Abstract
Purpose:
Emerging evidence indicates that gut microbiome plays a crucial role in the cancer pathogenesis. Although Fusobacterium nucleatum (F. nucleatum) is associated with poor prognosis in multiple cancers, its clinical significance in predicting response to chemotherapy in patients with esophageal squamous cell carcinoma (ESCC) remains unclear.
Experimental design:
The F. nucleatum levels were quantified by qPCR assays in tumor tissues from 551 ESCC patients from two independent cohorts, including 101 patients who received neoadjuvant chemotherapy prior to curative resection. Associations between F. nucleatum burden and recurrence free survival (RFS), as well with chemotherapeutic response were evaluated using response evaluation criteria in solid tumors (RECIST), primary tumor metabolic response defined by maximum standardized uptake value (SUVmax) changes in positron emission tomography - computed tomography (PET/CT), and pathological tumor regression grade (TRG).
Results:
High burden of F. nucleatum in ESCC patients associated with poor RFS in both training (log-rank p=0.02; Hazard Ratio [HR]=1.61; p=0.03) and validation cohorts (log-rank p=0.003; HR=1.96; p=0.004). Importantly, ESCC patients with high levels of F. nucleatum displayed poor chemotherapeutic response for all three evaluation methods: RECIST (p=0.04), SUVmax change in PET/CT (p=0.0004), and TRG (p=0.003).
Conclusions:
We conclude that high levels of intratumoral F. nucleatum have a prognostic significance for predicting poor RFS in ESCC patients. More importantly, our data indicates that higher F. nucleatum burden correlates with poor response to neoadjuvant chemotherapy, suggesting the possibility that an antibiotic intervention against this bacterium may significantly improve therapeutic response in ESCC patients.
Keywords: Fusobacterium nucleatum, esophageal cancer, prognosis, neoadjuvant chemotherapy, response
Introduction
Esophageal cancer is the sixth most common cause of cancer-related deaths worldwide (1). In spite of advances in multimodal therapies, including surgical removal of tumors, chemotherapy, radiotherapy and chemoradiotherapy, esophageal cancer remains a malignancy with high degree of fatality and overall 5-year survival rates of 15 to 20% (2, 3). Globally, esophageal squamous cell cancer (ESCC) is the predominant histological subtype of esophageal cancer (4). In particular, ESCC cases make up to 80% of all esophageal cancers in developing countries (5). The standard treatment strategy for locally advanced esophageal cancer in western and Asian countries comprise of neoadjuvant chemoradiotherapy or chemotherapy (NAC), followed by surgery (6, 7). Previous studies have shown that patients who respond well to NAC often exhibit improved overall survival (8, 9). A combination of cisplatin and 5-fluorouracil (5-FU) is currently used as standard chemotherapeutic regimen for esophageal cancer patients; however, the reported response rates remain relatively poor (10, 11). A recent study reported that addition of docetaxel to this regimen significantly improved the therapeutic response in patients with node-positive esophageal cancer (12). Nevertheless, most tumors acquire resistance to chemotherapeutic agents with subsequent treatment failure. Furthermore, there is currently no effective molecularly-targeted therapy available for esophageal cancer, and the efficacy of immunotherapy in these patients remains unclear.
In order to improve treatment response in esophageal cancer patients, it is of paramount importance to elucidate the underlying mechanism(s) that confer chemotherapeutic resistance in these patients. It has been postulated that cancer chemoresistance is attributed to complex interplay between gene regulation and external environmental factors. In this context, in recent years, gut microbiota has garnered a lot of attention in various malignancies, and it has been linked to both initiation and progression of gastrointestinal cancers through modulation of intestinal inflammation (13–15) and tumor-related signaling pathways (16). Recent studies have demonstrated that composition of the gut microbiome can significantly influence response to immunotherapy (17) and chemotherapy (18). Two recent independent studies identified an overabundance of Fusobacterium nucleatum (F. nucleatum) in colorectal cancer (CRC) tissues using metagenomic analysis (19, 20) and a high prevalence of F. nucleatum in these tissues associated with worse overall survival (21). In line with these observations, we identified that even in ESCC patients, the presence of intratumoral F. nucleatum in neoplastic tissues was significantly associated with poor patient survival (22). Interestingly, building upon this growing evidence, a recent study reported that CRC patients who experienced increased incidence of tumor recurrence also possessed significantly higher burden of intratumoral F. nucleatum in their primary cancer tissues compared to those who did not exhibit tumor recurrence (23). In functional studies, F. nucleatum has been shown to enhance CRC chemoresistance through modulation of autophagy (23). In spite of the collective evidence highlighting the clinical importance of F. nucleatum in gastrointestinal cancers, whether changes in its expression levels contributes to patient prognosis and chemotherapeutic response in ESCC patients has not yet been explored.
Accordingly, in the present study we report that increased levels of intratumoral F. nucleatum associate with advanced tumor stage and poor survival. We also observed that higher burden of this microorganism in ESCC tissues predicted recurrence-free survival (RFS), as well as associated with poor response to NAC in patients with ESCC.
