Abstract
Background:
Esophageal cancer is considered a disease of the elderly. Although the incidence of esophageal adenocarcinoma in young patients is increasing, guidelines for endoscopic evaluation of gastroesophageal reflux disease and Barrett’s esophagus include age as a cutoff. There is a paucity of data on the presentation and treatment of esophageal cancer in young patients. Most studies are limited by small sample sizes and conflicting findings are reported regarding delayed diagnosis and survival compared to older patients.
Methods:
A retrospective cohort study was performed utilizing the National Cancer Database between 2004 and 2015. Patients with esophageal adenocarcinoma were divided into quartiles by age (18–57, 58–65, 66–74, 75+) for comparison. Clinicopathologic and treatment factors were compared between groups.
Results:
101,596 patients were identified with esophageal cancer. The youngest patient group (18–57 years) had the highest rate of metastatic disease (37%). No difference in tumor differentiation was observed between age groups. Younger patient groups were more likely to undergo treatment despite advanced stage at diagnosis. Overall 5-year survival was better for younger patients with local disease, but the difference was less pronounced in locoregional and metastatic cases.
Conclusion:
In this study, young patients were more likely to have metastatic disease at diagnosis. Advanced stage in young patients may reflect the need for more aggressive clinical evaluation in high-risk young patients.
INTRODUCTION
Esophageal cancer is the 8th most common cancer worldwide and the 6th most common cause of cancer-related death.1 Although considered a highly fatal disease, therapeutic advancements have resulted in improved 5-year overall survival from 5.1% to 19.2% over the last four decades.2, 3
Esophageal cancer occurs primarily in the 6th and 7th decades of life; as such, it is considered a disease of the elderly. However, esophageal cancer does occur in young patients and patients younger than 55 years currently account for almost 13% of new diagnoses in the United States.3 Citing low risk of disease in young patients, current guidelines from the American College of Gastroenterology and American Gastroenterologic Association recommend against endoscopy for evaluation of dyspepsia in patients <60 years old, even in the setting of alarm symptoms.4 (Table 1) Furthermore, guidelines specify that age > 50 is considered a high risk indication for endoscopic evaluation of gastroesophageal reflux symptoms or to screen for Barrett’s Esophagus5–9 Similarly, national guidelines in other western countries recommend against urgent endoscopic evaluation of dyspepsia in patients age <55.10, 11 This is concerning as studies suggest that the incidence of esophageal cancer among young patients is increasing.12, 13 Furthermore, perceptions of esophageal cancer as a rare disease in young patients may result in lower clinical suspicion; as such, some studies have suggested that patients <50 years old are more likely to have delayed diagnosis and consequently, more advanced disease.14–18 Given that 5-year overall survival for advanced disease is <25% and <5% for regional and distant disease, respectively, earlier identification has a significant impact on prognosis.3
Table 1.
Current guidelines for endoscopic evaluation of gastroesophageal reflux disease and Barrett’s Esophagus (BE)
| Indications for Endoscopic Evaluation | High Risk Features | |
|---|---|---|
| American Society of Gastrointestinal Endoscopy (2015)5 | Screening EGD for select patients with prolonged history of GERD | Age ≥ 50, chronic GERD symptoms (>5 years), white, male, nocturnal reflux |
| American Gastroenterological Association (AGA) (2008, 2011)6, 7 | Consider EGD to screen for BE in patients with high risk | Age ≥50, male, white, hiatal hernia, elevated BMI, intra-abdominal fat distribution |
| American College of Gastroenterology (ACG) (2016)8 | Consider screening for BE in high risk patients | Chronic GERD symptoms (>5 years) and 2+ BE/cancer risk factors: age ≥ 50, white, central obesity, current/past history of smoking, family history |
| American College of Gastroenterology (ACG) (2013)9 | Individualized EGD for GERD diagnosis in select patients for alarm symptoms and high risk | Elderly, those at risk for BE, noncardiac chest pain, patients unresponsive to PPI |
| AGA and ACG (2017)4 | EGD should be performed in patients age> 60 with dyspepsia to rule out neoplasia | NA |
EGD: esophagogastroduodenoscopy; GERD: gastroesophageal reflux disease; BE: Barrett’s Esophagus; NA: not applicable.
To date, esophageal cancer in young patients has received minimal attention in the literature. Most studies are single-institution experiences limited by small sample sizes and conflicting findings relating to the presentation, treatment and prognosis of this population; as such, the clinicopathologic presentation and outcomes of esophageal cancer in young patients is not well defined.14–25 Further complicating current understanding of this disease in young patients is the lack of consensus as to what constitutes a “young patient”, with many studies arbitrarily selecting age under 50.14–21
Given that survival for this disease remains poor, esophageal cancer in young patients merits further investigation to better guide diagnosis and management in this population. The purpose of this study was to compare the clinicopathologic characteristics and survival of patients with esophageal cancer between age groups utilizing a national cancer cohort in the United States.
METHODS
Data Source and Study Population
A population-based retrospective review was performed using data from the National Cancer Database (NCDB) Participant User File (PUF). All patients diagnosed between 2004 to 2015 with adenocarcinoma of the esophagus (ICD-0 Code C15.0–15.9) and esophagogastric junction (C16.0, cardia NOS) with a single primary malignancy were included. The NCDB is a cancer registry from the American College of Surgeons and American Cancer Society which collects data from more than 1,500 Commission on Cancer accredited centers. (21) Approximately 70% of all newly diagnosed cancer cases are captured annually. This study was exempt from institutional IRB review.
