Abstract
Small fluctuations in striatal glutamate and dopamine are required to establish goal-directed behaviors and motor learning, while large changes appear to underlie many neuropsychological disorders, including drug dependence and Parkinson’s disease. A better understanding of how variations in neurotransmitter availability can modify striatal circuitry will lead to new therapeutic targets for these disorders. Here, we examined dopamine-induced plasticity in prefrontal cortical projections to the nucleus accumbens core. We combined behavioral measures of male mice, presynaptic optical studies of glutamate release kinetics from prefrontal cortical projections, and postsynaptic electrophysiological recordings of spiny projection neurons within the nucleus accumbens core. Our data show that repeated amphetamine promotes long-lasting but reversible changes along the corticoaccumbal pathway. In saline-treated mice, coincident cortical stimulation and dopamine release promoted presynaptic filtering by depressing exocytosis from glutamatergic boutons with a low-probability of release. The repeated use of amphetamine caused a frequency-dependent, progressive, and long-lasting depression in corticoaccumbal activity during withdrawal. This chronic presynaptic depression was relieved by a drug challenge which potentiated glutamate release from synapses with a low-probability of release. D1 receptors generated this synaptic potentiation, which corresponded with the degree of locomotor sensitization in individual mice. By reversing the synaptic depression, drug reinstatement may promote allostasis by returning corticoaccumbal activity to a more stable and normalized state. Therefore, dopamine-induced synaptic filtering of excitatory signals entering the nucleus accumbens core in novice mice and paradoxical excitation of the corticoaccumbal pathway during drug reinstatement may encode motor learning, habit formation, and dependence.
Keywords: Striatum, glutamate, dopamine, amphetamine
Graphical Abstract
Small changes in striatal glutamate and dopamine establish goal-directed behaviors, while large changes underlie many neuropsychological disorders. We identify long-lasting but reversible changes along the corticoaccumbal pathway that occur following repeated amphetamine. This synaptic plasticity corresponds with behaviors in mice and may encode motor learning, habit formation, and dependence.
1 ∣. INTRODUCTION
Addiction is a chronic condition characterized by drug seeking behavior and relapse following long periods of withdrawal. Psychostimulant drugs have a high potential for abuse as they acutely increase brain dopamine levels (Sulzer 2011) and can produce long-lasting changes at glutamatergic synapses in the striatum. These synaptic changes are critical for the expression of behavioral and motoric responses (Day and Carelli 2008; Pessiglione et al. 2006). Understanding how altered corticostriatal activity can lead to dependency is required to identify new therapeutic targets (Dackis 2004).
The striatum represents the main input for cortical signals entering the corticostriatal-basal ganglia-thalamocortical feedback loop (Albin et al. 1989; Parent and Hazrati 1995). The striatum is a continuum of largely similar neural networks and is broadly partitioned into anatomical regions that are defined by both function and anatomy of glutamatergic inputs from the cortex and dopamine inputs from the midbrain. The dorsal striatum participates in motoric control and motor learning and contributes to decision-making, action selection, and initiation (Balleine et al. 2007; Bamford and Bamford 2019; Darvas and Palmiter 2009; Darvas and Palmiter 2011; Wang et al. 2013). The ventral striatum, which includes the nucleus accumbens (NAc) core and a surrounding shell, is commonly associated with rewarding behaviors (Haber 2011). The NAc shell responds to a broad variety of physiological and pharmacological stimuli, including psychostimulant drugs, whereas the NAcore may encode the reinstatement of drug seeking behavior (Kalivas and Volkow 2005; Zahm 1999). However, there is significant functional overlap between these regions, and reward responses and habits appear to be encoded by plasticity that is generated throughout the striatum (Bamford and Bamford 2019; Haber 2011).
The NAc consists of a tripartite network where excitatory glutamatergic signals from the prefrontal cortex (PFC) and modulatory dopamine inputs from the ventral tegmental area (VTA) form synapses on spiny projection neurons (SPNs) (Nirenberg et al. 1997). The NAc also contains a relatively low number of modulatory cholinergic and GABAergic interneurons that participate in synaptic function and plasticity (Bamford and Cepeda 2009; Cepeda et al. 2010).
While the modulation of cortical signals by dopamine contributes to motor learning and cue-dependent behaviors (Darvas and Palmiter 2009; Darvas and Palmiter 2011; Wang et al. 2013), long-lasting plasticity in corticostriatal activity generated by too much or too little dopamine contributes to the signs and symptoms of many neuropsychological diseases, including addiction, Parkinson’s disease, and schizophrenia (Bamford et al. 2018; Cepeda et al. 2010; Goto and Grace 2007; Kalivas and Volkow 2005). The repeated use of psychostimulants in rodents has been shown to reduce corticoaccumbal activity (McFarland and Kalivas 2001; McFarland et al. 2003), suggesting that long-lasting alterations in glutamate transmission within the NAcore may participate in locomotor sensitization.
In the present study, we combined electrophysiological recordings in SPNs and optical recordings from individual cortical boutons within the NAcore of male mice to determine if repeated amphetamine in a regimen that produces robust locomotor sensitization (Beutler et al. 2011) can modify corticoaccumbal neurotransmission. We found that the repeated non-contingent use of amphetamine promoted a long-lasting, frequency-dependent plasticity in corticoaccumbal neurotransmission during withdrawal. Glutamate release from presynaptic boutons in the NAcore was enhanced at low frequencies and reduced at higher frequencies. This high-frequency chronic presynaptic depression was reversed by a frequency-dependent presynaptic potentiation during drug reinstatement that was mediated through D1-type dopamine receptors (D1Rs). The synaptic potentiation developed through a specific subset of cortical boutons and was proportional to the expression of locomotor sensitization in individual animals. These data suggest that this long-lasting presynaptic plasticity may contribute to addictive behaviors by promoting sensitized responses and allostatic normalization during relapse (Ahmed and Koob 2005).
2 ∣. MATERIALS AND METHODS
2.1 ∣. Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington and Yale University. C57BL/6 male mice (n=96), aged 30 to 60 days, were obtained from Jackson Labs (Bar Harbor, Maine) and housed together in a modified specific pathogen-free vivarium with a normal 12-hr light/dark cycle. Male mice were used to avoid potential confounds that might be associated with ovulation. Mice were provided access to food and water ad libitum, except during locomotor recording. For terminal procedures, mice were anesthetized using Beuthanasia (270 mg/kg i.p.) or Ketamine (650 mg/kg i.p.) with Xylazine (44 mg/kg i.p.) prior to sacrifice.
2.2 ∣. Experimental design
Mice were randomly assigned to amphetamine and saline experimental groups. For the electrophysiological experiments, mice received either amphetamine or saline for 5 days and were sacrificed on withdrawal day (WD) 10 or WD 21. The data collecting timepoints for the saline-treated control mice were the same as those for the amphetamine-treated mice. For experiments used to compare results from WD 10 and WD 21, the data obtained from saline-treated mice sacrificed on WD 10 or WD 21 were compared and the results were pooled together, if similar. For the optical experiments, mice received either amphetamine or saline for 5 days. They received additional amphetamine or saline treatments on experiment days corresponding to WD 10 and WD 21 and were sacrificed on WD 50. When possible, the experimenters were blind to group assignment and outcome. Sample-size estimates were determined by a power analysis. Data Handling: The study included 96 male mice. All mice used in the study were included in the data set. During the electrophysiology experiments, “outliers” were defined as cells demonstrating >20% change in holding current or evidence of polysynaptic activity (see below). For the optical studies, outliers were defined by slice movement that could not be compensated by automated software (see Methods). Data that were defined as an outlier were deemed failed experiments and were removed from all subsequent analyses. Replicates: All physiological recordings were replicated in 4 or more mice. The number of experimental repetitions is indicated in the results section. Results were pooled and averaged together as one “n.”
2.3 ∣. Behavior
Locomotor responses were determined using animal activity monitor cages (San Diego Instruments, CA). Four infrared beams, separated by 8.8 cm, that cross the width of each chamber were connected to an IBM computer, which recorded the number of times each beam was broken. Locomotor activity was measured in ambulations (2 consecutive beam interruptions) summated over 5 min intervals. On each test day, animals were acclimated to individual activity chambers for 90 min to allow the animal to become accustomed to its behavioral cage before subsequent injections of either amphetamine or saline. Following the injection, each animal was placed back into their respective activity chamber and ambulations were recorded over 90 min. To separate the effects of novelty from the pharmacological effects of the drug, animals were injected with saline and acclimated to the locomotor chambers for two hours in the two days prior to measuring locomotor activity.
2.4 ∣. Electrophysiology
Evoked (e) and miniature (m) excitatory postsynaptic currents (EPSCs) were recorded in 185 SPNs from 65 mice. Standard techniques were used to prepare 300 μm slices for electrophysiology (Joshi et al. 2009). Sagittal sections containing the PFC and NAcore were cut at an interaural distance range of 0.72 mm to 1.44 mm from midline (Paxinos and Franklin 2005). Brains were dissected, submerged in ice-cold, carbogenated (95% O2, 5% CO2) cutting solution containing (in mM): KCl 2.8, NaHCO3 26, NaH2PO4 1.25, MgSO4 2, MgCl2 1, CaCl2 1, sucrose 206, and glucose 10 (pH 7.2-7.4, 290-310 mOsm). Slices were prepared on a vibratome then transferred to an incubating chamber containing carbogenated artificial cerebrospinal fluid solution (aCSF) containing (in mM): NaCl 124, KCl 5, NaHCO3 26, NaH2PO4 1.25, MgCl2 2, CaCl2 2, and glucose 10 (pH 7.2-7.4, 290-310 mOsm) at room temperature. After 1 hr, slices were placed on the stage of an upright Zeiss Axioskop FS or an Olympus BX51WI microscope and submerged in continuously flowing carbogenated aCSF (3 ml/min), warmed to 35°C.
Whole-cell patch clamp recordings in voltage clamp mode were obtained from SPNs in the NAcore. Cells were visualized in slices with the aid of infrared videomicroscopy coupled with differential interference contrast optics. SPNs were identified by size (~8-12 μm) and typical passive membrane properties (Joshi et al. 2009). Electrophysiological properties were monitored throughout the recording and cells were removed from the analysis if the series resistance changed by >20%. Passive membrane properties of SPNs were determined in voltage clamp mode by applying a depolarizing step voltage command (10 mV) and using the membrane test function integrated in the pClamp software. For voltage clamp recordings, the patch pipette (4-6 MΩ) contained the following internal solution (in mM): Cs-methanesulfonate 125, KCl 3, NaCl 4, MgCl2 1, MgATP 5, EGTA 5, HEPES 8, Tris-GTP 1, Di-sodium phosphocreatine 10, leupeptin 0.1, and N-(2,6-Dimethylphenylcarbamoylmethyl) triethylammonium bromide 4 (QX-314; pH 7.2-7.3, 270-280 mOsm). eEPSCs were isolated by blocking GABAA receptors with bicuculline (10 μM). Cells were held at −70 mV to minimize further the contribution of GABAA-mediated events and that of voltage-gated conductances. Amphetamine was used to elevate striatal dopamine concentrations to ~3 μM after superfusion for 5-10 min (Bamford et al. 2004b), via a reversal of the dopamine transporter (Sulzer 2011).
Synaptic currents were evoked by electrical stimulation of the deep cortical layers at strengths adjusted to 1.5x threshold. A twisted tungsten bipolar electrode (Plastics One, Roanoke, VA) was placed over the dorsal PFC, which preferentially projects to the NAcore (Gorelova and Yang 1997). Single or paired 20 Hz current pulses (200 μs duration) were presented every 30 sec. Each cell received 10 paired-pulses in background aCSF before amphetamine or receptor ligands were added to the bath. eEPSC amplitudes and the paired pulse ratios (PPR) were averaged and compared 2.5 min before and 5 to 7.5 min after bath application of amphetamine or a receptor ligand. The PPR was determined by dividing the amplitude of the second pulse by the first pulse and then multiplying by 100. Cells demonstrating eEPSCs with variable latencies or prolonged durations suggesting polysynaptic responses were rejected from all analyses. Currents were Bessel filtered at 2 kHz and digitized at 50 μs using an IBM computer equipped with Digidata 1440A data acquisition and pClamp10.2 software (Molecular Devices). Peak currents were measured by subtracting the baseline from the eEPSC peak.
mEPSCs were recorded in gap-free mode with the Na+ channel blocker, tetrodotoxin, for 90 sec, both before and 10 min following bath-application of amphetamine and/or a receptor ligand. The membrane current was filtered at 1 kHz and digitized at 100 μs using Clampfit 10.1 (Molecular Devices, Inc., Foster City, CA). Spontaneous synaptic events were analyzed off-line using the Mini Analysis Program (Jaejin Software, Leonia, NJ). The threshold amplitude for the detection of an event was adjusted to at least 2 times above root mean square noise level (~2-3 pA at −70 mV). Synaptic events could be prevented by adding NBQX (10 μM) to the bath solution, indicating that they arose from activation of glutamatergic receptors.
