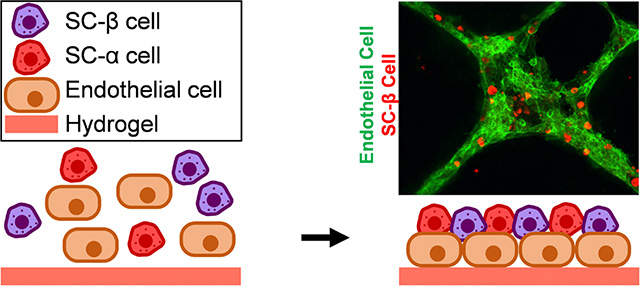
Abstract
Differentiation of stem cells into functional replacement cells and tissues is a major goal of the regenerative medicine field. However, one limitation has been organization of differentiated cells into multi-cellular, three-dimensional assemblies. The islets of Langerhans contain many endocrine and non-endocrine cell types, such as insulin-producing β cells and endothelial cells. Despite the potential importance of endothelial cells to islet function, facilitating interactions between endothelial cells and islet endocrine cell types already differentiated from human embryonic stem cells has been difficult in vitro. We have developed a strategy of assembling human embryonic stem cell-derived islet cells with endothelial cells into three-dimensional aggregates on a hydrogel. The resulting islet organoids express β cell and other endocrine markers and are functional, capable of undergoing glucose-stimulated insulin secretion. This assembly was not observed on traditional tissue culture plastic and in aggregates generated in suspension culture, highlighting how physical culture conditions greatly influence the interactions among these cell types. These results provide a platform for evaluating the effects of the islet tissue microenvironment on human embryonic stem cell-derived β cells and other islet endocrine cells to develop tissue engineered islets.
Graphical Abstract
1. Introduction
In diabetes, insulin-producing β cells, which are located within islets of Langerhans in the pancreas, are dysfunctional or destroyed by high levels of metabolites, such as glucotoxicity or lipotoxicity, or by autoimmune attack. The rapid rise in diabetes prevalence has generated much attention towards the development of technologies to better study and treat this disease. However, there is no cure for diabetes, and current treatments are insufficient in controlling the disease for many patients. A small number of patients have been transplanted with cadaveric human islets, which contain β cells, and remained insulin independent for years [1]. Unfortunately this approach is limited because of the scarcity and variability of isolated human islets available for patients, whom often require islets from multiple donors to achieve normal blood sugar levels [2].
Several reports by us and others have detailed approaches for making insulin-producing β-like cells from human embryonic stem cells (hESCs) with the goal that these cells could be used for both cell replacement therapy and drug screening for diabetes [3–8]. These hESC-derived β (SC-β) cells are capable of undergoing glucose-stimulated insulin secretion and express markers found in β cells. While current methodologies produce final cell populations containing SC-β cells, the cellular composition is significantly different than islets found within the body. Current SC-β cell differentiations create cellular clusters that, while visually resembling islets and having functional SC-β cells that are electrically coupled according to Ca2+ flux measurements [4, 9], differ significantly from human islets. The differentiation process does produce cells expressing hormones indicative of other islet endocrine cell types, such as glucagon and somatostatin, but endothelial cells (ECs) are absent. The native islet microenvironment, which is influenced in part by ECs, is highly specialized in supporting islet architecture and function [10]. This three-dimensional islet environment facilitates β cell-β cell and β cell-EC interactions which altogether support β cell survival and insulin secretion. Specifically, ECs produce extracellular matrix (ECM) proteins to provide such interactions [11, 12].
Modulating the microenvironment that SC-β cells experience to be more islet-like is technologically challenging with current approaches. In particular, facilitating interactions between ECs and already differentiated SC-β cells and other SC-islet endocrine cell types has been difficult in vitro, limiting study of the influence of ECs on SC-islet maturation and function. As SC-β cells are derived from the endoderm germ layer and endothelial cells from mesoderm, robust simultaneous differentiation of both cell types in the same culture is not possible. This is because current directed differentiation protocols specify only one germ layer. Approaches focused on assembly of multiple cell types during or after differentiation are therefore preferable. However, currently culture of aggregates containing SC-β cells in suspension is commonly performed on shaker plates or spinner flasks [5], which requires expensive water-, heat-, and CO2-resistant equipment and expertise with hESC culture in reactors, or on air-liquid-interfaces [8], which requires manual formation of each individual aggregate and are not amenable to addition of islet components.
Here we established a platform for the assembly of islet-like organoids with already differentiated SC-β cells and ECs. These parameters allow for SC-β cells to assemble and interact with ECs when cultured on Matrigel hydrogel slabs. The heterozygous cell assembles express markers found in islets and are capable of undergoing glucose-stimulated insulin secretion. In contrast, SC-β cells and ECs did not assemble when plated in two-dimensional culture on standard tissue culture plastic coated with dilute Matrigel or with aggregation in suspension culture. This platform facilitates the study of SC-β cell/EC interactions as well as tissue engineering of islet organoids to further improve SC-islet performance.
2. Materials and methods
2.1. Stem cell culture
The HUES8 hESC line was generously provided by Dr. Douglas Melton (Harvard University) and has been previously published [4, 5]. These cells were cultured in an undifferentiated state in mTeSR1 (StemCell Technologies; 05850) in 100-mL or 30-mL spinner flasks (REPROCELL; ABBWVS10A or ABBWVS03A) on a stirrer plate (Chemglass) set at 60 RPM in a humidified incubator set at 5% CO2 and 37 °C. Accutase (StemCell Technologies; 07920) was used to passage cells every 3 d. The Vi-Cell XR (Beckman Coulter) was used to quantify viable cell counts and 6 × 105 cells/mL in mTeSR1+ 10 μM Y27632 (Abcam; ab120129) were seeded back into the flasks.
2.2. Differentiation to SC-β cells
Differentiation was performed as developed and described by us in Velazco-Cruz et al [5]. Undifferentiated hESCs were seeded into 30-mL spinner flasks, cultured for 3 d in mTeSR1, and then cultured in the following conditions in order:
Stage 1: 3 days in S1 basal media with 100 ng/ml Activin A (R&D Systems; 338-AC) and 3 μM CHIR99021 (Stemgent; 04–0004-10) for 1 day followed by S1 basal media with 100 ng/ml Activin A for 2 days.
Stage 2: 3 days in S2 basal media with 50 ng/ml KGF (Peprotech; AF-100–19).
