Abstract
Selenium is an essential dietary micronutrient. Ingested selenium is absorbed by the intestines and transported to the liver where it is mostly metabolized to selenocysteine (Sec). Sec is then incorporated into selenoproteins, including selenoprotein P (SELENOP), which is secreted into plasma and serves as a source of selenium to other tissues of the body. Herein, we provide an overview of the biology of selenium from its absorption and distribution to selenoprotein uptake and degradation, with a particular focus on the latter. Molecular mechanisms of selenoprotein degradation include the lysosome-mediated pathway for SELENOP and endoplasmic reticulum-mediated degradation of selenoproteins via ubiquitin-activated proteasomal pathways. Ubiquitin-activated pathways targeting full-length selenoproteins include the peroxisome proliferator-activated receptor gamma-dependent pathway and substrate-dependent ubiquitination. An alternate mechanism is utilized for truncated selenoproteins, in which cullin-RING E3 ubiquitin ligase 2 targets the defective proteins for ubiquitin-proteasomal degradation. Selenoproteins, particularly SELENOP, may have their Sec residues reutilized for new selenoprotein synthesis via Sec decomposition. This review will explore these aspects in selenium biology, providing insights to knowledge gaps that remain to be uncovered.
Keywords: selenium, selenoprotein, degradation, ubiquitination, selenocysteine
1. Introduction
Selenium is a trace element necessary for cellular functions. Our understanding of selenium requirements and homeostasis hinges on our knowledge of how selenium is metabolized. Selenium is ingested from food sources, absorbed in the gut, carried to the liver to be metabolized, and transported and distributed to the tissues of the body. Selenium compounds are mostly metabolized to selenocysteine (Sec), an amino acid that is incorporated into peptide chains to form selenoproteins. There are 25 genes encoding for selenoproteins in humans, and 24 in rodents [1]. Selenium absorption, transport, distribution and use in selenoprotein biosynthesis have been extensively studied, as has the biology of individual selenoproteins [2, 3]. Nevertheless, the degradation pathways of most selenoproteins have received modest attention.
This review will provide a brief overview of dietary selenium absorption, transport, and distribution to tissues, delving further into mechanisms of selenoprotein degradation. We will also discuss selenocysteine decomposition as an important step to provide selenide to new selenoprotein synthesis, a recycling process which may occur after selenoprotein degradation.
2. Selenium Absorption
Selenium enters the food chain through plants and microorganisms, which take up inorganic and organic selenium from the soil via sulfate transporters [4, 5]. Following ingestion, organic forms of selenium such as selenomethionine and selenocysteine (Sec) are absorbed in the small intestine. Studies in rats have demonstrated that selenomethionine absorption in the gut occurs through the same active sodium-dependent transport system as its sulfur-containing counterpart, methionine [6, 7]. Selenocysteine may also be absorbed similarly to cysteine.
Inorganic selenate crosses the intestinal brush border membrane where absorption occurs via the sodium-facilitated and energy-dependent system utilized by sulfate, as observed in pigs, sheep, and rats [8, 9]. Alternatively, selenite is absorbed by non-mediated passive diffusion, with a slow absorption rate compared to amino-acid bound selenium compounds [10, 11]. In terms of bioavailability, selenate and selenite are well-absorbed in the intestines but are not as well-retained compared to organic selenomethionine and Sec. A supplemental dose of selenomethionine of 37 μg/day is sufficient to raise human plasma glutathione peroxidase (GPX) levels to a maximum of 150 U/L, while 66 μg/day of sodium selenite is required to achieve the same level [12, 13]. Human stable isotope studies also indicated 96% gut absorption of ingested selenomethionine compared to 34–47% of selenite [14]. When comparing inorganic selenium sources, 90% of ingested selenate is absorbed in humans compared to 50% absorption of selenite [15]. After intestinal absorption, selenium forms enter the bloodstream and are predominantly taken up into the liver from the portal vein [16], where they are further metabolized.
3. Tissue Distribution of Selenium via Selenoprotein P
Tissue selenium supply is dependent on the plasma selenium transporter selenoprotein P (SELENOP). The liver, a key organ in whole-body selenium homeostasis, is where the majority of SELENOP is produced. SELENOP is a glycoprotein with a C-terminal domain composed of nine Sec residues in mice, rats, and humans, and another Sec residue in the N-terminal region in a redox-active domain [17, 18]. Four isoforms of SELENOP have been identified in rats; a full-length form with 10 Sec residues and shorter variants that terminate at the second, third, and seventh Sec residues [19, 20]. In humans, at least two isoforms of approximately 60 kDa (full-length) and 50 kDa are present in the plasma [21]. The full-length form is generally accepted to be a selenium transporter for tissues of the body while the shorter isoforms are speculated to be involved in redox reactions or signaling [22].
Hepatically-synthesized SELENOP is secreted to the plasma for selenium delivery to peripheral tissues. Selenium is differentially distributed in the body, with some tissues, such as the brain and the testes, acquiring a higher percentage of the total selenium content than others, particularly in times of selenium deficiency. These two organs preferentially sequester selenium as both depend on a steady selenium supply for function [15,16]. Preferential sequestration of selenium leads to a tissue hierarchy in selenium distribution [23], a hierarchy that is also extended to intracellular mechanisms to prioritize the synthesis of a subgroup of selenoproteins [24–27]. SELENOP transport is particularly paramount to tissue-specific selenium hierarchy as evidenced by the effects of its deletion.
Whole-body SELENOP knockout mice exhibit loss of motor coordination and reduced viability, surviving on average about 15 days after weaning [28]. Survival rate and neurological and reproductive impairments are circumvented by supplementing diets with an established nutritional requirement of 0.1 mg/kg selenium [28, 29]. Selenium concentrations are severely reduced in SELENOP knockout mice testes and brains compared to SELENOP homozygous wild-type mice, even with selenium supplementation. A moderate reduction in selenium concentration is observed in the kidneys but not in the liver or heart [28]. These results indicate SELENOP is the primary supplier of selenium to the testes, brain, and, to a lesser extent, kidney.
Interestingly, increasing dietary selenium to 2 mg/kg is able to further raise brain selenium concentrations of SELENOP knockout mice in comparison to wild-type mice. A similar dietary selenium increase does not raise the selenium concentration in the testes of SELENOP knockout mice [28]. One possibility is that the brain can uptake a different chemical form of selenium through a second transport mechanism while the testes rely chiefly on SELENOP to deliver selenium. Inorganic selenium or small-molecule selenium metabolites such as dimethyl selenide, trimethylselenonium, or 1β-methylseleno-N-acetyl-D-galactosamine could serve as alternative sources of selenium. Nevertheless, these small molecules have only been observed as urinary metabolites under conditions of toxic selenium levels [30].
Similar tissue selenium loss is found when SELENOP is deleted only in the liver of mice. This targeted abrogation results in decreased whole-body selenium when compared to wild-type mice. These mice exhibit a reduction in selenium concentrations in most tissues, with comparatively higher retention in the liver. Hepatocyte-specific deletion of SELENOP also increased urinary excretion of selenium metabolites when mice were fed adequate selenium diets, as occurred in mice with whole-body deletion of SELENOP [31]. Intriguingly, when mice with hepatic deletion of SELENOP were fed selenium-deficient diets, their urinary excretion of selenium metabolites was very low. Hence, the hepatic retention of selenium under selenium adequacy is mostly targeted towards synthesizing SELENOP, the selenoprotein with the highest requirement for selenium in its molecule [32].
