Abstract
The prostate is an important organ for the maintenance of sperm health with prostate cancer being a common disease for which there is a critical need to distinguish indolent from aggressive disease. Several selenium containing proteins have been implicated in prostate cancer risk or outcome due to either enzyme function, the reduced levels of these proteins being associated with cancer recurrence after prostatectomy or their corresponding genes containing single nucleotide polymorphisms associated with increased risk. Moreover, experimental data obtained from the manipulation of either cultured cells or animal models have indicated that some of these proteins are contributing mechanistically to prostate cancer incidence or progression. Among these are selenocysteine-containing proteins selenoprotein P (SELENOP), glutathione peroxidase (GPX1) and selenoprotein 15 (SELENOF) and the selenium-associated protein selenium binding protein 1 (SBP1). Genotyping of some of these genes for these proteins have identified functional single nucleotide polymorphisms that are associated with prostate cancer risk and the direct quantification of these proteins in human prostate tissues has not only revealed associations to clinical outcomes but have also identified unique properties that are different from what is observed in other tissue types. The location of GPX1 in the nucleus and SELENOF in the plasma membrane of prostate epithelial cells indicate that these proteins may have functions in normal prostate tissue that are distinct from that of the other tissue types.
Introduction
The prostate is a highly specialized organ among whose functions is to accumulate and secrete large amounts of citrate as a component of semen, thus supporting sperm health. Prostate cancer (PCa) remains a significant clinical problem in the USA with an estimated 180,890 men being diagnosed with the disease and 26,120 men dying from PCa in 2016 according to the American Cancer Society, making death from PCa the second leading cause of death among American men. The most significant risk factor for prostate cancer is aging, with the average age of being diagnosed with the disease being approximately 66 years old. Because of its prevalence and long latency period, prostate cancer is considered to be a strong candidate as a target for a chemoprevention strategy.
Interest in selenium in the prevention of cancer in general was sparked by reports of an inverse association between multiple cancer types and selenium levels in the 1970’s [1]. What followed was an extensive period of experimentation with selenium in tissue culture and animal models of carcinogenesis with promising results. Selenium was able to inhibit the growth of cancer cells and was effective in reducing the growth of a wide variety of tumors induced by carcinogens in rodents [2]. While the growth of prostate cancer cells could be inhibited with selenium compounds, only a single paper reported data on the effective suppression of prostate lesions, this occurring in the TRAMP mouse, a rodent model where prostate cancer occurs due to the expression of the SV40 T antigen oncogene from a prostate-specific promoter, resulting in tumors of neuroendocrine origin and not the much more common tumors which as adenocarcinomas of epithelial origin [3]. Selenium was shown to be ineffective in reducing prostate cancer incidence using rats in which prostate tumors were induced either by combination of testosterone and estradiol or by exposure to N-methyl-N-nitrosurea [4,5]. And while several observational cohort studies have detected an association between reduced selenium status and prostate cancer risk, this result has not been consistently found among different study populations [6].
The interest in selenium as a means to reduce prostate cancer risk increased in 1997 when the results of National Prevention of Cancer trial were reported [7]. While the primary endpoint of the selenium supplementation trial was the prevention of the recurrence of skin cancers, the secondary analyses indicated a protective effect of selenium against prostate cancer among those participants with the lowest baseline selenium levels [8]. With promising results from cell culture, animal and human research, the National Institutes of Health embarked on the largest prostate prevention trial ever conducted, the Selenium and Vitamin E in Cancer Prevention Trial (SELECT). SELECT was terminated early due to the increased risk of prostate cancer in the vitamin E arm of the study and an apparent lack of benefit of selenium supplementation [9]. Several commentaries provided opinions as to why selenium was not effective in SELECT. The authors of these have suggested that the form of selenium used may have been a factor: the previous study from 1997 used a selenized yeast supplement while SELECT used selenomethionine, and others have suggested that the differences in baseline levels of selenium in the different cohorts was a factor [10–12], In spite of the disappointing results from SELECT, the interest in selenium’s impact on prostate cancer has evolved into the study of selenium-containing proteins implicated in the biology of both the normal and cancerous prostate, this being the focus of this review.
Selenoproteins Implicated in Cancer
Selenium-containing proteins fall into two basic categories: a small group of selenium-containing proteins with selenium covalently attached, i.e. selenium binding protein 1 (SBP1), and a better characterized group including proteins containing selenium as the amino acid selenocysteine, which is encoded by an in-frame UGA codon. For this second group, the process of selenoprotein biosynthesis begins with the aminoacylation of a unique tRNA with serine and the subsequent synthesis of selenocysteine via a phosphoseryl tRNA intermediate [13,14], Recognition of the in-frame UGA codon as the triplet for selenocysteine occurs if there is a structurally conserved sequence, called the Selenocysteine Insertion Sequence (SECIS) element, in the 3’-untranslated region (UTR) of the selenoprotein mRNA [15]. While selenoproteins were initially identified by their ability to incorporate radioactive Se75, Gladyshev and colleagues developed an algorithm to accurately detect all selenoprotein genes encoding selenocysteine in any sequenced genome, including the 25 present in humans [16].
With few exceptions, selenocysteine, when present in proteins, occurs within the active site of enzymes that participate in oxidation/reduction reactions. Reactions catalyzed when selenocysteine is present proceed at speeds orders of magnitude higher than when cysteine is present at that position. Many selenoproteins are anti-oxidants with the ability to detoxify reactive oxygen species (ROS) or oxidized proteins, and this created early interest in the possibility that reduced selenoprotein levels could increase the risk of prostate cancer [17]. In support of this possibility was data resulting from the cross between mice with reduced selenoprotein levels with mice engineered to develop prostate cancer [18]. In this study, bigenic mice that expressed lower levels of several selenoproteins due to the expression of an altered selenocysteine tRNA gene as well as the simian virus 40 (SV40) early-region large T and small t oncogenes (C3(1)Tag) were created. These mice exhibited accelerated prostatic hyperplasia and nuclear atypia, lesions associated with prostate cancer progression compared to the C3(1)Tag mice. Using a different genetic approach, the specific deletion of the selenocysteine tRNA in mouse prostate epithelium, resulted in the appearance of intraepithelial neoplasia which progressed to high grade dysplasia and carcinomas [19].
