Abstract
Initial studies on cancer primarily focused on malignant cells themselves. The overarching narrative of cancer revolved around unchecked and rapidly proliferating cells. Special attention was given to the molecular, genetic and metabolic profiles of isolated cancer cells in hopes of elucidating a critical factor in malignancy. However, the scope of cancer research has broadened over the past few decades to include the local environment around cancer. It has become increasingly apparent that the immune cells, vascular networks, and the extracellular matrix all have a part in cancer progression. The impact of the extracellular matrix is particularly fascinating and key stromal changes have been identified in various cancers. Pioneering work studying laminin and hyaluronate has shown that these molecules have vital roles in cancer progression. More recently, fibronectin has been included as an extracellular driver of malignancy. Fibronectin is thought to play a considerable, albeit poorly understood, role in cancer pathogenesis. In this review, we present fundamental studies that have investigated the impact of fibronectin in cancer. As an abundant component of the extracellular matrix, understanding the effect of this molecule has the potential to elucidate cancer biology.
Keywords: Cancer, extracellular matrix, fibronectin, ECM, Extra Domain A, Extra Domain B, cancer signaling
Introduction
The past 20 years have given rise to a rapidly expanding perspective on cancer. Whereas the formative studies on cancer biology focused on the tumor cells themselves, there is now a growing appreciation for the importance of the environment in which the tumor cells thrive [1]. These insights have shifted the paradigm of cancer from a homogenous mass of tumor cells into a complex organ composed of interacting elements [1–3]. Tumors can then be thought of as cooperating vascular, immunological and stromal elements and due attention can be directed to the system as a whole [1,4,5]. This process has provided tremendous insight, namely the considerable role that macrophages play in cancer pathogenesis and how the complex angiogenic orchestra can provide nutrients to growing tumors [4,6,7]. There has also been growing interest in the components and structure of the extracellular matrix (ECM) itself [8–10]. The ECM is composed of numerous polysaccharides, proteins, proteoglycans and glycoproteins [11]. These components mesh together to provide the structural foundation for cell-to-cell communication and homeostasis [11]. However, alterations in the ECM are becoming increasingly implicated in cancer pathogenesis, including alterations in laminin, hyaluronan and fibronectin [9,12–14]. The goal of this review is to update the reader on the part that fibronectin plays in cancer biology. Recent work has identified some key changes that occur in fibronectin in the context of cancer that can provide insight into biology and may inspire new therapeutic approaches.
The Extracellular Matrix
Tissues are composed of cells embedded in an extracellular matrix [15]. This network holds neighboring cells together and facilitates cell-to-cell communication. As a structural element, the ECM helps orient the polarity of cells and affix them to a mutual platform. A diverse body of cellular integrin dimers bind to the ECM and mediate forces between the ECM and the intracellular cytoskeleton. The ECM reinforces direct cellular connections (adherens junctions, desmosomes, etc.) and allows non-neighboring cells to adhere to a common framework. This physical property of the ECM also allows it to serve as an anti-neoplastic barrier [1]. For cells that do become dysregulated, the ECM is a barrier against cellular invasion and suspends the malignant progression of cancer. It also provides tensile support against injury. In wound repair the ECM is promptly created and then acts as a foundation for further wound healing and cell migration. In addition to its physical importance, the ECM provides a medium for cell-to-cell communication [16]. While neighboring cells can communicate with direct cellular connections, it is essential for entire tissues to react to changes. Tensile forces distributed via extracellular elements can manipulate integrins to produce large, multi-cellular changes and potentiate cytokine signaling from local sources. Therefore, the ECM is responsible for providing tissues their the structural integrity and for enabling rapid cell-to-cell communication.
Fibronectin in Health: A Coagulant and a Scaffold
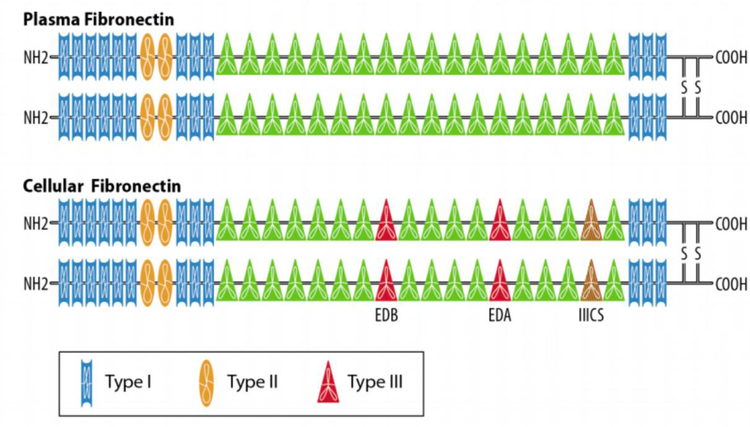
Fibronectin is a large, 440 kDa, glycoprotein composed of two smaller, 230–250 kDa, monomers (Figure 1) [17]. Every monomeric strand is composed of a combination of Type I, II and III repeats, and each of these repeats generates an anti-parallel beta sheet [17]. The monomers are composed of 12 Type I repeats (40 amino acids each), 2 Type II repeats (60 amino acids each) and 15–17 Type III repeats (90 amino acids each). Structural variation is introduced via alternative splicing, whereby certain repeats are excluded from the final mRNA structure [17]. The most notable of these splice variations are Extra-domain A (EDA, located after the 11th Type III repeat) and Extra-domain B (EDB, located after the 7th Type III repeat) [17]. As will be discussed later, these differentially spliced sites help distinguish plasma from cellular fibronectin. The last notable source of variation is the IIICS, or the variable region, which can introduce additional partial Type III repeats [17]. As often occurs in biology, this small structural variation has disproportionately large functional implications, namely a role for the EDA as a region for specialized integrin binding. Different fibronectin forms integrate differently with particular receptors. Overall, the process of splice variation results in 20 unique isoforms of fibronectin and there is a great diversity of possible ligand-receptor interactions [17].
Figure 1.