Variables
Demographic characteristics of interest included age, gender, race, comorbidities (Charlson Comorbidity Index > 0), insurance status (uninsured, private or government), cancer center type (academic versus non-academic), education level (percent of population within patient zip code without high school education ≥ 21% or <21%), year of diagnosis, region (grouped into 4 geographic areas, including: Northeast, Midwest, South and West), distance traveled to facility (miles), location (population ≥ 250,000 vs <250,000) and year of diagnosis (2004–2007, 2008–2011, 2012–2015). Histopathologic data included tumor location (lower third/cardia NOS/abdominal, Middle Third/Thoracic, Upper Third/Cervical, Overlapping, Esophagus NOS), histology (adenocarcinoma, squamous cell carcinoma or carcinoma NOS), and differentiation grade (well/moderate, poor/undifferentiated or unknown). Treatment was defined as having received any recommended therapy for esophageal cancer, including chemotherapy, radiation, surgery, or any combination of these interventions. As previously described elsewhere, treatments were categorized into surgery, definitive chemoradiation, induction + surgery (surgery and neoadjuvant chemotherapy ± radiation and/or adjuvant chemotherapy ± radiation), palliative (chemotherapy or radiation alone), and no treatment.26 (Appendix 1) For patients that underwent surgery, postoperative 30-day mortality and readmission rate was recorded.
The American Joint Committee on Cancer (AJCC) staging criteria are recorded in concordance with diagnosis year. To account for different categorization of staging between the 6th and 7th AJCC editions, stage was divided into three categories using clinical data, including: local, locoregional, and metastatic, as previously described by Wong et al.27, 28 (Appendix 2)
Statistical Analysis
Patients were divided into 4 groups based on age quartiles. Baseline patient demographics and clinical factors were compared between age groups using chi-square for categorical variables and Wilcoxon rank sum test for continuous variables. Data was reported as counts (percentages) and medians (interquartile range), respectively. Overall survival was defined as time (in months) from diagnosis to death or last follow-up. Survival analysis was performed using the Kaplan-Meier method. A multivariate model using Cox regression analysis was included to determine whether age independently impacted survival after adjusting for treatment and stage. A sub-analysis was performed among patients <50 and ≥ 50 as well as <60 and ≥ 60 as well as, to reflect the guidelines discussed in Table 1.
Given the large sample size of this study (>100,000), all comparisons between groups were statistically significant to p<0.0001. As such, the p values should be interpreted with caution as statistical significance may not always correspond with clinical significance. All data analyses were performed using SAS v9.4 (SAS Institute, Carey, North Carolina) and R v3.3.3.
RESULTS
There were 101,596 patients included in the study cohort. The median age was 65 (57–74). Patients were grouped by quartiles of age distribution, which included: 1) 18–57 years (n=26,440) 2) 58–65 (n=25,162) 3) 66–74 (n=26,135) and 4) 75+ (n=23,589). The median and IQR by age group was 52 (IQR 47–55), 62 (IQR 60–64), 70 (IQR 67–72), and 80 (IQR 77–84). In this population 34.7% of patients were under age 60.
Clinicopathologic Characteristics
Comparison of baseline demographics between age groups is given in Table 2. The youngest age quartile had the highest rate of non-white patients compared to other age groups. Gender was similar amongst the younger three age quartiles (13.5%, 13.1%, and 15.7% respectively); however, there were 24.7% females in the 75 and older group. Patients in the youngest quartile were more likely to be uninsured and live in less educated areas than other age groups. With increasing age, distance from the hospital and rate of treatment at an academic center decreased. Patients had similar tumor location and grade.
Table 2.
Comparison of Demographic Characteristics by Age Group
| Characteristic, N (%) | Age Quartile Groups | |||||
|---|---|---|---|---|---|---|
| 18–57 | 58–65 | 66–74 | 75 + | p overall |
||