Stage 3: 1 day in S3 basal media with 50 ng/ml KGF, 200 nM LDN193189 (Reprocell; 040074), 500 nM PdBU (MilliporeSigma; 524390), 2 μM Retinoic Acid (MilliporeSigma; R2625), 0.25 μM Sant1 (MilliporeSigma; S4572), and 10 μM Y27632.
Stage 4: 5 days in S3 basal media with 5 ng/mL Activin A, 50 ng/mL KGF, 0.1 μM Retinoic Acid, 0.25 μM SANT1, and 10 μM Y27632.
Stage 5: 7 days in S5 basal media with 10 μM ALK5i II (Enzo Life Sciences; ALX-270–445-M005), 20 ng/mL Betacellulin (R&D Systems; 261-CE-050), 0.1 μM Retinoic Acid, 0.25 μM SANT1, 1 μM T3 (Biosciences; 64245), and 1 μM XXI (MilliporeSigma; 595790). At the end of this stage, clusters were reaggregated by dispersion with TrypLE Express (ThermoFisher; 12604013) and replating in a 6-well plate on an Orbi-Shaker (Benchmark).
Stage 6: 12–22 days in enhanced serum-free media (ESFM).
The basal media formulations are as follows:
S1 basal media: 500 mL MCDB 131 (Cellgro; 15–100-CV) plus 0.22 g glucose (MilliporeSigma; G7528), 1.23 g sodium bicarbonate (MilliporeSigma; S3817), 10 g bovine serum albumin (BSA) (Proliant; 68700), 10 μL ITS-X (Invitrogen; 51500056), 5 mL GlutaMAX (Invitrogen; 35050079), 22 mg vitamin C (MilliporeSigma; A4544), and 5 mL penicillin/streptomycin (P/S) solution (Cellgro; 30–002-CI).
S2 basal media: 500 mL MCDB 131 plus 0.22 g glucose, 0.615 g sodium bicarbonate, 10 g BSA, 10 μL ITS-X, 5 mL GlutaMAX, 22 mg vitamin C, and 5 mL P/S.
S3 basal media: 500 mL MCDB 131 plus 0.22 g glucose, 0.615 g sodium bicarbonate, 10 g BSA, 2.5 mL ITS-X, 5 mL GlutaMAX, 22 mg vitamin C, and 5 mL P/S.
S5 media: 500 mL MCDB 131 plus 1.8 g glucose, 0.877 g sodium bicarbonate, 10 g BSA, 2.5 mL ITS-X, 5 mL GlutaMAX, 22 mg vitamin C, 5 mL P/S, and 5 mg heparin (MilliporeSigma; A4544).
ESFM: 500 mL MCDB 131 plus 0.23 g glucose, 10.5 g BSA, 5.2 mL GlutaMAX, 5.2 mL P/S, 5 mg heparin, 5.2 mL MEM nonessential amino acids (Corning; 20–025-CI), 84 μg ZnSO4 (MilliporeSigma; 10883), 523 μL Trace Elements A (Corning; 25–021-CI), and 523 μL Trace Elements B (Corning; 25–022-CI).
2.3. Light microscopy
Images of cell clusters unstained or stained with 2.5 μg/mL dithizone (MilliporeSigma; 194832) were taken with an inverted light microscope (Leica DMi1).
2.4. Immunohistochemistry
Clusters were fixed with 4% paraformaldehyde (Electron Microscopy Science; 15714) overnight at 4 °C, embedded in Histogel (Thermo Scientific; hg-4000–012), and paraffin-embedded and sectioned by the Division of Comparative Medicine (DCM) Research Animal Diagnostic Laboratory Core at Washington University. Immunostaining was performed by paraffin removal with Histoclear (Thermo Scientific; C78–2-G), rehydration by treatment with increasing ratios of water to ethanol, antigens retrieved by treatmetn with 0.05 M EDTA (Ambion; AM9261) in a pressure cooker (Proteogenix; 2100 Retriever). Non-specific antibody binding was blocked with a 30-min treatment in staining buffer (5% donkey serum (Jackson Immunoresearch; 017–000-121) and 0.1% Triton-X 100 (Acros Organics; 327371000) in PBS), followed by overnight staining with 1:300 dilutions of rat-anti-C-peptide (DSHB; GN-ID4-S) and mouse-anti-glucagon (ABCAM; ab82270) primary antibodies or only with buffer. Samples were stained with donkey secondary antibodies containing Alexa Fluor fluorophores (Invitrogen) for 2 hr at 4 °C, and treated with DAPI in the mounting solution Fluoromount-G (SouthernBiotech; 0100–20). Imaging was performed on a Nikon A1Rsi confocal microscope.
2.5. Whole-mount immunostaining
Cells or cell assemblies were fixed within the well using 4% paraformaldehyde treatment overnight at 4 °C. Non-specific antibody binding was blocked with a 30-min treatment in staining buffer (5% donkey serum (Jackson Immunoresearch; 017–000-121) and 0.1% Triton-X 100 (Acros Organics; 327371000) in PBS) followed by overnight staining with 1:300 dilutions of rat-anti-C-peptide (DSHB; GN-ID4-S), mouse-anti-CD31 (Dako; M082329–2), goat-anti-PDX1 (R&D Systems; AF2419), and/or mouse-anti-NKX6–1 (University of Iowa, Developmental Hybridoma Bank; F55A12-supernatant) primary antibodies. Samples were stained with donkey secondary antibodies containing Alexa Fluor fluorophores (Invitrogen) for 2 hr at 4 °C and treated with DAPI. Imaging was performed on a Nikon A1Rsi confocal microscope or Leica DMI4000 microscope. Quantification was done with manual counting.
2.6. Assembly of SC-β cells and ECs in suspension
Human umbilical vein endothelial cells (HUVECs) were purchased form Lonza (C2519A) and cultured in EGM-2 media (Lonza; CC-3162). HUVECs and Stage 6 clusters containing SC-β cells were dispersed with TripLE Express and plated into a 6-well plate on an Orbi-Shaker at 100 rpm. Two conditions were tested: 3×106 Stage 6 cells only (control) and 2.5×106 Stage 6 cells mixed with 0.5×106 HUVECs. Cells were cultured in a media of 90% ESFM and 10% EGM-2. The presence of these cell types was assessed after 48 hr by dispersion and plating into a 96-well plate followed by immunostaining.