Materials and methods
Patients and sample collection procedures
This study analyzed a total of 551 cases with ESCC, which consisted of two independent clinical cohorts. A first patient cohort (training) included 207 ESCC patients, who were surgically treated at the Nagoya University Hospital, Nagoya, Japan, between October 2001 and October 2015. The second patient cohort (validation) comprised of 344 ESCC patients who underwent surgical resections, including 316 with radical surgeries, at the Kumamoto University Hospital, Kumamoto, Japan, between 2005 and 2016. Furthermore, this cohort included 187 patients that experienced surgery alone, 41 who received neoadjuvant chemoradiation therapy and 116 patients with NAC. Among these 116 patients in the NAC group, 101 patients were treated with two cycles of docetaxel, cisplatin and 5-FU (DCF) regimen. The study workflow is summarized in Supplementary Figure S1. Tumor depth (clinical T1–3) and regional lymph node involvement without distant metastases (N1) were used as the selection criteria for selecting patients for NAC treatment. Recurrence-free survival (RFS) was defined as the time period between the date of surgery to the time of tumor recurrence or death. Our study was conducted in accordance with the Declaration of Helsinki. A written informed consent was obtained from each patient, and the institutional review boards of all participating institutions approved this study. The patient characteristics are summarized in Table 1. The median follow-up duration for all cases after surgery was 20.4 months in the training cohort and 31.5 months in the validation cohort. The pathological diagnosis of all ESCC tumor tissue specimens was confirmed histologically, and the tumor node-metastasis (TNM) staging was determined according to the American Joint Committee on Cancer staging handbook (7th edition) (24), prior to and after surgery.
Table 1:
F. nucleatum expression levels and relationship with various clinicopathological features in ESCC patients
| Training cohort (n=207) | Validation cohort (n = 344) | |||||
|---|---|---|---|---|---|---|
| F. nucleatum expression | F. nucleatum expression | |||||
| Low | High | P | Low | High | P | |
| Age (range) | 66 (44–84) | 66 (44–83) | 0.26 | 66 (41–86) | 65 (49–89) | 0.82 |
| Sex | 0.62 | > 0.99 | ||||
| Male | 76 | 83 | 228 | 72 | ||
| Female | 21 | 27 | 34 | 10 | ||
| Location | 0.29 | 0.42 | ||||
| Upper | 14 | 10 | 171 | 50 | ||
| Lower | 83 | 100 | 91 | 32 | ||
| Tumor size, cm | 4.5(1.5 – 17.0) | 4.5 (2.2 – 14.0) | 0.78 | 3.5 (1.1 – 15.0) | 4.2 (1.2 – 14.5) | 0.004 |
| SCC, ng/mL | 1.2(0.2 – 22.8) | 1.2 (0.2 – 7.3) | 0.44 | NA | NA | NA |
| T category | 0.03 | <0.0001 | ||||
| T1 | 26 | 17 | 126 | 17 | ||
| T2 – 4 | 69 | 93 | 136 | 65 | ||
| Undefined | 2 | 0 | NA | NA | ||
| Lymph node metastasis | 0.88 | 0.12 | ||||
| Absent | 34 | 38 | 141 | 33 | ||
| Present | 63 | 72 | 121 | 49 | ||
| Tumor Stage | > 0.99 | 0.03 | ||||
| I – II | 42 | 48 | 169 | 40 | ||
| III – IV | 53 | 62 | 93 | 42 | ||
| Undefined | 2 | 0 | NA | NA | ||
| Differentiation | 0.16 | NA | ||||
| well-mod | 79 | 93 | NA | NA | ||
| poor- | 17 | 13 | NA | NA | ||
| Undefined | 1 | 4 | NA | NA | ||
| Preoperative treatment | 0.03 | 0.01 | ||||
| Present | 41 | 59 | 108 | 49 | ||
| Absent | 58 | 49 | 154 | 33 | ||
Patient treatments
The NAC regimen consisted of 2 hour intravenous administration of 60 mg/m2 docetaxel beginning on day 1, a 24 hour continuous intravenous infusion of 350 mg/m2 5-FU from days 1 through 5, and 1 hour intravenous administration of 6 mg/m2 cisplatin from days 1 through 5. Two scheduled courses of NAC regimen were administered 3 weeks apart prior to esophagectomy. Surgery was carried out within 4 to 6 weeks following the final treatment day of preoperative chemotherapy, when curative resection was considered feasible.
DNA extraction and quantitative polymerase chain reaction (qPCR) assays
Genomic DNA from fresh frozen tissues in the training cohort were extracted using AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Hilden, Germany). Likewise, genomic DNA from the formalin-fixed paraffin-embedded (FFPE) tissues in the validation cohort were extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen). The amount of F. nucleatum DNA was quantified by use of a qPCR assay. The nus G gene of F. nucleatum and the reference human gene SLCO2A1 were amplified using custom TaqMan primer/probe sets (Applied Biosystems, Carlsbad, CA, USA) in 384-well optical PCR plates, as described previously (22).
Evaluation of response to chemotherapy using RECIST
The response to chemotherapy was assessed in the validation cohort using response evaluation criteria in solid tumors (RECIST) Version 1.1 (25). Briefly, computed tomography (CT) images were analyzed using with the following definitions: complete response (CR), as disappearance of all clinical and radiological evidence of the tumor; partial response (PR), as decrease of 30% or more in the sum of longest diameters of all target measurable lesions; progressive disease (PD), as increase of more than 20% of the sum of longest diameters of all target measurable lesions or the appearance of new lesions; and stable disease (SD), as all other indications. Patients with CR or PR were defined as responders, while PD and SD were classified as non-responders.