In terms of the impact of SELENOP in selenium delivery in humans, the SELENOP polymorphism rs3877899 (G/A, Ala234Thr) presents an association between its Ala homozygote version and higher plasma selenium concentrations in response to supplementation. Ala/Ala homozygosity has a frequency of approximately 60% in the population, with plasma selenium levels of Ala homozygotes affected by gender and body mass index [33]. However, breast tissues with the Thr/Thr homozygote version for this polymorphism have a significant positive association with higher selenium concentration compared to either the Ala/Ala homozygote or heterozygotes [34]. In addition, rs3877899 polymorphism of SELENOP has been shown to modulate the risk of developing breast cancer, affecting GPX activity in erythrocytes [35]. Interestingly, pregnant women with the A allele of the rs3877899 polymorphism are able to maintain selenium status during pregnancy better [36].
4. Selenoprotein P Uptake
The differential tissue distribution of selenium may occur as a result of differential SELENOP uptake. Studies using mice expressing only a truncated form of SELENOP (SELENOPΔ240−361) lacking the C-terminal Sec-rich domain of the protein determined that plasma levels of the selenoprotein GPX3 were maintained, but SELENOP levels were elevated compared to wild-type mice. Interestingly, a severe decrease in selenium concentration in testes and brain occurred in these mutant mice, even after selenium supplementation. Moreover, selenium concentration in the kidneys was inversely proportional to dietary selenium supplementation. In contrast, whole-body selenium levels were preserved [37]. Due to inefficient uptake of SELENOP in brain and testes, the SELENOPΔ240−361 mice presented reduced fertility and brain function, especially when fed selenium-deficient diets, though not at the same severity as observed with the SELENOP knockout mice. These results indicated that a differential mechanism for SELENOP uptake was at play, with the C-terminal Sec-rich domain of SELENOP necessary for uptake into selenium-demanding tissues, such as brain and testes, constituting a crucial supply of selenium to these tissues. Additionally, the truncated SELENOP with only one Sec residue was sufficient for supplying selenium to the kidneys [37].
In rodent testis, SELENOP is localized in Sertoli cells of the seminiferous epithelium. In this tissue, apolipoprotein E receptor-2 (ApoER2) was uncovered as one of the SELENOP binding partners [38]. ApoER2 is a low-density lipoprotein (LDL) receptor also localized in the Sertoli cells of the testes. Interestingly, ApoER2 knockout mice are infertile, presenting sperm abnormalities that are similar to those observed in the SELENOP knockout mouse [39, 40]. SELENOP is not taken up by Sertoli cells of ApoER2 knockout mice. Hence, this mouse model has reduced selenium levels in the testis, which jeopardizes spermatogenesis and explains its infertility [38].
Selenium concentrations in ApoER2 knockout mice decrease sharply not only in the testes but also in the brain when compared to heterozygous or homozygous wild-type mice. In addition to sperm defects, these mice also develop neurological abnormalities leading to death if not selenium supplemented [41]. Although deletion of ApoER2 in mice does not disrupt whole-body selenium concentrations, brain function is affected to a similar extent as with disruption of SELENOP in mice, which suggests that the selenium supply to the brain also depends on appropriate binding of both these proteins to facilitate the endocytosis of SELENOP as in the testis. ApoER2 has also been established to interact with SELENOP at the blood-brain barrier, providing a steady selenium supply from the circulatory system to the brain [42]. Interestingly, ApoER2 has different splice variants. The brain isoform possesses five ligand-binding repeats in its extracellular domain compared to four ligand-binding repeats in the testes isoform [32]. The isoform distinction could play a mechanistic role in differences and efficiency of SELENOP binding or uptake into cell.
Binding of SELENOP to ApoER2 depends on the recognition of the Sec-rich C-terminal part of SELENOP, with residues Cys324, Gln 325 and Cys 326 determined to be essential to the binding. These residues are evolutionarily conserved. Although in some species, including humans, either Cys 324 or Cys 326 can be replaced by Sec, which suggests that the chemical characteristics of this sequence are crucial for the binding of SELENOP to ApoER2 [43]. It is postulated that through disulfide or diselenide bridges these residues contribute to a conformation favorable for recognition, especially considering SELENOP is taken up in its oxidized state [44]. SELENOP binds to the YWTD beta-propeller domain of ApoER2 and undergoes endocytosis, likely in a clathrin-dependent manner with the involvement of adaptor protein Dab2, as occurs with other ApoER2 substrates [43, 45]. It remains to be determined, however, if SELENOP and ApoER2 are part of a multi-protein complex that allows for the endocytosis of SELENOP.
While ApoER2 is postulated as the mediator of SELENOP uptake in testes and neurons of mice, kidney cells utilize a different mediator for the uptake of SELENOP. This is evidenced as neither ApoER2 nor C-terminal Sec-rich region were needed for uptake of SELENOP by the proximal tubule epithelial cells, where SELENOP is mostly found in the kidneys. Instead, lipoprotein receptor megalin, which is involved in re-uptake of several plasma proteins, mediates the uptake of SELENOP from the glomerular filtrate into proximal tubule epithelial cells of the kidneys [46]. Whole-body deletion of the megalin gene in mice is perinatally lethal. In megalin knockout fetuses, neither megalin nor SELENOP are detected in the proximal tubule epithelium [46]. Mice expressing a nonsense mutation in the extracellular domain of megalin have significant urinary loss of selenium and SELENOP, implicating this receptor region in the reuptake of SELENOP to serve as selenium supply for the kidneys [47].
Interestingly, megalin is also found in the ependymal cells of the choroid plexus [48], an area where the cerebrospinal fluid is produced to supply the ventricles of the brain, and where SELENOP is also expressed [49]. As in the kidneys, megalin in the choroid plexus may be mediating the SELENOP uptake from plasma to ependymal cells to degrade and re-synthesize it, replenishing a different compartment. The molecular mechanism by which the uptake of SELENOP via megalin occurs at the initial portion of the proximal tubule is not characterized. However, it is puzzling that both members of the LDL receptor family, ApoER2 and megalin, are expressed in brains of mice. Either localization, uptake mechanism or Sec-richness of the SELENOP isoform perhaps prevail for SELENOP to differentiate among its receptors.
It is not implausible to hypothesize that ApoER2-mediated SELENOP uptake is prevalent in other tissues as well. In fact, SELENOP was determined to serve as the selenium supply for rat myoblast L8 and H9c2 cardiac myoblast cells, through an endocytosis mechanism also involving ApoER2. Besides the testis and brain, ApoER2 mRNA is expressed in the bone marrow, placenta, thymus, quadriceps, and heart of mice [50], which suggests that, in all these tissues, ApoER2 could contribute to supplying selenium by mediating SELENOP uptake. It remains to be understood whether the mechanism of ApoER2-dependent SELENOP uptake and endocytosis is universal or if other selenium forms are more predominant and physiologically relevant as selenium supply to these cells, bypassing SELENOP availability.
5. Selenoprotein Degradation
Protein degradation is carried out in cells via lysosome or endoplasmic reticulum (ER)-mediated degradation pathways. Among degradation mechanisms, protein ubiquitination and subsequent ubiquitin-dependent proteasomal degradation is regarded as the most important, often functioning as a control mechanism for the activation or inactivation of signaling pathways, regulating the levels of rate-limiting enzymes [51–53]. Conjugation of at least four monomers of the small polypeptide ubiquitin to lysine residues of proteins targets these proteins to be destroyed by the proteasome, a protein complex comprised of several proteolytic enzymes that degrade target proteins, releasing amino acids to be recycled by the cell. Attachment of the ubiquitin moiety is performed by a cascade of enzymatic events involving a ubiquitin-activating protein (E1), a ubiquitin-conjugating protein (E2), and a ubiquitin ligase (E3) [54]. An array of proteins have been described to have either E1, E2 or E3 activities; hence pathways for degrading different proteins may vary in their players according to characteristics of the targeted protein.