Further evidence of the role of selenoproteins in a wide range of diseases comes from studies in which naturally occurring genetic variations in several of the corresponding genes were shown to associate with an increased risk of disease in humans. In the case of prostate cancer, single nucleotide polymorphisms (SNPs) in the genes for a subset of selenoproteins expressed in that organ have been linked to either the risk or dying of prostate cancer. In some of these, the functionality of the SNP in altering either the primary sequence of the encoded protein, the transcription of the gene or translation of the protein have provided evidence for the role of that selenoprotein in the development or progression of the disease [20].
Selenium transport to the prostate and selenoprotein P (SELENOP)
Selenoprotein P (SELENOP) is the major form of selenium in plasma, contains multiple selenocysteines and transports selenium to organs where the amino acid selenocysteine is degraded by sec-β-lyase to release the selenium for selenoprotein synthesis [21,22]. Using tissue microarrays (TMAs), lower levels of SELENOP have been reported in prostate cancers as compared to adjacent benign prostate tissue [23]. Whether levels of SELENOP are associated with the risk of prostate cancer was investigated in a Danish population which has a relatively low selenium status [24], The results indicated that here was an inverse association between SELENOP levels and the risk of advanced disease. Additional consideration for a role of SELENOP in prostate cancer has been pursued by assessing if functional polymorphisms in the SELENOP gene are associated with prostate cancer risk or mortality. Two SELENOP SNPs have been focused upon, one resulting in a coding region alanine/threonine variation (rs3877899) and another resulting in either a G or A in the 3’ UTR (rs7579) that regulates selenocysteine insertion into the SELENOP peptide in response to in-frame UGA codons. Both of these SNPs have been shown to be functional, impacting the amount of selenium, SELENOP and selenoproteins in a variety of cell types [25,26]. However, there have been mixed results regarding the association of these SNPs and prostate cancer risk or stage at presentation [20]. One study involved subjects participating in the Adiposity and Outcomes of Clinically Localized Prostate Cancer Study, a prospective study of men who had undergone prostatectomy and designed to investigate whether obesity was associated with recurrence of the disease after surgery [27]. Genotyping DNA obtained from these men indicated that subjects with the SELENOP rs3877899 genotype resulting in a threonine rather than an alanine at that position were approximately 6-fold more likely to experience a rise in prostate specific antigen (PSA) levels, an indication of the recurrence of their disease, within the first 2 years post-surgery [27].
While it is possible that the anti-oxidant function attributed to SELENOP or some yet unknown activity mechanistically accounts for some of these data, the transport of selenium into the prostate by SELENOP may determine the levels of other selenoproteins whose transcription or translation are selenium-dependent. Each tissue and cell type has its own repertoire of selenoproteins, and among these, there is a subset of selenoproteins that are very sensitive to fluctuations in selenium availability and those that are not. This “hierarchy” of selenium responsiveness that occurs in the prostate [28] may indicate selenoproteins which are regulated by the import of SELENOP and can attenuate prostate cancer initiation or progression. Transport of SELENOP to the prostate may also be highly regulated as indicated by the lack of a statistically significant association between selenium levels assessed in both the serum and prostate tissue obtained from the same men following prostatectomy [29]. In addition, a significant association was found between SELENOP rs3877899 and an at-risk polymorphism in the gene for the NKX3.1 tumor suppressor and the increased risk of prostate cancer recurrence following prostatectomy, although no association was found between the NKX3.1 variant and plasma selenium levels [30]. These results may indicate that SELENOP transport proteins play an important function in ultimately determining the impact of selenium levels on prostate cancer. While polymorphisms in the gene for megalin, one such transporter present in the prostate that imports SELENOP to the tissue, were shown to be associated with prostate cancer outcome after diagnosis, megalin also transports many other molecules and hormones making it uncertain what role megalin contributes to the impact of SELENOP on the disease [31].
Glutathione Peroxidase 1 (GPX1)
One protein that is regulated by selenium availability is glutathione peroxidase 1 (GPX1), the first discovered and best characterized selenoprotein that functions to reduce lipid and hydrogen peroxides to water [32], A role for GPX1 in cancer was initially considered due to its anti-oxidant function and subsequent work investigating the association between GPX1 SNPs and cancer risk or outcome. The GPX1 allele containing a leucine at codon 198 has been associated with increased risk of several types of cancers, including those of the lung, breast, bladder, liver as well as lymphomas (reviewed in [33,32]). Evidence for the functionality of this polymorphism has been provided by studies in which either the GPX1Leu or GPX1Pro is exclusively expressed in human MCF7 breast carcinoma cells that do not express detectable GPX1 levels [34,35]. These in vitro studies indicated that the GPX1Leu variation would likely result in reduced amounts of GPX1 being synthesized when selenium levels are low [34]. Consistent with these in vitro results, there have been several reports indicating that the individuals with lower selenium status and the GPX1Leu allele exhibited lower GPX activity than those expressing GPX1Pro, although none of these studies determined GPX enzyme activity in the prostate [36–39]. GPX1 resides primarily in two different subcellular compartments, the cytoplasm and the mitochondria, and using the same in vitro approach, it was also determined that GPX1Leu expressed in MCF7 cells differentially partitioned more to the cytoplasm as compared to GPX1Pro [35]. The functional consequences of the subcellular location was investigated by targeting GPX1 to the mitochondria in these cells by the addition of a mitochondrial localization signal and demonstrating that GPX1 cellular location affected cellular bioenergetics, mitochondrial function and response to oxidative stress [35,40]. However, the impact of GPX1 allelic identity on prostate cancer risk or progression has been looked at in several populations with conflicting results. Some studies have reported increased risk associated with GPX1Leu, others indicated increased risk associated with the GPX1Pro allele while yet others showing no genotype effect [41–48].
