Abstract
Background and Objective:
Sleep is an emerging risk factor for cardiovascular disease (CVD) that is not currently included as a cardiovascular health (CVH) metric in the American Heart Association’s Life’s Simple 7 (AHA LS7). Our objective was to evaluate the association of sleep with CVH in women and examine differences by menopausal status and race/ethnicity.
Methods:
Baseline data from the Columbia University AHA Go Red for Women Strategically Focused Research Network were examined. Sleep habits were self-reported using validated questionnaires. A CVH score was computed using AHA LS7 criteria for smoking, diet, physical activity, BMI, blood pressure(BP), total cholesterol, and fasting glucose. Women received a score of 2(ideal), 1(intermediate), or 0(poor) based on their level of meeting each AHA LS7 metric. Multivariable-adjusted regression models were used to evaluate associations of sleep with meeting overall and individual CVH metrics.
Results:
The analytical sample consisted of n=507 women (62% minority/Hispanic, mean age:37y). Participants with adequate sleep duration(≥7h), good sleep quality, no insomnia nor snoring, and low risk for OSA were more likely to meet >4 of the AHA LS7 metrics(p<0.01). Poorer sleep quality (β=−0.08,p=0.002), higher insomnia severity(β=−0.05,p=0.002), snoring(β=−0.77,p=0.0001), and higher risk for OSA(β=−1.63,p<0.0001) were associated with poorer CVH. Insomnia, snoring, and high OSA risk were associated with 69% to >300% higher odds of having poor CVH (p≤0.03). Associations were stronger in post-menopausal and racial/ethnic minority women.
Conclusions:
Better sleep habits were associated with more favorable CVH among women, suggesting that there may be benefit in incorporating sleep assessment into CVD risk screening.
Keywords: Sleep, AHA Life’s Simple 7, Cardiovascular Health, Menopausal Status, Race/Ethnicity
INTRODUCTION:
The American Heart Association’s (AHA) Life’s Simple 7 (AHA LS7) is a measure of cardiovascular health (CVH) that permits tracking the health status of populations and individuals in relation to the 2020 strategic goal to improve CVH among all Americans.1 This construct encompasses both health behaviors and health factors that are established risk factors for cardiovascular disease (CVD) such as smoking, diet, physical activity (PA), body mass index (BMI), blood pressure (BP), total cholesterol, and fasting glucose.1 A score based on the seven metrics of CVH represents the extent of meeting the established criteria for CVD risk reduction. Indeed, a higher AHA LS7 composite score indicative of more favorable CVH has been linked to lower incidence of cardiovascular outcomes, such as heart failure and stroke, and other non-cardiovascular outcomes.2–7
Sleep is an important pillar of health and suboptimal sleep, both in duration and quality, has been associated with increased risk of CVD, stroke, diabetes and hypertension8, with suggestive evidence of potentially stronger associations among women.9 Women may be particularly vulnerable to sleep problems due to life events including pregnancy, motherhood, and menopause, as well as lifestyle factors, circadian rhythm abnormalities, medical conditions, and changing reproductive hormone levels across the female life course.10,11 Based on 2005 National Health and Nutrition Examination Survey (NHANES) data, women are more likely than men to have a very short sleep duration (≤5 h/night).12 Furthermore, a recent examination of secular trends in insomnia using 2002–2012 NHANES, showed that, although prevalence rates have increased in both men and women over time, approximately one quarter of women have insomnia, and the prevalence of insomnia was consistently greater in women compared to men over time.13
In a 2016 AHA statement8, insufficient sleep, poor quality sleep, obstructive sleep apnea (OSA) and insomnia were linked to increased risk of obesity, type 2 diabetes, hypertension, coronary heart disease and stroke. However, despite the observed associations between sleep and lifestyle mediators of CVD risk factors, namely diet and PA, as well as clinical biomarkers of CVH, sleep has not been included in formal CVD prevention guidance. The purpose of this analysis was to examine associations of sleep characteristics with meeting the AHA LS7 in a diverse sample of women and to explore potential differences in these associations by menopausal status and race/ethnicity. To our knowledge, this represents the first investigation into sleep characteristics in relation to overall CVH in a diverse sample of women encompassing different life stages. This research may provide important information on the role of sleep habits in achieving favorable CVH and may inform public health efforts to promote CVH, particularly among women.
PARTICIPANTS AND METHODS
Design and Participants
This was a cross-sectional analysis using data from the baseline examination of an ongoing community-based prospective cohort of n=507 women, aged 20–79y, recruited as part of the AHA Go Red for Women (GRFW) Strategically Focused Research Network (SFRN) population study at Columbia University Irving Medical Center (CUIMC). The goal of the AHA GRFW SFRN award at CUIMC is to investigate associations of sleep patterns with cardiometabolic risk factors in a community-based cohort of women. Participants were a community-based sample of women living in the neighboring communities. Participants included: 1) family members and friends of patients hospitalized at a large urban medical center in Northern Manhattan (Columbia University Irving Medical Center/New York Presbyterian Hospital) and visitors to the medical center (10%), 2) women recruited at neighborhood community centers (~1%), 3) women recruited using media brochures and recruitment flyers (19%), 4) women who responded to online postings (40%), 5) participant friend referrals and referrals from other studies (23%), and 6) referrals from affiliated physicians (7%). Pregnant women were excluded. Bilingual study personnel recruited both English- and Spanish-speaking women, and all study forms were available in English and Spanish. The study was approved by the Institutional Review Board of CUIMC and was performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. All participants gave written informed consent prior to their inclusion in the study.
