Abstract
Purpose:
Metastasis requires malignant cell circulation from the primary to a distant tissue. Elevated levels of circulating tumor cells (CTC) portend a poor prognosis in breast and other cancers. Recent studies have suggested that CTC clusters may be a factor in the metastatic process. We conducted a prospective retrospective study of the SWOG0500 clinical trial to test whether CTC clusters are associated with poorer prognosis.
Experimental Design:
CTC CellSearch® galleries from SWOG0500 trial were re-read using pre-specified criteria for CTC clusters, doublets, and enumeration. Survival analysis methods include Kaplan-Meier plots and log-rank tests.
Results:
Patients were classified into three prognostic subgroups based on baseline CTC/7.5 ml whole blood (WB): Arm A: <5CTC; Arm B/C: ≥5CTC and then B (<5CTC) and C (≥5CTC)/7.5 ml WB at first follow-up. At baseline, 19% of patients had CTC doublets or clusters, which were more likely in Arm B/C vs. Arm A (38% vs 1.4%; p<0.0001). Furthermore, doublets or clusters were significantly more common in patients who were ultimately assigned to Arm C vs. B (54% vs 25%; p<0.0001). In Arm C, doublets and clusters were associated with worse OS than only doublets, clusters, or no doublets nor clusters at baseline (p=0.008) and first follow-up (p=0.010). When compared to enumeration alone, doublets, clusters, or both were not prognostic in patients who had 5–19 or ≥20 CTC/7.5 ml WB.
Conclusions:
In metastatic breast cancer patients starting first line chemotherapy, mortality is independent of the presence of CTC clusters, but rather depends on the number of CTC/7.5 ml WB.
Keywords: Circulating Tumor Cells (CTC), CTC cluster, overall survival (OS), metastatic breast cancer (MBC), CellSearch® System
Introduction
The metastatic process is complex, requiring several distinct biological steps, including invasion of surrounding normal tissue, intravasation into the circulation, extravasation into distant organs, and establishment of viable clones (1). In this regard, recently reported studies have suggested that clusters of circulating tumor cells (CTC), rather than elevated levels of single cells, are the driving force for subsequent metastases and death (2). The proposed mechanisms for this hypothesis are based on propensity of clusters for survival in a hostile environment and ability to extravasate and establish metastases (2). In addition, heterotypic clusters consisting of CTC and leukocytes appear to have higher viability and confer advantage to the metastatic process(3, 4). More recently, a comprehensive genome-wide analysis of DNA-methylation events has shown that CTC found in clusters have hypomethylation of critical stemness- and proliferation-related sites (5). Furthermore, CTC clusters may play a key role in the metastatic process and may also reflect resistance to chemotherapy (2, 6–12). Indeed, preliminary clinical investigatations have suggested that the presence of CTC clusters are prognostic in metastastic breast, prostate and small cell lung cancers (SCLC) (2, 6, 11, 12).
Several assays have been developed over the last two decades to identify, enumerate, and characterize circulating tumor cells (13). Of these, the most widely used is the CellSearch® system, which is based on anti-EpCAM capture and subsequent validation with immunofluorescent staining for DAPI and cytokeratin (14). Elevated CTC levels using this assay are highly prognostic in breast, prostate, colorectal, and SCLC as well as in early stage breast cancer (6, 15–19). However, for the most part, prognosis in these studies was based on enumeration of CTC, without regard to the presence of CTC-clusters.
SWOG S0500, a prospective randomized clinical trial, addressed whether patients with metastatic breast cancer (MBC) who had residual CTC [≥5/7.5 ml whole blood (WB)] after one cycle of first line chemotherapy (indicating lack of a CTC-response) benefit from continuing that therapy or changing to an alternate chemotherapy. Although switching chemotherapy regimens did not improve OS, S0500 demonstrated that lack of CTC response after only a single cycle of chemotherapy portends chemo-resistant MBC (18).
Based on the previously published pre-clinical and clinical data, we hypothesized that CTC doublets or clusters might identify relatively poorer prognosis in patients with MBC who participated in S0500 (18).
Material and methods
S0500 Conduct/Study design
This was a prospectively designed retrospective translational medicine study of S0500, for which the conduct and primary results have been previously reported (18). S0500 was conducted in accordance with the Declaration of Helsinki, and all applicable laws. All subjects provided written informed consent approved by local Institutional Review Boards. In S0500, Arm A included patients with <5 classic CTC/7.5 ml WB at baseline, whereas Arms B and C had ≥5 classic CTC/7.5 ml WB at baseline, but either had <5 classic CTC/7.5 ml WB (Arm B) or ≥5 classic CTC/7.5 ml WB (Arm C) after one cycle of single agent chemotherapy. A prospectively written study plan of this study was approved by the SWOG Breast Translational Medicine Working Group (Supplementary material).
Patient staging and follow-up
Details regarding patient eligibility, accrual, and overall conduct of the trial have been reported (18). As previously described, estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor 2 (HER2) status were determined locally by routine pathology at the treating institutions(18).
Blood draw, Isolation and enumeration of CTC
Blood draw, mailing, and processing for CTC using the CellSearch® System (Menarini, Silicon Biosystems, Inc.) has been previously described (18). If a patient had <5 classic CTC/7.5 ml WB (Arm A), no further blood draws were collected. Patients with ≥5 classic CTC/7.5 ml WB at baseline (Arm B/C) had additional blood draws after 1 cycle of chemotherapy (C2D1).