Splice Variation Produces Differences in Fibronectin. Cellular fibronectin (bottom) differs from plasma fibronectin (top) by a number of additional repeats. Both types of fibronectin have 12 Type I repeats (rectangles) and 2 Type II repeats (ovals). Plasma fibronectin has 15 Type III repeats and cellular fibronectin typically has 17 Type III repeats (triangles). The IIICS splice site can introduce partial Type III repeats as well. EDA sits between the 11th and 12th Type III repeat and EDB sits between the 7th and 8th Type III repeat. These extra domains allow cellular fibronectin to interact with various integrin heterodimers.
Plasma fibronectin, which lacks EDA and EDB, is secreted by hepatocytes into the blood stream [18,19]. While in the plasma, fibronectin is soluble and inactive [19]. However, when fibronectin approaches a site of injury it can be rapidly integrated into a fibrin clot [19]. In this role it helps build a fibrillary network to stabilize the injury [19]. Given the plentiful quantities found in the blood, there has been an interest in using plasma fibronectin as a test for cancer or other pathology [20,21]. To date, the most notable correlation between serum fibronectin levels and disease is in hepatocellular carcinoma [22]. In a recent study, Kim et al. noted that serum fibronectin was elevated in patients suffering from hepatocellular cancer with levels falling in treated patients [22]. Therefore, this marker may be useful in screening patients or evaluating the effect of treatment. However, plasma fibronectin has not been associated with other diseases and it does not appear to have a role in the pathogenesis of cancer.
On the other hand, cellular fibronectin, which differs from plasma fibronectin by the inclusion of the EDA/EDB and IIICS splice sites, has a more robust biological role [18,23]. It is produced by a variety of cell types, but most cellular fibronectin originates from fibroblasts [24]. It is highly upregulated in pathologic angiogenesis and is a component of forming neovasculature [10,25]. The EDA/EDB moieties interact strongly with specific pairs of alpha/beta integrins (namely α4β1, α4β7, and α9β1 interacting with EDA; and α5β1 interacting with EDB) that are not stimulated by plasma fibronectin [26]. These unique integrin-fibronectin interactions promote myofibroblast differentiation, which directs wound constriction [18]. Additionally, cellular fibronectin can be assembled into fibers to allow directional cell growth [27,28]. This function as a scaffold is particularly valuable as cellular fibronectin is a vital component of embryogenesis and is essential for the organization of tissue [27]. However, this embryonic cellular fibronectin can also be identified in malignancy – hence cellular fibronectin has the alternative name of oncofetal fibronectin [29].
Cellular Fibronectin in Cancer
Cellular fibronectin was identified in cancer samples over 20 years ago [30]. The most intriguing question arising from this discovery was the cellular origin of fibronectin in cancer. Initial work postulated that oncofetal fibronectin originated largely from the tumor cells themselves [31]. It was observed that many tumors would produce fibronectin when samples were cultured ex vivo [31]. However, as it became increasingly apparent that tumors were composed of many cell types, the search for the actual source of this glycoprotein began in earnest. Macrophages, endothelial cells and fibroblasts have all been shown to produce cellular fibronectin in the adult, and each of these cell types is found in tumors [32]. Subsequent studies teasing apart these sources has led to the predominant theory that most cellular fibronectin in cancer actually originates from unique tumoral fibroblasts — termed cancer-associated fibroblasts (CAFs). Molecular analysis of CAFs extracted from tumors has determined that they are highly active and quite distinct from non-cancer fibroblasts [33]. The cellular origin of CAFs is currently under active investigation, with many intriguing theories being pursued. The first, and most straightforward, hypothesis suggests that CAFs are altered resident fibroblasts, which exist in healthy tissue. This hypothesis is supported by the work of Kojima et al. who demonstrated conversion of breast fibroblasts to CAFs in xenograft studies [34]. These investigators were able to show that treatment with TGF-β and SDF-1 was sufficient to drive resident myofibroblasts to a more CAF-like phenotype. However, because CAFs differ significantly from resident fibroblasts in genetic and antigenic signature, other hypotheses about their origins have garnered support [35]. For example, a second hypothesis advocates that CAFs originate from adipose stem cells or bone marrow-derived stem cells [36,37]. The support for bone marrow-derived stem cells is particularly robust, with some studies suggesting that at least 20% of CAFs originate from the bone marrow [36,38]. This hypothesis has been driven by the fact that mice implanted with human tumor cell lines have CAFs that are derived from the host bone marrow [36,39]. In both murine and human tumor samples, CAFs express markers found on host marrow-derived cells [36,39]. A third CAF origin hypothesis is that CAFs are derived from the local tumor environment via epithelial-to-mesenchymal or endothelial-to-mesenchymal transitions [40,41]. This hypothesis asserts that the origin of CAFs is from native structures that have been transformed by cancer. Similar to what was done with resident fibroblasts, Zeisberg et al. showed that exposing endothelial cells to TGF-β resulted in CAF-like cells [41]. This hypothesis is intriguing as cancer progression involves proliferating vasculature that could be a ready source of CAFs [41,42]. A fourth hypothesis about the origin of CAFs is that carcinoma associated stem cells are the origin of CAFs [43]. These stem cells have been found in a variety of cancers and, when given the opportunity to differentiate, can transform into CAFs in culture. This theory will be of great interest as our understanding of the origin and role of cancer-associated stem cells grows. In short, the origin of CAFs is complex and there is room in the present narrative for these fibroblasts to be derived from heterogeneous lineages. Although the origin of CAFs is an interesting and evolving story, CAFs are generally regarded to be the source of cellular fibronectin in tumors [25,44,45].