| N | 26440 | 25162 | 26135 | 23859 | ||
| Female Gender | 3567 | 3289 | 4101 | 5903 | <0.001 | |
| (13.5%) | (13.1%) | (15.7%) | (24.7%) | |||
| White Race | 23197 | 22855 | 23989 | 21850 | <0.001 | |
| (87.7%) | (90.8%) | (91.8%) | (91.6%) | |||
| Any Comorbidities | 5215 | 6921 | 8403 | 7672 | <0.001 | |
| (19.7%) | (27.5%) | (32.2%) | (32.2%) | |||
| Median Distance to Hospital, miles (IQR) | 13.4 | 13.4 | 12.8 | 8.80 | <0.001 | |
| [1.50; 143] | [1.50; 148] | [1.40; 146] | [1.10; 104] | |||
|
Education (≥21% in zip code without high school diploma) |
4117 | 3507 | 3513 | 2769 | <0.001 | |
| (15.6%) | (13.9%) | (13.4%) | (11.6%) | |||
| Insurance Status | <0.001 | |||||
| Government | 6145 | 8202 | 21526 | 21021 | ||
| (23.2%) | (32.6%) | (82.4%) | (88.1%) | |||
| Private | 17630 | 15183 | 3806 | 2246 | ||
| (66.7%) | (60.3%) | (14.6%) | (9.41%) | |||
| None | 1774 | 1027 | 170 | 117 | ||
| (6.71%) | (4.08%) | (0.65%) | (0.49%) | |||
| Insurance Status Unknown | 891 | 750 | 633 | 475 | ||
| (3.37%) | (2.98%) | (2.42%) | (1.99%) | |||
| Facility Type | <0.001 | |||||
| Academic | 10964 | 10926 | 10656 | 8173 | ||
| (44.3%) | (43.4%) | (40.8%) | (34.3%) | |||
| Non-Academic | 13784 | 14236 | 15479 | 15686 | ||
| (55.7%) | (56.6%) | (59.2%) | (65.7%) | |||
| Location | <0.001 | |||||
| East | 5428 | 5703 | 6021 | 5999 | ||
| (21.9%) | (22.7%) | (23.0%) | (25.1%) | |||
| South | 7211 | 7233 | 7306 | 7000 | ||
| (29.1%) | (28.7%) | (28.0%) | (29.3%) | |||
| Midwest | 8551 | 8406 | 8639 | 6967 | ||
| (34.6%) | (33.4%) | (33.1%) | (29.2%) | |||
| West | 3558 | 3820 | 4169 | 3893 | ||
| (14.4%) | (15.2%) | (16.0%) | (16.3%) | |||
|
Patient Location (Combine into ≥250,000) |
20717 | 19603 | 20252 | 19085 | <0.001 | |
| (78.4%) | (77.9%) | (77.5%) | (80.0%) | |||
| Diagnosis Year | <0.001 | |||||
| 2004–2007 | 8194 | 6844 | 7124 | 7411 | ||
| (31.0%) | (27.2%) | (27.3%) | (31.1%) | |||
| 2008–2011 | 8867 | 8377 | 8404 | 7836 | ||
| (33.5%) | (33.3%) | (32.2%) | (32.8%) | |||
| 2012–2015 | 9379 | 9941 | 10607 | 8612 | ||
| (35.5%) | (39.5%) | (40.6%) | (36.1%) | |||
| Tumor Site | <0.001 | |||||
| Abdominal/Lower/Stomach | 22715 | 21614 | 22647 | 20089 | ||
| (85.9%) | (85.9%) | (86.7%) | (84.2%) | |||
| Overlapping | 740 | 751 | 667 | 611 | ||
| (2.80%) | (2.98%) | (2.55%) | (2.56%) | |||
| Thoracic/Middle | 962 | 966 | 1011 | 1219 | ||
| (3.64%) | (3.84%) | (3.87%) | (5.11%) | |||
| Unspecified | 1837 | 1663 | 1654 | 1727 | ||
| (6.95%) | (6.61%) | (6.33%) | (7.24%) | |||
| Upper/Cervical | 186 | 168 | 156 | 213 | ||
| (0.70%) | (0.67%) | (0.60%) | (0.89%) | |||
| Differentiation Grade | <0.001 | |||||
| Unknown | 4801 | 4585 | 4925 | 4883 | ||
| (18.2%) | (18.2%) | (18.8%) | (20.5%) | |||
| Well/Moderate | 9930 | 9639 | 10112 | 9140 | ||
| (37.6%) | (38.3%) | (38.7%) | (38.3%) | |||
| Poor/Undifferentiated | 11709 | 10938 | 11098 | 9836 | ||
| (44.3%) | (43.5%) | (42.5%) | (41.2%) | |||
Staging
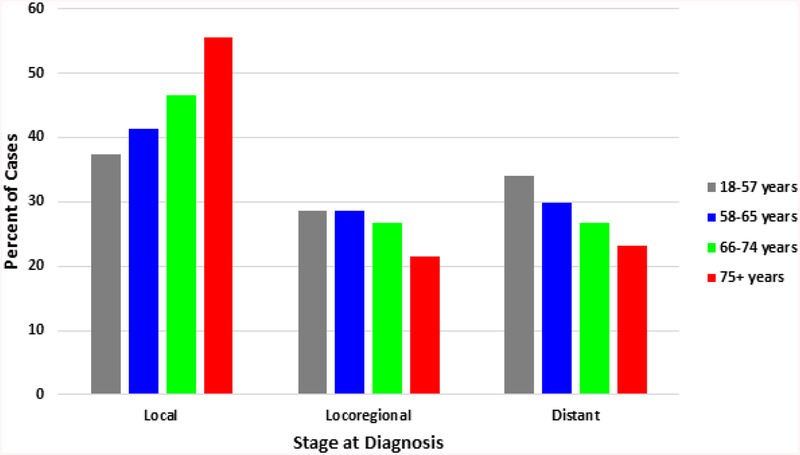
Distribution of tumor stage by age group is presented in Figure 1. Patients aged 18–57 had the lowest rate of local disease (37.3%), with incidence rising with increasing age group (41.4%, 46.5% and 55.6%, respectively). Rates of locoregional disease were similar between the youngest three agree groups, and lowest in the oldest patients. Distant disease at diagnosis decreased with increasing age; 33.9% of patients age 18–57 had metastasis, followed by 29.8%, 26.8% and 23.1% in the respective increasing age groups. The rate of advanced disease at diagnosis, including locoregional and distant, was highest in the youngest age group (62.5%) and lowest in the patients aged 75+ (44.4%).
Figure 1.
Distribution of stage at diagnosis by age quartile at diagnosis
Treatment
Disease treatment by age quartile is demonstrated in Table 3. Non-treatment was twice as likely in patients age 75+ (32%) compared to patients in other age groups (14%, 15% and 17%, respectively by increasing age quartile). The rate of patients receiving definitive chemoradiation and surgery alone were similar between all groups. Patients in the youngest two age quartiles underwent induction + surgery more frequently (22.3% and 21.7% respectively) than patients in the third and fourth quartiles (17.3% and 5.2%, respectively).
Table 3.