Re-analyses of S0500 Galleries
Classic versus revised algorithm
In the CellSearch® System, candidate fluorescence-generated images from each of the filters, plus a composite image, are arranged as thumbnail images in a gallery format for classification by reviewers (Figures S6A–B as examples). A CTC event is defined as DAPI positive, expresses cytokeratin, but CD45 negative (10, 15, 18, 20). In the “classic” algorithm for the FDA-approved CellSearch® System, thumbnail images are only counted as one CTC event, even if they contain several single cells or a doublet or cluster of cells (Fig. S1A–B).
In order to determine the presence or absence of CTC doublets or clusters, and to compare the incidence of these to absolute numbers of CTC/7.5 ml WB, we applied a “revised” CellSearch CTC enumeration algorithm, in which CTC enumeration was calculated by counting each individual CTC, or each CTC within a doublet or a cluster, if captured in one thumbnail image. Thus, a patient may have originally had only 1–4 CTC/7.5 ml WB, which is below the cutoff for positivity in the classic algorithm, and yet, in the revised algorithm, might have more CTC (Fig. S1A–B).
Doublet and Cluster Analysis
CTC clusters were defined as a group of CTC containing three or more distinct nuclei, and with contiguous cytoplasmic membranes, as previously described (6, 7, 10). However, in order to maximize our sensitivity, we included 2 cells containing 2 distinct nuclei with contiguous cytoplasmic membranes as a “doublet”. Examples of single CTC, CTC cluster, and CTC doublet are provided in Table S1.
Existing, archived CellSearch® images were re-read blindly by two operators (EMD or EPD) without knowledge of which arm the patient was assigned or clinical outcomes. To test inter-laboratory variability, images from 20 samples were read in parallel by Hayes laboratory (EMD; EPD), and Menarini Silicon Biosystems’ laboratory (MR).
Statistical analysis
Results were returned to SWOG Statistical Office for clinical associations. Patients were re-assigned to Arm A, B or C based on CTC values under the revised algorithm and analyses investigated the association between CTC enumeration, doublets, clusters and clinical outcomes (OS). Survival curves were estimated using Kaplan-Meier method, 95% confidence intervals for median (m) OS were calculated using the method of Brookmeyer and Crowley, and were compared by using log-rank test. Chi-square testing was performed to find the proportional differences among groups. All tests performed were two-sided.
Results
Patient characteristics
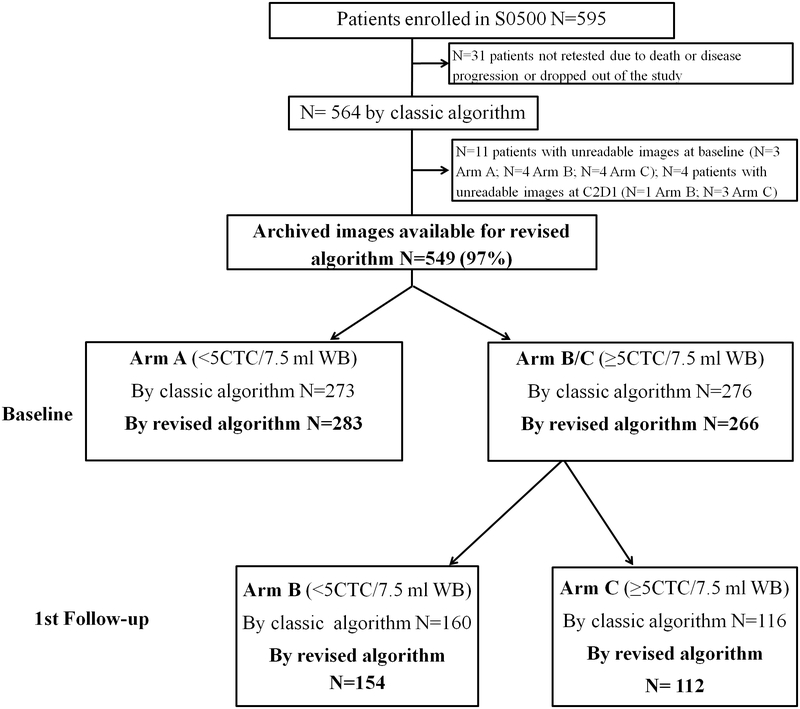
595 evaluable patients were enrolled in S0500 (18)(Fig. 1). Of these, 31 patients (10%) were not retested due to death, disease progression, or patient withdrawal before the second blood draw was due, accounting for a total of 564 patients. For this translational medicine study, using the revised algorithm, 15 patients had unreadable images (11 at baseline and four at C2D1); thus, 549/564 (97%) of the original enrolled patients were eligible (Fig. 1). Using the classic CellSearch® algorithm, 273 patients (50%) did not have increased CTC levels at baseline (Arm A) and 276 patients had ≥5 classic CTC/7.5 ml WB at baseline (Arm B/C). Patients in Arm B/C were then divided in Arms B (N=160) and Arm C (N=116) according to reduction of CTC to <5 or ≥5 classic CTC/7.5 ml WB at first follow-up, respectively.
Fig. 1.
