Abstract
Pancreatic ductal adenocarcinoma (PDAC) is associated with significant fibrosis. Recent findings have highlighted the pro-fibrotic activity of tissue-resident macrophages in the pancreatic cancer microenvironment. Here, we showed that neoplastic pancreatic epithelium, as well as a subset of tissue-resident macrophages, express the prolactin receptor (PRLR). HMGB1-induced prolactin expression in the pancreas maintained FAK1 and STAT3 phosphorylation within the epithelium and stroma. Gain-of-function and loss-of-function experiments demonstrated the essential role of prolactin in promoting collagen deposition, and fibrosis. Finally, the signaling cascade downstream of Prolactin/PRLR activated STAT3 rather than STAT5 in PDAC. These findings suggest that targeting prolactin together with IL-6, a known major activator of STAT3, could represent a novel therapeutic strategy for treating pancreatic cancer.
Introduction
PDAC is an almost uniformly fatal disease, arising from mutant KRAS-expressing precursors following a prolonged period of local inflammation and stress (1). A characteristic feature of PDAC is its intense stromal content, accounting for up to 90% of the tumor volume, and is composed of cancer associated fibroblasts, pancreatic stellate cells, vascular endothelium, and immune cells, including tumor-associated macrophages (TAMs), one of the most abundant myeloid-derived cell types in the stroma (2).
Macrophages are monocyte-derived myeloid cells that develop from a common myeloid progenitor cell residing within the bone marrow (BM) (3). Upon tissue damage or in pathological conditions, inflammatory monocytes are recruited across the endothelium, where they differentiate into macrophages (3). Another subset of macrophages, called tissue resident macrophages, originally derived from primitive hematopoiesis in the embryonic yolk sac are deposited early in development (4). These embryonic-derived resident macrophages persist into adulthood, and self-maintain independently of the bone marrow (4, 5). Recently, it was reported that in addition to the BM-derived macrophages, embryonic-derived tissue-resident macrophages contributed significantly to the TAM population in PDAC (6). This newly identified TAM sub-population can expand in situ, displaying a pro-fibrotic expression profile and facilitating PDAC progression (6). Macrophages exist across an M1–M2 polarization state, in which M1 cells initiate and sustain inflammation through production of high levels of pro-inflammatory cytokines, reactive nitrogen species and oxygen intermediates, while the more heterogeneous M2 cells are characterized by alternative mechanisms of arginine metabolism, exhibit a different chemokine expression profile, and are associated with wound healing or smoldering chronic inflammation (7).
Expansion and progression of noninvasive pancreatic precursor lesions, as well as pancreatic cancer, relies heavily on soluble factors produced by individual cell populations within the stroma. Together these factors exert effects on a wide range of cell types (8). For instance, while IL-6, IL-17, TGF-β and Notch pathway ligands primarily influence the malignant epithelium, other factors such as GM-CSF are implicated in the recruitment and expansion of both immunosuppressive elements as well as antigen cross-presenting dendritic cells (9–14). The stroma also displays an abundant and rigid extra cellular matrix (ECM), which promotes increases in tumor interstitial fluid, induces vascular compression and impedes access of therapeutic agents (15, 16). Depleting stroma could in theory enhance drug delivery while disrupting stroma-cancer cell interactions (13–15, 17, 18). However, targeting stroma alone may actually favor a more aggressive form of PDAC (17, 18), thus arguing for therapeutic approaches that specifically target the cancer cells.
Prolactin (PRL) is a polypeptide hormone that is predominantly synthesized in, and secreted from, lactotroph cells of the anterior pituitary gland (19). Although it is best known as the hormone that elicits lactation in mammals, it is present in males and females in all vertebrates and in evolutionary terms, is an ancient hormone (19, 20). Prolactin has many functions that extend beyond reproduction and lactation, with approximately 300 identifiable biological actions in vertebrates (21). Binding of prolactin to its membrane-associated receptor, the prolactin-receptor (PRLR), induces several intracellular oncogenic signaling cascades including JAK/STAT signaling that stimulate proliferation of cancer cells and promote tumor growth (19, 21, 22). Although PRL-induced JAK2/STAT5 activation has been considered the hallmark of PRLR signaling, multiple intracellular kinases, such as Src-Family Kinases (SFKs), focal adhesion kinase (FAK), mitogen activated protein kinase (MAPK), and PI3K are also induced by PRL (22, 23). Altered JAK/STAT activity in pancreatic cancer cells stimulates cell proliferation and malignant transformation, inhibits apoptosis, and may be responsible for promoting resistance to therapy (24). Mounting evidence suggests that blocking JAK/STAT signaling pathways inhibits pancreatic cancer growth (25–27). Nevertheless, despite the oncogenic activity of the PRL/PRLR axis in general, and the involvement of JAK/STAT in PDAC in particular, the role of PRL and its cognate receptor in pancreatic cancer remains poorly defined.
In this study we investigated the role of PRL/PRLR axis in pancreatic intraepithelial neoplasia (PanIN) and PDAC progression to determine the mechanism by which PRL promotes pancreatic tumorigenesis, and to identify the source of prolactin within the tumor microenvironment.
Material and Methods
Human tissues
Human pancreatic adenocarcinoma specimens were collected from patients who underwent surgical procedure. The studies were conducted in accordance with the ethical guidelines of the Declaration of Helsinki and were approved by the University of Pittsburgh Institutional Review Board. All surgically removed pancreatic tissues were collected in accordance with the University of Pittsburgh Institutional Review Board-approved protocols and used for analysis under written informed consent from the patients.
Mice
Mice used in these studies were maintained according to protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The Ptf1aCre (28), KrasG12D (29), and the prolactin−/− strains (30) were obtained from the Mutant Mouse Regional resource Centers, National Cancer Institute Repository, and the Jackson laboratories, respectively.
