Abstract
INTRODUCTION
Sleep-wake disturbances are a common and early feature in Alzheimer’s disease (AD). The impact of early tau-pathology in wake-promoting neurons (WPNs) remains unclear.
METHODS
We performed stereology in postmortem brains from AD individuals and healthy controls to interrogate quantitative differences in morphological metrics in WPNs. Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) were included as disease-specific controls.
RESULTS
The three nuclei studied accumulate considerable amounts of tau inclusions and showed a decrease in neurotransmitter-synthetizing neurons in AD, PSP, and CBD. However, substantial neuronal loss was exclusively found in AD.
DISCUSSION
WPNs are extremely vulnerable in AD but not to 4-repeat tauopathies. Considering that WPNs are involved early in AD, such degeneration should be included in the models explaining sleep-wake disturbances in AD and considered when designing a clinical intervention. Sparing of WPNs in PSP, a condition featuring hyperinsomnia, suggest that interventions to suppress the arousal system may benefit PSP patients.
Keywords: Alzheimer’s disease, human, tauopathies, progressive supranuclear palsy, corticobasal degeneration, wake-promoting, locus coeruleus, orexin, histamine, sleep, wakefulness, unbiased stereology, autopsy
1. Background
Sleep-wake disturbances are common in Alzheimer’s disease (AD). Decreased sleep quality is associated with greater cognitive decline and lower quality of life, and it is one of the leading causes of institutionalization[1]. Sleep-wake disturbances occur early in AD and involve an increase in nocturnal awakenings, a prominent decrease in slow wave sleep (SWS), a modest decline in total sleep and rapid eye movement (REM) sleep time, and an increased propensity for daytime sleep[2–4]. Arousal deficiencies, such as excessive daytime sleepiness and sundowning, are significant complaints in AD patients and may precede the onset of cognitive decline[5, 6].
Arousal state requires the coordinated activity of variously interconnected neurons. The classic sleep model postulate that wake-promoting neurons (WPNs) and sleep-promoting neurons (SPNs), both subcortically located, compete for network dominance through mutual inhibition, creating a systematic “switch” that results in either the sleep or awake state[7, 8]. During wakefulness, WPNs exhibit high neuronal firing rates and suppress SPNs, whereas, during sleep, WPNs are inhibited by SPNs[7, 8]. WPNs include, among others, noradrenergic locus coeruleus (LC) neurons, orexin/hypocretin-producing neurons in the lateral hypothalamic area (LHA), and histaminergic neurons in the tuberomammillary nucleus (TMN). Many WPNs send excitatory projections to the cerebral cortex to stimulate cortical activation and behavioral arousal[9].
The neurological basis of arousal deficiencies in AD remains unclear. Animal and clinical imaging studies point to β-amyloid as the main contributor, particularly in disrupting SWS[10]. Moreover, decreasing sleep escalates β-amyloid production and cortical deposition, as sleep normally promotes β-amyloid clearance[10].
Besides β-amyloid, which first accumulates in the cortex, a non-random accumulation of p-tau positive inclusions (AD-tau) constitutes AD’s neuropathological hallmarks. AD-tau shows a stronger association with neuronal loss and clinical outcomes[11]. Nevertheless, little is known about the role of AD-tau in sleep-wake disturbances. Contrary to β-amyloid pathology, AD-tau accumulates first in the brainstem and subcortical regions, later reaching allocortex and neocortex[12]. AD-tau inclusions often precede β-amyloid accumulation. We and others have shown that AD-tau inclusions in WPNs are among the first identifiable AD lesions in humans[13–15], which beg the question of whether a possible early and progressive degeneration of WPNs by AD-tau pathology contributes to arousal disturbances in AD. Unfortunately, the resolution achieved by tau-PET is still inadequate for enabling visualization of tau pathology in small subcortical nuclei in vivo and systematic and quantitative neuropathological investigations of the arousal centers in AD remain elusive.
Here, we used design-based stereology in well-characterized post-mortem brain tissues from AD patients and healthy controls to quantitatively assess the differences in phospho-tau neuronal burden, neuronal loss, and neuronal ability to synthesize neurotransmitters in the wake-promoting network, focusing on the LC, LHA, and TMN. To further validate the clinical significance of our results in AD, we investigated the same brain areas in individuals with corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). CBD and PSP are tauopathies in which neurons from the arousal system develop tau inclusions, but unlike AD, they seldom present with arousal deficiencies[16, 17]. In fact, PSP patients experience hyperarousal[18]. Thus, we hypothesize that despite accumulating tau inclusions in arousal areas, WPNs will show smaller rates of neuronal and neurotransmitter loss in PSP and CBD than in AD.
2. Methods
2.1. Participants and Neuropathological Diagnosis
Disease subjects were selected based on a primary neuropathological diagnosis of AD, CBD, or PSP and absence of any other significant neurodegenerative or cerebrovascular changes. Normal control subjects (NC) were free of any cognitive impairment (CDR = 0); neurological or neuropathological diagnosis. PSP, CBD and NC subjects scored as A≤1B≤1C0 according to the NIA-AA guidelines for the neuropathological assessment of AD[19]. Tissues were sourced from the Neurodegenerative Disease Brain Bank (NDBB) from the University of California, San Francisco (UCSF) and Brazilian BioBank for Aging Studies (BBAS) from the University of Sao Paulo[20] (Table 1). The NDBB receives brains from patients seen at the UCSF Memory and Aging Center. BBAS is population-based and hosts a high percentage of NC that are not available in NDBB. Neuropathological assessments were performed using standardized protocols and followed the internationally accepted criteria for neurodegenerative diseases[21–23].
