Abstract
Background:
Sevoflurane administered to neonatal rats induces neurobehavioral abnormalities and epigenetic reprogramming of their germ cells; the latter can pass adverse effects of sevoflurane to future offspring. As germ cells are susceptible to reprogramming by environmental factors across the lifespan, we hypothesized that sevoflurane administered to adult rats could induce neurobehavioral abnormalities in future offspring, but not in the exposed rats themselves.
Methods:
Sprague-Dawley rats were anesthetized with 2.1% sevoflurane for 3 h every other day between postnatal days 56 and 60. Twenty five days later, exposed rats and non-exposed controls were mated to produce offspring.
Results:
Adult male but not female offspring of exposed parents of either sex exhibited deficiencies in elevated plus maze (mean ± SD, offspring of control parents vs. offspring of both exposed parents, 35 ± 12 vs. 15 ± 15 seconds, P < 0.001) and prepulse inhibition of acoustic startle (offspring of control parents vs. offspring of both exposed parents, 46.504 ± 13.448 vs. 25.838 ± 22.866 %, P = 0.009), and increased methylation and reduced expression of the K+-Cl− co-transporter KCC2 gene (Kcc2) in the hypothalamus. Kcc2 was also hyper-methylated in sperm and ovary of the exposed rats. Surprisingly, exposed male rats also exhibited long-term abnormalities in functioning of the hypothalamic-pituitary-gonadal and -adrenal axes, reduced expression of hypothalamic and hippocampal Kcc2, and deficiencies in elevated plus maze (control vs. sevoflurane, 40 ± 24 vs. 25 ± 12 seconds, P = 0.038) and prepulse inhibition of startle (control vs. sevoflurane, 39.905 ± 21.507 vs. 29.193 ± 24.263 %, P < 0.050).
Conclusions:
Adult sevoflurane exposure affects brain development in male offspring by epigenetically reprograming both parental germ cells, while induces neuroendocrine and behavioral abnormalities only in exposed males. Sex steroids may be required for mediation of the adverse effects of adult sevoflurane in exposed males.
TOC Statement
The neurobehavioral abnormalities in male offspring are accompanied by increased methylation and decreased expression of the K-Cl co-transporter KCC2 gene that regulates neuronal chloride homeostasis, and, thereby, the functional modalities of GABAergic neurotransmission. Sevoflurane exposure also induces hypermethylation of the KCC2 gene in both male and female parental germ cells. These observations suggest that epigenetic reprograming of parental germ cells is involved in transmitting the adverse effects of sevoflurane exposure of adult rats to their male progeny.
Introduction
The United States Food and Drug Administration recommends avoiding anesthesia in children under age 3 whenever possible, as it may negatively affect their brain development.1 This recommendation is based largely on an earlier body of work in animals, particularly rodents, showing that neonates are especially susceptible to lasting adverse effects of anesthesia during the first two postnatal weeks.2 In rodents the first two weeks of life are characterized by unique physiological properties, such as excitatory signaling through γ-aminobutyric acid type A receptors (GABAARs),3,4 a major substrate for the inhibitory (sedative) effects of GABAergic anesthetics in the more mature brain.5–7 GABAAR signaling is excitatory during early life due to elevated levels of intraneuronal Cl−, maintained by relatively low and high levels of the K+-Cl− co-transporter KCC2 and Na+-K+-Cl− cotransporter NKCC1, respectively.3,4 During the second postnatal week in rats, GABAAR signaling gradually becomes inhibitory, primarily due to age-dependent increases in KCC2.3,4 The magnitude of GABAAR excitatory signaling and the proper timing of its transition from excitatory to inhibitory are key for normal brain development, and impairments in KCC2 and resulting shifts in GABAAR signaling toward excitation have been linked to neuropsychiatric disorders in both humans and animal models.8,9 It appears that exposure to GABAergic anesthetics early in life may induce developmental abnormalities at least in part by enhancing the magnitude of GABAAR excitatory signaling at the time of anesthesia, and by disrupting normal developmental shifts in Kcc2 expression.10–12 In support of this contention, exposure of neonatal rats to GABAergic anesthetics, such as sevoflurane, causes electroencephalography-detectable epileptic seizures and multifold increases in corticosterone secretion at the time of anesthesia.12 After maturing to adulthood, these anesthetic-exposed rats have down-regulated Kcc2 levels, exacerbated hypothalamic-pituitary-adrenal (HPA) axis responses to stress, and behavioral abnormalities.10,11 Inhibition of NKCC1 at the time of anesthesia ameliorates acute seizure activities, as well as many of the lasting developmental effects of GABAergic anesthetics,11,12 suggesting that anesthetic-exacerbated GABAAR-mediated excitation in neonatal brain is an important initiating step in anesthetic-induced neurobehavioral abnormalities in the exposed animals.
Surprisingly, neonatal parental sevoflurane also impairs Kcc2 expression in the brain of future male offspring by initiating DNA methylation at the 5′ position of cytosine residues adjacent to guanines (CpG sites) in the Kcc2 gene in parental gamete cells.10 These findings suggest that neonatal sevoflurane can induce two distinct types of adverse effects in the exposed animals – excitatory GABAAR signaling-dependent effects in the exposed neonatal brain and epigenetic reprogramming of their germ cells, which can pass adverse effects of neonatal parental sevoflurane to future unexposed offspring.10 Epidemiological studies and research in animal models suggest that parental germ cells can be susceptible to reprogramming by environmental factors across the lifespan.13–15 Therefore, here we tested the hypothesis that sevoflurane can affect future offspring even when administered to adult rats. We further hypothesized that the primarily inhibitory GABAAR signaling in the brain of young adult rats3,16 renders them resistant to the adverse neuroendocrine and behavioral effects of sevoflurane.
Materials and Methods
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Sprague-Dawley rats were bred at the University of Florida animal care facility. The rats were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (23–24 °C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched pairs for the remainder of the study.
Treatment groups
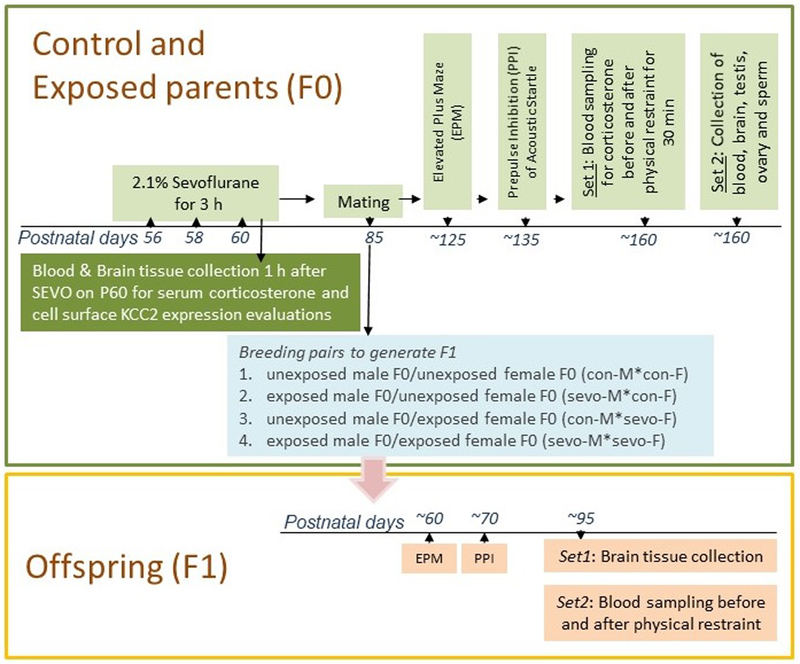
Male and female rats (generation F0) were randomized for treatment and breeding pairs using a randomization plan generator (http://www.randomization.com/) and the investigators were blind to group assignments. The F0 rats underwent anesthesia on postnatal days 56, 58 and 60. During this period, rats were held in a temperature-controlled chamber to maintain body temperature at ~+37 °C with a continuous supply of 30% oxygen in air (1.5 L/min) during anesthesia, which was induced by 6% sevoflurane for 3 min followed by 2.1% sevoflurane for 177 min for anesthesia maintenance (the Sevoflurane group). Gas monitoring was performed using a calibrated Datex side stream analyzer (Datex-Ohmeda, Helsinki, Finland), which sampled from the animal chamber interior. Rats in the F0 control group (Control) were not subjected to anesthesia. A subset of the rats in the F0 Control and Sevoflurane groups was sacrificed 1 h after the last exposure to sevoflurane (or equivalent timepoint in the Control group) to collect blood and brain tissue samples to measure serum levels of corticosterone and to evaluate expression of KCC2 in the paraventricular nucleus (PVN) of the hypothalamus (See Fig. 1 for schematic of experimental design). The remaining Control and Sevoflurane F0 male and female rats were mated on P85 to produce offspring (generation F1). Only rats from different litters were mated. F1 rats were categorized as the offspring of: 1) control males + control females (con-M*con-F); 2) exposed males + control females (sevo-M*con-F); 3) control males + exposed females (con-M*sevo-F); and 4) exposed males + exposed females (sevo-M*sevo-F). The females were housed individually throughout the entire gestation and post-partum rearing periods.
Figure 1.
Study design.
Sixty-four F0 rats (32 control and 32 sevoflurane exposed) were used as breeders to produce 122 F1 rats, which were not exposed to sevoflurane anesthesia and were subjected to animal facility rearing only. The F0 rats were sequentially evaluated in the elevated plus maze starting on ~P125 and prepulse inhibition of the acoustic startle response on ~P135 (Fig. 1). A subset of these animals were sacrificed on ~P160 to collect trunk blood, brain and testis and ovarian tissues for further analyses. The rest of these animals were physically restrained for 30 min on ~P160 to measure corticosterone responses, followed by collection of tissue samples for further analyses. The F1 rats were evaluated in the elevated plus maze starting on P60 and prepulse inhibition of startle on P70 (Fig. 1). One half of these animals were sacrificed on ~P95 to collect trunk blood and brain tissue samples for further analyses, and the rest of these animals were tested for the corticosterone responses to physical restraint for 30 min on ~P95 before collecting the brain tissue samples.
