Abstract
Purpose:
PD-L1 status by immunohistochemistry (IHC) is prognostic in metastatic renal cell carcinoma (mRCC) and its role as a potential predictive biomarker is under investigation. Using tumor tissue from the METEOR () and CABOSUN () clinical trials, we explored whether PD-L1 expression and the extent of the immune cell infiltrate can serve as prognostic and/or predictive biomarkers for cabozantinib and other targeted agents.
Experimental Design:
IHC double-staining for PD-L1 and CD45/CD163 (immune cell markers) was performed on tumor tissue from METEOR (n=306) and CABOSUN (n=110) clinical trials. Immune cell density and MET expression levels were also analyzed. Our primary aim was to correlate progression-free survival (PFS) by independent central review with PD-L1 status in patients treated with cabozantinib, everolimus (METEOR) or sunitinib (CABOSUN). Overall survival (OS) was also interrogated.
Results:
TC PD-L1 expression (≥1% cutoff) was detected in 29% and 23% of tumors from patients in the METEOR and CABOSUN trials, respectively. On univariate analysis, patients with PD-L1-positive TC had poorer PFS and OS than patients with PD-L1-negative TC on both trials, independent of therapy. On multivariable analysis and when combining the two trials, the association between TC PD-L1 expression and OS was statistically significant for all patients (p=0.034) and for patients treated on cabozantinib only (p=0.038). Cabozantinib was associated with improved PFS (HR<0.70) and OS (HR<0.85) compared to everolimus and sunitinib irrespective of PD-L1 expression.
Conclusions:
Higher PD-L1 expression results in worse clinical outcomes in mRCC treated with targeted therapy. Furthermore, PD-L1 expression is not predictive of response to cabozantinib therapy.
Keywords: Cabozantinib, Sunitinib, Everolimus, PD-L1, renal cell carcinoma, predictive biomarkers
INTRODUCTION
Over the past decade, the therapeutic landscape for metastatic renal cell carcinoma (mRCC) has witnessed a dramatic expansion(1, 2). With the advent of Vascular Endothelial Growth Factor (VEGF)- Tyrosine Kinase Inhibitors (TKI)(3-7), mechanistic target of rapamycin (mTOR) inhibitors(8, 9), and more recently immune checkpoint blockade (ICB)(10-14), there is now an extensive therapeutic armamentarium across the world for the treatment of mRCC. However, with this plethora of therapeutic options, a fundamental challenge facing clinicians is selecting the most efficacious therapy for each individual patient. Therefore, the development of predictive biomarkers to aid clinicians in choosing the right drug for the right patient has become a pressing need.
Expression of the programmed death-ligand 1 (PD-L1), by immunohistochemistry (IHC) has been mostly studied as a potential biomarker in the setting of ICB. In Checkmate-025 trial (Study of Nivolumab [BMS-936558] vs. Everolimus in Pre-Treated Advanced or Metastatic Clear-Cell Renal Cell Carcinoma), PD-L1 positivity in tumor cells (TC) was associated with a worse overall survival independent of receipt of the ICB nivolumab or the mTOR inhibitor everolimus(11). On the other hand, in the front-line CheckMate-214 trial (Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma), patients with PD-L1 expression in TC were more likely to derive clinical benefit from the combination of nivolumab (anti-PD1) and ipilimumab (anti-CTLA4) versus the VEGF inhibitor sunitinib(12). Recently, in KEYNOTE-426 (assessing pembrolizumab in combination with axitinib versus sunitinib monotherapy as a first-line treatment in patients with mRCC), better overall survival was observed with pembrolizumab plus axitinib compared to sunitinib regardless of PD-L1 expression. However, in the subgroup analysis for progression free survival (PFS), a lower hazard ratio for disease progression or death was observed with combination pembrolizumab plus axitinib in the PD-L1 positive group compared to the PD-L1 negative group. (13). Moreover, in the recent JAVELIN RENAL 101 study, which compared avelumab plus axitinib versus sunitinib as first-line treatment in mRCC, only patients with positive PD-L1 expression had a statistically significant increase in PFS with avelumab plus axitinib (with a non-significant trend towards improved PFS in the PD-L1 negative group)(14). Results from another front-line study comparing the PD-L1 inhibitor atezolizumab (as monotherapy or in combination with bevacizumab) to the VEGF-TKI sunitinib, showed that clinical outcomes may be affected by PD-L1 expression in tumor infiltrating immune cells (IC); though response was seen in both PD-L1 positive and negative tumors(15). Relevant to VEGF TKIs, we have previously interrogated PD-L1 expression in a large phase 3 trial comparing sunitinib and pazopanib and showed that PD-L1 positivity confers a worse PFS and OS to both agents(16) . Overall, these data suggest that in clear cell RCC (ccRCC), the role of PD-L1 expression as a potential predictive therapeutic biomarker remains controversial and needs further investigation.
Cabozantinib is a multi-target tyrosine kinase inhibitor, with activity against vascular endothelial growth factor receptors (VEGFR), MET, and other kinases(17, 18). However, there is evidence that cabozantinib also possesses immunomodulatory properties that might contribute to its antitumor activity. For instance, this agent has been shown to reduce the function of regulatory T cells and CD14 positive immunosuppressive monocytes, increase cytokine production from effector T cells in response to antigen stimulation(19, 20), and activate the innate immune response(21). The METEOR () and CABOSUN () trials have established cabozantinib as an active agent for mRCC in both previously-treated and treatment-naïve populations(3, 4). Currently, there is no specific biomarker used to select for cabozantinib efficacy; however, since cabozantinib exhibits immunomodulatory activity, it is possible that patients with tumors displaying markers of inflammation, including PD-L1 expression in TC and/or IC, may achieve improved clinical outcomes when treated with cabozantinib compared to other targeted therapies. In addition, since combinations of cabozantinib and ICB are currently being developed in clinical trials (CheckMate 9ER; )(22), there is increased interest in studying the role of PD-L1 as predictive biomarker for cabozantinib-based therapy.