Table 1:
Demographics stratified by diagnostic group and grouped by nucleus. P-value calculated by a Kruskal-Wallis rank sum test comparing across the diagnostic groups unless otherwise stated.
| Characteristic | Controls (n=7) | AD (n=13) | PSP (n=7) | CBD (n=7) | P value |
|---|---|---|---|---|---|
| Locus Coeruleus | |||||
| N | 6 | 8 | 5 | 4 | |
| Age, mean (SD), y | 61 (10.56) | 66.12 (8.95) | 68 (5.52) | 69 (6.88) | 0.429 |
| Males, No. (%) | 5 (83.33) | 6 (75) | 4 (80) | 3 (75) | 1 |
| Education, mean (SD), y † | 8.33 (3.39) | 16.38 (2.39) | 17.33 (1.15) | 18 (2.16) | 0.005 |
| CDR-SOB, median (IQR) | 0 (0) | 3 (0.25) | 0.5 (0) | 1.75 (2.5) | 0.001 |
| Brain weight, mean (SD), g‡ | 1305 (132) | 1122 (105) | 1322 (175) | 1177 (218) | 0.053 |
| PMI, mean (SD), h | 16 (4) | 10 (5) | 15 (13) | 11 (8) | 0.22 |
| Lateral hypothalamic area | |||||
| N | 5 | 12 | 5 | 6 | |
| Age, mean (SD), y | 59.4 (11.1) | 64.75 (7.71) | 66.4 (6.88) | 66.5 (6.66) | 0.396 |
| Males, No. (%) | 3 (60) | 9 (75) | 4 (80) | 4 (66.67) | 0.94 |
| Education, mean (SD), y † | 9.2 (4.76) | 16.92 (2.39) | 16.5 (1.91) | 16.2 (3.49) | 0.019 |
| CDR-SOB, median (IQR) § | 0 (0) | 3 (1.5) | 0.5 (0) | 0.5 (0) | 0.002 |
| Brain weight, mean (SD), g ‡ | 1236 (82) | 1108 (109) | 1303 (172) | 1177 (144) | 0.051 |
| PMI, mean (SD), h | 14 (3) | 12 (9) | 13 (14) | 10 (6) | 0.321 |
| Tuberomammillary nucleus | |||||
| N | 5 | 6 | 5 | 5 | |
| Age, mean (SD), y | 57.8 (8.58) | 65.83 (9.99) | 65.8 (7.05) | 66.8 (7.4) | 0.332 |
| Males, No. (%) | 3 (60) | 4 (66.67) | 5 (100) | 3 (60) | 0.581 |
| Education, mean (SD), y† | 10.4 (2.88) | 16.5 (2.81) | 16 (2) | 15 (2.58) | 0.042 |
| CDR-SOB, median (IQR) § | 0 (0) | 2.5 (1.75) | 0.5 (0.5) | 0.5 (0) | 0.002 |
| Brain weight, mean (SD), g‡ | 1270 (103) | 1120 (131) | 1350 (142) | 1177 (144) | 0.055 |
| PMI, mean (SD), h | 15 (3) | 13 (4) | 7 (2) | 7 (2) | 0.007 |
NOTE. P value calculated by a Kruskal-Wallis rank sum test comparing across the diagnostic groups unless otherwise stated. Controls sourced from BBAS; AD, CBD, and PSP sourced from UCSF NDBB.
P value for sex composition computed by Fisher’s exact test.
Data on education missing for two cases examined for the locus coeruleus, two cases for the lateral hypothalamus, and three cases for the tuberomammillary nucleus.
Brain mass missing for one case examined for the locus coeruleus, two cases for the lateral hypothalamus, and one case for the tuberomammillary nucleus.
CDR-SOB missing for two cases examined for the lateral hypothalamus and one case for the tuberomammillary nucleus. Abbreviations: CDR-SOB, Clinical Dementia Rating Sum of Boxes score. PMI: Postmortem Interval
2.2. Tissue Processing and Immunohistochemistry
The specific protocol for tissue processing has been previously described (see [14] and Supplementary material). Basically, celloidin-embedded brainstem and diencephalon blocks were cut in coronal or horizontal serial sections. Section orientation does not affect the optical fractionator probe in stereology. LC neurons were quantified in 60 μm-thick sections double-immunostained for tyrosine hydroxylase (TH) and phospho-Ser202 tau (CP13) at 1200 μm intervals. LHA (or TMN) were quantified in 30 μm-thick sections double-immunostained for orexin A (or histidine decarboxylase (HDC) and CP13 at 300 μm intervals. Sections were counterstained using Gallocyanin stain (Figure 1).
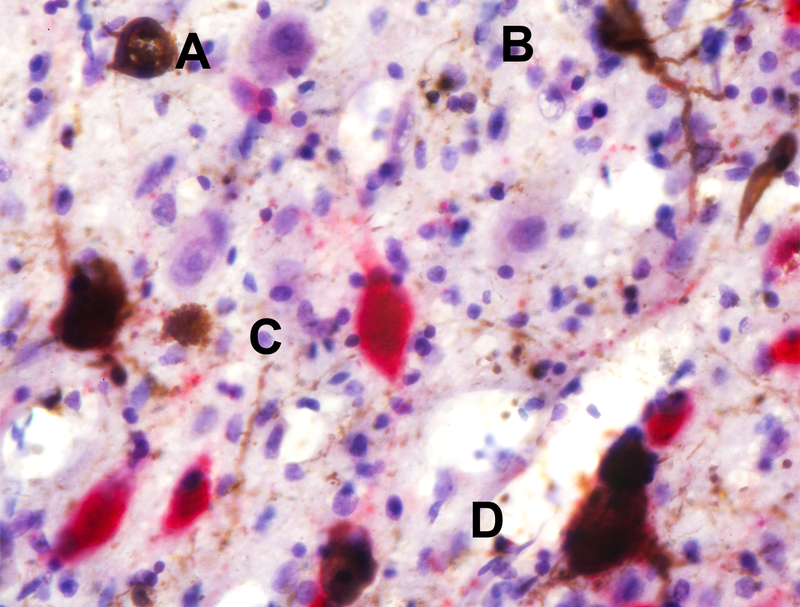
Figure 1. Neuronal Classification Using Double Staining Immunohistochemistry.