2.5 ∣. Optical recordings
Optical recordings of cortical afferents in the NAcore were obtained from 151 slices from 31 mice, as described (Bamford et al. 2004b). Sagittal sections were prepared, as for the electrophysiology studies, and recovered for 1 hr in carbogenated aCSF containing (in mM): NaCl 109, KCl 5, NaHCO3 35, NaH2PO4 1.25, HEPES 20, MgCl2 1.2, CaCl2 2, and Glucose 10 (pH 7.3-7.4, 295-305 mOsm) at RT. During the experiment, slices were held in a RC-27L incubation chamber (56 μL/mm; Warner Instruments, Hamden, CT) and were perfused at 3 mL/min with the aCSF warmed to 35°C. To ensure equilibrium, sections were exposed to pharmacological agents for 10 min before stimulation-mediated unloading.
The endocytic tracer FM1-43 (N-[3-(triethylammonio)propyl]-4-(4-dibutylaminostyryl) pyridinium dibromide; Molecular Probes; 8 μM in aCSF) was loaded into presynaptic boutons by stimulating cortical layers V-VI at 10 Hz for 10 min with 400 μA, 200 μs pulses. This loading method ensures the absence of provoked striatal dopamine release and that changes in the release of FM1-43 are not due to inadequate FM1-43 loading of the recycling synaptic vesicle pool (Bamford et al. 2004a; Bamford et al. 2004b; Joshi et al. 2009). The stimulating electrode was of the same type and was placed in the same location as the one used for the electrophysiology studies. Similarly, the recording regions were the same as those in the electrophysiological experiments. Following loading, slices were superfused in ADVESEP-7 (1 mM in aCSF; CyDex, Overland Park, KS) for 2 min to remove adventitious staining. For stimulation-dependent destaining, pulse trains were again delivered to the cortex. During unloading, aCSF was supplemented with ADVASEP-7 (100 μM) to prevent recurrent endocytosis of dye into synaptic boutons. Electrical stimulation was provided by a Grass Stimulator (West Warwick, RI) through a stimulation isolator (AMPI, Jerusalem, Israel) and monitored by a Tektronix TDS 3014B digital oscilloscope (Beaverton, OR).
Fluorescent boutons in the NAcore were visualized using an LSM 510 NLO multiphoton laser-scanning microscope equipped with a titanium-sapphire laser (excitation 810 nm/emission 625 nm) and a 40x inverted oil objective (Zeiss). Images were captured in 8-bit, 123 × 123 μm regions of interest (ROI) at 512 × 512 pixel resolution and acquired at 22.5 s intervals using Zeiss LSM 510 software. To compensate for z-axis shift, a z-series of five images, separated by 1 μm in the z-axis, was obtained for each imaging period. The time series of images was analyzed for changes in fluorescence using Image J (National Institutes of Health, Rockville, MD) and custom software written in Interactive Data Language (Research Systems, Boulder, CO) (Bamford et al. 2004b). The software identified fluorescent puncta measuring 0.5-1.5 μm in diameter. The criteria for punctum inclusion were (1) spherical in shape, (2) fluorescence two standard deviations above the background, and (3) stimulation-dependent destaining. The IDL software aligned and combined the five-image z-series for each time interval and the overall intensity of the FM1-43 fluorescence was measured over the course of the time series. Image J was used to subtract background fluorescence of the tissue from the fluorescence intensity of each individual punctum. The results were then normalized by the maximal puncta fluorescence just prior to application of destaining stimulation. The half-time of fluorescence intensity decay during destaining (t1/2) was determined using a software algorithm written on SigmaPlot software (SPSS, Chicago, IL).
2.6 ∣. Drug administration
Bicuculline methiodide and NBQX were dissolved in dimethylsulfoxide and diluted to final concentrations in the aCSF. Amphetamine sulfate was dissolved in 0.9% normal saline (0.2 mg/mL) and injected i.p. Unless specified in the text, chemical and drugs were obtained from either Sigma (St. Louis, MO) or Abcam Biochemicals (Cambridge, MA).
2.7 ∣. Statistical analysis
Values given in the text and in the figures are mean ± standard error (SEM). “n” represents the number of mice, cells, or puncta indicated in the text. Differences in mean values were assessed by 2-tailed Student’s t-tests (two groups), or repeated measures ANOVAs (multiple groups). Bonferroni t-tests were used for data requiring multiple comparisons. Cumulative probability data was compared using the non-parametric Kolmogorov-Smirnov test. The non-parametric Mann-Whitney test was used to validate differences between cases where the data may have failed to meet the assumptions of parametric testing. Testing for normally-distributed data in the optical studies was determined graphically using normal probability plots. When individual half-times are presented in a normal probability plot, a straight line was used to indicate a normally-distributed population (Bamford et al. 2004a). To correlate electrophysiological results with behavioral data, numbers obtained in multiple cells from a single animal were averaged and treated as one “n” to minimize the possibility of obtaining a type I error caused by individual differences. Statistical analyses were performed with Statistica 6.1 (StatSoft, Tulsa, OK) and differences were considered significant if p<0.05.
3 ∣. RESULTS
3.1 ∣. The repeated use of amphetamine promotes a depression in corticoaccumbal activity during withdrawal
We examined the effect of repeated amphetamine use on corticoaccumbal activity by performing whole-cell voltage-clamp recordings of SPNs within the NAcore. C57B/6 mice, aged 4-8 weeks, were treated with amphetamine (2 mg/kg/d; i.p.) or an equivalent volume of 0.9% saline (10 μl/g mouse) once per day for 5 consecutive days. Mice were then sacrificed on withdrawal day (WD) 10 or WD 21 and acute sagittal slices encompassing the PFC and NAcore were prepared for electrophysiology (Figure 1a-b). Experiments in 4-8 week-old C57B/6 mice mice allowed comparisons with prior work in the NAcore (Wang et al. 2012) while the experimental time points of WD 10 and WD 21 allowed comparisons with earlier work in SPNs from the dorsal striatum following withdrawal from contingent and non-contingent use of psychostimulants (Bamford et al. 2008; Beutler et al. 2011; Storey et al. 2016; Wang et al. 2013). The passive cell membrane properties of SPNs from saline-treated mice (n=13) were compared with those from amphetamine-treated mice (n=26). The series resistance of each cell was <20 MΩ (11±0.7 MΩ and 13±0.6 MΩ for saline- and amphetamine-treated mice, respectively; mean ± SEM). Compared to SPNs from saline-treated mice, SPNs from amphetamine-treated mice demonstrated an increase in the membrane capacitance (93±6 pF, n=37 cells for saline vs. 122±7 pF, n=43 cells on WD 10; t(78)=3.15, p=0.002, 2-tailed Student’s t-test) and in the time constant (1.1±0.1 ms, n=11 for saline vs. 2.4±0.1 ms, n=31 on WD 10; t(40)=5.6, p<0.001, 2-tailed Student’s t-test). There was no change in resting membrane resistance (227±31 MΩ, n=37 cells for saline vs. 232±23 MΩ, n=43 cells for WD 10). The length of drug withdrawal had no effect on the membrane capacitance (121±7 pF, n=31 cells on WD 21) or resting membrane resistance (245±28 MΩ, n=19 on WD 21). However, the time constant was slightly higher on WD 10 than on WD 21 (1.8 ± 0.2 ms, n=19; t(48)=3.09, p=0.003, 2-tailed Student’s t-test). There was no difference in the passive membrane parameters of SPNs from saline-treated controls that were sacrificed on WD 10 or WD 21, so the results were pooled together. Thus, withdrawal from repeated amphetamine modifies SPNs by increasing their membrane capacitance and the time needed for cells to reach their resting state.
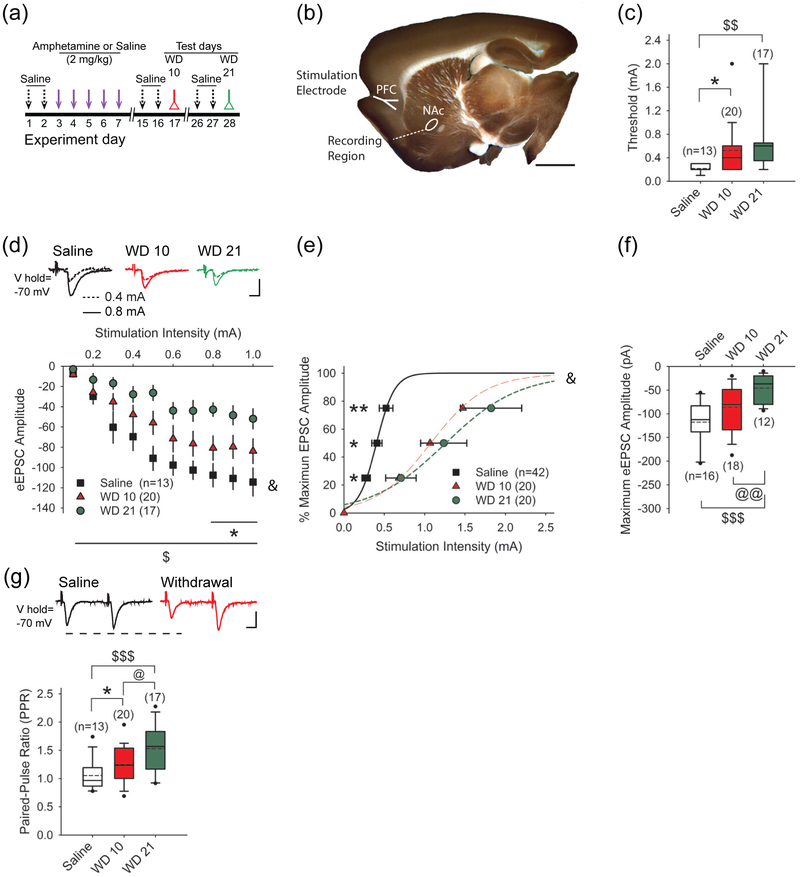
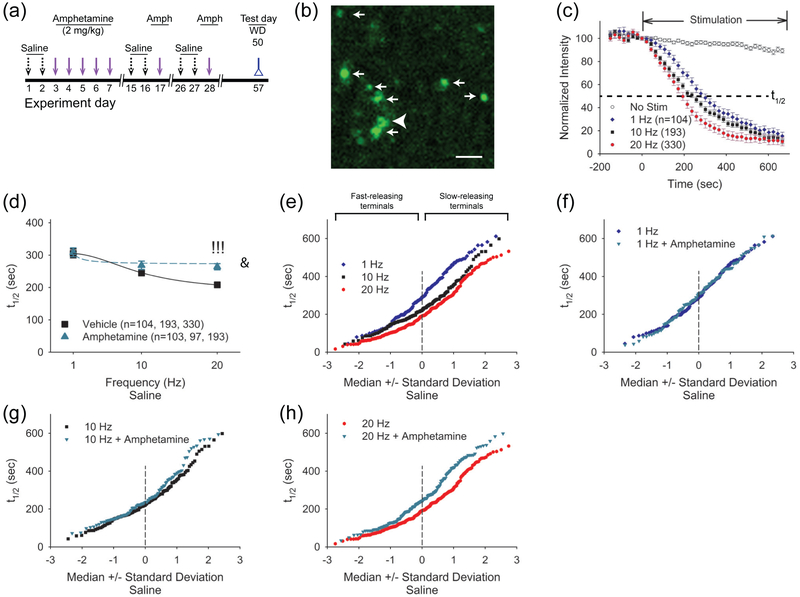
FIGURE 1.