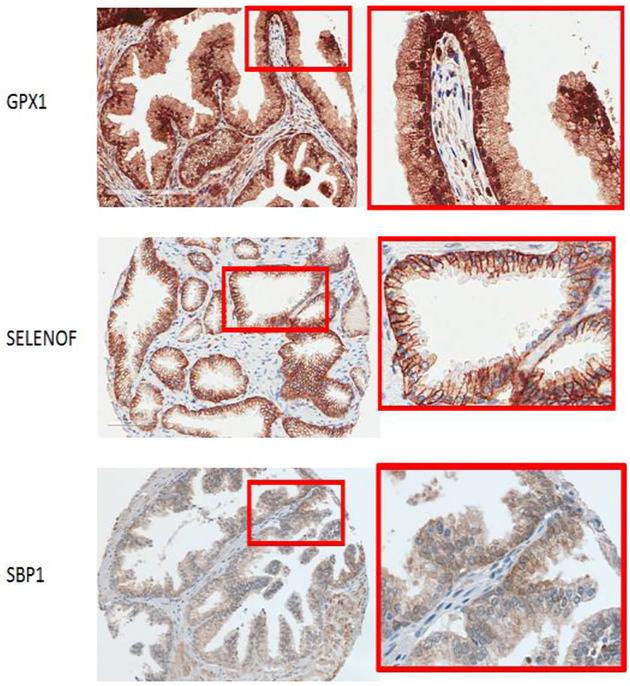
In order to determine if GPX1 levels in the prostate were associated with cancer recurrence following prostatectomy, a cancer tissue microarray (TMA) specifically designed to address this question was used [49]. The TMA was composed of tissues obtained from 200 men whose prostate cancer returned, as defined by rising PSA after prostatectomy (referred to as biochemical recurrence), matched by age and year of surgery, race, Gleason sum score and pathological stage to tissues from 200 men who cancer did not return. An unexpected observation was the localization of GPX1 in the nucleus of the prostate epithelial cells (Figure 1), in contrast to previously published data indicating that GPX1 distributes predominantly between the cytoplasm and the mitochondria. Nuclear localization of GPX1 was also observed in both primary prostate epithelial cells and the RWPE-1 immortalized prostate epithelial cell line, but not in either PC-3 or LNCaP prostate cancer-derived cell lines [49]. However, there was no association of cellular location or levels of GPX1 with prostate cancer biochemical recurrence, tumor stage or grade.
Fig.1.
Immunochemistry showing the location of the indicated selenoproteins in human prostate epithelial tissue. Boxed regions are shown at higher magnification on the right.
What function GPX1 might provide in the nucleus of prostate epithelial cells is unknown. Treating LNCaP cells with selenium under conditions that result in increased GPX1 activity can reduce oxidative damage to DNA by stimulating the repair of the damage [50] and knocking down GPX1 with siRNA sensitized the cells to UV-induced micronuclei formation, an assay of susceptibility to DNA damage [51]. Over-expression of GPX1 in MCF7 cells stimulated the activation of H2AX, a DNA repair protein critical in promoting the assembly of DNA repair complexes at the site of DNA double strand breaks [52]. GPX1 was also required in MCF7 cells for the activation of CHK2, a checkpoint protein that functions as a tumor suppressor by regulating the progression of the cell cycle after DNA damage, by phosphorylation following exposure of the cells to ionizing radiation [52]. Whether these results reflect a nuclear function of GPX1 or are a consequence of the effect of GPX1 activity on non-nuclear ROS-responsive signaling pathways has not been investigated.
Selenoprotein F (SELENOF) and the disparity in prostate cancer among African American men
Another selenoprotein whose levels are regulated by selenium availability [28,53] is selenoprotein 15 (SELENOF, previously referred to as SEP15 [54]), which was originally identified as a human T cell 15-kDa selenocysteine-containing protein that can bind 75Se and is expressed at high levels in the prostate [55]. The function of SELENOF is still being investigated, but is known to physically associate with the UDP-glucose:glycoprotein glucosyltransferase (UGGT) in the endoplasmic reticulum (ER) and likely plays an important role in disulfide bond formation and protein quality control in that organelle [56–59]. SELENOF is unusual as it contains an ER-localization sequence, but does not contain an ER-retention signal; retention of SELENOF in the ER is postulated to occur due to its interaction with UGGT [56].
The SELENOF gene is polymorphic in the 3’-untranslated region that determines the recognition of in-frame UGA codons as the amino acid selenocysteine during protein synthesis [55]. The polymorphisms at positions 811 (rs5845) and 1125 (rs5859) form a haplotype where a C at 811 always corresponds to a G at 1125 and a T at 811 always corresponds to an A at 1125 [60]. Using two different specialized reporter constructs, these genetic variations have been shown to be functional and likely contribute to determining the amount of SELENOF protein made as a function of selenium availability [61,60]. Genetic data examining the frequency of these SNPs have implicated SELENOF in prostate cancer etiology. The SELENOF811 SNP was associated with prostate cancer risk in a cohort of men in New Zealand [48]. SELENOF’s possible role in prostate cancer was also supported by there being a significant association between polymorphisms in the SELENOF gene and prostate cancer mortality among the 22,000 participants of the Physician’ Health Study [62], Similarly, genotyping a cohort of 126 men from the Chicago area from the Adiposity Study indicated that there was a five-fold decrease in the odds of presenting with a higher Gleason sum group, an indication of tumor aggressiveness, among participants with a CC genotype at position 1125, as compared to those with the CC +CT genotypes [27].