Behavioral Tests
Assessment of behavior in the elevated plus maze
The elevated plus maze studies were performed using an elevated plus maze apparatus and BIO-EPM 3C video tracking software (EB Instruments, Pinellas Park, FL) during the light phase of the dark–light cycle as previously described by our laboratory.10,12,17 If a fall occurred, the animal was removed from the study (one F0 male rat was removed from the study for this reason).
Assessment of prepulse inhibition of startle
The prepulse inhibition of startle tests were performed using a SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory.10,12,17 The % of prepulse inhibition of startle for each prepulse level was calculated using the following formula: % prepulse inhibition of startle =100x [(pulse alone) – (prepulse + pulse)]/pulse alone. Data were collected as Vmax amplitude.
Basal and stress-induced activity of the hypothalamic-pituitary-adrenal axis
Blood samples (~300 μl) were collected using the “tail clip” method at rest and 10, 60, and 120 min after the restraint. Physical restraint was administered using rodent holders (Kent Scientific Corporation, Torrington, CT). Serum corticosterone was measured using commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Tissue collection
After decapitation, the trunk blood samples were collected and centrifuged at 4°C, 1000 g for 15 min, and then kept at −80°C for hormone assays. The brain was removed from the skull onto ice pads. The hypothalamus was isolated by making an anterior cut at the level of the optic chiasm, a posterior coronal section anterior to the mammillary bodies, two sagittal cuts parallel to the lateral ventricles and a dorsal horizontal cut at the level of the anterior commissure, as described previously.18 The hippocampus was isolated from the respective blocks. All brain tissue samples were placed in vials filled with RNAlater solution (Invitrogen, Carlsbad, CA) and then stored at −80°C. Testis tissue was removed and washed with 0.9% saline before weighing. Sperm were isolated from the caudal epididymis of adult males into phosphate-buffered saline with 1% bovine serum albumin solution at 37°C using a swim-up assay. After settling for 30 min, sperm-containing supernatant was centrifuged for 5 min at 4000 rpm. Sperm pellets were stored at −80°C. After separation from the adipose tissues, ovaries were stored at −80°C.
Hormonal measurements
Hormone analyses in serum samples isolated from F0 and F1 rats were performed using commercially available kits according to the manufacturer’s instructions. Serum concentrations of follicle stimulating hormone (CSB-E06869r) and luteinizing hormone (CSB-E12654r) were quantified using ELISA kits (CUSABIO TECHNOLOGY LLC, Houston, TX, USA). Serum testosterone and estradiol concentrations were measured using ELISA kits (582701, Cayman Chemical Company, Ann Arbor, MI, USA and ES180S-100, Calbiotech, Spring Valley, CA, USA, respectively).
Quantitative mRNA measurements
Levels of mRNA for Nkcc1 and Kcc2 in the hypothalamus and hippocampus, for corticotropin-releasing hormone, estrogen receptor α, estrogen receptor β , aromatase, gonadotropin-releasing hormone in the hypothalamus, and for glucocorticoid receptor in the hippocampus were analyzed via reverse transcription-PCR (qRT-PCR) in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA) as previously described by our laboratory.10 RNA was extracted from the samples using an RNeasy Plus Kit (Qiagen, Valencia, CA), reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Hercules, CA), and then analyzed via qRT-PCR. Taqman probes specific for the above genes were obtained from Applied Biosystems (Carlsbad, CA): corticotropin-releasing hormone (Rn01462137_m1), glucocorticoid receptor (Rn00561369_m1), estrogen receptor α (Rn01430446_m1), estrogen receptor β (Rn00562610_m1), aromatase (Rn00567222_m1), gonadotropin-releasing hormone (Rn00562754_m1), Nkcc1 (Rn00582505_m1) and Kcc2 (Rn00592624_m1). Data were normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA (Rn01775763_g1). Gene expression was calculated using the ΔΔCT method and data are presented as relative fold change from that of control animals.
Bisulfite sequencing
Genomic DNA was extracted from the sperm pellet and ovaries of adult F0 rats and from hippocampal and hypothalamic tissues of F1 rats using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). The sodium bisulfite conversion was performed with EZ DNA Methylation kits (Zymo Research, Irvine, CA) following the manufacturer’s instructions. The primers (Kcc2: forward: GATTGTAAGTGTTTTTATTATTGAGTTGTATATT; reverse:AATAAACTTTTCCCCTTTTATACCC) were designed for the bisulfite-converted DNA sequences, using previously published sequences.10 PCR amplification was performed with HotStar Taq (Qiagen, Hilden, Germany). Amplicons were cloned into pCR4-TOPO vector with the TOPO TA cloning kit for sequencing (Life Technologies, Carlsbad, CA). Miniprep was performed on each positive clone using ZR Plasmid Miniprep kit (Zymo Research, Irvine, CA). Sanger sequencing was done by Genewiz (South Plainfield, NJ, USA) using M13R primers. The DNA methylation status of all CpG sites was analyzed using Benchling Molecular Biology 2.0 Software (Benchling, San Francisco, CA). The bisulfite sequencing analysis of the Kcc2 DNA was performed only in F1 male progeny of con-M*con-F and sevo-M*sevo-F matings.
Immunohistochemistry
Rats were anesthetized with sevoflurane and transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brains were collected and fixed in the 4% paraformaldehyde overnight and then dehydrated in 30% sucrose solution in phosphate-buffered saline at 4◦C for 2 days. The brains were cut into 18-um-thick coronal sections using a cryostat. After blocking with 10% normal goat serum for 1 h at room temperature, the slices were incubated with the primary antibody (rabbit anti-KCC2, 1:500; MilliporeSigma, Burlington, MA) in 10% normal goat serum at 4◦C overnight. After washing with PBS for 3 × 5 min, the slices were exposed to the secondary antibody, Alexa Fluor 549 goat anti-rabbit (Invitrogen, Carlsbad, CA). Slides were then washed with phosphate-buffered saline for 3 × 5 min and incubated for 10 min at room temperature with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI, 1:1000; Sigma, St. Louis, MO) in phosphate-buffered saline for 10 min. The sections were mounted and covered with coverslip after washing with phosphate-buffered saline for 3 × 5 min. A confocal microscope (IX2-DSU spinning disk confocal fluorescent microscope system, Olympus, Tokyo, Japan) was used to capture the fluorescent images. The immunofluorescence intensity of the KCC2-immunoreactive cells was measured using ImageJ software [National Institutes of Health]. Three images from the paraventricular nucleus of the hypothalamus of each animal were taken and the average intensity values were calculated to use for statistical analysis.
Statistical Analysis
Statistical analyses were conducted on raw data using SigmaPlot 14.0 software (Systat Software, Inc., San Jose, CA), which automatically checks if data set meets test criteria (Shapiro-Wilk for normality test and Brown-Forsythe for Equal Variance Test). Values are reported as mean ± SD. The primary outcome in this study was the neurobehavioral changes in offspring of rats exposed to sevoflurane in young adulthood. All other outcome measures were the secondary outcomes. One F0 male rat was removed from the elevated plus maze study because of a fall from the maze. Another F0 male rat in the control group was removed from the behavioral studies because of injury while mating with a female rat. Boxplots were used to identify outliers. No outliers were detected that were in not plausible range of values for the outcome (indicating measurement or recording error). Therefore, all values were maintained in analyses. An independent t-test was used to analyze F0 data for serum corticosterone levels and Kcc2 expression 1 h after the last exposure to sevoflurane, elevated plus maze, testis weight, hormone levels for testosterone, estradiol, luteinizing hormone and follicle stimulating hormone and gene expression for corticotropin-releasing hormone, glucocorticoid receptor, estrogen receptor α, estrogen receptor β, aromatase, gonadotropin-releasing hormone, Nkcc1 and Kcc2. To analyze F1 data for elevated plus maze, testis weight, testosterone level and gene expression for corticotropin-releasing hormone, glucocorticoid receptor, Nkcc1 and Kcc2, one-way ANOVA was used. A two-way repeated measures ANOVA was used to analyze the prepulse inhibition of startle data, with the treatment and prepulse intensity as independent variables. A two-way repeated measures ANOVA was used to analyze changes in serum corticosterone levels at rest and at 3 time points after the restraint, with experimental groups and time as the independent variables. To assess differences in total corticosterone concentrations, area under the curve with respect to baseline (levels of corticosterone at rest), was calculated and compared across experimental groups using one-way ANOVA. Two-way repeated measures ANOVA with treatment as ‘between’-subject factor and CpG site as ‘within’-subject factor was used to analyze data on the frequency methylation of CpG sites in the Kcc2 gene promoter in F0 and F1 tissue samples. An independent t-test was used to analyze DNA methylation level at all 6 CpG sites. All multiple pairwise comparisons were done with the Holm-Sidak method. All comparisons were run as two-tailed tests. P ≤ 0.05 was considered statistically significant. Statistical details are presented in text and in figure legends. The sample sizes in this study were based on previous experience with the same experimental techniques.10,11,17 This study was not designed to detect an anesthetic x sex interaction.
Results
Systemic abnormalities in male offspring of rats exposed to sevoflurane in young adulthood.