Repeated amphetamine produces a stimulation-dependent synaptic depression in the NAcore. (a) Paradigm for testing synaptic plasticity following repeated amphetamine. Amphetamine-treated mice were injected with saline for 2 days and amphetamine for 5 days, while saline-treated mice received saline instead of amphetamine. Amphetamine and saline-treated mice were sacrificed for experiments on WD 10 or WD 21. To separate the effects of novelty from the pharmacological effects of the drug, amphetamine- and saline-treated mice used for the combined behavioral and optical experiments also received saline injections on days 1, 2, 15, 16, 26, and 27. (b) A sagittal corticoaccumbal slice, stained with FM1-43 and 3,3’-diaminobenzidine, demonstrates the areas of stimulation and recording. Bar: 3 mm. (c) The cortical stimulation current required to reach eEPSC threshold in SPNs from amphetamine-treated mice on WD 10 and WD 21 was greater than that required in cells from saline-treated mice. Box-and-whisker plots: boundary, 25th and 75th percentiles; median, solid black line; mean, dashed line; whiskers, 10th and 90th percentiles; outlying points, circles. For all panels, *p<0.05, **p<0.01, saline vs. WD 10, 2-tailed Student’s t-test; $p<0.05, $$p<0.01, $$$p<0.001, saline vs. WD 21, 2-tailed Student’s t-test; @p<0.05, WD10 vs. WD 21, 2-tailed Student’s t-test. The number of cells (n) is indicated in parenthesis. (d) Representative traces (above) and input-output curves (below) show that cortical stimulation produced lower amplitude eEPSCs in SPNs on WD 10 and WD 21. &p<0.05, 2-way ANOVA for interaction between stimulation intensity and treatment. Bars: 100 pA, 5 ms. (e) The cortical stimulation intensity required to achieve 25%, 50%, and 75% of maximum eEPSC amplitude was greater in SPNs from amphetamine-treated mice on WD 10 and WD 21. &p<0.05, 2-way ANOVA for interaction between stimulation intensity and treatment. Curves were fit with a Hill equation. (f) The graph shows that the maximum eEPSC amplitude is lower in SPNs from amphetamine-treated mice on WD 10 and WD 21. Bars: 100 pA, 5 ms. (g) Representative traces (above) and graph show that the PPR is higher in SPNs from amphetamine-treated mice in withdrawal, compared to saline-treated mice.
Prior experiments in mice and rats have demonstrated that repeated psychostimulants modify glutamatergic activity in the dorsal and ventral striatum (Bamford et al. 2008; McFarland et al. 2003; Pierce and Kalivas 1997; Wang et al. 2013). To determine if withdrawal from repeated amphetamine can modify corticoaccumbal glutamatergic activity, we placed bipolar electrodes in the dorsal PFC and used single cortical pulses to elicit eEPSCs in SPNs within the NAcore. For these experiments, cortical stimulation was provided every 30 sec and the stimulation intensity was progressively increased until an eEPSC was detected in the SPN. Compared to SPNs from saline-treated mice (0.22±0.02 mA, n=13 cells from 5 mice), the stimulation intensity required to evoke an eEPSC amplitude above 10 pA was 144% higher in cells from amphetamine-treated mice on WD 10 (0.53±0.1 mA; n=20 cells from 6 mice; t(31)=2.48, p=0.019, 2-tailed Student’s t-test) and was 203% higher in SPNs from amphetamine-treated mice on WD 21 (0.65±0.13 mA; n=17 cells from 4 mice; t(28)=2.92, p=0.006, 2-tailed Student’s t-test; Figure 1c). While the threshold was 24% higher on WD 21 compared to WD 10, the difference was not significant (t(35)=0.79, p=0.4, 2-tailed Student’s t-test). The eEPSC threshold was similar in saline-treated mice sacrificed on WD 10 (0.22±0.02 mA; n=6 cells) and WD 21 (0.21±0.03 mA; n=7 cells) and the results were pooled together.
Cortical stimulation intensities above the firing threshold evoked larger eEPSC amplitudes in cells from saline-treated mice, compared to SPNs on WD 10 and on WD 21 (F(18,333)=1.98, p=0.010; n=50; 2-way ANOVA for interaction between stimulation intensity and treatment; Figure 1d). Additionally, a greater stimulation current was required to achieve a peak eEPSC amplitude following repeated amphetamine; the stimulation intensity (mA) required to obtain 25%, 50%, and 75% of maximum eEPSC amplitude (pA) in SPNs from saline-treated mice (0.27±0.05 mA, 0.41±0.06 mA, 0.52±0.08 mA, respectively; n=13 cells from 5 mice) was much lower than in cells from amphetamine-treated mice tested on WD 10 (0.71±0.29 mA, 1.24±0.49 mA, 1.82±0.68 mA, respectively; n=20 cells from 9 mice) and on WD 21 (0.68±0.09 mA, 1.07±0.15 mA, 1.47±0.24 mA, respectively; n=19 cells from 10 mice; F(4,98)=3.48, p=0.010, 2-way ANOVA for interaction between stimulation intensity and treatment; Figure 1e). The stimulation intensity required to obtain 25%, 50%, and 75% of maximum eEPSC amplitude in SPNs from saline-treated mice sacrificed on WD 10 (0.25±0.09 mA, 0.38±0.1 mA, 0.5±0.15 mA, respectively; n=6 cells) was similar to saline-treated mice sacrificed on WD 21 (0.29±0.06 mA, 0.44±0.08 mA, 0.54±0.09 mA, respectively; n=7 cells), so the results were pooled together.
To assess synaptic activity, we measured the maximum achievable peak eEPSC amplitude in response to a single cortical stimulus. The average maximum peak eEPSC amplitude in SPNs from saline-treated mice (−118±13 pA; n=13 cells from 4 mice) was higher than the peak amplitudes obtained in SPNs from amphetamine-treated mice on WD 10 (−86±12 pA; n=19 cells from 6 mice), but the results were not significant (t(30)=1.79, p=0.080, 2-tailed Student’s t-test; Figure 1f). The maximum peak eEPSC amplitude in saline-treated mice sacrificed on WD 10 (−119.34±19; n=6 cells) was similar to WD 21 (−116±19; n=7 cells), so the results were pooled together. The average peak eEPSC amplitude obtained in SPNs from amphetamine-treated mice on WD 21 (−46±7 pA; n=16 cells from 4 mice) was significantly lower when compared to saline (t(27)=5.14, p<0.001, 2-tailed Student’s t-test) or WD 10 (t(33)=2.83, p=0.008, 2-tailed Student’s t-test). Therefore, higher stimulation intensities were required to evoke and optimize EPSCs in SPNs from the NAcore during drug withdrawal, suggesting that a history of amphetamine exposure can cause a depression in corticoaccumbal activity.
Next, we used paired cortical pulses to determine if amphetamine can modify presynaptic corticoaccumbal activity (Mennerick and Zorumski 1995). We measured the paired-pulse ratio (PPR; amplitude of the second eEPSC /amplitude of the first eEPSC) in response to cortical stimulation with 50 ms paired-pulses applied every 30 sec. Paired-pulse depression can represent a Ca2+-dependent or Ca2+-independent reduction in presynaptic release and/or a postsynaptic release-dependent depression which may result from the unavailability of postsynaptic receptors (Kirischuk et al. 2002).
Compared to SPNs from saline-treated mice (1.05±0.07; n=16 cells from 7 mice), the PPR was 18% higher in cells from amphetamine-treated mice on WD 10 (1.25±0.08; n=18 cells from 7 mice; t(32)=2.2, p=0.030, 2-tailed Student’s t-test) and 45% higher in cells from amphetamine-treated mice on WD 21 (1.53±0.12; n=12 cells from 7 mice; t(26)=3.68, p<0.001, 2-tailed Student’s t-test; Figure 1g). The PPR was 22% higher on WD 21 compared to WD 10 (t(28)=2.17, p=0.023, 2-tailed Student’s t-test). The PPR was similar in saline-treated mice sacrificed on WD 10 (1.07±0.13; n=7 cells) and WD 21 (1.04±0.09; n=9 cells) and the results were pooled together. Thus, the data suggested that repeated use of amphetamine in mice can promote an enduring and progressive synaptic depression along the corticoaccumbal pathway. This corticoaccumbal depression was characterized by a reduction in postsynaptic responsiveness to single and paired stimuli, as well as an increase in the paired-pulse ratio.
3.2 ∣. An amphetamine challenge in withdrawal reverses corticoaccumbal depression through D1-type dopamine receptors
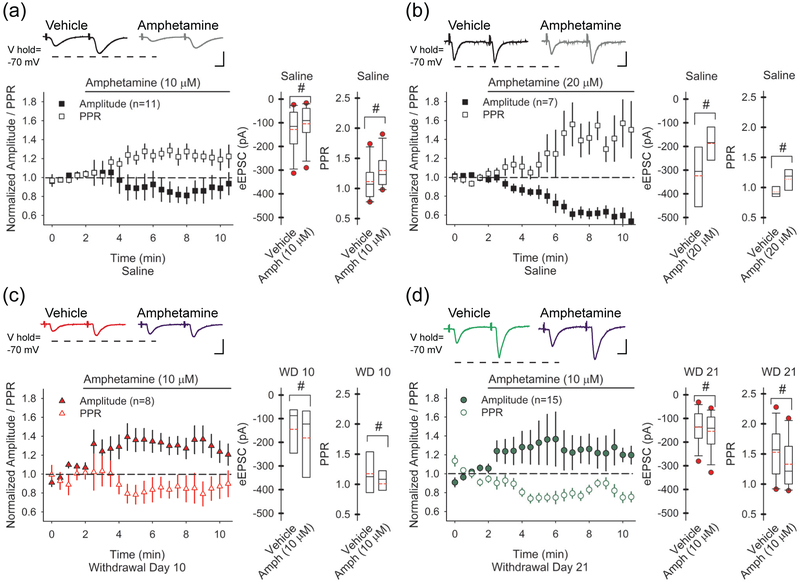
Since reinstatement of drug-seeking behaviors can be generated by changes in glutamatergic activity within the NAcore (Baker et al. 2003; Bell et al. 2000; McFarland et al. 2003; Pierce et al. 1996) as well as within the dorsal striatum (Bamford et al. 2008; Storey et al. 2016; Wang et al. 2013), we tested whether an amphetamine challenge in withdrawal would reverse or block the synaptic depression observed in the NAcore. Mice were treated with saline or amphetamine for 5 days (Figure 1a). On WD 10, slices were prepared and 50 ms paired pulses were applied to the dorsal PFC every 30 sec. In SPNs from saline-treated mice, amphetamine (10 μM) in vitro — which can elevate striatal dopamine concentrations to ~3 μM (Bamford et al. 2004b) via reversal of the dopamine transporter (Sulzer 2011) — decreased the average amplitude of the first eEPSC of the pair by 16±11% (−128±26 pA in vehicle vs. −104±23 pA with amphetamine; n=11 cells from 5 mice; t(10)=2.57, p=0.027, paired t-test) and increased the average PPR by 19±6% (1.11±0.09 in vehicle vs. 1.3±0.13 in amphetamine; t(10)=3.07, p=0.012, paired t-test; Figure 2a), indicating that novel exposure to amphetamine in vitro can reduce corticoaccumbal excitation. A higher concentration of amphetamine (20 μM) also reduced the probability of release from presynaptic boutons as the amplitude of the first eEPSC decreased (−43±5%; −324±76 pA in vehicle vs. −187±42 pA with amphetamine; n=5 cells from 4 mice; t(4)=3.73, p=0.020, paired t-test) while the PPR increased (0.92±0.05 in vehicle vs. 1.14±0.08 in amphetamine; t(4)=3.62, p=0.022, paired t-test; Figure 2b).
FIGURE 2.