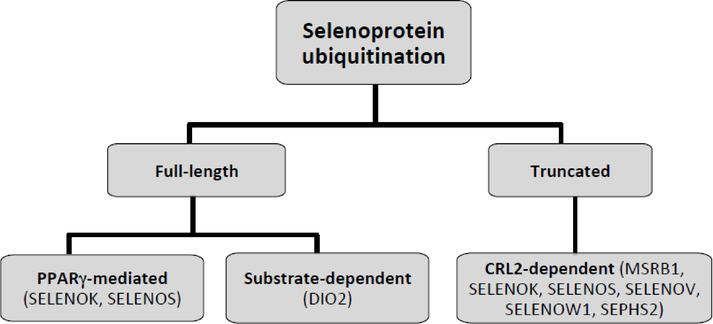
Two selenoproteins, selenoprotein K (SELENOK) and selenoprotein S (SELENOS), have been demonstrated to participate in mechanisms of protein degradation in the ER. In addition, selenoprotein degradation itself has been an element of selenium-dependent metabolism not yet fully explored. ERdependent degradation of type 2 iodothyronine deiodinase (DIO2), SELENOS and SELENOK has been investigated, with interesting results [55–61]. Hormonal control of ER-dependent degradation of selenoproteins has been demonstrated in the cases of DIO2 and SELENOS. Moreover, a mechanism by which truncated selenoproteins are targeted for proteolytic degradation has also been uncovered for SELENOS, SELENOK, selenophosphate synthethase 2 (SEPHS2), selenoprotein V (SELENOV) and methionine sulfoxide reductase B1 (MSRB1). In addition, lysosome-dependent degradation has been suggested for SELENOP. Molecular mechanisms of degradation in which selenoproteins either are degraded (figure 1) or participate are discussed below.
Figure 1.
Flow-chart of currently known ubiquitination mechanisms involved in the degradation of selenoproteins discussed in this review.
5.1 –. Selenoproteins involved in ER-associated protein degradation
Besides being a site for synthesis and trafficking of proteins, the ER is also involved in protein quality control via extraction and degradation of unfolded or misfolded proteins accumulated as a result of alterations in ER homeostasis [62]. ER-associated protein degradation (ERAD) is a mechanism activated by the unfolded protein response (UPR), which detects the accumulation of misfolded proteins as a result of stressful conditions disrupting cellular homeostasis. Moreover, UPR signaling leads to the upregulation of genes, such as ER-chaperones, to relieve ER stress. ERAD and UPR mechanisms are functionally coupled and occur to ensure that only properly folded proteins and protein complexes are conveyed to the cellular milieu [63]. Following recognition by the ERAD pathway, misfolded proteins are retrotranslocated to the cytosol, polyubiquitinated and degraded by the proteasome. ERAD components vary, depending on the subcellular location of the misfolded domain [64].
Seven selenoproteins are known to localize to the ER: DIO2, selenoprotein F (SELENOF), SELENOK, selenoprotein M (SELENOM), selenoprotein N (SELENON), SELENOS, and selenoprotein T (SELENOT). DIO2, SELENOK, SELENON and SELENOS are localized to the ER membrane, whereas SELENOF, SELENOT and SELENOM are present in the lumen of the ER [65, 66]. Interestingly, SELENOS participates in ERAD as a member of a transmembrane complex that retrotranslocates misfolded proteins to the cytosol [67, 68]. SELENOS interacts with multi-protein complexes, allowing anchoring of these complexes to the ER membrane [69]. Pharmacological induction of ER stress increased SELENOS expression in hepatocytes [70], suggesting SELENOS as important for the maintenance of ER homeostasis. Strikingly, SELENOS binds to valosin-containing protein/ATPase p97 (VCP/p97), a complex that binds to largely ubiquitinated proteins facilitating their degradation in the proteasome [71]. SELENOS-VCP/p97 binding is necessary for the binding of another selenoprotein, SELENOK, to VCP/97 as well [72]. Like SELENOS, SELENOK is also upregulated by ER stress, however it does not appear to have a universal role in ERAD. Moreover, both SELENOK and SELENOS are necessary for VCP/p97-dependent ERAD to occur [72]. Nevertheless, SELENOK participates in ERAD of misfolded glycosylated proteins by direct binding to Derlin1, while SELENOS binds to Derlin2 [73]. Since the Derlin subunit that SELENOK and SELENOS bind, as well as the characteristics of misfolded substrates for SELENOK and SELENOS-dependent ERAD, may differ, it is possible that these selenoproteins can determine the nature of the substrate being translocated through the channel.
5.2. ER-mediated degradation of selenoproteins
5.2.1. PPARγ-mediated ubiquitination of selenoproteins S and K
Peroxisome proliferator-activated receptor gamma (PPARγ) is a transcription factor critical for adipogenesis [74]. Interestingly, PPARγ was found in microarray studies of human colorectal adenocarcinoma to act on ubiquitin-mediated proteolysis [75]. This striking finding was further confirmed by the description of an E3 ubiquitin ligase activity for PPARγ. The E3 ubiquitin ligase activity of PPARγ involves a cysteine residue at position 139 (Cys139), which is in a zinc finger domain homologous to a typical E3 RING domain and is independent of PPARγ activity as a transcription factor [76, 77]. PPARγ-mediated ubiquitination and consequent proteolytic degradation of nuclear factor Kappa B (NFκB)/p65 and mucin-1 have been reported [77, 78]. For NFκB/p65 proteolysis, PPARγ ubiquitinates Lys48 in combined action with the associated ubiquitin-conjugating enzyme E2 R1 (UBCH3 or CDC34), and this polyubiquitination is the primary signal for ATP-dependent protein degradation by the 26S proteasome [79]. Inactivation of NFκB is crucial to self-regulation of inflammatory responses by the PPAR family of receptors [80].
An inverse relationship is found between levels of SELENOS and adipogenesis, as PPARγ expression increases [81, 82]. Obesity, a state in which PPARγ levels in adipose tissue soar, also reduces SELENOS levels. Moreover, knockdown of SELENOS in pre-adipocytes promoted adipogenesis as evidenced by increased lipid accumulation in these cells compared to control cells. This inverse relationship between SELENOS and adipogenesis occurs in response to the hormone dexamethasone, a corticosteroid inducer of adipogenesis that was demonstrated to increase SELENOS degradation in the early phase of adipogenesis [60].
SELENOS functions as part of the ERAD machinery, as described above [83]. Adipogenesis is a process that requires increased translation of proteins to maintain hyperplasia and hypertrophy of adipocytes. Elevated translational activity in adipocytes may lead to decreased degradation rates, which could trigger the degradation of the ERAD machinery itself, including SELENOS. This possibility is supported by recent evidence that PPARγ, the main driver of adipogenesis, directly binds to and ubiquitinates the lysine residue at position 150 (Lys150) of SELENOS via the Cys139 residue of PPARγ, which is key to its ubiquitin ligase activity but not to ligand binding [57]. Ubiquitination, and possibly poly-ubiquitination, of Lys150 of SELENOS could serve as a degradation signal induced by dexamethasone. Nevertheless, the specific molecular mechanism by which this corticosteroid participates in the ubiquitination activation remains unknown.
Interestingly, PPARγ was also found to directly bind and ubiquitinate another ER-resident selenoprotein, SELENOK, in adipocytes. In the case of SELENOK, two lysine residues, Lys47 and Lys48, have been recognized as PPARγ ubiquitination targets for subsequent proteasomal degradation. As with SELENOS, degradation of SELENOK was also required for adipocyte differentiation, which indicated that PPARγ-mediated adipogenesis is dependent on selenoprotein degradation [57]. SELENOK is involved in protein palmitoylation in macrophages [84, 85], but in adipocytes, it is unclear whether a similar role occurs. Considering palmitoylation is a fundamental regulatory post-translational modification in adipocyte physiology [86], it is intriguing that SELENOK degradation increases as adipogenesis progresses.