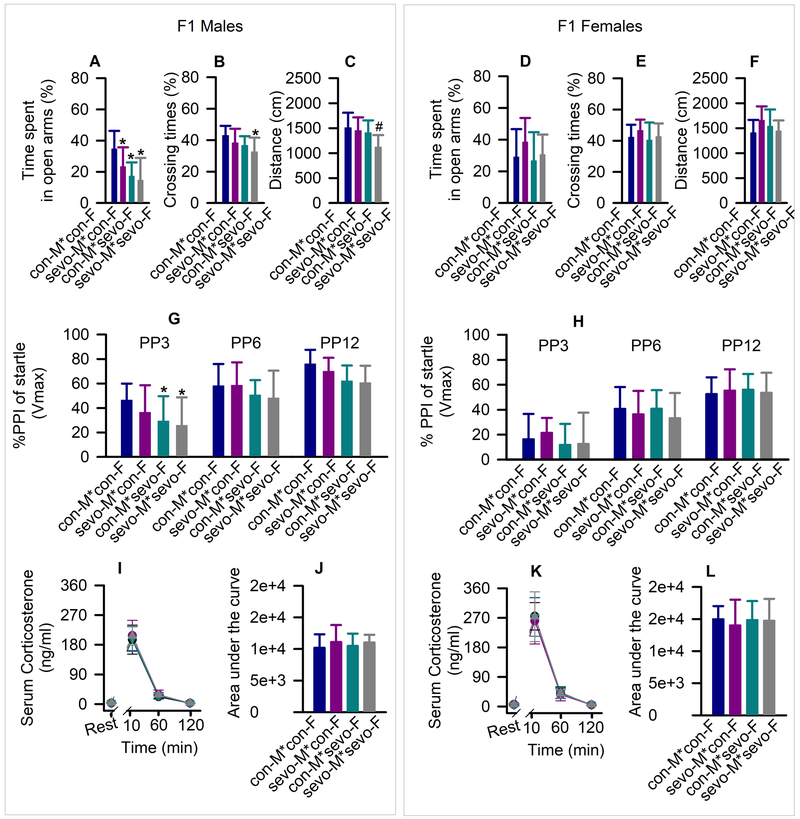
In the elevated plus maze test, there was a statistically significant between-subjects effect of young adult parental exposure to sevoflurane on time spent in open arms (F(3,58) = 8.514, P < 0.001; Fig. 2A) in F1 males. Specifically, F1 male progeny of sevoflurane exposed fathers and unexposed mothers (P = 0.040), unexposed fathers and exposed mothers (P < 0.001), and exposed fathers and exposed mothers (P < 0.001) spent less time in the open arms compared to F1 male offspring of control fathers and control mothers. Also, there were between-subjects effects of parental exposure to sevoflurane on number of crossings (F(3,58) = 4.657, P =0.006; Fig. 2B) and distance traveled (F(3,58) = 6.288, P <0.001; Fig. 2C) during the elevated plus maze test. Only F1 male offspring of both exposed parents made fewer crossings (P = 0.003) and traveled shorter distances (P = 0.001). All groups of F1 females were similar in respect to time spent in open arms (Fig. 2D), number of crossings (Fig. 2E) and distance traveled (Fig. 2F) during the elevated plus maze test.
Figure 2.
Systemic second-generation (F1) effects of young adult parental exposure to sevoflurane. (A-F) Time spent in open arms of the elevated plus maze, number of crossing the open arms, and distance traveled by male (A-C) and female (D-F) offspring. F1 rats were categorized as the offspring of: 1) control males + control females (con-M*con-F); 2) exposed males + control females (sevo-M*con-F); 3) control males + exposed females (con-M*sevo-F); and 4) exposed males + exposed females (sevo-M*sevo-F). Data are means ± SD from 15–16 rats/group. *P < 0.05 vs. con-M*con-F. (G,H) % of prepulse inhibition of startle responses at prepulse (PP) intensity of 3 dB, 6 dB and 12 dB in male (G) and female (H) offspring. Data are means ± SD from 14–16 rats/group. *P < 0.05 vs. con-M*con-F. (I-L) Plots showing the respective levels of serum corticosterone across each collection point, as well as the total corticosterone responses in male (I,J) and female (K,L) offspring. Serum levels of corticosterone at rest were taken as baselines for calculations of the total corticosterone responses. Data are means ± SD from 8 rats/group. Multiple pairwise comparisons were done with the Holm-Sidak method. Color coding of experimental groups in J and L is also applicable to I and K.
There was a statistically significant effect of young adult parental sevoflurane exposure on prepulse inhibition of startle responses in F1 male rats (F(3,174) = 7.590, P < 0.001; Fig. 2G). Multiple pairwise comparisons indicated that when compared to offspring of unexposed parents only F1 males of both exposed parents (P = 0.009) or those of control fathers and exposed mothers (P = 0.027) exhibited reduced prepulse inhibition of startle at PP3. In contrast to F1 males, there was no treatment effect on prepulse inhibition of startle in F1 female rats (F(3,168) = 0.584, P = 0.627; Fig. 2H). The startle amplitudes were similar among all experimental groups of F1 male (F(3,58) = 1.991, P = 0.125) and F1 female (F(3,56) = 0.514, P = 0.674) rats.
Measurements of serum levels of corticosterone in the ~P95 F1 male and female rats before and after physical restraint revealed no differences among experimental groups of the same sex (F(3,84) = 0.335, P = 0.800, males; Fig. 2I,J, and F(3,84) = 0.142, P = 0.934, females; Fig. 2K,L).
Reduction in the K+-Cl− co-transporter KCC2 gene (Kcc2) expression in male offspring of rats exposed to sevoflurane in young adulthood.
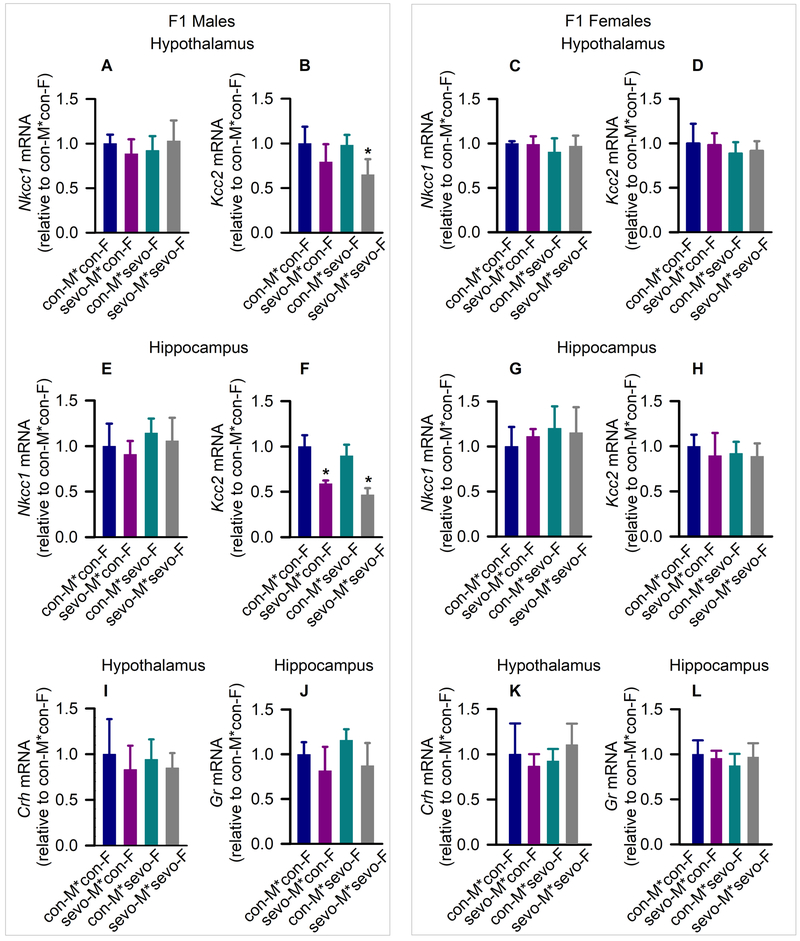
There were similar levels of Nkcc1 mRNA in the hypothalamus of all four treatment groups of the ~P95 F1 male rats (F(3,20) = 0.928, P = 0.446, Fig. 3A), but there was a between-subjects effect of parental sevoflurane exposure on hypothalamic Kcc2 mRNA levels (F(3,20) = 5.636, P = 0.006, Fig. 3B). Specifically, F1 male progeny of sevoflurane exposed fathers and mothers had lower levels of Kcc2 mRNA when compared to F1 male offspring of control fathers and control mothers (P = 0.012). In the hypothalamus of F1 females there were not between-subjects effects of parental sevoflurane exposure on Nkcc1 mRNA levels (F(3,19) = 0.886, P = 0.466, Fig. 3C) or Kcc2 mRNA levels (F(3,20) = 0.738, P = 0.542, Fig. 3D).
Figure 3.
Molecular second-generation (F1) effects of young adult parental exposure to sevoflurane. (A-H) The respective levels of Na+-K+-Cl− cotransporter NKCC1 gene (Nkcc1) mRNA and K+-Cl− co-transporter KCC2 gene (Kcc2) mRNA in the hypothalamus of F1 males (A,B) and F1 females (C,D) and in the hippocampus of F1 males (E,F) and F1 females (G,H). F1 rats were categorized as the offspring of: 1) control males + control females (con-M*con-F); 2) exposed males + control females (sevo-M*con-F); 3) control males + exposed females (con-M*sevo-F); and 4) exposed males + exposed females (sevo-M*sevo-F). Data normalized against control are means ± SD from 6 rats/group (n = 5, female hypothalamic Nkcc1 in con-M*con-F). *P < 0.05 vs. con-M*con-F. (I-L) The levels of corticotropin-releasing hormone mRNA in the hypothalamus and glucocorticoid receptor mRNA in the hippocampus in F1 males (I,J) and F1 females (K,L). Data normalized against control are means ± SD from 5–6 rats/group. Multiple pairwise comparisons were done with the Holm-Sidak method.
In the hippocampus of F1 males there were no effects of parental sevoflurane exposure on Nkcc1 mRNA levels (F(3,20) = 1.357, P = 0.284, Fig. 3E), but there was a significant effect on Kcc2 mRNA levels (F(3,20) = 41.375, P < 0.001, Fig. 3F). Only male offspring of both exposed parents (P < 0.001) and offspring of exposed fathers and control mothers (P < 0.001) had reduced hippocampal Kcc2 mRNA levels compared to offspring of two control parents. In the hippocampus of F1 females there were not significant between-subjects effects of parental neonatal sevoflurane exposure on Nkcc1 mRNA level (F(3,20) = 0.925, P = 0.447, Fig. 3G) or Kcc2 mRNA level (F(3,20) = 0.524, P = 0.671, Fig. 3H).