Herein, we conducted a correlative biomarker analysis in two independent clinical trials of cabozantinib (METEOR and CABOSUN), by examining associations between clinical outcomes and PD-L1 expression assessed by immunohistochemistry (IHC). We explored the utility of various PD-L1 expression measures as well as PD-L1 and/or MET co-expression in patients treated with cabozantinib versus other targeted therapies (control arms).
METHODS
Study design and Clinical Endpoints
PD-L1 expression was assessed on pre-treatment tumor tissue (archival nephrectomy specimens n= 359, or biopsied metastases n= 57) of patients from the METEOR and CABOSUN randomized clinical trials. METEOR was a randomized phase III clinical trial that compared cabozantinib versus everolimus in patients with mRCC who progressed after previous VEGFR tyrosine-kinase inhibitor treatment. CABOSUN was a randomized phase II trial comparing cabozantinib with sunitinib as first-line therapy in patients with intermediate and poor-risk mRCC. Study designs and clinical endpoints were previously described(3, 4). Patient baseline characteristics and treatment outcomes (Overall Response Rate (ORR, including complete response and partial response), Disease Control Rate (DCR, including complete response, partial response and stable disease), Progression Free Survival (PFS) and Overall Survival (OS)) were collected from the trial database. PFS and ORR (per RECIST 1.1) were determined by independent radiology review committee assessment. For both trials, PFS was defined as the time from randomization to radiographic progression or death from any cause; OS was calculated from randomization to date of death and censored at date of last follow up.
This study was approved by the institutional review board or ethics committee of the participating centers and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. All patients provided written informed consent.
Immunohistochemistry Staining
Immunohistochemistry studies were performed on formalin-fixed and paraffin-embedded (FFPE) tissue sections collected by the study sponsors at the time of the trials. An in-house double IHC staining assay was developed using an extensively validated antibody against PD-L1 (405. 9A11 mouse monoclonal antibody, 1:100, 13 micrograms/ml, Dr. Freeman laboratory, Dana-Farber Cancer Institute, Boston, MA, USA and commercially available through Cell Signaling Technology (CST))(23-27) and a cocktail of antibodies recognizing immune cells consisting of anti-CD45 (1:500, D9M8I XP, rabbit monoclonal antibody, CST) with anti-CD163 (1:5000, EPR19518, rabbit monoclonal antibody, Abcam). Tumor sections were stained with Bond Rx Autostainer (Leica Biosystems, Buffalo Grove, IL) using the Bond Polymer Refine Detection Kit (DS9800; Leica Biosystems) and Bond Polymer Refine Red Detection Kit (DS9390, Leica Biosystems). Antigen retrieval was performed with Bond Epitope Retrieval Solution 2 (EDTA, pH = 9.0) for 30 minutes. All slides were counterstained with hematoxylin, dehydrated in graded ethanol and xylene, mounted, and cover slipped (Supplementary Figure 1).
Scoring of IHC Staining by Image Analysis
Immunostained slides were scanned at 200x magnification using Aperio ScanScope (Leica Microsystems, Wetzlar, Rhine, Germany) and quantified using Indica Lab HALO platform algorithms. In each slide, tumor cells were identified using the HALO platform tissue classification module, and the number of PD-L1-positive tumor cells (TC) was determined using the HALO platform multiplex-IHC v1.2 algorithm. CD45/CD163 staining was used to identify tumor infiltrating IC. The HALO platform multiplex-IHC v1.2 algorithm was also utilized to determine the number of PD-L1-positive IC (Supplementary Figure 2). Results of the image analysis were validated through visual inspection by pathologists with expertise in the evaluation of PD-L1 staining in RCC (SS, AF, MF). Specifically, for each immunostained slide, pathologists confirmed that 1) the classifier correctly identified the TC and IC; 2) the algorithm correctly identified the PD-L1- positive cells (Supplementary Figure 2). Percentages of PD-L1-positive TC, PD-L1-positive IC and combined TC/IC score (defined as [(number of PD-L1-positive TC + number of PD-L1-positive IC) / (total number of TC)] x100)(28) were then calculated. For each tumor, positive TC PD-L1 expression was defined as ≥1% expression on TC. For PD-L1 positivity on IC and combined scores, two cutoffs (≥1% or ≥5%) were explored. For patients with multiple tissue samples analyzed, highest PD-L1 expression scores were used in subsequent analyses.
To measure the total amount of tumor infiltrating IC, we calculated immune cell density scores (ICD) defined as [(total number of IC / (Area occupied by tumor cells + stromal area)] for each tumor tissue specimen. Immune cell density scores were then divided into tertile groups (low, intermediate and high) using 33% and 66% as cutoffs from the joint distribution of ICD from the two trials. MET expression levels by IHC were previously assessed and reported for both METEOR(29) and CABOSUN(30) studies; a cutoff ≥ 50% of tumor tissue stained with an intensity of 2+ or 3+ was used to define positive MET expression.
Statistical analysis
To explore the prognostic value of PD-L1 expression, we compared clinical outcomes by PD-L1 expression status, regardless of therapies. The analysis was initially performed by trial and subsequently using a combined analysis if similar associations were observed in both trials. Fisher’s exact tests were used to compare ORR and DCR between PD-L1 positive versus negative tumors. The distributions of PFS and OS were estimated with the Kaplan-Meier methodology along with a 95% confidence interval (95%CI); comparisons between groups (PD-L1 positive versus negative) were conducted by the log-rank test. The associations of PD-L1 expression with clinical outcomes were also assessed in multivariable logistic regression (for ORR and DCR) and Cox regression (for PFS and OS), adjusted for treatment, International mRCC Database Consortium (IMDC) risk group, presence of bone metastases, and number of previous VEGFR TKI treatment (1 or ≥2, for the METEOR trial only). These clinical variables were chosen as they are known prognostic factors and have been used as stratification factors in these trials.