The figure depicts an example of how the neurons were classified. (A) A neuron negative for p-tau and not expressing the neurotransmitter of interest (shown in purple); (B) a neuron positive for phospho-tau only (in brown); (C) a neuron positive for neurotransmitter only (in this case, orexin; in red); (D) a neuron positive for both phospho-tau and neurotransmitter of interest (orexin). This photo was taken from the lateral hypothalamic area from a case with Alzheimer’s disease. The black bar represents 100 μm.
2.3. Stereological Quantification
Stereologically-determined estimates were made for the (1) neurotransmitter-producing (i.e., TH+, orexin+, or HDC+) neuronal population, (2) p-tau+ neuronal population and, (3) total neuronal population (Table 2; Supplemental Table). LC was identified using Olszewski’s “Cytoarchitecture of the Human Brain Stem” and was confirmed with TH staining. LHA and TMN were identified using online Allen Brain Atlas (Allen Institute for Brain Science) and were confirmed with orexin and HDC staining, respectively. Astroglial p-tau inclusions are enriched in cortical areas and rather scarce in subcortical nuclei in CBD and PSP[24], thus, we focused on neurons. The specific protocol for optical fractionator probe has been previously described[14, 25]. The Coefficient of Repeatability derived from the Bland-Altman plot showed consistency in counts between two trained, blinded investigators.
Table 2:
Mean (SD) stereological estimates for different charactaristics of the LC, LHA, and TMN, stratified by neuropathologic group.
| Characteristic | Controls | AD | PSP | CBD |
|---|---|---|---|---|
| Locus coeruleus | ||||
| Neuron population, mean (SD) | 77574 (6121) | 19516 (9700) | 36186 (12644) | 38491 (21542) |
| p-Tau+ neuron population, mean (SD) | 3082 (3031) | 7006 (4038) | 27271 (6676) | 22076 (18531) |
| TH+ neuron population, mean (SD) | 74636 (4013) | 14031 (7874) | 27747 (12193) | 36315 (25370) |
| Percentage of neurons with p-Tau, mean (SD), % | 4 (3) | 36 (16) | 77 (11) | 61 (50) |
| Percentage of neurons with TH, mean (SD), % | 96 (4) | 70 (12) | 76 (14) | 88 (45) |
| Lateral hypothalamic area | ||||
| Neuron population, mean (SD) | 71768 (35107) | 20194 (16656) | 57260 (25907) | 59529 (43447) |
| p-Tau+ neuron population, mean (SD) | 142 (203) | 8174 (8287) | 29887 (14182) | 23303 (12708) |
| Orexin+ neuron population, mean (SD) | 68576 (32625) | 14000 (11619) | 30677 (15785) | 38886 (30990) |
| Percentage of neurons with p-Tau, mean (SD), % | 0 (0) | 40 (15) | 51 (15) | 45 (12) |
| Percentage of neurons with orexin, mean (SD), % | 97 (6) | 68 (13) | 56 (22) | 63 (11) |
| Tuberomammillary nucleus | ||||
| Neuron population, mean (SD) | 154088 (35500) | 58468 (18604) | 125831 (20378) | 136469 (47036) |
| p-Tau+ neuron population, mean (SD) | 477 (682) | 14523 (10095) | 13004 (4603) | 24932 (26878) |
| HDC+ neuron population, mean (SD) | 152141 (35829) | 48218 (24672) | 116333 (16429) | 117467 (45656) |
| Percentage of neurons with p-Tau, mean (SD), % | 0 (0) | 30 (23) | 10 (3) | 18 (18) |
| Percentage of neurons with HDC, mean (SD), % | 99 (1) | 79 (18) | 93 (3) | 87 (14) |
Abbreviations: AD, Alzheimer’s disease; PSP, Progressive Supranuclear Palsy; CBD, Corticobasal Degeneration; TH, Tyrosine Hydroxylase; HDC, Histidine Decarboxylase.
2.4. Statistical Analyses
Pairwise differences in stereological estimates were assessed between disease groups for each nucleus as estimated size for each measured population and proportions of each subpopulation (e.g. TH+). The proportion of neurons positive for different subpopulations was determined by dividing the number of neurons positive for a given marker (estimates 1 or 2) by the total neuron population (estimate 3), represented as a percent. Pairwise differences were analyzed using the Wilcoxon signed-rank test with the alpha level set at 0.05. All analyses were conducted in the statistical computing program R (version 3.4.4; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Table 1 depicts demographics for the 34 cases in this study stratified by neuropathological diagnosis. Overall, the participants had an average (SD) age of 65.2 (7.9) and 76.5% were males. There were no differences between the groups regarding age and proportion of males (Table 1). In some cases, portions of the hypothalamus and brainstem had already been sampled for other studies and thus could not be included in the analysis because the unbiased stereological method requires the availability of the whole region of interest (Table 1). Table 2 gives absolute and relative numbers of the total neuronal population, neurons synthesizing neurotransmitter, and neurons bearing p-tau inclusions. Table 3 depicts pairwise comparisons between disease groups for all tested parameters.