2.7. Assembly of SC-β cells and ECs on tissue culture plastic
HUVECs and Stage 6 clusters containing SC-β cells were dispersed with TripLE Express and plated (1×105 cells each) into a 96-well plate pre-coated with diluted (1:80) Matrigel in MCDB 131. This low concentration of Matrigel will not form a three-dimensional gel like undiluted Matrigel, instead providing a thin coating that promotes two-dimensional cell attachment and growth [13]. Cells were cultured in a media of 90% ESFM and 10% EGM-2. After 24 hr, the cells were fixed with 4% paraformaldehyde for immunostaining assessment of both cell types. Assembly was quantified by manual counting of all C-peptide+ cells from three separate immunostained images of three separate attempted assemblies.
2.8. Assembly of SC-β cells and ECs on a slab of Matrigel hydrogel
Cold Matrigel (Fisher; 354277; 60 μL), which is qualified for stem cell culture and not growth factor reduced, was transferred into 96-well plates and then incubated at 37 °C for 1 hr to create gel slabs. After this time, HUVECs and Stage 6 clusters containing SC-β cells were dispersed with TripLE Express and plated on top of the gel slabs over a range of cell numbers (0.5−2×105 Stage 6 and 0–1.5×105 HUVECs). Cells were cultured in a media of 90% ESFM and 10% EGM-2. After 24 hr, the cells were fixed with 4% paraformaldehyde for immunostaining assessment of both cell types. Assembly was quantified by manual counting of all C-peptide+ cells from three separate immunostained images of three separate assemblies.
2.9. Viability assessment
Qualitative assessment of viability was performed using the Live/Dead Viability/Cytotoxicity Kit (Molecular Probes; L3224). Quantitative assessment of viability was performed by staining cells with dithizone (2.5 μg/mL) and trypan blue diluted 1:40 (Beckman Coulter; B94987) and manual counting.
2.10. Glucose-stimulated insulin secretion assay
This assay was performed by first washing cells twice with KRB buffer (128 mM NaCl, 5 mM KCl, 2.7 mM CaCl2 1.2 mM MgSO4, 1 mM Na2HPO4, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM HEPES (Gibco; 15630–080), and 0.1% BSA) and equilibrating cells in 2 mM glucose KRB for a 1 hr. The supernatant was removed and replaced with fresh 2 mM glucose KRB, discarding the old KRB solution. Assemblies were incubated for 1 hr, the supernatant removed and replaced with fresh 20 mM glucose KRB, retaining the old KRB solution. The assemblies were incubated for 1 hr, the supernatant removed and retained. Insulin concentration with the retained KRB supernatant was quantified with a Human Insulin ELISA (ALPCO; 80-INSHU-E10.1). Secretion was normalized to cell counts by single-cell dispersing assemblies with 10-min TrypLE Express treatment and quantifying viable cell count with a Vi-Cell XR instrument.
2.11. Real-time PCR
The RNeasy Mini Kit (Qiagen; 74016) with DNase treatment (Qiagen; 79254), was used to extract RNA from cell assemblies, hESCs, Stage 6 aggregates, and human islets purchased from Prodo Labs. The High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems; 4368814) was used to make cDNA for gene expression measurements. Real-time PCR was performed with a StepOnePlus (Applied Biosystems) instrument using PowerUp SYBR Green Master Mix (Applied Biosystems; A25741). Analysis was performed using ΔΔCt methodology and normalization to TBP. The following primer pairs used: CAATGCCACGCTTCTGC, TTCTACACACCCAAGACCCG; PDX1, CGTCCGCTTGTTCTCCTC, CCTTTCCCATGGATGAAGTC; TBP, GCCATAAGGCATCATTGGAC, AACAACAGCCTGCCACCTTA; NKX6–1, CCGAGTCCTGCTTCTTCTTG, ATTCGTTGGGGATGACAGAG; CHGA, TGACCTCAACGATGCATTTC, CTGTCCTGGCTCTTCTGCTC; NEUROD1, ATGCCCGGAACTTTTTCTTT, CATAGAGAACGTGGCAGCAA; NKX2–2, GGAGCTTGAGTCCTGAGGG, TCTACGACAGCAGCGACAAC; GCK, ATGCTGGACGACAGAGCC, CCTTCTTCAGGTCCTCCTCC; MAFB, CATAGAGAACGTGGCAGCAA, ATGCCCGGAACTTTTTCTTT; SOX9, GATTAGCACACTGATCACACGA, TTAACCCTCTTCAGAGCAAGC; KRT19, AGGATGCTGAAGCCTGGTT, GGTCAGTAACCTCGGACCTG
2.12. Statistical analysis
GraphPad Prism was used to determine statistical significance using two-sided unpaired and paired t-tests correcting for multiple comparisons using the Holm-Sidak method. Data is represented as mean±SEM.
3. Results
3.1. Development of platform for SC-β cells and EC assembly
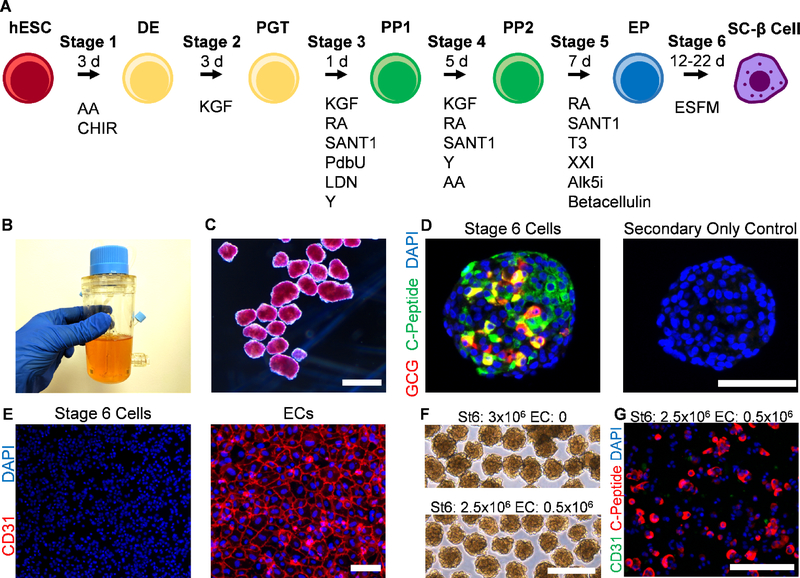
We have previously published a protocol for the generation of SC-β cells from hESCs [5] (Fig. 1A). This approach is done entirely in suspension culture with the cells grown as multicellular aggregates larger than 100 μm for between 31–41 d (Fig. 1B–C). Specific growth factors and molecules are given in different combinations as differentiation progresses to recapitulate pancreatic development. This protocol generates cellular aggregates containing populations of cells with varying degrees of staining for C-peptide, which produced by the INS gene and cleaved away from the mature insulin peptide during processing, as well as other islet hormones, including glucagon (Fig. 1D).