To explore the predictive value of PD-L1 expression, we summarized PFS, OS, ORR, and DCR by type of treatment and by PD-L1 expression. Treatment comparisons (cabozantinib versus everolimus or sunitinib) on PFS and OS were quantified by hazard ratios (95% CI) from the Cox regression, separately by PD-L1 positive and negative tumors. Hazard ratios reported for the subgroup analyses were from univariate analyses, consistent with previously described(29, 30). Test for interaction (p-interaction) was provided to assess whether treatment effects differed by PD-L1 expression status.
Similar analyses described for PD-L1 alone were performed for other PD-L1 expression parameters (i.e. PD-L1 TC and IC combined scores or using different cutoffs) as well as MET and PD-L1 combined expression, and the ICD score. These analyses were considered exploratory, with no adjustment for multiple comparisons.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided p-value <0.05 was considered statistically significant.
RESULTS
Patient Characteristics and Treatment
In the METEOR trial, 658 patients were randomly assigned 1:1 to receive cabozantinib (330 patients) or everolimus (328 patients) between August 2013 and November 2014. For the current analysis, data cutoff was May 22, 2015 for the PFS and response evaluation. For OS analysis, the data cutoff was December 31, 2015. PD-L1 expression on TC was assessed in 306 out of 658 patients (150 treated with cabozantinib and 156 treated with everolimus) (Supplementary Figure 3A). Patient demographic and clinical characteristics were similar in this subset of patients as compared to the overall trial population (Supplementary Table 1A). PD-L1 expression on tumor infiltrating IC was assessed in 301 patients; 5 patients with tumor tissue corresponding to metastatic lymph nodes were excluded due to difficulty in identifying tumor infiltrating IC.
The CABOSUN trial consisted of 157 patients randomly assigned 1:1 to receive cabozantinib (n=79) or sunitinib (n=78) between July 9, 2013 and April 6, 2015. Data cut-off was July 1, 2017 for the OS analysis and September 15, 2016 for the PFS analysis. PD-L1 expression on TC and IC was assessed in 110 patients (Supplementary Figure 3B). The patient demographic and clinical characteristics of this cohort were similar compared to the overall trial population (Supplementary Table 1B).
PD-L1 expression on TC and IC and their association with IMDC risk groups
In the METEOR cohort, TC PD-L1 expression (≥1% cutoff) was observed in 88 (29%) patients. PD-L1 positivity on IC (≥1% cutoff) was observed in 179 (59%) patients. Patients in the IMDC poor risk group were more likely to express PD-L1 on TC and IC than patients in the IMDC favorable or intermediate risk groups (Table 1, p=0.013 for TC and p=0.019 for IC).
Table 1.
PD-L1 expression: overall and by IMDC risk groups
| METEOR (N=306) | CABOSUN (N=110) | |||
|---|---|---|---|---|
| N | N(%) with PD-L1(+) (≥1% cutoff for TC and IC) |
N | N(%) with PD-L1(+) (≥1% cutoff for TC and IC) |
|
| Tumor cells | ||||
| All patients | 306 | 88(29) | 110 | 25(23) |
| By IMDC risk groups | ||||
| Favorable | 64 | 11(17) | - | - |
| Intermediate | 198 | 58(29) | 88 | 15(17) |
| Poor | 44 | 19(43) | 22 | 10(45) |
| P-value | 0.013 | 0.009 | ||
| Immune cells | ||||
| All patients | 301 | 179(59) | 110 | 67(61) |
| By IMDC risk groups | ||||
| Favorable | 61 | 28(46) | - | - |
| Intermediate | 196 | 119(61) | 88 | 50(57) |
| Poor | 44 | 32(73) | 22 | 17(77) |
| P-value | 0.019 | 0.092 | ||
IMDC: International Metastatic Renal Cell Carcinoma Database Consortium
In the CABOSUN cohort, PD-L1 expression on TC (≥1% cutoff) was observed in 25 (23%) patients and PD-L1 expression on IC was detected in 67 (61%) patients. In this cohort, PD-L1 positivity was associated with IMDC poor risk status (Table 1, p=0.009 for TC and p=0.092 for IC).
Association of PD-L1 expression with clinical outcomes
On univariate analysis, in both trials median PFS was significantly shorter in patients with positive TC PD-L1 expression (≥1%) compared to patients with negative TC PD-L1 expression (<1%) (METEOR: 5.3 versus 7.2 months, p=0.027; CABOSUN: 5.5 versus 8.3 months, p=0.051, Table 2, Figure 1A & 1C). The association did not persist in the multivariable analysis of either trial cohort or when combining the two trials. TC PD-L1 expression was not correlated with ORR or DCR in either trial (p>0.75, Supplementary Table 2).
Table 2.