Table 3:
P values from a Wilcoxon rank-sum test comparing the different stereological estimates between different pairs of the neuropathologic groups.
| Characteristic | Controls - AD | Controls - PSP | Controls - CBD | AD - PSP | AD -CBD | PSP -CBD |
|---|---|---|---|---|---|---|
| Locus coeruleus | ||||||
| Neuron population | 0.001 | 0.004 | 0.010 | 0.030 | 0.073 | 0.730 |
| p-Tau+ neuron population | 0.081 | 0.004 | 0.019 | 0.002 | 0.073 | 0.286 |
| TH+ neuron population | 0.001 | 0.004 | 0.010 | 0.045 | 0.214 | 0.730 |
| Percentage of neurons with p-Tau | 0.001 | 0.004 | 0.010 | 0.002 | 0.683 | 0.286 |
| Percentage of neurons with TH | 0.001 | 0.009 | 0.352 | 0.435 | 0.570 | 0.905 |
| Lateral hypothalamic area | ||||||
| Neuron population | 0.002 | 0.548 | 0.792 | 0.009 | 0.024 | 0.999 |
| p-Tau+ neuron population | 0.002 | 0.011 | 0.008 | 0.009 | 0.005 | 0.931 |
| Orexin+ neuron population | 0.001 | 0.056 | 0.247 | 0.014 | 0.067 | 0.999 |
| Percentage of neurons with p-Tau | 0.002 | 0.011 | 0.008 | 0.104 | 0.553 | 0.537 |
| Percentage of neurons with orexin | <0.001 | 0.016 | 0.004 | 0.195 | 0.180 | 0.429 |
| Tuberomammillary nucleus | ||||||
| Neuron population | 0.004 | 0.094 | 0.690 | 0.008 | 0.017 | 0.834 |
| p-Tau+ neuron population | 0.004 | 0.012 | 0.008 | 0.927 | 0.537 | 0.834 |
| HDC+ neuron population | 0.004 | 0.094 | 0.310 | 0.008 | 0.017 | 0.834 |
| Percentage of neurons with p-Tau | 0.004 | 0.012 | 0.008 | 0.410 | 0.537 | 0.402 |
| Percentage of neurons with HDC | 0.030 | 0.012 | 0.016 | 0.410 | 0.662 | 0.834 |
Abbreviations: AD, Alzheimer’s disease; PSP, Progressive Supranuclear Palsy; CBD, Corticobasal Degeneration; TH, Tyrosine Hydroxylase; HDC, Histidine Decarboxylase.
3.1. Noradrenergic Locus Coeruleus
3.1.1. The LC exhibits a profound neuronal loss in AD, whereas neuronal loss is milder in CBD and PSP
Compared to NC, we detected, 74.84% (p = .001) fewer LC neurons in AD, 50.38% (p = .010) fewer in CBD, and 53.35% (p = .004) fewer in PSP (Table 1). Within tauopathies, AD exhibited significantly fewer LC neurons when compared to PSP (p = .030) but not when compared to CBD (p = 0.073).
We found a similar pattern of loss when investigating the percentage of surviving neurons able to synthesize norepinephrine (measured by proxy using TH). The mean (±SD) percent of LC neurons with TH positivity in NC was 96% ± 4%. In AD, this percentage dropped to 70% ± 12%, whereas in PSP and CBD the percentages dropped to 76% ± 14% and 88% ±45%, respectively. These results indicate that in AD, not only neuronal loss in the LC is severe but a significant percentage of surviving neurons show signs of impaired norepinephrine synthesis. Interestingly, PSP also showed impaired norepinephrine synthesis despite having a milder neuronal loss than in AD (Figure 2; Figure 3A).
Figure 2. Representative Histological Images of Wake-Promoting Nuclei.
LC (top), LHA (middle), and TMN (bottom) tissue sections are depicted with their corresponding neurotransmitter/enzymes (TH, orexin, or HDC, respectively; in red) and tau (CP13; in brown). Notice smaller neuronal population in AD in all three wake- promoting nuclei. Some LC neurons contain neuromelanin and appear pigmented. The black bar represents 200 μm. From left to right column: Control, CBD, PSP, AD (20× magnification).
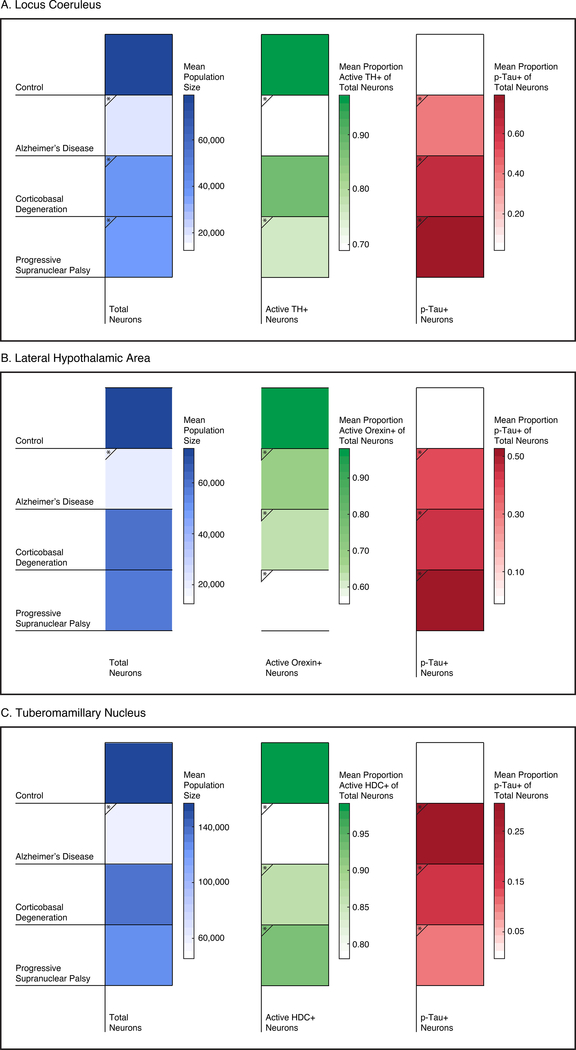
Figure 3. Stereological Comparison of Wake-Promoting Nuclei in Tauopathies.
The arousal network is extremely vulnerable to AD-tau toxicity as shown by the severe neuronal loss in LC, LHA, and TMN in AD, whereas the same network is relatively spared in PSP and CBD. Blue column depicts mean population size, green column depicts the mean proportion of neurotransmitter+ neurons, and the red column depicts the mean proportion of p-tau+ neurons. Scale bars are shown to the right. * denotes statistical significance compared to the control group.