PET/CT imaging
A total of 86 out of 101 patients who received NAC also underwent positron emission tomography - computed tomography (PET/CT) using a hybrid PET/CT imager, consisting of a dedicated GSO full-ring PET scanner and a 16-slice helical CT scanner (Gemini GXL16, Philips Medical Solutions, Amsterdam, Netherlands). All patients fasted for a minimum of 5 h prior to the examination. Emission scans were acquired in a 3D mode, with a 144×144 matrix, 60 min after intravenous injection of 185–300 MBq 18F-fluoro-deoxy-glucose (FDG), immediately after urination. PET/CT transmission data were acquired for the area defined from the base of the skull to the proximal thighs. Standardized uptake value (SUVmax) response was classified as follows (26): complete metabolic response (CMR), as complete resolution of FDG uptake within the measurable target lesion, with the appearance of no new lesion; partial metabolic response (PMR), with at least 30% reduction in SUVmax of FDG uptake; progressive metabolic disease (PMD), with more than 30% increase in the SUVmax of the FDG uptake or appearance of FDG avid new lesion/s that is/are morphologically typical of cancer; stable metabolic disease (SMD), as disease which did not qualify for CMR, PMR, or PMD. Patients with CMR or PMR were defined as responders.
Pathological tumor regression grading criteria
The histopathological response to NAC was classified into four categories according to the following criteria (27): grade 1, as no evidence of viable tumor cells; grade 2, with less than 10% viable tumor cells; grade 3, with 11–50% viable tumor cells; and grade 4, with more than 50% viable tumor cells. Subsequently, grade 1–3 tumors were combined as the group of patients with response (TRG 1–3), while grade 4 tumors were classified as non-responders (TRG 4).
Statistical analysis
All statistical analyses were carried out using JMP, version 10 (SAS Institute, Cary, NC, USA). Continuous variables were expressed as medians and were compared using a t-test or Mann–Whitney U test. Categorical variables were compared using chi-squared or Fisher’s exact test. All p-values were calculated using a two-sided test, and a p-value of <0.05 was considered statistically significant. For time-to-event analyses, survival estimates were calculated using the Kaplan-Meier analysis, and the survival differences between groups were compared using the log-rank test. Associations between RFS and clinicopathological features was evaluated by univariate Cox proportional hazards regression analysis. Parameters determined to be significant by univariate analysis were included in multivariate Cox proportional hazards regression analysis. Similarly, we analyzed associations between chemotherapeutic response and clinicopathological features using by univariate and multivariate logistic regression analysis.
Results
The levels of F. nucleatum are significantly higher in ESCC patients
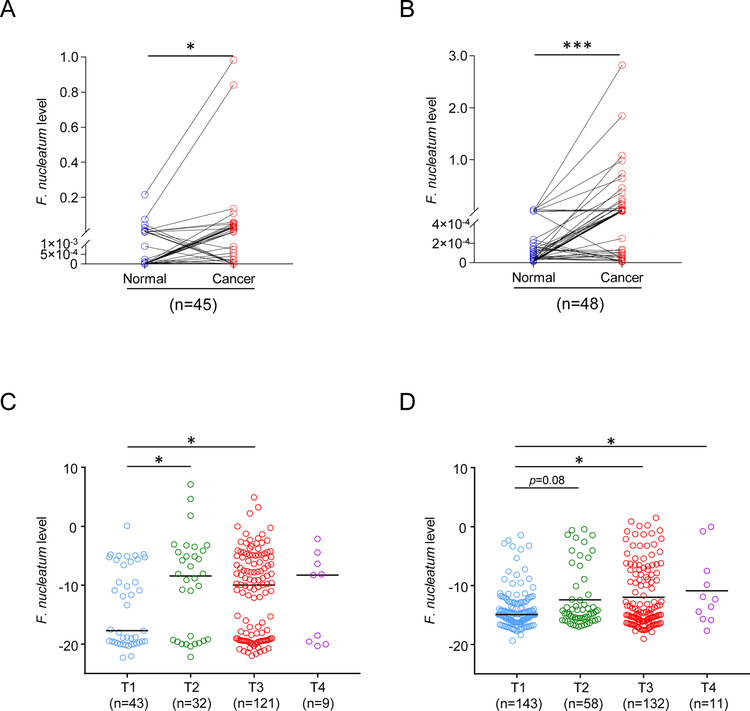
We first assessed the burden of F. nucleatum in ESCC tissues by a qPCR assay in two independent patient cohorts, where matched cancer and normal tissues were available. We observed that F. nucleatum DNA levels were significantly higher in cancer tissues compared to the paired adjacent normal tissues in both cohorts (training cohort; n=45, P=0.006; validation cohort; n = 48, P <0.0001; Figure 1A and 1B, respectively).
Figure 1: F. nucleatum expression in patients with ESCC.
(A) The expression of F. nucleatum in 45 pairs of ESCC and adjacent normal tissue in the training cohort, and (B) in 48 pairs of the validation cohort. (C) The relative amount of F. nucleatum in 207 ESCC tissue according to T category in training cohort, and (D) in 344 ESCC tissue in validation cohort. *p<0.05; **p<0.01, ***p<0.001
We next analyzed the abundance of F. nucleatum in the training (n=207) and validation (n=344) cohorts, based upon all tumor stages. Interestingly, we observed a marked enrichment of F. nucleatum in ESCC patients with advanced (T2-T4) vs. those with an earlier stage disease (T1), in both cohorts (P <0.05; Figure 1C and 1D).