Fig. 1.
Generation of SC-β cell aggregates. A. Schematic diagram of differentiation process, illustrating cell fate changes to generate SC-β cells. The entire procedure is done in cellular aggregates. B. Image of spinner flask approach used to grow and differentiate hESCs to SC-β cells. C. Micrograph of Stage 6 clusters stained with dithizone, a dye that stains β cells red, imaged under bright field. Scale bar = 500 μm. D. Immunostaining of Stage 6 cluster sectioned and stained for C-peptide, which is produced by β cells, and glucagon, which is produced by α cells. Both primary and secondary antibodies were used for the image on the left but only secondary antibodies for the image on the right. These images were taken with the same settings. Scale bar = 100 μm. E. Immunostaining of dispersed Stage 6 clusters (left) and ECs (right) plated for assessment for CD31, an EC marker. These images were taken with the same settings. Scale bar = 100 μm. F. Micrographs of unstained reaggregated Stage 6 clusters with or without the addition of ECs after 24 hr. Scale bar = 400 μm. G. Immunostaining of Stage 6 clusters reaggregated with ECs after 24 hr then dispersed and plated 24 hr for assessment. Scale bar = 150 μm. DE, definitive endoderm; PGT, primitive gut tube; PP1, pancreatic progenitor 1; PP2, pancreatic progenitor 2; EP, endocrine progenitor; AA, activin A; CHIR, CHIR9901; KGF, keratinocyte growth factor; RA, retinoic acid; Y, Y27632; LDN, LDN193189; PdbU, phorbol 12,13-dibutyrate; T3, triiodothyronine; Alk5i, Alk5 inhibitor type II; ESFM, enriched serum-free medium.
While this differentiation protocol produces SC-β cells, ECs are absent (Fig. 1E), in contrast to what is seen in native islets [14]. In order to develop a platform that enables study of SC-β cells and ECs, we first attempted to disperse the SC-β cell clusters our protocol normally generates, mix with a single-cell dispersion of ECs, and allow them to spontaneously reaggregate in a 6-well plate on an orbital shaker at 100 rpm, as we have used to previously reaggregated SC-β cell clusters [5]. The morphology of the resulting clusters was unaffected by the attempted inclusion of ECs (Fig. 1F). To check for the incorporation of ECs, we dispersed and plated the reaggregated clusters, then stained for C-peptide, to mark SC-β cells, and CD31, an endothelial cell marker (Fig. 1G). We observed little to no CD31+ cells, indicating this approach did not enable ECs to be incorporated with the SC-β cell clusters. This is likely due to death of the ECs during aggregation, which was not prevented by the presence of SC-β cell and other Stage 6 cells. Overall, we observed that hydrogel-free suspension-based aggregation did not result in significant assembly of SC-β cells with ECs.
3.2. Hydrogel platform enables SC-β cells and EC assembly
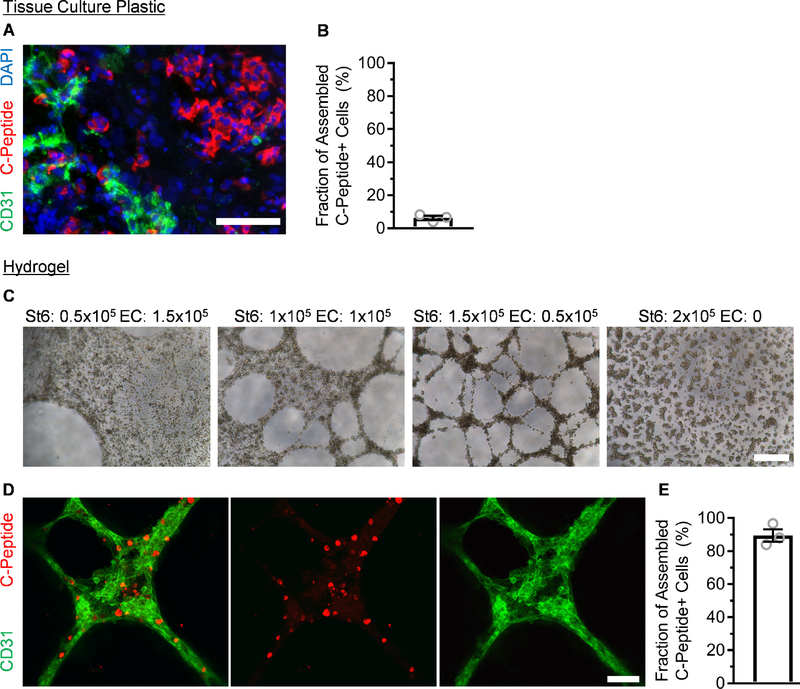
After observing the difficulty of facilitating C-peptide+ and CD31+ cell physical association with our standard cluster-based protocol, we turned to using Matrigel, which is a protein mixture derived from mouse sarcoma cells that consists in part of basement membrane extracellular matrix proteins. This material was chosen because it is widely availability and easy to use, using temperature to induce gelation. In addition, we chose to use standard, non-growth factor reduced Matrigel in the hopes of promoting assembly. As hESCs are commonly cultured on tissue culture plastic coated with dilute Matrigel, which does not result in a gel and instead provides a thin coating to promote attachment, we first attempted plating a single-cell dispersion of SC-β cells mixed with ECs on the bottom of a dilute Matrigel-coated tissue culture plate and assessed with immunostaining (Fig. 2A). While we observed both C-peptide+ and CD31+ cells, these populations tended to segregate away from each other, with only 6±1% of C-peptide+ cells touching a CD31+ cell (Fig. 2B). Next, we created slabs of undiluted Matrigel hydrogels and dispensed a mixture of single-cell dispersed SC-β cells and ECs at varying ratios on top. We observed assembly of cells after 24 hr (Fig. 2C). Both 1:1 and 3:1 ratios of SC-β cell to EC produced three-dimensional structures resembling tubule networks, and higher ratios of ECs tended to produce more sheet-like morphologies. ECs are likely secreting pro-migratory factors that attract SC-β cells to the network, since SC-β cells without ECs, while producing small aggregates, appeared fairly uniformly scattered across the hydrogel. This is also is interesting because this aggregation with ECs did not require the normal equipment used for SC-β cell culture and aggregation: Stirrer, shakers, and/or spinner flasks.