Comparison of Treatment Selection Between Age Groups
| Treatment Type | Age Quartile Groups | |||
|---|---|---|---|---|
| 18–57 | 58–65 | 66–74 | 75 + | |
| Definitive Chemoradiation | 6211 (23.5%) | 6062 (24.1%) | 6641 (25.4%) | 6038 (25.3%) |
| Induction + Surgery | 5907 (22.3%) | 5466 (21.7%) | 4521 (17.3%) | 1249 (5.23%) |
| Surgery | 5056 (19.1%) | 5108 (20.3%) | 5984 (22.9%) | 4459 (18.7%) |
| Palliative Care | 5698 (21.6%) | 4850 (19.3%) | 4649 (17.8%) | 4592 (19.2%) |
| No Treatment | 3568 (13.5%) | 3676 (14.6%) | 4340 (16.6%) | 7521 (31.5%) |
Among patients that underwent surgical treatment, patients aged 75+ received local endoscopic therapy most frequently and were least likely to receive esophagectomy. On operative pathology, nodal metastasis rates were similar between age groups; however, the incidence of advanced T stage decreased with increasing age group. Postoperative readmission rates were similar among all groups, but 30-day overall mortality rates increased with age (0.7%, 0.9%, 1.4% and 1.3%, respectively).
Survival
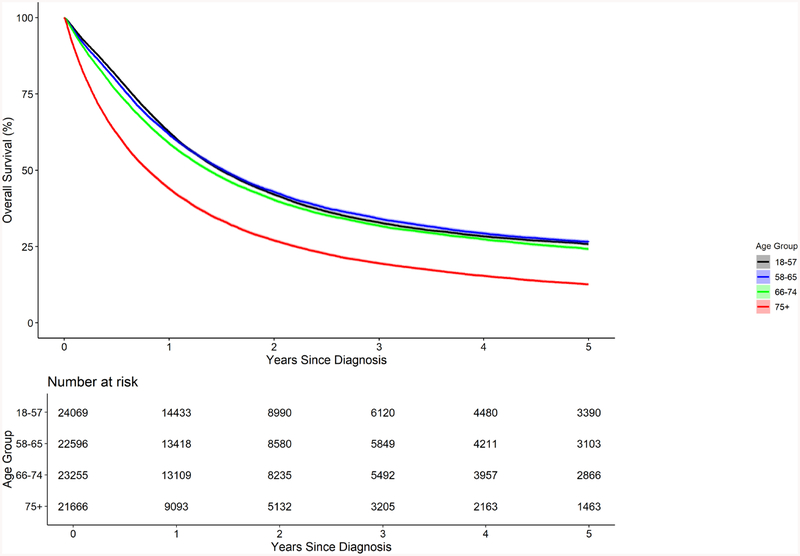
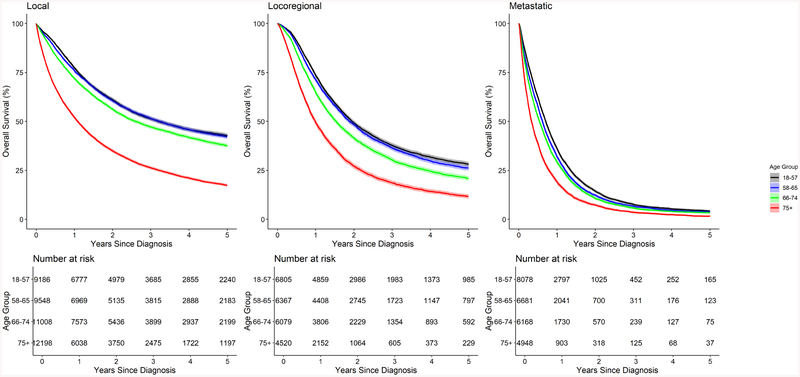
Comparison of overall survival by age quartile is presented in Figure 2. Median OS was similar in patients age 18–74 (16.5–18.3 months), and much lower in patients with age 75+ (9.6). Stratification of overall survival by age quartile and tumor stage is shown in Figure 3. In patients with local disease, median OS in patients age 18–57 and 58–65 was similar (38.4 and 38.7 months); however, for age 66–74 and 75+ OS decreased (31.2 months and 13.0, respectively). The differences between age groups were less apparent for patients with locoregional disease (23.5, 22.6, 18.4 and 11.9, respectively by increasing age quartile). Among patients with metastatic disease, median OS ranged from 4–8 months. 5-year overall survival by age and stage is presented in Supplemental Table 1.
Figure 2.
Comparison of overall survival by age quartile
Figure 3.
