Abstract
Sleep complaints are an early clinical symptom of neurodegenerative disorders. Patients with Parkinson’s disease (PD) experience sleep disruption (SD). The objective of this study was to determine if pre-existing, chronic SD leads to a greater loss of tyrosine hydroxylase (TH) within the striatum and substantia nigra following chronic/progressive exposure with the neurotoxin, MPTP. Male mice underwent chronic SD for 4 weeks, then injected with vehicle (VEH) or increasing doses of MPTP for 4 weeks. There was a significant decrease in the plasma corticosterone levels in the MPTP group, an increase in the SD group, and a return to the (VEH) levels in the SD+MPTP group. Protein expression levels for TH in the striatum (terminals) and substantia nigra pars compacta [dopamine(DA) cell counts] revealed up to a 78% and 38% decrease, respectively, in the MPTP and SD+MPTP compared to their relevant VEH and SD groups. DA transporter protein expression increased in the striatum in the MPTP vs VEH group and in the SN/midbrain between the SD+MPTP and VEH group. There was a main effect of MPTP on various gait measures (eg., braking) relative to the SD or VEH groups. In the SD+MPTP group, there were no differences compared to the VEH group. Thus, SD, prior to administration of MPTP, has effects on serum corticosterone and gait but more importantly does not potentiate greater loss of TH within the nigrostriatal pathway compared to the MPTP group, suggesting that in PD patients with SD, there is no exacerbation of the DA cell loss.
Keywords: corticosterone, tyrosine hydroxylase, dopamine transporter, striatum, substantia nigra pars compacta, gait
Introduction
Although there are currently no reliable biomarkers to predict the onset of Parkinson’s disease (PD) years or decades prior to when the classical motor symptoms first appear, it has been suggested that sleep disturbances may be an early marker of this disease (Chahine et al, 2017). PD patients have been reported to have significant nighttime sleep disruption and daytime sleepiness. Inherent to PD, and prevalent among any age or treatment status, is impaired nighttime sleep: Increased time to fall asleep, reduced sleep efficiency, and reduced rapid eye movement (REM) sleep (Breen et al, 2014; Buskova et al, 2011; Yong et al, 2011; Diederich et al, 2005; Chahine et al, 2013). Many studies report sleep fragmentation with only moderate reductions in total sleep time (Breen et al, 2014; Stocchi et al, 1998; Tandberg et al, 2004) that appear early and become more prominent as the disease progresses (Aldrich 2000). In a recent comprehensive review of criteria that may be used for prodromal PD, Berg et al (2015) detailed a lengthy list of markers and calculated the likelihood ratio of a specific diagnostic test that either increases or decreases the risk of developing PD. Amongst these potential prodromal markers, one marker related to sleep disruption is REM sleep behavior disorder (RBD). Although RBD is associated with a very specific sleep phenotype, sleep disruption (SD), in general, may still contribute to the pathogenesis of PD. Animal models of PD have found that fragmented sleep and REM sleep disturbance can emerge prior to the onset of motor impairments (Barraud et al, 2009; Hyacinthe et al, 2014, McDowell et al, 2014; but see Monaca et al, 2004); however, it remains to be determined whether disrupted sleep affects brain pathology as relevant to the development of PD. We hypothesized that chronic sleep disruption would increase or accelerate the loss of nigrostriatal dopamine (DA) and motor dysfunction in our mouse model of Parkinsonism.
In order to test this hypothesis, we used an animal model of parkinsonism that consisted of the chronic/progressive 1-methyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model which destroys the DA neurons within the substantia nigra pars compacta (SNpc) via transport of the active form (MPP+) by the dopamine transporter (DAT) (Dauer and Przedborksi 2003; Przedborski and Jackson-Lewis 1998). The majority of PD research conducted thus far has utilized acute/subacute models which have many benefits including lesioning a large number of DA neurons within a short period of time and robust behavioral changes (Meredith et al 2008). Although other chronic models of parkinsonism have been reported (Petroske et al), these lead to about a 30% loss of DA cells in the SNpc after 5 weeks (Shintu et al, 2009). However, one of the limitations of this study design is that SD was initiated prior to the administration of MPTP, with SD continuing during the time of the injection of the toxin. With PD patients, there is most likely the start of DA cell loss either prior to, or at least at, the time that SD is observed in these patients.
However, in humans, PD manifests over several years and is therefore a progressive disease. In our hands we find that acute intrastriatal bilateral infusion of 6-hydroxydopamine leads to a maximal loss of tyrosine hydroxylase (TH) neurons and terminals within only 10–12 days (Meshul, unpublished data), which is not a representative time frame for DA loss in PD patients. Therefore, we utilized a mouse model of parkinsonism, which consists of injecting MPTP in a graded manner over 4 weeks, to better mimic the development/progression of this disease. To mimic the prodromal sleep disturbances that PD patients may experience prior to the onset of the motor dysfunction, the mice were exposed to a well-established SD model in which sleep is fragmented by gentle agitation of the home cage on an orbital shaker (Sinton et al, 2009; Li et al, 2014; Jones et al, 2019) for 4 weeks prior to the onset of 4 weeks of progressive MPTP administration. In rodents, this method of sleep disruption chronically fragments sleep with brief arousals and reduces REM sleep (Li et al, 2014) without producing long term effects on total sleep time (studies report between a 10–50% reduction in total sleep in the first day (Li et al, 2014; Sinton et al, 2009; Jones et al, 2019) with no change in total sleep time after 4 weeks (Li et al, 2014), Importantly, this form of sleep disruption has been shown to only affect sleep during periods when the lights are on (Li et al, 2014), the time when mice are most likely to be asleep.
We report herein that 4 weeks of SD, followed by subsequent administration of a progressive dosing of MPTP for 4 weeks with continued SD during the toxin administration, leads to a similar loss of TH protein expression within the striatum, as measured by both immunohistochemistry and western immunoblotting, along with a nearly identical loss of TH labeled neurons within the SNpc, compared to the MPTP only group. Although there was an effect of MPTP on several gait measures, these values returned to the VEH level in the SD+MPTP group, suggesting that the continued loss of nigrostriatal TH was independent of the change in motor activity.
Methods
Animals
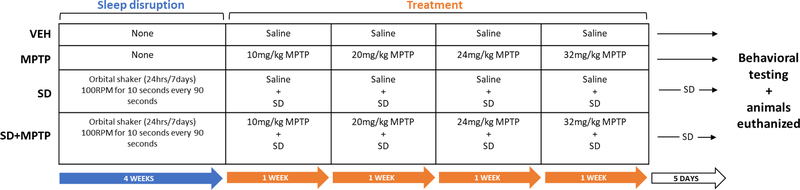
Mouse care and handling followed the federal guidelines of the Public Health Service Policy on the Humane Care and Use of Laboratory Animals. Protocols were approved by the Portland VA Center IACUC. Young adult (12 weeks) male C57BL/6J mice (Jackson Labs, Bar Harbor, Maine) were housed 4 to a cage, had ad libitum access to food and water and were on a 12 hour light/dark cycle, with lights on/off at 7AM/7PM. Male C57BL/6J mice were used in this study because they are more susceptible to MPTP than female mice (Meshul, unpublished findings). In addition, there is a significantly higher incidence rate of Parkinson’s disease in human males (Wooten et al, 2004). Mice were split into 4 groups: VEH (4 weeks of normal saline injections, euthanized 5 days after the last injection; n=12), MPTP (4 weeks of progressive MPTP administration, euthanized 5 days after the last injection; n=11), SD+VEH (8 weeks of sleep deprivation with normal saline injections administered the last 4 weeks, euthanized 5 days after the last injection; n=12), and SD+MPTP (8 weeks of sleep deprivation with progressive MPTP administration the last 4 weeks, euthanized 5 days after the last injection; n=14) (See Fig. 1 for a timeline).
Fig. 1: Timeline of experimental design.
During the first four weeks, mice were either sleep disrupted (SD and SD+MPTP groups) or not sleep disrupted (VEH and MPTP). Over the next four weeks mice were injected with either an increasing dose of MPTP or with saline, with the sleep disrupted groups (SD and SD+MPTP) continuing to be sleep disrupted during these four weeks of injections. Mice were behaviorally tested and euthanized 5 days after the last MPTP or saline injection. Sleep disrupted mice continued to be sleep disrupted for the 5 days between injections and euthanization.