Pro-tumoral effects of fibronectin
Organizing Cancer to Promote Invasion and Metastasis
In the context of embryogenesis, cellular fibronectin serves a vital and intuitive role as a structural scaffold [46,47]. It allows cells to organize, communicate, and lay down more permanent foundations [32]. Early in fetal development, the deposition of cellular fibronectin is highly dynamic, with different sources secreting and removing this molecule [32]. This process is mirrored in wound healing, where cellular fibronectin lays the groundwork for wound contraction and is then broken down to permit the growth of more permanent structures [48]. In the context of cancer, the orientation and pattern of cellular fibronectin, and whether it can serve as a scaffold in a pro-tumoral manner, has been a point of curiosity. Recent work by Erdogan et al. has demonstrated at sites of tumor invasion organizarion of fibronectin into anisotropic fibers [49]. These fibers extend outwards in many different directions and appear to guide cells outside of the original neoplasm. Thus fibronectin acts as a haptotactic guide and provides tensile support for cancer to pull itself outside the primary tumor[49]. Although cancer appears to be a highly disorganized process in certain regards, the alignment of fibronectin has a pathophysiologically significant order, namely in that it orients cancer cells to invade centrifugally away from the origin of malignancy [49]. Curiously, it appears that CAFS, acting through αvβ3-integrin, help organize fibronectin in this fashion. Therefore, CAFs are a central element that produce and organize this molecule into a proponent of invasion. Attieh et al. confirmed that the β3-integrin family of receptors had a role in this process and that the organization of cellular fibronectins was a key driver in invasion [50]. Indeed, this study determined that factors secreted by CAFs were insufficient to drive invasion, their presence is needed to organize the newly created extracellular matrix. When CAFs were selectively removed from cancer, the ECM was disordered at the tumor interface, and this translated into slower tumor invasion. However, when CAFs are included in the tumor milieu, cellular fibronectin is oriented perpendicularly to the expanding surface, which is ideal for anchoring and invading the local stroma. This order is partially maintained by the contractile quality of CAFs and orchestrated by β3-integrin acting as a tethering point for cellular fibronectin. Attieh et al. also discovered that CAFs produce much more fibronectin than fibroblasts taken from non-cancerous lineages [50]. This suggests that cancer has a role in transforming fibroblasts into proponents of invasion.
Apart from supplying the directionality needed for invasion, signaling triggered by fibronectin appears to prepare cells for widespread metastasis. In 2006 Zeng et al. determined that α5β1 signaling induced by fibronectin could rapidly activate Focal Adhesion Kinase (FAK), which would then provoke a downstream invasion cascade [51]. These investigators also identified that the invasive phenotype arising when fibronectin induces FAK activation in tumor cells was driven by MMP-1, an enzyme responsible for ECM breakdown. However, while the ECM is being restructured, cellular fibronectin appears to be sufficient to prevent anoikis via FAK/Src signaling, which is needed when cells detach from the ECM and lose supportive signaling [52]. Indeed, Balanis et al. determined that exposure to cellular fibronectin was enough to drive cancer cells into a fibronectin-dependent, EGF-independent epithelial-to-mesenchymal transition [53]. The impact of these intracellular changes has been assessed by a number of investigators. For example, after showing data that corroborated with the fibronectin-FAK narrative, Meng et al. were able to demonstrate that inhibiting the activation of FAK was enough to abrogate the ability of fibronectin to drive invasiveness in lung cancer [54]. This work has been supported by a study that demonstrated knocking down FAK, as opposed to preventing its activation, is also able to reduce the fibronectin-triggered invasive phenotype [55]. Taken together, these studies reveal an intriguing and unexpected role of cellular fibronectin. In addition to laying the path for cancer invasion, this protein is integral in programming cancer cells to make them more enduring and malignant when the ECM support structure is removed, as could occur when cancer cells metastasize to healthy tissue.
Increasing Cell Proliferation
Cellular fibronectin also appears capable of directly stimulating cell proliferation [56]. Illario et al. found that treating an immortalized cell line with cellular fibronectin doubled the proliferation rate [57]. Mitra et al. tested this finding in ovarian cancer and proposed that fibronectin signals through α5β1-integrin to activate FAK and Src [56]. FAK/Src signaling acts through c-Met to provide aconstitutive growth signal and escalate cell production. Mitra et al. were able to demonstrate reduced growth rates when the integrin-fibronectin pathway was disrupted, providing further support to the proliferative character of these signals. Therefore, fibronectin also promotes proliferation, lending credence to the possibility that cellular fibronectin signaling could incite tumor growth. An additional study by Kenny et al. corroborated this notion by reporting that knocking down fibronectin reduced proliferation by 54% in another ovarian cancer line [58]. Additionally, specifically blocking α5β1-integrin’s interaction with fibronectin reduced proliferation by 29–52%, depending on the cell line [58]. All of these results indicate cellular fibronectin helps some cancer lines proliferate and agree that α5β1-integrin signaling is a key component [23,57]. In addition to actively inducing proliferation in some cell types, it appears that fibronectin may be able to suppress apoptosis [59,60]. Han et al. reported decreased DNA fragmentation and a 50% reduction in Caspase 3/7 activity in cells treated with cellular fibronectin. The absence of DNA fragmentation was taken to indicate a lack of DNA breakdown, an essential step in apoptosis. These anti-apoptotic effects were independent of the previously described proliferative effects demonstrated with a thymidine incorporation assay that linearly increased with increasing cellular fibronectin [59]. It appears that this mechanism is also communicated via the α5β1-integrin pathway and stems from upregulation of NF-kB and a corresponding decrease in p21 [59,60]. Taken together, these studies suggest cellular fibronectin has a role in making cells rapidly proliferative and immortal – core attributes of malignancy.
Promoting Angiogenesis
Cellular fibronectin is a structural element in embryogenic angiogenesis as well as vascular neogenesis in the context of wound healing [12,61]. In the embryo, cellular fibronectin organizes into premature blood vessels that are subsequently epithelialized. During wound closure there is a similar leading-edge of vessel formation partially guided by fibronectin. Fibronectin can also promote pathologic angiogenesis such as in cancer or chronic inflammation [12]. The roles of fibronectin in embryonic angiogenesis have been explored by demonstrating reduced and unusual vessel density in mice with fibronectin knocked-out [61,62]. These knock-out models also have abnormalities in other mesodermal structures, which further supports cellular fibronectin as a scaffold for other structures [61]. Despite this compelling evidence confirming the role of fibronectin in embryonic angiogenesis, the impact of fibronectin in tumor angiogenesis remains incompletely defined. While fibronectin has been identified in forming tumor vessels [12,63,64], where it appears to lay down the framework for eventual vessel maturation [65], the role and value of fibronectin in tumor vessel formation remains unclear.