Comparison of overall survival by age quartile, stratified by tumor stage at diagnosis
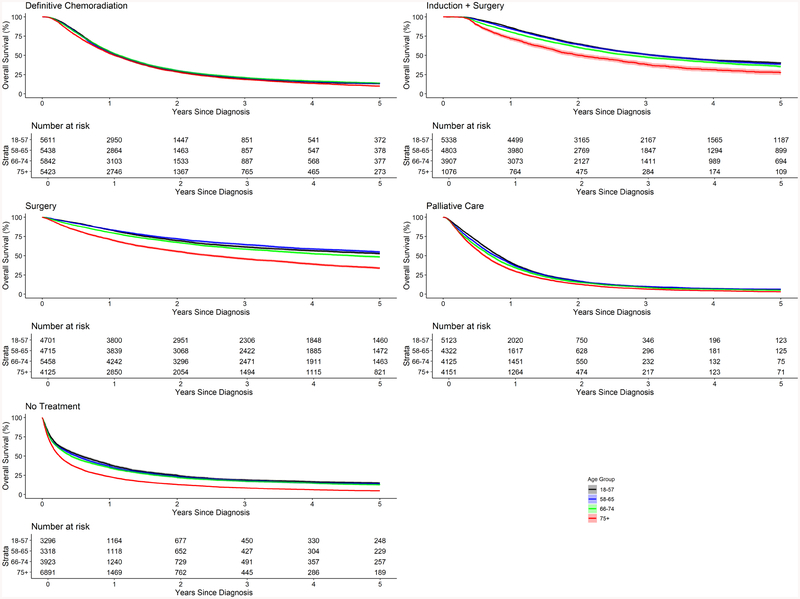
In Figure 4, median OS by age quartile was stratified by treatment selection. Among patients that underwent definitive chemoradiation, no difference in median OS was observed by age group (12.8–13.6 months). Similarly, minimal difference was observed among patients that underwent palliative treatment or received no treatment. However, among patients that underwent induction + surgery, median OS was much lower in patients in the oldest quartile (24.3 months) compared to younger groups (37.7, 37.3 and 33.4, respectively from quartile 1–3). Similarly, among patients that underwent surgical resection alone, patients age 75+ had lower median OS (30.1 months) compared to other groups (75.4, 81.9 and 55.3 months, respectively from quartile 1–3). Table 4 presents the results of multivariate cox proportional hazards assessing survival. After adjusting for stage and treatment, mortality risk increased with age quartile as compared to the youngest patients.
Figure 4.
Comparison of overall survival by age quartile, stratified by treatment selection
Table 4.
Adjusted model for overall survival
| HR | 95% CI | p value | |
|---|---|---|---|
| Age quartile | |||
| 18–57 | Ref | ||
| 58–65 | 1.05 | 1.03, 1.07 | <.001 |
| 66–74 | 1.15 | 1.13, 1.18 | <.001 |
| 75 + | 1.62 | 1.58, 1.65 | <.001 |
| Treatment | |||
| Definitive chemoradiation | Ref | ||
| Induction + surgery | 0.57 | 0.55, 0.5S | <.001 |
| No treatment | 1.79 | 1.75, 1.83 | <.001 |
| Palliative care | 1.18 | 1.15, 1.20 | <.001 |
| Surgery | 0.48 | 0.47, 0.50 | <.001 |
| Stage | |||
| Local | Ref | ||
| Locoregional | 1.27 | 1.24, 1.30 | <.001 |
| Metastatic | 2.53 | 2.48, 2.58 | <.001 |
HR, Hazard ratio; CI, confidence interval
Dichotomized Analysis
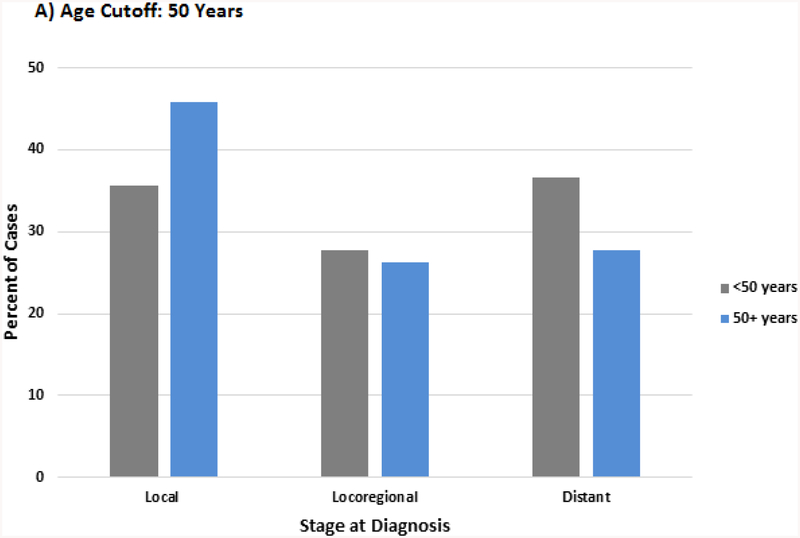
In order to account for the age cut-offs described in the guidelines in Table 1, separate analyses were conducted by dividing the population using the age cutoff of 50 and 60. (Supplemental Table 2 and 3) Stratification by age 50 demonstrated several differences from the quartile analysis. The racial difference between older and younger patients was greater, with the group <50 having a 68% higher rate of non-white patients than > 50. Patients <50 had a 31% higher rate of distant disease at diagnosis (36.6% vs 27.8%) and those <60 had a 28% higher rate (33.6% vs 26.2%). (Figure 5a and b) There was less variation in overall survival by stage and treatment between age groups as compared to the quartile analysis. (Supplemental Figure 1 and 2)
Figure 5A,B.
Distribution of stage at diagnosis by age cutoff A) 50 years B) 60 years
DISCUSSION
The presentation and outcomes of esophageal cancer in young patients is poorly understood. Our study is the largest to date to compare characteristics of esophageal cancer by age. The majority of available studies have been small and demonstrated conflicting results.9–12, 14–20 Furthermore, comparability is limited by varying definitions of “young”. As such, we utilized quartiles of age distribution (18–57, 58–65, 66–74, 75+) in this study to determine how young patients may differ compared to other age groups, but performed sub-analyses using age 50 and 60 as a cutoff to reflect current guideline cutoffs. (Table 1). Given that the incidence of esophageal cancer, a highly morbid disease, is rising among younger patients, there is an important need for consensus regarding the impact of age on the presentation and outcomes of esophageal cancer.7,8 Our findings support previous studies that demonstrated advanced disease presentation in younger patients.14, 15, 17–19 Furthermore, results of our analysis demonstrated that the incidence of metastatic disease at diagnosis decreased with increasing age quartile. Use of age 50 and 60 as a cutoff demonstrated that the rate of distant disease was around 30% higher for younger patients in both analyses. In the only other population-based study, Zeng et al recently used Surveillance, Epidemiology, and End Results (SEER) program data to evaluate 1385 patients age ≤ 50 and demonstrated that 48.5% presented with distant disease compared to 37.9%of older patients.18 The higher rate of patients with metastatic disease may reflect selection bias in the authors’ methodology, including the decision to not analyze patients with esophagogastric junction cancer and use of AJCC 6th edition for staging. However, their results confirm our conclusion that younger age is associated with more advanced disease and that this represents an important area for intervention.