Associations of PD-L1 expression on tumor or immune cells with treatment outcomes
| METEOR (N=306) | CABOSUN (N=110) | Combining two trials | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients (N=416) |
cabozantinib only (N=211) |
|||||||
| Total/ No. of Events |
Median months (95%CI) |
Adjusted* hazard ratio (95%CI) |
Total/ No. of Events |
Median months (95%CI) |
Adjusted* hazard ratio (95%CI) |
Adjusted* hazard ratio (95%CI) |
Adjusted* hazard ratio (95%CI) |
|
| Tumor cells (≥1% cutoff) | ||||||||
| PFS | ||||||||
| PD-L1(−) | 218/126 | 7.2 (5.6-7.5) |
1(reference) | 85/45 | 8.3 (5.4-12.9) |
1(reference) | 1(reference) | 1(reference) |
| PD-L1(+) | 88/60 | 5.3 (3.7-5.6) |
1.19 (0.86-1.63) |
25/20 | 5.5 (2.8-10.1) |
1.26 (0.72-2.23) |
1.21 (0.92-1.61) |
1.28 (0.83-1.99) |
| p-value | 0.027 | 0.301 | 0.051 | 0.419 | 0.173 | 0.265 | ||
| OS | ||||||||
| PD-L1(−) | 218/91 | 21.3 (18.0-NR) |
1(reference) | 85/44 | 28.1 (18.9-NR) |
1(reference) | 1(reference) | 1(reference) |
| PD-L1(+) | 88/52 | 15.1 (10.4-18.8) |
1.37 (0.97-1.94) |
25/18 | 20.8 (12.3-26.6) |
1.46 (0.81-2.65) |
1.39 (1.03-1.87) |
1.63 (1.03-2.60) |
| p-value | 0.003 | 0.078 | 0.047 | 0.209 | 0.034 | 0.038 | ||
| Immune cells (≥1% cutoff) | ||||||||
| PFS | ||||||||
| PD-L1(−) | 122/76 | 7.2 (5.6-7.5) |
1(reference) | 43/27 | 5.8 (4.3-12.9) |
1(reference) | 1(reference) | 1(reference) |
| PD-L1(+) | 179/107 | 5.5 (3.8-5.7) |
1.16 (0.86-1.57) |
67/38 | 8.3 (5.4-12.4) |
0.96 (0.57-1.61) |
1.09 (0.84-1.41) |
1.10 (0.74-1.62) |
| p-value | 0.136 | 0.324 | 0.698 | 0.864 | 0.512 | 0.649 | ||
| OS | ||||||||
| PD-L1(−) | 122/52 | 21.3 (17.3-NR) |
1(reference) | 43/22 | 35.4 (17.5-NR) |
1(reference) | 1(reference) | 1(reference) |
| PD-L1(+) | 179/89 | 18.4 (15.1-22) |
1.15 (0.81-1.62) |
67/40 | 26.0 (16.4-30.3) |
1.46 (0.86-2.50) |
1.24 (0.93-1.65) |
1.45 (0.93-2.26) |
| p-value | 0.101 | 0.443 | 0.237 | 0.165 | 0.145 | 0.103 | ||
PFS: Progression free survival; OS: Overall survival
All models were adjusted for treatment, IMDC risk groups and presence of bone metastases. For METEOR and combined analysis, the models were also adjusted for number of previous VEGFR TKI treatment (1 or ≥2 for METEOR, 0, 1, or ≥2 for the combined analysis).
Figure 1:
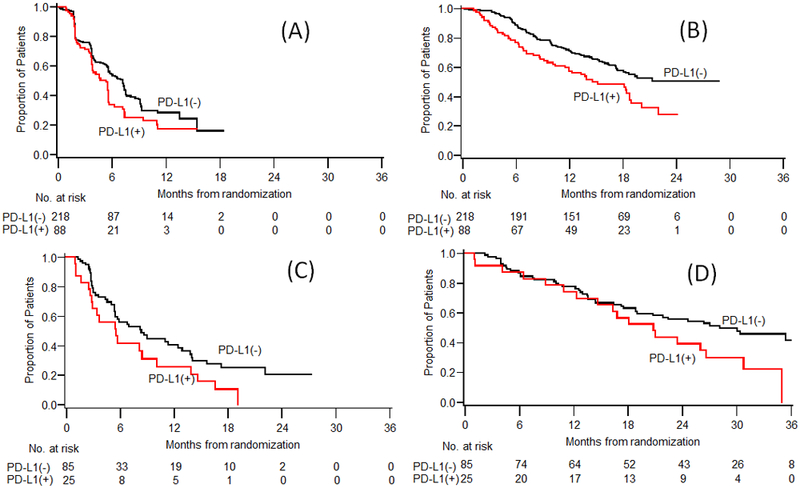
Kaplan Meier estimates of PFS and OS by PD-L1 expression on tumor cells: (A) PFS (B) OS in METEOR study; (C) PFS (D) OS in CABOSUN study.
In both trials, patients with positive TC PD-L1 expression (≥1%) had worse OS compared to patients with negative TC PD-L1 expression (<1%) (METEOR: median 15.1 versus 21.3 months, p=0.003, CABOSUN: 20.8 versus 28.1 months, p=0.047; Table 2, Figure 1B & 1D). The association between PD-L1 expression on TC and OS was statistically significant in the multivariable analysis when combining the two trials, with the adjusted hazard ratio of 1.39 (95%CI, 1.03–1.87; p=0.034) for all patients (N=416) and of 1.63 (95%CI, 1.03–2.60; p=0.038) for patients treated with cabozantinib only (N=211).
PD-L1 expression on IC (≥1% cutoff) was not associated with ORR, DCR, PFS, or OS in either trial or in the analysis of the combined cohorts (Table 2 & Supplementary Table 2).
Predictive Value of PD-L1 expression
Next, we assessed the predictive value of PD-L1 expression on TC or IC in patients receiving cabozantinib versus its comparator as part of the METEOR and CABOSUN trials. In both trials, treatment with cabozantinib was associated with improved PFS compared to everolimus (METEOR) and sunitinib (CABOSUN), irrespective of PD-L1 expression in TC (Table 3, Figure 2). In the METEOR trial, the median PFS in patients with negative TC PD-L1 expression (<1%) was 8.5 months with cabozantinib and 4.1 months with everolimus (HR, 0.46; 95% CI, 0.32–0.66). In patients with positive TC PD-L1 expression (≥1%), the median PFS was 5.6 months with cabozantinib and 3.7 months with everolimus (HR, 0.66; 95% CI, 0.40–1.11). Similarly, in the CABOSUN trial, the median PFS was longer in patients treated with cabozantinib than sunitinib, in both TC PD-L1-negative (<1%) (HR, 0.47; 95% CI, 0.26–0.86) and TC PD-L1-positive (HR, 0.46; 95% CI, 0.18–1.21) patient populations. When compared to everolimus and sunitinib, cabozantinib was associated with improved DCR irrespective of TC PD-L1 expression (Supplementary Table 3). Treatment comparison on ORR by PD-L1 expression was limited by the small number of patients available for this subgroup analysis (Supplementary Table 3).