Consistent with the observations of normal levels of corticosterone (Fig. 2 I,J), levels of corticotropin-releasing hormone mRNA in the hypothalamus (F(3,20) = 0.519, P = 0.674, Fig. 3I) and glucocorticoid receptor in the hippocampus (F(3,19) = 3.293, P = 0.043, Fig. 3J) of ~P95 F1 males were not different among all four treatment groups. In addition, there were no significant between-subjects effects of parental sevoflurane exposure on either hypothalamic corticotropin-releasing hormone mRNA (F(3,20) = 1.225, P = 0.327, Fig. 3K) or hippocampal glucocorticoid receptor mRNA levels (F(3,19) = 0.860, P = 0.479, Fig. 3L) in F1 female rats.
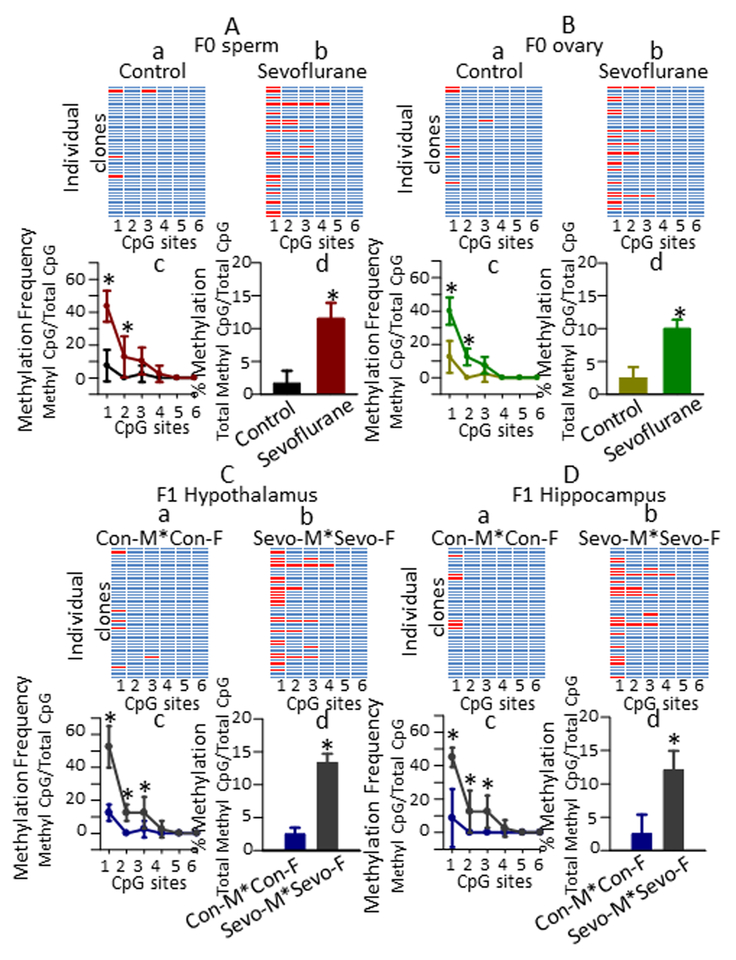
Elevated DNA methylation of the K+-Cl− co-transporter KCC2 gene (Kcc2) expression promoter region in sperm and ovary of rats exposed to sevoflurane in young adulthood and in the hypothalamus and hippocampus of their offspring.
Increased DNA methylation in the Kcc2 promoter in gamete cells of adult rats exposed to sevoflurane as neonates and in the hypothalamus and hippocampus of their male offspring has recently been described.10 We therefore sought to determine if similar effects would be observed in response to young adult exposure to sevoflurane. There was a significant effect of treatment (F(1,36) = 30.8, P < 0.001) and within-subjects effect of CpG site (F(5,36) = 20.066, P < 0.001) on the frequency of CpG methylation in the Kcc2 promoter region in sperm of the ~P160 F0 rats (Fig. 4A). There was also a statistically significant interaction between CpG site and treatment (F(5,36) = 10.036, P < 0.001). Pairwise multiple comparison analysis showed that young adult exposure to sevoflurane led to increased methylation in F0 male rats at CpG site 1 (P < 0.001 vs control), and CpG site 2 (P = 0.006 vs control). Similarly, there was a significant effect of treatment (F(1,36) = 34.714, P < 0.001) and within-subjects effect of CpG site (F(5,36) = 42.686, P < 0.001) on the frequency of CpG site methylation in the Kcc2 gene promoter in ovarian tissue of F0 rats (Fig. 4B). There was also a statistically significant interaction between CpG site and treatment (F(5,36) = 12.343, P < 0.001). Pairwise multiple comparison analysis showed that young adult exposure to sevoflurane led to increased methylation in F0 female rats at CpG site 1 (P < 0.001 vs control), and CpG site 2 (P < 0.001 vs control).
Figure 4.
DNA methylation in the promotor region of K+-Cl− co-transporter KCC2 gene (Kcc2) in parental (F0) sperm and ovary and in hypothalamus and hippocampus of their offspring (F1). (A,a-d) Bisulfite sequencing of the 5′ position of cytosine residues adjacent to guanines di-nucleotides (CpG sites) in the Kcc2 gene of 10 clones from four individual sperm DNA samples isolated from control and sevoflurane-exposed F0 male rats. Heat maps show distribution of unmethylated (blue cells) and methylated (red cells) CpG sites in sperm DNA samples isolated from control (A,a) and sevoflurane-exposed (A,b) F0 male rats. Histograms showing methylation frequency at each CpG site (A,c) and DNA methylation level at all 6 CpG sites (A,d). The results of similar analysis of bisulfite sequencing of CpG di-nucleotides in the Kcc2 gene of 10 clones from four individual ovary DNA samples isolated from sevoflurane-exposed and control F0 female rats shown in (B,a-d). Color coding of experimental groups in A,d and B,d is also applicable to A,c and B,c. (C,D) Shown are the respective methylation frequency at each CpG site and DNA methylation level at all 6 CpG sites in the Kcc2 gene of 10 clones in the hypothalamus (C,a-d) and hippocampus (D,a-d) of F1 male offspring of control sires and control dams (con-M*con-F) and of sevoflurane-exposed sires and dams (sevo-M*sevo-F). Data are means ± SD from 4 rats/group. *P < 0.05 vs. Control (A,B) and vs. con-M*con-F (C,D). Color coding of experimental groups in C,d and D,d is also applicable to C,c and D,c. Multiple pairwise comparisons were done with the Holm-Sidak method.
There was a between-subjects effect of parental treatment on CpG site methylation in the promoter of Kcc2 gene in the hypothalamus of the ~P95 male offspring of both exposed parents (F(1,36) = 48.286, P < 0.001), and a within-subject effect of CpG site (F(5,36) = 42.629, P < 0.001) (Fig. 4C). There was also a statistically significant interaction between CpG site and treatment (F(5,36) = 15.886, P < 0.001). Pairwise multiple comparison analyses showed that young adult parental exposure to sevoflurane led to increased DNA methylation in the Kcc2 gene promoter in the hypothalamus of F1 male progeny of both exposed parents at CpG site 1 (P < 0.001 vs F1 males from the con-M*con-F group), CpG site 2 (P = 0.002 vs F1 males from the con-M*con-F group) and CpG site 3 (P = 0.013 vs F1 males from the con-M*con-F group).
There was also a between-subjects effect of parental treatment on CpG site methylation in the promoter of Kcc2 gene in the hippocampus of male offspring of both exposed parents (F(1,36) = 21.740, P < 0.001, and within subject effect of CpG site (F(5,36) = 20.852, P < 0.001) (Fig. 4D). There was also a statistically significant interaction between CpG site and treatment (F(5,36) = 5.268, P < 0.001). Pairwise multiple comparison analyses showed that young adult parental exposure to sevoflurane led to increased DNA methylation in the Kcc2 gene promoter in the hippocampus of F1 male progeny of both exposed parents at CpG site 1 (P < 0.001 vs con-M*con-F), CpG site 2 (P = 0.018 vs con-M*con-F) and CpG site 3 (P = 0.018 vs con-M*con-F).
Systemic abnormalities in male rats exposed to sevoflurane in young adulthood.