Repeated amphetamine causes synaptic potentiation. (a) Representative traces (above) demonstrate the amplitudes of the paired-pulses in SPNs from saline-treated mice before (left) and 5 min to 7.5 min following bath-applied amphetamine (10 μM; right). The graphs (below) show the normalized amplitude of the first eEPSC (of the pair) and the normalized PPR. In saline-treated mice, amphetamine in vitro decreased the amplitude of the first eEPSC and increased the PPR (right). For all panels, #p<0.05, compared to saline, paired t-test. The number of cells (n) is indicated in parenthesis. Bars: 100 pA, 5 ms. (b) Representative traces (above) demonstrate the amplitudes of the paired-pulses in SPNs from saline-treated mice before (left) and 5 min to 7.5 min following bath-applied amphetamine (20 μM; right). The graphs (below) show that a higher concentration of amphetamine reduced the eEPSC amplitude and increased the PPR. (c) Representative traces (above) demonstrate the amplitudes of the paired-pulses in SPNs from amphetamine-treated mice on WD 10 before (left) and 5 min to 7.5 min following bath-applied amphetamine (10 μM; right). The graphs (below) show that on WD 10 and (d) WD 21, bath-applied amphetamine (10 μM) increased the eEPSC amplitude and decreased the PPR.
Next, we tested whether drug withdrawal would modify the inhibitory response of an amphetamine challenge. In SPNs from amphetamine-treated mice on WD 10, bath-applied amphetamine (10 μM) increased the amplitude of the first eEPSC by 30±9% (−145±41 pA in vehicle vs. −181±49 pA with amphetamine; n=8 cells from 4 mice; t(7)=2.56, p=0.037, paired t-test) and reduced the PPR by 14±6% (1.17±0.12 in vehicle vs. 0.97±0.1 with amphetamine; t(7)=2.80, p=0.026, paired t-test; Figure 2c). On WD 21, amphetamine in vitro also increased the amplitude of the first eEPSC (22±14%; −137±20 pA in vehicle vs. −154±22 pA with amphetamine; n=12 cells from 7 mice; t(11)=2.92, p=0.014, paired t-test) and reduced the PPR (−12±4%; 1.53±0.12 in vehicle vs. 1.33±0.12 with amphetamine; t(11)=2.99, p=0.012, paired t-test; Figure 2d). Thus, in novice mice, acute amphetamine, with concurrent cortical stimulation, promoted a presynaptic depression within the NAcore that was characterized by a reduction in the eEPSC amplitude and an increase in the PPR. This depression was maintained in withdrawal and could be reversed by an amphetamine challenge.
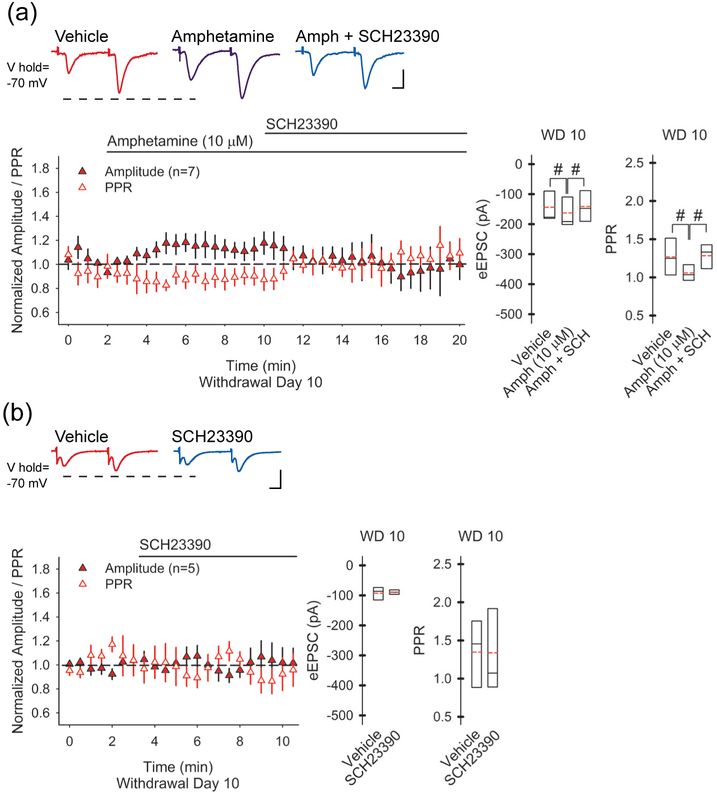
In a separate set of experiments, we determined if the synaptic potentiation that follows an amphetamine challenge in withdrawal might be generated through D1Rs, as occurs in the dorsal striatum (Bamford et al. 2008; Wang et al. 2013). In amphetamine-treated mice on WD 10, amphetamine (10 μM) in vitro increased the eEPSC amplitude (16±6%; −144±22 pA in vehicle vs. −163±33 pA with amphetamine; n=5 cells from 3 mice; t(4)=4.76, p=0.009, paired t-test) and reduced the PPR (−12±5%; 1.27±0.11 in vehicle vs. 1.1 ± 0.18 with amphetamine; t(4)=3.38, p=0.028, paired t-test; Figure 3a). The excitatory effect of an amphetamine challenge on corticoaccumbal activity was dependent on D1Rs, since the D1R antagonist SCH23390 (10 μM) blocked the increase in eEPSC amplitude (0.2±13%, compared to vehicle; −141±32 pA for amphetamine with SCH23390; t(4)=0.15, p=0.886, paired t-test compared to vehicle) and prevented the decrease in the PPR (3±9%, compared to vehicle; 1.28±0.1 for amphetamine with SCH23390; t(4)=0.13, p=0.899, paired t-test compared to vehicle). The D1R antagonist alone had no effect on the eEPSC amplitude (−0.7±9%; −93±10 pA in vehicle vs. −89±15 pA in SCH23390; n=5 cells from 3 mice; t(4)=0.46, p=0.670, paired t-test) or the PPR (−2±7%; 1.35±0.2 in vehicle vs. 1.34±0.2 in SCH23390; t(4)=0.09, p=0.934, paired t-test) on WD 10 (Figure 3b), suggesting that the synaptic potentiation which follows a drug challenge in withdrawal is mediated through D1Rs.
FIGURE 3.
Synaptic potentiation is mediated through D1Rs. (a) Representative traces demonstrate paired-pulse eEPSC amplitudes in SPNs from amphetamine-treated mice on WD 10 before (above, left), 5-7.5 min following bath application of amphetamine (above, center), and 5-7.5 min following perfusion of amphetamine with the D1R antagonist SCH23390 (10 μM; above, right). The graphs (below) show that amphetamine in vitro increased the amplitude of the first eEPSC and decreased the PPR, while bath-application of the D1R antagonist SCH23390 blocked these changes. For all panels, #p<0.05, compared to saline, paired t-test. The number of cells (n) is indicated in parenthesis. Bars: 100 pA, 5 ms. (b) On WD 10, bath application of SCH23390 alone had no effect on the eEPSC amplitude or the PPR.
3.3 ∣. Synaptic depression in withdrawal is dependent on cortical stimulation
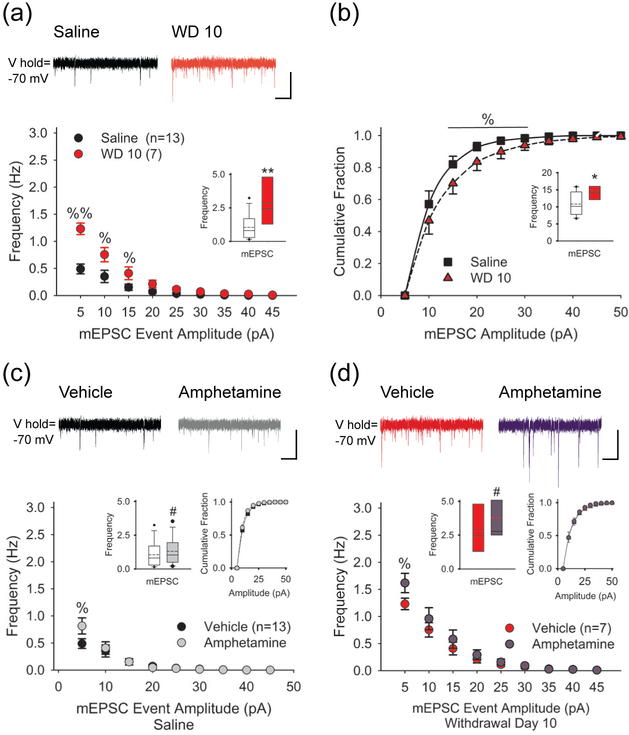
To determine if this synaptic plasticity within the NAcore is dependent on cortical stimulation, we measured spontaneous mEPSCs in SPNs in the absence of stimulation. Alterations in the frequency of mEPSCs can also help to determine if changes are generated presynaptically while a change in the mEPSC amplitude may signify an alteration in postsynaptic responsiveness (Van der Kloot 1991). The Na+ channel antagonist tetrodotoxin (TTX; 1 μM) was used to block spontaneous cortically-derived action potentials and isolate presynaptic activity. Compared to mEPSCs measured in SPNs from saline-treated mice (1.13±0.25; n=13 cells from 4 mice), the frequency of mEPSCs was enhanced on WD 10 (3.14±0.7; n=7 cells from 4 mice; t(17)=3.40, p=0.003, 2-tailed Student’s t-test), and there was a significant increase in the frequency-amplitude distribution (p<0.05, Kolmogorov-Smirnov test; Figure 4a). Similarly, the average mEPSC amplitude was increased on WD 10 (10.8±1 for saline vs.14.6±1.5 for WD 10; t(17)=2.22, p=0.040, 2-tailed Student’s t-test), and there was a significant change in the cumulative normalized amplitude-frequency distribution (p<0.05, Kolmogorov-Smirnov test; Figure 4b). Thus, under non-stimulated conditions, the results are consistent with an increase in pre-and postsynaptic responsiveness following repeated amphetamine (Van der Kloot 1991).
FIGURE 4.
Amphetamine enhances spontaneous mEPSCs. (a) Representative traces (above) show mEPSCs recorded in SPNs from saline- (left) and amphetamine-treated mice on WD 10 (right). The average frequency (inset) and the cumulative frequency distribution of mEPSCs was greater in withdrawal. For panels a and b: *p<0.05, **p<0.01, 2-tailed Student’s t-test; %p<0.05, %%p<0.01, 2-tailed Student’s t-test with Bonferroni correction. Bars: 10 pA, 1 sec. (b) The average mEPSC amplitude is greater on WD 10 (inset) and the cumulative amplitude distribution shows a greater fraction of mEPSCs in the 15-30 pA range. (c) Representative traces (above) show mEPSCs in SPNs from saline-treated mice in vehicle (upper left) and 5-7.5 min after bath-applied amphetamine (upper right). Amphetamine (10 μM) in vitro enhanced the frequency of mEPSCs (inset, left) by increasing the number of high-frequency, low-amplitude spontaneous inward currents but had no effect on the cumulative mEPSC amplitude distribution (inset, right). For panels c and d, #p<0.05, paired t-test. %p<0.05, %p<0.05, paired t-test with Bonferroni correction. (d) Representative traces (above) show mEPSCs in SPNs from amphetamine-treated mice on WD 10 in vehicle (upper left) and 5-7.5 min after bath-applied amphetamine (upper right). On WD 10, amphetamine (10 μM) in vitro boosted the frequency of mEPSCs (inset, left) by increasing high-frequency, low-amplitude inward currents, while having no effect on the cumulative amplitude distribution (inset, right).