The process of tumor formation places an increased vascular burden on expanding neoplasms. As cancers grow, cells near the center can become increasingly hypoxic due to reduced oxygen and nutrient perfusion. This reduced perfusion coupled with the high metabolic rates of many cancers make for a particularly inhospitable and oxygen starved environment. Therefore, the pathogenesis of many malignancies involves the formation of new blood vessels to support tumor expansion. Just as it does in embryogenic vessel formation, fibronectin appears to promote angiogenesis in cancer. Fibronectin’s role is two-fold: 1) supply a ridged structure to support lumen formation and 2) bind vascular signaling molecules (namely vascular endothelial growth factor, VEGF) and maintain a directional concentration gradient [66–68]. As a support structure, fibronectin interacts with other CAF-derived proteins in new vessels to produce competent vessels. Newman et al. demonstrated that ECM proteins derived from fibroblasts are required for angiogenesis and when these molecules were not present new blood vessels were poorly formed, erratically ordered, and did not have lumens [66]. They found that fibronectin was one of the 3 most abundant molecules in angiogenesis, but noted that it was only one of a number of ECM components responsible for making new blood vessels.
In addition to providing a ridged structure for future vessel development, fibronectin has a role in signaling endothelial cell migration. First, moieties in fibronectin, especially EDB, have been shown to bind VEGF thus helping to generate a stable concentration gradient [69]. This gives directionality to blood vessel formation by endothelial cells. The VEGF promotes proliferation in preexisting endothelial cells and encourages them to advance into ECM scaffolds [70]. However, in addition to attracting differentiated endothelial cells, fibronectin helps convert multipotent CD34+ cells into endothelial cell [70]. Thus, fibronectin contributes to the directionality of vessel formation and encourages undifferentiated cells to take on an endothelial lineage.
Multiple investigators have demonstrated that removing fibronectin from tumors does not clearly impact tumor angiogenesis or slow tumor growth [71]. This corroborates the disappointing results of clinical trials aimed at disrupting fibronectin-integrin binding[72,73]. This lack of clinical efficacy is assumed to be due to the fact that antibody mediated therapy does not completely disrupt all fibronectin-integrin signaling. To verify the impact of fibronectin on cancer pathogenesis, Murphy et al. showed, via inducible genetic knockdown, that if fibronectin was removed from an organism entirely a number of devastating vascular abnormalities ensued [74]. Taken together, these studies have shown that fibronectin is abundantly present in neovasculature but efforts to remove fibronectin from the equation do not seem to significantly abrogate vessel formation. Further work will be needed to understand the role of fibronectin in tumor angiogenesis and whether compensatory upregulation of alternative, fibronectin-independent, mechanisms of angiogenesis can explain the escape from anti-fibronectin therapy. However, as discussed above, the role of fibronectin in cancer extends well beyond vessel formation and this limitation may be of minimal consequence in therapy. The role that fibronectin has in vessel formation, both in health and disease, is highly intriguing and could advance our understanding of angiogenesis.
Potential Therapeutic Applications
Therapy targeting fibronectin is based on two facts: (1) EDA/EDB expression is primarily limited to sites of malignancy in adults and (2) cellular fibronectin appears to play a role in cancer pathogenesis. The first concept, that EDA/EDB is limited to tumors in otherwise healthy adults, has generated much enthusiasm for engineering a fibronectin-guided drug delivery mechanism [75]. This technology is particularly promising because specific EDA/EDB antibodies have already been developed and the process of linking drugs to antibodies is rapidly improving [75]. This has the potential to deliver targeted chemotoxic, biologic or radioactive agents directly to the cancer to impair tumor growth or augment imaging [75–77]. This approach fuses conventional cancer treatments with a cancer-specific target. Although this concept has had some strong pre-clinical results, human trials are still preliminary, although somewhat promising [78,79]. Johannsen et al. showed good effects in rat and monkey tumors when Il-2 was fused with a partial anti-EDB recombinant antibody (L19-IL2). This construct was taken to clinical trials and had a favorable side-effect profile. Fifty-one percent of patients, with diverse cancer histologies, treated with L19-IL2 had stable disease through the second cycle. The overall efficacy of this drug has yet to be determined. However, it is worth noting that this therapy is only one of numerous possibilities and that furthermore, there is potential to change the delivered agent (Il-2) to something with greater anti-cancer effect.
The other major approach to targeting cellular fibronectin in cancer involves directly suppressing its putative pro-tumoral effects. Whereas the fusion molecule approach uses EDA/EDB as a way to deliver other agents, it may be possible to directly interrupt the pro-tumoral effects of fibronectin. This has taken the form of antibodies and small molecule inhibitors directed at interrupting the interactions between fibronectin and its integrin partners [76]. At the present time, there is growing preclinical literature supporting the anti-tumor effects of α5β1 directed antibodies [80,81]. Ramakrishnan et al. explored the effect of Volociximab, an anti- α5β1 that disrupts the integrin-fibronectin binding using in vitro HUVEC models [82]. Volociximab showed strong anti-angiogenic effects and has been advanced to clinical trials. Subsequent work by Bell-McGuinn et al., who tested Volociximab in platinum-resistant ovarian cancer, found a favorable side-effect profile, but 13 of 14 patients progressed through treatment [83]. There is still a lot that needs to be understood about the role of fibronectin in cancer in order to determine whether fibronectin is a viable therapeutic target and the optimal way of targeting it.
Conclusion
As our understanding of oncology has expanded, there has been a growing appreciation for the impact of the ECM on cancer pathogenesis. Initial work on cancer cells has been incredibly informative, but our perspective has now broadened to include the environment around these cells. The assumption that the ECM around cancer was homogenous and insignificant has dissolved to reveal a rich and complex component of disease biology. Studying the ecosystem of interacting polysaccharides, proteins, proteoglycans and glycoproteins around tumors offers a unique and valuable perspective on cancer biology. In particular, the altered expression and atypical organization of fibronectin in cancer suggest it is an element in the progression of cancer. As has been revealed with other ECM molecules, fibronectin has broad and impactful effects on disease pathogenesis. We have reviewed studies that have shown fibronectin is capable of organizing the invasion of tumors, augmenting metastasis, increasing cell proliferation, and providing structure for budding neovascular elements. We also discussed that CAFs are responsible for creating fibronectin. CAFs are poorly understood, with unclear origins, but secrete and organize fibronectin into a pathophysiologically significant framework. Fibronectin then acts as a scaffold for invasion, foments rapid cell growth, and serves as a nidus for new vessel formation (Figure 2). At the present time, it is unclear if CAFs or fibronectin will be useful molecular targets for therapy. There is still much that needs to be understood about the role of these entities in cancer progression. Nonetheless, fibronectin has proven itself to be an intriguing element in cancer and demonstrates how far the field of oncology has progressed.