Higher levels of F. nucleatum associated with advanced stage disease in ESCC
Next, we determined the associations between F. nucleatum burden and various clinicopathological features in two independent ESCC patient cohorts (training cohort; n=207 and validation cohort; n=344). The cut-off thresholds to categorize tumors into the high and low groups were determined using ROC analysis and Youden’s index, based on the level of F. nucleatum that provided the highest sensitivity and specificity to predict ESCC recurrence in the training cohort. The same cut-off values were then applied to the patient in the validation cohort to evaluate survival. We observed that there was no effect of age (p=0.26), gender (p=0.62), tumor location (p=0.29), or N stage (p=0.88) on the expression of expression of F. nucleatum in the cancer tissues within the training cohort. Similar results were noted in the validation cohort for age (p=0.82), gender (p>0.99), location (p=0.42), and N stage (p=0.12).
However, in the training cohort, high intratumoral F. nucleatum levels were associated with higher T category (p=0.03) and in patients who received preoperative treatment (p=0.03). Similarly, high levels of intratumoral F. nucleatum were significantly associated with larger tumor size (p=0.004), higher T category (p<0.001), higher TNM stage (p=0.03), and in patients who underwent preoperative treatment (p=0.01) in the validation cohort (Table 1). Collectively, our results indicate that high levels of F. nucleatum associate with an invasion depth in ESCC patients.
Increased burden of F. nucleatum associate with higher tumor recurrence, poor RFS, and serve as a prognostic indicator for early stage ESCC patients
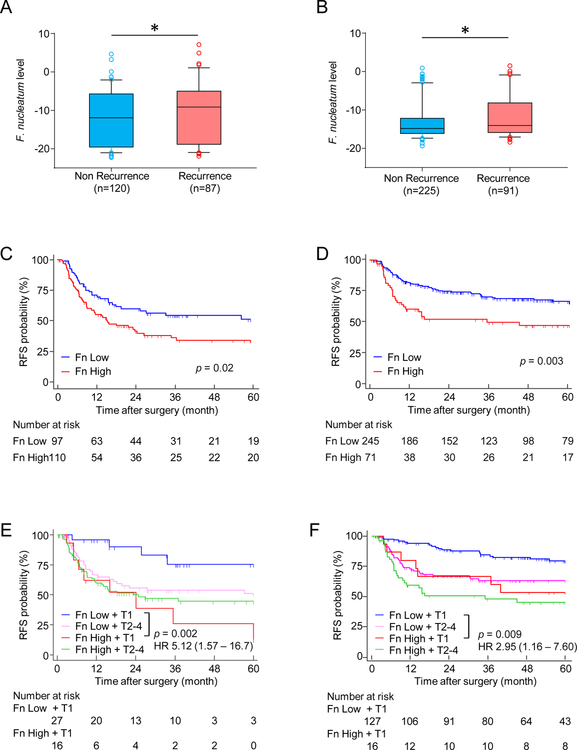
Considering that presence of F. nucleatum in cancer cells is associated with advanced disease, we were curious to interrogate its relationship with tumor recurrence in ESCC patients. Therefore, we determined the relationship between the F. nucleatum levels and cancer recurrence in the training cohort of 207 patients (87 patients with recurrence and 120 without recurrence), wherein we observed a significant association for higher F. nucleatum levels in patients with recurrence (P=0.04; Figure 2A). Likewise, these findings were subsequently confirmed in the validation cohort of 316 ESCC patients, which included 91 patients without recurrence and 225 with recurrence. Here again, we noted that the overall levels of F. nucleatum were significantly higher in neoplastic tissues in ESCC patients with recurrence vs. those without recurrence (P=0.01; Figure 2B).
Figure 2: High intratumoral F. nucleatum is associated with worse prognosis in ESCC.
Comparison of F. nucleatum expression levels in patients with or without recurrence in (A) the training and (B) the validation cohort. Kaplan-Meier analysis of RFS for ESCC patients with high (red) or low (blue) F. nucleatum levels in (C) the training and (D) the validation cohort. Kaplan-Meier analysis of RFS for ESCC patients with low F. nucleatum levels in T1 (blue) or T2–4 tumor (pink), or high F. nucleatum levels in T1 (red) or T2–4 tumor (green) in (E) the training and (F) the validation cohort. Fn indicates Fusobacterium nucleatum. *p<0.05
In order to determine whether intratumoral F. nucleatum burden in ESCC patients is associated with RFS, we performed Kaplan–Meier analysis in both cohorts. Interestingly, patients in the training cohort with high vs. low intratumoral F. nucleatum levels exhibited a significantly poor RFS (log-rank p = 0.02, Figure 2C); a finding which was also true when interrogated in the independent validation cohort (log-rank p = 0.003, Figure 2D).
Since we observed a higher burden of F. nucleatum in advanced ESCC patients (T2–4 vs. T1), we investigated whether the presence of this bacterium had any effect on patient survival, even in early ESCC. Advanced ESCC patients stratified by the T category alone (T2–4 vs. T1) exhibited poor prognosis in both patient cohorts (Supplementary Figure S2A and S2B). Importantly, however, when the T category was combined together with the F. nucleatum levels, we observed that even early stage T1 ESCC patients with high levels of this bacterium exhibited a worse RFS, which was similar to the one noted for patients with advanced disease, in both cohorts (training cohort: log-rank p = 0.002 and validation cohort: log-rank p = 0.009, Figure 2E and 2F, respectively). These findings highlight that presence of high levels of F. nucleatum indicate an important prognostic biomarker potential for this bacterium in ESCC patients.