3.1.2. The percentage of surviving neurons containing p-tau inclusions is higher in 4R tauopathies than in AD
Whereas our NC had p-tau inclusions in 4% ± 3% of neurons, these numbers grew significantly to 36% ± 16% in AD, 77% ± 11% in PSP, 61% ± 50% in CBD. Interesting, the percentage of surviving p-tau + neurons was significantly lower in AD than in PSP (p = .002).
3.2. Orexinergic Lateral Hypothalamic Area - the neuronal loss is significant in AD but not in PSP or CBD
Similar to LC, the neurons in LHA showed a severe 71.86% average loss in AD (p = .002) (Table 2). Whereas 97% ± 6% of neurons synthesize orexin in NC, this percentage fell to 68% ± 13% (p < .001) in AD.
Similar to AD, orexinergic neurons accumulate abnormal tau (51% ± 15% and 45% ± 12% of neurons in PSP and CBD, respectively) (Figure 2; Figure 3B). Also, the percentage of neurons synthetizing orexin diminished in PSP (56% ± 22%; p = .016) and CBD (63% ±11%; p = .004), but as oppose to AD, the total number of neurons remained similar to NC and significantly higher than in AD (PSP vs AD: p = .009 and CBD vs. AD: p = .024).
3.3. Histaminergic Tuberomammillary Nucleus - similar to LHA, TMN neuronal loss is specific to AD
In NC, almost all the TMN neurons were HDC positive (99% ± 1%). We detected, on average, a 62.06% (p = .004) reduction in the total number of neurons in AD (Table 1). Conversely, the total number of neurons in CBD and PSP remained similar to NC and significantly higher than in AD (PSP vs AD p=.008; CBD vs AD p=.017). In all three tauopathies, the percentage of remaining neurons containing HDC and p-tau were significantly lower and higher, respectively, than in NC (Table 2 and 3; Figure 2; Figure 3C).
4. Discussion
In this quantitative post-mortem study, we used design-based stereology to investigate morphological changes associated with tauopathies in three of the main brain nuclei containing WPNs – the LC, LHA, and TMN– to demonstrate that the arousal network is extremely vulnerable to AD-tau toxicity as shown by the severe neuronal loss, whereas the same network is relatively spared in PSP and CBD, even at late disease stages. Although all three nuclei substantially accumulate p-tau inclusions in AD, PSP, and CBD, we found greater than 60% neuronal loss of LC, LHA, and TMN neurons in AD whereas the neuronal loss in CBD and PSP was significantly mild in LC and undetectable in LHA and TMN. Nevertheless, despite being spared of neuronal loss, we detected a reduced percentage of neurons synthesizing their corresponding neurotransmitter (TH+, orexin+, or HDC+) in PSP and CBD, as we did in AD.
AD and PSP feature clear sleep-wake disturbances of different nature. While AD patient display increased sleep fragmentation with relatively preserved total sleep time and arousal deficiencies such as day-time napping and sundowning (late-day confusion)[26], PSP patients feature decreased NREM and REM sleep, elevated gamma activity in both wake and sleep EEG (hyperarousal), and prolonged sleep latencies on multiple sleep latency tests, meaning that individuals with PSP have much less total sleep time[18]. In CBD, sleep-wake disturbances have been suggested in clinical studies, but the features are unclear mainly because only 1/3 of patients with the corticobasal syndrome, the most common clinical manifestation of CBD, have an underlying CBD pathology.
Recent studies have been illuminating the mechanisms underlying sleep-wake disruption in AD. Experimental studies have shown that the accumulation of cortical β-amyloid can cause sleep disruption and in a positive feedback loop, further exacerbate β-amyloid deposition because β-amyloid is best metabolized during sleep[27]. Similar dynamics have been observed in human studies using CSF analysis and neuroimaging[27]. Individuals with β-amyloid deposition had a poorer sleep quality compared to those without β-amyloid deposition[28, 29] and cortical β-amyloid burden correlates with impaired generation of non-REM slow wave oscillation[30]. A recent study by Holtzman and colleagues showed that levels of interstitial fluid (ISF) tau fluctuates between sleep and wake states in mice and sleep deprivation increases levels of mice ISF tau and human CSF tau[31], suggesting a possible role of AD-tau in modulating sleep disturbances, such as sleep fragmentation. In summary, most models of sleep-wake disturbances in AD focus on sleep disturbances and consider arousal deficiencies as a secondary, compensatory consequence of sleep impairment. Very little data are available to examine the possibility that AD-related degeneration of the arousal system directly contributes to arousal deficiencies in AD.
Different neuronal populations modulate sleep (SPNs) and wake control (WPNs) and mutually inhibit each other’s activity, resulting in a behavioral wake or sleep state[9]. WPNs are distributed in specific nuclei in the brainstem, hypothalamus, and basal forebrain and discharge in an orchestrated manner to promote cortical arousal (for a comprehensive review, see Brown et al[9]). We focused on noradrenergic, orexinergic, and histaminergic WPNs because they are highly interconnected and play an integral part in governing consolidated wakefulness and sleep-to-wake transition.