Assessment of Sleep
Sleep duration and quality were assessed using the validated Pittsburgh Sleep Quality Index (PSQI).14 The PSQI measures seven domains of sleep: subjective sleep quality, sleep onset latency (time to fall asleep), duration, efficiency and disturbances, use of sleep medication, and daytime dysfunction over the last month to distinguish between poor and good sleepers. A global sum >5 indicates poor sleep quality. The Berlin Sleep Questionnaire was used to assess snoring status, using the response to the question “Do you snore?”. This questionnaire was also used to ascertain the prevalence of a high-risk phenotype for OSA, using responses to questions about snoring, daytime somnolence, hypertension and BMI, as previously described.15 The presence of insomnia was ascertained using the Insomnia Severity Index (ISI) Questionnaire.16 The ISI ranges between 0–28 and based on their responses to the ISI, participants were classified into the following categories: 1) no clinically significant insomnia (ISI: 0–7), 2) sub-threshold insomnia (ISI: 8–14), 3) clinical insomnia with moderate severity (ISI: 15–21), or 4) severe clinical insomnia (ISI: 22–28). Participants who reported having sub-threshold insomnia, moderate or severe clinical insomnia were considered to have some level of insomnia.
Assessment of Modifiable Lifestyle Behaviors and Clinical Risk Factors
Height was measured using a standardized stadiometer and weight was obtained using a research grade scale. BMI was calculated using the standard equation (BMI=weight(kg)/height(m2)). Systolic BP and diastolic BP were assessed using a hospital-grade automated BP monitor using a standard protocol (Omron® 5 Series Upper Arm (BP742)).17 Fasting blood samples were collected and analyzed at the CUMC Irving Institute Biomarkers Core Laboratory to assess fasting blood glucose and total cholesterol levels.
The International Physical Activity Questionnaire, a surveillance tool developed and tested for adults.18,19 was used to assess PA levels and sedentary behaviors. Habitual dietary intake over the past year was assessed using the validated Block Brief 2000 food frequency questionnaire20, which utilizes the USDA Food and Nutrient Database for Dietary Studies to estimate dietary intake.20 A standardized health questionnaire was used to evaluate smoking habits. Smoking status was ascertained from responses to the following the questions: “What is your smoking status?” and “When was your last cigarette?”. Participants responded whether they were a current, former, or never smoker, and former smokers reported whether their last cigarette was smoked <6mo, 6mo to 2y, 2–10y, or >10y ago. The standardized health questionnaire was also used to evaluate socio-demographic information including age, race/ethnicity, education, and medical history. Menopausal status was ascertained based on responses to the fosllowing questions: “Have you had a menstrual period in the last 12 months?” and “Have you ever had any of the following surgeries or procedures: a) uterus removed/hysterectomy, b) one ovary removed, c) two ovaries removed?”. Women who did not have a menstrual period in the last 12 months were considered post-menopausal.
Operationalization of the AHA Life’s Simple 7 Score
Published definitions1 used to assess AHA LS7 were adapted based on the data available in this study and consistently with previous literature on the AHA LS7 score as shown in Table 1.2–4,21 The individual components for the AHA LS7 score (smoking, diet, PA, BMI, BP, total cholesterol, and fasting glucose) were categorized as ideal (2 points), intermediate (1 point) or poor (0 point). The individual seven scores were then summed to create the total AHA LS7 score ranging from 0 to 14 such that 0 represented not meeting any CVH criteria and 14 represented meeting all CVH criteria.
Table 1.
Operationalization of the AHA Simple 7s Score*
| AHA Simple 7s Guideline | Operationalization | Scoring |
|---|---|---|
| Smoking | Never or former >2 year Former ≤2 year Current |
2 1 0 |
| Diet† | Meets 4 to 5 recommendations Meets 2 to 3 recommendations Meets 0 to 1 recommendations |
2 1 0 |
| Physical Activity‡ | ≥150 min/wk moderate intensity or ≥75 min/wk vigorous intensity or ≥150 min/wk moderate + vigorous intensity 1 to 149 min/wk moderate intensity or 1 to 74 min/wk vigorous intensity or 1 to 149 min/wk moderate + vigorous intensity No moderate or vigorous activity |
2 1 0 |
| BMI* | <25 kg/m2 25–29.9 kg/m2 ≥30 kg/m |
2 1 0 |
| Blood Pressure* | SBP<120 and DBP<80 mmHg untreated SBP: 120–129 or DBP: <80 mmHg or treated to ideal level SBP ≥130 or DBP ≥80 mmHg |
2 1 0 |
| Total Cholesterol | <200 mg/dl 200–239mg/dl ≥240 mg/dl |
2 1 0 |
| Fasting Glucose | <100 mg/dl 100–125 mg/dl ≥126 mg/dl |
2 1 0 |
AHA: American Heart Association; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
The diet score was derived from the Block Brief Food Frequency Questionnaire based on 5 healthy diet components, each worth 1 point. Scoring for this component was adapted from the American Heart Association definitions of the 5 healthy diet criteria, which were “(1) fruits and vegetables: ≥4.5 cups per day; (2) fish: ≥two 3.5-oz servings per week (preferably oily fish); (3) fiber-rich whole grains (≥1.1 g of fiber per 10 g of carbohydrate): ≥three 1-oz-equivalent servings per day; (4) sodium: <1500 mg per day; (5) sugar-sweetened beverages: ≤450 kcal (36 oz) per week.”
Vigorous activity and moderate physical activity were ascertained from the International Physical Activity Questionnaire. Participants were asked how many times per week they engage in vigorous and moderate physical activity and for how many hours and minutes.