High levels of F. nucleatum serve as an independent risk factor for RFS in ESCC patients
Next, we were curious to investigate the clinical significance of F. nucleatum levels in term of patient survival in the context of other clinicopathological features, using univariate and multivariate analysis, in both patient cohorts. In the training cohort, univariate Cox regression analysis revealed that patients with proximal location of tumors (HR=2.04; 95% CI, 1.10–3.50; p= 0.02), and those with higher TNM stages (III/IV vs. I/II; HR=3.32; 95% CI, 2.05–5.63; p<0.0001), and those with high levels of F. nucleatum were associated with poor RFS (HR=1.61; 95% CI, 1.06–2.52; p=0.03; Table 2). These findings were further evaluated in a multivariate Cox model adjusted for various clinicopathological features, which were in agreement with our univariate analysis and demonstrated that proximal location of tumors (HR=3.09; 95% CI, 1.64–5.45; p=0.001), and those with higher TNM stages (III/IV vs. I/II; HR=3.78; 95% CI, 2.30–6.46; p<0.0001), and those with high levels of F. nucleatum were associated with poor RFS (HR=1.72; 95% CI, 1.12–2.70; p=0.01), suggesting that this bacterium was indeed an independent risk factor for predicting poor RFS in the patients within the training cohort.
Table 2:
High levels of F. nucleatum serve as an independent risk factor for predicting RFS in SESCC patients
| Training cohort (n=207) | Validation cohort (n=316) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (< 65) | 0.71 (0.91 – 2.21) | 0.12 | 1.00 (0.66 – 1.53) | 0.99 | ||||
| Male | 1.42 (0.85 – 2.49) | 0.18 | 1.10 (0.60 – 2.25) | 0.78 | ||||
| Upper tumor | 2.04 (1.10 – 3.50) | 0.02 | 3.09 (1.64 – 5.45) | 0.001 | 0.95 (0.62 – 1.48) | 0.81 | ||
| Preoperative therapy | 1.09 (0.71 – 1.66) | 0.7 | 1.96 (1.29 – 3.00) | 0.002 | 0.87 (0.49 – 1.53) | 0.61 | ||
| TNM stage (III–IV) | 3.32 (2.05 – 5.63) | <0.0001 | 3.78 (2.30 – 6.46) | <0.0001 | 3.08 (2.03 – 4.72) | <0.0001 | 3.21 (1.81 – 5.70) | <0.0001 |
| F. nucleatum High | 1.61 (1.06 – 2.52) | 0.03 | 1.72 (1.12 – 2.70) | 0.01 | 1.96 (1.23 – 3.04) | 0.004 | 1.70 (1.06 – 2.65) | 0.03 |
We subsequently confirmed our findings in an independent validation cohort, wherein, once again we observed that in univariate analysis, preoperative therapy (HR=1.96; 95% CI, 1.29–3.00; p=0.002), TNM stages (HR=3.08; 95% CI, 2.03–4.72; p<0.0001), and higher burden of F. nucleatum (HR=1.96; 95% CI, 1.23–3.04; p=0.004) was significantly associated with worse RFS. Similarly, in multivariate analysis, TNM stages (HR=3.21; 95% CI, 1.81–5.70; p<0.0001) and high levels of F. nucleatum (HR=1.70; 95% CI, 1.06–2.65; p=0.03) were significantly associated with poor RFS. Collectively, these data demonstrate that high levels of intratumoral F. nucleatum are an independent risk factor for poor RFS in ESCC patients.
Intratumoral F. nucleatum burden correlates with worse chemotherapeutic response in ESCC patients
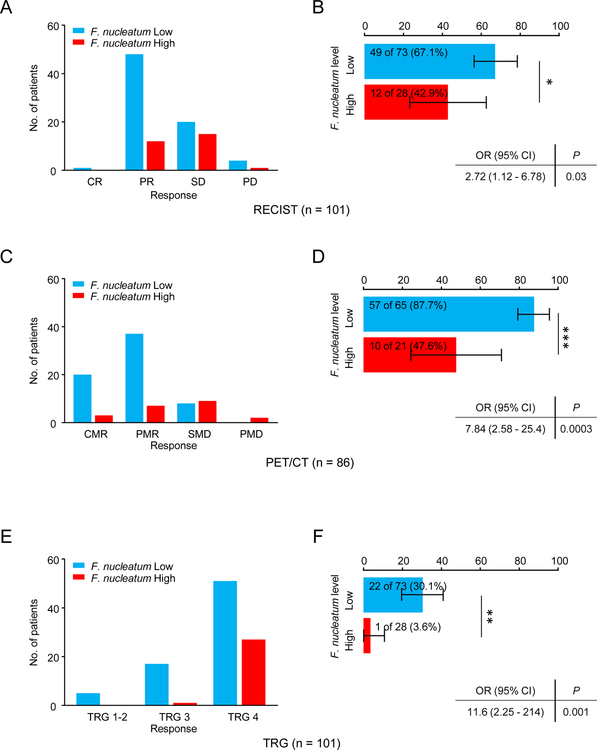
We examined whether higher burden of intratumoral F. nucleatum have any correlation with response to NAC in ESCC patients. We first investigated this association in the context of imaging data available to us from the CT scans. Of the 101 patients who underwent NAC treatment in the validation cohort, the F. nucleatum-high group had a significantly lower number of responders (i.e. patients with CR or PR; 42.9% (12/28) vs. 67.1% (49/73) in the F. nucleatum-low group; p = 0.04; Figure 3A and 3B).
Figure 3: Intratumoral F. nucleatum levels are associated with chemotherapeutic response.