To test the effect of an amphetamine challenge under non-stimulated conditions, mEPSCs (with TTX) were observed in SPNs before and following amphetamine (10 μM) in vitro. In SPNs from saline-treated mice, amphetamine in vitro enhanced the frequency of mEPSC by 42±13% (1.05±0.26 Hz in vehicle vs. 1.3±0.27 Hz in amphetamine; n=13 cells from 5 mice; t(12)=2.41, p=0.034, paired t-test) by selectively increasing high-frequency, low-amplitude inward currents (Figure 4c). Amphetamine had no effect on the average mEPSC amplitude (10.8±0.9 pA in vehicle vs. 10.3±0.6 pA in amphetamine; t(12)=0.98, p=0.347, paired t-test) or the amplitude distribution (p>0.1, Kolmogorov-Smirnov test). On WD 10, bath-applied amphetamine also increased the frequency of mEPSCs (63±21%; 2.9±0.69 Hz in vehicle vs. 3.8 ± 0.72 Hz in amphetamine; n=7 cells from 4 mice; t(6)=3.16, p=0.019, paired t-test), primarily by boosting high-frequency, low-amplitude currents (Figure 4d). An amphetamine challenge in withdrawal did not change the average mEPSC amplitude (14.6±1.6 pA in vehicle vs. 13.5±0.6 pA in amphetamine; t(6)=0.88, p=0.501, paired t-test) or the amplitude distribution (p>0.1, Kolmogorov-Smirnov test). Thus, under non-stimulated conditions, acute amphetamine had a small excitatory effect on presynaptic corticoaccumbal activity, with and without a history of psychostimulant exposure, and indicated that synaptic depression in drug withdrawal and the paradoxical potentiation that follows a drug challenge are dependent upon cortical stimulation.
3.4 ∣. Presynaptic depression and potentiation are frequency-dependent and modify a select subpopulation of presynaptic boutons in the NAcore
The data above indicate that synaptic depression was dependent on SPN activation. To investigate further, we directly measured the frequency dependence of presynaptic release by optical analysis using the endocytic tracer FM1-43 combined with multiphoton confocal microscopy (Bamford et al. 2004b; Wong et al. 2011). In these experiments, mice received saline or amphetamine (2 mg/kg/d; i.p.) for 5 consecutive days and were challenged with amphetamine on WD 10 and WD 21. To determine if presynaptic plasticity was long-lasting, optical recordings were performed on WD 50 (Figure 5a).
FIGURE 5.
Acute amphetamine produces a frequency-dependent presynaptic depression in saline-treated mice. (a) Model for measuring the long-term effects of repeated amphetamine (Amph) on corticoaccumbal activity. Amphetamine-treated mice were injected with saline for 2 days, amphetamine for 5 days and received an amphetamine challenge on challenge days 1 and 2 (experiment days 17 and 28), corresponding to WD 10 and WD 21. Saline-treated mice received saline instead of amphetamine. Mice were sacrificed for experiments on experiment day 57 (corresponding to WD 50). To separate the effects of novelty from the pharmacological effects of the drug, amphetamine- and saline-treated mice also received saline injections on days 1, 2, 15, 16, 26, and 27. (b) A multiphoton image of corticoaccumbal boutons obtained from NAcore. The arrows and arrowhead illustrate destaining and non-destaining fluorescent puncta, respectively. Bar: 2.5 μm. (c) In slices from saline-treated mice, stimulation of the motor cortex at 1 Hz, 10 Hz, and 20 Hz produced a frequency-dependent increase in FM1-43 release, reflected by a decrease in the half-time (t1/2) of FM1-43 destaining. Little FM1-43 destaining occurred when no stimulation was applied (No Stim). (d) Comparisons of the mean half-times (t1/2) of FM1-43 release in slices from saline-treated mice stimulated at 1 Hz, 10 Hz, and 20 Hz, with and without amphetamine (10 μM) in vitro. Amphetamine inhibited release at 20 Hz. The number of puncta (n) is indicated in parenthesis. !!!p<0.001, Mann-Whitney. &p<0.05, 2-way ANOVA for interaction between frequency and treatment. (e) The normal probability plot, where a straight line indicates a normally-distributed population (Bamford et al. 2004b), shows the release kinetics of individual cortical boutons in slices from saline-treated mice following cortical stimulation at 1 Hz, 10 Hz, and 20 Hz. Higher frequencies increase FM1-43 destaining, indicated by a reduction in the t1/2. (f) Destaining half-times of individual cortical boutons in slices from saline-treated mice, with and without bath-applied amphetamine at 1 Hz, (g) 10 Hz, and (h) 20 Hz. At 20 Hz, amphetamine preferentially decreases exocytosis (increases t1/2) from boutons with a low probability of release.
Stimulation of the dorsal PFC resulted in endocytosis of FM1-43 dye into recycling presynaptic vesicles in the NAcore, revealing fluorescent puncta that were distinctive of corticoaccumbal afferents (Bamford et al. 2004b; Wong et al. 2011) (Figure 5b). Following dye loading, cortical stimulation with a train of pulses produced exocytosis of FM1-43 dye from presynaptic boutons, characteristic of synaptic vesicle fusion (Bamford et al. 2004a). As FM1-43 destaining follows first-order kinetics (Joshi et al. 2009), corticostriatal release was characterized by the half-time of destaining (t1/2), defined as the time required for fluorescence to decay to half of its initial value (Figure 5c). In slices from saline-treated mice, an increase in cortical stimulation frequency at physiologically-relative rates (Charpier et al. 1999; Cowan and Wilson 1994; Fellous et al. 2003; Kasper et al. 1994; Stern et al. 1997) from 1 Hz to 20 Hz produced a corresponding increase in FM1-43 release from the majority of boutons, which was reflected by a decrease in the average half-time of FM1-43 destaining (t1/2= 305±14 sec at 1 Hz, n=104 puncta; t1/2= 246±10 sec at 10 Hz, n=193 puncta; and t1/2= 208±6 sec at 20 Hz, 330 puncta; F(2 ,564)=26.00, p=0.001, repeated measures (rm)-ANOVA; Figure 5c-d). When amphetamine was bath-applied, an increase in the stimulation frequency also accelerated release (t1/2= 309±14 sec at 1 Hz, n=103 puncta; t1/2= 269±13 sec at 10 Hz, n=97 puncta; and t1/2= 264±10 sec at 20 Hz, n=193 puncta; F(2, 387)=4.00, p=0.020, rm-ANOVA) but to a lesser extent than in vehicle (F(2, 951)=4.03, p=0.029, 2-way ANOVA for interaction between frequency and amphetamine; Figure 5d). Compared to vehicle, amphetamine in vitro caused a progressive, frequency-dependent reduction in exocytosis so that presynaptic inhibition by amphetamine was observed at 20 Hz (p<0.001, Mann-Whitney).
Prior investigations have shown the cortical boutons in the striatum have variable release kinetics (Bamford et al. 2004b). We used normal probability plots to determine if amphetamine differentially modifies a subset of corticoaccumbal boutons. When the half-time of FM-143 release is compared to the median ± standard deviation, a normally distributed population is reflected by a straight line (Bamford et al. 2004b). Increasing cortical stimulation frequencies from 1 Hz to 20 Hz produced a corresponding increase in FM-143 release with a relatively greater effect on slower-releasing boutons (Figure 5e). Bath application of amphetamine had little effect at 1 Hz and 10 Hz cortical stimulation. At 20 Hz, amphetamine inhibited corticoaccumbal release and produced a low-pass frequency filter with filtering applied specifically to a subset of boutons with a low probability of release (e.g. those with the highest t1/2; Figure 5f-h).
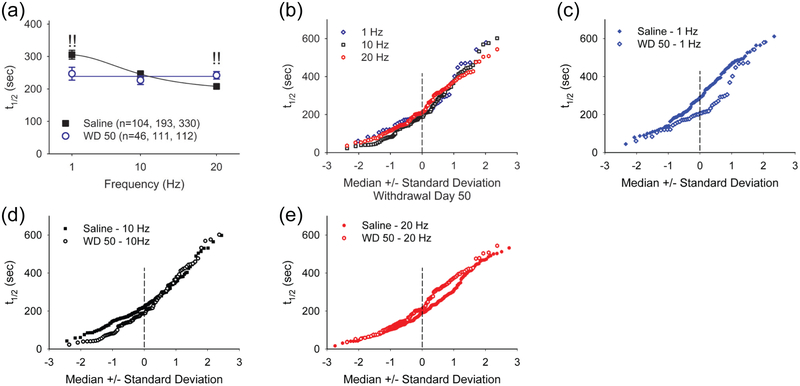
Next, we compared the release of FM-143 in brain slices from saline-treated mice with that obtained from amphetamine-treated mice on WD 50. In slices from amphetamine-treated mice on WD 50, an increase in cortical stimulation frequency produced little change in corticoaccumbal release (Figure 6a-b), suggesting that drug exposure caused a progressive frequency-dependent depression in the fractional release of FM1-43. Compared to saline-treated controls, corticoaccumbal release on WD 50 was augmented at 1 Hz (t1/2= 247±20 sec, n=46 puncta; p=0.01, Mann-Whitney), unchanged at 10 Hz (t1/2= 227±13 sec, n=111 puncta) and depressed at 20 Hz (t1/2= 242±12 sec, n=112 puncta; p=0.01, Mann-Whitney; Figure 6a and Figure 6c-e). Thus, under conditions of drug withdrawal, corticoaccumbal activity was enhanced during trains of low-frequency stimulation and was depressed at higher cortical frequencies. Evaluation of the kinetics of individual corticoaccumbal boutons showed that withdrawal from amphetamine increased release from boutons with intermediate kinetics at 1 Hz and from faster-releasing boutons at 10 Hz (Figure 6c-d). At 20 Hz, release was diminished only in those boutons with the lowest release probability (Figure 6e).
FIGURE 6.
Withdrawal from repeated amphetamine use produces presynaptic potentiation at low cortical stimulation frequencies and presynaptic depression at high stimulation frequencies. (a) Average half-times of FM1-43 release in slices from saline- and amphetamine-treated mice on WD 50 following cortical stimulation at 1 Hz, 10 Hz, and 20 Hz. Cortical stimulation produces a frequency-dependent increase in FM1-43 release (lower t1/2) in slices from saline-treated mice. Increasing frequencies have little effect on FM1-43 release kinetics in slices from amphetamine-treated mice on WD 50. Compared to saline-treated mice, presynaptic potentiation occurs at 1 Hz and presynaptic depression is seen at 20 Hz. !!p<0.01, Mann-Whitney. The number of puncta (n) is indicated in parenthesis. (b) The normal probability plot shows similar release kinetics of individual cortical boutons in slices from amphetamine-treated mice on WD 50 following cortical stimulation at 1 Hz, 10 Hz, and 20 Hz. (c) Destaining half-times of individual cortical boutons in slices from saline-treated mice are compared to those obtained in slices from amphetamine-treated mice on WD 50 in response to cortical stimulation at 1 Hz, (d) 10 Hz, and (e) 20 Hz. Presynaptic potentiation occurs at 1 Hz stimulation, while presynaptic depression occurs at 20 Hz.
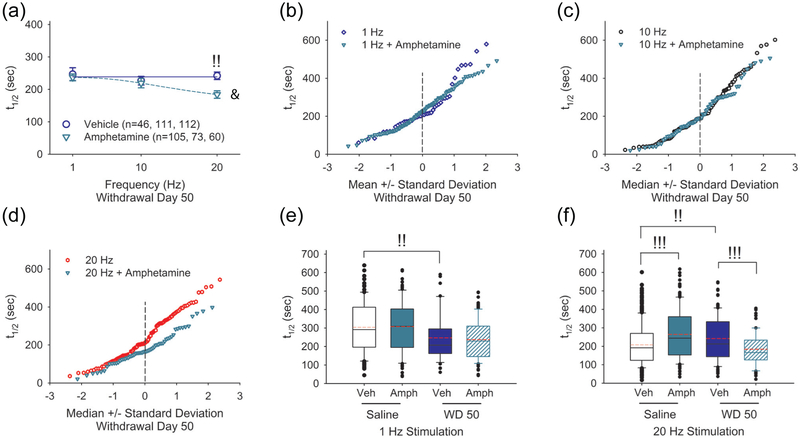
Next, we determined how an amphetamine challenge would modify frequency-dependent plasticity in corticoaccumbal release during withdrawal. Bath application of amphetamine in corticoaccumbal slices from amphetamine-treated mice on WD 50 caused a frequency-dependent increase in corticoaccumbal release (t1/2= 237±11 sec at 1 Hz, n=105 puncta; t1/2= 219±15 sec at 10 Hz, n=73 puncta; and t1/2= 184±12 sec at 20 Hz, n=60 puncta; F(2, 235)= 5.00, p=0.010, rm-ANOVA; Figure 7a). Compared to untreated slices on WD 50, amphetamine in vitro produced a frequency-dependent increase in release (F(2, 951)= 4.00, p=0.019, 2-way ANOVA for interaction between frequency and treatment). At lower cortical stimulation frequencies of 1 Hz and 10 Hz, amphetamine in vitro had little effect on presynaptic release in amphetamine-treated mice (Figure 7a-c). At higher stimulation frequencies (20 Hz), amphetamine boosted presynaptic release by assisting corticoaccumbal boutons with a low probability of release (Figure 7d). Therefore, the repeated use of amphetamine produced frequency-dependent plasticity in corticoaccumbal excitability. During withdrawal, presynaptic activity was enhanced at low frequencies (1 Hz) and depressed at high frequencies (20 Hz; Figure 7e-f). An amphetamine challenge in withdrawal had no effect on low-frequency potentiation but boosted corticoaccumbal activity at high frequencies to normalize synaptic function.