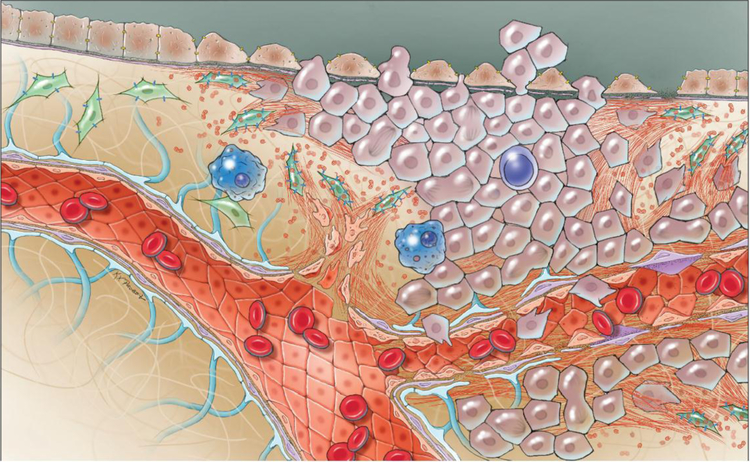
Figure 2.
Snapshot of the local tumor environment. This image depicts proliferating cancer cells (purple) co-opting their local environment to support metastasis, invasion, and angiogenesis. Cellular fibronectin is illustrated as red fibers. Fibronectin is produced by cancer-associated fibroblasts (CAFs, green) and then organized by these same cells. Fibronectin is assembled to produce a scaffold that is used by cancer cells to spread and invade local stroma. Fibronectin’s role in early angiogenesis is also depicted in forming neovasculature that will provide nutrients to the expanding neoplasm. Lastly, tumor immune cells (blue), which represent an important component of cancer pathogenesis, are also illustrated.
Table 1.
Outline of Key Literature on Fibronectin
| Author | Year | Findings | Comment |
|---|---|---|---|
| Adachi et al. | 1998 | Fibroblasts grown in 3D culture produce more fibronectin, and connect fibronectin into more stable structures | This is an early example of 3D culture, which would be imperative to understand fibronectin networks |
| Zand et al. | 2003 | Cancer cells attached and grew more robustly on cellular fibronectin than plasma fibronectin. | Although plasma and cellular fibronectin are structurally similar, a few splice variants can dramatically affect how this molecule interacts with cancer |
| Missirlis et al. | 2017 | Removing cellular fibronectin stops fibroblast migration. The receptors responsible for persistent cell invasion and invasion speed are independent, suggesting that cellular fibronectin acts on many different receptors to achieve different effects on cellular behavior | This work again highlights that cellular fibronectin is a key component in invasion, but it also shows that knocking out individual receptors incompletely removes the effects of this molecule |
| Sadlonova et al. | 2009 | Non-cancer associated fibroblasts (NAFs) and cancer associated fibroblasts (CAFs) are genetically distinct and express different factors | Mirroring the physiologic differences between CAFs and NAFs, the genetic signatures of these cell types are distinct |
| Kojima et al. | 2010 | Signaling via TGF-β and SDF-1 converts stroma fibroblasts into CAFs | This paper proposes that CAFs can originate from the local stroma, although not thought to be the primary source of CAFs, this identifies the heterogenetity of their origin |
| Ishii et al. | 2003 | For late stage tumors, the majority of CAFs seem to originate from the Bone Marrow | Currently, the bone marrow is the favored origin for most CAFs |
| Jotzu et al. | 2010 | Tumor signaling can induce local adipocytes to transform into CAFs | This notes another possible source of CAFs |
| Direkze | 2004 | Similar conclusion as Ishii et al. | -- |
| Quante et al. | 2011 | Similar conclusion as Ishii et al. This team used a mouse tumor model and identified that CAFs within the neoplasm were from donor bone marrow. | -- |
| Erdogan et al. | 2017 | CAFs organize fibronectin to establish tracts for cancer invasion | Fibronectin has a direct role in promoting cancer cell invasion into the stroma |
| Attieh et al. | 2017 | Similar conclusion as Erdogan et al. | -- |
| Nicosia et al. | 1993 | Fibronectin leads budding blood vessels | Fibronectin helps form new blood vessels and could have a role in tumor angiogenesis |
| Gopal et al. | 2017 | Fibronectin networks layed down by head and neck squamous cell cancers is a component of cancer migration into the local stroma, the integrins vβ6 and α9β1 are essential in this process | This work highlights that the fibronectin scaffold is a driver in malignant invasion, but also that many integrins interact with this molecule |
Acknowledgements
We would like to acknowledge Ken Probst for his outstanding artwork.
Funding: Jonathan Rick and Ankush Chandra are supported by the Howard Hughes Medical Institute (HHMI).
List of Abbreviations
- ECM
Extracellular Matrix
- EDA
Extra-Domain A
- EDB
Extra-Domain B
- KDa
Kilodaltons
- CAFs
Cancer-Associated Fibroblasts
- FAK
Focal Adhesion Kinase
- EGF
Epidermal Growth Factor
- IL
Interleukin
- FN
Fibronectin
- MMP
Matrix Metalloproteinase
- DNA
Deoxyribonucleic Acid
- HHMI
Howard Hughes Medical Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
No experiments were performed in the production of this manuscript and no ethics approval was required.
Consent for publication
All authors have consented this work for publication
Availability of data and material
We have the legal ownership of this text and the figures provided.
Competing interests
We declare no conflict of interest, financial or otherwise.
