Effects and Longitudinal Cognitive Change in Normal Aging vs. Incident Mild Cognitive Impairment and Dementia in The Mayo Clinic Study of Aging. Clin Neuropsychol 2013;27:1247–64. doi: 10.1080/13854046.2013.836567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2015;1:103–11. doi: 10.1016/J.DADM.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology 2015;29:940–8. doi: 10.1037/neu0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duff K, Horn KP, Foster NL, Hoffman JM. Short-Term Practice Effects and Brain Hypometabolism: Preliminary Data from an FDG PET Study. Arch Clin Neuropsychol 2015;30:264–70. doi: 10.1093/arclin/acv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Edmonds EC, Ard MC, Edland SD, Galasko DR, Salmon DP, Bondi MW. Unmasking the benefits of donepezil via psychometrically precise identification of mild cognitive impairment: A secondary analysis of the ADCS vitamin E and donepezil in MCI study. Alzheimer’s Dement Transl Res Clin Interv 2018;4:11–8. doi: 10.1016/j.trci.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subjective Cognitive Complaints Contribute to Misdiagnosis of Mild Cognitive Impairment. J Int Neuropsychol Soc 2014;20:836–47. doi: 10.1017/S135561771400068X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, et al. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J Int Neuropsychol Soc 2016;22:937–43. doi: 10.1017/S1355617716000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.