Sleep disruption
The sleep interruption method first described by Sinton et al (2009) was used to disrupt the sleep of mice in the SD+VEH and SD+MPTP groups. The home cages of the mice were placed on orbital shakers connected to automated timers for 8 weeks, set to rotate at 110RPM for 10 seconds every 110 seconds (10 seconds on, 100 seconds off), for 24 hours a day, 7 days a week. Water bottles were replaced with hydrogel to prevent spillage and cage card holders were left unsecured to provide auditory disruption in addition to gentle cage agitation. During this sleep disruption protocol we ensured that animals could still ambulate freely around the cage and continued to eat, drink, and groom as normal. Animals were checked daily for signs of distress or illness and maintained healthy appearance and behavior, consistent with non-sleep disrupted mice for the entire duration of SD. These mice underwent sleep disruption for eight weeks, with the addition of saline or MPTP treatment for the last four weeks. After stopping saline and MPTP injections, the mice continued to undergo sleep disruption for 5 days until they were behaviorally tested and euthanized.
MPTP Administration
The MPTP and SD+MPTP groups received intraperitoneal injections of MPTP (Santa Cruz Biotechnology, Dallas, TX, USA) as described by Churchill et al (2017, 2019) for 5 days a week for 4 weeks, after 4 weeks of undergoing either sleep disruption or no sleep disruption (Fig. 1). The dosages of MPTP (calculated as the base, dissolved in normal saline) used in our progressive model consisted of 10 mg/kg/day (week 1, 5d/wk), 20 mg/kg/day (week 2, 5d/wk), 24 mg/kg/day (week 3, 5d/wk) and 32 mg/kg/day (week 4, 5d/wk). The vehicle groups (VEH and SD) were injected with normal saline instead of MPTP for the full 4 weeks. 24 animals were analyzed by TH immunohistochemistry (IHC) and 25 animals were used for western immunoblot analysis.
Brain tissue harvesting and tissue fixation
All animals used for western immunoblotting were euthanized via cervical dislocation. The brains used for Western Immunoblots were micro-dissected for the dorsolateral striatum (anterior-posterior from Bregma: 2.0 mm to 3.5 mm; medial-lateral: 2.0 mm to 3.0 mm) and the ventral midbrain (anterior-posterior from Bregma: 4 mm to 5.5mm; medial lateral: 0.5 mm to 2 mm) (Paxinos and Franklin 2004). The dissected tissue was frozen at −80°C until western immunoblot protein analysis was performed. The animals designated for IHC were first anesthetized with ketamine/xylazine and perfused transcardially with 6 mls of 1000 units/ml of heparin (in 0.1M phosphate buffer, pH 7.3), followed by 50 mls of 2.5% paraformaldehyde, 0.5% glutaraldehyde and 0.1% picric acid. The brains were then cut coronally at the level of the hypothalamus and post-fixed for 30 minutes in 2.5% paraformaldehyde, 0.5% glutaraldehyde and 0.1% picric acid using a microwave tissue processor (Pelco Biowave, Ted Pella Inc, Redding, CA, US), as previously reported (Churchill et al, 2017, 2019). The brains were then individually placed in scintillation vials containing 0.1M PB at 4°C until IHC processing.
Tyrosine Hyrdoxylase Immunohistochemistry (IHC): striatum and SNpc
Animals from each of the four treatment groups (total n=24) were used for immunohistochemistry analysis. The fixed rostral half of the brain was sliced coronally using a vibratome (VT 1000S; Leica, Nussloch, Eisfield, Germany) at a thickness of 60 μm through the entire rostral-caudal extent of the striatum to the level of the anterior commissure (anterior-posterior from Bregma: +1.2 mm to +0.25 mm) as previously described (Churchill et al, 2017; Hood et al, 2016; Sconce et al, 2015a; Sconce et al, 2015b). The caudal half of the brain was sliced coronally at 40 μm through the entire rostral-caudal extent of the substantia nigra pars compacta (SNpc) (anterior-posterior from Bregma, −2.50 mm to −4.24 mm) and sections placed in serial order in 48 well plates. Six slices from each animal collected through the whole rostral-caudal extent of the striatum and SNpc (every 3rd slice) were used for immunolabeling with a monoclonal antibody raised against tyrosine hydroxylase (TH; Immunostar, 1:250, mouse monoclonal, #22941, AB_572268; Table 1). All tissue was processed for IHC on the same day using the Biowave (Ted Pella, Redding, CA) as previously described (Churchill et al, 2017; Hood et al, 2016; Sconce et al, 2015a; Sconce et al, 2015b). Once processed for TH immunoreactivity (TH-ir), the tissue was mounted onto gel-coated slides and left to dry at room temperature overnight. Striatal sections were coverslipped with Permount Mounting Medium (Fisher Scientific, Fair Lawn, NJ). SNpc sections were counterstained with cresyl violet (CV), as described by Churchill et al (2017, 2019), and then coverslipped similar to that of the striatal sections.
Table 1:
Primary antibodies used for immunohistochemistry and western blot.
| Antibody | RRID | Host species | Manufacturer, catalog #, clonality | Dilution used |
|---|---|---|---|---|
| Tyrosine hydroxylase (TH) | AB_572268 | Mouse | Immunostar, 22941, mouse monoclonal | IHC: 1:250 WB: 1:40,000 |
| Vesicular monoamine transporter 2 (VMaT2) | AB_887888 | Rabbit | Synaptic Systems, 138302, rabbit polyclonal | WB: 1:1000 |
| Dopamine transporter (DAT) | n/a | Rabbit | Proteintech, 22524–1-AP, rabbit polyclonal | WB: 1:1000 |
| Anti-β-Actin | AB_476744 | Mouse | Sigma-Aldrich, A5441, mouse monoclonal | WB: 1:6500 |
Striatal Microscopic Analysis
Striatal sections (n=6 from each animal) were imaged for TH optical density on the light microscope at 1.25x magnification (Axioplan Zeiss Microscope). ImagePro (ImagePro 9.2, Media Cybernetics) was used to determine the optical density in both the left and right sides of the striatum. Background was accounted for by taking optical density readings from the overlying cortex directly above the dorsolateral striatum. The background was subtracted from the optical density of TH in the dorsolateral striatum.
Surface Cell Counts
Treatment groups were analyzed in a blinded manner. For surface cell counts and determining the average number of TH+/CV+ neurons/sections, as previously reported (Churchill et al, 2017, 2019), SNpc sections were imaged using a light microscope (10x magnification). Areas were identified using Franklin and Paxinos (2004) mouse stereotaxic coordinates as a guide. ImagePro was then used to count the number of TH+/CV+ cells that were only at the in-focus surface plane of immunolabeled SNpc tissue. TH−/CV+ cells were also counted. Cell counts from both sides of the same SNpc sections were added together and then averaged. The mean number of TH+/CV+ cells per 6 sections was calculated for each animal and then an overall mean was calculated for each treatment group. The same analysis was carried out for the TH−/CV+ neurons in the SNpc. The total number of TH+/CV+ and TH−/CV+ labeled neurons/6 sections was re-evaluated and reported using the Abercrombie correction, which accounts for fragmented nuclei within each section and provides a more accurate estimate of neurons when tissue thickness exceeds soma thickness by more than 50% (Clarke. 1992, Smolen et al, 1983). Although this cell counting methodology may lead to an underestimation of the number of TH+/CV+ and TH−/CV+ neurons in the SNpc, it is an appropriate approach for measuring the mean number of neurons/section according to recent comparisons of 2D and 3D analyses of brain tissue (Baquet et al, 2009; Benes and Lange, 2001). We have previously found that the results from using this cell counting method significantly correlate (r = 0.8, p<0.0001) with results from using stereological cell counting (Churchill et al, 2017). We have reported that in our chronic progressive mouse model, there are no volume changes of the substantia nigra following MPTP treatment (Churchill et al, 2017).
Western Immunoblot Analysis
Tissue was prepared as previously described (Churchill et al., 2017, 2019, Hood et al., 2016, Sconce et al., 2015a, Sconce et al., 2015b). Primary antibodies used were (Table 1): tyrosine hydroxylase (TH; Immunostar, Mouse, 1:40,000, #22941, AB_572268), dopamine transporter (DAT; ProteinTech, Rabbit, 1:1000, #22524–1-AP), VMAT2 (Synaptic Systems, Rabbit, 1:1000, #138302, AB_887888) and β-actin (Sigma, Mouse, 1:6500, #A5441, AB-476744). The secondary antibodies used were alkaline phosphatase conjugated goat-anti-mouse (IgG H+L; Bio-Rad, 1:6000, #1706520), and alkaline phosphatase conjugated goat-anti-rabbit (IgG H+L; Bio-Rad, 1:5000, #1706518]. Enhanced chemifluoresence (ECF) substrate (GE Healthcare, Piscataway, NJ, US) was added to the membrane prior to visualization. Visualization and quantification of the antigen-antibody binding density was performed using the Bio-Rad ChemiDoc MP Imaging System and ImageJ, respectively. The intensity of the bands was analyzed and the resulting densitometry values were normalized using β-actin values. Quadruplicates of each sample were used in the analysis of all proteins.