Fig. 2.
ECs and SC-β cells assemble on top of Matrigel hydrogel but not tissue culture plastic. A. Immunostaining of Stage 6 clusters mixed with ECs and plated on tissue culture plastic for assembly after 24 hr. Scale bar = 150 μm. B. Quantification of the fraction of C-peptide+ cells in contact with CD31+ cells using the tissue culture plastic approach. n=3. C. Bright field micrographs of varying ratios of ECs and SC-β cells plated on a slab of Matrigel hydrogel for assembly after 24 hr. Scale bar = 400 μm. D. En face image of immunostained organoid produced with a 3:1 ratio of SC-β cells and ECs after 24 hr. Scale bar = 100 μm. E. Quantification of the fraction of C-peptide+ cells in contact with CD31+ cells after 24 hr using the hydrogel approach. n=3.
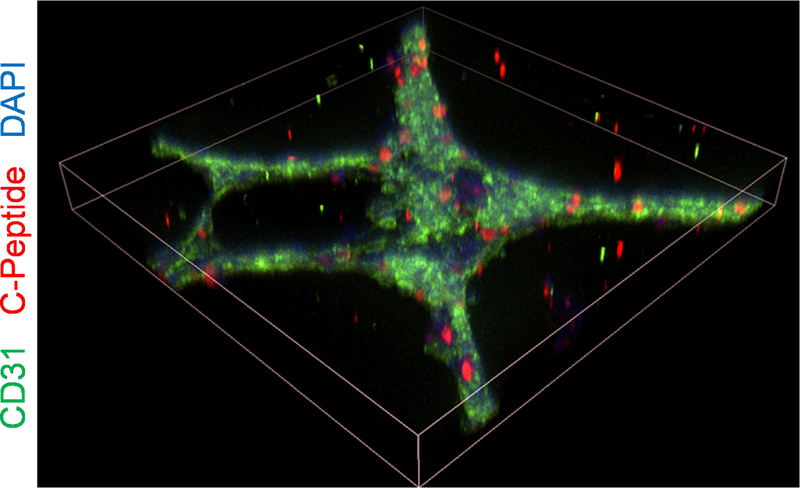
We whole-mount immunostained the resulting islet organoids made with the 3:1 ratio of SC-β cells to ECs and confirmed that the assembled tubule network was formed by the CD31+ and C-peptide+ cells (Fig. 2D). Most (84±4%) of C-peptide+ cells were incorporated into the aggregates (Fig. 2E), which is a significant increase (p<0.0001 by unpaired two-way t-test) compared to two-dimensional assembly (Fig. 2B). Confocal image construction confirmed that most C-peptide+ cells were incorporated into the assembly (Fig. 3). Taken together, these data demonstrate that three-dimensional assembly of already differentiated SC-β cells and ECs can be achieved using polymerized Matrigel.
Fig. 3.
Confocal image construction of islet organoid produced with 3:1 ratio of SC-β cells and ECs after 24 hr. Tilted view is constructed from three-dimensional z-stacks, and the shown dimensions are 636.4 × 636.4 × 93 μm.
3.3. Characterization of islet organoids
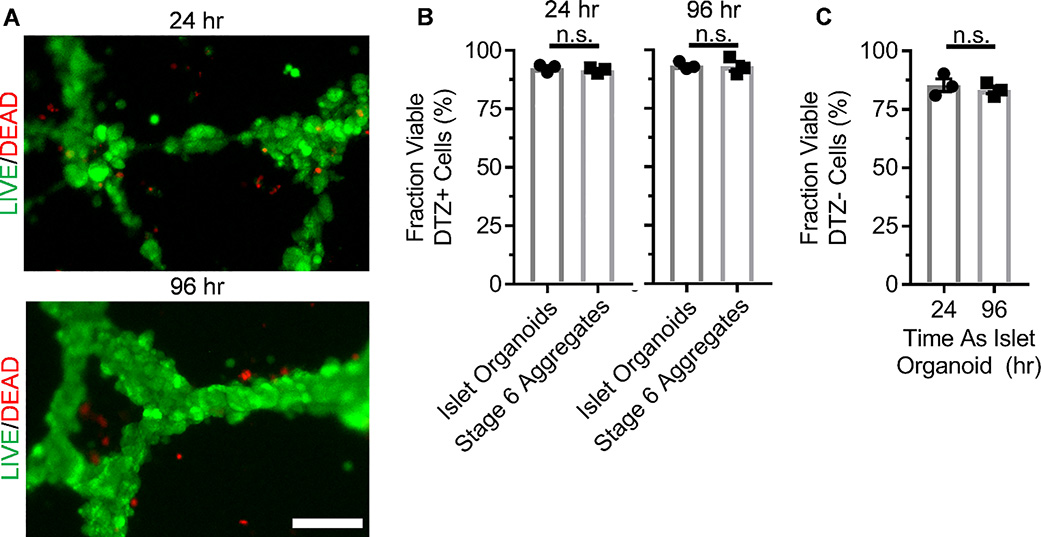
To characterize islet organoid assembly using our developed platform, we qualitatively assessed the viability of our constructs 24 and 96 hr after assembly using a Live/Dead Viability/Cytotoxicity Kit, which fluorescently stains live cells green and dead cells, defined as membrane-permeable, red (Fig. 4A). Minimal cell death was observed, which was mostly restricted to regions away from the cellular constructs. To quantify viability, we dispersed the organoids, stained with dithizone, a dye that stains β cells red, and trypan blue, a dye that stains dead (membrane permeable) cells blue, and quantified the fractions, comparing to stage 6 cells without ECs (Fig. 4B–C). Greater than 80% of dithizone+ cells and dithizone-, which would largely consist of ECs, cells were viable at both 24 and 96 hr from both organoids and Stage 6 aggregates, demonstrating that the platform did not negatively affect viability. These data show that our assembly platform enables expression of EC and islet endocrine markers while maintaining viability.
Fig. 4.