Whether the levels of SELENOF in tumors are associated with prostate cancer recurrence after prostatectomy was investigated using a TMA [27]. Although the tissue cores in this array are derived from prostatectomy specimens, most cores include adjacent, benign tissue as well. In the benign regions, SELENOF was predominantly located in the plasma membrane of basal and epithelial cells (Figure 1), reduced levels at the apical border and little detectable expression in the surrounding stroma. Localization to the plasma membrane was unexpected given all previous publications indicated predominantly ER localization. Plasma membrane staining was also observed in primary and immortalized prostate epithelial cells. In contrast to what was observed in benign tissue, tumors displayed mostly diffuse staining in the cytoplasm, as was the case when SELENOF location was examined in either PC-3 or LNCaP cells derived from human prostate cancers [27]. The role SELENOF plays in the outer membrane of prostate epithelial cells remains unknown. Knocking down SELENOF in HeLa cells resulted in cytoskeleton remodeling, non-apoptotic membrane blebbing, the relocation of focal adhesions and disruption of cell polarity [63,64], In addition, SELENOF knock-out mice were shown to exhibit elevated levels of circulating IgM, leading the authors to describe SELENOF as a “gatekeeper”, functioning in the retention of proteins [65]. It is unclear if any of these phenotypes reported for SELENOF are related to its function in the plasma membrane of prostate cells.
Although there was a dramatic difference in SELENOF staining between tumor and non-cancerous tissues in the TMA, there was no association between SELENOF levels and either tumor grade or cancer recurrence [27]. However, significant differences were observed between samples from African Americans and Caucasians. Among the samples in the TMA, 33 tissue cores were obtained from African American men and 295 were from Caucasian men. Comparing SELENOF levels between these two groups indicated that SELENOF levels in prostate tissues from African American was significantly lower than tissues from Caucasians. Likely contributing to this difference in SELENOF levels is the dramatic difference in the frequency of the SELENOF811 allele between African Americans and Caucasians, with the minor A allele being 4.4-fold more frequent among African Americans [61]. Similarly, genotyping a cohort of 126 men from the Chicago area from the Adiposity Study described above indicated that the difference in allele frequency was even greater, with the A allele being present at approximately 10-fold more frequent among African American men than Caucasians [27]. The problem of prostate cancer is disproportionally greater for African American men who have both the highest incidence and mortality from PCa world-wide as compared to other racial groups in the US [66]. The reasons for this disparity are likely multi-factorial, including reduced access to care and other socio-economic factors. In addition, there are a host of biological differences in disease presentation and clinical outcome which, along with environmental modifiers, are likely to account for the differences observed between African American and Caucasian men [67,68]. A genetic component in prostate cancer risk has been known for decades as individuals with a first-degree relatives diagnosed with PCa have a much higher risk of getting the disease, confirmed by a recent meta-analysis [69]. However, most men with PCa do not have a known family history which strongly suggests that high risk genetic factors are likely to be of relatively low penetrance and may be influenced by environmental variables. The data presented above is consistent with SELENOF contributing to the disparity in prostate cancer observed among African American men. The SELENOF A allele is associated with prostate cancer risk [70], more aggressive cancer [27] and is much more common among African Americans [27,61]. Moreover, based on functional reporter studies [61,60], the A allele is expected to result in lower SELENOF levels and consistent with these in vitro studies, tumors from African American men expressed less SELENOF. Additional studies with access to DNA, protein and individual clinical information from the same participants will be necessary to address this relationship.
Selenium Binding Protein 1 (SBP1, SELENBP1, hSP56) and prostate cancer
Most selenium-containing proteins contain selenium in the form of selenocysteine, an amino acid encoded by a UGA triplet that more typically is the translational termination signal in mRNAs [13]. SBP1 is a selenium-containing protein that does not contain selenocysteine and the nature of the selenium moiety in the protein is unknown. Because SBP1 levels are frequently lower in cancers as compared to the corresponding normal tissues, and lower levels are frequently associated with worse clinical outcome, (reviewed in [71]) SBP1 was examined in prostate cancer [72], SBP1 was distributed between the cytoplasm and nucleus (Figure 1) and levels in prostate tissue were determined using a TMA comparing matched tissues from men who experienced biochemical recurrence (rising PSA) following prostatectomy to that of men whose cancer did not return [72]. Cell-by-cell quantification of SBP1 in the nucleus and cytoplasm was performed and associations between SBP1 levels and tumor grade as well as recurrence were assessed. Both the nuclear levels of SBP1 and the nuclear to cytoplasmic ratio were inversely proportional to tumor grade and tumors in the lowest quartile of SBP1 were more than twice as likely to recur as compared to those in any of the other quartiles [72].
A tumor suppressor function for SBP1 is supported by observations made in several cell types [71]. Ectopic expression of SBP1 in colon, gastric and prostate cancer cell lines have resulted in reduced growth in semi-solid media and decreased tumorigenicity in xenograft models. For example, SBP1 was over-expressed in HCT116 human colon cancer cells that do not produce detectable SBP1 mRNA or protein. As a result of SBP1 over-expression, these cells exhibited reduced growth in soft agar along with significantly increased phosphorylation of p53 [72]. Others have shown that over-expression of SBP1 in these same cells altered cancer-related signaling pathways regulated by MAPK, Wnt, NFκB and Notch [73]. Very little is known about the role of SBP1 in prostate biology and what is known about SBP1 from other organs may not be the same as what occurs in the prostate.