Table 3.
Treatment comparison on PFS and OS, in subgroup analysis by PD-L1 expression on tumor cells (≥1% cutoff)
| METEOR (N=306) | CABOSUN (N=110) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CABOZANTINIB (C) |
EVEROLIMUS (E) |
C vs E | CABOZANTINIB (C) |
SUNITINIB (S) |
C vs S | |||||
| N | Median months (95%CI) |
N | Median months (95%CI) |
Hazard ratio (95%CI) |
N | Median months (95%CI) |
N | Median months (95%CI) |
Hazard ratio (95%CI) |
|
| PFS | ||||||||||
| PD-L1(−) | 112 | 8.5 (7.2-13.5) |
106 | 4.1 (3.7-6) |
0.46 (0.32-0.66) |
52 | 11.0 (6.8-15.6) |
33 | 5.0 (3-12.9) |
0.47 (0.26-0.86) |
| PD-L1(+) | 38 | 5.6 (4.5-7.4) |
50 | 3.7 (2-5.3) |
0.66 (0.40-1.11) |
9 | 8.4 (1.1-16.6) |
16 | 3.1 (1.6-10.1) |
0.46 (0.18-1.21) |
| P-interaction | 0.217 | 0.998 | ||||||||
| OS | ||||||||||
| PD-L1(−) | 112 | NR | 106 | 18.4 (15.1-NR) |
0.58 (0.38-0.88) |
52 | 30.3 (18.8-NR) |
33 | 22.4 (7.6-NR) |
0.71 (0.39-1.29) |
| PD-L1(+) | 38 | 18.4 (10.4-22) |
50 | 13.9 (8.7-18.9) |
0.82 (0.47-1.41) |
9 | 18.1 (1.1-35) |
16 | 21.0 (6.4-30.8) |
0.85 (0.31-2.31) |
| P-interaction | 0.359 | 0.372 | ||||||||
PFS: Progression free survival; OS: Overall survival
Figure 2:
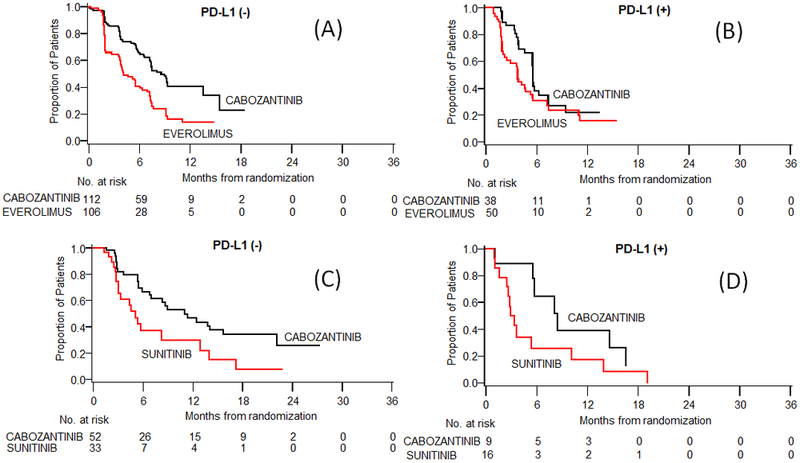
Kaplan Meier estimates of PFS according to treatment, subgroup by (A) TC PD-L1 (–), (B) TC PD-L1 (+) in METEOR study; (C) TC PD-L1 (–), (D) TC PD-L1(+) in CABOSUN study.
Analysis of the METEOR trial showed that the median OS was improved in patients treated with cabozantinib compared with everolimus, independent of TC PD-L1 status. In patients with negative TC PD-L1 expression (<1%) median OS was not reached with cabozantinib and was 18.4 months (95% CI,15.1-NR) with everolimus (HR, 0.58; 95% CI, 0.38–0.88) (Table 3). In patients with positive TC PD-L1 expression (≥1%), the median OS was 18.4 (95% CI, 10.4–22.0) and 13.9 (8.7–18.9), respectively (HR, 0.82; 95% CI, 0.47–1.41). A similar trend was observed in the CABOSUN trial, but the number of patients was small in these subgroup analyses (Table 3).
We additionally assessed the potential predictive value of PD-L1 expression on IC and that of the combined TC/IC PD-L1 score in both the METEOR and CABOSUN trials. Results were consistent with those obtained by evaluating TC PD-L1 expression, and were similar using different expression cutoff points (≥1% and ≥5%) (Supplementary Table 4).
Association of combined MET and PD-L1 expression with clinical outcomes
In order to assess the interplay between PD-L1 and MET pathways, we next assessed the association of PD-L1 and MET expression and the impact of the combined expression of these two targets on clinical outcomes. A total of 397 patients were stained for both MET and PD-L1 expression (CABOSUN: N=110; METEOR: N=287). Overall, expression of TC PD-L1 (≥1%) was higher in MET-positive tumors expression (47/115, 41%) compared to MET-negative tumors (63/282, 22%) (p=0.0003). Since patients with tumor cells expressing both MET and PD-L1 and patients with tumor cells expressing either MET or PD-L1 had similar median PFS and OS, the two groups were combined for further analysis (Supplementary table 5A). When analyzed as two groups, patients with tumor cells expressing either MET, or PD-L1 or both had significantly shorter OS (adjusted HR, 1.35; 95% CI, 1.02–1.80; p=0.039) but only a trend towards decreased PFS (adjusted HR, 1.27; 95% CI, 0.97–1.65; p=0.078) when compared to tumors negative for the expression of both proteins (Supplementary Table 5B). Nevertheless, MET and/or PD-L1 expression was not found to be a significant predictor of benefit for cabozantinib (p-interaction > 0.20; Supplementary Table 6).