Tissue processing and immunostaining
Tissue processing and immunostaining were conducted as previously described (31). Images were acquired on a Zeiss Imager Z1 microscope with a Zeiss AxioCam driven by Zeiss AxioVision Rel.4.7 software (Zeiss, Thornwood, NY). The antibodies used in this study are listed in Supplementary Table 1.
Macrophage isolation:
Generation of BMD macrophages as well as isolation of primary pancreatic macrophages were performed as described previously (32).
Prolactin treatment:
Recombinant human prolactin was obtained from R&D Systems Inc. (Minneapolis, MN). Cells were serum-starved overnight and then cultured with 10nM hrPRL for up to 60 minutes.
Metoclopramide treatment:
Mice were SC-injected once a day with 100 μg Metoclopramide for 3 weeks, as described elsewhere (33).
Cell lines:
PANC-1, and BxPC-3 cells were purchased from ATCC (Manassas, VA) in 2015, 2017 and 2019. Cell identity was verified by ATCC. Cells used for the experimental study were passaged within 10 to 20 passages after reviving from the frozen vials. Cell lines were screened at early and late passages for Mycoplasma. PANC-1 cells were cultured in a complete DMEM media (ATCC, Manassas, VA), BxPC-3 cells were cultured in RPMI 1640 media (ATCC, Manassas, VA), containing 10% FBS and 1% penicillin-streptomycin solution.
sh-PRLR Lentiviral Gene Delivery:
PRLR expression was silenced with commercially obtained shRNA- containing lentiviral particles (Origene, Rockville MD). Control shRNA (TR30021V), shRNA targeting PRLR (TL310169V) consisting of a four expression constructs each encoding target-specific 29 nt (plus hairpin) shRNA. The constructs were introduced into the cells by lentiviral transduction (200 μl of lentiviral shRNA supernatant), supplemented with 8 μg/ml Polybrene (Sigma-Aldrich). Cells were then replenished with fresh medium with 20nM PRL.
Cell Proliferation Assay
Cells were seeded in 96-wells plates at a density of 1×102 cells in 100 μl per well. The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was used as follows. Cells were allowed to grow for 72 hrs at 37°C and then mixed 1:1 with CellTiter-Glo reagent in a clear bottom white 96-wells plate and incubated in dark for 5 min to stabilize the signal. The luciferase signal, which is directly proportional to the amount of metabolically active cells in the sample, was measured on a Glomax luminometer (Promega).
Statistical analysis
Results in bar graphs are expressed as mean values ± SEM. The number of independently performed experiments is mentioned in the respective figure legends. To compare the experimental groups, Student’s t-test or one-way ANOVA followed by Bonferroni’s correction was performed using GraphPad Prism 5 software (Graphpad Software Inc., La Jolla, CA, USA). Differences with p<0.05 were considered statistically significant.
Results
Human PanINs and PDACs express PRLR:
Given the oncogenic nature of the PRL/PRLR axis, we first studied the expression of PRLR in specimens obtained from pancreatic cancer patients undergoing operative extirpation. PRLR could not be detected in normal human pancreas (Fig. 1A), or in early neoplastic lesions, however it was expressed in high grade PanINs (Fig. 1B). PRLR was expressed in all invasive cancer samples studied (15/15), although the extent of its expression by cancer cells varied within the cohort (Fig. 1C, D). A recent integrated genomic analysis has categorized PDAC into four distinct classes, namely aberrantly differentiated endocrine exocrine (ADEX), pancreatic progenitor-like, immunogenic, and squamous (34). Among these classes, the squamous sub-type is an independent prognostic factor and is associated with poor outcomes (34). We found lower PRLR expression levels in the squamous class compared to the other sub-types (Fig. 1E). Accordingly, patients with low PRLR expression survived less than patients with higher PRLR expression (Fig. 1F). Interestingly, closer examination revealed that in addition to the malignant epithelium, PRLR is expressed in a subset of stromal cells (Fig. 1G). Similarly, PRLR could be detected in mPanINs and a subset of surrounding stromal cells as well as PDACs in Ptf1aCre;KrasG12D mice (abbreviated KC mice) (Fig. 1H, I). The presence of PRLR in both PanINs and PDAC suggests that PRL/PRLR axis may play a role in the disease progression.
Figure 1: PRLR is expressed in human PDAC.
(A-D) Immunostaining against PRLR on tissues obtained from pancreatic cancer patients showed no PRLR expression in the normal pancreatic parenchyma (A), whereas it was expressed in high grade PanINs (B). PRLR could be expressed heterogeneously (C) or uniformly (D) in PDAC cells. Arrows in (C) highlight PRLR-negative PDAC cells. (E) Boxplot of PRLR gene expression stratified by class based on the study by Bailey et al. (34). (F) Kaplan-Meier analysis comparing survival of patients having either high or low PRLR expression (n=54, Median survival 27 vs 17 months). (G) PRLR was also expressed in subset of stromal cells (arrowheads). (H, I) Similar to their human counterparts, mouse PanIN (H) and PDAC (I) cells expressed PRLR. Arrows in (H) show a subset of PRLR-positive stromal cells in KC mice. N:normal pancreas, asterisks highlight early PanINs. Bars 20μm.