A biochemical function of SBP1 was only recently resolved as it was discovered that inactivating SBP1 mutations resulted in extraoral halitosis, bad breath [74]. The enzyme activity of SBP1 was determined to be a methanethiol oxidase (MTO) that converts methanethiol to H2O2 and hydrogen sulfide (H2S), both important signaling molecules, with the latter being able to suppress mitochondrial respiratory complex II at high concentration [75–77]. At least for the MTO activity of SBP1, removal of the likely selenium-binding cysteine did not alter the protein’s enzymatic function. Whether the MTO activity or some other function of SBP1 contributes to its putative tumor suppressor activity remains to be determined.
SBP1 may be indirectly regulated by selenium via its interaction with GPX1. An inverse relationship between the levels of SBP1 and GPX1 has been reported in several tissues, including the prostate [78,29].Consistent with this observation, knocking down SBP1 in liver cells results in an increase in GPX activity [79] and over-expressing SBP1 in colon cells caused a significant decline in GPX1 activity without altering protein levels [80]. Moreover, over-expressing GPX1 in breast carcinoma cells results in the transcriptional repression of SBP1 [80]. These results are consistent with there being a physical interaction between SBP1 and GPX1 that results in the inhibition of GPX1 and that GPX1 expression influences SBP1 transcription, possibly due to the presence of anti-oxidant response elements (AREs) in the SBP1 promoter. The significance of this interaction may involve reactive oxygen-sensitive signaling as reduced levels of SBP1 is likely to result in less H2O2 generated from its MTO activity while increasing the H2O2 scavenging activity of GPX1.
Conclusions
Interest in the potential use of selenium in the prevention of prostate cancer has declined with the negative results of SELECT, but there remains much research to be done to evaluate the role of selenoproteins in the normal prostate and cancers that develop in that organ. Genetic studies and the examination of the levels of several selenoproteins in the prostate and adjacent benign tissue have implicated these proteins in either the risk or aggressiveness of the disease. These results may ultimately lead to new therapeutic targets or predictive biomarkers to assist clinicians in diagnosing and/or managing aggressive cancer and distinguishing it from indolent ones. Moreover, the direct evaluation of selenoproteins in human prostate tissue has revealed unexpected results, such as the nuclear location of GPX1 and plasma membrane localization of SELENOF. The biological function of these well studied proteins in unique subcellular compartments in benign prostate epithelium raises the possibility that there are yet unidentified and unique functions of these proteins in the prostate that are yet to be resolved.
Acknowledgements:
This work was supported by grants from the National Institutes of Health (Grant # R21CA182103, R01CA193497) and a Department of Defense Prostate Cancer Research Program Health Disparity Research Award (PC 170236) to AMD. The author would like to acknowledge Lenny Hong for critical reading of the manuscript and preparation of the figure.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Schrauzer GN, White DA, Schneider CJ (1977) Cancer mortality correlation studies. III. Statistical association with dietary selenium intakes. Bioinorg Chem 7:23–34 [DOI] [PubMed] [Google Scholar]
- 2.El-Bayoumy K (ed) (1991) The role of selenium in cancer prevention Cancer Prevention. J.B. Lippincott Co., Philadelphia [Google Scholar]
- 3.Wang L, Bonorden MJ, Li GX, Lee HJ, Hu H, Zhang Y, Liao JD, Cleary MP, Lu J (2009) Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev Res (Phila) 2 (5):484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozten N, Schlicht M, Diamond AM, Bosland MC (2014) L-selenomethionine does not protect against testosterone plus 17beta-estradiol-induced oxidative stress and preneoplastic lesions in the prostate of NBL rats. Nutrition and Cancer 66 (5):825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick DL, Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE (2010) Null activity of selenium and vitamin e as cancer chemopreventive agents in the rat prostate. Cancer Prev Res (Phila) 3 (3):381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, Zeegers MP, Homeber M, D’Amico R, Crespi CM (2018) Selenium for preventing cancer. The Cochrane Database of Systematic Reviews 1:CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark LC, Combs GF Jr., Turnbull BW, Slate EH, Challp DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr., Park HK, Sanders BB Jr., Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 276 (24): 1957–1963 [PubMed] [Google Scholar]
- 8.Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF Jr., Park HK, Gross EG, Graham GF, Stratton MS, Marshall JR, Clark LC (2003) Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst 95 (19): 1477–1481 [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr., Baker LH, Coltman CA Jr. (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301 (1):39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatfield DL, Gladyshev VN (2009) The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv 9(1): 18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Zhang J, Jiang C, Deng Y, Ozten N, Bosland MC (2016) Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutrition and Cancer 68 (1): 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayman MP, Combs GF Jr., Waters DJ (2009) Selenium and vitamin E supplementation for cancer prevention. JAMA 30 (18): 1876; author reply 1877 [DOI] [PubMed] [Google Scholar]