Association of Immune cell density with clinical outcomes
Finally, we assessed whether tumor-infiltrating immune cells quantified as immune cell density (ICD) score was associated with clinical outcome. Median ICD score was 972 (range 12–6598) in the METEOR trial and 1087 (20–4034) in the CABOSUN trial. Median ICD was significantly higher in patients with positive TC PD-L1 expression (≥1%) in both trials (p<0.05; Supplementary Figure 4). When ICD was analyzed as tertile groups, there was no association of ICD with PFS, OS, ORR or DCR in either trial or in combined analysis of two trials (p-values≥0.20, Supplementary Table 7). Results were similar when ICD was analyzed as continuous values on the Log10 transformation (Supplementary Table 8). Furthermore, ICD was not found to be predictive of response in patients treated with cabozantinib (p-interaction >0.10; Supplementary Table 9).
DISCUSSION
Our analysis of two independent randomized clinical trials of cabozantinib (versus everolimus or sunitinib) shows that patients with mRCC expressing PD-L1 on their tumor cells experience shorter PFS and OS, independent of the type of therapy. Of note, we also demonstrated that treatment with cabozantinib is associated with improved PFS, OS and DCR compared to everolimus (METEOR) or sunitinib (CABOSUN), irrespective of PD-L1 expression or the amount of tumor infiltrating immune cells.
While the poor prognostic significance of TC PD-L1 expression in patients with mRCC treated with targeted agents has been previously reported by our group and others(12, 16), our results raise new important questions regarding patient selection for systemic therapy. Both cabozantinib and anti-PD-(L)1-based combination therapies (nivolumab plus ipilimumab, pembrolizumab plus axitinib, and avelumab plus axitinib) have been recently approved by the FDA as frontline treatments for advanced RCC(31-34). Recent data have consistently shown that patients with PD-L1 positive tumors have a greater PFS benefit with anti-PD-(L)1-based combination therapy compared to sunitinib (12-14), suggesting that PD-L1 expression might have some utility as a biomarker for these combination regimens. Our study provides evidence that cabozantinib is more effective than sunitinib or everolimus in the treatment of both PD-L1-positive and PD-L1-negative ccRCC tumors and thus supports the use of this agent in a PD-L1 unselected population. Notably, the efficacy of cabozantinib in patients with tumors displaying features generally associated with reduced clinical efficacy of ICB such as negative PD-L1 expression and absence of a significant intra-tumoral immune cell infiltrate (i.e. “cold” tumors), raises new clinical questions regarding whether cabozantinib or a cabozantinib-based combo with an anti-PD1, could possibly be another therapeutic option for this patient population. Prospective clinical trials are needed to answer these questions and define the optimal clinical setting for cabozantinib in mRCC.
Previously, MET protein expression assessed by IHC has not been shown to affect clinical outcomes with cabozantinib in patients treated on METEOR(29) and CABOSUN(30) although with a trend towards more benefit in MET-positive patients. We therefore aimed to determine whether combined expression of PD-L1 and/or MET in TC is a superior predictor of PFS and OS with cabozantinib than PD-L1 or MET expression alone in either trial. Subgroup analysis of PFS and OS based on PD-L1 and/or MET expression favored cabozantinib over everolimus or sunitinib irrespective of MET/PD-L1 status. These results likely reflect the wide target profile of cabozantinib, and might justify exploring other targets, such as AXL and VEGFR, as predictors of clinical outcomes to cabozantinib in patients with mRCC. It should be noted that recently, analyses of transcriptome data conducted on pre-treatment tumors from mRCC patients enrolled on the randomized IMmotion150 trial (), comparing the efficacy of atezolizumab (anti-PD-L1 antibody) alone or with bevacizumab (anti-VEGF antibody) versus sunitinib (VEGFR inhibitor), found that the expression of angiogenesis-associated genes might represent a predictive biomarker of response to sunitinib(15). Similarly, an angiogenesis gene expression program was associated with VEGF TKI response and survival in the phase III COMPARZ trial of sunitinib versus pazopanib(35). Given the well-recognized anti-angiogenic properties of cabozantinib, it will be of particular interest to test whether angionenesis gene signatures might also predict responses to this agent.
The biological variability in PD-L1 expression poses considerable challenges to establishing the PD-L1 status of a given tumor, with PD-L1 expression demonstrating substantial intra-tumoral heterogeneity, and differences between primary and metastatic sites(26, 36, 37). Moreover, accurately assessing PD-L1 expression by IHC is also inherently difficult due to challenges in both the analytical and post-analytical phases of the process. There are currently multiple commercially available anti-PD-L1 antibodies available for IHC analysis, which display different sensitivity and specificity. In our analysis, we utilized the 405.9A11 antibody that we have extensively validated in previous studies(24-27). Importantly, this antibody recognizes the cytoplasmic domain of PD-L1, is very selective for membranous PD-L1, and has excellent concordance with other commercially available PD-L1 antibodies that have been FDA-approved as companion or complementary diagnostic tests for ICB agents (including 28–8, 22C3, and SP263)(23, 38).
Recent studies have highlighted how the assessment of PD-L1 expression performed by pathologists is affected by substantial inter-observer variability, which is especially high when evaluating expression in IC(39-41). In order to overcome this problem, we developed a novel double IHC assay coupled with quantitative image analysis to simultaneously quantify PD-L1 expression on TC and IC. In line with previous studies(11, 12), we found that 29% and 23% of ccRCC tissues had positive TC PD-L1 expression (≥1%) in the METEOR and CABOSUN trials, respectively. PD-L1 expression in IC (≥1% cutoff) was observed in 59% of tumors in the METEOR cohort and in 67% of tumors in the CABOSUN cohort, highlighting the wide expression of PD-L1 in the ccRCC microenvironment, which may contribute to immune suppression and tumor escape. Our novel assay represents a first important step towards the standardization of PD-L1 quantification in both TC and IC using automated image analysis and has the potential of enhancing the reproducibility of PD-L1 assessment in future correlative studies of clinical trial cohorts.
