Abstract
A single exercise bout has been found to improve the retention of a skill-based upper extremity motor task up to a week post-practice. This effect is the greatest when exercise intensity is high and exercise is administered immediately after motor practice (i.e., early in consolidation). Whether exercise can affect other motor learning types (e.g., sensorimotor adaptation) and tasks (e.g., walking) is still unclear as previous studies have not optimally refined the exercise parameters and long-term retention testing. Therefore, we investigated whether a single high-intensity exercise bout during early consolidation would improve the long-term retention and relearning of sensorimotor adaptation during split-belt treadmill walking. Twenty-six neurologically intact adults attended three sessions; sessions 2 and 3 were one day and seven days after session 1, respectively. Participants were allocated either to Rest (REST) or to Exercise (EXE) group. In session 1, all groups walked on a split-belt treadmill in a 2:1 speed ratio (1.5:0.75 m/s). Then, half of the participants exercised for five minutes (EXE), while the other half rested for five minutes (REST). A short exercise bout during early consolidation did not improve retention or relearning of locomotor memories one or seven days after session 1. This result reinforces previous findings that the effect of exercise on motor learning may differ between sensorimotor locomotor adaptation and skilled-based upper extremity tasks; thus, the utility of exercise as a behavioral booster of motor learning may depend on the type of motor learning and task.
Keywords: sensorimotor adaptation, behavioral priming, consolidation, multiday motor learning, walking, gait rehabilitation
Introduction
In addition to the long-term benefits of exercise, an acute exercise bout may also affect the cognitive and motor function of the brain via neural processes at both molecular and functional level (Mang et al. 2013; El-Sayes et al. 2018). Based on that notion, numerous studies in the last few years have examined whether an acute exercise bout could improve the memory of motor tasks in neurologically intact persons and how the structure of that exercise impacts its effects (Roig et al. 2012; Skriver et al. 2014; Statton et al. 2015; Mang et al. 2016; Snow et al. 2016; Thomas et al. 2016a; Thomas et al. 2016b; Ferrer-Uris et al. 2017; Lundbye-Jensen et al. 2017; Stavrinos and Coxon 2017; Dal Maso et al. 2018; Ferrer-Uris et al. 2018; Neva et al. 2019). Most of these studies have tested the effect of exercise on motor learning by examining retention (i.e., maintenance of a newly learned task after a period delayed from practice of the same task (Christina and Shea 1993)) of the motor task one and seven days post-practice. The cumulative evidence from these studies suggests that exercise improve retention of upper extremity motor tasks in neurologically intact individuals and that the improved retention appears greatest when examined seven days post-practice. Several of these studies have examined how the parameters of the acute exercise impacts the improved retention; of the exercise parameters studied, intensity and timing have the greatest effect on motor learning (Roig et al. 2012; Thomas et al. 2016a; Thomas et al. 2016b; Thomas et al. 2017), while the type of exercise seems to have little effect. Specifically, retention is the greatest when exercise is administered at high intensities (i.e., post-exercise lactate levels greater than 10 mmol/l) and is performed immediately after motor practice (i.e., early in consolidation) (Roig et al. 2012; Thomas et al. 2016a; Thomas et al. 2016b).
The mechanism by which acute exercise improves motor learning it still unclear; however, it is believed that the benefits stem from exercise-induced upregulation of neurophysiological biomarkers, such as lactate and brain derived neurotrophic factor (BDNF) (Skriver et al. 2014). Though lactate has been mainly considered as a metabolic agent (Hall et al. 2016), the findings from the Skriver et al. study suggested that high concentrations of exercise-induced lactate may affect motor learning by enhancing brain functions, such as neuronal metabolism, neuroprotection, and long-term memory formation (Barros 2013; Proia et al. 2016; Steinman et al. 2016). Like lactate, cellular level BDNF is thought to be a potential link between exercise and learning (Cotman et al. 2007; Mang et al. 2013). However, results on whether the exercise-induced BDNF changes are associated with gains in motor learning in neurologically intact adults are still mixed (Mang et al. 2014; Skriver et al. 2014).