Corticosterone (CORT) Levels
Mice were euthanized 1–3 hours after being removed from the orbital shaker (between 10AM and 1PM) via decapitation, and trunk blood was collected into blood collection tubes with EDTA (BD Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ). Samples were centrifuged for 20-min at 914 × g to isolate plasma, and then stored at −18 °C until assayed. Steroid concentrations were measured using a commercially available 125I radioimmunoassay kit (ImmuChem Double Antibody Corticosterone for rodents; MP Biomedicals, Orangeburg, NY). The manufacturer supplied protocol was implemented, with minor modifications (e.g., Cozzoli et al, 2014). In brief, CORT concentration in plasma samples (5 μL) was single-determined. Counts per min were fit to a non-linear regression equation produced by log transformation of the six standards (ranging from 25 to 1000 ng/mL; i.e., 2.5 – 100 μg/dL). Mass of samples was calculated by interpolation of the standards. Intra- and inter-assay coefficients of variation were less than 10%, and the minimum detectable limit of CORT in the assay was ~ 1 μg/dL. The specificity of the assay is very high, with less than 0.5% cross-reactivity to other endogenous steroids.
Motor Behavior Analysis
A DigiGait apparatus (Mouse Specifics, Quincy, MA, USA) was used to assess gait as previously described (Goldberg et al, 2011b; Hood et al, 2016). The mice were tested between 10AM and 12PM. The gait of each mouse was captured by ventral plane videography through a transparent, motor-driven treadmill belt. Digital images of the paws of each mouse were taken at 150 frames/s while the mice ran at a velocity of 24 cm/s. The area of the underside of each paw relative to the area of the treadmill belt at each frame was used for spatial and temporal measurements. Mice in all four groups were tested using the DigiGait 5 days after the last MPTP or saline injection. Data were analyzed using DigiGait Analysis 15 software.
Statistical analysis
All data from the 4 treatment groups were analyzed by a one-way analysis of variance (ANOVA) using Graphpad Prism 6 (San Diego, CA, USA). We tested for and found a normal distribution with equal variances in our sampled distributions. In cases of a significant omnibus ANOVA, post-hoc Tukey’s multiple comparison testing, followed by post hoc Bonferroni-corrected t-tests, was then used to compare all possible pairs of means, for which P values are presented. All statistical analyses were considered significant when p<0.05 and all data were graphed using Graphpad Prism 6. Results from animals that were 2 standard deviations outside the mean were eliminated from the final analysis.
Results
Immunohistochemical analysis of TH within the striatum and SNpc
The overall study design was to investigate whether 8 weeks of SD would increase or accelerate the loss of the nigrostriatal dopamine pathway in animals following chronic/progressive treatment with the neurotoxin, MPTP (Figure 1). Since sleep disorders are a common feature of early or premorbid PD, we chose to perform sleep disruption first for 4 weeks, followed by neurotoxin treatment second. We then examined several outcome measures relevant to the nigrostriatal pathway in PD.
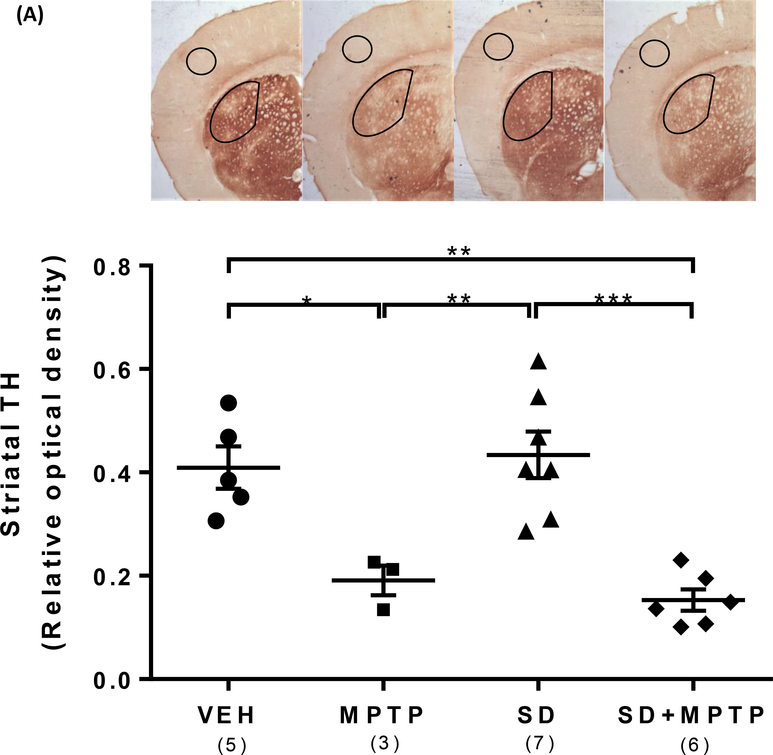
Tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of dopamine, is reduced in dopamine neurons/terminals within the substantia nigra pars compacta and striatum, respectively, in PD and is correlated with dopamine levels in our chronic/progressive MPTP model (Goldberg et al, 2011a). Representative images of TH labeling within the striatum are illustrated in Figure 2A. Analysis revealed a main effect of group upon the relative optical density of TH labeling within the dorsolateral striatum (Figure 2A; F(3,17)=14.36, p<0.001). Following 4 weeks of SD plus another 4 weeks of chronic MPTP treatment with continued SD (SD+MPTP), there was an equivalent loss of TH protein expression within the dorsolateral striatum in the MPTP only group vs the VEH group (53% loss, p=0.018) compared to the SD+MPTP group vs the SD only group (65% loss, p=0.005). There was no difference in TH striatal expression between the MPTP and SD+MPTP groups (p=0.932). SD alone for 8 weeks did not result in any change in TH protein expression within the striatum compared to the VEH group (p=0.964).
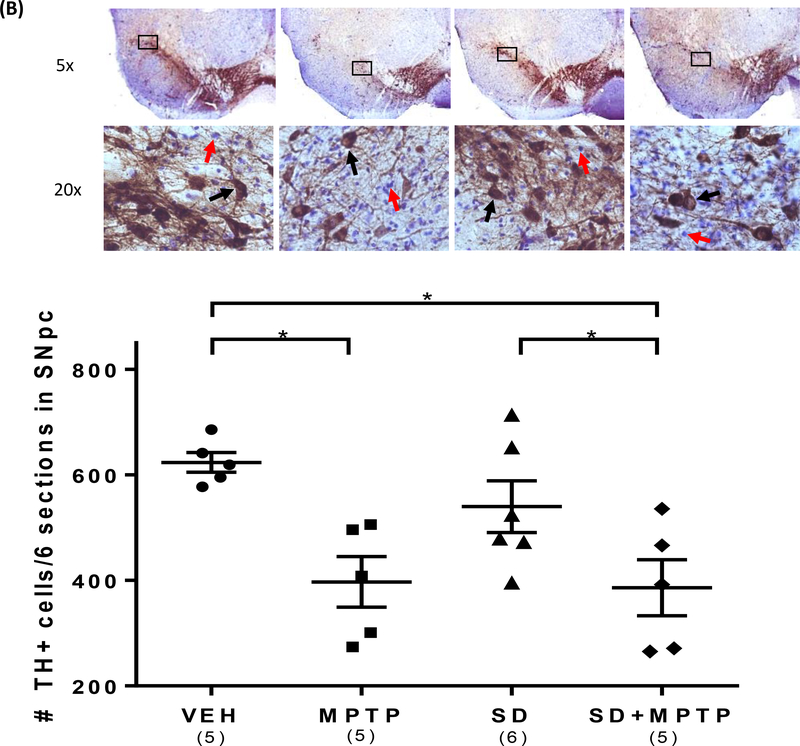
Fig. 2: TH Immunohistochemistry (IHC) of the striatum and substantia nigra pars compacta (SNpc).