- 13.Bulteau AL, Chavatte L (2015) Update on selenoprotein biosynthesis. Antioxid Redox Signal 23 (10):775–794 [DOI] [PubMed] [Google Scholar]
- 14.Hatfield DL, Gladyshev VN (2002) How selenium has altered our understanding of the genetic code. Molec Cell Biol 22 (11):3565–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry MJ, Banu L, Chen Y, Mandel SJ, Kiefer JD, Harney JW, Larsen PR (1991) Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3’ untranslated region. Nature 353:273–276 [DOI] [PubMed] [Google Scholar]
- 16.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300 (5624): 1439–1443 [DOI] [PubMed] [Google Scholar]
- 17.Diwadkar-Navsariwala V, Diamond AM (2004) The link between selenium and chemoprevention: a case for selenoproteins. J Nutr 134 (11):2899–2902 [DOI] [PubMed] [Google Scholar]
- 18.Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM (2006) Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci USA 103(21):8179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luchman HA, Villemaire ML, Bismar TA, Carlson BA, Jirik FR (2014) Prostate epithelium-specific deletion of the selenocysteine tRNA gene Trsp leads to early onset intraepithelial neoplasia. Amer J Path 184 (3):871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meplan C (2015) Selenium and chronic diseases: a nutritional genomics perspective. Nutrients 7 (5):3621–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burk RF, Hill KE (2009) Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 1790 (11): 1441–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seale LA, Ha HY, Hashimoto AC, Berry MJ (2018) Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism. Free Rad Biology Med 127:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Moreno O, Boque N, Redrado M, Milagro F, Campion J, Endermann T, Takahashi K, Saito Y, Catena R, Schomburg L, Calvo A (2011) Selenoprotein-P is down-regulated in prostate cancer, which results in lack of protection against oxidative damage. The Prostate 71 (8):824–834. [DOI] [PubMed] [Google Scholar]
- 24.Outzen M, Tjonneland A, Larsen EH, Friis S, Larsen SB, Christensen J, Overvad K, Olsen A (2016) Selenium status and risk of prostate cancer in a Danish population. Br J Nutr 115 (9):1669–1677 [DOI] [PubMed] [Google Scholar]
- 25.Meplan C, Crosley LK, Nicol F, Beckett GJ, Howie AF, Hill KE, Horgan G, Mathers JC, Arthur JR, Hesketh JE (2007) Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J 21 (12):3063–3074 [DOI] [PubMed] [Google Scholar]
- 26.Meplan C, Nicol F, Burtle BT, Crosley LK, Arthur JR, Mathers JC, Hesketh JE (2009) Relative abundance of selenoprotein P isoforms in human plasma depends on genotype, se intake, and cancer status. Antioxid Redox Signal 11 (11):2631–2640 [DOI] [PubMed] [Google Scholar]
- 27.Ekoue DN, Ansong E, Liu L, Macias V, Deaton R, Lacher C, Picklo M, Nonn L, Gann PH, Kajdacsy-Balla A, Prins GS, Freeman VL, Diamond AM (2018) Correlations of SELENOF and SELENOP genotypes with serum selenium levels and prostate cancer. The Prostate 78 (4):279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touat-Hamici Z, Bulteau AL, Bianga J, Jean-Jacques H, Szpunar J, Lobinski R, Chavatte L (2018) Selenium-regulated hierarchy of human selenoproteome in cancerous and immortalized cells lines. Biochim Biophys Acta S0304–4165(18)30105–3 [DOI] [PubMed] [Google Scholar]
- 29.Jerome-Morais A, Wright ME, Liu R, Yang W, Jackson MI, Combs GF Jr., Diamond AM (2012) Inverse association between glutathione peroxidase activity and both selenium-binding protein 1 levels and Gleason score in human prostate tissue. The Prostate 72 (9): 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donadio JLS, Liu L, Freeman VL, Ekoue DN, Diamond AM, Bermano G (2018) Interaction of NKX3.1 and SELENOP genotype with prostate cancer recurrence. The Prostate 79 (5):462–467 [DOI] [PubMed] [Google Scholar]
- 31.Holt SK, Karyadi DM, Kwon EM, Stanford JL, Nelson PS, Ostrander EA (2008) Association of megalin genetic polymorphisms with prostate cancer risk and prognosis. Clin Cancer Res 14 (12):3823–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15 (7): 1957–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuo P, Diamond AM (2009) Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochem Biophys Acta 115:227–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuo P, Goldberg M, Herman L, Lee BS, Wang H, Brown RL, Foster CB, Peters U, Diamond AM (2009) Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res 69 (20):8183–8190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bera S, Weinberg F, Ekoue DN, Ansenberger-Fricano K, Mao M, Bonini MG, Diamond AM (2014) Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function. Cancer Res 74 (18):5118–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jablonska E, Gromadzinska J, Reszka E, Wasowicz W, Sobala W, Szeszenia-Dabrowska N, Boffetta P (2009) Association between GPx1 Prol98Leu polymorphism, GPx1 activity and plasma selenium concentration in humans. Eur J Nutr 48 (6):383–386 [DOI] [PubMed] [Google Scholar]
- 37.Miller JC, Thomson CD, Williams SM, van Havre N, Wilkins GT, Morison IM, Ludgate JL, Skeaff CM (2012) Influence of the glutathione peroxidase 1 Pro200Leu polymorphism on the response of glutathione peroxidase activity to selenium supplementation: a randomized controlled trial. Am J Clin Nutr 96(4):923–931 [DOI] [PubMed] [Google Scholar]