Viability assessment of ECs and SC-β cells assembly formed on Matrigel. A. En face image of organoids stained with viability dye indicating live (green) and dead (red) cells. B. Quantification of the fraction of DTZ+ cells, marking the SC-β cell population, that are viable as assessed by trypan blue staining. p>0.05 by two-way unpaired t-test. n=3. C. Quantification of the fraction of DTZ- cells that are viable as assessed by trypan blue staining. n.s. p>0.05 by two-way unpaired t-test. n=3.
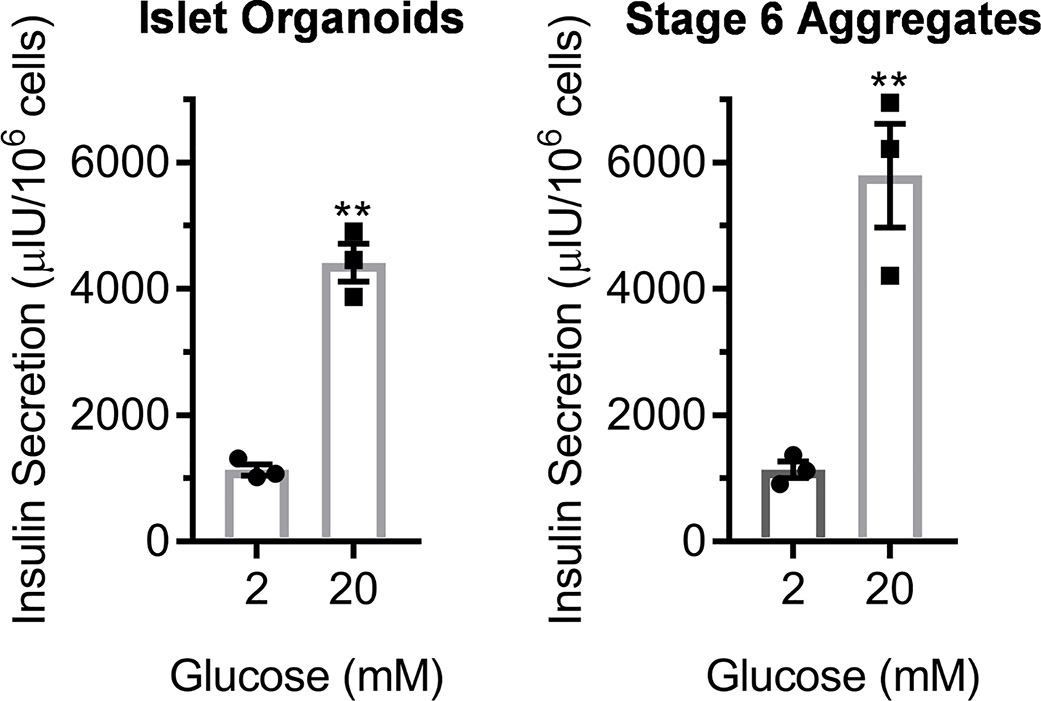
To evaluate the potential of this platform for islet organoid assembly for evaluating islet microenvironment parameters, we performed a glucose-stimulated insulin secretion assay of SC-β cell/EC assemblies (Fig. 5). The normal physiological function of pancreatic β cells in the body is to secrete insulin in response to high glucose stimulation. This in vitro assay involves treating cells first with low (2 mM) glucose for an hour, collecting the resulting supernatant, then subsequently treating cells with high (20 mM) glucose for an hour, collecting the resulting supernatant, and quantifying the amount of insulin released with ELISA. Testing three independent replicates of the islet organoid assembly revealed all three were robustly functional by secreting higher insulin at high glucose (p<0.01; two-way paired t-test). On average, insulin secretion increased by 3.9±0.3x by high glucose stimulation. This function was comparable to our Stage 6 aggregates. These data show that the assembled islet organoids are glucose-responsive and secrete insulin.
Fig. 5.
Glucose-stimulated insulin secretion assay of EC and SC-β cell assembly formed on Matrigel. 24 hr after (left) compared to Stage 6 aggregates containing SC-β cells without ECs (right). **p<0.01 by two-way paired t-test comparing low to high glucose. n=3.
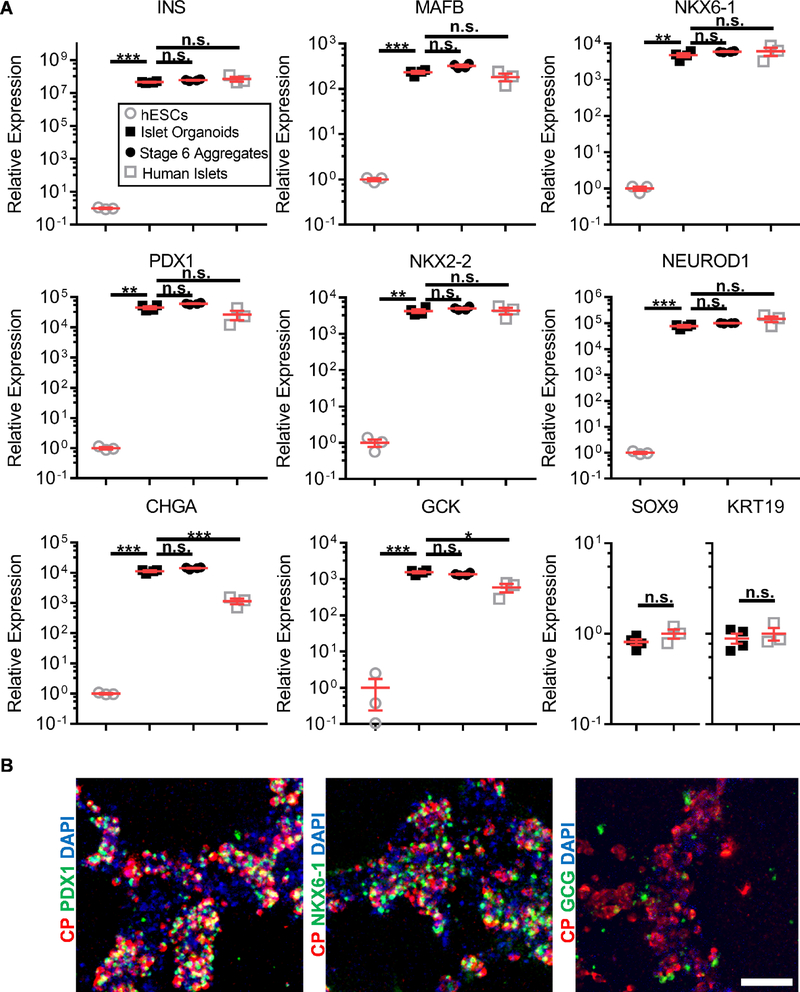
Finally, we evaluated our SC-β cell/EC assemblies by expression of β cell genes (Fig. 6A). Key genes associated with β cell identity, including insulin (INS), transcription factors (MAFB, PDX1, NKX6–1, NKX2–2, NEUROD1), and with function (CHGA, GCK) were all highly expressed in our assembled islet organoids compared to undifferentiated hESC controls and were overall comparable in expression with Stage 6 aggregates without ECs and human islets. CHGA and GCK were notable exceptions, with higher expression in islet organoids than human islets. Furthermore, we confirmed that the C-peptide+ cells co-expressed PDX1 and NKX6–1 and that other endocrine cells, indicated by being glucagon+, were present within the organoids (Fig. 6B). These data show that SC-β cell assemblies made with our platform maintain expression of β cell and islet markers.