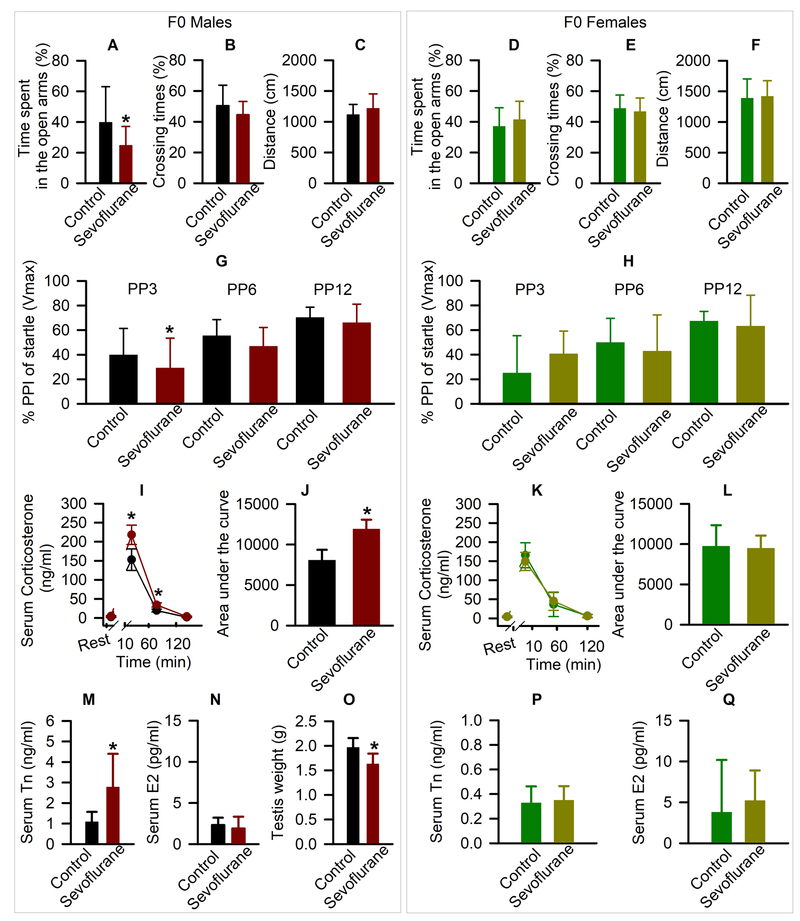
Unexpectedly, we found that more than two months after exposure to sevoflurane anesthesia, F0 male rats exhibited behavioral deficiencies. They spent less time in the open arms of the elevated plus maze (t(28) = 2.18; P = 0.038; Fig. 5A), but did not differ from their control counterparts in number of crossings (t(28) = 1.456; P = 0.157; Fig. 5B) or distance traveled (t(28) = −1.351; P = 0.188; Fig. 5C). In F0 females, there was no significant effect of sevoflurane on any of these elevated plus maze parameters (Fig. 5D-F).
Figure 5.
Systemic first generation (F0) effects of young adult exposure to sevoflurane. The % of time spent in open arms of the elevated plus maze, number of crossing the open arms, and distance traveled by male (A-C) and female (D-F) rats. Data are means ± SD from 15 male and 16 female rats/group. (G,H) The % of prepulse inhibition of startle responses at prepulse intensity (PP) of 3 dB, 6 dB and 12 dB in male (G) and female (H) rats. Data are means ± SD from 21 males in the Control group, 22 males in the Sevoflurane group and 16 females/group. *P < 0.05 vs. Control. (I-L) The respective levels of serum corticosterone across each collection point, as well as the total corticosterone responses in male (I,J) and female (K,L) rats. Serum levels of corticosterone at rest were taken as baselines for calculations of the total corticosterone responses. Data are means ± SD from 8 rats/group. Color coding of experimental groups in J and L is also applicable to I and K. (M-O) shown are serum levels of testosterone (M), estradiol (N) and testis weight (O) in male rats. (P,Q) Serum levels of testosterone and estradiol in female rats. Data are means ± SD from 8 rats/group. Data of testis weight are from 16 rats in control group and 13 rats in sevoflurane group. *P<0.05 vs. Control.
As in the elevated plus maze task, there was a significant effect of sevoflurane exposure on prepulse inhibition of startle in adult F0 male rats (F(1,123) = 6.765; P = 0.010; Fig. 5G), but not in F0 female rats (F(1,60) = 0.049; P = 0.827; Fig. 5H). Multiple pairwise comparisons indicated that exposure to sevoflurane led to impaired prepulse inhibition of startle responses in F0 male rats at prepulse intensity of 3 dB (P = 0.042 vs control). Startle stimuli by themselves caused similar responses in the control and sevoflurane groups of F0 male (t(41) = −0.969; P = 0.338) and F0 female (t(30) = 1.465; P = 0.153) rats.
Male F0 rats had higher total corticosterone responses to physical restraint on ~P160 when compared to their control counterparts (t(14) = −6.209; P < 0.001; Fig. 5I,J). This increase was due to higher levels of corticosterone at 10 min (P <0.001 vs control) and 60 min (P =0.036 vs control) after restraint, as serum levels of corticosterone before the restraint (P = 0.736 vs control) and 120 min (P = 0.787 vs control) post restraint were not different in control and sevoflurane-exposed rats (Fig. 5I). There was no difference in serum corticosterone levels between control and sevoflurane-exposed F0 female rats (Fig. 5K,L).
During collection of sperm for the DNA methylation studies we incidentally found that sevoflurane-exposed F0 males had reduced testis weight (t(27) = 4.494; P < 0.001; Fig. 5O) more than 3 months after sevoflurane exposure. Counterintuitively, their serum levels of testosterone were increased (t(14) = −2.839; P = 0.013; Fig. 5M), although their serum levels of estradiol were normal (t(14) = 0.703; P = 0.494; Fig. 5N). F0 female rats in the sevoflurane and control groups were not different with respect to serum levels of testosterone (P = 0.743; Fig. 5P) or estradiol (P = 0.600; Fig. 5Q).
Reduction in the K+-Cl− co-transporter KCC2 expression in male rats 1 h after the last exposure to sevoflurane.
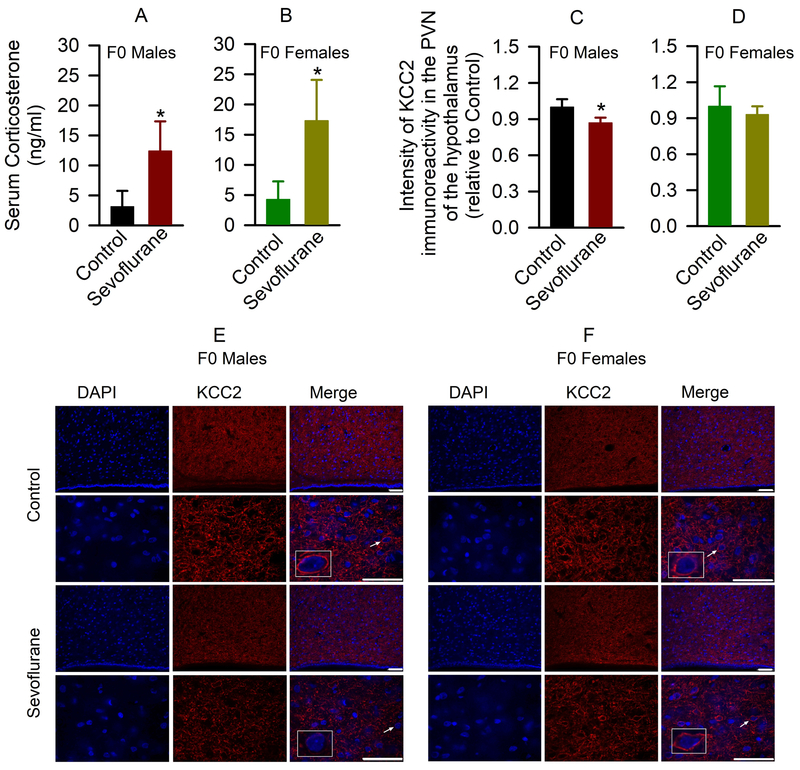
Our current and recently-published findings10 indicate that rats exposed to sevoflurane in young adulthood and in the early postnatal period develop comparable systemic abnormalities. Given that sevoflurane acts as a stressor in neonatal rats and initiates developmental abnormalities, at least in part, by potentiating excitatory GABAAR signaling,10–12,17 here we tested whether sevoflurane can induce a stress-like19–22 reduction in KCC2 expression in young adult rats. To measure acute effects of sevoflurane, rats were exposed to 2.1% sevoflurane anesthesia for 3 h on P56, P58 and P60. Brain tissue and trunk blood samples were collected 1 h after sevoflurane anesthesia on P60. Consistent with the stress-like effects of sevoflurane reported in previous work,10–12,17 the exposed male and female rats had increased serum levels of corticosterone compared to controls (t(6)= −3.313; P=0.016, males, Fig. 6A; and t(8)= −3.949; P= 0.004, females, Fig. 6B). Despite similar increases in corticosterone levels in sevoflurane-exposed males and females, immunofluorescence evaluations of KCC2 expression in the PVN of the hypothalamus region found reductions in KCC2 level in male (t(6)= 3.343; P= 0.016), but not female (t(6)= 0.773; P= 0.469), F0 rats (Fig. 6C-F).
Figure 6.
Acute effects of young adult sevoflurane exposure. (A,B) The respective levels of serum corticosterone in male (A) and female (B) rats. Data are means ± SD (n=4 and n=5 per treatment group in males and females, respectively). *P<0.05 vs. Control. (C-F) Representative confocal images and quantitative analysis of the K+-Cl− co-transporter KCC2 immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus of the control and sevoflurane-exposed male (C,E) and female (D,F) rats. (E) Representative confocal images of the PVN from the control and sevoflurane-exposed male rats, immunostained for 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (left; blue) and KCC2 (middle; red); the merge column shows colocalized images (right; red and blue). The arrowheads indicate the cells shown in the boxed areas at higher magnifications. The KCC2 immunoreactivity, located on the periphery of the neurons (red color), decreased in the PVN neurons from the sevoflurane-exposed male rats. Similar representative confocal images of the PVN from the control and sevoflurane-exposed female rats shown in (F). Scale bars, 50 μm. The sevoflurane-exposed males, but not sevoflurane-exposed females, had reduced KCC2 expression (*P<0.05 vs. Control; Fig. 6C,E). Data are means ± SD (n=4 rats/group).
Abnormalities in the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal axes in male rats exposed to sevoflurane in young adulthood.