REMARK diagram for doublet/clusters analysis of S0500. Of the 564 patients originally eligible for S0500, 549 had images stored and readable for analysis for doublets and clusters. When the CellSearch galleries were re-read, 10 patients originally assigned to Arms B/C were reassigned to Arm A due to revised total CTC <5 due to inter-laboratory variability, and likewise, 4 patients originally assigned to Arm C were assigned to Arm B due to revised total CTC <5 at 1st follow-up. Two patients originally assigned to Arm A were revised to ≥5 CTC/7.5 ml WB, but they could not be reassigned to Arm B or C due to the lack of assessments at first follow up and therefore, they were still assigned to Arm A (N=1 due to interlaboratory variability; N=1 due revised algorithm)(see text for details).
We used the revised CellSearch® algorithm to permit counting of all CTC, regardless of whether they were in one or several CellSearch® thumbnail images, and regardless of whether they are present singly, in doublets, or in clusters. 283/549 eligible patients (52%) were placed in Arm A. Ten patients originally in Arm B/C were deemed to have <5 CTC/7.5 ml WB upon re-reading, and were moved to Arm A. Two patients (0.4%) originally assigned to Arm A were determined to have ≥5 revised CTC/7.5 ml WB, either due to inter-laboratory reading variability (N=1) or to the revised algorithm (N=1). Since they only had baseline and not subsequent follow-up blood draw, they could not be reassigned to Arm B or C and were included in the revised Arm A. Of the 266 patients who had ≥5 revised CTC/7.5 ml WB at baseline (Arm B/C), 154 were assigned to Arm B and 112 were assigned to Arm C. Four patients from the original Arm C were moved to Arm B due to inter-laboratory reading variability. Overall, assignment of only 2.9% (16/549) of patients differed between the original and revised arm due to inter-laboratory variability (N=15) or due the revision of the algorithm (N=1).
Incidence of single CTC, doublet(s), and cluster(s).
For analysis of outcomes based on CTC-clusters, we divided the patients into five groups: 1) no clusters or doublets, 2) doublets only, 3) clusters only, 4) doublets and clusters, and 5) any doublets or clusters (sum of groups 2–4).
Baseline
The incidence of single CTC, CTC doublet(s) only, CTC cluster(s) only, CTC doublet(s) and cluster(s), and any CTC doublet(s) or cluster(s) across Arms A, B/C, B, and C are shown in Table 1A. Of those in Arm A, using the revised algorithm, 158/283 patients (56%) had zero CTC (Table 1A). Among 125 (44%) patients in Arm A with at least 1 CTC, four (1.4%) had CTC doublet(s) or cluster(s).
Table 1.
Incidence of single CTC, doublets(s), and cluster(s) at baseline
| Baseline | Single CTC (no Doublets or Clusters) | CTC with Doublets or Clusters present | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 CTC | 1–4 CTC | ≥5 CTC | Doublet(s) only | Cluster(s) only | Doublet(s) and Cluster(s) | Any Doublet(s) or Cluster(s) | ||
| All | N=549 | 158 (29%) | 120 (22%) | 167 (30%) | 58 (11%) | 11 (2%) | 35 (6%) | 104 (19%) |
| Arm A | N=283 | 158 (56%) | 120 (42%) | 1 (<1%) | 3 (1%) | 0 | 1 (<1%) | 4 (1.4%) |
| Arm B/C | N=266 | NA | NA | 166 (62%) | 55 (21%) | 11 (4%) | 34 (13%) | 100 (38%) |
| Arm B | N=154 | NA | NA | 115 (75%) | 20 (13%) | 4 (2%) | 15 (10%) | 39 (25%) |
| Arm C | N= 112 | NA | NA | 51 (46%) | 35 (31%) | 7 (6%) | 19 (17%) | 61 (54%) |
| Baseline | Single CTC (no Doublets nor Clusters) | CTC with Doublets or Clusters present | ||||||
| 0 CTC | 1–4 CTC | ≥5 CTC | Doublet(s) only | Cluster(s) only | Doublet(s) and Cluster(s) | Any Doublet(s) or Cluster(s) | ||
| All | N=545a | 157 (29%) | 120 (22%) | 164 (30%) | 58 (11%) | 11 (2%) | 35 (6%) | 104 (19%) |
| HR pos; HER2 neg | N=320 | 81 (25%) | 72 (22%) | 101 (32%) | 37 (12%) | 10 (3%) | 19 (6%) | 66 (21%) |
| HER2 pos | N=95 | 30 (32%) | 23 (24%) | 26 (27%) | 11 (12%) | 1 (1%) | 4 (4%) | 16 (17%) |
| Triple negative | N=130 | 46 (35%) | 25 (19%) | 37 (29%) | 10 (8%) | 0 | 12 (9%) | 22 (17%) |
| Baseline | Single CTC (no Doublets nor Clusters) | CTC with Doublets or Clusters present | ||||||
| 0 CTC | 1–4 CTC | ≥5 CTC | Doublet(s) only | Cluster(s) only | Doublet(s) and Cluster(s) | Any Doublet(s) or Cluster(s) | ||
| All | N=547a | 156 (29%) | 120 (22%) | 167 (30%) | 58 (11%) | 11 (2%) | 35 (6%) | 104 (19%) |
| Bone only | N=66 | 20 (30%) | 15 (22%) | 21 (32%) | 4 (6%) | 3 (5%) | 3 (5%) | 10 (15%) |
| Visceral | N=372 | 97 (26%) | 74 (20%) | 123 (33%) | 43 (11%) | 6 (2%) | 29 (8%) | 78 (21%) |
| Other | N=109 | 39 (36%) | 31 (28%) | 23 (21%) | 11 (10%) | 2 (2%) | 3 (3%) | 16 (15%) |
Two patients with missing data. Chi-square test for presence of doublets according sites of metastases: p=0.10 Chi-square test for presence of any doublets or clusters according to sites of metastases: p=0.24.