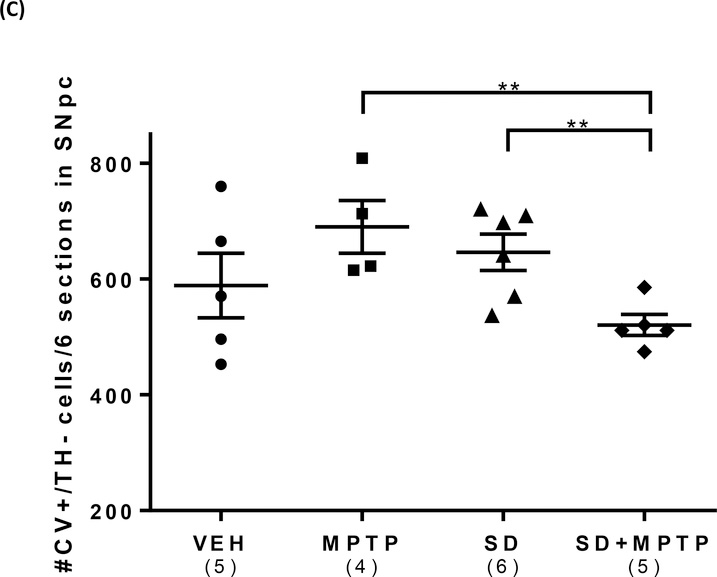
(A) IHC analysis of the dorsolateral striatum showed a 53% decrease (p=0.018) and a 65% decrease (p=0.005) in TH optical density in both the MPTP and SD+MPTP groups compared to the VEH and SD groups, respectively. Representative photos of the striatum from each group are shown above the graph with the analyzed area of the striatum and cortex circled. (B) TH+/dopamine cell counts of the SNpc showed a 36% decrease (p=0.0036) and a 38% decrease (p=0.0021) in both the MPTP and SD+MPTP groups compared to the VEH and SD groups, respectively. Photos of TH labeled SNpc from each treatment group are shown above the graph at 5x and 20x magnification (black arrows point to TH-ir SNpc neurons and red arrows point to Cresyl Violet SNpc neurons). (C) Cell counts of cresyl violet positive (CV+)/TH negative (TH−) neurons within the SNpc. There was a significant decrease in the number of CV+/TH− neurons in the SD and SD+MPTP groups compared to the MPTP only group (p<0.03). Overall, there was no evidence of enhanced striatal TH terminal or TH+ cell loss in the SNpc in the SD+MPTP group compared to the MPTP group. Values are presented as the mean ± SEM of group averages. The symbols in each column refer to values from each animal. The number of animals used is in parentheses below the treatment group. * − p<0.05; ** − p<0.01; *** − p<0.001.
We have previously reported that the analysis of TH+ cell surface counts within the substantia nigra pars compacta (SNpc) have been comparable to findings seen using stereology (Churchill et al, 2017) and that this progressive MPTP protocol did not result in any changes in the volume (i.e. shrinkage) of the substantia nigra compared to that commonly observed in the more acute nigrostriatal lesion models (Churchill et al, 2017). In addition, our previous studies have shown that there was no further TH+/SNpc dopamine cell loss following 4 weeks of MPTP treatment (Goldberg et al, 2012; Churchill et al, 2017, 2019). Representative images of TH labeling within the SNpc are illustrated in Figure 2B. Analysis of TH+ surface cell counts within the SNpc revealed a main effect of group (Figure 2B; F(3,17)= 6.26, p=0.0046). The time course of the 4 weeks of TH+ cell loss following MPTP was previously reported (Goldberg et al, 2011a). There was a 36% decrease in the number of TH+ surface cell neurons in the MPTP compared to the VEH group (p=0.0036) and a nearly equivalent 38% decrease in the SD+MPTP compared to the VEH group (p=0.0021). There was no statistical difference between the MPTP and SD+ MPTP groups (p=0.99), suggesting that pre-exposure to SD did not result in any additional TH+ cell loss compared to the group treated with just MPTP. There was no difference in the number of TH+ surface cell neurons between the VEH and SD only group (p=0.55)(Figure 2B). There was a significant difference between the SD and SD+MPTP groups (p=0.025), resulting in a 30% loss of TH+ cells within the SNpc due to MPTP treatment.
The SNpc/TH labeled sections were also counterstained with Cresyl Violet (CV+), to allow quantification of non-TH labeled cells within the SNpc to determine if there had been a loss of TH+ neurons as opposed to just a loss of TH expression. Analysis of CV+/TH− cells (i.e., non-TH labeled cells in the SNpc) revealed a main effect of group (Fig.2C; F(3,15)=3.38, p=0.046). There was no change in the CV+/TH− surface cell counts between the VEH and MPTP groups (p=0.11), but there was a significant difference (27% decrease) between SD+MPTP group compared to the MPTP (p=0.0099) and the SD only group (22% decrease) (p=0.026). What is of particular interest is that prior sleep deprivation, followed by chronic/progressive MPTP treatment, resulted in even fewer CV+/TH− labeled cells in the SNpc, compared to either group alone (i.e., MPTP or SD). However, there was no difference between the MPTP or SD groups compared to the VEH group (p>0.11). It has been reported that within the SNpc, the TH− neurons are GABAergic (Nair-Roberts et al, 2008) and project to the striatum (Rodriguez and Gonzalez-Hernandez, 1999).
Western immunoblot analysis of TH, DAT and VMAT2 levels in the striatum and SN/midbrain
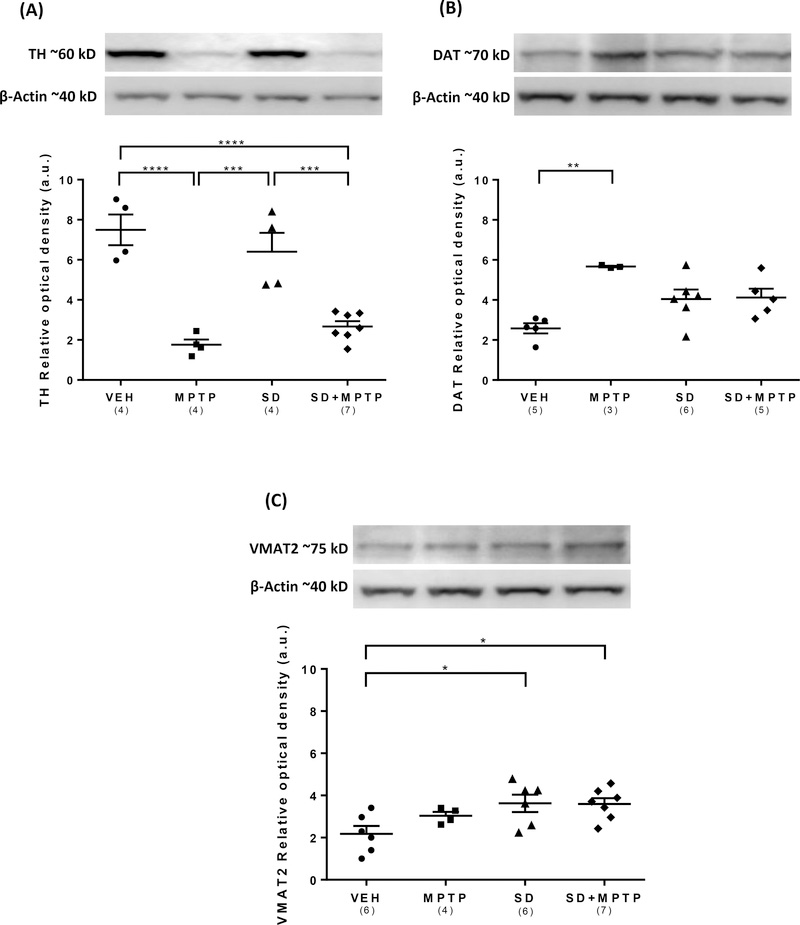
Analysis of TH protein expression within the dorsolateral striatum via western immunoblotting revealed a main effect of group on TH levels (Fig. 3A; F(3,15)= 20.9, p<0.0001). There was a 70% decrease in striatal TH expression following MPTP treatment compared to the VEH group (p=0.004) compared to a comparable 56% loss in the SD+MPTP group compared to the SD only group (p=0.003). There was no difference between the MPTP and SD+MPTP groups (p=0.243). Analysis of DAT protein expression within the dorsolateral striatum revealed a main effect of group on DAT levels (Fig. 3B; F(3,15)= 7.74, p=0.023). There was a statistically significant increase in striatal DAT expression in the MPTP compared to the VEH group (p=0.013). There was also an increase in DAT expression in the SD compared to the VEH group, but this difference was not statistically different (p=0.06). Although there was a 28% decrease in DAT expression in the SD+MPTP treated group compared to the MPTP group, this change was not statistically different (p=0.125). Analysis of VMAT2 protein expression within the dorsolateral striatum revealed a main effect of group on VMAT2 levels (Fig. 3C; F(3,19)= 4.14, p=0.021). There was a significant increase in VMAT2 levels in the SD compared to the VEH group (p=0.032). There was no change in VMAT2 levels in the MPTP compared to the VEH group (p=0.39), but there was an increase in VMAT2 protein expression in both the SD (39%; p = 0.032) and SD+MPTP groups (64%; p=0.028) compared to the VEH group. There was no difference in VMAT2 expression between the MPTP and SD+MPTP groups (p = 0.70).