- 38.Jablonska E, Gromadzinska J, Peplonska B, Fendler W, Reszka E, Krol MB, Wieczorek E, Bukowska A, Gresner P, Galicki M, Zambrano Quispe O, Morawiec Z, Wasowicz W (2015) Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer 15:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoso BR, Busse AL, Hare DJ, Cominetti C, Horst MA, McColl G, Magaldi RM, Jacob-Filho W, Cozzolino SM (2016) Prol98Leu polymorphism affects the selenium status and GPx activity in response to Brazil nut intake. Food Funct 7 (2):825–833 [DOI] [PubMed] [Google Scholar]
- 40.Aashique M, Roy A, Diamond A, Bera S (2019) Subcellular compartmentalization of glutathione peroxidase 1 allelic isoforms differentially impact parameters of energy metabolism. J Cell Biochem 120 (3):3393–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arsova-Sarafinovska Z, Matevska N, Eken A, Petrovski D, Banev S, Dzikova S, Georgiev V, Sikole A, Erdem O, Sayal A, Aydin A, Dimovski AJ (2008) Glutathione peroxidase 1 (GPX1) genetic polymorphism, erythrocyte GPX activity, and prostate cancer risk. Int Urol Nephrol 41(1):63–70 [DOI] [PubMed] [Google Scholar]
- 42.Erdem O, Eken A, Akay C, Arsova-Sarafinovska Z, Matevska N, Suturkova L, Erten K, Ozgok Y, Dimovski A, Sayal A, Aydin A (2012) Association of GPX1 polymorphism, GPX activity and prostate cancer risk. Hum Exp Toxicol 31 (1):24–31 [DOI] [PubMed] [Google Scholar]
- 43.Steinbrecher A, Meplan C, Hesketh J, Schomburg L, Endermann T, Jansen E, Akesson B, Rohrmann S, Linseisen J (2010) Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiol Biomarkers Prev 19 (11):2958–2968 [DOI] [PubMed] [Google Scholar]
- 44.Liwei L, Wei Z, Ruifa H, Chunyu L (2012) Association between genetic variants in glutathione peroxidase 1 gene and risk of prostate cancer: a meta-analysis. Mol Biol Rep 39 (9):8615–8619. [DOI] [PubMed] [Google Scholar]
- 45.Men T, Zhang X, Yang J, Shen B, Li X, Chen D, Wang J (2014) The rsl050450 C > T polymorphism of GPX1 is associated with the risk of bladder but not prostate cancer: evidence from a meta-analysis. Tumour Biol 35 (1):269–275 [DOI] [PubMed] [Google Scholar]
- 46.Choi JY, Neuhouser ML, Barnett M, Hudson M, Kristal AR, Thomquist M, King IB, Goodman GE, Ambrosone CB (2007) Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev 16 (6):1115–1120 [DOI] [PubMed] [Google Scholar]
- 47.Abe M, Xie W, Regan MM, King IB, Stampfer MJ, Kantoff PW, Oh WK, Chan JM (2011) Single-nucleotide polymorphisms within the antioxidant defence system and associations with aggressive prostate cancer. BJU Int 107 (1):126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karunasinghe N, Han DY, Goudie M, Zhu S, Bishop K, Wang A, Duan H, Lange K, Ko S, Medhora R, Kan ST, Masters J, Ferguson LR (2012) Prostate disease risk factors among a New Zealand cohort. J Nutrigenet Nutrigenomics 5 (6):339–351 [DOI] [PubMed] [Google Scholar]
- 49.Ekoue DN, Ansong E, Hong LK, Nonn L, Macias V, Deaton R, Rupnow R, Gann PH, Kajdacsy-Balla A, Diamond AM (2018) GPX1 Localizes to the Nucleus in Prostate Epithelium and its Levels are not Associated with Prostate Cancer Recurrence. Antioxidants (Basel) 7 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Rosa V, Erkekoglu P, Forestier A, Favier A, Hincal F, Diamond AM, Douki T, Rachidi W (2012) Low doses of selenium specifically stimulate the repair of oxidative DNA damage in LNCaP prostate cancer cells. Free Rad Res 46 (2):105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliga MS, Diwadkar-Navsariwala V, Koh T, Fayad R, Fantuzzi G, Diamond AM (2008) Selenoprotein deficiency enhances radiation-induced micronuclei formation. Mol Nutr Food Res 52 (11): 1300–1304 [DOI] [PubMed] [Google Scholar]
- 52.Jerome-Morais A, Bera S, Rachidi W, Gann PH, Diamond AM (2013) The effects of selenium and the GPx-1 selenoprotein on the phosphorylation of H2AX. Biochem Biophys Acta 1830 (6):3399–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schomburg L, Schweizer U (2009) Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochem Biophys Acta 1790 (11): 1453–1462 [DOI] [PubMed] [Google Scholar]
- 54.Gladyshev VN, Arner ES, Berry MJ, Brigelius-Flohe R, Bruford EA, Burk RF, Carlson BA, Castellano S, Chavatte L, Conrad M, Copeland PR, Diamond AM, Driscoll DM, Ferreiro A, Flohe L, Green FR, Guigo R, Handy DE, Hatfield DL, Hesketh J, Hoffmann PR, Holmgren A, Hondal RJ, Howard MT, Huang K, Kim HY, Kim IY, Kohrle J, Krol A, Kryukov GV, Lee BJ, Lee BC, Lei XG, Liu Q, Lescure A, Lobanov AV, Loscalzo J, Maiorino M, Mariotti M, Prabhu KS, Rayman MP, Rozovsky S, Salinas G, Schmidt EE, Schomburg L, Schweizer U, Simonovic M, Sunde RA, Tsuji PA, Tweedie S, Ursini F, Whanger PD, Zhang Y (2016) Selenoprotein Gene Nomenclature. J Biol Chem 291(46):24036–24040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gladyshev VN, Jeang K-T, Wootton JC, Hatfield DL (1998) A new human selenium-containing protein. Purification, characterization and cDNA sequence. J Biol Chem 273 (15):8910–8915 [DOI] [PubMed] [Google Scholar]
- 56.Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN (2001) Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem 276 (18):15330–15336 [DOI] [PubMed] [Google Scholar]
- 57.Labunskyy VM, Hatfield DL, Gladyshev VN (2007) The Sep15 protein family: roles in disulfide bond formation and quality control in the endoplasmic reticulum. IUBMB Life 59 (1): 1–5 [DOI] [PubMed] [Google Scholar]