5.2.2. Substrate-dependent ubiquitination of type 2 iodothyronine deiodinase
Type 2 iodothyronine deiodinase (DIO2) is a selenoprotein localized in the ER membrane, with its N-terminus facing the ER lumen and the C-terminus facing the cytosol. DIO2 removes outer ring iodines from thyroid hormones, catalyzing the conversion of biologically inactive thyroxine (T4) to active triiodothyronine (T3), as well as the deiodination of inactive reverse T3 (rT3) to 3,3’-diiodothyronine (3,3’-T2) [87, 88]. DIO2 activity determines tissue-specific T3 concentrations, and is thus a crucial enzyme in regulating not only cellular levels of the hormone but also contributing to the negative feedback loop of the hypothalamic-pituitary-thyroid axis.
DIO2 has a short half-life, under one hour, being proteolytically degraded after ubiquitination and targeting to the proteasomes. Experiments using either the proteasome inhibitor carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (MG132) or inhibiting the ubiquitin-activating enzyme E1 (UBA1) demonstrated stabilization of DIO2 activity [58, 89]. DIO2 ubiquitination was first demonstrated in a ts20 Chinese hamster ovary cell line expressing a temperature-sensitive E1 enzyme. At temperatures that inactivate E1, DIO2 activity and protein levels are stable, even with the addition of substrate rT3 [58].
Interestingly, the degradation of DIO2 is accelerated after its interaction with substrates T4 and rT3, shortening its half-life significantly [59, 90, 91]. Mutation of the Sec133 in the DIO2 active center to cysteine leads to a 1000-fold increase of rT3 (30 pM) required to observe the same downregulation as the native form. Moreover, substitution of the same Sec133 residue with an alanine eliminates DIO2 catalytic activity and abrogates rT3-induced degradation. Substrate interaction at the Sec133 residue in the active site, therefore, induces DIO2 degradation, decreasing its half-life [59]. Interestingly, selenoprotein type 1 iodothyronine deiodinase (DIO1), which also converts T4 into T3, is inactivated by the substrate, but not ubiquitinated in its presence [58]. This specific substrate-regulated ubiquitination of DIO2 allows for control of DIO2 activity and thyroid hormone levels for a distinctive regulation of the negative feedback mechanism via proteolysis [92].
Curiously, the expression of a C-terminal FLAG-tagged DIO2 resulted in significant increases in DIO2 activity and ubiquitinated DIO2 levels. This finding uncovered that the exposure of DIO2 to the cytosol is necessary for proteasomal degradation to occur [58]. DIO2 works as a dimer that associates with Hedgehog-inducible ubiquitin ligase WD repeat and SOCS box-containing 1 (WSB-1) [93], ubiquitin conjugases UBC6 and UBC-7 [94], and DIO2-specific deubiquitinating enzymes ubiquitin-specific peptidase 20 (USP20), and ubiquitin-specific peptidase 33 (USP33) [95]. T4 binding to the DIO2 catalytic site allows for ubiquitination with the participation of WSB-1, as the consequent conformational change that occurs exposes two lysine residues, Lys237 and Lys244, to ubiquitination. This generates a state of transient inactivity for the enzyme, with its rate of reactivation dependent then on the actions of USP20 and USP33 [92]. T4 binding and consequent ubiquitination of DIO2 then allows for retrotranslocation of the selenoprotein to the cytosol via an interaction with the p97/ATPase complex of the 19S proteasome subunit S5a, and terminal degradation [96].
WSB-1 and membrane-associated ring-CH-type finger 6 (MARCH6, also called TEB4) are recognized DIO2-specific E3 ubiquitin ligases [97, 98]. Knockdown of either WSB-1 or MARCH6 significantly increases DIO2 activity, protein levels, half-life, and decreases ubiquitination. DIO2, but not DIO1 and DIO3, contains an 18 amino acid loop required for the interaction with the WD-40 propeller domain of WSB-1 but not the interaction with MARCH6 [93, 97]. Interestingly, WSB-1 and MARCH6 have distinct patterns of tissue gene expression. WSB-1 expression occurs in brown adipose tissue, while MARCH6 is expressed in the heart and pituitary. Both are expressed at high levels in the skeletal muscle and the brain, and both are not expressed in the thyroid [97]. In addition, prolonged iodine intake increases mRNA and protein expression levels of both ubiquitin ligases in the pituitary of rats [99]. This differential expression possibly poses a fascinating distinction between the intricacies of the DIO2 mechanism of proteolysis in different tissues, which ultimately could affect its activity, half-life and consequently physiological control of the negative feedback of hypothalamic-pituitary-thyroid axis.
5.3. Degradation of truncated selenoproteins
Full-length selenoproteins DIO2, SELENOK, and SELENOS have been observed to be degraded through the ubiquitin-proteasome pathway, as discussed above. In addition, truncated selenoproteins are also recognized for degradation by ubiquitin-proteasome mechanisms, however with different molecular players.
The cullin-RING E3 ubiquitin ligase 2 (CRL2) complex is found to ubiquitinate the truncated forms of selenoproteins SELENOK, SELENOS, SELENOV, SEPHS2, SELENOW1, and MSRB1, targeting them for degradation [55]. CRL2 is a modular ubiquitin ligase complex consisting of a cullin 2 (Cul2) scaffold, a RING catalytic domain, an elongin B/C adaptor, and an E3 ubiquitin ligase. The E3 subunit contains a VHL-box that complexes to both the Cul2 and elongin B/C adaptor proteins. Moreover, a RING box protein (Rbx1) connected to Cul2 will complex with interchangeable sets of BC-box proteins functioning as substrate receptors [100]. A diverse array of substrates has been reported to be proteolytic degraded by CRL complexes, including insulin receptor substrate 1 (IRS1), USP18 and JUN, while substrates that are specifically degraded by CRL2 include RNA polymerase II subunit BP1 (RBP1), the SOCS substrate adaptors, and the hypoxia-inducible-factor 2 alpha (HIF2α) [101, 102].
In the case of selenoproteins, CRL2 targets only the truncated forms while the full-length forms remain stable, even as truncated SELENOK and SELENOS only differ from corresponding full-length forms by three and two amino acids, respectively. Furthermore, genetic or pharmacological inhibition of CRL2 stabilized selenoproteins with no effect on their non-Sec containing paralogs, such as selenoprotein W2 (SELW2) and selenophosphate synthetase 1 (SEPHS1), leading to accumulation of these truncated selenoproteins.
CRL2 binds and degrades truncated selenoproteins via recognition of the glycine immediately N-terminal to the absent Sec during translation. This finding was determined by maintenance of CRL2 binding after knockdown of cis and trans factors of selenoprotein translation, including the 3’UTR SECIS element, SECIS binding protein (SBP2), the elongation factor for Sec (EFSec), or any other 3’ sequences downstream of the UGA codon, despite decreases in Sec incorporation [55]. CRL2 recognizes a glycine residue at the −1 or −2 positions of the C-end degron, which complexes with the CRL2 substrate receptors [61]. Strikingly, a critical −1 glycine residue relative to the Sec position at SELENOK and SELENOS and a −2 glycine of SELENOV, were identified as necessary for CRL2-dependent degradation, instead of glycine residues at the C-terminus [55]. Moreover, CRL2 substrate receptors used by truncated selenoproteins do not share a substrate recognition motif, raising the possibility of diverse mechanisms for the degradation of truncated selenoproteins [55, 100, 103].