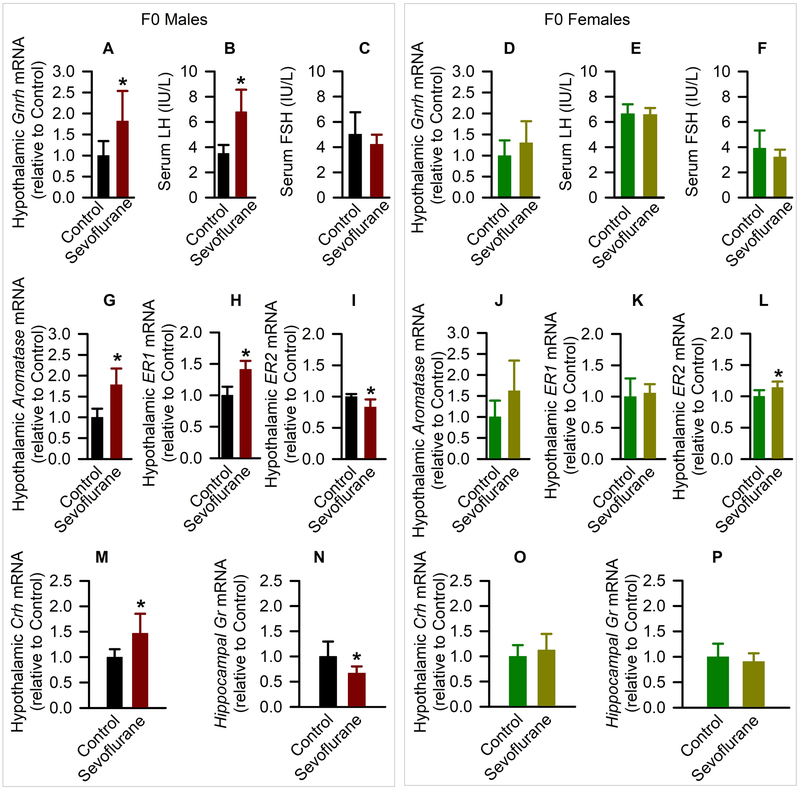
Acute KCC2 and long-term systemic effects of young adult sevoflurane in the exposed males only suggest an involvement of sex steroids. To test whether sevoflurane-induced increases in serum testosterone levels involve changes in functioning of the entire hypothalamic-pituitary-gonadal axis, we measured the expression of gonadotropin-releasing hormone in the hypothalamus and serum levels of follicle stimulating hormone and luteinizing hormone. Consistent with the finding that sevoflurane increased systemic levels of testosterone in ~P160 F0 male rats, these same rats also had increased hypothalamic levels of gonadotropin-releasing hormone mRNA (t(10) = −2.519; P = 0.030; Fig. 7A) and serum levels of luteinizing hormone (t(14) = −4.932; P < 0.001; Fig. 7B), while serum levels of follicle stimulating hormone (t(10) = 1.026; P = 0.329; Fig. 7C) were not different from those in F0 control male rats. There were no treatment effects on the hypothalamic levels of gonadotropin-releasing hormone mRNA (Fig. 7D) or serum levels of luteinizing hormone (Fig. 7E) and follicle stimulating hormone (Fig. 7F) in F0 female rats.
Figure 7.
Molecular first generation (F0) effects of young adult exposure to sevoflurane. (A-F) The levels of hypothalamic gonadotropin-releasing hormone mRNA, serum levels of luteinizing hormone and follicle stimulating hormone of male (A-C) and female (D-F) rats. Data are means ± SD from 8 rats/group (n=6 rats/ group in follicle stimulating hormone). *P < 0.05 vs. Control. (G-L) shown are levels of aromatase mRNA, estrogen receptor α mRNA, and estrogen receptor β mRNA in the hypothalamus of male (G-I) and female (J-L) rats. Data normalized against control are means ± SD from 6 rats/group. *P < 0.05 vs. (M-P) Shown are the respective levels of hypothalamic corticotropin-releasing hormone mRNA and hippocampal glucocorticoid receptor mRNA in males (M,N) and females (O,P). Data normalized against control are means ± SD from 6 rats/group (n=8, male corticotropin-releasing hormone; n=5 in the Control group and n=4 in the Sevoflurane group in female glucocorticoid receptor). *P < 0.05 vs. Control.
The elevated testosterone may modulate the hypothalamic-pituitary-adrenal axis responses to stress through estrogen receptors after testosterone aromatization to estradiol in the brain. One such mechanism includes modulation by estradiol of the glucocorticoid receptor-mediated negative feedback effect of corticosterone on the hypothalamic-pituitary-adrenal axis activity. Estradiol can both inhibit and enhance the negative feedback effects of glucocorticoids by activating estrogen receptor α and estrogen receptor β, respectively.23,24 In further support of involvement of testosterone/estradiol in exacerbated hypothalamic-pituitary-adrenal axis responses to stress, we found that the hypothalamic levels of aromatase mRNA were increased in F0 sevoflurane-exposed males (t(10) = −4.333; P = 0.002; Fig. 7G). In addition, the F0 male rats exposed to sevoflurane had increased and decreased hypothalamic levels of estrogen receptor α mRNA (t(10) = −5.144; P < 0.001; Fig. 7H) and estrogen receptor β mRNA (t(10) = 3.156; P = 0.010; Fig. 7I), respectively. Again, consistent with the normal hypothalamic-pituitary-adrenal axis responses to stress in the exposed F0 females, the increase in the hypothalamic aromatase mRNA in sevoflurane-exposed F0 female rats did not achieve statistical significance (t(10) = −1.878; P = 0.090; Fig. 7J). Moreover, in the F0 females the hypothalamic levels of estrogen receptor α mRNA (Fig. 7K) were not different between Sevoflurane and Control groups, while those of estrogen receptor β mRNA were slightly, though significantly increased in the Sevoflurane group (t(10) = −2.521; P = 0.030; Fig. 7L) when compared to the Control group.
In agreement with the exacerbated corticosterone responses to stress in sevoflurane-exposed F0 male rats at ~P160, these rats had increased hypothalamic corticotropin-releasing hormone mRNA levels 2 hours after the restraint (t(14) = −3.181; P = 0.007; Fig. 7M), as well as reduced levels of glucocorticoid receptor mRNA in the hippocampus (t(10) = 2.493; P = 0.032; Fig. 7N). Neither hypothalamic corticotropin-releasing hormone mRNA levels (Fig. 7O) nor hippocampal glucocorticoid receptor mRNA levels (Fig. 7P) were different in sevoflurane-exposed and control F0 female rats.
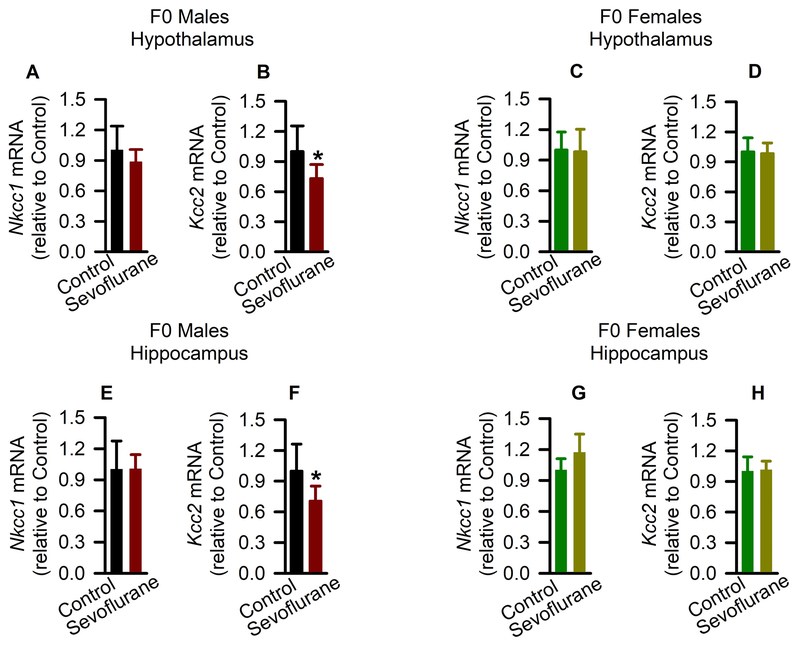
Finally, considering the controlling roles of GABAAR signaling in hypothalamic gonadotropin-releasing hormone and corticotropin-releasing hormone neuronal activity, it is plausible that sevoflurane initiates enhancements of gonadotropin-releasing hormone and corticotropin-releasing hormone neuronal activity and, hence, the entire reproductive and stress axes, by downregulating Kcc2 expression and rendering GABAAR signaling less inhibitory or even excitatory. To assess whether exposure of young adult rats to sevoflurane led to persistent alterations in expression of Cl− transporters, brain hypothalamic and hippocampal tissue samples were collected more than 3 months after exposure (~P160). The F0 male rats from the Sevoflurane group had normal Nkcc1 mRNA levels (t(10) = 1.065; P = 0.312; Fig 8A), but decreased Kcc2 mRNA levels (t(10) = 2.273; P = 0.046; Fig. 8B) in the hypothalamus. In contrast, the F0 female rats from the Sevoflurane group had unaltered levels of both Nkcc1 mRNA (t(10) = 0.155; P = 0.880; Fig. 8C) and Kcc2 mRNA (t(10) = 1.346; P = 0.208; Fig. 8D) in the hypothalamus. In the hippocampus of F0 male rats from the Sevoflurane group, Kcc2 mRNA levels, but not Nkcc1 mRNA levels, were significantly reduced (t(10) = 2.387, P = 0.038, Fig. 8E,F). The hippocampal mRNA levels for Nkcc1 and Kcc2 were similar in control and sevoflurane-exposed F0 female rats (Fig. 8G,H).
Figure 8.
Effects of sevoflurane exposure on hypothalamic and hippocampal K+-Cl− co-transporter KCC2 gene (Kcc2) and Na+-K+-Cl− cotransporter NKCC1 gene (Nkcc1) mRNA in F0 rats. (A-H) Shown are the respective levels of Nkcc1 mRNA and Kcc2 mRNA in the hypothalamus of F0 males (A,B) and F0 females (C,D) and in the hippocampus of F0 males (E,F) and F0 females (G,H). Data normalized against control are means ± SD from 6 rats/group (n = 5, female hippocampus in sevoflurane group). *P < 0.05 vs. Controls.