Prolactin promotes STAT3 activation but not STAT5:
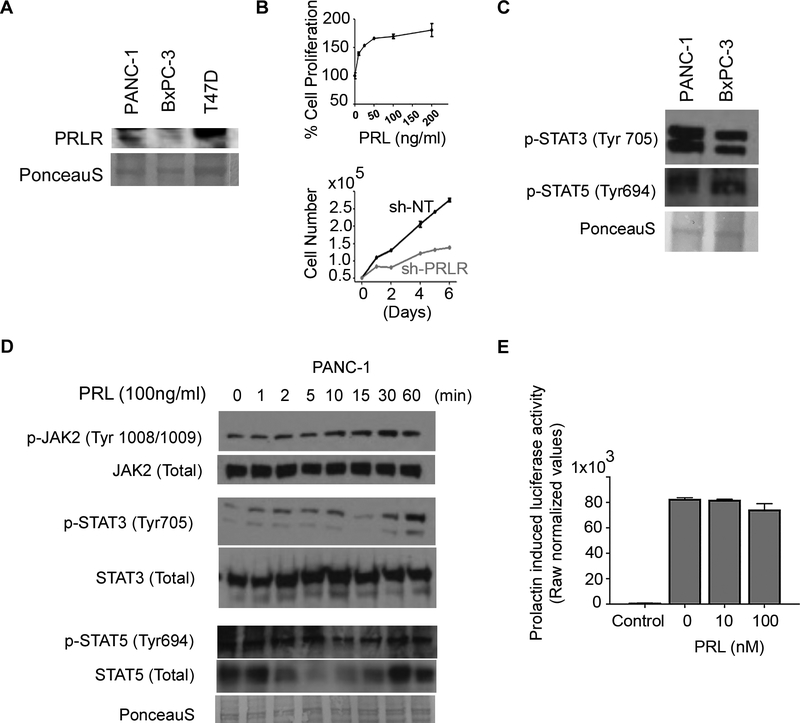
As the first step to study the PRL/PRLR axis in vitro, we examined two different pancreatic carcinoma cell lines, which either expressed PRLR (PANC-1), or lacked PRLR expression (BxPC-3) (Fig. 2A). Moreover, we found that activation or inactivation of the pathway by recombinant prolactin or sh-RNA targeting PRLR in PANNC-1 cells resulted in increase or decline in cell proliferation, respectively (Fig. 2B). Furthermore, regardless of PRLR expression, known PRLR downstream signaling molecules STAT3, and STAT5 (23) were phosphorylated in both cell lines (Fig. 2C). Next, we studied the ability of PRL/PRLR intracellular signaling through activation of JAK2 signaling cascade. Stimulation of PANC-1 cells with human recombinant prolactin resulted in a time-dependent increase in pJAK2, and pSTAT3, whereas surprisingly the level of pSTAT5 remined unchanged (Fig. 2D). To further confirm this finding, we transfected Panc-1 cells with a 6xSTAT5-Luc reporter construct (35), and examined the prolactin induced STAT5 transcriptional activity. As shown in figure 2E, prolactin treatment did not increase 6xSTAT5-Luc promoter activity, indicating that STAT5 activation in human pancreatic cancer cells is not dependent on PRL/PRLR signaling. Noteworthy, we could not detect any changes in p-STAT3 or p-STAT5 levels in BxPC3 cells following prolactin treatment (Supplemental Fig. S1). These results confirm the data presented in figure 1 in that pancreatic cancer cells express PRLR in a heterogenous manner. Moreover, the PRL/PRLR axis activates STAT3 through a JAK2 signaling cascade. Finally, phosphorylation of STAT5 in these cells is likely to be independent of PRL/PRLR pathway.
Figure 2: PRL promotes STAT3.
(A) PRLR is detected in PANC-1 but not in BxPC-3 pancreatic cell lines. Lysates from T47D breast cancer cell line was used as positive control for PRLR detection. (B) Prolactin can stimulate proliferation while silencing of PRLR even in the presence of prolactin leads to a decline in the number of cells. Schematic illustration of known oncogenic pathways activated by PRL/PRLR axis. (C) Both STAT3 and STAT5 are activated in the PDAC cell lines. (D) Western blot analyses of Serum-starved PANC-1 cells that had been cultured with 100ng/ml hrPRL for up to 60 minutes. (E) Luciferase assay on PANC-1 cells transfected with STAT5 reporter and stimulated with the increasing amount of rhPRL as indicated.
Pregnancy and prolactin accelerate PanIN progression in mice:
As shown in figure 1, in addition to being present in PDAC cells, PRLR could be detected in PanINs, and also in the surrounding stromal cells. Pregnancy and lactation are two conditions associated with high systemic prolactin levels. To test the ability of PanINs to respond to prolactin, 6–8 week old KC females were mated and subsequently sacrificed three weeks post-partum (at the time of weaning). This approach exposed PanINs to high levels of prolactin for almost 6 weeks, which resulted in an intense stromal response, as well as a higher incidence of both acinar-to-ductal metaplasia (ADM) and PanIN1 lesions, although still in the context of intact lobule architecture (Fig. 3A–C).
Figure 3: Prolactin can promote PanIN progression:
(A-G) Tissues obtained from 3 (A-C) or 4 month old KC mice (D-G) without (A, D) or with one previous pregnancy (B, E) or two pregnancies (F) were histologically analyzed. Higher ADM and early PanIN incidences were observed in mice with previous pregnancy (C, G). (H) ELISA analysis for serum prolactin in control KC males or KC males treated with metoclopramide confirmed that metoclopramide treated mice have increased blood prolactin levels. (I-K) 4 week old KC mice were treated with saline (I) or metoclopramide (J) for three weeks. The mice were sacrificed immediately after treatment. Higher systemic prolactin levels appeared to accelerate the progression of PanINs (K). (C, G, K,) Columns, percentages of normal ducts (ND), metaplastic ducts (ADM), and PanINs by grade per total ductal structures in different conditions. (n=3–5 mice per condition). Results in (C, G, K) are expressed as mean ± standard error of the mean (SEM) and analyzed statistically by Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Bars 20μm.