Direct evidence of ubiquitination of a specific Lys residue and proteasome-mediated degradation of truncated selenoproteins as a result of the association between CRL2 and the C-end degron has not yet been reported. In addition, the E2 ubiquitin-conjugating enzyme associated with CRL2 in truncated selenoprotein ubiquitination has not been identified. These unknowns of the molecular mechanism of ubiquitination of truncated selenoproteins provide an exciting avenue for future studies.
5.4. Lysosome-mediated proteolysis of selenoprotein P
Lysosomes are widely known as the terminal organelles of the endocytic pathway that maintain their acidic lumen via the vacuolar H+-ATPase [104, 105]. The maintenance of this pH, between 4.5 and 5.5, is ideal for the activity of hydrolytic enzymes required for proteolysis [106]. Lysosome-mediated degradation occurs for various reasons including the elimination of pathogens, degradation of organelles, and digestion of macromolecules via receptor-mediated endocytosis, autophagy, and phagocytosis [104].
As previously discussed, SELENOP uptake provides various tissues with the selenium needed for selenoprotein synthesis, but we have yet to learn the specific mechanism by which the selenium in SELENOP is released for cell utilization. It is established that LDL receptors mediate SELENOP uptake in various tissues including testes, brain, and kidney [38, 41, 46]. These receptors probably follow classical receptor-mediated endocytosis for internalization and degradation [107], and some of the molecular details of SELENOP binding with ApoER2 have already been defined, as discussed in section 4 of this review. Nevertheless, specific steps and regulatory players for the endocytosis mechanism still need further clarification.
Lysosomal acidification is essential for utilization of SELENOP as a source of selenium. Incubation of rat myoblast L8 cells in serum containing only SELENOP 75Se-labeled as a selenium source, followed by pharmacological inhibition of lysosomal acidification by either 100 nM bafilomycin A1 or 100 μM chloroquine, led to a reduction in selenoprotein GPX activity, without differences in the amount of selenium uptake. This confirmed that SELENOP was endocytosed in these cells, but it could not be digested to be utilized for GPX production. Moreover, purification of the 75Se-labeled SELENOP in these cells using an antibody recognizing the SELENOP N-terminal region further revealed an accumulation of full-length SELENOP (~50 kDa) in the cells when lysosomal acidification is blocked. In contrast, myoblast cells that were not treated with chloroquine displayed much smaller amounts of the full-length SELENOP, with an 18 kDa fragment as the predominant form. This form was also reduced in comparison to the level of undigested SELENOP in chloroquine-treated cells. Combined, these results confirm that SELENOP digestion occurs in the conventionally acidic lysosomes [50].
A recent investigation expanded on the lysosomal degradation of SELENOP [44]. HeLa cells provided with 75Se-labeled SELENOP were incubated with either chloroquine, lysosomal protease inhibitor leupeptin, or proteasomal inhibitor MG-132. Neither MG-132 nor leupeptin affected the degradation of SELENOP, while chloroquine, as expected, was able to inhibit SELENOP digestion. Since leupeptin specifically inhibits lysosomal serine, cysteine and threonine proteases, the result suggests that these proteases are most likely not involved in the breakdown of SELENOP. This same study provides further evidence of the conditions needed for SELENOP proteolysis in the lysosome. By testing the stability of 75Se-SELENOP in the presence of acidifying and reducing conditions, it was found that 75Se-labeled SELENOP only degraded when media contained 0.5% acetic acid and 2 mM dithiothreitol (DTT), a potent reducing reagent. Moreover, this susceptibility to degradation was completely eliminated when the histidine stretch of SELENOP, responsible for binding to divalent cations and contributing to its structure stabilization, was blocked [44]. Combined, these results confirmed that acidic and reducing conditions without changes in the conformational structure of the histidine stretch are needed for SELENOP proteolysis in the lysosome.
In summary, SELENOP endocytosis upon binding to the receptor is likely followed by fusion of the early SELENOP-containing endosomes with lysosomes. If the receptor is ApoER2, the endocytosis is probably via a clathrin-dependent mechanism. Acidification of the lysosome primes its catalytic enzymes for the digestion of SELENOP. This limited evidence suggests that proper lysosomal conditions are essential for SELENOP breakdown and consequently utilization of its Sec residues as a source of selenium, but additional research is required to elucidate the molecular intricacies of this mechanism further.
6. Selenocysteine Decomposition as a Source of Selenium
SELENOP is delivered to the cell and possibly degraded by endosome fusion with a lysosome, making its reserve of Sec residues available to the synthesis of new selenoproteins. The Sec residues of SELENOP, as well as the ones released after degradation of other selenoproteins, may also become a pool of selenium to be recycled and utilized for selenoprotein synthesis.
Sec residues coming from degradation mechanisms are not directly incorporated into selenoproteins. Sec is synthesized on its own tRNA[Ser]Sec after aminoacylation with a seryl moiety and its phosphorylation. Selenide produced by the metabolism of various selenocompounds, including the decomposition of Sec, is used to form selenophosphate via the actions of the selenoprotein SEPHS2. Selenophosphate is then used by the enzyme Sec synthase to synthesize Sec attached to the tRNA[Ser]Sec using the phosphoseryl moiety as a backbone [3]. Hence, to be utilized in selenoprotein synthesis, Sec derived from degradation requires first to be decomposed into selenide.
Sec decomposition is catalyzed by the enzyme selenocysteine lyase (Scly) [108, 109]. Scly is mostly expressed in tissues that rely on SELENOP as a selenium source, such as liver, kidneys, and testes. Although Scly is found in the spermatids of mature testes while SELENOP is mostly taken up by Sertoli cells [108, 110]. Targeted knockdown of Scly in cultured cells or knockout in selenium-deficient mice both decrease selenoprotein expression [110–112], implicating Scly-mediated production of selenide as a source of selenium for new selenoprotein synthesis, especially when selenium is limiting.
The specific molecular mechanism by which lysosomally-degraded SELENOP releases its Sec residues and these become available for selenoprotein synthesis has not been elucidated. Possibly, Sec-rich SELENOP could work in tandem with Scly to deliver selenide to cells after its degradation. Nevertheless, whole-body deletion of Scly in mice did not lead to infertility or neurological disturbances as with SELENOP knockout mice [111]. Interestingly, whole-body Scly knockout mice fed selenium-deficient diets become obese with glucose intolerance and hyperinsulinemia, displaying severe declines in liver GPX1 and SELENOS and plasma SELENOP [112]. These animals additionally present mild neurological dysfunctions in spatial learning [111], and diminished hypothalamic expression of selenoproteins SELENOM, SELENOS, and GPX1 [113].
The development of a mouse model lacking both Scly and SELENOP led to a new perspective regarding the role of SELENOP as a source of selenium for cells with the action of Scly. These double knockout mice exhibit more severe neurological impairments than the SELENOP knockout mice, requiring selenium supplementation of dams and pups to survive past the onset of puberty [114]. Brain selenium is undoubtedly supplied by the combined efforts of Scly and SELENOP. Interestingly, neurological function in these mice can be rescued by castration [115], which uncovers a fascinating competition between the brain and testes for selenium availability. The fact that castration demonstrated such a dramatic rescue of neurological deficits underscores the critical nature of appropriate tissue selenium distribution, and the importance of the testis demand on selenium supply. Ultimately, experimental models such as those described herein are highly illuminating in improving our knowledge of selenium metabolism, function and nutritional requirements.