Statistical Analysis
All data were collected on standardized forms, entered into a secure RedCap database, and exported to SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) for statistical analyses. Participant demographic, lifestyle and medical characteristics were described using mean ± standard deviation (SD) for continuous variables and frequencies for categorical variables. We computed the mean and median AHA LS7 score and the percentage of participants with high (11–14), moderate (9–10), and low scores (0–8) as defined previously.21 T-tests were used to compare the mean AHA LS7 scores by sleep duration (sufficient: ≥7 vs. insufficient: <7 h/night)8, sleep quality (poor: PSQI>5 vs. good: PSQI≤5), insomnia (ISI≥8 vs. ISI<8), snoring (yes vs. no), OSA risk (high-risk vs. low-risk). Fisher’s Exact test used to examine sleep duration (sufficient: ≥7 vs. insufficient: <7 h/night), sleep quality (poor: PSQI>5 vs. good: PSQI≤5), insomnia (ISI≥8 vs. ISI<8), snoring (yes vs. no), OSA risk (high-risk vs. low-risk) in relation to meeting >4 of the AHA LS7 metrics (AHA LS7 score: 9–14 vs. 0–8) as well as meeting vs. not meeting (ideal vs. intermediate or poor) the individual AHA LS7 criteria (smoking, diet, PA, BP, BMI, cholesterol and glucose).
Multivariable-adjusted linear and logistic regression models were used to examine associations between sleep characteristics and the AHA LS7 score and its components. Sleep duration (hours) and quality (PSQI score) and insomnia (ISI) were evaluated as continuous and categorical variables (sleep duration ≥7h vs. <7h; sleep quality: good vs. poor; insomnia: none vs. sub-threshold, moderate or severe). Snoring (yes vs. no) and risk for OSA (high-risk vs. low-risk) were assessed as categorical variables. The AHA LS7 score was evaluated as a continuous variable in linear regression models and as a binary variable (moderate to high CVH: 9–14 vs. low CVH: 0–8) in logistic regression models. The component scores were examined as binary variables (meeting ideal criterion for CVH metric (i.e. component score=2) vs. not meeting ideal criterion for CVH metric (i.e. component score=0 or 1)) in logistic regression models. Models were adjusted for age (years), race/ethnicity (non-Hispanic white vs. Hispanic or racial minority), education (college or more vs. less than college), health insurance (yes vs. no), nativity (born in US vs. foreign-born) and menopausal status (pre-menopausal vs. post-menopausal).
Given that poor sleep characteristics may cluster, we created a composite sleep score based on having short sleep duration, poor sleep quality, high risk for OSA, and insomnia. Women who had 3–4 of these sleep problems received a score of 0, while those who had 1–2 and 0 sleep problems received a score of 1 and 2, respectively. We then examined the three-category sleep score (1 vs. 2 and 0 vs. 2) and a binary sleep score (0–1 vs. 2) in relation to the AHA LS7 score in multivariable-adjusted logistic regression models to determine how the clustering of poor sleep characteristics influences CVH.
We also tested for interactions of sleep characteristics with menopausal status and race/ethnicity. A multiplicative term was introduced for these potential interactions in each model. In exploratory analyses, results were stratified by menopausal status and by race/ethnicity with the caveat of limited power. Stratified analyses were also adjusted for age, education, health insurance, and nativity. A p-value<0.05 was considered significant for all analyses.
RESULTS
Descriptive Characteristics of Study Population
Descriptive characteristics of the study population at baseline are shown in Table 2. The mean age was 37±16y and about one third of the women were postmenopausal. This was a racially and ethnically diverse sample with more than half identifying as Hispanic ethnicity (28.6%) and/or racial minority (43%). Participants reported engaging in moderate-to-vigorous intensity PA for ~5h/wk. Less than one quarter of the study sample (22.7%) reported being a current or former smoker. Approximately half of the women had a normal BMI, while 30.2% and 19.0% were in the overweight and obese BMI categories, respectively; additionally, 4% had diabetes, 31.8% had hypertension, and 20.3% had hypercholesterolemia.
Table 2.
Descriptive Characteristics of Study Population at Baseline (N=507)
| Mean ± SD/ Percentage (N) | |
|---|---|
| Demographic Characteristics | |
| Age (years) | 37 ± 16 |
| Race White Black American Indian or Alaskan Native Native Hawaiian or Pacific Islander Asian Other |
57% (289) 19.9% (101) 0.2% (1) 0.2% (1) 18.7% (95) 3.9% (20) |
| Hispanic Ethnicity | 28.6% (145) |
| Married | 29.2% (148) |
| Have Health Insurance | 76% (385) |
| Employed/Student | 86.6% (433) |
| Education ≥ College | 77% (389) |
| Born In USA | 64% (323) |
| Clinical/Medical Characteristics | |
| Postmenopausal | 28.8% (146) |
| History of Chronic Disease Diabetes Hypertension Hypercholesterolemi a |
42.6%(216) 4% (22) 31.8% (161) 20.3% (103) |
| BMI (kg/m2)* | 26.0 ± 5.7 |
| Overweight or obese BMI | 52% (263) |
| Waist Circumference>35 inches | 42.2% (214) |
| Systolic BP (mmHg)* | 117.4 ± 14.3 |
| Diastolic BP (mmHg)* | 73.0 ± 10.8 |
| Fasting Glucose (mg/dl) | 88.9 ± 17.7 |
| Total Cholesterol (mg/dl) | 182.2 ± 36.4 |
| Total AHA Simple 7s Score | 10.2 ± 2.3 |
| Sleep Habits | |
| Sleep Duration (hours/night) | 6.8 ± 1.2 |
| Sleep Duration<7 hours | 43.2% (219) |
| Poor Sleep Quality (PSQI>5)* | 51% (257) |
| Insomnia (Somewhat, Moderate, Severe) | 37.8% (189) |
| High-Risk for OSA* | 17.0% (86) |
| Reported Snoring | 30% (152) |
| Lifestyle Habits | |
| Current or Former Smoker | 22.7% (115) |
| Meets 5 AHA LS7 diet guidelines* | 10.2% (97) |
| Moderate/vigorous PA (min/week)* | 281.5 ± 556.8 |
AHA LS7: American Heart Association Life Simple 7; BMI: body mass index; BP: blood pressure; OSA: obstructive sleep apnea; PA: physical activity; PSQI: Pittsburg Sleep Quality Index
An assessment of sleep habits revealed that the mean nightly sleep duration in this sample was 6.8 ± 1.2 h/night and 43.2% of women reported sleeping <7 h/night. Half of the sample reported having poor sleep quality (51.0%) and 37.8% reported having sub-clinical, moderate or severe insomnia. The prevalence of a high-risk profile for OSA (including snoring and other factors) was 17.0% whereas the prevalence of snoring was 30.0%. In addition, 63% of the women exhibited at least one of these poor sleep characteristics, to a maximum of 4. The clustering of poor sleep characteristics was such that 25% and 38% of our sample reported 3–4 and 1–2 sleep problems, respectively.