Discussion
The results of these experiments show that sevoflurane administered to young adult rats of either sex can induce abnormalities in their male offspring, as well as in the exposed male rats themselves. The reduction in Kcc2 expression in the hypothalamus and hippocampus of the F1 male progeny of the exposed parents, as well as hypermethylation of the Kcc2 promoter in F0 male and female gamete cells and F1 male hypothalamus and hippocampus, support the involvement of epigenetic mechanisms in transmitting adverse effects of sevoflurane exposure in young adult rats to the next generation. The findings that both the physiology and behavior of sevoflurane-exposed F0 females were normal, while their ovarian Kcc2 promoter was hyper-methylated and male progeny of exposed F0 females exhibited neurobehavioral abnormalities, suggest that sevoflurane affects somatic and germ cells via different mechanisms.
Intergenerational effects of young adult sevoflurane
Human and animal studies provide evidence that exposure to alcohol, endocrine disruptors, and stress can affect future generations.10,12,13–15,25–27 Surprisingly, the heritable effects of general anesthetics are poorly studied, despite the fact that anesthetic agents share molecular mechanisms of action with alcohol and may act as both environmental stressors and endocrine disruptors.10–12,17,27–29 Furthermore, anecdotal observations point to the possibility of heritable effects of anesthesia/surgery in humans.30 Finally, indirect evidence of heritable effects of general anesthetics comes from clinical surveys indicating that anesthesia care providers may have altered female/male offspring ratios.31–35 Importantly, such sex ratio effects have been linked to alcohol, stress and endocrine disruptors as well.36–38 Our recently published10 and present findings support the possibility that sevoflurane can induce intergenerational effects. Obviously, sevoflurane-induced epigenetic modulation of Kcc2 in F0 gamete cells and F1 male brain may contribute to the intergenerational effects of young adult sevoflurane exposure, but it is unlikely that sevoflurane-induced changes in Kcc2 are the only mediating mechanism. Indeed, this study found that male progeny of exposed dams and control sires had normal Kcc2 mRNA levels in the hypothalamus and hippocampus, but exhibited deficiencies in the elevated plus maze and prepulse inhibition of startle behavioral tests. It is possible that Kcc2 DNA methylation in gamete cells affects other epigenetic mechanisms that program offspring brain development, and/or that multiple genes are independently epigenetically modified by sevoflurane in F0 gamete cells and F1 brain. Furthermore, the epigenetic effects of GABAergic anesthetics may not be limited to DNA methylation, as we and others recently reported experimental evidence for involvement of not only DNA methylation,10,39 but also histone acetylation40,41 in adverse effects of neonatal sevoflurane exposure in rats.
Despite different anesthesia regimens in our recent study of intergenerational effects of neonatal anesthesia10 and the young adult anesthesia in the present study, similarities in the intergenerational effects of sevoflurane outweighed differences between the two models. These findings are also consistent with those reported by Rodgers et al. that 42-day exposure of male rats to stress either throughout puberty or in adulthood leads to similar blunted hypothalamic-pituitary-adrenal axis responses in their progeny.14 Moreover, repeated exposure of 11 week old male mice to the anesthetic agent enflurane negatively affected their offspring’s behavior.42 The susceptibility of germ cell maturation to sevoflurane over an extended period of the lifespan, from the early postnatal period10 through young adulthood (this study), suggests that supporting cells, such as granulosa cells in ovaries and Sertoli cells in testis, may be the primary target for epigenetic modifications initiated by sevoflurane. It will be important in future studies to analyze epigenetic effects of parental exposure to anesthetics in F0 oocytes, which represent only a minor part of total ovarian tissue that was tested in this study.
Irrespective of whether fathers, mothers, or both parents were exposed to sevoflurane as young adults, only male F1 progeny exhibited epigenetic, gene expression, and systemic abnormalities. Given that Kcc2 was hyper-methylated in both male and female gamete cells in F0 rats, it is plausible that parental sevoflurane initiates mechanisms leading to disruption of embryonic DNA methylation reprograming in F1 males, but not in F1 females, resulting in affected F1 males but normal F1 females. Our findings that the exposed but physiologically unaffected dams, similar to exposed and affected sires, pass deleterious effects of sevoflurane to unexposed male offspring raise the possibility that male offspring may be affected even when anesthesia level/duration is not sufficient to induce significant abnormalities in the exposed parents.
Adverse effects of young adult sevoflurane in the exposed rats
The long-term adverse effects of general anesthesia in early childhood and at advanced ages are a widely recognized health-related concern and the subject of extensive clinical and laboratory research.43 Investigations of such effects in young adults in their prime reproductive age, however, are relatively scarce. Several studies have assessed effects of isoflurane in young adult rats, primarily using young adult rats as comparisons to other age groups. Isoflurane administered to P60 rats affected progenitor proliferation and improved spatial memory in one study and had no effect in other one.44,45 Crosby and colleagues also observed improvement in spatial memory in rats anesthetized with 1.2% isoflurane-70% nitrous oxide at 6 month of age.46 Aside from the fact that two studies found long-term effects of isoflurane in young adult rats, different isoflurane concentrations and exposure regimens make it difficult to compare the effects across these studies. Such comparison is even more problematic with our current findings in F0 males, given that we tested not only a distinct anesthesia regimen, but also a different anesthetic agent. Clearly, further research is needed to elucidate the full range of long-term effects of young adult sevoflurane anesthesia and mechanisms that mediate such effects.
The GABAAR/testosterone/aromatase/estradiol/KCC2 pathway may be a key mediator of sevoflurane’s long-term neuroendocrine effects in F0 male rats. Because many gonadotropin-releasing hormone neurons are excited by GABAAR signaling even under basal conditions,47,48 sevoflurane may initially stimulate gonadotropin-releasing hormone neuronal activity and, hence, the entire reproductive axis. Male-specific factors may be required for sevoflurane’s actions to acutely reduce KCC2 expression, as similar increases in corticosterone levels in sevoflurane-exposed female rats were not accompanied by a reduction in KCC2 expression. The GABAAR/testosterone/aromatase/estradiol/KCC2 pathway in F0 male rats may function as a system with a positive feedback effect leading to a persistently up-regulated hypothalamic-pituitary-gonadal axis. Sevoflurane-induced increases in systemic testosterone and in brain aromatase expression may lead to reductions in Kcc2 expression, and in turn to diminished inhibitory or increased stimulatory control of gonadotropin-releasing hormone neurons by KCC2/GABAAR signaling. The plausibility of this scenario is supported by literature demonstrating that estradiol increases expression of aromatase and decreases expression of KCC2 in the brain.49,50 Interestingly, consistent with our findings, Galanopoulou and Moshé found that estradiol reduced KCC2 expression in males only.50
The GABAAR/testosterone/aromatase/estradiol/KCC2 pathway is also likely to be involved in dysregulated (exacerbated) hypothalamic-pituitary-adrenal axis responses to stress and stress-dependent behavioral abnormalities. One such mechanism includes modulation by estradiol of the glucocorticoid receptor-mediated negative feedback effect of corticosterone on hypothalamic-pituitary-adrenal axis activity. Estradiol can both inhibit and enhance the negative feedback effects of glucocorticoids by activating estrogen receptor α and estrogen receptor β, respectively.23,24 In support of this mechanism are our findings that hypothalamic levels of aromatase and estrogen receptor α mRNA were elevated, whereas hypothalamic levels of estrogen receptor β mRNA and hippocampal levels of glucocorticoid receptor mRNA were down-regulated. Higher estradiol concentrations are thought to contribute to higher corticosterone at rest or after stress exposure in adult female rats when compared to their male counterparts.51–53 Hence, our findings of exacerbated corticosterone responses to stress in F0 males, but not F0 females, suggest that exposure to sevoflurane in young adulthood induces a persistent transformation of the male stress response to a more “female-like” form. Upregulated expression of hypothalamic aromatase and resulting elevated levels of estradiol may also explain why in sevoflurane-exposed males, elevated levels of testosterone were accompanied by exacerbated hypothalamic-pituitary-adrenal axis responses to stress, because it was previously shown that gonadectomy of male rats elevated, while androgen replacement blunted the corticosterone response to stress.54 The persistent down-regulation of Kcc2 expression and resulting GABAAR depolarizing/stimulatory signaling in the hypothalamic PVN is likely to further contribute to dysregulated stress responses and behavioral abnormalities by impairing fundamental mechanisms of HPA axis functioning; e.g., the neuroactive steroid/GABAAR negative feedback-based mechanism of desensitization to stress.55 Finally, it is plausible that the negative feedback effect of elevated testosterone contributed to the reduced testis weight in exposed F0 males.
In conclusion, our results demonstrate that parental exposure to sevoflurane in young adulthood epigenetically reprograms germ cells, leading to neurobehavioral abnormalities in adult male progeny. These findings also provide evidence that sevoflurane administered even in young adulthood induces neurobehavioral deficits, profound alterations in the hypothalamic-pituitary-gonadal axis and dysregulated hypothalamic-pituitary-adrenal axis responses to stress in exposed male rats. Exposed young adult female rats exhibited no long-term physiological or behavioral abnormalities, but together with the exposed males, passed the adverse effects of sevoflurane exposure to their unexposed male offspring. These differential effects of sevoflurane exposure in males and females could suggest that distinct mechanisms mediate the somatic and germ cell effects of young adult sevoflurane exposure. It is important to note, however, that the current study was not powered to detect such sex differences, and hence it will be important in future work to conduct direct comparisons between males and females.
Boxed Summary
What we already know about this topic:
Exposure to environmental stressors and endocrine disruptors can inducemultigenerational effects resulting in neurobehavioral or other abnormalities in the offspring.
Early life anesthesia exposure in rodents alters neurocognitive function in their offspring but whether exposure of adult animals affects offspring has not been previously reported.What this article tells us that is new:
Repeated exposures of adult rats to sevoflurane (2.1%, 3 times, 3 hours on everysecond day) induce neurobehavioral abnormalities in the exposed males and in male but not female progeny.