7. Degradation of Damaged or Abnormal Selenoproteins in Plants
While plants serve as an important source of dietary selenium to animals, higher plants mostly do not require selenium to survive [116]. Plants growing in a selenium-rich environment can accumulate up to <100 mg of selenium per kg of dry matter [117]. This trace element enters the plant via its root system utilizing the sulfate transporters SULTR1 and SULTR2, accumulate in vacuoles, and may reach toxic levels in plant tissues such as shoots [118–120]. In cellular excess, selenium analogs of sulfur-containing amino acids cysteine and methionine, i.e. selenocysteine and selenomethionine, can be produced and misincorporated in place of the former in the primary structure, usually resulting in malformed proteins with selenium atom substituted for sulfur [121].
Ubiquitination of mistranslated Sec-containing proteins has been observed in the selenium-accumulator plant Stanleya pinnata where levels of 26S proteasome unit increase upon selenate treatment. Moreover, selenium non-accumulator Arabidopsis presenting 26S proteasome defects showed decreased selenium tolerance, possibly due to the accumulation of misfolded Sec-containing proteins. Combined, these results substantiate that selenoprotein degradation in plants also includes ubiquitin-proteasomal recognition [56].
There is also evidence of selenium-stress induced ubiquitination and proteasome activity in the green algae Chlamydomonas reinhardtii that is both dose- and time-dependent. A moderate treatment with 50 ^M sodium selenite provided to C. reinhardtii led to two-fold increased proteasomal activity with ubiquitin accumulation. Nevertheless, after 28 hours of moderate selenite exposure, a decrease in proteasomal activity occurred as well as the disappearance of accumulated ubiquitin [122]. This is attributed to severe selenite toxicity that overwhelms the capacity of the C. reinhardtii ubiquitin-proteasome pathway. Overall, these findings suggest that the mechanism to curb the increase of misfolded Sec-containing proteins in selenium toxicity in plants involves ubiquitin-proteasome degradation. More studies are required for unveiling specific mechanisms by which ubiquitination and consequent proteolysis of selenoproteins occur in plants.
8. Conclusion
Dietary selenium is essential to mammalian viability, as this trace element is central to pathways that curb oxidative stress and regulate endocrine physiology, as well as its critical role in neuronal function and male fertility. We have reviewed here essential aspects of selenium nutrition in mammalian physiology, from absorption to the degradation of its main bioactive products, selenoproteins, and the potential recycling of the amino acid Sec. The molecular mechanism by which most selenoproteins are degraded is a topic still in its scientific infancy, yet published findings and ongoing studies are a prelude for a fascinating future lying ahead, which will certainly enlighten our understanding of selenium metabolism.
Acknowledgments
Supported by United States National Institutes of Health grants R01DK47320 (NIDDK), R01DK47320-S1 (Office of Dietary Supplements) and G12MD007601 (NIMHD) to Marla J. Berry, and U54MD007601 - subproject 5544 (NIMHD) to Lucia A. Seale.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Kryukov GV, et al. , Characterization of mammalian selenoproteomes. Science, 2003. 300(5624): p. 1439–43. [DOI] [PubMed] [Google Scholar]
- 2.Davis CD, Tsuji PA, and Milner JA, Selenoproteins and cancer prevention. Annu Rev Nutr, 2012. 32: p. 73–95. [DOI] [PubMed] [Google Scholar]
- 3.Labunskyy VM, Hatfield DL, and Gladyshev VN, Selenoproteins: molecular pathways and physiological roles. Physiol Rev, 2014. 94(3): p. 739–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sors TG, et al. , Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J, 2005. 42(6): p. 785–97. [DOI] [PubMed] [Google Scholar]
- 5.Dumont E, Vanhaecke F, and Cornelis R, Selenium speciation from food source to metabolites: a critical review. Anal Bioanal Chem, 2006. 385(7): p. 1304–23. [DOI] [PubMed] [Google Scholar]
- 6.Schrauzer GN, Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr, 2000. 130(7): p. 1653–6. [DOI] [PubMed] [Google Scholar]
- 7.Vendeland SC, et al. , Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. Biometals, 1994. 7(4): p. 305–12. [DOI] [PubMed] [Google Scholar]
- 8.Wolffram S, Grenacher B, and Scharrer E, Transport of selenate and sulphate across the intestinal brush-border membrane of pig jejunum by two common mechanism. Q J Exp Physiol, 1988. 73(1): p. 103–11. [DOI] [PubMed] [Google Scholar]
- 9.Cherest H, et al. , Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics, 1997. 145(3): p. 627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiry C, et al. , An in vitro investigation of species-dependent intestinal transport of selenium and the impact of this process on selenium bioavailability. Br J Nutr, 2013. 109(12): p. 2126–34. [DOI] [PubMed] [Google Scholar]
- 11.Reasbeck PG, et al. , Selenium absorption by canine jejunum. Dig Dis Sci, 1985. 30(5): p. 489–94. [DOI] [PubMed] [Google Scholar]
- 12.Burk RF, et al. , Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomarkers Prev, 2006. 15(4): p. 804–10. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, et al. , Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr, 2005. 81(4): p. 829–34. [DOI] [PubMed] [Google Scholar]
- 14.Van Dael P, et al. , Selenium absorption and retention from a selenite- or selenate-fortified milk- based formula in men measured by a stable-isotope technique. Br J Nutr, 2001. 85(2): p. 157–63. [DOI] [PubMed] [Google Scholar]
- 15.Mangels AR, et al. , Selenium utilization during human lactation by use of stable-isotope tracers. Am J Clin Nutr, 1990. 52(4): p. 621–7. [DOI] [PubMed] [Google Scholar]
- 16.Kato T, et al. , Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am J Physiol, 1992. 262(5 Pt 1): p. G854–8. [DOI] [PubMed] [Google Scholar]
- 17.Burk RF and Hill KE, Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr, 2005. 25: p. 215–35. [DOI] [PubMed] [Google Scholar]
- 18.Burk RF and Hill KE, Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta, 2009. 1790(11): p. 1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himeno S, Chittum HS, and Burk RF, Isoforms of selenoprotein P in rat plasma. Evidence for a full-length form and another form that terminates at the second UGA in the open reading frame. J Biol Chem, 1996. 271(26): p. 15769–75. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, et al. , Mass spectrometric characterization of full-length rat selenoprotein P and three isoforms shortened at the C terminus. Evidence that three UGA codons in the mRNA open reading frame have alternative functions of specifying selenocysteine insertion or translation termination. J Biol Chem, 2002. 277(15): p. 12749–54. [DOI] [PubMed] [Google Scholar]
- 21.Meplan C, et al. , Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, se intake, and cancer status. Antioxid Redox Signal, 2009. 11(11): p. 2631–40. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa S, et al. , Sepp1(UF) forms are N-terminal selenoprotein P truncations that have peroxidase activity when coupled with thioredoxin reductase-1. Free Radic Biol Med, 2014. 69: p. 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burk RF and Hill KE, Regulation of Selenium Metabolism and Transport. Annu Rev Nutr, 2015. 35: p. 109–34. [DOI] [PubMed] [Google Scholar]
- 24.Sunde RA, et al. , Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep, 2009. 29(5): p. 329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low SC, et al. , SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J, 2000. 19(24): p. 6882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry MJ, Insights into the hierarchy of selenium incorporation. Nat Genet, 2005. 37(11): p. 1162–3. [DOI] [PubMed] [Google Scholar]
- 27.Kuhbacher M, et al. , The brain selenoproteome: priorities in the hierarchy and different levels of selenium homeostasis in the brain of selenium-deficient rats. J Neurochem, 2009. 110(1): p. 13342. [DOI] [PubMed] [Google Scholar]
- 28.Hill KE, et al. , Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem, 2003. 278(16): p. 13640–6. [DOI] [PubMed] [Google Scholar]