Our study has several potential limitations. First, this is retrospective analysis of tissue samples collected from multiple institutions and it is well recognized that variability in tissue processing and handling protocols can affect the immunogenicity of the tissues, and thus affect the results of immunohistochemical analysis(42, 43) . These issues are common to most correlative analyses performed in the context of multi-institutional studies and a major advance in this area would require substantial efforts directed towards the standardization of the tissue collection and processing procedures within individual pathology laboratories, which is undoubtedly a complex task. An additional potential limitation of the study is that the analysis of a single tumor specimen per patient might not adequately address the heterogeneity of PD-L1 expression that has been previously documented in ccRCC(26). However, since we have previously demonstrated that PD-L1 is mostly expressed in high grade areas of the tumor(26), and areas containing the highest tumor grade are usually selected by pathologists for correlative studies, the chance of false negative results is reduced.
In conclusion, our multiplex IHC analysis on tumor samples from the METEOR and CABOSUN trials demonstrated that PD-L1 expression on tumor cells is associated with shorter survival in patients with mRCC, irrespective of the type of targeted therapy received, and that neither PD-L1 expression nor immune cell infiltrate is predictive of response to cabozantinib.
Supplementary Material
Statement of translational relevance:
Both cabozantinib and anti-PD-(L)1-based combination therapies (nivolumab plus ipilimumab pembrolizumab plus axitinib, and avelumab plus axitinib) are approved frontline treatments for metastatic renal cell carcinoma (mRCC). The magnitude of benefit from anti-PD-(L)1-based combination therapies does appear to be consistently greater in the patients with PD-L1 positive tumors. As cabozantinib is a multi-target tyrosine-kinase inhibitor with immunomodulatory functions, it is important to assess whether PD-L1 expression also predicts responses to cabozantinib. Using baseline tumor tissue from the METEOR and CABOSUN clinical trials, we demonstrate that patients expressing PD-L1 on TC experience worse clinical outcome, independent of the type of targeted therapy they received. Our data also show that cabozantinib is more effective than everolimus or sunitinib in the treatment of both PD-L1-positive and PD-L1-negative mRCC, supporting the use of this drug in a PD-L1 unselected population. As cabozantinib shows excellent efficacy in PD-L1-negative tumors, our findings argue that cabozantinib-based therapies should be evaluated as part of a first-line therapeutic option for patients with negative TC PD-L1 expression.
Acknowledgements
TKC and SS have received support from NIH/NCI DF/HCC Kidney Cancer SPORE P50-CA101942 and a Sponsored Research Agreement from Exelixis. TKC is supported in part by the Kohlberg chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at the Dana-Farber Cancer Institute. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Grant Support: NIH/NCI DF/HCC Kidney Cancer SPORE P50CA101942 (TKC, SS, GF), U10CA180821, U10CA031946, and Sponsored Research Agreement from Exelixis (TKC, SS).
Footnotes
Disclosure of Potential Conflicts of Interest: D.A. Braun is a consultant/advisory board member for Octane Global, Defined Health, Dedham Group, Adept Field Solutions, Slingshot Insights, Blueprint Partnership, Charles River Associates/KC2 Medical, and Schlesinger Trinity Group. B. Escudier is a consultant/advisory board member for Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, and Roche, and reports receiving speaker’s bureau honoraria from Pfizer and Novartis. D.J. George reports receiving commercial research support from Acerta Pharmaceuticals, Astellas, Bayer H/C Pharmaceuticals, Bristol-Meyers Squibb, Calithera, Dendreon, Exelixis, Innocrin, Janssen Pharmaceuticals, Novartis, Pfizer, and Sanofi, speakers bureau honoraria from Bayer H/C Pharmaceuticals, Sanofi, and Exelixis, and is a consultant/advisory board member for Astellas, Astrazeneca, Bayer H/C Pharmaceuticals, Bristol-Meyers Squibb, Capio Biosciences, Exelixis, Genentech, Innocrin, Janssen Pharmaceuticals, Merck Sharp & Dohme, Myovant Sciences, Pfizer, and Sanofi, receives other honorarium from EMD Serono, Michael J. Hennessey Associates, OncLive, Pfizer, and UroToday, is an independent contractor for Axess Oncology, is on the steering committees of NCI and Pfizer, and is on the independent data monitoring committees of Acceleron Pharmaceuticals and Janssen Pharmaceuticals. R.J. Motzer reports receiving commercial research grants from Bristol-Myers Squibb, Pfizer, Genentech/Roche, and Eisai, and is a consultant/advisory board member for Pfizer, Genentech/Roche, Novartis, Eisai, and Exelixis. M.J. Morris reports receiving research funding from Bayer (Inst) Corcept Therapeutics (Inst), Endocyte (Inst), Progenics (Inst), Genentech/Roche (Inst), and Sanofi (Inst); and receiving funds for travel, accommodations, and expenses from Bayer and Endocyte; and is a consulting/advisory board member for Advanced Accelerator Applications, Astellas Pharma, Bayer, Blue Earth Diagnostics, Endocyte, Tokai Pharmaceuticals, and Tolmar Pharmaceuticals. T. Powles is a consultant/advisory board member for Novartis, Pfizer, and GlaxoSmithKline; has received company speaker honoraria from Novartis, Pfizer, GlaxoSmithKline, and Genentech; has participated in trials for GlaxoSmithKline, Pfizer, BMS, Genentech, and Genetech; and has received grants/research support from GlaxoSmithKline, Pfizer, and Novartis. E. Wang is an employee of Exilexis. G.J. Freeman reports receiving commercial research grants from Bristol-Myers Squibb, Roche/ Genentech, Novartis, VCB, and Ipsen; holds ownership interest (including patents) in Novartis, Roche/Genentech, Bristol-Myers Squibb, Amplimmune/ AstraZeneca, Merck, EMD Serono, Beohringer Ingelheim, and