AHA Life Simple 7s in this Population
The mean and median AHA LS7 scores in this population were 10.2 and 10 (range: 3 to 14). Approximately 22.7%, 28.6% and 48.7% of participants had low, moderate and high AHA LS7 scores, respectively, suggesting that the majority of this sample met >4 of the AHA LS7 guidelines. Extent of meeting the individual health behaviors metrics of AHA LS7 was also examined. Three quarters of the sample met the metric for smoking (77.5%), and half met the metric for PA (50.3%). On the other hand, only 20.2% of the sample met the ideal criterion for diet, and approximately two thirds of the sample only met 2–3 of the AHA dietary guidelines (63.4% in intermediate group). When health factors were examined, more than half of the sample had normal BMI (51.0%) and BP (55.8%); 70.6% and 88.9% had normal fasting cholesterol and glucose, respectively, and were therefore classified into the ideal category for these AHA LS7 metrics.
Sleep Characteristics in relation to the AHA LS7 Score and its Components
Women who slept ≥7 versus <7 h/night had significantly higher AHA LS7 scores (10.6 vs. 9.6;p<0.0001)(Table 3). Women with good sleep quality also had higher AHA LS7 scores compared to women with poor sleep quality (10.4 vs. 9.9;p=0.010). Participants who reported sub-threshold, moderate, and severe insomnia had significantly lower AHA LS7 scores compared to those who reported none (9.7 vs. 10.5;p<0.0001). Similarly, women who snored and those with a high-risk profile for OSA had significantly lower AHA LS7 scores (9.2 vs. 10.6;p<0.0001 and 8.2 vs. 10.6;p<0.0001 for snoring and OSA-risk, respectively).
Table 3.
| Sleep Characteristics | Mean Score (SD) | P-value | |
|---|---|---|---|
| Sleep Duration | <7 hours/night | ≥7 hours/night | |
| 9.6 ± 2.4 | 10.6 ± 2.1 | <0.0001 | |
| Sleep Qualitya | Poor (PSQI>5) | Good (PSQI≤5) | |
| 9.9 ± 2.4 | 10.4 ± 2.1 | 0.01 | |
| Insomniaa | Sub-threshold, moderate, severe insomnia (ISI≥8) | None (ISI<8) | |
| 9.7 ± 2.5 | 10.5 ± 2.0 | <0.0001 | |
| Snoring | Yes | No | |
| 9.2 ± 2.3 | 10.6 ± 2.1 | <0.0001 | |
| OSAa | High-Risk | Low-Risk | |
| 8.2 ±1.9 | 10.6 ± 2.1 | <0.0001 | |
PSQI: Pittsburg Sleep Quality Index, ISI: Insomnia Severity Index; OSA: obstructive sleep apnea
T-tests were used to compare the mean AHA LS7 score by categories of sleep characteristics
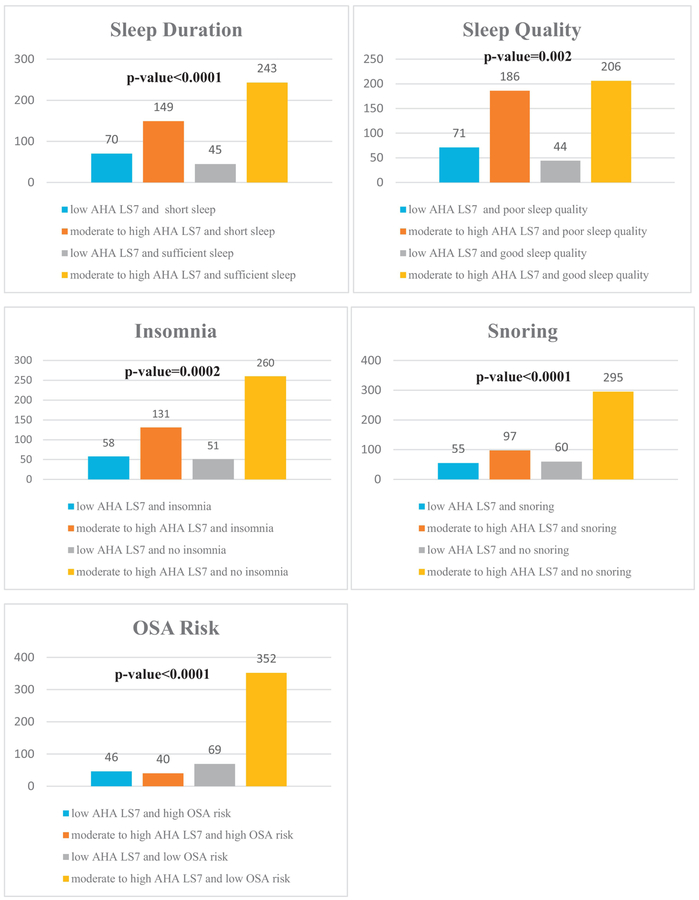
When univariate associations were examined using Fisher’s exact test (Figure 1), participants who slept ≥7 versus <7 h/night were more likely to meet >4 of the AHA LS7 metrics (AHA LS7 score of 9–14 i.e. have a moderate to high CVH)(p<0.0001). Participants were also more likely to meet >4 of the AHA LS7 metrics if they reported good sleep quality (p=0.002), no insomnia (p=0.0002) or snoring (p<0.0001) and had a low vs. high risk for OSA (p<0.0001). When sleep characteristics were evaluated in relation to individual AHA LS7 components, participants who reported sleeping ≥7 h/night, good sleep quality, no insomnia, no snoring, and low OSA risk were more likely to be in the ideal category for BMI (p≤0.05) and BP (p≤0.001). Those with sleep duration ≥7h/night, no insomnia or snoring, and low OSA risk were also more likely to be in the ideal category for the glucose criterion (p≤0.03). Snoring and high OSA risk were additionally associated with being less likely to be in the ideal category for the cholesterol (p≤0.03) and smoking (p≤0.003) metrics; only high OSA risk was associated with lower likelihood of adhering to the PA criterion (p=0.03).