- 29.Weiss Sachdev S and Sunde RA, Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and −4 in rat liver. Biochem J, 2001. 357(Pt 3): p. 851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi Y, et al. , Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc Natl Acad Sci U S A, 2002. 99(25): p. 15932–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burk RF, et al. , Deletion of selenoprotein P upregulates urinary selenium excretion and depresses whole-body selenium content. Biochim Biophys Acta, 2006. 1760(12): p. 1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill KE, et al. , Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J Biol Chem, 2012. 287(48): p. 40414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meplan C, et al. , Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGENstudy). Faseb J, 2007. 21(12): p. 3063–74. [DOI] [PubMed] [Google Scholar]
- 34.Ekoue DN, et al. , Selenium levels in human breast carcinoma tissue are associated with a common polymorphism in the gene for SELENOP (Selenoprotein P). J Trace Elem Med Biol, 2017. 39: p. 227–233. [DOI] [PubMed] [Google Scholar]
- 35.Meplan C, et al. , Association between polymorphisms in glutathione peroxidase and selenoprotein P genes, glutathione peroxidase activity, HRT use and breast cancer risk. PLoS One, 2013. 8(9): p. e73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao J, et al. , Genetic polymorphisms that affect selenium status and response to selenium supplementation in United Kingdom pregnant women. Am J Clin Nutr, 2016. 103(1): p. 100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill KE, et al. , The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J Biol Chem, 2007. 282(15): p. 10972–80. [DOI] [PubMed] [Google Scholar]
- 38.Olson GE, et al. , Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J Biol Chem, 2007. 282(16): p. 12290–7. [DOI] [PubMed] [Google Scholar]
- 39.Andersen OM, et al. , Differential binding of ligands to the apolipoprotein E receptor 2. Biochemistry, 2003. 42(31): p. 9355–64. [DOI] [PubMed] [Google Scholar]
- 40.Andersen OM, et al. , Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem, 2003. 278(26): p. 23989–95. [DOI] [PubMed] [Google Scholar]
- 41.Burk RF, et al. , Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J Neurosci, 2007. 27(23): p. 6207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burk RF, et al. , Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J, 2014. 28(8): p. 3579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurokawa S, et al. , Isoform-specific binding of selenoprotein P to the beta-propeller domain of apolipoprotein E receptor 2 mediates selenium supply. J Biol Chem, 2014. 289(13): p. 9195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shetty S, Marsicano JR, and Copeland PR, Uptake and Utilization of Selenium from Selenoprotein P. Biol Trace Elem Res, 2018. 181(1): p. 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuitino L, et al. , ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its Rafts’ association. Traffic, 2005. 6(9): p. 820–38. [DOI] [PubMed] [Google Scholar]
- 46.Olson GE, et al. , Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem, 2008. 283(11): p. 6854–60. [DOI] [PubMed] [Google Scholar]
- 47.Chiu-Ugalde J, et al. , Mutation of megalin leads to urinary loss of selenoprotein P and selenium deficiency in serum, liver, kidneys and brain. Biochem J, 2010. 431(1): p. 103–11. [DOI] [PubMed] [Google Scholar]
- 48.Zheng G, et al. , Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J Histochem Cytochem, 1994. 42(4): p. 531–42. [DOI] [PubMed] [Google Scholar]
- 49.Scharpf M, et al. , Neuronal and ependymal expression of selenoprotein P in the human brain. J Neural Transm, 2007. 114(7): p. 877–84. [DOI] [PubMed] [Google Scholar]
- 50.Kurokawa S, et al. , Long isoform mouse selenoprotein P (Sepp1) supplies rat myoblast L8 cells with selenium via endocytosis mediated by heparin binding properties and apolipoprotein E receptor-2 (ApoER2). J Biol Chem, 2012. 287(34): p. 28717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myung J, Kim KB, and Crews CM, The ubiquitin-proteasome pathway andproteasome inhibitors. Med Res Rev, 2001. 21(4): p. 245–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glickman MH and Ciechanover A, The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev, 2002. 82(2): p. 373–428. [DOI] [PubMed] [Google Scholar]
- 53.Dunn WA Jr., Autophagy and related mechanisms of lysosome-mediatedprotein degradation. Trends Cell Biol, 1994. 4(4): p. 139–43. [DOI] [PubMed] [Google Scholar]
- 54.Lee MJ and Yaffe MB, Protein Regulation in Signal Transduction, in Signal Transduction - Principles, Pathways, and Processes, Cantley LC, et al. , Editors. 2014, Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY: p. 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin HC, et al. , SELENOPROTEINS. CRL2 aids elimination of truncated selenoproteins produced by failed UGA/Sec decoding. Science, 2015. 349(6243): p. 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabbagh M and Van Hoewyk D, Malformed selenoproteins are removed by the ubiquitin-proteasome pathway in Stanleya pinnata. Plant Cell Physiol, 2012. 53(3): p. 555–64. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, et al. , Degradation of selenoprotein S and selenoprotein K through PPARgamma-mediated ubiquitination is required for adipocyte differentiation. Cell Death Differ, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gereben B, et al. , Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol, 2000. 14(11): p. 1697–708. [DOI] [PubMed] [Google Scholar]
- 59.Steinsapir J, et al. , Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme’s active center. Endocrinology, 2000. 141(3): p. 1127–35. [DOI] [PubMed] [Google Scholar]
- 60.Kim CY and Kim KH, Dexamethasone-induced selenoprotein S degradation is required for adipogenesis. J Lipid Res, 2013. 54(8): p. 2069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rusnac DV, et al. , Recognition of the Diglycine C-End Degron by CRL2(KLHDC2) Ubiquitin Ligase. Mol Cell, 2018. 72(5): p. 813–822 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotamisligil GS and Davis RJ, Cell Signaling and Stress Responses, in Signal Transduction - Principles, Pathways, and Processes, Cantley LC, et al. , Editors. 2014, Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY: p. 345–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai YC and Weissman AM, The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer, 2010. 1(7): p. 764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X and Rapoport TA, Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol, 2018. 53: p. 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reeves MA and Hoffmann PR, The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci, 2009. 66(15): p. 2457–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baqui MM, et al. , Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology, 2000. 141(11): p. 4309–12. [DOI] [PubMed] [Google Scholar]
- 67.Ye Y, et al. , A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature, 2004. 429(6994): p. 841–7. [DOI] [PubMed] [Google Scholar]
- 68.Schulze A, et al. , The ubiquitin-domain protein HERP forms a complex with components of the endoplasmic reticulum associated degradation pathway. J Mol Biol, 2005. 354(5): p. 1021–7. [DOI] [PubMed] [Google Scholar]
- 69.Turanov AA, et al. , Selenoprotein S is involved in maintenance and transport of multiprotein complexes. Biochem J, 2014. 462(3): p. 555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao Y, et al. , Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress - SelS is a novel glucose-regulated protein. FEBS Lett, 2004. 563(1–3): p. 185–90. [DOI] [PubMed] [Google Scholar]
- 71.van den Boom J and Meyer H, VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol Cell, 2018. 69(2): p. 182–194. [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, et al. , Selenoprotein S-dependent Selenoprotein K Binding to p97(VCP) Protein Is Essential for Endoplasmic Reticulum-associated Degradation. J Biol Chem, 2015. 290(50): p. 29941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shchedrina VA, et al. , Selenoprotein K binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis. J Biol Chem. 286(50): p. 42937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lowell BB, PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell, 1999. 99(3): p. 239–42. [DOI] [PubMed] [Google Scholar]