Dako; and is a consultant/advisory board member for Novartis, Lilly, Roche/Genentech, Bristol-Myers Squibb, Bethyl Laboratories, Xios Therapeutics, Quiet Therapeutics, and Seattle Genetics. T.K. Choueiri reports receiving commercial research grants from AstraZeneca, Alexion, Bayer, Bristol-Myers Squibb, Cerulean, Eisai, Foundation Medicine, Exelixis, Ipsen, Tracon, Genentech, Roche, Hoffman-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Prometheus, Corvus, Calithera, Analysis Group, Sanofi/Aventis, and Takeda; and is a consultant/advisory board member for AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol-Myers Squibb, Cerulean, Eisai, Foundation Medicine, Exelixis, Genentech, Heron Therapeutics, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus, Corvus, Ipsen, Up-to Date, NCCN, and Analysis Group. S. Signoretti reports receiving commercial research grants from Bristol-Myers Squibb, AstraZeneca, and Exelixis; is a consultant/advisory board member for Merck, AstraZeneca, Bristol-Myers Squibb, AACR, and NCI; and receives royalties from Biogenex. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med. 2017;376(4):354–66. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35(6):591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. [DOI] [PubMed] [Google Scholar]
- 10.Escudier B, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur Urol. 2017;72(6):962–71. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015;21(5):1071–7. [DOI] [PubMed] [Google Scholar]
- 17.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308. [DOI] [PubMed] [Google Scholar]
- 18.Tannir NM, Schwab G, Grunwald V. Cabozantinib: an Active Novel Multikinase Inhibitor in Renal Cell Carcinoma. Cur Onc Rep. 2017;19(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apolo AB, Tomita Y, Lee M-J, Lee S, Frosch A, Steinberg SM, et al. Effect of cabozantinib on immunosuppressive subsets in metastatic urothelial carcinoma.J Clin Oncol 2014;32(15_suppl):4501–. [Google Scholar]
- 20.Kwilas AR, Ardiani A, Donahue RN, Aftab DT, Hodge JW. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J Transl Med. 2014;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patnaik A, Swanson KD, Csizmadia E, Solanki A, Landon-Brace N, Gehring MP, et al. Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity. Cancer Discov. 2017;7(7):750–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri TK, Apolo AB, Powles T, Escudier B, Aren OR, Shah A, et al. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER). J Clin Oncol 2018;36(15_suppl):TPS4598–TPS. [Google Scholar]
- 23.Mahoney KM, Sun H, Liao X, Hua P, Callea M, Greenfield EA, et al. PD-L1 Antibodies to Its Cytoplasmic Domain Most Clearly Delineate Cell Membranes in Immunohistochemical Staining of Tumor Cells. Cancer Immunol Res. 2015;3(12):1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015;26(4):812–7. [DOI] [PubMed] [Google Scholar]
- 26.Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, et al. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015;3(10):1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wankowicz SAM, Werner L, Orsola A, Novak J, Bowden M, Choueiri TK, et al. Differential Expression of PD-L1 in High Grade T1 vs Muscle Invasive Bladder Carcinoma and its Prognostic Implications. J Urol. 2017;198(4):817–23. [DOI] [PubMed] [Google Scholar]
- 28.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med. 2018. [DOI] [PubMed] [Google Scholar]
- 29.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17(7):917–27. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma [press release]. US Food and Drug Administration https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm604685.htm., April 16 2018.
- 32.FDA grants regular approval to Cabometyx for first-line treatment of advanced renal cell carcinoma [press release]. US Food and Drug Administration https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm589842.htm., December 19 2017.
- 33.FDA approves avelumab plus axitinib for renal cell carcinoma [press release]. US Food and Drug Administration https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma, May 15 2019.
- 34.wj/>FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma [press release]. US Food and Drug Administration https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma, April 22 2019.
- 35.Hakimi AA, Voss MH, Kuo F, Sanchez A, Liu M, Nixon BG, et al. Transcriptomic Profiling of the Tumor Microenvironment Reveals Distinct Subgroups of Clear cell Renal Cell Cancer - Data from a Randomized Phase III Trial. Cancer Discov. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of pd-l1 expression in non–small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura S, Hayashi K, Imaoka Y, Kitamura Y, Akazawa Y, Tabata K, et al. Intratumoral heterogeneity of programmed cell death ligand-1 expression is common in lung cancer. PloS One. 2017;12(10):e0186192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaule P, Smithy JW, Toki M, Rehman J, Patell-Socha F, Cougot D, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol 2016: 10.1001/jamaoncol.2016.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12(2):208–22. [DOI] [PubMed] [Google Scholar]
- 40.Rehman JA, Han G, Carvajal-Hausdorf DE, Wasserman BE, Pelekanou V, Mani NL, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol. 2017;30(3):340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilie M, Hofman P. Reproducibility of PD-L1 assessment in non-small cell lung cancer-know your limits but never stop trying to exceed them. Transl Lung Cancer Res. 2017;6(Suppl 1):S51–s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Napoli A, Signoretti S. Tissue biomarkers in renal cell carcinoma: issues and solutions. Cancer. 2009;115(10 Suppl):2290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Signoretti S, Bratslavsky G, Waldman FM, Reuter VE, Haaga J, Merino M, et al. Tissue-based research in kidney cancer: current challenges and future directions. Clin Cancer Res. 2008;14(12):3699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