All of the previous studies that have found a positive effect of exercise on motor learning have studied upper extremity tasks, either procedural motor skills or motor sequences (Roig et al. 2012; Skriver et al. 2014; Ostadan et al. 2016; Thomas et al. 2016a; Thomas et al. 2016b; Lundbye-Jensen et al. 2017; Thomas et al. 2017; Dal Maso et al. 2018). The effects of exercise on learning and retention of a walking task, however, are far less positive (Helm et al. 2017; Charalambous et al. 2018b). Helm et al. (Helm et al. 2017) examined whether a short bout of arm cycling exercise performed immediately prior to split-belt treadmill learning would improve the 24-hour retention of locomotor learning in healthy individuals. In that study, learning was quantified using spatial (step length) and temporal (limb phasing) gait parameters; two measures that are widely used to quantify sensorimotor-based locomotor learning (Reisman et al. 2005; Choi and Bastian 2007; Reisman et al. 2007; Malone and Bastian 2010; Torres-Oviedo and Bastian 2010). Despite the significant exercise-induced changes in BDNF and lactate, no significant effect of exercise on locomotor learning was demonstrated (Helm et al, 2017). The non-significant exercise effect has been posited to be: a) the result of a differing effect of exercise on locomotor sensorimotor adaptation and/or b) due to the non-optimal selection of exercise parameters (e.g., intensity, timing) and time points of retention testing (e.g., 1 day vs. 7 days).
As previously described, previous work suggest that acute exercise should be performed at high-intensity (i.e., post-exercise lactate levels greater than 10 mmol/l) and immediately after motor practice (i.e., early phase of consolidation) to achieve maximal effects. In Helm et al., the exercise group had significantly greater lactate changes than those who just rested (Helm et al. 2017). Yet, exercise-induced lactate changes did not reach the level (> 10 mmol/l) which has been reported in the previous studies (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b). Moreover, the exercise was administered immediately before the motor practice, a time point that might not maximize the learning gains as much as if exercise was administered immediately after (Roig et al. 2012; Thomas et al. 2016a). Additionally, Helm et al. only tested the effects of exercise on retention 1 day post-learning, whereas previous studies of the effects of acute exercise on the learning of upper extremity tasks have found the greatest effects seven days post-learning (Helm et al. 2017). This noteworthy “7-day” effect of exercise on long-term motor memory was consistent across studies (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b; Lundbye-Jensen et al. 2017) and has been suggested to be related to the longer-term structural and network changes associated with memory consolidation (Thomas et al. 2016b).
Thus, it is possible that in the past studies of locomotor learning, the acute exercise timing and intensity were not optimally selected and the effects were not tested at optimal time points, resulting in the negative effect. Understanding whether this is the case is important because if true, it would suggest that there may not be a fundamental difference in the effects of acute exercise on differing forms of motor learning (skill-based vs. sensorimotor adaptation) or types of motor tasks (upper extremity isometric contraction vs. walking).
In the present study, therefore, we aimed to determine whether exercise would improve locomotor learning (i.e., retention and relearning) of a novel locomotor task (i.e., split-belt treadmill walking) one day and seven days post-learning in neurologically intact adults by targeting post-exercise lactate levels ≥ 10 mmol/l (exercise intensity: high) and administering exercise during the early consolidation phase (exercise timing: immediately after practice). We hypothesized that those who exercised with these two exercise parameters would show greater long-term retention (i.e., less asymmetric gait at the beginning of session 2 and 3 compared to session 1) and relearning (i.e., return faster toward baseline symmetry in session 2 and 3) than those who did not exercise, and that this difference would be the greatest at seven days after session 1 (i.e., session 3).