By definition, all patients in Arms B/C, had ≥5 single CTC/7.5 ml WB, at baseline. Of note, 100 patients (38%) had CTC doublet(s) or cluster(s) (Table 1A). In those ultimately assigned to Arm B, 39/154 (25%) patients had CTC doublet(s) or cluster(s) (Table 1A). In those ultimately assigned to Arm C, 61/112 (54%) patients had CTC doublet(s) or cluster(s) (Table 1A). Overall, we found that doublets(s) or cluster(s) or both were more likely to be present in those with ≥5 CTC/7.5 ml WB (Arm B/C) vs. <5 CTC/7.5 ml WB (Arm A) (38% vs. 1.4%; p<0.0001). Additional data are in Table S2A. Since the revised algorithm permits counting every cell within a single composite image regardless of whether it is single, in doublet or cluster, we examined the level of CTC as determined with the classic CellSearch® algorithm according to the presence or absence of doublets and clusters (Fig. S2). In this analysis, CTC doublets and cluster were more likely in specimens with higher CTC values (p<0.0001).
At baseline, there was no significant difference in the incidence of CTC doublet(s) and/or cluster(s) among different breast cancer subtypes. In particular, 21%, 17%, and 17% of patients with HR-positive/HER2-negative, HER2-positive, and triple negative had doublet(s) and/or cluster(s), respectively (p= 0.55) (Table 1B). Likewise, there was no significant difference in the incidence of CTC doublet(s) and/or cluster(s) between different sites of disease. Fifteen percent, 21%, and 15% of patients with bone only, visceral, and other disease sites had an incidence of any doublet(s) or cluster(s), respectively (p= 0.24) (Table 1C). More extensive data regarding CTC number and doublets and clusters by hormone receptor status and HER2 status of primary tumor as well as by disease site are further reported in Tables S2B and S2C.
First Follow-up
By definition, at first follow-up none of the 160 patients originally assigned to Arm B had ≥5 CTC/7.5 ml WB with the classic algorithm. Using the revised algorithm, 154 patients were assigned to the revised Arm B. Of these, at first follow-up, 89 (58%) had 0 and 65 (42%) had 1–4 single CTC/7.5 ml WB. None had any doublet(s) or clusters(s) in their first follow-up blood specimens (Table S3A).
At first follow-up, using the revised algorithm, 112 patients were assigned to the revised Arm C. Of these, at first follow-up blood draw, in total, 35 (31%) patients in Arm C had CTC doublet(s) or cluster(s)(Table S3A). There was a statistically significant difference in the incidence of CTC clusters at first follow-up between patients in Arm B vs. Arm C (Arm B: 0%; Arm C: 31%; p<0.0001).
The incidence of doublets or clusters was higher in patients who, using the classic algorithm, had ≥20 vs. 5–19 CTC/7.5 ml WB at baseline (≥20 CTC/7.5 ml WB=54%; 5–19 CTC/7.5 ml WB=9%; p<0.0001). Similar results were observed using the revised algorithm (≥20 revised CTC/7.5 ml WB =55%; 5–19 revised CTC/7.5 ml WB=10%; p<0.0001) (Table 2).
Table 2.
Correlation between CTC cluster(s) and/or doublet(s) in patients with 5–19 vs. ≥20 CTC/7.5 ml WB using the revised algorithm.
| Baseline | Single CTC (no Doublets or Clusters) | CTC with Doublets or Clusters present Any Doublet(s) or Cluster (s) | ||||
|---|---|---|---|---|---|---|
| ≥5 CTC | Doublet(s) only | Cluster(s) only | Doublet(s) and Cluster(s) | Any Doublet(s) or Cluster (s) | ||
| 5–19 CTC | N=103 | 93 (90%) | 5 (5%) | 2 (2%) | 3 (3%) | 10 (10%) |
| ≥20 CTC | N=165 | 74 (45%) | 50 (30%) | 9 (6%) | 32 (19%) | 91 (55%) |
p-value of presence of doublets or clusters comparing 5–19 vs. ≥20 CTC/7.5 ml WB: < 0.0001
There were significant differences in the incidence of CTC doublet(s) or cluster(s) among different breast cancer subtypes at first follow-up in Arm B and C. Fourteen and 19% of patients with HR-positive/HER2-negative or triple negative disease, respectively, had doublets or clusters (p =0.31). However, only eight patients with HER2-positive disease had ≥5 CTC/7.5 ml WB at first follow-up, and none of them had CTC clusters or doublets (p=0.005 comparing HER2+ to other subsets) (Table S3B). There was no significant difference in the incidence of CTC doublet(s) and/or cluster(s) between different sites of disease. Twelve percent, 13%, and 14% of patients with bone only, visceral, and other had doublet(s) or cluster(s), respectively (p= 0.95) (Table S3C).