FIGURE 7.
An amphetamine challenge on WD 50 promotes a paradoxical presynaptic potentiation at high-frequency stimulation. (a) Average half-times of FM1-43 release in slices from amphetamine-treated mice on WD 50 before and following an amphetamine (10 μM) challenge in vitro. An amphetamine challenge has no effect on FM1-43 destaining during cortical stimulation at 1 Hz and 10 Hz. Amphetamine produced a frequency-dependent increase in FM1-43 release (lower t1/2) at 20 Hz. For all panels, !!p<0.01, !!!p<0.001, Mann-Whitney. &p<0.05, 2-way ANOVA for interaction between frequency and treatment. The number of puncta (n) is indicated in parenthesis. (b) In slices from amphetamine-treated mice on WD 50, the half-time responses of individual boutons are compared with and without an amphetamine challenge at 1 Hz, (c) 10 Hz, and (d) 20 Hz. At 20 Hz, amphetamine increases exocytosis (decreased t1/2) from boutons with a low probability of release. (e) Summary graph compares the half-times of FM1-43 release in saline-treated and amphetamine-treated mice on WD 50 in response to low-frequency (1 Hz) stimulation of the PFC. Compared to saline-treated controls, exposure to repeated amphetamine increases presynaptic release on WD 50. An amphetamine challenge has little effect on the average half-time of FM1-43 release in saline-treated or amphetamine-treated mice on WD 50. Veh, vehicle. (f) Summary graph compares the half-times of FM1-43 release in saline-treated and amphetamine-treated mice on WD 50 in response to high-frequency (20 Hz) stimulation of the PFC. Compared to saline-treated controls, withdrawal from repeated amphetamine decreases release from presynaptic corticoaccumbal boutons. Acute amphetamine in vitro reduces the average release of FM1-43 in slices from saline-treated mice but boosts FM1-43 release in slices from amphetamine-treated mice on WD 50.
3.5 ∣. Synaptic potentiation correlates with locomotor sensitization
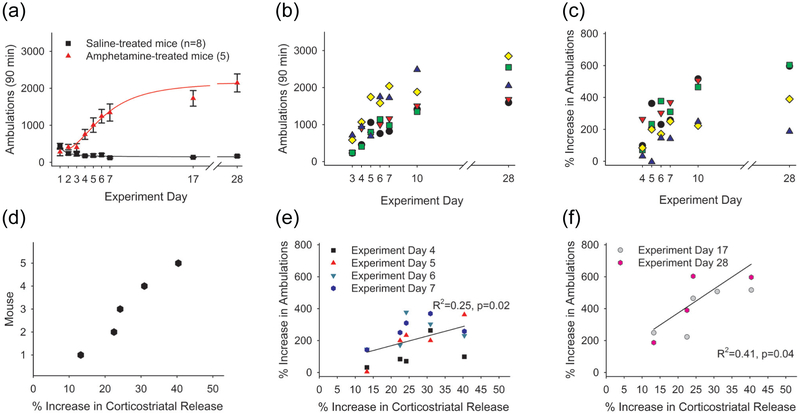
Evidence suggests that amphetamine-induced adaptations in glutamatergic signaling may underlie locomotor sensitization (Cornish et al. 1999; Ghasemzadeh et al. 2003; Knackstedt and Kalivas 2009; Li et al. 1999; Storey et al. 2016; Wang et al. 2013). To investigate further, we treated mice with saline (n=8) or amphetamine (n=5) for 5 consecutive days and monitored their locomotor activity. Locomotor sensitization, defined as a progressive increase in locomotion with each treatment, was tested by an amphetamine challenge on challenge day 1 (corresponding to WD 10) and challenge day 2 (corresponding to WD 21; Figure 5a). Mice were sacrificed on experiment day 57 (WD 50) for optical measurements of corticoaccumbal release. We then compared locomotor ambulations in individual mice with the change in FM1-43 release following an amphetamine challenge in vitro.
Amphetamine in vivo produced locomotor sensitization with increasing ambulations following each amphetamine injection (Figure 8a). Locomotor ambulations varied widely between animals (Figure 8b) and generally increased following each treatment (292±36%; range, 3%-603%; Figure 8c). On WD 50, the mice were sacrificed and we measured FM1-43 destaining in response to 20 Hz cortical stimulation. Amphetamine (10 μM) in vitro depressed FM1-43 release in saline-treated mice. In slices from amphetamine-treated mice, an amphetamine challenge in vitro increased FM1-43 release. The percent increase in corticoaccumbal release varied widely between amphetamine-treated mice (28.2±3.3%; range, 22.5%-40.3%; Figure 8d). A linear regression comparison between the percent increase in corticostriatal release and the percent change in ambulations for each mouse revealed a significant correlation during the initial 5 days of treatment (R2=0.25, F(1, 18)=6.08, p=0.020, Figure 8e) and when challenged with amphetamine on challenge day 1 and challenge day 2 (R2=0.41, F(1, 8)=5.72, p=0.040; Figure 8f), indicating a correlation between the degree of presynaptic potentiation and sensitized locomotor responses.
FIGURE 8.
The degree of locomotor sensitization correlates with synaptic potentiation in individual mice. (a) Locomotor ambulations in response to treatment with saline or amphetamine. The number of mice (n) is indicated in parenthesis. (b) Locomotor ambulations of individual amphetamine-treated mice from the behavioral experiments shown in Figure 8a. (c) The percent increase in locomotor ambulations for individual mice shown in Figure 8a, when normalized to ambulations on the first day of amphetamine (experiment day 3). (d) The percent increase in corticostriatal release (potentiation) following an amphetamine challenge in vitro on WD 50, measured in slices from amphetamine-treated mice (shown in Figure 8a). (e) Percent increase in ambulations on experiment days 4-7 is compared to the percent increase in corticostriatal release on WD 50. (f) The percent increase in ambulations following an amphetamine challenge in vivo on challenge days 1 and 2 (experiment day 17 and 28) is compared to the percent increase in corticostriatal release (potentiation) on WD 50.
4 ∣. Discussion
Excitatory glutamatergic signals from the PFC and modulatory dopamine inputs from the VTA converge on SPNs in the NAcore, which initiate and direct signaling through “direct” and ‘indirect” pathways to generate go / no-go actions through striatal-thalamic-cortical pathways (Albin et al. 1989; Parent and Hazrati 1995). While small changes in the availability of glutamate and dopamine are putatively required to establish goal-directed behaviors, motor learning, and habits through functional and structural alterations in striatal circuitry, large changes in these neurotransmitters appear to underlie many neuropsychological disorders, including drug dependence (Bamford et al. 2018; Kalivas et al. 2003; Kalivas and Volkow 2005). Our data show that repeated psychostimulants, when delivered in a paradigm that releases dopamine (Bamford et al. 2004b), while allowing simultaneous measurements of behavior, promotes long-lasting, but reversible changes along the corticoaccumbal pathway (Figure 9).
FIGURE 9.
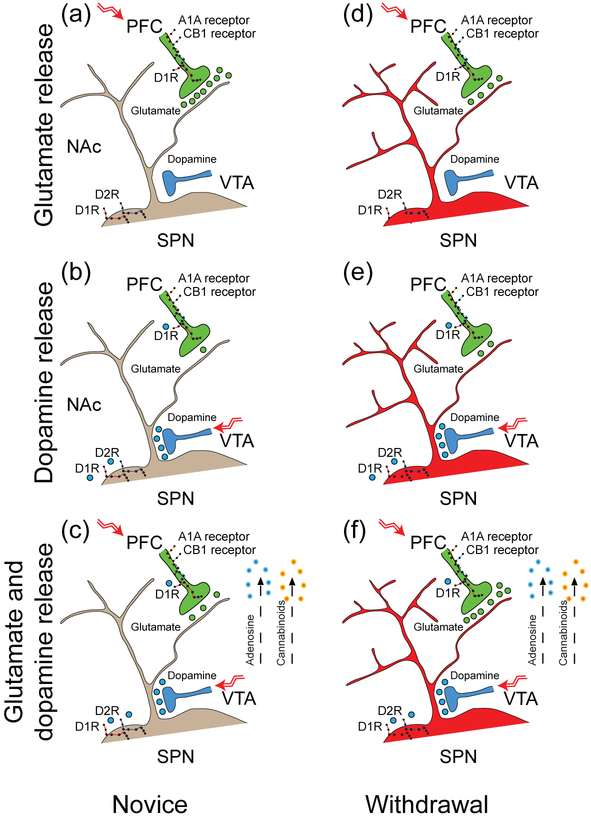
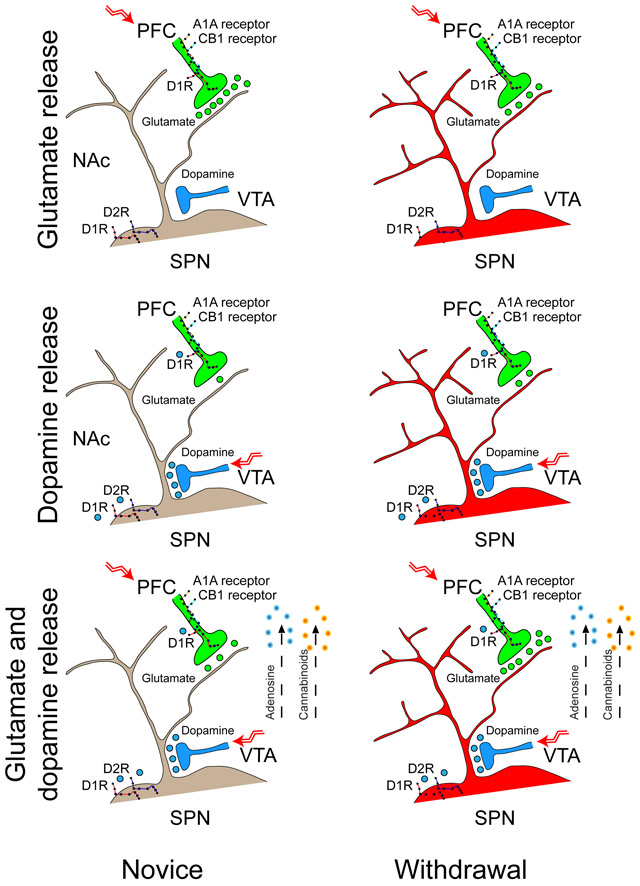
Proposed mechanism of corticoaccumbal presynaptic plasticity in the NAcore (a) The illustration depicts a D1R- or D2R-expressing SPN within the NAcore. A dopamine afferent from the VTA and a glutamatergic projection from the PFC form synapses on the dendritic spine of an SPN (Nirenberg et al. 1997; Totterdell and Smith 1989). (b) When the cortex is quiescent, dopamine released by amphetamine increases glutamate release from corticoaccumbal afferents by interacting with presynaptic D1Rs (Wang et al. 2012), manifesting in an increase in the frequency (but not the amplitude) of mEPSCs. (c) When the PFC is activated by high-frequency impulses, coincident glutamate and dopamine occludes the excitation provided by presynaptic D1Rs and reduces glutamate release, evident by a reduction in the eEPSC amplitude, an increase in the PPR, and a reduction in FM1-43 release. The simultaneous release of dopamine and glutamate promotes presynaptic inhibition via the release of endocannabinoids (Grueter et al. 2010) and adenosine (Dunwiddie and Masino 2001; Harvey and Lacey 1997) that are putatively produced by SPNs (Di Marzo et al. 1994) and interact with CB1 cannabinoid and A1A adenosine receptors that are located on presynaptic glutamatergic terminals (Ciruela et al. 2006; Wang et al. 2012). (d) Exposure to repeated amphetamine increases the dendritic length and spine density (Robinson and Kolb 1997) and reduces glutamate neurotransmission, evident by a reduction in the eEPSC amplitude and threshold, an increase in the PPR, and an increase in the cell’s capacitance and time constant. A longer withdrawal time further reduces glutamate neurotransmission, manifesting in a greater reduction in the maximal eEPSC amplitude and a larger increase in the PPR. A train of low-frequency impulses from the PFC promotes presynaptic potentiation during withdrawal. (e) With the cortex at rest, pre- and postsynaptic potentiation is evident by a small increase in the frequency and amplitude of mEPSCs. Dopamine release by amphetamine increases spontaneous glutamate release by interacting with presynaptic D1Rs, demonstrated by an increase in the frequency but not the amplitude of mEPSCs. (f) With PFC activation by high-frequency impulses, coincident dopamine enhances glutamate release from presynaptic boutons, evident by an increase in the eEPSC amplitude, a decrease in the PPR, and an increase in FM1-43 release.