Next, 6–8 week-old KC females were again mated but, instead they were sacrificed either one month post-weaning, or alternatively they were subjected to a second round of pregnancy and lactation (Fig. 3D–G). Here, the KC dams were all sacrificed at 16–17 weeks of age. Compared to control 4 month-old unmated KC mice (Figure 3D), both experimental cohorts showed a significant decrease in the number of normal ducts, and an increase in PanIN1 lesions (Fig. 3E–G). Furthermore, a robust desmoplastic response, secondary loss of the lobular acinar parenchyma, as well as increased duct ectasia could be observed in these mice (Fig. 3E). Mice with two back-to-back pregnancies also showed significant higher numbers of ADMs and PanIN2s (Fig. 3F, G).
Pregnancy is associated with numerous hormonal changes, thus the observed acceleration in PanIN progression as the result of pregnancy and lactation could be the combined effect of many hormones. Prolactin secretion is inhibited by dopamine, therefore metoclopramide, a dopamine antagonist, has been used to stimulate prolactin secretion (19, 33). To exclude the potential influence of other hormones, we next studied PanIN progression in response to metoclopramide treatment. To do so, 4 week-old male KC mice were treated with metoclopramide for three weeks as described elsewhere (33), and were sacrificed immediately after the treatment. As expected, we detected increased serum prolactin levels in mice treated with metoclopramide (Fig. 3H). In the control untreated KC mice, except for only scattered early lesions the pancreas looked morphologically normal (Fig. 3I). In contrast, the metoclopramide-treated mice displayed a higher incidence of both ADMs and PanIN1 lesions (Fig. 3J, K). Together, these findings suggest that elevated systemic prolactin levels promote PanIN progression.
Prolactin is necessary for PanIN progression:
Binding to PRLR is not specific for prolactin, as placental lactogen in pregnant females also has some affinity for this receptor (23). Thus, the ability of PanINs to respond to high systemic prolactin levels does not necessarily mean that prolactin is part of disease progression. To dissect the potential role of prolactin in pancreatic tumorigenesis in vivo, we crossed the KC mice with prolactin-deficient mice (Prl−/− mice) to generate a Ptf1aCre;KrasG12D;Prl−/− transgenic line (abbreviated KC;Prl−/−). In Prl−/− mice mammary gland development is impaired, however they have a normal hematopoietic system (30). More importantly, the Prl−/− mice show normal pancreatic gross morphology and differentiation (Supplementary Fig. S2). As in the KC mice (Fig. 4A) although, we could find areas with desmoplastic reaction and neoplastic lesions throughout the 6 month-old KC; Prl−/− pancreas (Fig. 4B), many lobes displayed generally normal gross morphology (Fig. 4C). In the areas containing lesions, while the number of ADMs was similar to the age-matched KC mice, there was a decrease in PanIN1 and PanIN2 lesions (Fig. 4 A–D). It should be noted that throughout this study, KC mice showed classic stepwise PanIN formation and in some cases (2/5) transition to PDAC (Figs. 3A, D, & 4A, E, F). Interestingly, no further PanIN progression nor PDAC formation could be detected in one year old KC; Prl−/− mice (Fig. 4G, H). Considering that Prl−/− female mice are infertile (30), it was not feasible to study the effect of pregnancy on PanINs in KC; Prl−/− mice. Nevertheless, these findings suggest that prolactin is dispensable for initiation of PanINs but is required for PanIN progression.
Figure 4: Prolactin is essential for PanIN progression.
(A-H) Histological analyses of representative tissues obtained from KC (A, E, F) and KC; Prl−/− mice (B, C, G) were collected at 6 (A-C), or 12 (E-G) months showed significantly slowed PanIN progression in prolactin deficient KC mice. (D, H) Columns, percentages of normal ducts (ND), metaplastic ducts (ADM), and PanINs by grade per total ductal structures in different genotypes. (n=3–5 mice per genotype). Results in (D, H) are expressed as mean ± standard error of the mean (SEM) and analyzed statistically by Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Bars 20μm.
Loss of prolactin results in reduced pSTAT3 and decreased fibrosis:
Our in vitro studies identified STAT3 as downstream effector of PRL/PRLR axis in PDAC (Figs. 2 & 3). To gain insight into the mechanism by which prolactin contributes to PanIN progression, we analyzed the status of pSTAT3 in 6 month-old KC, and KC; Prl−/− mice, as well as in 4 month-old KC dams with two previous pregnancies (Fig. 5A–D). In the pancreas pSTAT3 is present in metaplastic ducts, a subset of premalignant lesions as well as in F4/80+ macrophages (11, 12, 36, 37). In accordance with these reports, in control KC mice we could detect pSTAT3 in ADMs, PanINs (60%) and a subset of surrounding stromal cells (Fig. 5A). In KC; Prl−/−pancreas, while total Stat3 could be detected in PanINs (Supplemental Fig. S3A) the number of pSTAT3-positive PanINs had declined significantly (Fig. 5B). Moreover, pStat3 was absent in stromal cells, whereas it could still be detected in ADMs (Fig. 5B inset). In mice with previous pregnancies, the number of pSTAT3-positive cells increased both in the stromal compartment and the lesions (Fig. 5C, D).
Figure 5: Prolactin promotes fibrosis.