Outcomes According to Presence or Absence of Doublets or Clusters
Benefit from randomization
We explored whether OS for patients in Arm C differed according to clusters or not between those who were assigned to stay on the original chemotherapy (C1) vs. those who were assigned to switch to an alternative chemotherapy regimen (C2). For those without cluster(s) at first follow-up, we observed no difference in OS between Arm C1 and C2 (mOS: C1 = 13.3 months; C2 = 12.5 months; p=0.58) (Fig. S3A). For patients with cluster(s), there was a non-significant trend (mOS: C1 = 3.5 months; C2 = 11.0 months; p=0.49) (Fig. S3B). In addition, for those without clusters and doublets at first follow-up, we observed no difference in OS between Arm C1 and C2 (p=0.78). Similar results were found for patients with doublets and clusters (p=0.63) (Fig. S3C–D).
CTC-clusters and Overall Survival
Since S0500 and this study differ by only 14/549 patients (2.6%), the study population is almost identical to the original for the primary endpoint: OS [original cohort: mOS Arm A, B, and C=34.8, 22.9, and 13.1 months, respectively; revised CTC cohort: mOS Arm A, B, and C=34.2 (95% CI, 29.0 to 37.3), 22.9 (95% CI, 18.6 to 28.1), and 12.4 (95% CI, 9.1 to 14.1) months, respectively](18).
Baseline
Arm A:
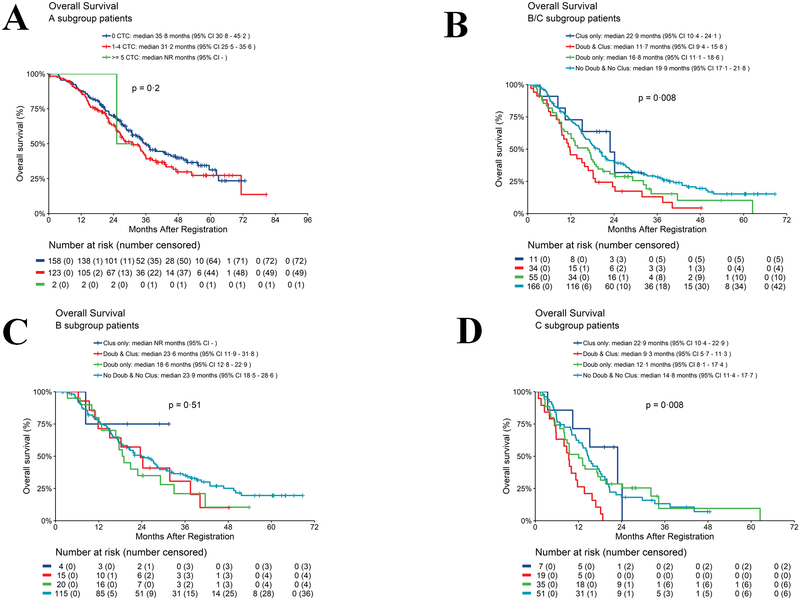
There was a non statistically significant trend towards longer OS for patient with 0 vs. 1–4 CTC/7.5 ml WB (mOS: 0 CTC/7.5 ml WB =35.8 months; 1–4 CTC/7.5 ml WB =31.2 months; p=0.20) (Fig. 2A). Only three patients had clusters so no statistically meaningful conclusions could be drawn.
Fig. 2.
Overall survival according to CTC enumeration or doublets and clusters at baseline. 2A. Arm A: OS for patients with 0 vs 1–4 CTC/7.5 ml WB using revised algorithm. 2B. Arm B/C: OS according to presence or absence of clusters. 2C. Arm B: OS according to presence or absence of clusters. 2D. Arm C: OS according to presence or absence of clusters (See text for definition of Arm A, B, and C).
Arm B/C:
In Arm B/C, patients with doublets and clusters at baseline had statistically significantly worse OS (11.7 months) compared to doublets only (16.8 months) or clusters only (22.9 months) or no doublets or clusters (19.9 months) (p=0.008 comparing the 4 groups) (Fig. 2B). Pairwise comparison between no doublets and no clusters vs. doublets and clusters was highly statistically significant (p=0.002).
In patients ultimately assigned to Arm B, there was no statistically significant difference in OS between patients who had doublets and clusters (23.6 months) compared to patients with only doublets (18.6 months) or patients with no doublets or clusters (23.9 months) (p=0.51 comparing the 4 groups) (Fig. 2C). The group with clusters only was too small to conduct any analysis. In Arm C, patients with doublets and clusters had statistically significantly worse OS (9.3 months) compared to those with only doublets (12.1 months) or only clusters (22.9 months) or no doublets or clusters (14.8 months) (p=0.008 comparing the 4 groups) (Fig. 2D). Pairwise comparisons between doublets and clusters vs. doublets only, clusters only, and no doublets or clusters was highly statistically significant (p=0.02, p=0.006, and p=0.0008, respectively). See Fig. S4–6 for additional KM curves of OS.