Chemotherapeutic response and the rate of responders in the validation cohort by comparing F. nucleatum high (red) and low (blue) patients using RECIST (A and B), PET/CT (C and D) and TRG (E and F). RECIST, response evaluation criteria in solid tumors. PET/CT, Positron Emission Tomography - Computed Tomography. TRG, Tumor Regression Grade. *p<0.05; **p<0.01, ***p<0.001
Next, we interrogated this correlation as determined by the metabolic response rates determined by SUVmax values obtained from PET/CT imaging. Reassuringly, these analyses also revealed that patients with higher burden of F. nucleatum had a significantly fewer responders (i.e. patients with CMR or PMR; 47.6% (10/21) vs. 87.7% (57/65) in the low F. nucleatum group; p = 0.0004; Figure 3C and 3D).
Finally, we performed the pathological assessment of all patients based upon tumor regression grade (TRG) analysis. In these analyses, we noted that F. nucleatum levels were significantly higher in ESCC patients with a low vs. high pathological response (TRG 4 vs. TRG 1, 2 and 3; p = 0.003; Figure 3E and 3F). Taken together, these results illustrate that patients with high intratumoral levels of F. nucleatum appear to have greater resistance to NAC treatment.
High levels of F. nucleatum serve as an independent risk factor for predicting response to neoadjuvant chemotherapy in ESCC patients
Next, we analyzed the results of CT (RECIST), PET/CT and TRG in univariate and multivariate settings to determine the clinical significance of F. nucleatum as a potential biomarker of chemotherapeutic response in ESCC patients belonging to the validation cohort. The univariate logistic regression analysis revealed that higher levels of F. nucleatum associated with an overall poor chemotherapeutic response to NAC in all three approaches (RECIST: Odds Ratio [OR], 2.72; 95% CI 1.12–6.78, p = 0.03; PET/CT: OR, 7.84; 95% CI 2.58–25.4, p = 0.0003; and TRG: OR, 11.6; 95% CI 2.25–214, p = 0.001; Table 3).
Table 3:
Intratumoral F. nucleatum burden correlates with worse chemotherapeutic response in ESCC patients
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| RECIST | ||||
| Age (vs >= 65) | 1.47 (0.65 – 3.41) | 0.35 | ||
| Male (vs Female) | 2.42 (0.68 – 11.3) | 0.18 | ||
| Upper tumor location (vs lower) | 1.14 (0.48 – 2.74) | 0.77 | ||
| T category, 3–4 (vs 1–2) | 0.83 (0.34 – 2.03) | 0.68 | ||
| Lymph node metastasis | 5.88 (1.02 – 111) | 0.04 | 6.95 (1.19 – 135) | 0.03 |
| F. nucleatum High (vs Low ) | 2.72 (1.12 – 6.78) | 0.03 | 2.97 (1.19 – 7.73) | 0.02 |
| PET/CT | ||||
| Age (vs >= 65) | 1.65 (0.57 – 5.18) | 0.35 | ||
| Male (vs Female) | 2.10 (0.34 – 40.6) | 0.47 | ||
| Upper tumor location (vs lower) | 0.92 (0.32 – 2.93) | 0.89 | ||
| T category, 3–4 (vs 1–2) | 10.0 (1.89 – 186) | 0.004 | 9.74 (1.66 – 187) | 0.008 |
| Lymph node metastasis | 0.43 (0.09 – 2.27) | 0.3 | ||
| F. nucleatum High (vs Low) | 7.84 (2.58 – 25.4) | 0.0003 | 7.66 (2.37 – 26.8) | 0.0006 |
| TRG | ||||
| Age (vs >= 65) | 0.56 (0.20 – 1.49) | 0.25 | ||
| Male (vs Female) | 0.25 (0.01 – 1.38) | 0.13 | ||
| Upper tumor location (vs lower) | 1.53 (0.57 – 4.02) | 0.39 | ||
| T category, 3–4 (vs 1–2) | 2.56 (0.95 – 6.85) | 0.06 | 2.05 (0.74 – 5.70) | 0.17 |
| Lymph node metastasis | 1.88 (0.36 – 7.48) | 0.45 | ||
| F. nucleatum High (vs Low) | 11.6 (2.25 – 214) | 0.001 | 10.3 (1.96 – 190) | 0.003 |
Likewise, multivariate analysis also revealed that high levels of intratumoral F. nucleatum burden was an independent risk factor for poor response to NAC in all three criteria (RECIST: OR, 2.97; 95% CI 1.19–7.73, p = 0.02; PET/CT: OR, 7.66, 95% CI 2.37–26.8, p = 0.0006; and TRG: OR, 10.3; 95% CI 1.96–190, p = 0.003). Collectively, these results illustrate that F. nucleatum is an important independent risk factor and a potential biomarker for predicting response to NAC in ESCC patients.
Discussion
With the growing recognition for the role of microbiome in human disease, over the last decade, one such organism, F. nucleatum, has been identified as an important bacterium linked to the pathogenesis of multiple human cancers. In this present study, we for the first time, interrogated the clinical significance of F. nucleatum as a potential prognostic and predictive biomarker of response to neoadjuvant chemotherapy in large, multiple, independent cohort of ESCC patients, and for the first time, investigated F. nucleatum as a potential predictive biomarker of response to neoadjuvant chemotherapy. In this study, we make several novel observations. First, we demonstrate that F. nucleatum burden is significantly higher in ESCC patients with advanced disease stage. Second, we describe that higher levels of this bacterium are present in patients with recurrence, and are an independent risk factor for predicting poor RFS in ESCC. Third, we illustrate that using RECIST, PET/CT and TRG analysis, higher burden of F. nucleatum predicts poor response to neoadjuvant chemotherapy (NAC) in ESCC patients; collectively highlighting the potential possibility of its prognostic and predictive biomarker utility, as well as suggest the possibility of using an antibiotic intervention to target this bacterium for improving the therapeutic response rates to chemotherapy in ESCC patients. In addition as we developed a PCR-based cut-off to measure the F. nucleatum levels in the training cohort patients, and subsequently applied these in an independent validation cohort, we are enthused that our findings can be further validated in a prospective settings for response prediction to neoadjuvant chemotherapy.