(A-H) Immunohistochemical analyses of 6 month old KC (A, E,), KC; Prl−/− (B, F), or KC mice with previous pregnancy (C, G) pancreas using antibodies against pSTAT3 (A-C), pFAK1 (E-G) showed a significant decrease or increase of pSTAT3 (D) and pFAK1 (H) in KC; Prl−/− mice or as the result of pregnancy, respectively. Inset in (B) shows specific loss of pSTAT3 in PanIN cell, whereas it can be detected in the adjacent ADM. Insets in (E-G) highlight pFAK1-posotive stromal cells. (I-P) Sirius red (I-L) and collagen staining (M-P) revealed a relationship between collagen deposition and prolactin levels. (n=3–5 mice per condition, or genotype). Results in (D, H, L and P) are expressed as mean ± standard error of the mean (SEM) and analyzed statistically by Student’s t-test, *p<0.05, **p<0.01. Bars 20μm.
In addition to induction of Jak2/Stat pathway, prolactin may also promote phosphorylation of FAK through activation of Src (23). Recent studies have shown that high FAK1 activity in PDAC is associated with extensive collagen deposition and fibrosis (38). Given the absence of desmoplastic reaction in relatively large areas in the KC; Prl−/−pancreas, and increased fibrosis as the result of pregnancy, we next compared the pFAK1 status, the collagen deposition as well as the abundance of hyaluronic acid (HA) in these settings. In control KC mice, pFAK1 could be detected in ADMs, PanINs (80%) and also in some stromal cells (Fig. 5E). While prolactin deficiency did not alter total FAK1 (Supplemental Fig. S3B) or pFAK1 in ADMs, pFAK1 was barely detectable in PanINs and the stroma (Fig. 5F). In contrast, previous pregnancy led to a more prominent pFAK1 presence within the stromal cells, and a significant increase in the epithelial pFAK1 levels compared to the age-matched control KC mice (Fig. 5FG, H. Furthermore, we found that KC; Prl−/−mice had reduced levels of fibrosis as seen by Sirius Red (Fig. 5I–L) and collagen staining (Fig. 5M–P). Interestingly, the difference in collagen deposition appeared more prominent in the area surrounding the lesions (Fig. 5J–L, insets). The abundance of HA on the other hand, confirmed by staining with HA-binding protein (HABP) was similar in KC and KC; Prl−/−mice (Supplemental Fig. S3C–F). Together, these findings indicate that prolactin influences both the neoplastic lesions and the surrounding stroma.
HMGB1 induces prolactin expression in macrophages:
Pancreatic cancer is somewhat more common in men than in women. Pancreatic cancer patients also have normal blood prolactin levels (39). Therefore, it is unlikely that high systemic prolactin levels would be driving progression of the disease. Thus, the source of prolactin may be found within the pancreas. We have previously reported the role of macrophages during pancreatic regeneration (32). In search of the putative cell type responsible for prolactin production in the pancreas, we examined F4/80 and prolactin expression in KC pancreas and found prolactin-positive macrophages surrounding the PanIN lesions (Fig. 6A, B).
Figure 6: Prolactin is expressed by macrophages in the pancreas.
(A, B) Immunostaining of tissues obtained from KC pancreas using antibodies against E-cadherin and F4/80 (A) or prolactin and F4/80 (B) showed expression prolactin in macrophages surrounding the PanIN lesions (dashed lines). Inset in (B) highlights prolactin-expressing macrophages. (C) Wild type BMD-macrophages were cultured with conditioned media collected from activated wild type or Hmgb1−/− platelets showed that prolactin expression in macrophages can be induced by HMGB1. (D) Immunostaining of tissues obtained from KCH pancreas using antibodies against prolactin and F4/80 showed lower expression of prolactin in macrophages surrounding the PanIN lesions (dashed lines). Inset in (D) highlights prolactin-expressing macrophages. (E) Representative flow cytometric analysis of PRLR expression on cells gated for CD11b and F4/80 expression showed no PRLR expression in bone marrow derived macrophages. (F-H) Representative flow cytometric analysis of CD11c, CD206 or PRLR expression on cells gated for CD11b and F4/80 collected from non-injured pancreas (F), on day 3 post-caerulein treatment (G), or KC mice (H) showed that PRLR+ macrophages could be exclusively found within CD11b+/F4/80+/CD11c+/CD206+ population. (I) Quantitative analysis of different macrophage populations in the non-injured adult pancreas, day 3 post-caerulein treated, or KC pancreas. Results in (I) are expressed as mean ± standard error of the mean (SEM) and analyzed statistically by Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, ****p≤0.0001. (n=3 mice, and 3 replicates for each flow). Bars 20μm.
The high mobility group box 1 (HMGB1) protein regulates DNA damage responses within the nucleus (40), and serves to promote access to various transcriptional complexes including hormone/nuclear receptor complexes (41). However, under stressful situations, such as in pancreatitis or PDAC, HMGB1 is released into the cytosol where it promotes autophagy and mitochondrial quality control as well as into the extracellular space, enhancing the inflammatory response (42). Given the abundance of macrophages in proximity to PanINs, and also the ability of extracellular HMGB1 to bind to TLR2, TLR4 and the receptor for advanced glycation endproducts (RAGE) in macrophages (43) we speculated that HMGB1 might induce prolactin expression in macrophages. To test this hypothesis, conditioned media (CM) collected from activated wild type or HMGB1-deficient platelets were added to the BMD macrophage cultures. Macrophages cultured in the absence of CM did not express prolactin, whereas we could detect prolactin expression in those cultured with CM obtained from wild type platelets (Fig. 6C). Interestingly, HMGB1-deficient CM failed to stimulate similar prolactin expression in macrophages (Fig. 6C). To confirm our in vitro study, we next examined PdxCre;KrasG12D;Hmgb1fl/fl mice (KCH mice) (44) and found reduced prolactin expressed by F4/80+ cells (Fig. 6D). These data imply that macrophages present in the tumor environment express prolactin, and that this prolactin expression can be stimulated by extracellular HMGB1.