Fig. 6.
Marker analysis of EC and SC-β cell assembly formed on Matrigel. A. Ten genes associated with the β cells and the pancreas were measured, comparing the islet organoids 24 hr after (n=4) to undifferentiated hESCs (n=3), Stage 6 aggregates containing SC-β cells without ECs (n=4), and human islets (n=3). n.s. p>0.05, *p<0.05, **p<0.01, and ***p<0.001 by two-way unpaired t-test. B. En face image of immunostained organoid. CP=C-Peptide. Scale bar = 100 μm.
4. Discussion
The islets of Langerhans are complex, multicellular tissues that are responsible for maintaining glucose tolerance within humans through their ability to sense glucose and secrete hormones. Islets consist of β cells and other endocrine cell types along with ECs. Despite the potential importance of ECs to islet function, facilitating direct interactions between ECs and already differentiated and functional SC-β cells has been difficult in vitro. Here we developed a platform that enables the assembly of already differentiated SC-β cells and other endocrine cells with ECs. The assembled heterogenous cell mixture were capable of undergoing glucose-stimulated insulin secretion, a key β cell functional feature, and expressed a panel of β cell genes that are associated with its identity and function. We encountered difficulties successfully assembling SC-β cells with ECs using other approaches, indicating that the conditions for this phenotype are limited. In particular, we observed that SC-β cells are capable of assembling with ECs when cultured on top of a slab of polymerized Matrigel but not on traditional tissue culture plastic coated with dilute Matrigel, highlighting how physical culture conditions can greatly influence the interaction between these two cell types. Importantly, this platform facilitates the study of potential improvements to SC-β cells and SC-islets maturation and function by studying and improving interactions with ECs.
Current protocols produce SC-β cells that resemble their in vivo counterparts in many important parameters but are still different in other aspects. While SC-β cells are capable of undergoing glucose-stimulated insulin secretion and controlling blood sugar levels in mice, how glucose-responsive the cells are and how much insulin the cells secrete is still lower than primary cadaveric human islets [4–6, 8, 15]. SC-β cells express many markers found in primary cadaveric human islets, but several genes associated with maturation continue to be under expressed, such as MAFA and UCN3 [5, 15]. We hope our reported platform for assembling key islet components enables future studies to identify the parameters to make more mature SC-β cells in tissue engineered islets by utilizing ECs, as this was not pursued in this study. A practical feature of our platform is that it can be achieved without specialized reagents, equipment, or training, which will facilitate future studies and is in contrast with current SC-β cell culture methodologies [5, 8].
Approaches have been developed in order to generate SC-β cell-containing aggregates that better resemble native islets. Optimization of the timing and combinations of soluble small molecules and growth factors have led to increases in differentiation yield [5]. Resizing of clusters has led to increased function both in vitro and in vivo [5, 6, 15, 16], in part by limiting hypoxia [16]. Sorting based on a transgenic reporter [6] or surface marker [15] has allowed further increases in the purity of SC-β cells to better define the cellular population present in aggregates. Combining 3D printing with fibrin gels has better enabled transplantation of resized SC-β cell clusters [16]. Our current work presents a hydrogel platform that now allows for the physical interaction of ECs with already differentiated SC-β cells that can take advantage of current and future improvements in SC-β cell differentiation and maturation approaches, which will bring stem cell technology closer to clinical translation and increase our understanding of β cell biology and diabetes pathology.
Prior studies that have investigated ECs combined them with pluripotent stem cells or pancreatic progenitors as they are differentiating to insulin-expressing cells, using the ECs as a differentiation factor, and observed benefits of the ECs in differentiation yield and insulin secretion [17–19]. However, a major difference in our study and development of the hydrogel platform compared to these prior EC studies is that we started with SC-β cells that we had already differentiated without using ECs using a chemical method [5]. In particular, we used our chemical differentiation factor approach that generates 73% C-peptide+ cells that secretes approximately 500 μIU of insulin per 105 cells while also displaying first and second phase insulin secretion and controlling diabetes in mice [5]. In contrast, Candiello et al. reported a system of combining ECs with differentiating hESCs [18] but only achieved insulin secretion per cell of 3–5 μIU of insulin per 105 cells, about 102 times lower than achieved with our new platform here containing ECs or with our reported chemical differentiation factor based-approach [5]. Our chemical differentiation factor based-approach possibly already provides the same or similar signaling that ECs provided in the prior studies [17–19]. Because of the high differentiation yields and function our SC-β cell chemical differentiation factor approach can already achieve, we were motivated to build our new cell assembly platform described here using these already differentiated and functional SC-β cells to enable development of improved SC-β cells and islets compared to both current ECbased and differentiation factor based-approaches.
While we did not find success with several approaches that we attempted, alternative methodologies could be developed to assemble ECs and SC-β cells. We recently used microwells to resize and enable embedding of SC-β cell aggregates into fibrin gels within a 3D-printed macroporous device before transplantation, but introduction of ECs was not studied [16]. The specific factors promoting assembly was not identified in this study. However, we do demonstrate that the physical culture conditions were crucial to SC-β cell/EC interactions. Specifically, only culture on top of Matrigel hydrogel slab supported physical interaction between the two cell types but not on top of traditional tissue culture polystyrene with a thin coat of Matrigel. Additional study of the successes and failures of islet organoid assembly could yield additional insights, such as the studying the role of growth factors and particular ECM proteins normally found in Matrigel in assembly success. As Matrigel contains laminin [20], laminin has been found to be beneficial for islets [21], and laminin promotes vascular network formation in scaffolds [22], further mechanistic studies into the role of laminin in the islet organoid assembly process is warranted. There are also likely alternative hydrogels or materials, including other basement membrane matrixes formulations, that would work in assembling islet organoids and reduce cost or non-biological materials [23].