Methods
Participants
Twenty-six neurologically intact participants participated in the study and met the following criteria: 1) age 18–40, 2) no previous experience with the split-belt treadmill paradigm, 3) resting heart rate between 40–100 beats per minute, and 4) resting blood pressure between 90/60 to 170/90. Participants were excluded if they had any of the following criteria: 1) history of neurologic conditions, 2) coronary artery bypass graft, myocardial infarction, or other major cardiovascular intervention within past 3 months, 3) acute systemic infection, accompanied by fever, body aches, or swollen lymph glands, 4) musculoskeletal pain that limited walking or exercising with upper and lower extremities, 5) inability to communicate with investigators, 6) unexplained dizziness in last 6 months, 7) intermittent claudication, or 8) greater than two risk factors in accordance with the American Heart Association and American College of Sports Medicine Health/Fitness Facility Pre-Participation Screening Questionnaire (Balady et al. 1998). All participants read and signed a written informed consent approved by the University of Delaware Institutional Review Board and adhered to the tenets of the Declaration of Helsinki.
Experimental Procedures
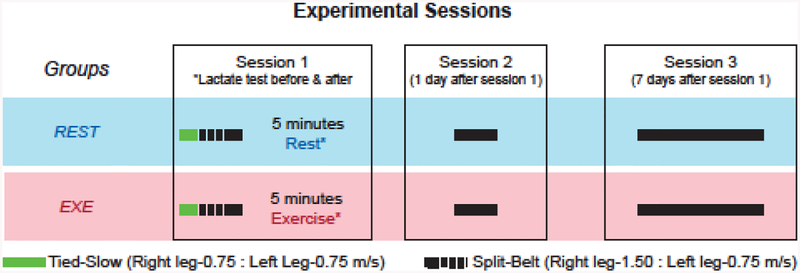
All participants attended three experimental sessions; session 2 and 3 were one day and seven days after session 1, respectively. All 3 sessions were carried out at the same time of the day. Participants were allocated to one of the four groups that differed based on whether they rested (REST) or exercised (EXE) after learning and whether they immediately walked on split-belts at the start of sessions 2 and 3 (NO_WASH) or whether they performed tied-belt walking (i.e. washout) before re-introduction to the split-belt treadmill paradigm in sessions 2 and 3 (WASH). Combining these conditions (REST, EXE × NO_WASH, WASH) resulted in four groups (N): RESTNO_WASH (5), EXENO_WASH (5), RESTWASH (8), and EXEWASH (8). Because there were no differences in the EXE or REST groups regardless of the inclusion of a washout period, only the results comparing EXE and REST are presented (e.g., not broken into subgroups based on presence of washout).
Exercise protocol
Previous work demonstrated that exercise may have the greatest effect on motor learning when participants exercised at high-intensity immediately after motor practice (Roig et al. 2012; Skriver et al. 2014; Roig et al. 2016; Thomas et al. 2016a; Thomas et al. 2016b). Therefore, in the present study, high-intensity exercise was performed shortly after practice of the novel locomotor task (~15–20 minutes). Specifically, the goal for the exercise group (EXE) was to exercise continuously at the high-intensity target range, which was defined as 77–94% heart rate max (HRmax= 207 – (0.7 × age)(Pescatello 2014), and to reach post-exercise lactate levels greater than 10 mmol/l (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b; Lundbye-Jensen et al. 2017). Both exercise and rest bout duration was 5 minutes. Exercise duration used in this study was much shorter than previous priming studies in neurologically intact adults, where exercise duration ranged from 12 to 28 minutes of high-intensity interval training. Given the potential applications of this priming strategy in neurorehabilitation (Stoykov and Madhavan 2015; Stoykov et al. 2017), we chose an exercise duration of only 5 minutes as this duration would be feasible within the context of rehabilitation in neurologically impaired individuals (Charalambous et al. 2018a). We used the same total body exercise protocol previously used in our lab (Charalambous et al. 2018a; Charalambous et al. 2018b). Briefly, participants cycled on the total body exerciser (SCIFIT, Tulsa, OK, USA) using both arms and legs. Compared to fast running (Winter et al. 2007), we found via pilot work that using this protocol, healthy participants can exercise at high intensities and show lactate changes greater than 10 mmol/l within 5 minutes of exercise. Participants in REST group sat quietly on a chair for five minutes. The intensity during exercise and rest was quantified using HR and peripheral concentration of blood lactate. HR was taken in real time every 15 seconds (i.e., total of 17 measurements) using a HR monitor (Polar Electro Inc., Lake Success, NY, USA), while blood lactate was collected 1 minute immediately before and 1 minute immediately after the 5 minutes of exercise or rest using a finger-stick automated portable Lactate Plus analyzer and test strips (Noval Medical, Waltham, MA, USA)(Hart et al. 2013).