What this article tells us that is new:
The neurobehavioral abnormalities in male offspring are accompanied by increased methylation and decreased expression of the K-Cl co-transporter KCC2 gene that regulates neuronal chloride homeostasis, and, thereby, the functional modalities of GABAergic neurotransmission.
Sevoflurane exposure also induces hypermethylation of the KCC2 gene in both male and female parental germ cells.
These observations suggest that epigenetic reprograming of parental germ cells is involved in transmitting the adverse effects of sevoflurane exposure of adult rats to their male progeny.
Summary Statement:
Sevoflurane administered to young adult rats can induce two types of adverse effects – the germ cell effects and the somatic cell effects, which lead to abnormalities in future offspring and the exposed animals, respectively.
Acknowledgement:
The authors would like to thank Terrie Vasilopoulos, Ph.D., Department of Anesthesiology, University of Florida College of Medicine, Gainesville, Florida, for her advice and help with the statistical analyses.
Funding Statement:
Supported by the National Institutes of Health (R01NS091542 and R01NS091542-S to A.E.M.), the Escher Autism Fund (A.E.M.), the I. Heermann Anesthesia Foundation (L.S.J), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (N.G.).
Footnotes
Conflicts of Interest:
The authors declare no competing interests.
References
- 1.FDA Drug Safety Communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children. US Food and Drug Administration. 4–27-2017. https://www.fda.gov/Drugs/DrugSafety/ucm554634.htm [Google Scholar]
- 2.Stratmann G Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011; 113: 1170–9. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y: The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 2014; 279:187–219. [DOI] [PubMed] [Google Scholar]
- 4.Khazipov R, Valeeva G, Khalilov I: Depolarizing GABA and developmental epilepsies. CNS Neurosci Ther 2015; 21:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antkowiak B, Rudolph U. New insights in the systemic and molecular underpinnings of general anesthetic actions mediated by γ-aminobutyric acid A receptors. Curr Opin Anaesthesiol. 2016; 29: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotani N, Akaike N. The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission. Brain Res Bull. 2013; 93: 69–79. [DOI] [PubMed] [Google Scholar]
- 7.Khan KS, Hayes I, Buggy DJ. Pharmacology of anaesthetic agents II: inhalation anaesthetic agents. Cont. Educ. Anaesth. Critical Care & Pain. 2014; 14: 106–11. [Google Scholar]
- 8.Lemonnier E, Villeneuve N, Sonie S, Serret S, Rosier A, Roue M, Brosset P, Viellard M, Bernoux D, Rondeau S, Thummler S, Ravel D, Ben-Ari Y: Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl Psychiatry 2017; 7:e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang X, Kim J, Zhou L, Wengert E, Zhang L, Wu Z, Carromeu C, Muotri AR, Marchetto MC, Gage FH, Chen G: KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci U S A 2016; 113:751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju LS, Yang JJ, Morey TE, Gravenstein N, Seubert CN, Resnick JL, Zhang JQ, Martynyuk AE: Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth 2018; 121: 406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju LS, Yang JJ, Gravenstein N, Seubert CN, Morey TE, Sumners C, Vasilopoulos T, Yang JJ, Martynyuk AE: Role of environmental stressors in determining the developmental outcome of neonatal anesthesia. Psychoneuroendocrinology 2017; 81: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Xu C, Puentes DL, Seubert CN, Gravenstein N, Martynyuk AE. Role of steroids in hyperexcitatory adverse and anesthetic effects of sevoflurane in neonatal rats. Neuroendocrinology 2016; 103: 440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R, Acharya K, Boudreau MH, Price TM, Raburn DJ, Corcoran DL, Lucas JE, Mitchell JT, McClernon FJ, Cauley M, Hall BJ, Levin ED, Kollins SH. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018; 13: 1208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013; 33: 9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, Meaney MJ. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry. 2014; 171: 872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehorter N, Vinay L, Hammond C, Ben-Ari Y. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci. 2012; 35: 1846–56. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Tan S, Zhang J, Seubert CN, Gravenstein N, Sumners C, Vasilopoulos T, Martynyuk AE: Anesthesia with sevoflurane in neonatal rats: Developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(−) importer antagonists. Psychoneuroendocrinology 2015; 60: 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM: Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology 2001; 152:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron 2016; 92: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009; 12: 438–43. [DOI] [PubMed] [Google Scholar]
- 21.Miller S and Maguire J. Deficits in KCC2 and activation of the HPA axis lead to depression like behavior following social defeat. Horm Stud. 2014; 2:2. [Google Scholar]
- 22.Tsukahara T, Masuhara M, Iwai H, Sonomura T, Sato T. The effect of repeated stress on KCC2 and NKCC1 immunoreactivity in the hippocampus of female mice. Data Brief 2016; 6: 521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiser MJ, Handa RJ: Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 2009; 159:883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiser MJ, Foradori CD, Handa RJ: Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res 2010; 1336:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis SW, Conneely KN, Marder ME, Terrell ML, Marcus M, Smith AK. Intergenerational effects of endocrine-disrupting compounds: a review of the Michigan polybrominated biphenyl registry. Epigenomics. 2018; 10: 845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill SY, Tessner KD, McDermott MD. Psychopathology in offspring from families of alcohol dependent female probands: a prospective study. J Psychiatr Res. 201; 45: 285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan CP, Chan JC, Bale TL. Driving the Next Generation: Paternal Lifetime Experiences Transmitted via Extracellular Vesicles and Their Small RNA Cargo. Biol Psychiatry. 2019; 85: 164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren LQ, Sun XX, Guan Y. Effects of sevoflurane or propofol combined with remifentanil anesthesia on clinical efficacy and stress response in pregnant women with pregnancy-induced hypertension. Eur Rev Med Pharmacol Sci. 2018; 22: 1825–29. [DOI] [PubMed] [Google Scholar]
- 29.Das W, Bhattacharya S, Ghosh S, Saha S, Mallik S, Pal S. Comparison between general anesthesia and spinal anesthesia in attenuation of stress response in laparoscopic cholecystectomy: A randomized prospective trial. Saudi J Anaesth. 2015; 9: 184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escher J Bugs in the program: can pregnancy drugs and smoking disturb molecular reprogramming of the fetal germline, increasing heritable risk for autism and neurodevelopmental disorders? Environ Epigenet. 2018; 4: dvy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt R, Wilson AM. Children of anaesthetists. Br Med J 1973; 1: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta D, Kaminski E, McKelvey G, Wang H. Firstborn offspring sex ratio is skewed towards female offspring in anesthesia care providers: A questionnaire-based nationwide study from United States. J Anaesthesiol Clin Pharmacol 2013; 29: 221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagella AB, Ravishankar M, Hemanth Kumar VR. Anaesthesia practice and reproductive outcomes: facts unveiled. Indian J Anaesth 2015; 59: 706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagella AB, Ravishankar M, Hemanth Kumar VR. Anaesthesia practice and reproductive outcomes: facts unveiled. Indian J Anaesth 2016; 60: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta D Firstborn female offspring are significantly more common among Indian anaesthesiologists as compared to national child sex ratio. Indian J Anaesth 2016; 60: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson H, Parker L. Do alcohol and lead change the sex ratio? J Theor Biol 1994; 169: 313–5. [DOI] [PubMed] [Google Scholar]
- 37.James WH. Offspring sex ratios at birth as markers of paternal endocrine disruption. Environ Res 2006; 100: 77–85. [DOI] [PubMed] [Google Scholar]
- 38.Navara KJ. Programming of offspring sex ratios by maternal stress in humans: Assessment of physiological mechanisms using a comparative approach. J Comp Physiol B. 2010;180:785–96. [DOI] [PubMed] [Google Scholar]
- 39.Ju LS, Jia M, Sun J, Sun XR, Zhang H, Ji MH, Yang JJ, Wang ZY. Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res. 2016; 29: 243–55. [DOI] [PubMed] [Google Scholar]
- 40.Jia M, Liu WX, Yang JJ, Xu N, Xie Z, Ju LS, Ji MH, Martynyuk AE, Yang JJ. Role of histone acetylation in long-term neurobehavioral effects of neonatal exposure to sevoflurane in rats. Neurobiol Dis. 2016; 91: 209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalla Massara L, Osuru HP, Oklopcic A, Milanovic D, Joksimovic SM, Caputo V, DiGruccio MR, Ori C, Wang G, Todorovic SM, Jevtovic-Todorovic V. General Anesthesia Causes Epigenetic Histone Modulation of c-Fos and Brain-derived Neurotrophic Factor, Target Genes Important for Neuronal Development in the Immature Rat Hippocampus. Anesthesiology. 2016; 124: 1311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang CK, Chalon J, Markham JP, Ramanathan S, Turndorf H: Exposure of sires to enflurane affects learning function of murine progeny. Anesth Analg 1984; 63: 729–30. [PubMed] [Google Scholar]
- 43.Vutskits L, Xie Z: Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 2016; 17:705–17. [DOI] [PubMed] [Google Scholar]
- 44.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009; 110: 834–48. [DOI] [PubMed] [Google Scholar]
- 45.Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010; 30: 1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg. 2005; 101: 1389–92. [DOI] [PubMed] [Google Scholar]
- 47.DeFazio RA, Heger S, Ojeda SR, Moenter SM: Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 2002; 16:2872–91. [DOI] [PubMed] [Google Scholar]
- 48.Herbison AE, Moenter SM: Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol 2011; 23:557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015; 18: 690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003; 184: 1003–9. [DOI] [PubMed] [Google Scholar]
- 51.Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R: Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci 1998; 16:289–95. [DOI] [PubMed] [Google Scholar]
- 52.Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP: Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 2007; 292:E1173–82. [DOI] [PubMed] [Google Scholar]
- 53.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T: Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 2009; 34:226–37. [DOI] [PubMed] [Google Scholar]
- 54.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR: Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav 1994; 55:117–24. [DOI] [PubMed] [Google Scholar]
- 55.Camille Melón L, Maguire J: GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J Steroid Biochem Mol Biol 2016; 160:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]