The noradrenergic LC sends dense excitatory projections to extensive brain areas and inhibitory projections to SPNs in the intermediate nucleus of the hypothalamus (analog of the ventrolateral preoptic nucleus in rodents)[9]. LC neurons exhibit the highest firing rate during waking and silence during NREM or REM sleep in animal models[32]. LC neurons are among the first to accumulate p-tau in AD and suffer a catastrophic neuronal loss along AD progression[13, 14]. The LC-norepinephrine system becomes highly dysfunctional in AD pathology. Here, we detected a milder LC neuronal loss in PSP and CBD than in AD, confirming our previous results from semi-quantitative methods[16]. These findings either represent the fact that LC involvement in PSP and CBD may occur later in the disease progression than in AD, or that CBD-tau and PSP-tau are less toxic to LC neurons than AD-tau. Nonetheless, both hypotheses reflect a relatively simplistic model in which tau neurotoxicity is a product of a time of exposure and/or “toxicity of a strain” with little influence of intrinsic neuronal factors. Interestingly, the percentage of surviving p-tau + neurons was significantly higher in PSP than in AD (p = .002). Considering the more severe LC neuronal loss in AD, this finding may be seen paradoxical, begging the question of whether intrinsic factors make LC neurons are more resistant to PSP-tau. Curiously, we showed previously that PSP-tau correlates with a more severe neuronal loss in substantia nigra than AD-tau[16], despite the fact that SN also accumulates AD-tau from Braak stage 0[15]. Studies including cases at progressive PSP and CBD stages might help to clarify this question and answer whether the surviving neurons seen in the LC in AD may represent the subpopulation resilient to p-tau. In addition, LC neuronal loss has been previously shown to be topographically arranged in AD[33–35]. For instance, some studies indicate that heaviest neuronal loss occurs in the central area of LC (projecting to temporal cortex/hippocampus) whereas neurons in the rostral and caudal areas of LC (projecting mainly to frontal/occipital regions) are relatively spared[34]. Given these topographical characteristics in AD, future studies examining whether CBD and PSP show a similar pattern of neuronal loss in LC will further inform us of the heterogeneous nature of the noradrenergic system. Regardless, our results highlight the need to quantify multiple parameters to understand the impact of a neurodegenerative condition. A stand-alone measurement of tau (or any other misfolded protein) burden is a poor indicator of the neurodegeneration status.
Orexinergic neurons in the LHA play an important role in wakefulness. Orexin knockout mice, orexin-receptor knockout mice, and orexinergic neurons-ablated transgenic mice all show a pattern resembling human narcolepsy[36]. Orexinergic neurons accumulate AD-tau from Braak stage 0[15] but the impact of AD on the orexinergic system remains unclear. Most studies measured orexin cerebrospinal-fluid (CSF) levels and show contradicting results. Schmidt et al. showed an unaltered CSF orexin level in moderate to severe AD patients compared to healthy controls[37], whereas Liguori et al. reported increases in CSF orexin levels in AD featuring moderate to severe cognitive decline[38]. In a previous post-mortem study examining orexinergic neurons in AD, Fronczek et al. reported a 40% decrease in neuronal number in late-stage AD, using semi-quantitative methods[39]. Here, using unbiased stereology, we report an even greater neuronal loss in AD- with 71.85% reduction. To put this into another perspective, patients with narcolepsy (a chronic sleep disorder that causes overwhelming daytime drowsiness) have been reported to show 85–95% reduction in the number of orexinergic neurons[40], almost comparable to what we see in AD. While direct comparisons should be examined with caution due to differences in the methodologies employed, this highlights an abnormally high magnitude of orexinergic loss in AD.
Little is known about LHA neurons in 4R-tauopathies. Yasui et al. reported a significantly lower orexin CSF level in patients with probable CBD and PSP when compared to Parkinson’s Disease[41] and speculated that such lower levels reflect a loss of orexinergic neurons or impaired orexin transmission. In our pioneering study investigating LHA neuronal numbers in PSP and CBD, we found a decrease in neuronal ability to synthesize orexin but failed to detect an orexinergic neuronal loss. Like the LC, LHA neurons accumulated CBD-tau and PSP-tau in large proportions and future studies should explore the biological properties that confer resistance to death in orexinergic neurons in 4R tauopathies but not in AD, especially because these neurons are, in theory, rescuable. Regardless, an intriguing issue here is how to reconcile our findings of substantial LHA neuronal loss in AD, but not in CBD and PSP, with findings of increased CSF orexin levels in AD but decreased in PSP and CBD.
Histamine is a critical wake-promoting neurotransmitter. Almost all histaminergic neurons are located in the hypothalamic TMN. Previous neuropathological reports describe TMN vulnerability to AD-tau starting at Braak stage 0 with accompanying neuronal loss[15, 42–44]. However, those studies were either qualitative, did not use histaminergic-specific neuronal markers, or only analyzed neurons at rostral TMN level. An early study using single-label immunostaining found no significant differences in the number of histaminergic neurons between AD (n=3) and cases with milder AD pathology lacking clinical decline[45]. That study preceded the Braak stage system, thus it is unclear if results point to a lack of neuronal loss in TMN or AD or if losses occurred already in early AD stages. Interestingly, CSF studies have indicated either no change or only a modest decrease in CSF histamine/tele-methylhistamine level in AD clinical syndrome[46, 47]. However, these studies could have been confounded by the fact that brain immune cells can synthesize histamine[48]. Here, we assessed the full extent of TMN using a histaminergic neuronal marker, and demonstrate a significant 62% reduction in TMN neurons in late stages AD. In CBD and PSP, although 18% and 10% of TMN neurons contained tau inclusions respectively, TMN neuronal numbers were comparable to NC. But similar to LHA neurons, in CBD and PSP (and also in AD), the proportion of histamine synthetizing neurons were smaller than in controls, again suggesting that CBD-tau and PSP-tau have a degree of neurotoxicity to this neuronal subpopulation, that could be, theoretically, reversed.
In general, our results contrast with findings in noradrenergic, orexinergic and histaminergic levels from CSF and in vivo studies. Some studies indicate that levels of CSF norepinephrine and orexin[38, 49], TH-mRNA expression in LC neurons[50], and histamine release at axon terminals[51] are increased in advanced AD. In our study, however, the percentage of neurotransmitter-producing neurons were decreased in all tauopathies. Evidence suggests that a significant neuronal loss of neurons may lead to compensatory adaptations, including an increase in neurotransmitter expression per cell or turnover rate[52]. Our findings, especially regarding the massive neuronal loss in all three nuclei in AD, warrants for a re-interpretation of these CSF findings.