This study demonstrated similar tumor grade between groups, suggesting that observed differences in stage are not a reflection of a more aggressive disease process but rather delayed diagnosis. In support of this observation, several studies noted a longer period of reported symptoms and slower referral to endoscopy among young patients.14, 15 The high rate of adenocarcinoma in the youngest age quartile is consistent with the demonstrated rising incidence of adenocarcinoma in the United States.12, 13 Currently, gastroesophageal reflux disease is the only well accepted risk factor for development of esophageal adenocarcinoma. Recent data has reported that up to 40% of people in the United States report symptoms of gastroesophageal reflux.29 It has previously been described that the time for progression from reflux to Barrett’s esophagus is approximately 10 years.30, 31 As such the decision to perform endoscopic evaluation in a symptomatic patient should focus on length of time they have had gastroesophageal reflux disease rather than age. People with known gastroesophageal reflux disease as children or adolescents should be considered at risk. As the rates of premature birth, childhood obesity and high consumption of high fat foods in the United States continue to increase, it is possible that increasing rates of esophageal adenocarcinoma may be observed in the young.32–34 Recent data from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON) assessing age-specific risk profiles demonstrated that the associations between reflux disease and obesity were stronger among those who developed cancer at a younger age.35 Our findings support the warning by Hamouda et al against over reliance on age cutoffs and alarm symptoms based on previous studies that have questioned the efficacy of these factors as underlying predictors of cancer.23,36 Instead, clinicians should focus on the patient as a whole. Age quartile analysis demonstrated minimal differences in the demographic “profile” of patients <75 years old. However, evaluation using age 50 as a cutoff demonstrated that patients < 50 years old had a significantly higher rate of non-white race and were more likely to be uninsured as compared to older patients This is important given the guidelines in Table 1 mention White race as a risk factor. Previous studies have demonstrated racial disparities in stage at diagnosis, treatment selection and prognosis among non-White patients, which may in part reflect differences in socioeconomic status.26, 37–39 Further research may investigate whether there are underlying genetic factors that may contribute to earlier development of esophageal cancer in certain patients. However, the absence of distinguishing findings support the conclusion that physicians must maintain a high clinical suspicion in patients with prolonged histories of reflux symptoms, and recognize that not all patients present in the “typical” fashion (elderly white males).
Previous studies have suggested that more aggressive treatment selection among young patients results in similar overall survival despite more advanced stage at diagnosis.14, 15, 17, 23 While some studies demonstrate improved 5-year overall survival among younger patients, others fail to replicate these findings.14–19, 23 In this series, patients < 75 had a similar rate of any surgical therapy, although patients < 65 were more likely to get aggressive treatment with induction therapy + surgerythan older patients.. However, stratification by stage demonstrated that the magnitude of survival benefits among younger patients decreased with increasing stage. This highlights the importance of early diagnosis for esophageal cancer, especially in younger patients who have less comorbidities and can better tolerate aggressive curative therapy. Once distant disease is diagnosed, overall prognosis is very poor regardless of age.
Interpretation of our results are subject to the limitations associated with utilization of NCDB data. It is not possible to determine whether young patients in this series had a delay in diagnosis because of lower clinical suspicion as it relates to patient age. However, this hypothesis is supported by current understanding of perceptions of esophageal cancer amongst providers, endoscopic screening guidelines, smaller studies that did identify delayed presentation in younger patients, and the absence of markers of more aggressive tumor pathology as an underlying etiology for advanced stage at diagnosis. Additionally, NCDB does not provide information regarding postoperative complications or toxicity because of chemotherapy or radiation. As such, it is difficult to determine why a survival difference was observed between age groups among patients undergoing surgical treatments. Furthermore, the specific chemotherapy agents given are not included, and therefore it is not possible to determine whether patients that underwent treatment at non-academic centers received the most updated standard of treatment. Finally, it is not possible to assess the impact of birth cohort on observed differences between age groups with this dataset. However, the increasing incidence of esophageal adenocarcinoma has still been demonstrated in more recent birth cohorts.13
CONCLUSIONS
The results of this demonstrate that in a large cohort compromising the majority of cases of esophageal cancer in the United States between 2004 to 2015, young patients with esophageal adenocarcinoma had more advanced disease at diagnosis despite similar clinicopathologic tumor characteristics. Given that stage at diagnosis is the most important determinant of overall survival for this highly lethal disease, it is important that clinicians consider esophageal cancer in the differential diagnosis of symptomatic patients with high-risk histories,, such as obesity and prolonged reflux. Patient symptoms and risk factors, rather than age alone, should be considered when deciding whether to perform endoscopic evaluation. Esophageal cancer is not only a disease of older patients. (Figure 5)
Supplementary Material
Figure 6.