Figure 1. Univariate Associations of Sleep Characteristics with the AHA LS7 Score.
This figure displays results from Fisher’s exact test to examine whether the number of participants with a low American Heart Association Life Simple 7 score (0–8) versus a moderate or high score (9–14) varies significantly across the categories of sleep characteristics. In general, short sleepers (<7 h/night) and those with poor sleep quality, insomnia, snoring and a high risk of obstructive sleep apnea were more likely to have a low AHA LS7 score.
In multivariable-adjusted linear regression models (Table 4), there was a borderline significant association between longer sleep duration and a higher AHA LS7 score (β=0.14;p=0.057). Poorer sleep quality (β=−0.08;p=0.002) and more severe insomnia (β=−0.05;p=0.002) were associated with a lower AHA LS7 score indicative of poorer CVH. Snoring and a high vs. low OSA risk were both associated with a lower AHA LS7 score (β=−0.77;p=0.0001 and β=−1.63;p<0.0001, respectively). In logistic regression models (Table 4), sleeping <7 vs. ≥7 h/night and poor vs. good sleep quality were not significantly associated with odds of having a poor AHA LS7 score (AHA LS7 score: 0–8), though the OR>1 indicative of greater odds were observed. In contrast, insomnia was significantly associated with 69% greater odds of having a poor AHA LS7 score (OR(95%CI): 1.69(1.05–2.71)). Similarly, snoring and high vs. low OSA risk were associated with 87% and >3-fold greater odds of having a poor AHA LS7 score (OR(95%CI): 1.92(1.06–3.47) and 4.60(2.4–8.66), respectively).
Table 4.
Multivariable-Adjusted Linear and Logistic Regression Models for Associations of Sleep Characteristics with the AHA LS7 Score (n=507)*
| Linear Model†, § | Logistic Model‡, § | ||||
|---|---|---|---|---|---|
| Sleep Characteristics | B(SE) | p-value | Sleep Characteristics | OR (95%CI) | p-value |
| Sleep Duration (Hours) | 0.14 (0.08) | 0.057 | Sleep Duration (<7 vs. ≥7h) | 1.60 (0.99–2.60) | 0.06 |
| Sleep Quality (PSQI score) | −0.08 (0.02) | 0.002 | Sleep Quality (PSQI >5 vs. ≤5) | 1.51 (0.93–2.45) | 0.095 |
| Insomnia Severity Index | −0.05 (0.02) | 0.002 | Insomnia (Yes vs. No) | 1.69 (1.05–2.71) | 0.031 |
| Snore (Yes vs. No) | −0.77 (0.20) | 0.0001 | Snore (Yes vs. No) | 1.87 (1.14–3.06) | 0.013 |
| OSA Risk (High vs. Low) | −1.63 (0.24) | <0.0001 | OSA Risk (High vs. Low) | 3.89 (2.23–6.81) | <0.0001 |
OSA: obstructive sleep apnea; PSQI: Pittsburg Sleep Quality Index
Results of linear regression models represent the increase in the AHA LS7 score per one-hour increase in sleep duration, per one unit increase in the PSQI or ISI scores, for snoring vs. no snoring, and for high vs. low risk of OSA
Logistic regression models examine the odds of having a poor AHA LS7 score (0–8) by category of sleep characteristics
Linear and logistic regression models are adjusted for age, race/ethnicity, education, health insurance, employment, menopausal status, and nativity (born in USA)
In sensitivity analyses, we examined the clustering of sleep problems in relation to the AHA LS7 score. In multivariable-adjusted logistic regression models, women who had 1–4 versus 0 poor sleep characteristics had 2-fold higher odds of having poor CVH (OR(95%CI: 2.00(1.05–3.82)). Women who had 3–4 poor sleep characteristics were particularly prone to having poor CVH (OR(95%CI): 2.79(1.37–5.69) compared to women with adequate sleep duration and no sleep problems.
In multivariable logistic regression models that evaluated sleep characteristics in relation to meeting individual AHA LS7 metrics, null results were observed for smoking, diet, PA, cholesterol and glucose (data not shown). However, sleeping <7 h/night was associated with 91% higher odds of having BMI ≥25kg/m2 (OR(95%CI): 1.91(1.28–2.85)). Snoring and high risk OSA risk were associated with 2-fold and >8-fold higher odds of having an overweight or obese BMI (OR(95%CI): 2.00(1.29–3.11) and 8.41(3.99–17.74), respectively) and with 83% and ~3-fold higher odds of having BP ≥130/80 mmHg (OR(95%CI): 1.83(1.16–2.88) and 2.81(1.54–5.11), respectively).