- 58.Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN (2009) Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry 48 (35):8458–8465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasaikina MV, Fomenko DE, Labunskyy VM, Lachke SA, Qiu W, Moncaster JA, Zhang J, Wojnarowicz MW Jr., Natarajan SK, Malinouski M, Schweizer U, Tsuji PA, Carlson BA, Maas RL, Lou MF, Goldstein LE, Hatfield DL, Gladyshev VN (2011) Roles of the 15-kDa selenoprotein (Sep15) in redox homeostasis and cataract development revealed by the analysis of Sep15 knockout mice. J Biol Chem 286 (38):33203–33212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumaraswamy E, Malykh A, Korotkov KV, Kozyavkin S, Hu Y, Kwon SY, Moustafa ME, Carlson BA, Berry MJ, Lee BJ, Hatfield DL, Diamond AM, Gladyshev VN (2000) Structure-expression relationships of the 15-kDa selenoprotein gene. Possible role of the protein in cancer etiology. J Biol Chem 275 (45):35540–35547 [DOI] [PubMed] [Google Scholar]
- 61.Hu YJ, Korotkov KV, Mehta R, Hatfield DL, Rotimi CN, Luke A, Prewitt TE, Cooper RS, Stock W, Vokes EE, Dolan ME, Gladyshev VN, Diamond AM (2001) Distribution and functional consequences of nucleotide polymorphisms in the 3’-untranslated region of the human Sep15 gene. Cancer Res 61 (5):2307–2310 [PubMed] [Google Scholar]
- 62.Penney KL, Schumacher FR, Li H, Kraft P, Morris JS, Kurth T, Mucci LA, Hunter DJ, Kantoff PW, Stampfer MJ, Ma J (2010) A large prospective study of SEP15 genetic variation, interaction with plasma selenium levels, and prostate cancer risk and survival. Cancer Prev Res (Phila Pa) 3 (5):604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bang J, Jang M, Huh JH, Na JW, Shim M, Carlson BA, Tobe R, Tsuji PA, Gladyshev VN, Hatfield DL, Lee BJ (2015) Deficiency of the 15-kDa selenoprotein led to cytoskeleton remodeling and non-apoptotic membrane blebbing through a RhoA/ROCK pathway. Biochem Biophys Res Commun 456 (4):884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bang J, Huh JH, Na JW, Lu Q, Carlson BA, Tobe R, Tsuji PA, Gladyshev VN, Hatfield DL, Lee BJ (2015) Cell Proliferation and Motility Are Inhibited by G1 Phase Arrest in 15-kDa Selenoprotein-Deficient Chang Liver Cells. Mol Cells 38 (5):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yim SH, Everley RA, Schildberg FA, Lee SG, Orsi A, Barbati ZR, Karatepe K, Fomenko DE, Tsuji PA, Luo HR, Gygi SP, Sitia R, Sharpe AH, Hatfield DL, Gladyshev VN (2018) Role of Selenof as a Gatekeeper of Secreted Disulfide-Rich Glycoproteins. Cell Rep 23 (5): 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A (2016) Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 66(4):290–308 [DOI] [PubMed] [Google Scholar]
- 67.Martin DN, Starks AM, Ambs S (2013) Biological determinants of health disparities in prostate cancer. Curr Opin Oncol 25 (3):235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrell J, Petrovics G, McLeod DG, Srivastava S (2013) Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. International J Mol Sci 14 (8):15510–15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeegers MP, Jellema A, Ostrer H (2003) Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis. Cancer 97 (8): 1894–1903. [DOI] [PubMed] [Google Scholar]
- 70.Geybels MS, van den Brandt PA, Schouten LJ, van Schooten FJ, van Breda SG, Rayman MP, Green FR, Verhage BA (2014) Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J Natl Cancer Inst 106 (3):dju003 [DOI] [PubMed] [Google Scholar]
- 71.Elhodaky M, Diamond AM (2018) Selenium-Binding Protein 1 in Human Health and Disease. Int J Mol Sci 19 (11) pii: E3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ansong E, Ying Q, Ekoue DN, Deaton R, Hall AR, Kajdacsy-Balla A, Yang W, Gann PH, Diamond AM (2015) Evidence that selenium binding protein 1 is a tumor suppressor in prostate cancer. PloS one 10 (5):e0127295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ying Q, Ansong E, Diamond AM, Lu Z, Yang W, Bie X (2015) Quantitative proteomic analysis reveals that anti-cancer effects of selenium-binding protein 1 in vivo are associated with metabolic pathways. PloS One 10 (5):e0126285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pol A, Renkema GH, Tangerman A, Winkel EG, Engelke UF, de Brouwer APM, Lloyd KC, Araiza RS, van den Heuvel L, Omran H, Olbrich H, Oude Elberink M, Gilissen C, Rodenburg RJ, Sass JO, Schwab KO, Schafer H, Venselaar H, Sequeira JS, Op den Camp HJM, Wevers RA (2018) Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat Genet 50 (1):120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Modis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C (2013) Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharm 86 (9): 1311–1319 [DOI] [PubMed] [Google Scholar]
- 76.Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F (2014) Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British J Pharm 171 (8):2099–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A, Hellmich MR (2013) Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Nat Acad Sci (USA) 110 (30): 12474–12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ansong E, Yang W, Diamond AM (2014) Molecular cross-talk between members of distinct families of selenium containing proteins. Mol Nut Food Res 58 (1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang C, Ding G, Gu C, Zhou J, Kuang M, Ji Y, He Y, Kondo T, Fan J (2012) Decreased Selenium-Binding Protein 1 Enhances Glutathione Peroxidase 1 Activity and Downregulates HIF-1alpha to Promote Hepatocellular Carcinoma Invasiveness. Clin Cancer Res 18:3042–3053 [DOI] [PubMed] [Google Scholar]
- 80.Fang W, Goldberg ML, Pohl NM, Bi X, Tong C, Xiong B, Koh TJ, Diamond AM, Yang W (2010) Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 31 (8): 1360–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]