We combined presynaptic optical studies with postsynaptic electrophysiological recordings in the NAcore of male mice treated with repeated amphetamine. The results of these experiments show that under normal conditions, dopamine released by amphetamine promotes synaptic filtering by causing a frequency-dependent depression in a subset of corticoaccumbal boutons. The repeated use of amphetamine promoted a long-lasting and progressive depression in corticoaccumbal activity. This corticoaccumbal depression was characterized by activity- and frequency-dependent plasticity during withdrawal that was partially relieved by a drug challenge. The increase in glutamate release from excitatory corticoaccumbal boutons in response to the drug challenge was generated though D1Rs and corresponded with the degree of locomotor sensitization in individual animals. Thus, the boost in glutamate release during drug reinstatement promotes allostasis (Ahmed and Koob 2005), an attempt to return corticoaccumbal activity to a more stable and normalized state.
These experiments were performed in young adolescent mice and at time points that corresponded to our earlier work in the dorsal and ventral striatum (Bamford et al. 2008; Beutler et al. 2011; Storey et al. 2016; Wang et al. 2013). Limitations of the study include the use of young mice rather than older rats that have been used extensively to analyse the effects of contingent psychostimulant use but see {Storey, 2016 #4623}. The electrophysiology and optical data were collected in the slice preparation and may differ from plasticity that occurs in vivo, therefore presenting an avenue for future research. Another limitation is our exclusive use of male mice. While the data are not confounded by any possible effects of ovulation, sex-based comparisons were not available.
4.1 ∣. Dopamine promotes synaptic filtering
Our experiments in saline-treated control mice showed that novel exposure to amphetamine in vitro modulates corticoaccumbal activity. In the absence of stimulation, amphetamine provoked a small excitatory response in cortical boutons with a low probability of release, characterized by an increase in the frequency but not the amplitude of mEPSCs. This combination of changes mEPSCs suggests that dopamine has a small excitatory effect on presynaptic boutons (Van der Kloot 1991) in the NAcore and is consistent with the presence and function of D1Rs on corticoaccumbal axons (Dumartin et al. 2007; Wang et al. 2012). The responses to amphetamine under non-stimulated conditions were similar in saline- and amphetamine-treated mice and suggested that any plasticity provoked by drug exposure had no effect on excitation provided by presynaptic D1Rs.
When SPNs from saline-treated mice became activated by stimulation of the PFC, amphetamine in vitro reduced corticoaccumbal activity. Corticoaccumbal depression was present at 20 Hz, but not at lower stimulation frequencies of 1 Hz and 10 Hz. This frequency-dependent inhibition suggests the presence of presynaptic inhibition by the neuromodulators, adenosine and endocannabinoids. These neuromodulators are putatively released by SPNs in response to activation and provide retrograde inhibition of glutamate release (Wang et al. 2012). Frequency-dependent filtering of a subset of presynaptic excitatory afferents by dopamine also occurs in the dorsal striatum and may act as a mechanism to focus attention on meaningful information by boosting strong signals while inhibiting the weak (Bamford et al. 2004b; Dani and Zhou 2004).
4.2 ∣. Stimulation-dependent synaptic depression increases during drug withdrawal and is generated though pre- and postsynaptic plasticity
We determined if the depression in corticoaccumbal activity provoked by amphetamine in novice, saline-treated mice might persist in mice exposed to several amphetamine treatments. The repeated use of amphetamine in mice promoted a synaptic depression along the corticoaccumbal pathway that lasted over 50 days. The depression was characterized by a reduction in postsynaptic responsiveness to single and paired (20 Hz) stimuli. Higher cortical stimulation intensities were required to evoke and optimize eEPSCs, and the maximal amplitude of eEPSCs progressively declined with the duration of withdrawal.
We found that the synaptic depression found in amphetamine withdrawal was dependent on excitation of the PFC. Under non-stimulated conditions, SPNs from amphetamine-treated mice manifest an elevated frequency and a higher amplitude of mEPSCs compared to SPNs from saline-treated mice. This increase in frequency and amplitude suggests enhanced presynaptic release and post-synaptic responsiveness following repeated amphetamine (Van der Kloot 1991). There was additional evidence of post-synaptic plasticity. Recordings in SPNs from amphetamine-treated mice demonstrated increased cellular capacitance and time constant, while the input resistance was unchanged. These findings are consistent with the increase in the dendritic length and spine density (Isokawa 1997) of SPNs in the NAcore that follows repeated amphetamine (Robinson and Kolb 1997). Therefore, changes in the membrane properties of SPNs likely represent a postsynaptic alteration in consequence of repeated exposure to the psychostimulant. Structural changes in the dendritic spines of SPNs in the NAc can persist for months after the last drug treatment (Li et al. 2003) and may underlie or compensate for long-lasting alterations in glutamatergic and/or dopaminergic synaptic transmission (Garcia et al. 2010; Villalba and Smith 2013).
There was also evidence of presynaptic depression, manifesting in an increase in the PPR (Mennerick and Zorumski 1995). Although paired-pulse facilitation can also be generated by changes in postsynaptic receptors (Kirischuk et al. 2002), the results are similar to the presynaptic depression found in the dorsal striatum following contingent and non-contingent use of methamphetamine (Bamford et al. 2008) and amphetamine (Storey et al. 2016; Wang et al. 2013). The observed depression was not due to current spread, because 1) eEPSCs in SPNs required intact corticostriatal axons, 2) the resting membrane potential of SPNs was unchanged by cortical stimulation, and 3) similar methods produced opposing responses in saline-treated controls (Wong et al. 2015).
Neurotransmitter release from subpopulations of presynaptic boutons is reliant on neuronal firing frequencies (Bamford et al. 2004a; Bamford et al. 2004b; Sulzer 2011), and this dependence is altered in disease (Bamford et al. 2004a; Joshi et al. 2009). Our optical recordings of FM1-43 release from presynaptic boutons in the NAcore validated the frequency-dependent plasticity found in post-synaptic recordings. By providing trains of cortical stimuli at physiologically-relevant frequencies (Charpier et al. 1999; Cowan and Wilson 1994; Fellous et al. 2003; Kasper et al. 1994; Stern et al. 1997), we measured the kinetics of vesicle fusion at presynaptic boutons in the NAcore. Compared to slices from saline-treated mice, FM1-43 release in slices from amphetamine-treated mice was elevated 1 Hz and became depressed at 20 Hz. Therefore, presynaptic corticoaccumbal activity in withdrawal was enhanced at no or low cortical stimulation frequencies and was depressed at higher cortical frequencies.
Different stimulation frequencies also differentially modulated certain subsets of corticoaccumbal boutons. In saline-treated controls, amphetamine had little effect at lower frequencies but inhibited boutons with a low probability of release at high frequencies. In amphetamine withdrawal, low-frequency stimulation enhanced exocytosis from boutons with high probability of release while higher frequencies depressed boutons with a low probability of release. These findings suggest that the synaptic filtering provided by dopamine in novice mice may persist during drug withdrawal.
4.3 ∣. Amphetamine reinstatement generates a frequency-dependent potentiation in corticoaccumbal activity
Synaptic potentiation likely plays a key role in the motivational circuitry underlying learning and dependence since supra-physiological glutamatergic drive promotes compulsive drug-seeking in addicts by decreasing the value of natural rewards (Kalivas and Volkow 2005). In slices from saline-treated mice, acute amphetamine decreased the amplitude of the first eEPSC (of the pair) and increased in PPR, consistent with a decrease in glutamate release from corticoaccumbal boutons. Conversely, in amphetamine-treated mice, an amphetamine challenge in withdrawal increased the EPSC amplitude and decreased the PPR, suggesting that dopamine promotes synaptic filtering in novice mice but potentiates glutamate release in withdrawal.
The optical recordings confirmed a primary presynaptic mechanism of synaptic potentiation due to a drug challenge in withdrawal. In saline-treated mice, an amphetamine challenge in vitro produced a frequency-dependent decrease in release from boutons with a low probability of release. In drug withdrawal an amphetamine challenge produced a frequency-dependent boost in exocytosis from this same population of presynaptic boutons.
Synaptic potentiation in response to a drug challenge was not dependent on dopamine release kinetics (Bamford et al. 2008) and could not depend on changes in dopamine neuronal firing since it was measured in the striatal slice from which dopamine cell bodies were absent. The boost in presynaptic activity during a drug challenge appeared to be mediated though presynaptic mechanisms and was unchanged over the length of drug withdrawal. In comparison, the synaptic depression appears to be generated through both pre-and postsynaptic plasticity and worsened with the length of drug withdrawal, suggesting that drug reinstatement which attempts to normalize the synapse can become less effective over time.
4.4 ∣. Synaptic potentiation correlates with the degree of locomotor sensitization
Prior experiments in mice and rats have demonstrated that repeated psychostimulants modify glutamatergic activity in the dorsal and ventral striatum (Bamford et al. 2008; McFarland et al. 2003; Pierce and Kalivas 1997; Wang et al. 2013). This plasticity modifies behavioral responses to subsequent drug treatments and appears to encode drug-seeking behavior (McFarland et al. 2003; Storey et al. 2016).
We tested whether the reversal of corticoaccumbal synaptic depression by an amphetamine challenge in withdrawal might encode sensitized locomotor responses. Comparisons between the percent increase in corticostriatal release and the percent change in ambulations for each mouse revealed a significant correlation between behavior and excitatory synaptic function. Therefore, drug reinstatement in withdrawal which moves synaptic activity from a depressed level to a potentiated state may be one mechanism involved in the initiation and maintenance of behavioral sensitization.
4.5 ∣. Mechanisms of synaptic filtering
SPNs exhibit a low tonic activity (~1 Hz) in vivo due to presynaptic inhibition of cortical inputs by tonic eCBs (Wang et al. 2012) and the prominent potassium currents found in mature SPNs (Wilson and Kawaguchi 1996). Evidence indicates that both glutamate and dopamine need to be present simultaneously for the SPN to fire (Bamford et al. 2018). Our data suggests that under quiescent conditions when there is little cortical activity, dopamine can provoke a small excitatory response in SPNs which would bring the cells closer toward threshold. Once the PFC becomes active, dopamine provides a frequency-dependent suppression of corticoaccumbal boutons with a low probability of release. This filtering of cortical information by dopamine may then act to suppress extraneous information and provide a mechanism whereby the animal might focus attention on meaningful and relative information that leads to rewarding behaviors (Bamford and Bamford 2019; Bamford et al. 2018; Bamford et al. 2004b; Wong et al. 2015). A similar filtering mechanism by dopamine has been described in the dorsal striatum (Bamford et al. 2004b) and has been more fully described within the NAcore (Wang et al. 2012). Published work demonstrates that synaptic filtering in the NAcore is mediated through presynaptic dopamine receptors as well as retrograde presynaptic inhibition that is generated via the stimulation-dependent release of adenosine and endocannabinoids (Wang et al. 2012). Adenosine receptors are present on D1- and D2-type (D2R) receptor expressing SPNs (Dunwiddie and Masino 2001; Harvey and Lacey 1997), while cannabinoid CB1 receptors may primarily regulate D2R-expressing SPNs (Grueter et al. 2010; Wang et al. 2012). Adenosine and endocannabinoids are considered necessary for the establishment of reward and attention (Harvey and Lacey 1996; Kalivas and Volkow 2005; Wang et al. 2012). When dopamine and glutamate are released simultaneously, adenosine and endocannabinoids appear to focus excitatory signals toward D1-receptor expressing SPNs that drive behaviors and movements via the “direct” striatal pathway (Wang et al. 2012). This mechanism may persist in drug withdrawal, but excitation of the direct pathway would be significantly amplified by the potentiation of glutamate release that occurs in response to a drug challenge.