First Follow-up
Arm B:
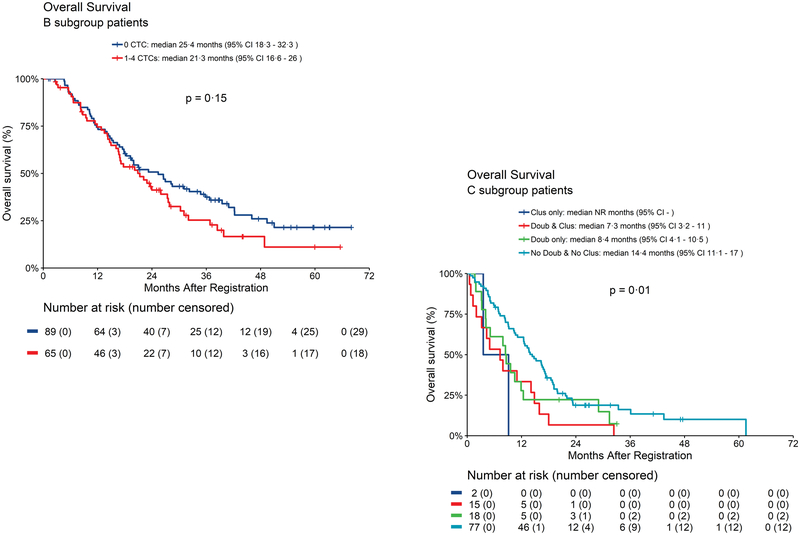
There was a non statistically significant difference in OS between patients with 0 vs. those with 1–4 CTC/7.5 ml WB at first follow-up (mOS: 0 CTC/7.5 ml WB =25.4 months; 1–4 CTC/7.5 ml WB =21.3 months; p=0.15) (Fig. 3A). No patients in Arm B had doublets or clusters at first follow-up.
Fig. 3.
Overall survival according to CTC enumeration or doublets and clusters at first follow-up. 3A. Arm B: OS for patients with 0 vs 1–4 CTC/7.5 ml WB using revised algorithm. 3B. Arm C: OS according to presence or absence of clusters (See text for definition of Arm A, B, and C).
Arm C:
Patients with doublets and clusters at first follow-up had statistically significantly worse OS (7.3 months) compared with those with only doublets (8.4 months) or no doublets or clusters (14.4 months) (p=0.01) (Fig. 3B). Pairwise comparison between no doublets or clusters vs. doublets and clusters was highly statistically significant (p=0.004). Additional KM curves are shown in Fig. S7A–C.
Prognosis According to Number of CTC vs. CTC Clusters
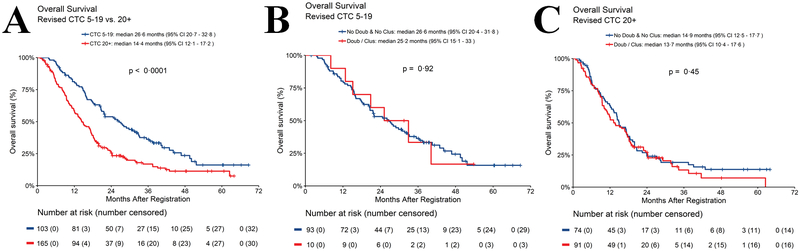
We hypothesized that perhaps the worse OS observed with clusters was due to the presence of more CTC/7.5 ml WB, in clusters or not, since the number of CTC would be increased by virtue of the revised algorithm. OS was longer in patients who had 5–19 vs. ≥20 CTC/7.5 ml WB at baseline, regardless of which algorithm was used (classic: mOS in patients with 5–19 CTC/7.5 ml WB =24.6 months; ≥20 CTC/7.5 ml WB =14.2 months; p<0.0001; revised: mOS in patients with 5–19 CTC/7.5 ml WB =26.6 months; ≥20 CTC/7.5 ml WB =14.4 months; p<0.0001) (Fig. 4A; Table S4). However, within each of these subgroups using the revised algorithm, mOS was not statistically different for presence of clusters and doublets compared to no clusters or doublets (revised: mOS in patients with 5–19 CTC/7.5 ml WB with doublets or clusters=25.2 months; without doublets or clusters=26.6 months; p=0.92; Fig. 4B); (revised: mOS in patients with ≥20 CTC/7.5 ml WB with doublets or clusters=13.7 months; without doublets or clusters=14.9 months; p=0.45; Fig. 4C). Taken together, these data suggest the worse prognosis in patients with clusters may be a function of the number of CTC/7.5 ml WB, which is increased with clusters using the revised algorithm.
Fig. 4.
Overall survival according to CTC enumeration in patients with 5–19 vs ≥20 CTC/7.5 ml WB at baseline using the revised algorithm. 4A. OS for patients with 5–19 vs. ≥20 CTC/7.5 ml WB; 4B. OS of patients with 5–19 CTC/7.5 ml WB divided by doublets or clusters vs. no doublets or clusters; 4C. OS of patients with ≥20 CTC/7.5 ml WB divided by doublets or clusters vs no doublets or cluster.
Discussion
In this prospective-retrospective translational medicine study, we observed that using the CellSearch® system, CTC-doublet(s) and cluster(s) were commonly identified in patients with MBC starting first line chemotherapy and who had elevated CTC at baseline. As expected, CTC clusters were associated with a worse prognosis, regardless of whether they were detected at baseline or at first follow-up.
However, unexpectedly, CTC clusters were not independent of the number of CTC enumerated using the revised CellSearch® algorithm. Rather, the negative association of clusters with OS was likely due to the number of CTC present, which is increased by the high presence of doublets or clusters using the revised algorithm, since the latter permits counting every cell within a single thumbnail composite image. Even though patients with ≥20 CTC/7.5 ml WB at baseline had a shorter OS than those with 5–19 CTC/7.5 ml WB (p<0.0001), when we revisited the importance of doublets and clusters within these two groups there was no difference in outcomes according to the presence of doublets or clusters. Even though CTC counts are higher by using the revised algorithm, the observation that doublets and clusters are more common in specimens with high CTC counts using the classic algorithm (Fig. S2) further suggests that it is the number of cells, regardless of whether they are singlets or in clusters, that drives a worse prognosis in MBC patients starting first line chemotherapy. Our results are consistent with a recently reported study that suggested that CTC clusters were not significantly prognostic when examined in patients with MBC who had the cutoff of ≥20 CTC/7.5 ml WB (21).