Our findings may inform on therapeutic strategies to improve sleeping quality in PSP. PSP patients experience profound sleep deprivation without recuperation, suggesting a diminished homeostatic sleep drive[18]. Since LHA and TMN are relatively preserved in PSP, it is possible that the lack of inhibition of WPN may underlie hyperarousal. Thus, strategies using orexin or histamine antagonists may help to restore WPN-SPN balance.
4.1. Limitation
Our study has a number of limitations. First, our sample size is relatively small due to the rarity of CBD and PSP cases, made even more difficult by our criterion to exclude cases with co-pathology and our requirement of whole hypothalamic and brainstem availability for the study. In addition, our AD cohort is relatively young (average age of death: 66 years), especially because we aimed to match the age of death across groups and excluded cases with mixed pathology. Although all included cases were sporadic, some evidence suggests that AD pathology in presenile and senile age groups may vary in certain aspects. Thus, our results may not be generalizable to a cohort of older participants with AD. Still, our study is one of the largest quantitative studies of its kind to date. Also, the disadvantage of our relatively small sample size is partially compensated by our in-depth clinical and postmortem characterization of the cases and the use of unbiased stereological methods. Second, our cohort has a greater proportion of male, leading to potential sex-related bias. Although we and others failed to detect sex differences in LC[13, 53], sex-differences in LHA and TMN neuronal numbers have not been well addressed. Third, despite our effort to only include cases with short postmortem intervals, we cannot rule out a possibility of hypoxia-induced neuropathological changes, including the degradation of our proteins of interest. Fourth, LHA neurons are heterogeneous in nature and thus, our counts may include some surrounding non-orexinergic neurons. However, we minimized this risk by accurately delineating LHA using cytoarchitecture, 3-dimensional reconstruction images, and orexin staining. Fifth, while all cases used in the study were Caucasians (in Brazilian cases, ethnicity is determined by DNA ancestry markers to avoid bias in such an admixed population) and underwent exactly same tissue processing, our disease cohort (UCSF NDBB) and control cohort (BBAS) were sourced from two different geographical regions. Sixth, while we conducted multiple tests without correcting for multiple comparisons, many of our tests were not truly independent. Nevertheless, this underscores the need for a larger sample and a limitation on the generalizability of these results. Another limitation is the unknown status of obstructive sleep apnea, a sleep disorder associated with hypoxia-reoxygenation that has been associated with dementia and may lead to daytime somnolence. Lastly, our study is cross-sectional by nature and only includes cases that are at late stages, which makes it difficult to determine when pathological changes begin.
5. Conclusion
In conclusion, our study provides compelling evidence that WPNs are extremely vulnerable to AD-tau and relatively resistant to CBD-tau and PSP-tau. Our findings do not rebuke the bidirectional role of β-amyloid and sleep disturbances in AD but argue that AD-tau driven degeneration of the arousal system should be included in the models explaining sleep-wake disturbances in AD and considered when designing a clinical intervention. A longitudinal, clinicopathological study combining objective sleep measurements with unbiased post-mortem evaluation may further clarify the contribution of tau pathology to arousal deficiencies in AD. Also, our study corroborates the hypothesis that selective neuronal vulnerability to tauopathies at the cellular level is not a simple result of exposure time and “strain” toxicity. Wake-promoting nuclei accumulate PSP-tau and CBD-tau in a large proportion without proportional terminal neuronal loss. Investigating selective vulnerability to tau using nuclei with a relatively homogeneous neuronal population as a framework may inform on the intrinsic neuronal factors influencing vulnerability to tau toxicity.
Supplementary Material
Research in Context.
Systematic Review
The authors thoroughly reviewed the literature using PubMed and cited appropriate papers. Only a small number of human studies have been conducted on the wake-promoting neurons in AD, and systematic and quantitative neuropathological investigations of the arousal centers still remain elusive. Very little is known about wake-promoting neurons in 4 repeat-tauopathies.
Interpretation
Our study provides compelling evidence that WPNs are extremely vulnerable to AD-tau while relatively resistant to CBD-tau and PSP-tau. Furthermore, our study corroborates the hypothesis that selective neuronal vulnerability to tauopathies at the cellular level is not a simple result of exposure time and “strain” toxicity. For instance, unlike AD, wake-promoting nuclei accumulate PSP-tau and CBD-tau in a large proportion without proportional terminal neuronal loss.
Future Directions
Tau-driven degeneration of the arousal system should be included in the models explaining sleep-wake disturbances in AD and considered when designing a clinical intervention.
Acknowledgments
The authors thank the patients and their families for their invaluable contribution to brain aging neurodegenerative disease research. This study was supported by the Tau Consortium/Rainwater Charity Foundation and grants from the Institutional grants P50AG023501, P01AG019724, and K24AG053435 (LTG). Elisa de Paula França Resende is an Atlantic Fellow for Equity in Brain Health at the Global Brain Health Institute and thanks the fellowship for supporting her work. This article was also supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation – FAPESP) grant #2016/02224–1-2 (JCB). JCB is also a recipient of a Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (Agency for the Advancement of Higher Education) and Comité Français d’Evaluation de la Coopération Universitaire avec le Brésil (French Committee for the Evaluation of Academic and Scientific Cooperation with Brazil) grant CAPES|COFECUB #848/15. JCB is an Investigator with the Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development – CNPq) with grants #301035/2015–6 and #426378/2016–4. We also thank Eva Larsen, Alex Burr, Wenda Lee (UCSF) for human tissue processing and Alexandria Lai (Massachusetts General Hospital) for manuscript editing.