It has been recognized that F. nucleatum is frequently present in the human oral cavity, and acts as a pathogen in periodontal disease (28). Recently, several studies have reported that high F. nucleatum burden correlates with poor prognosis in colorectal cancer (21, 29, 30). Moreover, we previously reported a similar positive correlation between high F. nucleatum levels and poor overall and cancer specific survival in ESCC patients (22). The potential role of F. nucleatum in gastrointestinal cancers is poorly understood. Experimental evidence in colorectal cancer has provided mechanistic insights that F. nucleatum expresses adhesin protein, FadA, on the bacterial cell surface. FadA can bind to E-cadherin, activates b-catenin signaling, and promotes colorectal cancer cell proliferation (31). In this study, F. nucleatum burden was significantly higher in ESCC patients with advanced stage. However, T1 ESCC patients with high levels of F. nucleatum exhibited a worse RFS, analogous to patients with advanced disease, suggesting that this bacterium, even in early stage ESCC patients promotes aggressive tumor behavior and could impact patient prognosis.
To further investigate the role of F. nucleatum, it was recently demonstrated that mice bearing colorectal cancer and treated with an antibiotic were found to have lower levels of this baterium and exhibited reduced cell proliferation and tumor growth, suggesting that antibiotics may be helpful in the treatment of F. nucleatum-associated cancers (32). Accumulating evidence suggests that the gut microbiota modulates local immune response, and in turn might alter the efficacy of chemotherapy (18, 33) and immunotherapy (34, 35). In one such study, chemotherapeutic response was modulated by adaptive immunity in ovarian cancer (36). Although these preclinical evidences indicates that microbiota appears to modulate chemotherapeutic response in multiple cancer types (37, 38), to the best of our knowledge, none of the studies have thus far evaluated the clinical significance of F. nucleatum in the context of responsiveness to chemotherapeutic treatment in cancer patients. Herein, we fill this important gap in knowledge, and evaluated therapeutic response using three commonly used and well-established approaches for drug resistance in ESCC patients. In spite of the use of RECIST as one of the most widely used tumor response metric (25), it has several limitations due to its dependence on morphologic changes (39). RECIST criteria can often select lymph nodes as target lesions in ESCC patients. In contrast, 18F-FDG PET is considered as a superior method which overcomes the limitations of RECIST. Since metabolic changes are thought to be more closely related to malignant potential of tumors (40), PET/CT is emerging as a more accurate non-invasive imaging modality for initial staging and response assessment in ESCC patients (41). Based on these findings, PET response criteria in solid tumors (PERCIST), which is RECIST using 18F-FDG PET, has recently been proposed as an optimal method for standardized evaluation of the metabolic tumor response rates (39).
In the present study, we observed significant differences between response classifications and F. nucleatum levels in ESCC tissues. Interestingly, PET response and tumor regression grade (TRG) were more strongly associated with F. nucleatum levels compared to RECIST, in ESCC patients receiving NAC treatment. While RECIST in ESCC patients primarily evaluates shrinkage of lymph nodes, PET/CT and TRG reflect the response of the primary tumor itself. In this study, since F. nucleatum levels in tumor tissues correlate with higher T category, our data imply that this bacterium might be involved in modulating chemotherapeutic response more directly. While specific mechanism(s) underlying chemotherapeutic response of F. nucleatum in cancer remain unclear, several studies have investigated bacteria-induced drug resistance using in vitro and in vivo models. Yu et al. reported that F. nucleatum activates autophagy-related pathways in colorectal cancer through modulation of TLR4 and MYD88 innate signaling, along with certain miRNAs which subsequently promote chemoresistance (23). Likewise, Geller and colleagues reported that intratumoral gamma-proteobacteria modulated the chemotherapeutic response by converting gemcitabine into an inactive metabolite through regulation of cytidine deaminase in pancreatic cancer (42). Nonetheless, further studies are required to interrogate and validate the findings of the current study, and elucidate the mechanisms by which F. nucleatum modulates the chemotherapeutic response in ESCC patients.
Although our results indicate that F. nucleatum levels could serve as a potential biomarker for predicting survival and response to NAC in ESCC patients, there are certain limitations of our study. The detection rates of F. nucleatum were different between the training and validation cohorts. We analyzed frozen tissues in the training cohort of patients, and FFPE tissues in the validation cohort. The qPCR method is currently the most commonly used method for the quantification of F. nucleatum levels; however, most the detection rates of F. nucleatum in frozen tissues are generally higher vs. FFPE tissues (43, 44). There is a possibility that tissue fixation during the processing of FFPE tissues might be an important factor for the reduced detection rates of F. nucleatum (45) in clinical specimens.
In conclusion, we demonstrate that high intratumoral F. nucleatum levels associated with tumor recurrence and poor RFS in two large, independent cohorts of ESCC patients. More importantly, our results indicate that high burden of F. nucleatum in ESCC is predictive of response to neoadjuvant chemotherapy. Collectively, our data highlight that F. nucleatum is not only an important predictive biomarker of chemotherapeutic response, but might be a potential target of antibiotic intervention for improving the therapeutic response rates in ESCC patients.