Our results are dissimilar with those of other investigators, who have suggested that, in contrast to individual CTC, CTC clusters in breast as well as lung cancers have distinct biological properties and mediate the metastatic process (2, 7, 11). These discrepancies could be due to the the use of different assays. One concern might be that the CellSearch® system is not ideal for identifying or enumerating doublets or clusters, since the blood specimens are subjected to multiple manipulations prior to CTC analyses. The CellSearch® platform might potentially disrupt CTC doublets and clusters, underestimating their biological/prognostic effect compared to other assays. However, the incidence of CTC doublets and clusters in MBC in our study (~19%) is similar to that previously reported in MBC detected either by the CellSearch® (10–12) or other assays (2, 22). Similar to other studies, we found that CTC doublets and/or clusters were more likely to be associated with ≥5 CTC/7.5 ml WB (10, 11). However, new technologies specifically designed to isolate CTC clusters have been developed (23–25) and might provide further insight into this issue.
Another concern is that other investigators have reported that CTC clusters are prognostic when identified in longitudinally sampled specimens (12, 21). Since our study focuses on CTC enumeration and evaluation of CTC doublets and clusters at baseline and first follow-up, we cannot exclude that longitudinal evaluation of CTC doublets and clusters could provide additional prognostic information for patients with MBC.
Our study was exclusively conducted in patients with MBC starting first line chemotherapy (18). It is possible that clusters are important biologically in different types of breast cancer, such as inflammatory breast cancer (11) or in completely different cancers, such as small cell lung cancer (7). Whereas it is possible that clusters do indeed play a critical role in inflammatory breast and small cell lung cancer, we are confident from this blinded prospective retrospective study that CTC clusters do not provide substantial prognostic information over CTC enumeration, either at baseline or first follow-up, in patients starting first line chemotherapy for MBC.
Other investigators have reported that heterotypic clusters consisting of CTC and leukocytes might be more prognostic than homotypic clusters of CTC alone (3, 4). However, the CellSearch® system is not designed to analyze heterotypic clusters, and therefore we cannot address this issue.
Finally, patients enrolled in S0500 might not have been typical or representative of other studies. However, S0500 was a pragmatic trial, in which patients with MBC regardless of intrinsic subtype (ER and HER2 status) were enrolled, and the treatment was the choice of standard of care single agent chemotherapy at the treating doctor’s discretion. The incidence of having elevated CTC (≥5/7.5 ml WB) at baseline in S0500 was approximately 50%, in line with several other studies of CTC in patients with MBC (15). Moreover, there was no difference in the incidence of CTC doublets or clusters according to clinical or pathological subtypes or site of disease (Table 1B and 1C). Indeed, a strength of this study is that it was conducted using images from 97% of participants in the S0500 clinical trial (N=595). Pre-specified definitions of clusters and doublets were used to reread the images without knowledge of clinical outcomes and clinical correlation was conducted in the SWOG statistical center.
Nonetheless, although we interrogated data from 97% of the more than 500 patients enrolled in S0500, subgroup size limits the power of our analyses. However, results obtained in Figure 4B and C, suggest that it is very unlikely that our results would be substantially different with larger numbers. In addition, although all the patients entered in the trial had received single agent first line chemotherapy, chemotherapy regimens were not assigned, rather they were chosen by the treating physician limiting the power to attempt subgroup analyses on the effect of doublets and clusters versus not according to treatment type.
Taken together, these considerations suggest that S0500 is very representative of the average patient starting first line chemotherapy in MBC and an ideal setting in which to test the biological and clinical role of CTC-clusters.
Perhaps the only suggestion that doublets or clusters might have some biological or clinical effect was raised by the observation that they were present more frequently at baseline in patients ultimately assigned to Arm C compared to Arm B. By definition, Arm B represents patients who had a “CTC response”, while Arm C contains those who did not. Taken together, these data might suggest that CTC doublets or clusters mediate resistance to first line chemotherapy in MBC. However, within Arm C, there was no statistically significant difference in OS between patients with or without doublets or clusters for those assigned to stay on initial chemotherapy (C1) vs. switching to an alternative chemotherapy (C2).
In a series of secondary analyses, we also interrogated whether the classic cutoff (≥5 CTC/7.5 ml WB) chosen for determination of elevated vs. not elevated CTC in prior studies is optimal (15). As noted, the prognosis for patients in Arm A (those with <5 CTC/7.5 ml WB), was quite favorable using the classic algorithm, with a median OS = 36 months(18). In this regard, OS using the revised algorithm was essentially no different for patients in Arm A who had 0 vs. 1–4 CTC/7.5 ml WB. Further, there was no difference in OS in Arm B at first follow-up between patients with 0 CTC/7.5 ml WB vs. 1–4 CTC/7.5 ml WB (mOS 0 CTC/7.5 ml WB = 25.4 months; 1–4 CTC/7.5 ml WB = 21.3 months p=0.15). These data further validate the cut-off of ≥5 CTC/7.5 ml WB as the correct absolute cut-off for CellSearch® assay in MBC(15). Incidentally, OS did not differ according to presence or absence of doublets or clusters in Arm A at baseline, or in Arm B at first follow-up, although the incidence of doublets or clusters in these two subgroups was quite low.