References
- [1].Bissell MJ, Radisky D, Putting tumours in context, Nat. Rev. Cancer 1 (2001) 46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Qin Y, Rodin S, Hollande F, Laminins and cancer stem cells: Partners in crime?, Semin. Cancer Biol 45 (2017) 3–12. doi: 10.1016/J.SEMCANCER.2016.07.004. [DOI] [PubMed] [Google Scholar]
- [3].Venning FA, Wullkopf L, Erler JT, Targeting ECM Disrupts Cancer Progression., Front. Oncol 5 (2015) 224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Noy R, Pollard JW, Tumor-associated macrophages: from mechanisms to therapy., Immunity 41 (2014) 49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kalluri R, Zeisberg M, Fibroblasts in cancer, Nat. Rev. Cancer 6 (2006) 392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- [6].Yang L, Zhang Y, Tumor-associated macrophages: from basic research to clinical application, J. Hematol. Oncol 10 (2017) 58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang H, Yang L, Wang D, Zhang Q, Zhang L, Pro-tumor activities of macrophages in the progression of melanoma., Hum. Vaccin. Immunother 13 (2017) 1556–1562. doi: 10.1080/21645515.2017.1312043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lu P, Weaver VM, Werb Z, The extracellular matrix: a dynamic niche in cancer progression., J. Cell Biol 196 (2012) 395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Givant-Horwitz V, Davidson B, Reich R, Laminin-induced signaling in tumor cells., Cancer Lett 223 (2005) 1–10. doi: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- [10].Ruoslahti E, Fibronectin and Its Integrin Receptors in Cancer, Adv. Cancer Res 76 (1999) 1–20. doi: 10.1016/S0065-230X(08)60772-1. [DOI] [PubMed] [Google Scholar]
- [11].Frantz C, Stewart KM, Weaver VM, The extracellular matrix at a glance., J. Cell Sci 123 (2010) 4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D, Extracellular matrix modulates angiogenesis in physiological and pathological conditions., Biomed Res. Int 2014 (2014) 756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A, High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat, Nature 499 (2013) 346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gopal S, Veracini L, Grall D, Butori C, Schaub S, Audebert S, Camoin L, Baudelet E, Radwanska A, Beghelli-de la Forest Divonne S, Violette SM, Weinreb PH, Rekima S, Ilie M, Sudaka A, Hofman P, Van Obberghen-Schilling E, Fibronectin-guided migration of carcinoma collectives, Nat. Commun 8 (2017) 14105. doi: 10.1038/ncomms14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frantz C, Stewart KM, Weaver VM, The extracellular matrix at a glance, J. Cell Sci 123 (2010) 4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim S-H, Turnbull J, Guimond S, Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor, J. Endocrinol 209 (2011) 139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- [17].White ES, Muro AF, Fibronectin splice variants: Understanding their multiple roles in health and disease using engineered mouse models, IUBMB Life 63 (2011) 538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- [18].To WS, Midwood KS, Plasma and cellular fibronectin: distinct and independent functions during tissue repair, Fibrogenesis Tissue Repair 4 (2011) 21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maurer LM, Ma W, Mosher DF, Dynamic structure of plasma fibronectin., Crit. Rev. Biochem. Mol. Biol 51 (2015) 213–27. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zerlauth G, Wolf G, Plasma fibronectin as a marker for cancer and other diseases., Am. J. Med 77 (1984) 685–9. http://www.ncbi.nlm.nih.gov/pubmed/6385694 (accessed February 16, 2018). [DOI] [PubMed] [Google Scholar]
- [21].To WS, Midwood KS, Plasma and cellular fibronectin: distinct and independent functions during tissue repair, Fibrogenesis Tissue Repair 4 (2011) 21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim H, Park J, Kim Y, Sohn A, Yeo I, Jong Yu S, Yoon J-H, Park T, Kim Y, Serum fibronectin distinguishes the early stages of hepatocellular carcinoma, Sci. Rep 7 (2017) 9449. doi: 10.1038/s41598-017-09691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zand L, Feng Q, Roskelley C, Leung P, Auersperg N, Differential effects of cellular fibronectin and plasma fibronectin on ovarian cancer cell adhesion, migration and invasion, Vitr. Cell. Dev. Biol 39 (2003) 178–82. doi: 10.1290/0302011. [DOI] [PubMed] [Google Scholar]
- [24].Adachi Y, Mio T, Takigawa K, Striz I, Romberger DJ, Spurzem JR, Rennard SI, Fibronectin production by cultured human lung fibroblasts in three-dimensional collagen gel culture., In Vitro Cell. Dev. Biol. Anim 34 (1998) 203–10. http://www.ncbi.nlm.nih.gov/pubmed/9557937 (accessed March 25, 2018). [DOI] [PubMed] [Google Scholar]
- [25].Gopal S, Veracini L, Grall D, Butori C, Schaub S, Audebert S, Camoin L, Baudelet E, Radwanska A, Beghelli-de la Forest Divonne S, Violette SM, Weinreb PH, Rekima S, Ilie M, Sudaka A, Hofman P, Van Obberghen-Schilling E, Fibronectin-guided migration of carcinoma collectives, Nat. Commun 8 (2017) 14105. doi: 10.1038/ncomms14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].White ES, Muro AF, Fibronectin splice variants: Understanding their multiple roles in health and disease using engineered mouse models, IUBMB Life 63 (2011) 538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- [27].Singh P, Carraher C, Schwarzbauer JE, Assembly of fibronectin extracellular matrix., Annu. Rev. Cell Dev. Biol 26 (2010) 397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Missirlis D, Haraszti T, Kessler H, Spatz JP, Fibronectin promotes directional persistence in fibroblast migration through interactions with both its cell-binding and heparin-binding domains, Sci. Rep 7 (2017) 3711. doi: 10.1038/s41598-017-03701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE, Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast., J. Submicrosc. Cytol. Pathol 25 (1993) 285–95. http://www.ncbi.nlm.nih.gov/pubmed/7686813 (accessed February 23, 2018). [PubMed] [Google Scholar]
- [30].Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K, George AJT, Oncodevelopmental regulation of the alternative splicing of fibronectin pre-messenger RNA in human lung tissues., Cancer Res 50 (1990) 1075–8. http://www.ncbi.nlm.nih.gov/pubmed/2297755 (accessed February 23, 2018). [PubMed] [Google Scholar]
- [31].Ruoslahti E, Fibronectin in cell adhesion and invasion., Cancer Metastasis Rev 3 (1984) 43–51. http://www.ncbi.nlm.nih.gov/pubmed/6324988 (accessed March 25, 2018). [DOI] [PubMed] [Google Scholar]