Fig. 3: Western blot analysis of TH, DAT, and VMAT2 protein expression in the dorsolateral striatum.
(A) The dorsolateral striatum showed a decrease in TH protein expression in the MPTP and MPTP+SD groups when compared to the VEH group (p<0.0001), but no significant decrease in the SD group when compared to the VEH group. (B) There was an increase in DAT protein expression in the MPTP group compared to the VEH group (p<0.01), and a decrease in the SD+MPTP group towards the VEH values. (C) Protein expression of VMAT2 increased in the SD+MPTP and SD groups compared to the VEH group (p<0.05). Overall, there was no added loss or increase in any of the markers in the SD+MPTP compared to the MPTP group. Values are presented as the mean ± SEM of group averages. The symbols in each column refer to values of each animal. The number of animals used is in parentheses below the treatment group. * − p<0.05; ** − p<0.01; *** − p<0.001.
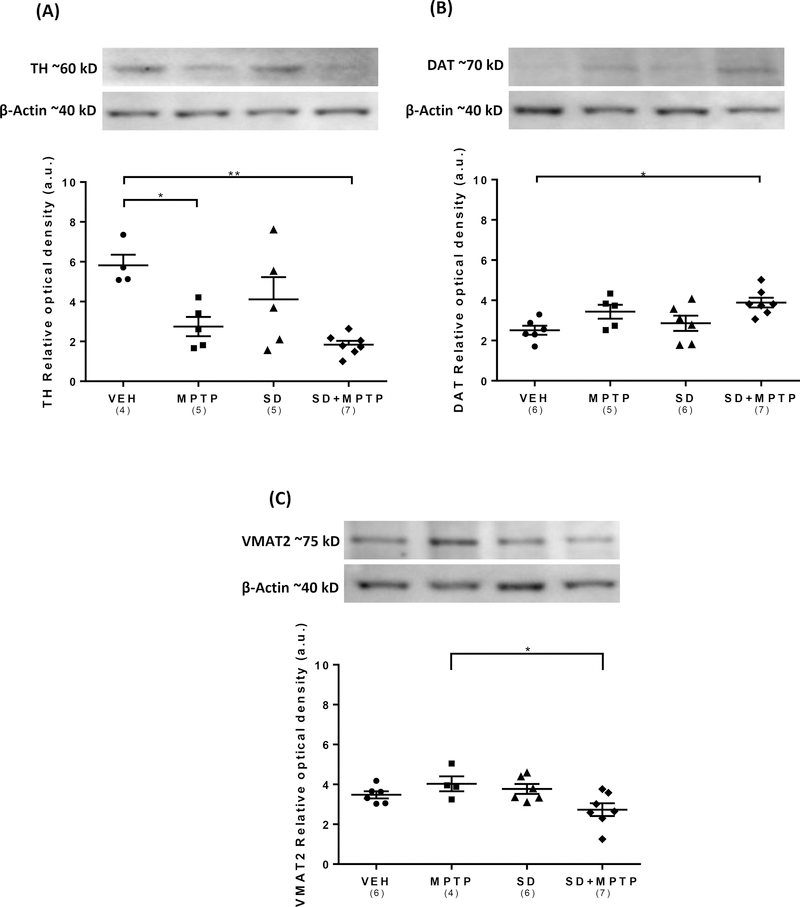
Analysis of TH protein expression within the SN/midbrain revealed a main effect of group on TH levels (Fig. 4A; F(3,17)= 7.37, p=0.0023). There was a 53% decrease in TH protein expression in the MPTP compared to the VEH group which was statistically significant (p=0.024) and a 69% decrease in the SD+MPTP compared to the VEH group (p=0.002). There was no difference in TH protein expression between the MPTP and SD+MPTP groups (p=0.71), the SD+MPTP and SD groups (0.064) or between the SD and VEH groups (p=0.31). Analysis of DAT protein expression within the SN/midbrain revealed a main effect of group on DAT levels (Fig. 4B; F(3,20)= 4.54, p=0.015). There was a statistically significant increase (54%) in DAT protein expression in the SD+MPTP compared to the VEH group (p=0.014). The 36% increase in DAT levels in the MPTP compared to the VEH group was not statistically significant (p=0.19). There were no other significant differences between the other groups, including a comparison between the MPTP and SD+MPTP groups (p=0.125). Analysis of VMAT2 protein expression within the SN/midbrain revealed a main effect of group (Fig. 4C; F(3,19)= 4.01, p=0.022). There was a statistically significant decrease in VMAT2 levels in the SD+MPTP compared to the MPTP group (p=0.031). These data suggest that SD had a differential effect on synaptic vesicles located within the remaining dopamine cell dendrites following MPTP-induced dopamine loss. There was no effect on VMAT2 expression following SD alone. There were no other significant changes between any of the other treatment groups. With regard to VMAT2 expression within the SN/midbrain, it is well established that the three major neuronal cell types in the SNpc are dopaminergic, GABAergic and glutamatergic (Nair-Roberts et al, 2008). The later are only located in the ventral tegmental area and not in the SNpc. There is no evidence that the GABAergic neurons also express VMAT2 protein. Therefore, the primary source of VMAT2 is that located in the dendrites, where dopamine release can occur.
Fig. 4: Western blot analysis of TH, DAT, and VMAT2 protein expression in the substantia nigra/midbrain.
(A) TH protein expression in the MPTP and SD+MPTP groups decreased (53% and 69%) when compared to the VEH group (p<0.05). (B) There was an increase in DAT expression in the SD+MPTP group compared to the VEH group (54%, p=0.014) and an increasing trend in the MPTP group (36%, p=0.19). (C) The SN/midbrain showed a significant decrease in VMAT2 protein expression in the SD+MPTP group compared to the MPTP group (p=0.03), but there was no significant decrease in VMAT2 in any other group when compared to the VEH group. Overall, there was no added loss or increase in any of the markers in the SD+MPTP compared to the MPTP group. Values are presented as the mean ± SEM of group averages. The symbols in each column refer to values from each animal. The number of animals used is in parentheses below the treatment group. * − p<0.05; ** − p<0.01.
SD and MPTP treatment have differential effects on blood corticosterone levels
Although TH protein expression within either the dorsolateral striatum or SN/midbrain was not significantly altered when comparing the MPTP and SD+MPTP groups, it is possible that these two treatments might differentially affect the stress axis. This leads to the question as to whether the loss of nigrostriatal dopamine results in added stress to these animals. This was evaluated by determining the levels of circulating corticosterone within the blood. Analysis of corticosterone levels within the blood revealed a main effect of group (Fig. 5; F(3,17)= 18.8, p<0.0001). There was a statistically significant decrease in corticosterone levels between the MPTP and VEH groups (p=0.001), a significant increase between the SD and VEH groups (p=0.023), but no difference between the SD+MPTP and VEH groups (p=0.823). In addition, there was a significant decrease (56%) in corticosterone levels between the MPTP and the SD+MPTP groups (p=0.0035). These data suggest that following MPTP treatment, with which there is a decrease in nigrostriatal TH, and perhaps a comparable decrease in dopamine levels within the hypothalamus, results in a decrease in activity of the stress axis, as measured by peripheral corticosterone levels, while SD results in an increase in stress. The combination of the two treatments, SD+MPTP, brings the levels of corticosterone back to the control/VEH values.
Fig. 5: Analysis of corticosterone blood levels.
There was a significant decrease in the plasma corticosterone level in the MPTP group and an increase in the SD group compared to the VEH group. There was a significant decrease in the SD+MPTP group compared to the SD only group, while the levels in the SD+MPTP group returned back to the VEH levels. Values are presented as the mean ± SEM of group averages. The symbols in each column refer to values from each animal. The number of animals used is in parentheses below the treatment group. * − p<0.05; ** − p<0.01; *** − p<0.001; **** − p<0.0001.