5. Conclusions
Our findings show that assembly of already differentiated SC-β cells with ECs will only occur under specific culture conditions. Specifically, suspension aggregation into clusters or assembly on tissue culture plastic coated with dilute Matrigel were unable to induce interactions between SC-β cells and ECs. However, we observed that dispersing and plating SC-β cells with ECs on top of Matrigel induced the physical interaction between the two cell types. These assembled islet organoids were able to undergo glucose-stimulated insulin secretion and expressed a panel of β cell and other endocrine markers. Our described approach provides a platform for the study of key microenvironmental components for the development of tissue engineered islet for diabetes cell replacement therapy and drug screening.
Acknowledgements
This work was supported by the NIH (R01DK114233), JDRF Career Development Award (5-CDA-2017–391-A-N), Washington University Center of Regenerative Medicine, and startup funds from Washington University School of Medicine Department of Medicine. L.V.C. was supported by the NIH (R25GM103757). Microscopy was performed through the Washington University Center for Cellular Imaging (WUCCI), which is supported by Washington University School of Medicine, CDI (CDI-CORE-2015–505) and the Foundation for Barnes-Jewish Hospital (3770). The Washington University Diabetes Research Center (P30DK020579) provided support for the microscopy. We thank Madeleine Goedegebuure, Nicholas White, and Shriya Swaminathan for technical assistance. We thank Dr. Nathaniel J. Hogrebe for reading of this manuscript.
Footnotes
Disclosure Statement
L.V.C., J.S., and J.R.M. are inventors on patent filings for the stem cell technology.
Differentiation of insulin-producing cells and tissues from human pluripotent stem cells is being investigated for diabetes cell replacement therapies. Despite successes generating β cells, the cell type responsible for glucose-stimulated insulin secretion within the islets of Langerhans found in the pancreas, successful assembly with other non-endocrine cell types, particularly endothelial cells, has been technically challenging. The present study provides a platform for the assembly of endothelial cells with SC-β and other endocrine cells, producing islet organoids that are functional and express β cell markers, that can be used to study the islet microenvironment and islet tissue engineering.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ, Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes, Am J Transplant 12(6) (2012) 1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McCall M, Shapiro AM, Update on islet transplantation, Cold Spring Harb Perspect Med 2(7) (2012) a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA, Generation of stem cell-derived beta-cells from patients with type 1 diabetes, Nat Commun 7 (2016) 11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA, Generation of functional human pancreatic beta cells in vitro, Cell 159(2) (2014) 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, Millman JR, Acquisition of Dynamic Function in Human Stem Cell-Derived beta Cells, Stem cell reports 12(2) (2019) 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, Juang C, Li ML, Nguyen VQ, Giacometti S, Puri S, Xing Y, Wang Y, Szot GL, Oberholzer J, Bhushan A, Hebrok M, Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells, Nature cell biology 21(2) (2019) 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghazizadeh Z, Kao DI, Amin S, Cook B, Rao S, Zhou T, Zhang T, Xiang Z, Kenyon R, Kaymakcalan O, Liu C, Evans T, Chen S, ROCKII inhibition promotes the maturation of human pancreatic beta-like cells, Nat Commun 8(1) (2017) 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ, Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells, Nature biotechnology 32(11) (2014) 1121–33. [DOI] [PubMed] [Google Scholar]
- [9].Srivatsava AR, Shahan ST, Gutgesell LC, Velazco-Cruz L, Millman JR, Assessment of the in vitro function of human stem cell-derived β cells, bioRxiv (2019) 656785. [Google Scholar]
- [10].Pagliuca FW, Melton DA, How to make a functional beta-cell, Development (Cambridge, England) 140(12) (2013) 2472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kragl M, Lammert E, Basement membrane in pancreatic islet function, Adv Exp Med Biol 654 (2010) 217–34. [DOI] [PubMed] [Google Scholar]
- [12].Stendahl JC, Kaufman DB, Stupp SI, Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation, Cell Transplant 18(1) (2009) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thorne CA, Chen IW, Sanman LE, Cobb MH, Wu LF, Altschuler SJ, Enteroid Monolayers Reveal an Autonomous WNT and BMP Circuit Controlling Intestinal Epithelial Growth and Organization, Dev Cell 44(5) (2018) 624–633 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olsson R, Carlsson PO, The pancreatic islet endothelial cell: emerging roles in islet function and disease, Int J Biochem Cell Biol 38(5–6) (2006) 710–4. [DOI] [PubMed] [Google Scholar]
- [15].Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gurtler M, Pagliuca FW, Peterson QP, Melton DA, Charting cellular identity during human in vitro beta-cell differentiation, Nature 569(7756) (2019) 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Song J, Millman JR, Economic 3D-printing approach for transplantation of human stem cellderived beta-like cells, Biofabrication 9(1) (2016) 015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jaramillo M, Mathew S, Mamiya H, Goh SK, Banerjee I, Endothelial cells mediate islet-specific maturation of human embryonic stem cell-derived pancreatic progenitor cells, Tissue Eng Part A 21(1–2) (2015) 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Candiello J, Grandhi TSP, Goh SK, Vaidya V, Lemmon-Kishi M, Eliato KR, Ros R, Kumta PN, Rege K, Banerjee I, 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform, Biomaterials 177 (2018) 27–39. [DOI] [PubMed] [Google Scholar]
- [19].Talavera-Adame D, Woolcott OO, Ignatius-Irudayam J, Arumugaswami V, Geller DH, Dafoe DC, Effective endothelial cell and human pluripotent stem cell interactions generate functional insulin-producing beta cells, Diabetologia 59(11) (2016) 2378–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR, Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma, Biochemistry 21(24) (1982) 6188–93. [DOI] [PubMed] [Google Scholar]
- [21].Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E, The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation, Dev Cell 10(3) (2006) 397–405. [DOI] [PubMed] [Google Scholar]
- [22].Stamati K, Priestley JV, Mudera V, Cheema U, Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake, Exp Cell Res 327(1) (2014) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Youngblood RL, Sampson JP, Lebioda KR, Shea LD, Microporous scaffolds support assembly and differentiation of pancreatic progenitors into beta-cell clusters, Acta Biomater (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]