- 75.Cekanova M, et al. , Gene alterations by peroxisome proliferator-activated receptor gamma agonists in human colorectal cancer cells. Int J Oncol, 2008. 32(4): p. 809–19. [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe M, et al. , The E3 ubiquitin ligase TRIM23 regulates adipocyte differentiation via stabilization of the adipogenic activator PPARgamma. Elife, 2015. 4: p. e05615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou Y, Moreau F, and Chadee K, PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65. Nat Commun, 2012. 3: p. 1300. [DOI] [PubMed] [Google Scholar]
- 78.Hou Y, et al. , PPARgamma E3 ubiquitin ligase regulates MUC1-C oncoprotein stability. Oncogene, 2014. 33(49): p. 5619–25. [DOI] [PubMed] [Google Scholar]
- 79.Xu P, et al. , Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell, 2009. 137(1): p. 133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korbecki J, Bobinski R, and Dutka M, Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim CY, et al. Selenium inhibits adipogenesis through suppression of ER-stress and induction of selenoprotein S in Experimental Biology 2011. 2011. Washington, DC: FASEB. [Google Scholar]
- 82.Olsson M, et al. , Expression of the selenoprotein S (SELS) gene in subcutaneous adipose tissue and SELS genotype are associated with metabolic risk factors. Metabolism, 2011. 60(1): p. 11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitts MW and Hoffmann PR, Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium, 2018. 70: p. 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fredericks GJ, et al. , Selenoprotein K Increases Efficiency of DHHC6 Catalyzed Protein Palmitoylation by Stabilizing the Acyl-DHHC6 Intermediate. Antioxidants (Basel), 2017. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Norton RL, et al. , Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient FcgammaR-mediated phagocytosis. J Leukoc Biol, 2017. 101(2): p. 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren W, Jhala US, and Du K, Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte, 2013. 2(1): p. 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bianco AC, et al. , Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev, 2002. 23(1): p. 38–89. [DOI] [PubMed] [Google Scholar]
- 88.Marsili A, et al. , Physiological role and regulation of iodothyronine deiodinases: a 2011 update. J Endocrinol Invest, 2011. 34(5): p. 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steinsapir J, Harney J, and Larsen PR, Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J Clin Invest, 1998. 102(11): p. 1895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.St Germain DL, The effects and interactions of substrates, inhibitors, and the cellular thiol- disulfide balance on the regulation of type II iodothyronine 5’-deiodinase. Endocrinology, 1988. 122(5): p. 1860–8. [DOI] [PubMed] [Google Scholar]
- 91.Silva JE and Leonard JL, Regulation of rat cerebrocortical and adenohypophyseal type II5’- deiodinase by thyroxine, triiodothyronine, and reverse triiodothyronine. Endocrinology, 1985. 116(4): p. 1627–35. [DOI] [PubMed] [Google Scholar]
- 92.Sagar GD, et al. , Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol, 2007. 27(13): p. 4774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dentice M, et al. , The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol, 2005. 7(7): p. 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Botero D, et al. , Ubc6p and ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol Endocrinol, 2002. 16(9): p. 1999–2007. [DOI] [PubMed] [Google Scholar]
- 95.Curcio-Morelli C, et al. , Deubiquitination of type 2 iodothyronine deiodinase by von HippelLindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest, 2003. 112(2): p. 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arrojo EDR, et al. , The type II deiodinase is retrotranslocated to the cytoplasm and proteasomes via p97/Atx3 complex. Mol Endocrinol, 2013. 27(12): p. 2105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zavacki AM, et al. , The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol Cell Biol, 2009. 29(19): p. 5339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fekete C, et al. , Expression patterns of WSB-1 and USP-33 underlie cell-specific posttranslational control of type 2 deiodinase in the rat brain. Endocrinology, 2007. 148(10): p. 4865–74. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, et al. , Effect of Prolonged Iodine Overdose on Type 2 lodothyronine Deiodinase Ubiquitination-Related Enzymes in the Rat Pituitary. Biol Trace Elem Res, 2016. 174(2): p. 377–386. [DOI] [PubMed] [Google Scholar]
- 100.Cai W and Yang H, The structure and regulation of Cullin 2 based E3 ubiquitin ligases and their biological functions. Cell Div, 2016. 11: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Emanuele MJ, et al. , Global identification of modular cullin-RING ligase substrates. Cell, 2011. 147(2): p. 459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petroski MD and Deshaies RJ, Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol, 2005. 6(1): p. 9–20. [DOI] [PubMed] [Google Scholar]
- 103.Kamura T, et al. , VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev, 2004. 18(24): p. 3055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saftig P and Klumperman J, Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol, 2009. 10(9): p. 623–35. [DOI] [PubMed] [Google Scholar]
- 105.Johnson DE, et al. , The position of lysosomes within the cell determines their luminal pH. J Cell Biol, 2016. 212(6): p. 677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mellman I, Fuchs R, and Helenius A, Acidification of the endocytic and exocytic pathways. Annu Rev Biochem, 1986. 55: p. 663–700. [DOI] [PubMed] [Google Scholar]
- 107.Hussain MM, Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front Biosci, 2001. 6: p. D417–28. [DOI] [PubMed] [Google Scholar]
- 108.Esaki N, et al. , Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J Biol Chem, 1982. 257(8): p. 4386–91. [PubMed] [Google Scholar]
- 109.Mihara H, et al. , cDNA cloning, purification, and characterization of mouse liver selenocysteine lyase. Candidate for selenium delivery protein in selenoprotein synthesis. J Biol Chem, 2000. 275(9): p. 6195–200. [DOI] [PubMed] [Google Scholar]
- 110.Kurokawa S, et al. , Mammalian selenocysteine lyase is involved in selenoprotein biosynthesis. J Nutr Sci Vitaminol (Tokyo), 2011. 57(4): p. 298–305. [DOI] [PubMed] [Google Scholar]
- 111.Raman AV, et al. , Absence of selenoprotein P but not selenocysteine lyase results in severe neurological dysfunction. Genes Brain Behav, 2012. 11(5): p. 601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seale LA, et al. , Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol Cell Biol, 2012. 32(20): p. 4141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogawa-Wong AN, et al. , Sexual Dimorphism in the Selenocysteine Lyase Knockout Mouse. Nutrients, 2018. 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Byrns CN, et al. , Mice lacking selenoprotein P and selenocysteine lyase exhibit severe neurological dysfunction, neurodegeneration, and audiogenic seizures. J Biol Chem, 2014. 289(14): p. 9662–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pitts MW, et al. , Competition between the Brain and Testes under Selenium-Compromised Conditions: Insight into Sex Differences in Selenium Metabolism and Risk of Neurodevelopmental Disease. J Neurosci, 2015. 35(46): p. 15326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhuo P and Diamond AM, Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta, 2009. 1790(11): p. 1546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.White PJ, Selenium accumulation by plants. Ann Bot, 2016. 117(2): p. 217–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El Kassis E, et al. , Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol, 2007. 143(3): p. 1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terry N, et al. , Selenium in Higher Plants. Annu Rev Plant Physiol Plant Mol Biol, 2000. 51: p. 401–432. [DOI] [PubMed] [Google Scholar]
- 120.Zhu YG, et al. , Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci, 2009. 14(8): p. 436–42. [DOI] [PubMed] [Google Scholar]
- 121.White PJ, Selenium metabolism in plants. Biochim Biophys Acta Gen Subj, 2018. [DOI] [PubMed] [Google Scholar]
- 122.Vallentine P, et al. , The ubiquitin-proteasome pathway protects Chlamydomonas reinhardtii against selenite toxicity, but is impaired as reactive oxygen species accumulate. AoB Plants, 2014. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]