Tissue-resident macrophages express PRLR:
The presence of PRLR+ cells in the stroma (Figs. 1 & 4) in conjunction with the observed effect of prolactin on stromal pFAK1 and collagen deposition (Fig. 5), suggests that in addition to the neoplastic epithelium, prolactin may also impact stromal cells directly. Since embryonic-derived resident macrophages are implicated in PDAC fibrogenesis (6), we hypothesized that the resident macrophages with pro-fibrotic activities may express PRLR. To test this hypothesis, we first generated macrophages from the bone marrow in vitro, and evaluated PRLR expression. As shown in figure 6E, the monocyte-derived F4/80+/CD11b+ macrophages do not express PRLR. Next, we isolated primary macrophages from the adult pancreas. F4/80+/CD11b+/CD11c+/CD206+ cells are embryonic-deposited resident macrophages in the pancreas (45). In the uninjured pancreas, we found that the vast majority of macrophages were CD11c+/CD206+ resident macrophages, whereas around 10% displayed pure M1 feature (Fig. 6F). Notably, at the baseline, macrophages did not express PRLR (Fig. 6F). To explore the impact of tissue damage and inflammation on CD11c+/CD206+/PRLR+ macrophages, we analyzed the status of these cells in wild type animals following caerulein treatment (Fig. 6G) or KC mice (Fig. 6H). As anticipated, the overall number of F4/80+/CD11b+ cells were higher in these two conditions compared to the uninjured pancreas (Fig. 6G–I). However, we observed a shift from a M1/M2 to a M1 phenotype within the macrophage population (Fig. 6G–I). Moreover, a subpopulation of PRLR+ macrophages could be found exclusively within the CD11c+/CD206+ macrophages (Fig. 6G–I). Interestingly, in KC mice, despite an overall decline in the number of CD11c+/CD206+ M1/M2 cells, the ratio of PRLR-expressing cells among resident macrophages had increased (Fig. 6H, I).
Together, our findings suggest the PRLR-expressing resident macrophages appear to have a more prominent presence in the pancreatic tumor microenvironment compared to non-injured or caerulein-treated pancreas.
Discussion
Prolactin is produced by many tissues outside the pituitary, including the breast and prostate (23, 46). These findings suggest that prolactin participates in tumor growth, via a paracrine or autocrine mechanism (23, 46). Here, we report that in the pancreas, prolactin is expressed by TAMs. Thus, it appears that prolactin functions in a paracrine manner during individual stages of pancreatic tumorigenesis. While it is well established that the pituitary prolactin secretion is regulated by the inhibitory action of dopamine (33, 47), the mechanisms behind the extra-pituitary prolactin expression or release remain poorly understood. In experimental pancreatitis, damaged/dead acinar cells or activated macrophages release HMGB1 into the extracellular space, where it triggers an innate immune response (42). Given that we observed reduced prolactin in macrophages in KCH mice in which HMGB1 is conditionally knocked-out in the pancreatic epithelium, it is likely that the main source for HMGB1 in this context may be damaged/dead neoplastic cells. Regardless of the origin, HMGB1-mediated prolactin expression in the macrophages may be part of the nascent immune response.
The physiological functions of prolactin are facilitated through its cognate receptor, which we found to be expressed in premalignant and malignant lesions in the human and murine KC pancreas. However, PRLR was expressed as early as in PanIN1s in mice, whereas in patient samples we first detected PRLR only in more advanced PanINs. The difference in the onset of PRLR expression may reflect the differences in species.
In the current study, we provide evidence that prolactin plays a key role in PanIN progression. Our conclusion is based on gain-of-function (pregnancy or metoclopramide treatment) and loss-of-function (KC;Prl−/− mice) approaches, where we found an interesting relationship between PanIN acceleration and prolactin levels. Cytokines favor the expansion and development of noninvasive pancreatic precursor lesions (8, 9, 15). Accordingly, we could observe an overall increase in the number of pSTAT3 cells as the result of high systemic prolactin. Moreover, in KC; Prl−/− mice, pSTAT3 declined both in PanINs and stromal cells. Interestingly, the status of pSTAT3 was unchanged in ADMs, suggesting that the ADM to PanIN transition coincides with a switch from a prolactin-independent to a prolactin-dependent mechanism for STAT3 activation. IL-6 has been shown to stimulate STAT3 activity in PanINs and myeloid cells (10, 11, 36). Thus, while inactivation of Stat3 in KC mice prevents ADM to PanIN transformation (11), in KC;Prl−/− pancreas PanINs are initially formed but they fail to advance further.
An intriguing finding in this study was the effect of prolactin on fibrogenesis and collagen deposition. We have found that the number of pSTAT3+ and pFAK+ stromal cells correlate with prolactin levels. Furthermore, we have identified a sub-population of embryonic-derived pancreatic resident macrophages that express PRLR during tumorigeneis. These findings indicate that prolactin (i) regulates STAT3 and FAK1 phosphorylation in PanINs and a subset of stromal cells, and (ii) induces pro-fibrotic activities in resident TAMs. Given the oncogenic nature of PRL/PRLR pathway, we were surprised to find that patients with high PRLR expression displayed better survival than those with lower PRLR levels. However, this can be partially explained by the fact that the PRLR low-expressing cells belong to the squamous class which is generally associated with poor survival (34). Moreover, the extent of PRLR-expressing resident macrophages that can respond to prolactin may also contribute to the overall survival of the patients.