4.6 ∣. Potential mechanisms of synaptic plasticity
Evidence of synaptic depression during psychostimulant withdrawal has been found in the dorsal striatum (Bamford et al. 2008) and NAc (Bell et al. 2000; McFarland et al. 2003; Pierce et al. 1996), while potentiation at glutamatergic synapses during drug reinstatement has been found in several brain areas (Gass and Olive 2008), including the NAc (Bell et al. 2000; McFarland et al. 2003; Pierce et al. 1996; Reid and Berger 1996) and the dorsal striatum (Bamford et al. 2008; McKee and Meshul 2005; Storey et al. 2016; Wang et al. 2013). The precise mechanism(s) underlying these changes remains unclear but may develop in part through plasticity generated in SPNs or striatal interneurons.
Infusion of AMPA into the NAcore induces reinstatement behavior (Ping et al. 2008) while PFC lesions, or blockade/ inactivation of AMPA, NMDA or metabotropic glutamate receptors in this region attenuate sensitization and cue-induced reinstatement (Backstrom and Hyytia 2006; Beutler et al. 2011; Ghasemzadeh et al. 2003; Li et al. 1999). In the dorsal striatum, synaptic potentiation and locomotor sensitization can be blocked by D1R antagonists in withdrawal (Bamford et al. 2008; Kuribara 1995; Wang et al. 2013).
Cholinergic interneurons have also been implicated in the mechanism of amphetamine-induced locomotor sensitization and drug seeking behavior. Experiments have revealed that both corticostriatal depression in withdrawal and subsequent potentiation by drug reinstatement rely on tonic excitation and inhibition by acetylcholine at nicotinic and muscarinic receptors located on corticostriatal axons (Bamford et al. 2008). Synaptic depression (Bamford et al. 2008) and locomotor sensitization (Kelly et al. 2008) are dependent on D2Rs, which act to reduce acetylcholine efflux (Bamford et al. 2008; Bickerdike and Abercrombie 1997). Potentiation and locomotor sensitization are contingent on D1Rs (Bamford et al. 2008; Kuribara 1995), which act to boost acetylcholine release (Bamford et al. 2008; Bickerdike and Abercrombie 1997). These shifts in acetylcholine availability can then modulate corticostriatal activity by interacting with nicotinic receptors on corticostriatal axons (Bamford et al. 2008; Wang et al. 2013). In this way, the increase in glutamate might boost locomotor activity by activating SPNs that have a diminished capacity to respond (Gass and Olive 2008) and promote an imbalance between striatal pathways by creating a net shift from a D2R-generated reduction motor activity along the “indirect” pathway to D1R-mediated excitation along the “direct” pathway (Bamford et al. 2018; Beutler et al. 2011; Wang et al. 2013).
Therapeutic approaches that target upstream interneurons might increase synaptic glutamate in withdrawal and facilitate extinction of drug-seeking behavior, while those that prevent potentiation would potentially act to disrupt the reinforcing effects of drugs (Gass and Olive 2008). Since dopamine modifies corticoaccumbal activity by changing the kinetics of different subpopulations of excitatory boutons, our results may also explain how dopamine released by salient experiences might encode learned behaviors and movements and how disruption of corticoaccumbal filtering triggered by too much or too little dopamine would lead to habits and movement disorders like Parkinson’s disease (Bamford and Cepeda 2009; Cepeda et al. 2010).
Supplementary Material
Significance.
The striatum is a subcortical structure within the brain that helps to execute learned movements and goal-directed behaviors. Drug addiction, Parkinson’s disease, and other neuropsychiatric disorders are produced by transient or permanent changes in the neurotransmitter dopamine. These investigations seek new targets and alternative pharmacological treatments for drug dependence by showing how the repeated use of the psychostimulant amphetamine can promote long-lasting alterations in striatal function which might encode habit learning and addictive behaviors.
ACKNOWLEDGEMENTS
The authors wish to thank Professor Michael S. Levine for his mentorship, support, and encouragement throughout the years.
Funding information: This work was supported by NIH NS060803
Abbreviations:
- D1R
D1-type dopamine receptors
- D2R
D2-type dopamine receptors
- eEPSCs
evoked excitatory postsynaptic currents
- mEPSC
miniature excitatory postsynaptic currents
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- PPR
paired-pulse ratio
- rm
repeated measures
- SEM
mean ± standard error
- SPN
spiny projection neuron
- TTX
tetrodotoxin
- VTA
ventral tegmental area
- WD
withdrawal day
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Ahmed SH, Koob GF. 2005. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 180(3):473–490. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci 12(10):366–375. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. 2006. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology 31(4):778–786. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. 2003. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6(7):743–749. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. 2007. The role of the dorsal striatum in reward and decision-making. J Neurosci 27(31):8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford IJ, Bamford NS. 2019. The Striatum’s Role in Executing Rational and Irrational Economic Behaviors. Neuroscientist:1073858418824256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Cepeda C. 2009. The Corticostriatal Pathway in Parkinson’s Disease In: Tseng KY, editor. Cortico-Subcortical Dynamics in Parkinson’s Disease. New York: Humana Press; p 87–104. [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. 2004a. Dopamine modulates release from corticostriatal terminals. J Neurosci 24(43):9541–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Wightman RM, Sulzer D. 2018. Dopamine’s Effects on Corticostriatal Synapses during Reward-Based Behaviors. Neuron 97(3):494–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohen R, Cepeda C, Levine MS, Harleton E, Sulzer D. 2008. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron 58(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. 2004b. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42(4):653–663. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. 2000. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology 23(3):335–344. [DOI] [PubMed] [Google Scholar]
- Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD. 2011. Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization. Proc Natl Acad Sci U S A 108(10):4206–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerdike MJ, Abercrombie ED. 1997. Striatal acetylcholine release correlates with behavioral sensitization in rats withdrawn from chronic amphetamine. J Pharmacol Exp Ther 282(2):818–826. [PubMed] [Google Scholar]
- Cepeda C, Bamford NS, Andre VM, Levine MS. 2010. Alterations in Corticostriatal Synaptic Function in Huntington’s and Parkinson’s Diseases In: Steiner H, Tseng KY, editors. Basal Ganglia Structure and Function. San Diego: Elsevier; p 607–623. [Google Scholar]
- Charpier S, Mahon S, Deniau JM. 1999. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience 91(4):1209–1222. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. 2006. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26(7):2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. 1999. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93(4):1359–1367. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. 1994. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol 71(1):17–32. [DOI] [PubMed] [Google Scholar]
- Dackis CA. 2004. Recent advances in the pharmacotherapy of cocaine dependence. Curr Psychiatry Rep 6(5):323–331. [DOI] [PubMed] [Google Scholar]
- Dani JA, Zhou FM. 2004. Selective dopamine filter of glutamate striatal afferents. Neuron 42(4):522–524. [DOI] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. 2009. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc Natl Acad Sci U S A 106(34):14664–14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. 2011. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol Psychiatry 69(7):704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. 2008. Methamphetamine induces chronic corticostriatal depression: too much of a bad thing. Neuron 58(1):6–7. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. 1994. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372(6507):686–691. [DOI] [PubMed] [Google Scholar]
- Dumartin B, Doudnikoff E, Gonon F, Bloch B. 2007. Differences in ultrastructural localization of dopaminergic D1 receptors between dorsal striatum and nucleus accumbens in the rat. Neurosci Lett 419(3):273–277. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. 2001. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Rudolph M, Destexhe A, Sejnowski TJ. 2003. Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience 122(3):811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BG, Neely MD, Deutch AY. 2010. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb Cortex 20(10):2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. 2008. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75(1):218–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. 2003. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci 18(6):1645–1651. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Yang CR. 1997. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience 76(3):689–706. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. 2007. The Dopamine System and the Pathophysiology of Schizophrenia: A Basic Science Perspective. Int Rev Neurobiol 78C:41–68. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13(12):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. 2011. Neuroanatomy of Reward: A View from the Ventral Striatum In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Boca Raton (FL). [PubMed] [Google Scholar]
- Harvey J, Lacey MG. 1996. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol 492 (Pt 1):143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Lacey MG. 1997. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. Journal of Neuroscience 17(14):5271–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M 1997. Membrane time constant as a tool to assess cell degeneration. Brain Res Brain Res Protoc 1(2):114–116. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. 2009. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci 29(8):2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. 2003. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci 1003:169–175. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. 2005. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162(8):1403–1413. [DOI] [PubMed] [Google Scholar]
- Kasper EM, Larkman AU, Lubke J, Blakemore C. 1994. Pyramidal neurons in layer 5 of the rat visual cortex. II. Development of electrophysiological properties. J Comp Neurol 339(4):475–494. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. 2008. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav 7(5):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Clements JD, Grantyn R. 2002. Presynaptic and postsynaptic mechanisms underlie paired pulse depression at single GABAergic boutons in rat collicular cultures. J Physiol 543(Pt 1):99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. 2009. Glutamate and reinstatement. Curr Opin Pharmacol 9(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H 1995. Dopamine D1 receptor antagonist SCH 23390 retards methamphetamine sensitization in both combined administration and early posttreatment schedules in mice. Pharmacol Biochem Behav 52(4):759–763. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. 1999. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse 34(3):169–180. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. 2003. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology 28(6):1082–1085. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21(21):8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23(8):3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. 2005. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience 133(2):605–613. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. 1995. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol 488 (Pt 1):85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. 1997. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci 17(14):5255–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. 1995. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev 20(1):91–127. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin J. 2005. The Mouse Brain in Stereotaxic Coordinates. London: Academic Press. [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. 2006. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442(7106):1042–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. 1996. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16(4):1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. 1997. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci 17(9):3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. 2008. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res 1215:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Berger SP. 1996. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport 7(7):1325–1329. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 1997. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17(21):8491–8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. 1997. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol 77(4):1697–1715. [DOI] [PubMed] [Google Scholar]
- Storey GP, Gonzalez-Fernandez G, Bamford IJ, Hur M, McKinley JW, Heimbigner L, Minasyan A, Walwyn WM, Bamford NS. 2016. Nicotine Modifies Corticostriatal Plasticity and Amphetamine Rewarding Behaviors in Mice(1,2,3). eNeuro 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D 2011. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69(4):628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. 1989. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 2(5):285–298. [PubMed] [Google Scholar]
- Van der Kloot W 1991. The regulation of quantal size. Prog Neurobiol 36(2):93–130. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. 2013. Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: a key role of dopamine? Neuroscience 251:2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Darvas M, Storey GP, Bamford IJ, Gibbs JT, Palmiter RD, Bamford NS. 2013. Acetylcholine encodes long-lasting presynaptic plasticity at glutamatergic synapses in the dorsal striatum after repeated amphetamine exposure. J Neurosci 33(25):10405–10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Dever D, Lowe J, Storey GP, Bhansali A, Eck EK, Nitulescu I, Weimer J, Bamford NS. 2012. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J Physiol 590(Pt 16):3743–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. 1996. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16(7):2397–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Borgkvist A, Choi SJ, Mosharov EV, Bamford NS, Sulzer D. 2015. Dopamine-dependent corticostriatal synaptic filtering regulates sensorimotor behavior. Neuroscience 290:594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Sulzer D, Bamford NS. 2011. Imaging presynaptic exocytosis in corticostriatal slices. Methods Mol Biol 793:363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. 1999. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci 877:113–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.