Sleep Characteristics in relation to the AHA LS7 Score by Menopausal Status
A statistically significant interaction was observed for all sleep characteristics with menopausal status (p interaction <0.001). In exploratory analyses stratified by menopausal status, only snoring and a high risk for OSA were associated with a lower AHA LS7 score among pre-menopausal women, in linear regression models (β=−0.88;p<0.0001 and β=−1.78;p<0.0001, respectively)(Table 5). However, in post-menopausal women, every one-hour increase in sleep duration was associated with a higher AHA LS7 score (β=0.33; p=0.013) and therefore with more favorable CVH. Poorer sleep quality (β=−0.11;p=0.004) and more severe insomnia (β=−0.07;p=0.006) were associated with a lower AHA LS7 score. A high vs. low OSA risk, but not snoring, was associated with a lower AHA LS7 score (β=−1.35;p=0.0004).
Table 5.
Multivariable-Adjusted Linear Regression Models for Associations of Sleep Characteristics with the AHA LS7 Score Stratified by Race/Ethnicity and by Menopausal Status (n=507)*, †
| Racial/Ethnic Minority (n=311)‡ | Non-Hispanic White (n=196)‡ | Pre-menopausal (n=361)§ | Post-menopausal (n=146)§ | |||||
|---|---|---|---|---|---|---|---|---|
| B(SE) | p-value | B(SE) | p-value | B(SE) | p-value | Β(SE) | p-value | |
| Sleep Duration (Hours) | 0.19 (0.09) | 0.038 | 0.19 (0.12) | 0.122 | 0.06 (0.09) | 0.517 | 0.33 (0.13) | 0.013 |
| Sleep Quality (PSQI score) | −0.13 (0.03) | 0.0001 | −0.04 (0.04) | 0.255 | −0.05 (0.03) | 0.169 | −0.11 (0.04) | 0.004 |
| Insomnia Severity Index | −0.06 (0.02) | 0.001 | −0.04 (0.02) | 0.133 | −0.03 (0.02) | 0.127 | −0.07 (0.02) | 0.006 |
| Snore (Yes vs. No) | −1.14 (0.24) | <0.0001 | −0.06 (0.31) | 0.834 | −0.88 (0.22) | <0.0001 | −0.36 (0.36) | 0.333 |
| OSA Risk (High vs. Low) | −2.03 (0.27) | <0.0001 | −0.68 (0.43) | 0.119 | −1.78 (0.30) | <0.0001 | −1.35 (0.37) | 0.0004 |
OSA: obstructive sleep apnea; PSQI: Pittsburg Sleep Quality Index
Results of linear regression models represent the increase in the AHA LS7 score per one-hour increase in sleep duration, per one unit increase in the PSQI or Insomnia Severity Index, for snoring vs. no snoring, and for high vs. low risk of OSA
Models are adjusted for age, education, insurance, employment, menopausal status, and nativity (born in USA)
Models are adjusted for age, race/ethnicity, education, insurance, employment, and nativity (born in USA)
Sleep Characteristics in relation to the AHA LS7 Score by Race/Ethnicity
A statistically significant interaction was observed for all sleep characteristics with race/ethnicity (p interaction <0.05). In exploratory analyses stratified by race/ethnicity, associations of sleep characteristics with the AHA LS7 score were not significant in non-Hispanic white women (Table 5). However, in Hispanic and racial minority women, longer sleep duration was associated with higher AHA LS7 scores (β=0.19;p=0.038), while poorer sleep quality (β=−0.13;p=0.0001) and greater insomnia severity (β=−0.06;p=0.001) were associated with lower AHA LS7 scores. Similarly, snoring (β=−1.14;p<0.0001) and having a high risk for OSA (β=−2.02;p<0.0001) were associated with lower AHA LS7 scores indicative of poorer CVH among racial/ethnic minority women only.
DISCUSSION
This study uniquely evaluated sleep characteristics in relation to CVH, assessed using an AHA LS7 score, among a diverse cohort of women and demonstrated that sleep habits were associated with the total AHA LS7 score and several of its components. In particular, longer sleep duration, better sleep quality, lack of insomnia or lower insomnia severity, and a lower risk profile for OSA were associated with a higher AHA LS7 score and therefore a more favorable CVH profile. The clustering of poor sleep characteristics was associated with greater odds of having poor CVH. Our findings also demonstrate that meeting the AHA LS7 may vary by sleep characteristics, as participants reporting adequate sleep duration (≥7 h/night), good sleep quality (PSQI≤5), no insomnia or snoring, and a low risk for OSA had significantly higher AHA LS7 scores and a greater likelihood of meeting >4 of the AHA LS7 metrics. These participants were more likely to meet the AHA LS7 metrics on smoking, PA, BMI, BP, cholesterol and fasting glucose. Furthermore, sleep duration <7h/night, snoring, and a high risk for OSA were associated with lower odds of meeting the BMI and BP metrics of the AHA LS7.