In summary, the results of this translational medicine study suggest that neither doublets nor clusters plays a major role in the outcome of MBC patients starting first line chemotherapy, raising questions regarding their role in progression of metastatic disease. Rather, our data suggest that absolute number of CTC may be more important than the physical presence of clusters. Therefore, analysis of CTC doublets or clusters is unlikely to provide additional information to direct patient care in standard or investigational clinical settings in patients with MBC starting first line chemotherapy. Additional trials in non-metastatic breast cancer patients, or using different CTC enrichment assays specifically designed to capture clusters, warrant further investigation. In addition, our data confirm the classic clinical CellSearch® algorithm and cut-off of ≥5 CTC/7.5 ml WB, as determined in prior studies, is appropriate.
Supplementary Material
Translational Relevance.
Several assays have been developed to enumerate circulating tumor cells (CTC). Elevated CTC levels portend a poor prognosis in several cancers including metastatic and early stage breast cancer. Recent studies have suggested that, beyond elevated numbers of CTC, CTC clusters may be a driving force in the metastatic process. In this work, we tested the prognostic significance of CTC clusters compared to elevated CTC alone within the SWOG0500 clinical trial. Our findings suggest that the presence of doublets or clusters contributed little, if any, added prognostic information beyond the absolute number of CTC. We conclude that cluster evaluation in patients with MBC starting 1st line chemotherapy has little or no clinical significance and it is unlikely to provide additional information to direct patient care in standard or investigational clinical settings. Additional trials in non-metastatic patients, or using different CTC enrichment assays specifically designed to capture clusters, warrant further investigation.
Financial Support:
This work was supported by the National Institutes of Health (NIH) (Award: CA180888, CA180819, CA180801, CA180858); and by Immunicon, Veridex, and the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale ™ (D.F.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest: C.P. received travel paid by Menarini Silicon Biosystems, the maker of CellSearch, the system used to capture and enumerate CTC and research funding from AstraZeneca, Pfizer, outside the submitted work. M.R. is an employee of MSB and has patents US 8,940,493 B2 and US 7,863,012 B2 issued. J.R.G. is a steering committee, data safety monitoring committee (DSMC) member for Roche/Genentech, DSMC member for Merck, Novartis, Immunomedics, advisory board for AstraZeneca, Puma, Pfizer, consultant for Sandoz/Hexal AG and Genomic Health, outside the submitted work. D.F.H. reports grants and research support from MSB, during the conduct of the study; D.F.H has patent US 8,790,878 B2. D.H.F. is designated as inventor/co-inventor with royalties paid to MSB. Stock options from OncImmune LLC, InBiomotion, advisory board for Cepheid, research funding from Merrimack, Eli Lilly, Puma Biotechnology, Pfizer, AstraZeneca, outside the submitted work; The rest of the co-authors have nothing to disclose.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–7. [DOI] [PubMed] [Google Scholar]
- 5.Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell. 2019;176:98–112 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–32. [DOI] [PubMed] [Google Scholar]
- 7.Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kats-Ugurlu G, Roodink I, de Weijert M, Tiemessen D, Maass C, Verrijp K, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009;219:287–93. [DOI] [PubMed] [Google Scholar]
- 9.Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7:4080–5. [PubMed] [Google Scholar]
- 10.Paoletti C, Li Y, Muniz MC, Kidwell KM, Aung K, Thomas DG, et al. Significance of Circulating Tumor Cells in metastatic triple negative breast cancer patients within a randomized, phase II trial: TBCRC 019. Clin Cancer Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu Z, Wang C, Ye Z, Austin L, Civan J, Hyslop T, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat. 2015;154:563–71. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat. 2017;161:83–94. [DOI] [PubMed] [Google Scholar]
- 13.Paoletti C, Hayes DF. Circulating Tumor Cells. Adv Exp Med Biol. 2016;882:235–58. [DOI] [PubMed] [Google Scholar]
- 14.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. [DOI] [PubMed] [Google Scholar]
- 18.Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating Tumor Cells and Response to Chemotherapy in Metastatic Breast Cancer: SWOG S0500. J Clin Oncol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–63. [DOI] [PubMed] [Google Scholar]
- 20.Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14. [DOI] [PubMed] [Google Scholar]
- 21.Larsson AM, Jansson S, Bendahl PO, Levin Tykjaer Jorgensen C, Loman N, Graffman C, et al. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu Z, Benali-Furet N, Uzan G, Znaty A, Ye Z, Paolillo C, et al. Detection and Characterization of Circulating Tumor Associated Cells in Metastatic Breast Cancer. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods. 2015;12:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au SH, Edd J, Stoddard AE, Wong KHK, Fachin F, Maheswaran S, et al. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci Rep. 2017;7:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SB, Xie M, Chen Y, Xiong J, Liu Y, Chen Z, et al. Three-Dimensional Scaffold Chip with Thermosensitive Coating for Capture and Reversible Release of Individual and Cluster of Circulating Tumor Cells. Anal Chem. 2017;89:7924–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.