Motor (gait) function following SD and MPTP treatments
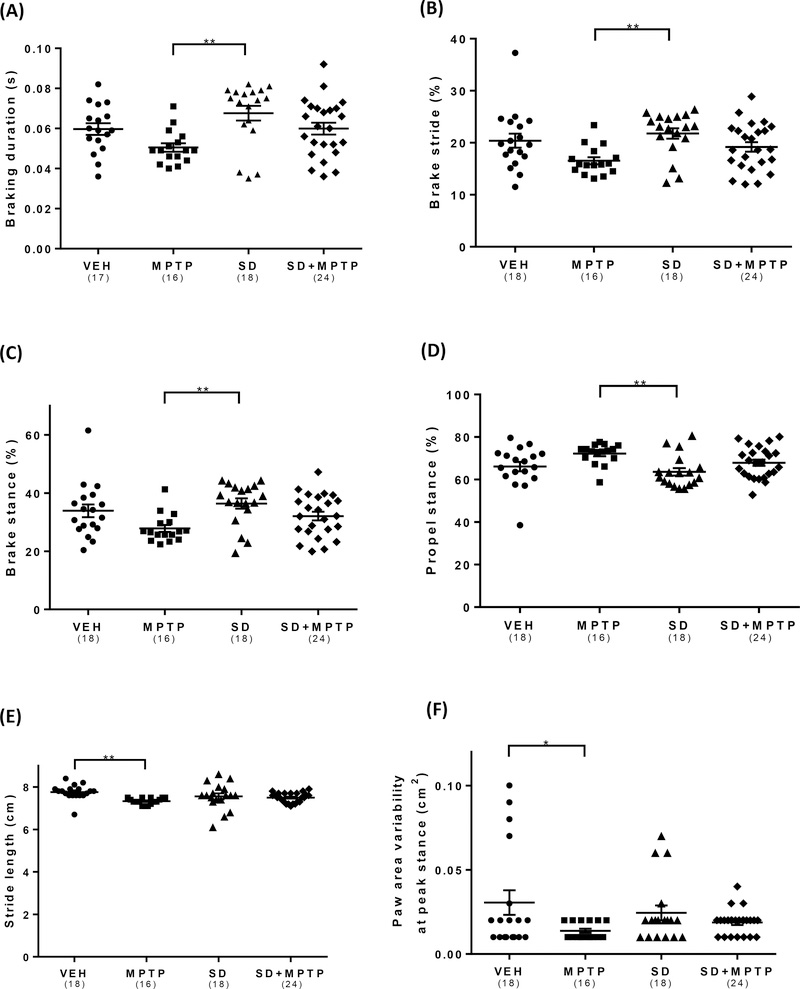
To determine if SD resulted in changes in motor function in the MPTP treated group, mice were tested for gait function using a DigiGait apparatus at the conclusion of 4 weeks of toxin administration, as previously reported (Goldberg et al, 2011b, Churchill et al, 2019). Analysis of the time duration of the braking phase for the mice (initial paw contact to maximum paw contact, commencing after the swing phase, in seconds) revealed a main effect of group (Fig. 5A; F(3,72)= 3.92, p=0.012). There was a significant decrease in the time to brake in the MPTP compared to the SD group (p=0.007). There was an interesting reversal in brake time between the MPTP and SD+MPTP groups, with the latter group showing a nearly 20% improvement in brake time (p=0.05), a value which was now similar to that seen in the VEH group. The same findings were seen in the measure for the percentage of time the animal was in the brake phase (% of the total stride duration that the paw is in the braking phase). Analysis of the % brake stride revealed a main effect of group (Fig. 5B; F(3,72)= 4.16, p=0.0089). There was a 25% decrease in the percentage the animal was in the brake phase in the MPTP compared to the SD group (p=0.0058). This effect was reversed in the SD+MPTP group compared to the VEH group (p=0.83). There was no difference in % brake stride between the MPTP and SD+MPTP group (p=0.07). A somewhat similar finding was seen when the percent brake stance [percentage of the stance phase (i.e., paw contact) that the paw is in the braking phase] was analyzed. Analysis of the % brake stance revealed a main effect of group (Fig. 5C; F(3,72)= 3.94, p=0.0116). There was a 25% decrease in the % brake stance in the MPTP compared to the SD group (p=0.0072). The findings in the SD+MPTP group were nearly equal to that of the VEH group (p=0.84), suggesting that SD, in the MPTP treated group, had resulted in an interesting recovery of this gait measure compared to that of the VEH/control group. There was no difference in % brake stance between the MPTP and SD+MPTP groups (p=0.299). Analysis of the % propel stance [the percentage of the stance phase (i.e., paw contact) in which the animal’s limbs are in the propulsion phase (i.e., paw coming off of the belt) revealed a main effect of group (Fig. 5C; F(3,72)= 3.94, p=0.0116). There was a small, but significant, increase in the % propel stance in the MPTP compared to the SD group (p=0.007). There was no change between the SD+MPTP and VEH groups (p=0.86) or the MPTP vs SD+MPTP groups (p=0.279). Similar to that seen in patients with PD (Blin et al., 1990; Stolze et al., 2001; Peppe et al., 2007) there was a main effect of group on stride length (the spatial length that a paw traverses through a given stride) (Fig. 5D; F(3,72)= 3.91, p=0.012), as we have previously reported (Goldberg et al, 2011b). There was a small but significant decrease in stride length in the MPTP compared to the VEH group (p=0.0067). There was no statistical difference between the SD+MPTP compared to the VEH group (p=0.1114), suggesting that there was an interesting recovery of this measure due to SD treatment. In addition, there was no difference in stride length between the MPTP and SD+MPTP groups (p=0.523). As the animal runs on the treadmill, the deviation of the paw area as the animal is moving (i.e., striding) and touching the belt can be analyzed. There was a main effect of group on paw area variability (Fig. 5F; F(3,72)= 2.77, p=0.047). There was a significant 54% decrease in the paw area variability (animal is more rigid) in the MPTP compared to the VEH group (p=0.041). In addition, there was no difference in paw area variability between the MPTP and SD+MPTP groups (p=0.83).
Discussion
Our initial hypothesis was that prior sleep disruption for several weeks, followed by subsequent administration of a progressive/chronic dosing of the neurotoxin, MPTP, would increase the nigrostriatal dopamine cell and terminal loss compared to toxin administration alone. Since it is well documented that PD patients, prior to the time of developing the motor symptoms of this disease, experience some form of nighttime sleep disruption (Chahine et al, 2017), it was reasonable to suggest that sleep disturbances may in fact play an additive/negative role in the dopamine degenerative process. In the current study, 4 weeks of sleep disruption using an orbital shaker to mirror the nocturnal sleep impairments frequently observed in PD patients (e.g. fragmented sleep and reduced REM) did not significantly accelerate/enhance the MPTP-induced loss of either TH positive terminals or cells within the striatum or SNpc, respectively, or TH protein levels within either of these two regions, although there were promising statistical trends in some conditions. There was a statistically significant effect of MPTP on some measures of gait when compared to the SD only group, suggesting that disturbances of sleep alone affect some aspects of motor performance in the opposite direction as MPTP. Consequently, there were no differences in the gait measures collected here between SD+MPTP and the VEH/control group. Interestingly, the loss of TH in the nigrostriatal pathway, even in those mice previously experiencing SD, was not correlated with improvement in those gait measures. With the hypothesis that SD exposure in the MPTP treated animals would augment the alterations due to MPTP alone, we report that for DAT protein expression in the striatum, VMAT2 levels in the SN/midbrain, and plasma corticosterone concentration, SD actually resulted in a reversal of those changes back towards the values seen in the VEH group.
Considering that SD may be a possible risk factor to the development of PD, could this increased sensitivity to dopamine loss be detected using an animal model of PD? One question was whether treatment with MPTP alone could result in such a large loss of nigrostriatal TH that it would be nearly impossible to detect any further loss following prior exposure to SD. As most likely seen in humans with PD, there is a large range of dopamine cell loss within the SNpc (Kordower et al, 2013). It has been suggested that in order to detect the motor symptoms of the disease, there must be at least 80% dopamine tissue loss within the striatum, which equates to about a 50–60% dopamine cell loss (Bernheimer et al, 1973; Riederer and Wuketich, 1976). In the current study, there was between a 50–60% loss in TH protein expression within the striatum by IHC and 70% by western immunoblotting, with a loss of about 40% of the dopamine/TH labeled nerve cells in the SNpc. These findings suggest that there is definitely the capacity to detect even further loss of TH within the nigrostriatal pathway following exposure to SD. The fact that exposure to SD prior to the start of the MPTP treatment did not result in any further loss of nigrostriatal TH suggests that at least in this study design, chronic sleep disruption as the experimental model did not result in any further deleterious effects on the nigrostriatal dopamine pathway following MPTP treatment.