Sleep characteristics, including short sleep duration, poor sleep quality, insomnia, sleep disordered breathing, and snoring, are associated with higher incidence of cardiovascular outcomes in a number of cohort studies.8,22,23 Among women, short sleep duration and insomnia predicted elevated risk for coronary heart disease, CVD, and CVD-specific mortality in prospective cohort studies within the Women’s Health Initiative.24,25 To our knowledge, associations between sleep characteristics and the AHA LS7 score, as a measure of overall CVH, have not been previously evaluated, particularly among women. Yet, our findings of significant associations between sleep duration, quality and insomnia in relation to the AHA LS7 score are consistent with the growing literature demonstrating that sleep is associated with modifiable lifestyle behaviors and biomarkers that constitute the AHA LS7 and influence the risk for CVD.8 For instance, in a recent meta-analysis26, short sleep duration was associated with 55% greater risk of obesity, and for every additional hour of sleep, BMI was reduced by 0.35 points. Furthermore, cross-sectional evidence has shown that shorter sleep is consistently associated with metabolic syndrome, including higher risk of increased adiposity, dyslipidemia, elevated fasting glucose and BP.8,27–29 Similarly, insomnia and OSA have been linked to the development of hypertension and diabetes mellitus8,30–32, and poor sleep quality has been associated with greater odds for obesity and hypertension.33
Beyond their potential influence on clinical cardio-metabolic risk factors, there is limited emerging evidence that sleep characteristics may be related to other modifiable lifestyle behaviors, including diet, PA, and smoking and that these associations are likely bi-directional. Sleep restriction increases food intake in intervention studies34, supporting cross-sectional associations between short sleep and higher energy intake and low dietary quality.35,36 In an analysis of 495 postmenopausal women enrolled in the Women’s Health Initiative, actigraphy-assessed sleep duration was negatively associated with energy and fat intake.37 The influence of sleep on PA is less clear, but short-term intervention studies indicate that restricting sleep reduces engagement in high-intensity PA suggesting a possible behavioral mechanism for the health-impairing influence of insufficient sleep.34,38
Data from the 2004–2006 National Health Interview Survey also showed that individuals with shorter sleep, particularly non-Hispanic white adults, are more likely to be current smokers.39 Smoking has also been linked to poor quality sleep and snoring in U.S. men and women40, and insomnia has been shown to increase smoking heaviness and hamper cessation.41 In fact, there is emerging evidence for genetic correlations and bidirectional, causal effects between smoking and sleep behaviors that point to sleep as a potential smoking treatment target.41
In exploratory analyses stratified by menopausal status, associations between sleep characteristics and the AHA LS7 score appeared to be stronger among postmenopausal women. While high OSA risk was associated with poor CVH in both pre- and post-menopausal women, shorter sleep duration, poorer sleep quality, and greater insomnia severity were associated with a lower AHA LS7 score among postmenopausal women only in linear models. Previous research has demonstrated that the menopausal transition, independent of other potential explanatory factors, is associated with poor sleep42, and may therefore modify the association of sleep with cardiovascular risk.
In analyses stratified by race/ethnicity, longer sleep duration was associated with more favorable CVH, while poorer sleep quality, greater insomnia severity, snoring, and having a high risk for OSA were associated with poorer CVH in racial/ethnic minority women only. These findings are consistent with the growing literature demonstrating stronger associations between sleep characteristics and cardiometabolic risk in racial/ethnic minorities that may contribute to the CVD disparities observed at the population level.8,43 One potential explanation for these differences is hypothesized to be the higher prevalence of poor sleep among racial/ethnic minorities.44 Indeed, in the present cohort, racial/ethnic minority women had shorter sleep duration, poorer sleep quality, and greater insomnia severity and were more likely to have high risk for OSA compared to non-Hispanic white women.
Strengths of this study include the rigorous measures of lifestyle and clinical risk factors for CVD, which were evaluated using standardized, widely used questionnaires with demonstrated validity and reliability. Anthropometric and BP measures were also obtained by trained personnel, and biomarkers were assessed using standardized protocols. Furthermore, this cohort of women was racially and ethnically diverse, addressing an important knowledge gap that has been highlighted in a recent statement on sleep and cardiometabolic health by the AHA.8
Limitations of this analysis include the cross-sectional nature of the study, which lacks temporality and therefore limits causal inference. The use of questionnaires to assess lifestyle habits is also potentially a limitation due to the measurement error associated with self-reported data. Another limitation of this analysis is the moderate sample size, which limited power for stratified analyses by menopausal status and by race/ethnicity. Therefore, these sub-group analyses were exploratory. Furthermore, given that our sample consisted of 70% pre-menopausal women who volunteered to participate in a health study, our findings on the associations between sleep and AHA LS7 components may be conservative estimates. Approximately 86% of the women adhered to 4 or more of the AHA LS7 in this study, compared to 30–40% previously reported among men and women in previous studies within NHANES45,46. It is possible that the null results observed for sleep characteristics in relation to adherence to some of the individual AHA LS7 criteria may be due to insufficient variability in the outcomes. Hence, the effects of sleep on overall CVH and adherence to the individual criteria may be underestimated compared to the general population. Finally, despite the hypothesized U-shaped association between sleep duration and cardiometabolic risk factors8, we were unable to decipher the influence of long sleep on CVH in this cohort due to the low prevalence of a sleep duration ≥9 h in this cohort (~3%).
CONCLUSIONS
In conclusion, these results provide evidence that sleep may be a key factor associated with the maintenance of optimal CVH. We observed that sufficient sleep duration, good sleep quality, the absence of insomnia, and a lower risk for OSA are associated with higher AHA LS7 scores and consequently better overall CVH in a diverse sample of women. Our findings add to the emerging body of evidence indicating that sleep characteristics may be important to consider when assessing cardiometabolic risk among women. However, additional research is warranted to further examine these associations by menopausal status and race/ethnicity in larger samples and to detect sex differences. Importantly, prospective studies should be conducted to determine whether sleep plays an independent, additive or mediator role in associations of health behaviors with CVD and subsequently whether adding sleep characteristics into CVH lifestyle metrics would provide added value in predicting CVD risk. Positive findings could justify incorporating sleep into public health messaging and strategic efforts to prevent CVD, akin to existing guidance on other modifiable lifestyle behaviors such as diet, PA, and smoking.
Acknowledgements:
The authors would like to thank project coordinators Stephanie Byun, MS, Ashley Rodriguez, MS, and Kelly Naranjo, BA and research assistants Jessica Oleas Astudillo, BS, Jasmine Taylor, MS, and Zara Mayat, MS, for their assistance with recruitment and data collection. The critical review of this research by Dr. Lori Mosca, MD, PhD, MPH is greatly appreciated.