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27:478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. [DOI] [PubMed] [Google Scholar]
- [3].Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–96. [DOI] [PubMed] [Google Scholar]
- [4].Lee JH, Bliwise DL, Ansari FP, Goldstein FC, Cellar JS, Lah JJ, et al. Daytime sleepiness and functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:620–6. [DOI] [PubMed] [Google Scholar]
- [5].Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD, et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis. 2018;66:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsapanou A, Gu Y, Manly J, Schupf N, Tang MX, Zimmerman M, et al. Daytime Sleepiness and Sleep Inadequacy as Risk Factors for Dementia. Dement Geriatr Cogn Dis Extra. 2015;5:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oh J, Petersen C, Walsh CM, Bittencourt JC, Neylan TC, Grinberg LT. The role of co-neurotransmitters in sleep and wake regulation. Mol Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016;39:552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP, et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017;13:236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol. 2017;43:393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stratmann K, Heinsen H, Korf HW, Del Turco D, Ghebremedhin E, Seidel K, et al. Precortical Phase of Alzheimer’s Disease (AD)-Related Tau Cytoskeletal Pathology. Brain Pathol. 2016;26:371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eser RA, Ehrenberg AJ, Petersen C, Dunlop S, Mejia MB, Suemoto CK, et al. Selective Vulnerability of Brainstem Nuclei in Distinct Tauopathies: A Postmortem Study. J Neuropathol Exp Neurol. 2018;77:149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246 Suppl 2:II6–15. [DOI] [PubMed] [Google Scholar]
- [18].Walsh CM, Ruoff L, Walker K, Emery A, Varbel J, Karageorgiou E, et al. Sleepless Night and Day, the Plight of Progressive Supranuclear Palsy. Sleep. 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, et al. Brain bank of the Brazilian aging brain study group - a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007;8:151–62. [DOI] [PubMed] [Google Scholar]
- [21].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suemoto CK, Ferretti-Rebustini RE, Rodriguez RD, Leite RE, Soterio L, Brucki SM, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study. PLoS Med. 2017;14:e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kovacs GG. Tauopathies. Handb Clin Neurol. 2017;145:355–68. [DOI] [PubMed] [Google Scholar]
- [25].West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. [DOI] [PubMed] [Google Scholar]
- [26].Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. [DOI] [PubMed] [Google Scholar]
- [27].Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–26. [DOI] [PubMed] [Google Scholar]
- [33].Marcyniuk B, Mann DM, Yates PO. The topography of cell loss from locus caeruleus in Alzheimer’s disease. J Neurol Sci. 1986;76:335–45. [DOI] [PubMed] [Google Scholar]
- [34].Marcyniuk B, Mann DM, Yates PO. Loss of nerve cells from locus coeruleus in Alzheimer’s disease is topographically arranged. Neurosci Lett. 1986;64:247–52. [DOI] [PubMed] [Google Scholar]
- [35].Marcyniuk B, Mann DM, Yates PO. The topography of nerve cell loss from the locus caeruleus in elderly persons. Neurobiol Aging. 1989;10:5–9. [DOI] [PubMed] [Google Scholar]
- [36].Inutsuka A, Yamanaka A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne). 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, et al. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. PLoS One. 2013;8:e63136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–505. [DOI] [PubMed] [Google Scholar]
- [39].Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, et al. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33:1642–50. [DOI] [PubMed] [Google Scholar]
- [40].Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yasui K, Inoue Y, Kanbayashi T, Nomura T, Kusumi M, Nakashima K. CSF orexin levels of Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci. 2006;250:120–3. [DOI] [PubMed] [Google Scholar]
- [42].Saper CB, German DC. Hypothalamic pathology in Alzheimer’s disease. Neurosci Lett. 1987;74:364–70. [DOI] [PubMed] [Google Scholar]
- [43].Nakamura S, Takemura M, Ohnishi K, Suenaga T, Nishimura M, Akiguchi I, et al. Loss of large neurons and occurrence of neurofibrillary tangles in the tuberomammillary nucleus of patients with Alzheimer’s disease. Neurosci Lett. 1993;151:196–9. [DOI] [PubMed] [Google Scholar]
- [44].Shan L, Bossers K, Unmehopa U, Bao AM, Swaab DF. Alterations in the histaminergic system in Alzheimer’s disease: a postmortem study. Neurobiol Aging. 2012;33:2585–98. [DOI] [PubMed] [Google Scholar]
- [45].Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, Suomalainen R, et al. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991;44:465–81. [DOI] [PubMed] [Google Scholar]
- [46].Motawaj M, Peoc’h K, Callebert J, Arrang JM. CSF levels of the histamine metabolite tele-methylhistamine are only slightly decreased in Alzheimer’s disease. J Alzheimers Dis. 2010;22:861–71. [DOI] [PubMed] [Google Scholar]
- [47].Gabelle A, Jaussent I, Hirtz C, Vialaret J, Navucet S, Grasselli C, et al. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol Aging. 2017;53:59–66. [DOI] [PubMed] [Google Scholar]
- [48].Katoh Y, Niimi M, Yamamoto Y, Kawamura T, Morimoto-Ishizuka T, Sawada M, et al. Histamine production by cultured microglial cells of the mouse. Neurosci Lett. 2001;305:181–4. [DOI] [PubMed] [Google Scholar]
- [49].Raskind MA, Peskind ER, Halter JB, Jimerson DC. Norepinephrine and MHPG levels in CSF and plasma in Alzheimer’s disease. Arch Gen Psychiatry. 1984;41:343–6. [DOI] [PubMed] [Google Scholar]
- [50].Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus. 2015;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hoogendijk WJ, Feenstra MG, Botterblom MH, Gilhuis J, Sommer IE, Kamphorst W, et al. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol. 1999;45:82–91. [DOI] [PubMed] [Google Scholar]
- [53].Theofilas P, Dunlop S, Heinsen H, Grinberg LT. Turning on the Light Within: Subcortical Nuclei of the Isodentritic Core and their Role in Alzheimer’s Disease Pathogenesis. J Alzheimers Dis. 2015;46:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.