In summary, we propose that extracellular HMGB1, either passively released by damaged/dying neoplastic cells, or actively secreted by TAMs stimulates prolactin expression by macrophages (Figure 7A). The macrophage-derived prolactin may promote fibrosis through PRLR-expressing resident macrophages. In addition, prolactin binds to its cognate receptor on PanIN cells, where it maintains FAK1 and STAT3 activity. Since collagen density or stiffness could also result in FAK1 activation in normal or malignant cell types including the pancreas (38, 48), it is possible that the effect of prolactin on pFAK1 in PanINs is the result of the overall desmoplastic status of the environment. The canonical PRL/PRLR signaling may result in phosphorylation of cSrc or JAK2, which in turn can directly activate FAK1 and STAT5, respectively (23). JAK2 can also activate STAT3, although not directly, but through JAK1 as an intermediate (Fig 7B) (23). Our study shows that STAT5 is active in pancreatic cancer, but its activity is not dependent on PRL/PRLR, as the current literature would suggest. Instead, prolactin appears to regulate STAT3 activity (Fig. 7C). This finding is clinically relevant, as it would suggest that inactivation of STAT3 in pancreatic cancer patients could potentially include IL-6 in conjunction with prolactin as therapeutic targets.
Figure 7: Schematic illustration of the proposed mechanism by which prolactin promotes PanIN progression.
(A) Tissue resident embryonic-derived macrophages release HMGB1 during stress/inflammation, recruiting bone marrow-derived inflammatory macrophages. These TAMs release prolactin acting back on the tissue resident macrophages expressing neoplastic endothelium as well as nascent tumor cells. B. Conventional Prolactin Receptor signaling involves downstream phosphorylation of JAK2 and subsequently STAT5 and STAT3. C. PRLR signaling in pancreatic tumorigenesis involves phosphorylation of JAK2 and STAT3.
We have demonstrated that the source of prolactin in pancreatic tumorigenesis is locally derived within the pancreas. Nevertheless, given the relatively higher systemic prolactin in females, one would expect a higher PDAC incidence among women compared to men. It has been suggested that it may take up to 20 years from the time of overt tumor initiation to the time of a patient’s death (49). The average age for PDAC diagnosis in both sexes is in the mid-seventies, which implies that the average age for tumor initiation should be above 50 years. Clearly, in individuals younger than 50 years of age, prolactin concentrations are higher in women than men (47, 50), however, post-menopausal women (typically over 50 years old) exhibit a 40% decrease in prolactin secretion, whereas men above age 50 display an 18% increase (47, 51). Thus, the differences in systemic prolactin between sexes declines around the putative time of pancreatic tumor initiation.
Prolactin is not a carcinogen, but it appears to be required for the normal progression of the disease. Nonetheless, high levels of prolactin in conditions such as obesity (52, 53) may accelerate the oncogenic events. Together, these findings indicate that prolactin signaling is a key mediator of the crosstalk between the stroma and the neoplastic epithelium. Further studies are required to determine whether it may represent an intriguing and novel target for therapy in this disease.
Supplementary Material
Statement of significance.
Prolactin is a key factor in the crosstalk between the stroma and neoplastic epithelium, functioning to promote fibrosis and PDAC progression.
Acknowledgements
NIH/NIDDK grant DK101413 (to F.E.), National Pancreas Foundation (to F.E.), The Children’s Hospital of Pittsburgh of UPMC (to F.E.). Cancer Research UK C29717/A17263, C29717/A18484, C596/A18076, C596/A20921, A23526 (to A.V.B.), Wellcome Trust Senior Investigator Award: 103721/Z/14/Z (to A.V.B.), Pancreatic Cancer UK Future Research Leaders Fund FLF2015_04_Glasgow (to A.V.B.), Scottish Genome Partnership SEHHD-CSO 1175759/2158447 (to A.V.B.), MRC/EPSRC Glasgow Molecular Pathology Node (to A.V.B.), The Howat Foundation (to A.V.B.). We acknowledge the Comparative Pathology and Mouse Phenotyping Shared Resource (CPMPSR) of The Ohio State University Comprehensive Cancer Center for excellent immunohistochemical (Alan Flechtner and Florinda Jaynes) support. The work was supported in part by Cancer Center Support Grant P30 CA016058, National Cancer Institute, Bethesda, Maryland. We would like to thank Joshua Michel for helping us with the flow cytometry analyses.
Footnotes
Conflict of Interest: The authors disclose no conflicts.