Grant Support: This research was supported by an American Heart Association Go Red for Women Strategically Focused Research Network Award (Grant # AHA16SFRN27960011) awarded to Dr. Aggarwal and is also supported in part by Columbia University’s CTSA Grant #UL1TR001873 from NCATS, NIH. Dr. Makarem is supported by an AHA Soter Collaborative Award 16SFRN27880000–1. Dr. St-Onge is supported by AHASFRN27950012 and R01 HL128226.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
Financial disclosure: None.
Non-financial disclosure: None.
References:
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Shah AM, Lutsey PL, et al. American Heart Association’s life’s simple 7: Avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970–976.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogunmoroti O, Oni E, Michos ED, et al. Life’s simple 7 and incident heart failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6(6):10.1161/JAHA.116.005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulshreshtha A, Vaccarino V, Judd SE, et al. Life’s simple 7 and risk of incident stroke: The reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM. Cardiovascular health and protection against CVD: More than the sum of the parts? Circulation. 2014;130(19):1671–1673. [DOI] [PubMed] [Google Scholar]
- 6.Younus A, Aneni EC, Spatz ES, et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. 2016;91(5):649–670. [DOI] [PubMed] [Google Scholar]
- 7.Ogunmoroti O, Allen NB, Cushman M, et al. Association between life’s simple 7 and noncardiovascular disease: The multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2016;5(10):10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge M, Grandner MA, Brown D, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makarem N, Aggarwal B. Gender differences in associations between insufficient sleep and cardiovascular disease risk factors and endpoints: A contemporary review. Gender and the Genome. 2017;1(2):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jehan S, Masters-Isarilov A, Salifu I, et al. Sleep disorders in postmenopausal women. Journal of sleep disorders & therapy. 2015;4(5). [PMC free article] [PubMed] [Google Scholar]
- 11.National Sleep Foundation. Sleep in America report. 2007. Washington, DC. [Google Scholar]
- 12.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: Results from the national health interview survey. Sleep. 2010;33(8):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among US adults from 2002 to 2012. Sleep Med. 2015;16(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 15.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45(1):142–161. [DOI] [PubMed] [Google Scholar]
- 18.Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;195(9131/03):3508–1381. [DOI] [PubMed] [Google Scholar]
- 19.Curi HP, Gomes VC. Reliability and validity of the international physical activity questionnaire (IPAQ). Med Sci Sports Exerc. 2004;36(3):556–556. [DOI] [PubMed] [Google Scholar]
- 20.Nutritionquest. development and validation of block FFQs and screeners. Website: http://www.nutritionquest.com/company/our-research-questionnaires/.
- 21.Thacker EL, Gillett SR, Wadley VG, et al. The American Heart Association life’s simple 7 and incident cognitive impairment: The REasons for geographic and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2014;3(3):e000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219(2):963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon Y, Gharib SA, Biggs ML, et al. Association of sleep characteristics with atrial fibrillation: The multi-ethnic study of atherosclerosis. Thorax. 2015;70(9):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the women’s health initiative. J Women Health. 2013;22(6):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabat GC, Xue X, Kamensky V, et al. The association of sleep duration and quality with all-cause and cause-specific mortality in the women’s health initiative. Sleep Med. 2018;50:48–54. [DOI] [PubMed] [Google Scholar]
- 26.Cappuccio FP, Taggart FM, Kandala N, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J, Kim M, Kim J, et al. Association between short sleep duration and high incidence of metabolic syndrome in midlife women. Tohoku J Exp Med. 2011;225(3):187–193. [DOI] [PubMed] [Google Scholar]
- 28.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaput J, McNeil J, Després J, Bouchard C, Tremblay A. Short sleep duration as a risk factor for the development of the metabolic syndrome in adults. Prev Med. 2013;57(6):872–877. [DOI] [PubMed] [Google Scholar]
- 30.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertens Res. 2013;36(11):985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122(12):1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montag SE, Knutson KL, Zee PC, et al. Association of sleep characteristics with cardiovascular and metabolic risk factors in a population sample: The Chicago area sleep study. Sleep Health. 2017;3(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossavar-Rahmani Y, Jung M, Patel SR, et al. Eating behavior by sleep duration in the Hispanic community health study/study of Latinos. Appetite. 2015;95:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28(11–12):1146–1150. [DOI] [PubMed] [Google Scholar]
- 37.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11(2):180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90(6):1476–1482. [DOI] [PubMed] [Google Scholar]
- 39.Schoenborn CA, Adams PF. Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United states, 2004–2006. Book Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United States, 2004–2006. 2008. [Google Scholar]
- 40.Mehari A, Weir NA, Gillum RF. Gender and the association of smoking with sleep quantity and quality in American adults. Women Health. 2014;54(1):1–14. [DOI] [PubMed] [Google Scholar]
- 41.Gibson M, Munafo MR, Taylor A, Treur JL. Evidence for genetic correlations and bidirectional, causal effects between smoking and sleep behaviors. bioRxiv. 2018:258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 43.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch Intern Med. 2009;169(11):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, socio-demographics, and socioeconomic position. Sleep. 2014;37(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Gillespie C, Merritt R, Yang Q. Association Between Cardiovascular Health and Depression Among US Adults: NHANES 2007–2014. Circulation. 2017;136(suppl_1):A14562. [Google Scholar]
- 46.Caleyachetty R, Echouffo-Tcheugui JB, Muennig P, Zhu W, Muntner P, Shimbo D. Association between cumulative social risk and ideal cardiovascular health in US adults: NHANES 1999–2006. Int J Cardiol. 2015;191:296–300. [DOI] [PubMed] [Google Scholar]