There was a differential regulation of DAT within the striatum and SN/midbrain following MPTP and SD+MPTP. DAT is thought to be crucial for terminating dopamine action by clearing extraneuronal dopamine (Iversen, 1971). DAT is also heavily glycosylated protein (Li et al, 2004). Generally, including carbohydrates, post-translational modifications of proteins can contribute to protein folding and its stability at that conformation, regulating protein trafficking, and protecting against proteolysis (Lis and Sharon, 1998). Li et al (2004) carefully characterized DAT stability and activity when all or individual glycosylation sites were mutated and found that prevention of N-glycosylation decreased DA transport efficiency but did not terminate function. The increase in glycosylated DAT within the striatum following MPTP suggests that following nigrostriatal dopamine loss, this up-regulation of the dopamine transporter may be related to greater membrane stability and would lead to an increase in dopamine uptake to compensate for the loss of dopamine terminals and neurons. This up-regulation following MPTP was seen within the striatum, with DAT expression returning back towards the control levels in the SD+MPTP group. Considering that the SD was initiated prior to the start of the MPTP treatment, these results could also be interpreted as SD providing some aspect of protection against the effects of MPTP. Research in rats suggests that dopaminergic neurons in the SNpc may play a role in promoting REM sleep (Lima et al, 2007) and one of the long lasting effects of our chronic SD paradigm is a reduction in REM sleep during lights on with no change in wakefulness during lights off (Li et al, 2014). Within the SN/midbrain, there was a non-significant increase of 36% in DAT expression in the MPTP compared to the VEH group, with a further increase to 54% in the SD+MPTP group. During exposure to SD, the brain may be compensating for this disturbance and the effects are most noticeably seen following subsequent loss of dopamine due to MPTP. So there appears to be differential regulation of DAT within the striatum and midbrain, with the effects of SD producing opposite results following nigrostriatal dopamine loss.
For VMAT2, there is also a differential regulation within the striatum and SN/midbrain due to SD. We find increased protein expression in the SD+MPTP group within the striatum compared to the VEH group and decreased expression in the SD+MPTP group compared to the MPTP only group.
It has been reported that there is an increase in striatal dopamine D2 receptors following SD (Lim et al, 2011). According to the model of basal ganglia function (Albin et al, 1989, Obeso et al, 2008), following dopamine release in the striatum, activation of these dopamine D2 receptors could eventually result in inhibition of the external globus pallidus, leading to increased activity of the thalamocortical pathway and motor movement. These findings would be consistent with our observation of gait function returning to that seen in the VEH group in the previously exposed SD group that was subsequently treated with MPTP.
One of the more intriguing findings in this study was the effect of SD and MPTP on corticosterone levels. Although we only investigated the effects of MPTP within the nigrostriatal pathway, there is a dopamine input to the hypothalamus and pituitary, where the release of dopamine can regulate the levels of ACTH and subsequently that of corticosterone (Di et al, 2019). The depletion of dopamine following MPTP resulted in a decrease in plasma corticosterone levels, possibly due to the decrease in dopamine within the hypothalamus and effecting the stress axis. The increase in corticosterone following SD alone is also quite interesting, considering that it was reported that SD for up to 4 weeks did not result in any change in the levels of this hormone (Li et al, 2014). One major difference was that in the current study, the SD only mice were handled and injected with vehicle for 4 weeks during the time that the SD+MPTP group was injected with the toxin compared to the Li et al study in which the mice were not handled. Additionally, in our study, SD included an additional auditory disruption (cage card holder) that was not included in the study conducted by Li et al (2014). Regardless, the combination of SD and MPTP administration resulted in the levels of corticosterone now being equivalent to that of the VEH control group. It’s apparent that at least in terms of the nigrostriatal dopamine pathway, SD did not enhance or augment the loss of TH following MPTP. As we have reported, there can be motor improvement following exercise with there being no recovery of nigrostriatal dopamine (Sconce et al, 2015b, Hood et al, 2016; Churchill et al, 2017). Therefore, the findings of the current study would be consistent with the fact that some motor improvements occur even with the lack of nigrostriatal dopamine recovery.
Fig. 6: Gait analysis of MPTP and SD treated animals.
Testing of mice using a DigiGait apparatus showed decreases in several gait measures in animals treated with MPTP, followed by near recovery to the VEH values in the SD+MPTP group. (A) There was a significant decrease in braking time (s) in the MPTP group compared to the SD group (p<0.01). A shorter braking time may indicate less precise control and weight load. There was a reversal in brake time between the MPTP and SD+MPTP groups, with the latter group showing a nearly 20% improvement in brake time, similar to that seen in the VEH group. (B) The MPTP group showed a decrease in brake stride (%) when compared to the SD group (p<0.01). This effect was reversed in the SD+MPTP group compared to the VEH group. (C) There was a decrease in % brake stance [i.e. percentage of the stance phase (i.e., paw contact) that the paw is in the braking phase] in the MPTP compared to the SD group (p<0.01), which recovered in the SD+MPTP group to the level of the VEH group. (D) There was an increase in the % propel stance [the percentage of the stance phase (i.e., paw contact) in which the animals’ limbs are in the propulsion phase (i.e., paw coming off of the belt) in the MPTP group compared to the SD group (p<0.01). There was no change between the SD+MPTP compared to the VEH group. (E) There was a decrease in stride length between the MPTP and VEH group (p<0.01), which recovered in the SD+MPTP group to the level of the VEH group. (F) There was a significant decrease in paw area variability between the MPTP and VEH group (p<0.05), while there was no difference between the SD+MPTP group and the VEH group. Overall, there was a main effect of MPTP on various gait measures but in the SD+MPTP group, there were no differences compared to the VEH group. Values are presented as the mean ± SEM of group averages. The symbols in each column refer to values from each animal. The number of animals used is in parentheses below the treatment group. * − p<0.05; ** − p<0.01; *** − p<0.001; **** − p<0.0001.
Highlights.
MPTP resulted in a significant decrease in striatal and midbrain TH expression, which was not further affected in the SD+MPTP group.
MPTP resulted in a significant decrease in the number of TH labeled cells in the SNpc, which was not further affected in the SD+MPTP group.
MPTP resulted in a significant increase in midbrain DAT protein expression, which was not further affected in the SD+MPTP group.
Corticosterone serum levels were significantly decreased in the MPTP group, significantly increased in SD only group and returned to normal VEH levels in the SD+MPTP group.
In several measures of gait, there was an effect of MPTP, which then returned to the VEH levels in the SD+MPTP group.
Significance Statement.
Patients with Parkinson’s disease (PD) report significant nighttime sleep disruption (SD). The question is whether SD augments/accelerates the loss of nigrostriatal dopamine in an animal model of parkinsonism. Using a progressive/chronic toxin (MPTP) mouse model of parkinsonism, with prior exposure to SD before treatment with MPTP, we find that SD did not result in a greater loss of the dopamine biomarker, tyrosine hydroxylase, compared to the MPTP only treated group. Using this model of SD in chronically MPTP treated mice, our data suggests that prior exposure to SD does not accelerate the loss of nigrostriatal dopamine. As an extension to PD patients who experience SD, this does not lead to an exacerbated loss of dopamine.
Acknowledgments
This work was supported by Merit Review Grant #1BX 001643 and #1BX 000552 to CKM, Merit Review Grant #1 BX002966 and NIH grant R01 AA012439 to DAF, and VA Career Development Award # IK2 BX002712 to MML, from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development. This work was also supported with resources and the use of facilities at the VA Portland Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Additional funding was provided by NIH grant R01 AA012439 to DAF and NIH EXITO Institutional Core, # UL1GM118964 to MML, and NIH T32 5T32HL083808-10 to CEJ.
Footnotes
CONFLICTS OF INTEREST:
The authors declare that they have no competing interests.
Data Availability Statement: All datasets generated for this study are included in the manuscript.
DECLARATION OF TRANSPARENCY:
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
I have read and abided by the statement of ethical standards for manuscripts submitted to Journal of Neuroscience Research.