Graphical Abstract. Esophageal cancer diagnosed at median age of 68 and is considered a disease of the elderly. In this large series of patients with esophageal cancer from the National Cancer Database (2004–2015), although clinicopathologic similarities suggest similar tumor biology between age groups, the youngest age quartile had the highest rate of distant disease at diagnosis (34% versus 29.9, 26.8 and 23.1%, respectively with increasing age group). Risk factors, such as obesity and prolonged history of reflux, should dictate evaluation of esophageal cancer rather than age.
CENTRAL MESSAGE.
Perceptions of esophageal cancer as a disease of the elderly may result in the higher rate of advanced diagnostic stage in younger patients. Risk factors, rather than age, should guide evaluation.
PERSPECTIVE STATEMENT.
This population-based study showed similar clinicopathologic characteristics but more advanced disease among young patients diagnosed with esophageal adenocarcinoma.. Given poor prognosis associated with metastatic esophageal cancer, itmay be time to rethink the approach for evaluation of young high-risk patients.
FUNDING SOURCES:
This study was supported, in part, by the National Institutes of Health/National Cancer Institute Cancer Support Grant P30 CA008748. Tamar Nobel is supported, in part, by a grant from the American Cancer Society. Thank you to Todd Litinsky for inspiring this work and supporting Daniela Molena’s research fund.
GLOSSARY OF ABBREVIATIONS
- OS
Overall Survival
- DFS
Disease Free Survival
- DSS
Disease Specific Survival
- EAC
Esophageal Adenocarcinoma
APPENDIX
Appendix 1. Definition of treatment groups
| SURGERY | ||
| Surgery in first 180 days from Dx | ||
| No radiation or chemo within first 180 days | ||
| Surgery in first 180 days from Dx | ||
| Radiation and/or chemo in first 180 days | ||
| Number of days to surgery < days to chemo/rad | ||
| PALLIATIVE CARE | ||
| Chemo Only | Chemo in first 180 days from Dx | |
| No radiation within first 180 days | ||
| No surgery within first 180 days | ||
| Rads Group B | Radiation in first 180 days from Dx | |
| No chemo within first 180 days | ||
| No surgery within first 180 days | ||
| DEFINITIVE CHEMORADIATION | ||
| Subsequent | Chemo within first 180 days from Dx | |
| Radiation within first 365 days | ||
| Days between start of chemo and start of radiation is >7 | ||
| Concurrent | Chemo within first 180 days from Dx | |
| Radiation within first 365 days | ||
| Days between start of chemo and start of radiation is <7 | ||
| INDUCTION + SURGERY | ||
| Chemo alone + surgery | Chemo in first 180 days | |
| Surgery in first 180 days | ||
| Number of days to chemo < number of days to surgery | ||
| Chemo starts more than 7 days before surgery | ||
| Chemo + radiation concurrent + surgery | Chemo and radiation in first 180 days | |
| Surgery in first 180 days | ||
| Number of days to chemo/rad < number of days to surgery | ||
| Number of days between start of chemo and start of rad is < 7 | ||
| Other Induction + surgery (radiation alone or chemorad subsequent) | Chemo and/or radiation in first 180 days from Dx | |
| Surgery in first 180 days | ||
| Number of days to chemo/rad < number of days to surgery | ||
| Number of days between start of chemo and start of rad is > 7 | ||
| Radiation Group A | Radiation in first 180 days from Dx | |
| No chemo within first 180 days | ||
| Radiation occurs before any surgery (surg would be salvage) | ||
| NO TREATMENT | ||
| No Treatment | No treatment within 180 days | |
Appendix 2. Staging
| Stage | Definition |
|---|---|
| Local | Unknown or a tumor of any size, unknown or no nodes, and unknown or no metastatic disease (Tx-4, Nx-0, Mx-0) |
| Locoregional | Unknown or a tumor of any size, positive nodes, and unknown or no metastatic disease (Tx-4, N1–3, Mx-1A) |
| Distant | Unknown or a tumor of any size, unknown nodes or positive nodes, and having a metastatic diagnosis (Tx-4, Nx-3, M1-M1B) |
Adapted from Wong et al28
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: No conflicts of interest to disclose
IRB APPROVAL: Exempt
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: 2018. [DOI] [PubMed] [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. (eds). SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 3.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 4.Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG Clinical Guideline: Management of Dyspepsia. American J Gastroenterol. 2017;112:988–1013. [DOI] [PubMed] [Google Scholar]
- 5.Muthusamy VR, Lightdale JR, Acosta RD, et al. The role of endoscopy in the management of GERD. Gastrointestinal Endoscopy. 2015;81:1305–1310. [DOI] [PubMed] [Google Scholar]
- 6.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Medical Position Statement on the Management of Gastroesophageal Reflux Disease. Gastroenterology. 2008;135:1383–1391.e1385. [DOI] [PubMed] [Google Scholar]
- 7.American Gastroenterological Association Medical Position Statement on the Management of Barrett’s Esophagus. Gastroenterology. 2011;140:1084–1091. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–328. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. https://www.nice.org.uk/guidance/ng12/chapter/1-Recommendations-organised-by-site-of-cancer#upper-gastrointestinal-tract-cancers. Published 2015, updated 2017.