- [32].To WS, Midwood KS, Plasma and cellular fibronectin: distinct and independent functions during tissue repair, Fibrogenesis Tissue Repair 4 (2011) 21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sadlonova A, Bowe DB, Novak Z, Mukherjee S, Duncan VE, Page GP, Frost AR, Identification of molecular distinctions between normal breast-associated fibroblasts and breast cancer-associated fibroblasts., Cancer Microenviron 2 (2009) 9–21. doi: 10.1007/s12307-008-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A, Autocrine TGF- and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts, Proc. Natl. Acad. Sci 107 (2010) 20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H, Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth., Cancers (Basel) 7 (2015) 2443–58. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, Emura M, Ochiya T, Ochiai A, Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction., Biochem. Biophys. Res. Commun 309 (2003) 232–40. http://www.ncbi.nlm.nih.gov/pubmed/12943687 (accessed February 25, 2018). [DOI] [PubMed] [Google Scholar]
- [37].Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song Y-H, Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors., Anal. Cell. Pathol. (Amst) 33 (2010) 61–79. doi: 10.3233/ACP-CLO-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA, Bone Marrow Contribution to Tumor-Associated Myofibroblasts and Fibroblasts, Cancer Res 64 (2004) 8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- [39].Quante M, Tu SP, Tomita H, Gonda T, Wang SSW, Takashi S, Baik GH, Shibata W, DiPrete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC, Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth, Cancer Cell 19 (2011) 257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Potenta S, Zeisberg E, Kalluri R, The role of endothelial-to-mesenchymal transition in cancer progression, Br. J. Cancer 99 (2008) 1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R, Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma-Associated Fibroblasts, Cancer Res 67 (2007) 10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- [42].Huang M, Liu T, Ma P, Mitteer RA, Zhang Z, Kim HJ, Yeo E, Zhang D, Cai P, Li C, Zhang L, Zhao B, Roccograndi L, O’Rourke DM, Dahmane N, Gong Y, Koumenis C, Fan Y, Fan Y, c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma., J. Clin. Invest 126 (2016) 1801–14. doi: 10.1172/JCI84876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AKK, Hurley L, Vaidyanath A, Seno A, Masuda J, Iwasaki Y, Tanaka H, Kasai T, Seno M, A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment, Sci. Rep 7 (2017) 6838. doi: 10.1038/s41598-017-07144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang K, Seo BR, Fischbach C, Gourdon D, Fibronectin Mechanobiology Regulates Tumorigenesis., Cell. Mol. Bioeng 9 (2016) 1–11. doi: 10.1007/s12195-015-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang JP, Hielscher A, Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting., J. Cancer 8 (2017) 674–682. doi: 10.7150/jca.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].V Pulina M, Hou S-Y, Mittal A, Julich D, Whittaker CA, Holley SA, Hynes RO, Astrof S, Essential roles of fibronectin in the development of the left-right embryonic body plan., Dev. Biol 354 (2011) 208–20. doi: 10.1016/j.ydbio.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].de Almeida PG, Pinheiro GG, Nunes AM, Gonçalves AB, Thorsteinsdóttir S, Fibronectin assembly during early embryo development: A versatile communication system between cells and tissues, Dev. Dyn 245 (2016) 520–535. doi: 10.1002/dvdy.24391. [DOI] [PubMed] [Google Scholar]
- [48].Lenselink EA, Role of fibronectin in normal wound healing, Int. Wound J 12 (2015) 313–316. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, Hayward SW, Li D, Webb DJ, Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin., J. Cell Biol 216 (2017) 3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, Elkhatib N, Betz T, Gurchenkov B, Vignjevic DM, Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly., J. Cell Biol 216 (2017) 3509–3520. doi: 10.1083/jcb.201702033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zeng Z-Z, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL, Role of Focal Adhesion Kinase and Phosphatidylinositol 3′-Kinase in Integrin Fibronectin Receptor-Mediated, Matrix Metalloproteinase-1–Dependent Invasion by Metastatic Prostate Cancer Cells, Cancer Res 66 (2006) 8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- [52].Bouchard V, Demers M-J, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, Beaulieu J-F, Gauthier R, Vézina A, Villeneuve L, Vachon PH, Fak/Src signaling in human intestinal epithelial cell survival and anoikis: Differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways, J. Cell. Physiol 212 (2007) 717–728. doi: 10.1002/jcp.21096. [DOI] [PubMed] [Google Scholar]
- [53].Balanis N, Wendt MK, Schiemann BJ, Wang Z, Schiemann WP, Carlin CR, Epithelial to Mesenchymal Transition Promotes Breast Cancer Progression via a Fibronectin-dependent STAT3 Signaling Pathway, J. Biol. Chem 288 (2013) 17954–17967. doi: 10.1074/jbc.M113.475277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu J, Zhao YZ, Wang Z, Chen F, Lee K-Y, Fu SB, Characterisation of fibronectin-mediated FAK signalling pathways in lung cancer cell migration and invasion, Br. J. Cancer 101 (2009) 327–334. doi: 10.1038/sj.bjc.6605154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yousif NG, Fibronectin promotes migration and invasion of ovarian cancer cells through up-regulation of FAK-PI3K/Akt pathway, Cell Biol. Int 38 (2014) 85–91. doi: 10.1002/cbin.10184. [DOI] [PubMed] [Google Scholar]
- [56].Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E, Ligand-independent activation of c-Met by fibronectin and α5β1-integrin regulates ovarian cancer invasion and metastasis, Oncogene 30 (2011) 1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Illario M, Cavallo AL, Monaco S, Di Vito E, Mueller F, Marzano LA, Troncone G, Fenzi G, Rossi G, Vitale M, Fibronectin-Induced Proliferation in Thyroid Cells Is Mediated by αvβ3 Integrin through Ras/Raf-1/MEK/ERK and Calcium/CaMKII Signals, J. Clin. Endocrinol. Metab 90 (2005) 2865–2873. doi: 10.1210/jc.2004-1520. [DOI] [PubMed] [Google Scholar]
- [58].Kenny HA, Chiang C-Y, White EA, Schryver EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K, Montag A, Wroblewski K, Yamada SD, Mazar AP, Bowtell D, Lengyel E, Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion, J. Clin. Invest 124 (2014) 4614–4628. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Han SW, Roman J, Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: pro-oncogenic effects mediated by PI3-kinase and NF-κB, Oncogene 25 (2006) 4341–4349. doi: 10.1038/sj.onc.1209460. [DOI] [PubMed] [Google Scholar]