All Authors have approved the final article
References
- Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375. [DOI] [PubMed] [Google Scholar]
- Aldrich MS. Parkinsonism In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia: WB Saunders, 2000: 1051–1057 [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ (2009) A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience 161:1082 1090. 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I (2009). Sleep disorders in Parkinson’s disease: the contribution of the MPTP non-human primate model. Exp Neurol 219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lange N (2001) Two-dimensional versus three dimensional cell counting: a practical perspective. Trends Neurosci 24:11–17. 10.1016/S0166-2236(00)01660-X. [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday H, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2015). MDS research criteria for prodromal Parkinson’s disease. Movement Dis 30:1600–1609. DOI: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973). Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455. [DOI] [PubMed] [Google Scholar]
- Blin O, Ferrandez AM, Serratrice G (1990). Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci 98:91–97. [DOI] [PubMed] [Google Scholar]
- Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA (2014) Sleep and Circadian Rhythm Regulation in Early Parkinson Disease, JAMA Neurol 71:589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bušková J, Klempíř J, Majerovaá V, Picmausova J, Sonka K, Jech K, Roth J, Ruzicka E (2011) Sleep disturbances in untreated Parkinson’s disease. J Neurol 258:2254–2259. doi: 10.1007/s00415-011-6109-7 [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA (2014). Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 48:741–754. doi: 10.1016/j.alcohol.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Daley J, Horn S, Duda JE, Colcher A, Hurtig H, Cantor C, Dahodwala N (2013) Association between dopaminergic medications and nocturnal sleep in early-stage Parkinson’s disease. Parkinsonism Relat Disord 19:859–63. doi: 10.1016/j.parkreldis.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Chahine LM, Amara AW, Videnovic A (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Medicine Reviews 35:33–50. doi.org/ 10.1016/j.smrv.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Pflibsen L, Sconce MD, Moore C, Kim K, Meshul CK (2017) Exercise in an animal model of Parkinson’s disease: motor recovery but not restoration of the nigrostriatal pathway. Neurosci 359: 224–247. doi: 10.1016/j.neuroscience.2017.07.031 [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Cantu MA, Katanga EA; Moore C, Salvatore MF, Meshul CK (2019) Glatiramer acetate reverses motor dysfunction and the decrease in tyrosine hydroxylase levels in a mouse model of Parkinson’s disease. Neurosci 414: 8–27. doi: 10.1016/j.neuroscience.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Clarke PGH (1992). How inaccurate is the Abercrombie correction factor for cell counts? Trends Neurosci 15:211–212. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003). Parkinson’s disease: mechanisms and models. Neuron 39:889–909. [DOI] [PubMed] [Google Scholar]
- Di T, Chen P, Yuan Z, Sang Y, Sha S, Chen L (2019). Dorsal hypothalamic dopaminergic neurons play an inhibitory role in the hypothalamic-pituitary-adrenal axis via activation of D2R in mice. Acta Physiol 225:e13187 10.1111/apha.13187 [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Vaillant M, Mancuso G, Lyen P, Tiete J (2005) Progressive sleep ‘destructuring’ in Parkinson’s disease: a polysomnographic study in 46 patients. Sleep Med 6:313–318. [DOI] [PubMed] [Google Scholar]
- Goldberg NRS, Haack AK, Lim NS, Janson OK, Meshul CK (2011a). Dopaminergic and behavioral correlates of progressive lesioning of the nigrostriatal pathway with MPTP. Neurosci 180:256–271. [DOI] [PubMed] [Google Scholar]
- Goldberg NRS, Hampton T, McCue S, Kale A, Meshul CK (2011b). Profiling changes in gait dynamics due to progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal lesioning. Journal of Neuroscience Research 89:1698–706. [DOI] [PubMed] [Google Scholar]
- Goldberg NRS, Fields V, Pflibsen L, Salvatore MF, Meshul CK (2012). Social enrichment attenuates nigrostriatal lesioning and reverses motor impairment in a progressive 1-methyl-2-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiology of Disease 45:1051–1067. [DOI] [PubMed] [Google Scholar]
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM (2019). The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood RL, Liguore WA, Moore C, Pflibsen L, Meshul CK (2016). Exercise intervention increases spontaneous locomotion but fails to attenuate dopaminergic system loss in a progressive MPTP model in aged mice. Brain Res 1646:535–542. 10.1016/j.brainres.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Hyacinthe C, Barraud Q, Tison F, Bezard E, Ghorayeb I (2014) D1 receptor agonist improves sleep-wake parameters in experimental parkinsonism. Neurobiology of Disease, 63, 20–24. [DOI] [PubMed] [Google Scholar]
- Iversen LL (1971). Role of transmitter uptake mechanisms in synaptic neurotransmission. Brit J Pharmacol 41:571–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Opel RA, Kaiser ME, Chau AQ, Quintana JR, Nipper MA, Finn DA, Hammock EAD, Lim MM (2019). Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Science Adv 5:eaav5188. doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136:2419–2431. 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Panossian LA, JZhang J, Zhu Y, Zhan G, Chou Y-T, Fenik P, Bhatnagar S, Piel DA, Beck SG, Sigrid Veasey S (2014). Effects of Chronic Sleep Fragmentation on Wake-Active Neurons and the Hypercapnic Arousal Response. Sleep 37:51–64. 10.5665/sleep.3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L-B, Chen N, Ramamoorthy S, Chi L, Cui X-N, Wang LC, Reith MEA (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem 279:21012–21020. [DOI] [PubMed] [Google Scholar]
- Lim MM, Xu J, Holtzman DM Mach RH (2011). Sleep deprivation differentially affects dopamine receptor subtypes in mouse striatum. Neuroreport 22:489–493. doi: 10.1097/WNR.0b013e32834846a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima MM, Andersen ML, Reksidler AB, Vital MA, Tufik S (2007) The role of the substantia nigra pars compacta in regulating sleep patterns in rats. PloS one, 2(6), e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H, Sharon N (1998) Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem Rev 98:637–674. [DOI] [PubMed] [Google Scholar]
- McDowell KA, Shin D, Roos KP, Chesselet MF (2014) Sleep dysfunction and EEG alterations in mice overexpressing alpha-synuclein. J Parkinsons Dis 4:531–539. doi: 10.3233/JPD-140374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ (2008). Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord 14 (Suppl 2):S112–S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaca C, Laloux C, Jacquesson JM, Gelé P, Maréchal X, Bordet R, Destee A, Derambure P (2004). Vigilance states in a parkinsonian model, the MPTP mouse. Eur J Neurosci 20:2474–2478. [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW (2008). The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol 64 Suppl 2:S30–46. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, eds (2004) The mouse brain in stereotaxic coordinates, 2nd ed. San Diego, CA: Gulf Professional Publishing. [Google Scholar]
- Peppe A, Chiavalon C, Pasqualetti P, Crovato D, Caltagirone C (2007). Does gait analysis quantify motor rehabilitation efficacy in Parkinson’s disease patients? Gait Posture 26:452–462. [DOI] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau Y S (2001). Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neurosci 106, 589–601. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V (1998). Mechanisms of MPTP toxicity. Mov Disord 13 (Suppl 1):35–38. [PubMed] [Google Scholar]
- Riederer P, Wuketich S (1976). Time course of nigrostriatal degeneration in Parkinson’s disease. J Neural Transm 38:277–301. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Gonzalez-Hernandez T (1999). Electrophysiological and morphological evidence for a GABAergic nigrostriatal pathway. J Neurosci 19:4682–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR (2009). Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res 16:127–139. [DOI] [PubMed] [Google Scholar]
- Sconce MD, Churchill MJ, Moore C, Meshul CK (2015a). Intervention with 7,8-dihyroxyflavone blocks further striatal terminal loss and restores motor deficits in a progressive mouse model of Parkinson’s disease. Neuroscience 290:454–471. [DOI] [PubMed] [Google Scholar]
- Sconce MD, Churchill MJ, Greene RE, Meshul CK (2015b). Intervention with exercise restores motor deficits but not nigrostriatal loss in a progressive MPTP mouse model of Parkinson’s disease. Neuroscience, 299:156–174 (2015). [DOI] [PubMed] [Google Scholar]
- Sinton CM, Kovakkattu D, Friese RS (2009). Validation of a novel method to interrupt sleep in the mouse. J Neurosci Meth 184:71–78. [DOI] [PubMed] [Google Scholar]
- Smolen AJ, Wright LL, Cunningham TJ (1983) Neuron numbers in the superior cervical ganglion of the rat: a critical method of cell counting. J Neurocytol 12:739–750. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S (1998) Sleep disorders in Parkinson’s Disease. J Neurol 245 (Suppl 1):S15–18. [DOI] [PubMed] [Google Scholar]
- Stolze H, Kuhtz-Buschbeck JP, Drucke H, Johnk K, Illert M, Deuschl G (2001). Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry 70:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandberg E, Larsen JP, Karlsen K (2004). A community-based study of sleep disorders in patients with Parkinson’s disease. Movement Disorders 13:895–899 [DOI] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J (2004). Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry 75:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science 342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK (2011) Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One 6(7):e22511. [DOI] [PMC free article] [PubMed] [Google Scholar]