- 11.Gisbert JP, Calvet X, Ferrandiz J, Mascort J, Alonso-Coello P, Marzo M. [Clinical practice guideline on the management of patients with dyspepsia. Update 2012]. Atencion Primaria. 2012;44:e721–e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guardino JMKF, Lopez R, Wachsberger DM, Richter JE, Falk GW. Barrett’s esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol. 2006;101:2187–2193. [DOI] [PubMed] [Google Scholar]
- 13.Thrift APWD. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. [DOI] [PubMed] [Google Scholar]
- 14.Portale GPJ, Hsieh CC, Tamhankar AP, Almogy G, Hagen JA, et al. Esophageal adenocarcinoma in patients < 50 years old: Delayed diagnosis and advanced disease at presentation. Am Surg. 2004;70:954–958. [PubMed] [Google Scholar]
- 15.Hashemi N, Loren D, DiMarino AJ, Cohen S. Presentation and Prognosis of Esophageal Adenocarcinoma in Patients Below Age 50. Digestive Dis Sci. 2008;54:1708. [DOI] [PubMed] [Google Scholar]
- 16.Markar SR, Karthikesalingam A, Low DE. Outcomes assessment of the surgical management of esophageal cancer in younger and older patients. Ann Thorac Surg. 2012;94:1652–1658. [DOI] [PubMed] [Google Scholar]
- 17.van Nistelrooij AM, Andrinopoulou ER, van Lanschot JJ, Tilanus HW, Wijnhoven BP. Influence of young age on outcome after esophagectomy for cancer. World J Surg. 2012;36:2612–2621. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y, Ruan W, Liu J, et al. Esophageal cancer in patients under 50: a SEER analysis. J Thorac Dis. 2018;10:2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Nistelrooij AM, van Steenbergen LN, Spaander MC, et al. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Onc. 2014;109:561–566. [DOI] [PubMed] [Google Scholar]
- 20.Kasagi Y, Morita M, Otsu H, et al. Clinicopathological characteristics of esophageal squamous cell carcinoma in patients younger than 50 years. Ann Surg Onc. 2015;22:311–315. [DOI] [PubMed] [Google Scholar]
- 21.Yoon HY, Kim CB. Gastroesophageal junction adenocarcinoma of young patients who underwent curative surgery: A comparative analysis with older group. Surgery Today. 2011;41:203–209. [DOI] [PubMed] [Google Scholar]
- 22.Mehta SP, Bailey D, Davies N. Comparative outcome of oesophagogastric cancer in younger patients. Annals of the Royal College of Surgeons of England. 2010;92:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamouda A, Forshaw M, Rohatgi A, Mirnezami R, Botha A, Mason R. Presentation and survival of operable esophageal cancer in patients 55 years of age and below. World J Surg. 2010;34:744–749. [DOI] [PubMed] [Google Scholar]
- 24.Adam DJ, Craig SR, Sang CT, Walker WS, Cameron EW. Oesophagogastrectomy for carcinoma in patients under 50 years of age. J Roy Coll Surg Edin. 1996;41:371–373. [PubMed] [Google Scholar]
- 25.Mori M, Ohno S, Tsutsui S, Matsuura H, Kuwano H, Sugimachi K. Esophageal carcinoma in young patients. Ann Thorac Surg. 1990;49:284–286. [DOI] [PubMed] [Google Scholar]
- 26.Molena D, Stem M, Blackford AL, Lidor AO. Esophageal Cancer Treatment Is Underutilized Among Elderly Patients in the USA. J Gastrointest Surg. 2017;21:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice TW, Blackstone EH, Rusch VW. 7th Edition of the AJCC Cancer Staging Manual: Esophagus and Esophagogastric Junction. Ann Surg Onc. 2010;17:1721–1724. [DOI] [PubMed] [Google Scholar]
- 28.Wong AT, Shao M, Rineer J, Osborn V, Schwartz D, Schreiber D. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esoph. 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 29.Bai Y, Li ZS, Zou DW, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722–728. [DOI] [PubMed] [Google Scholar]
- 30.Cohen E, Bolus R, Khanna D, et al. GERD symptoms in the general population: prevalence and severity versus care-seeking patients. Dig Dis Sci. 2014;59:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kountourakis P, Ajani JA, Davila M, Lee JH, Bhutani MS, Izzo JG. Barrett’s Esophagus: A Review of Biology and Therapeutic Approaches. Gastrointestinal Cancer Research. 2012;5:49–57. [PMC free article] [PubMed] [Google Scholar]
- 32.Ong CA, Shapiro J, Nason KS, et al. Three-gene immunohistochemical panel adds to clinical staging algorithms to predict prognosis for patients with esophageal adenocarcinoma. J Clin Oncol. 2013;31:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin JAOM. Describing the increase in preterm births in the United States, 2014–2016. NCHS Data Brief. 2018;312:1–8. [PubMed] [Google Scholar]
- 34.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–843. [DOI] [PubMed] [Google Scholar]
- 35.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017;288: 1–8. [PubMed] [Google Scholar]
- 36.Drahos J, Xiao Q, Risch HA, Freedman N, Abnet CC, Andersen LA, et al. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int J Cancer. 2016;138:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyerberg EW, Earle CC, Neville BA, Weeks JC. Racial Differences in Surgical Evaluation, Treatment, and Outcome of Locoregional Esophageal Cancer: A Population-Based Analysis of Elderly Patients. J Clin Oncol. 2005;23:510–517. [DOI] [PubMed] [Google Scholar]
- 38.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, McGinn TG, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Onc. 2008;15:881–888. [DOI] [PubMed] [Google Scholar]
- 39.Nobel T, Lavery J, Barbetta A, Gennarelli R, Lidor AO, Jones DR, Molena D. National guidelines may reduce socioeconomic disparities in treatment selection for esophageal cancer. Dis Esophagus. 2018: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munasinghe A, Markar SR, Mamidanna R, et al. Is It Time to Centralize High-risk Cancer Care in the United States? Comparison of Outcomes of Esophagectomy Between England and the United States. Ann Surg. 2015;262:79–85. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc. 2017;31:2491–2497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.