- [60].Scott G, Cassidy L, Bllsacco A, Fibronectin Suppresses Apoptosis in Normal Human Melanocytes Through an Integrin-Dependent Mechanism, J. Invest. Dermatol 108 (1997) 147–153. doi: 10.1111/1523-1747.ep12332650. [DOI] [PubMed] [Google Scholar]
- [61].George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO, Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin., Development 119 (1993) 1079–91. http://www.ncbi.nlm.nih.gov/pubmed/8306876 (accessed March 24, 2018). [DOI] [PubMed] [Google Scholar]
- [62].Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO, Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies., Arterioscler. Thromb. Vasc. Biol 22 (2002) 927–33. http://www.ncbi.nlm.nih.gov/pubmed/12067900 (accessed March 24, 2018). [DOI] [PubMed] [Google Scholar]
- [63].Schaffner F, Ray AM, Dontenwill M, Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors., Cancers (Basel) 5 (2013) 27–47. doi: 10.3390/cancers5010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Astrof S, Hynes RO, Fibronectins in vascular morphogenesis., Angiogenesis 12 (2009) 165–75. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nicosia RF, Bonanno E, Smith M, Fibronectin promotes the elongation of microvessels during angiogenesis in vitro, J. Cell. Physiol 154 (1993) 654–661. doi: 10.1002/jcp.1041540325. [DOI] [PubMed] [Google Scholar]
- [66].Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CCW, The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation., Mol. Biol. Cell 22 (2011) 3791–800. doi: 10.1091/mbc.E11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D, Extracellular matrix modulates angiogenesis in physiological and pathological conditions., Biomed Res. Int 2014 (2014) 756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mongiat M, Andreuzzi E, Tarticchio G, Paulitti A, Extracellular Matrix, a Hard Player in Angiogenesis., Int. J. Mol. Sci 17 (2016). doi: 10.3390/ijms17111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen S, Chakrabarti R, Keats EC, Chen M, Chakrabarti S, Khan ZA, Regulation of Vascular Endothelial Growth Factor Expression by Extra Domain B Segment of Fibronectin in Endothelial Cells, Investig. Opthalmology Vis. Sci 53 (2012) 8333. doi: 10.1167/iovs.12-9766. [DOI] [PubMed] [Google Scholar]
- [70].Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M, Fibronectin promotes VEGF-induced CD34+ cell differentiation into endothelial cells, J. Vasc. Surg 39 (2004) 655–660. doi: 10.1016/J.JVS.2003.10.042. [DOI] [PubMed] [Google Scholar]
- [71].Astrof S, Crowley D, George EL, Fukuda T, Sekiguchi K, Hanahan D, Hynes RO, Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis., Mol. Cell. Biol 24 (2004) 8662–70. doi: 10.1128/MCB.24.19.8662-8670.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mateo J, Berlin J, de Bono JS, Cohen RB, Keedy V, Mugundu G, Zhang L, Abbattista A, Davis C, Gallo Stampino C, Borghaei H, A first-in-human study of the anti-α5β1 integrin monoclonal antibody PF-04605412 administered intravenously to patients with advanced solid tumors, Cancer Chemother. Pharmacol 74 (2014) 1039–1046. doi: 10.1007/s00280-014-2576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong Y-K, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam D-H, Hau P, Weyerbrock A, Taphoorn MJB, Shen C-C, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann R-D, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim C-Y, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M, European Organisation for Research and Treatment of Cancer (EORTC), Canadian Brain Tumor Consortium, CENTRIC study team, Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial, Lancet Oncol 15 (2014) 1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- [74].Murphy PA, Begum S, Hynes RO, Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors., PLoS One 10 (2015) e0120872. doi: 10.1371/journal.pone.0120872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kumra H, Reinhardt DP, Fibronectin-targeted drug delivery in cancer, Adv. Drug Deliv. Rev 97 (2016) 101–110. doi: 10.1016/j.addr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- [76].Kaspar M, Zardi L, Neri D, Fibronectin as target for tumor therapy, (n.d.). doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- [77].Rekers NH, Zegers CM, Germeraad WT, Dubois L, Lambin P, Long-lasting antitumor effects provided by radiotherapy combined with the immunocytokine L19-IL2., Oncoimmunology 4 (2015) e1021541. doi: 10.1080/2162402X.2015.1021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, González-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, Lovato V, Kaspar M, Trachsel E, Menssen HD, Neri D, Garbe C, A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma., Clin. Cancer Res 17 (2011) 7732–42. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- [79].Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, Miller K, Berndt A, Kosmehl H, Trachsel E, Kaspar M, Lovato V, González-Iglesias R, Giovannoni L, Menssen HD, Neri D, de Braud F, The tumour-targeting human L19-IL2 immunocytokine: Preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma, Eur. J. Cancer 46 (2010) 2926–2935. doi: 10.1016/J.EJCA.2010.07.033. [DOI] [PubMed] [Google Scholar]
- [80].Schaffner F, Ray AM, Dontenwill M, Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors., Cancers (Basel) 5 (2013) 27–47. doi: 10.3390/cancers5010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bell-McGuinn KM, Matthews CM, Ho SN, Barve M, Gilbert L, Penson RT, Lengyel E, Palaparthy R, Gilder K, Vassos A, McAuliffe W, Weymer S, Barton J, Schilder RJ, A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer, Gynecol. Oncol 121 (2011) 273–279. doi: 10.1016/j.ygyno.2010.12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ramakrishnan V, Bhaskar V, Law DA, Wong MHL, DuBridge RB, Breinberg D, O’Hara C, Powers DB, Liu G, Grove J, Hevezi P, Cass KM, Watson S, Evangelista F, Powers RA, Finck B, Wills M, Caras I, Fang Y, McDonald D, Johnson D, Murray R, Jeffry U, Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent., J. Exp. Ther. Oncol 5 (2006) 273–86. http://www.ncbi.nlm.nih.gov/pubmed/17024968 (accessed October 18, 2018). [PubMed] [Google Scholar]
- [83].Bell-McGuinn KM, Matthews CM, Ho SN, Barve M, Gilbert L, Penson RT, Lengyel E, Palaparthy R, Gilder K, Vassos A, McAuliffe W, Weymer S, Barton J, Schilder RJ, A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer., Gynecol. Oncol 121 (2011) 273–9. doi: 10.1016/j.ygyno.2010.12.362. [DOI] [PMC free article] [PubMed] [Google Scholar]