Supplementary Material
Supplementary Figure S1: Overview of the study design
ESCC, Esophageal squamous cell carcinoma. CRT, Chemoradiation therapy. DCF. Docetaxel, cisplatin and 5-FU. NAC, Neoadjuvant chemotherapy. RECIST, response evaluation criteria in solid tumors. SUVmax, maximum standardized uptake value.
Supplementary Figure S2: Patient survival of ESCC according to T category.
Kaplan-Meier analysis of RFS for ESCC patients with T2 – 4 (red) or T1 (blue) in (A) the training cohort and (B) the validation cohort.
Translational relevance.
Esophageal squamous cell carcinoma (ESCC) is a disease with high mortality rates. The standard treatment strategy for locally advanced ESCC comprises of neoadjuvant chemoradiotherapy or chemotherapy (NAC), followed by surgery; however, there is lack of availability of adequate biomarkers to predict chemotherapeutic response. Fusobacterium nucleatum (F. nucleatum) has been identified as an important bacterium linked to the pathogenesis of multiple human cancers. In this study, we investigated its clinical significance in ESCC patients and demonstrated that higher levels of F. nucleatum are an independent risk factor for predicting poor recurrence free survival. Furthermore, we illustrate that using RECIST, PET/CT and TRG analysis, higher burden of F. nucleatum predicts poor response to NAC. Our data highlight that F. nucleatum levels serve as an important prognostic and predictive biomarker, and suggest the possibility of using an antibiotic intervention to target this bacterium for improving the chemotherapeutic response in ESCC patients.
Acknowledgements
We thank Yuko Ogata, Keisuke Miyake and Kazuo Okadome for collecting clinical samples and information. We thank Shusuke Toden for editing the manuscript. We thank Lauren J Patterson, Preethi Ravindranathan and Divya Pasham for help with performing various experiments.
Funding: The present work was supported by the grants CA72851, CA181572, CA184792, CA202797 and CA187956 from the National Cancer Institute; a grant (RP140784) from the Cancer Prevention Research Institute of Texas (CPRIT), pilot grants from the Baylor Sammons Cancer Center, as well as funds from the Baylor Scott & White Research Institute.
Footnotes
Conflicts of interest: None of the authors has any potential conflicts to disclose.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499–509. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–7. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 7.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 8.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–34. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita K, Hosoda K, Moriya H, Katada C, Sugawara M, Mieno H, et al. Prognostic Advantage of Docetaxel/Cisplatin/ 5-Fluorouracil Neoadjuvant Chemotherapy in Clinical Stage II/III Esophageal Squamous Cell Carcinoma due to Excellent Control of Preoperative Disease and Postoperative Lymph Node Recurrence. Oncology 2017;92:221–28. [DOI] [PubMed] [Google Scholar]
- 10.Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91:2165–74. [PubMed] [Google Scholar]
- 11.Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol 2001;31:419–23. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M, Baba Y, Yoshida N, Ishimoto T, Nagai Y, Iwatsuki M, et al. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by esophagectomy in patients with resectable node-positive esophageal cancer. Ann Surg Oncol 2014;21:2838–44. [DOI] [PubMed] [Google Scholar]
- 13.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- 14.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012;338:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett WS. Cancer and the microbiota. Science 2015;348:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013;13:800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2017. [DOI] [PubMed] [Google Scholar]
- 18.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin Cancer Res 2016;22:5574–81. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017;170:548–63.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721–4. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 26.Izumi D, Yoshida N, Watanabe M, Shiraishi S, Ishimoto T, Kosumi K, et al. Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol 2016;51:788–95. [DOI] [PubMed] [Google Scholar]
- 27.Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347–55. [DOI] [PubMed] [Google Scholar]
- 28.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. Isme j 2012;6:1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida T, Fujimoto M, Watanabe M, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol 2018;53:517–24. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, Liska V, et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis 2014;33:1381–90. [DOI] [PubMed] [Google Scholar]
- 31.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016;165:1092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep 2015;5:14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panebianco C, Adamberg K, Jaagura M, Copetti M, Fontana A, Adamberg S, et al. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol 2018;81:773–82. [DOI] [PubMed] [Google Scholar]
- 39.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122s–50s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496–507. [DOI] [PubMed] [Google Scholar]
- 41.Sloof GW. Response monitoring of neoadjuvant therapy using CT, EUS, and FDG-PET. Best Pract Res Clin Gastroenterol 2006;20:941–57. [DOI] [PubMed] [Google Scholar]
- 42.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta RS, Nishihara R, Cao Y, Song M, Mima K, Qian ZR, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 2017;3:921–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res 2014;74:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Han SW, Kang JK, Bae JM, Kim HP, Won JK, et al. Association Between Fusobacterium nucleatum, Pathway Mutation, and Patient Prognosis in Colorectal Cancer. Ann Surg Oncol 2018;25:3389–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Overview of the study design
ESCC, Esophageal squamous cell carcinoma. CRT, Chemoradiation therapy. DCF. Docetaxel, cisplatin and 5-FU. NAC, Neoadjuvant chemotherapy. RECIST, response evaluation criteria in solid tumors. SUVmax, maximum standardized uptake value.
Supplementary Figure S2: Patient survival of ESCC according to T category.
Kaplan-Meier analysis of RFS for ESCC patients with T2 – 4 (red) or T1 (blue) in (A) the training cohort and (B) the validation cohort.