References
- 1.Maitra A, Fukushima N, Takaori K and Hruban RH, Precursors to invasive pancreatic cancer. Adv Anat Pathol, 2005. 12(2): p. 81–91. [DOI] [PubMed] [Google Scholar]
- 2.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N and Tuveson DA, The pancreas cancer microenvironment. Clin Cancer Res, 2012. 18(16): p. 4266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon S and Taylor PR, Monocyte and macrophage heterogeneity. Nat Rev Immunol, 2005. 5(12): p. 953–64. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F and Guilliams M, Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity, 2016. 44(3): p. 439–449. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. , Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity, 2013. 38(4): p. 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. , Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity, 2017. 47(3): p. 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sica A and Mantovani A, Macrophage plasticity and polarization: in vivo veritas. J Clin Invest, 2012. 122(3): p. 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waghray M, Yalamanchili M, di Magliano MP and Simeone DM, Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol, 2013. 29(5): p. 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu GC, Kimmelman AC, Hezel AF and DePinho RA, Stromal biology of pancreatic cancer. J Cell Biochem, 2007. 101(4): p. 887–907. [DOI] [PubMed] [Google Scholar]
- 10.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. , Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell, 2011. 19(4): p. 456–69. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, et al. , Stat3 and MMP7 Contribute to Pancreatic Ductal Adenocarcinoma Initiation and Progression. Cancer Cell, 2011. 19(4): p. 441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. , Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell, 2014. 25(5): p. 621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. , Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell, 2012. 21(6): p. 822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G and Bar-Sagi D, Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell, 2012. 21(6): p. 836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, et al. , Stromal biology and therapy in pancreatic cancer. Gut, 2011. 60(6): p. 861–8. [DOI] [PubMed] [Google Scholar]
- 16.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. , Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science, 2009. 324(5933): p. 1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. , Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell, 2014. 25(6): p. 735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. , Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell, 2014. 25(6): p. 719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald P and Dinan TG, Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol, 2008. 22(2 Suppl): p. 12–9. [DOI] [PubMed] [Google Scholar]
- 20.Riddle O, Prolactin in Vertebrate Function and Organization. J Natl Cancer Inst, 1963. 31: p. 1039–110. [PubMed] [Google Scholar]
- 21.Soares MJ, The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol, 2004. 2: p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rui H, Kirken RA and Farrar WL, Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem, 1994. 269(7): p. 5364–8. [PubMed] [Google Scholar]
- 23.Damiano JS and Wasserman E, Molecular pathways: blockade of the PRLR signaling pathway as a novel antihormonal approach for the treatment of breast and prostate cancer. Clin Cancer Res, 2013. 19(7): p. 1644–50. [DOI] [PubMed] [Google Scholar]
- 24.Yu JH and Kim H, Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver, 2012. 6(4): p. 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, et al. , Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Lett, 2013. 341(2): p. 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ and Ou JR, miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Lett, 2015. 589(17): p. 2224–32. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Chen X and Tang M, MicroRNA-216a inhibits pancreatic cancer by directly targeting Janus kinase 2. Oncol Rep, 2014. 32(6): p. 2824–30. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ and Wright CV, The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet, 2002. 32(1): p. 128–34. [DOI] [PubMed] [Google Scholar]
- 29.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. , Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell, 2003. 4(6): p. 437–50. [DOI] [PubMed] [Google Scholar]
- 30.Horseman ND, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Engle SJ, et al. , Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J, 1997. 16(23): p. 6926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Criscimanna A, Duan LJ, Rhodes JA, Fendrich V, Wickline E, Hartman DJ, et al. , PanIN-Specific Regulation of Wnt Signaling by HIF2alpha during Early Pancreatic Tumorigenesis. Cancer Res, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD and Esni F, Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and beta-cell regeneration in mice. Gastroenterology, 2014. 147(5): p. 1106–18 e11. [DOI] [PubMed] [Google Scholar]
- 33.Durant S, Alves V, Coulaud J, El Hasnaoui A, Dardenne M and Homo-Delarche F, Attempts to pharmacologically modulate prolactin levels and type 1 autoimmune diabetes in the non-obese diabetic (NOD) mouse. J Autoimmun, 1995. 8(6): p. 875–85. [DOI] [PubMed] [Google Scholar]
- 34.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. , Genomic analyses identify molecular subtypes of pancreatic cancer. Nature, 2016. 531(7592): p. 47–52. [DOI] [PubMed] [Google Scholar]
- 35.Goffin V, Kinet S, Ferrag F, Binart N, Martial JA and Kelly PA, Antagonistic properties of human prolactin analogs that show paradoxical agonistic activity in the Nb2 bioassay. J Biol Chem, 1996. 271(28): p. 16573–9. [DOI] [PubMed] [Google Scholar]
- 36.Long KB, Tooker G, Tooker E, Luque SL, Lee JW, Pan X, et al. , IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther, 2017. 16(9): p. 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Amico S, Shi J, Martin BL, Crawford HC, Petrenko O and Reich NC, STAT3 is a master regulator of epithelial identity and KRAS-driven tumorigenesis. Genes Dev, 2018. 32(17–18): p. 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. , Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med, 2016. 22(8): p. 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, et al. , Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One, 2014. 9(4): p. e94928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange SS, Mitchell DL and Vasquez KM, High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A, 2008. 105(30): p. 10320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang R, Zhang Q, Zeh HJ, 3rd, Lotze MT and Tang D, HMGB1 in cancer: good, bad, or both? Clin Cancer Res, 2013. 19(15): p. 4046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, et al. , Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology, 2014. 146(4): p. 1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, et al. , Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med, 2013. 19: p. 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, et al. , Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res, 2017. 27(7): p. 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, et al. , The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med, 2015. 212(10): p. 1497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goffin V, Touraine P, Culler MD and Kelly PA, Drug Insight: prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat Clin Pract Endocrinol Metab, 2006. 2(10): p. 571–81. [DOI] [PubMed] [Google Scholar]
- 47.Roelfsema F, Pijl H, Keenan DM and Veldhuis JD, Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS One, 2012. 7(2): p. e31305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae YH, Mui KL, Hsu BY, Liu SL, Cretu A, Razinia Z, et al. , A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci Signal, 2014. 7(330): p. ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. , Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature, 2010. 467(7319): p. 1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaji T, Shimamoto K, Ishibashi M, Kosaka K and Orimo H, Effect of age and sex on circulating and pituitary prolactin levels in human. Acta Endocrinol (Copenh), 1976. 83(4): p. 711–9. [DOI] [PubMed] [Google Scholar]
- 51.Veldhuis JD, Changes in pituitary function with ageing and implications for patient care. Nat Rev Endocrinol, 2013. 9(4): p. 205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kok P, Roelfsema F, Frolich M, Meinders AE and Pijl H, Prolactin release is enhanced in proportion to excess visceral fat in obese women. J Clin Endocrinol Metab, 2004. 89(9): p. 4445–9. [DOI] [PubMed] [Google Scholar]
- 53.Kok P, Roelfsema F, Langendonk JG, de Wit CC, Frolich M, Burggraaf J, et al. , Increased circadian prolactin release is blunted after body weight loss in obese premenopausal women. Am J Physiol Endocrinol Metab, 2006. 290(2): p. E218–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.