Locomotor Learning Task
In all three sessions, participants walked on a split-belt treadmill in which the belts moved at the same (tied-belt mode) or different (split-belt mode) speeds (Reisman et al. 2005) (Fig. 1). At the beginning of session 1, all participants walked for 2 minutes while both belts moved at 0.75 m/s (i.e., tied-slow mode; 1:1 speed ratio). This data was used to quantify the baseline walking pattern (i.e., step length) of each participant. After baseline, all participants walked in a split-belt mode (2:1 speed ratio; right belt – 1.5 m/s, left belt: 0.75 m/s) for 5 minutes. To test how much participants retained and relearned the motor patterns practiced during split-belt treadmill walking, participants returned to the lab one day (session 2) and seven days (session 3) after session 1 to again walk on the treadmill in the split-belt mode at the same 2:1 speed ratio for 5 minutes (session 2) or 15 minutes (session 3) The duration walked in the split-belt mode was selected due to a recent study in which a short bout (7.5 minutes) of split-belt training resulted in the same retention as a longer, 30 minute, bout of training (Day et al. 2018).
Fig. 1.
Schematic of experimental sessions. Participants were allocated either to REST or to EXE group and attended three experimental sessions, including session 1 and two sessions, which were one day and seven days after session 1. Session 1 consisted of 2 minutes of baseline walking, 5 minutes of split-belt treadmill walking, and five minutes of either rest or exercise. Lactate was collected immediately before and after this 5-minute rest or exercise bout (see asterisk). On session 2, participants repeated only the 5 minutes of split-belt walking. On session 3, experimental procedures were the same as session 2 with the exception that the duration of the split-belt treadmill walking was 15 minutes. Green solid line and black dashed lines indicate tied-slow and split-belt walking, respectively.
During treadmill trials in all three sessions, participants wore a ceiling mounted harness without body weight support and held onto the front horizontal bar. During all walking trials, no form of demonstration, verbal instructions, or augmented feedback was provided. To prevent participants from using visual feedback of the belts’ speeds, we instructed them to look straight ahead and avoid looking down. We also blocked the view of the treadmill by covering the top part of the treadmill. Between tied-slow and split-belt mode, the treadmill was stopped for 15-seconds. The onset, offset, and the speed of the treadmill belts were controlled by a custom-written script in Matlab (Mathworks, Inc.).
All walking trials were performed on a dual-belt treadmill instrumented with 2 independent 6-degree of freedom force platforms (Bertec Corporation, Columbus, OH, USA). Kinetic and kinematic data were continuously acquired using Vicon Nexus (Vicon Motion Systems Ltd, Oxford, UK) as described in our previous work (Helm et al. 2016; Helm et al. 2017; Charalambous et al. 2018a). Briefly, force data were recorded by the 2 force-plates and sampled at 1000 Hz, while the three-dimensional coordinates of a total of six retroreflective markers, which were placed on bilateral heels, lateral malleoli, and fifth metatarsals, recorded with 8 motion capture Vicon cameras (Vicon Motion Systems Ltd, Oxford, UK) and sampled at 100 Hz.
Data Analyses
Exercise-Derived Measures
Similar to our previous work (Charalambous et al. 2018a; Charalambous et al. 2018b), we calculated three measures. To quantify whether participants exercised at the target high-intensity range, we averaged the exercise intensity at the target high-intensity range (averageHRtargetmtensity) and then divided by the HRmax ([averageHRtargetmtensity/HRmax]*100; % HRmax). Then, to quantify how long participants exercised at the target high-intensity range, we calculated the time spent (minutes) at that range. For the participants in the rest groups, a value of 0 was assigned for both measures if the HR did not reach the high-intensity target range. Finally, we calculated the magnitude of blood lactate change (after exercise – before exercise; mmol/l).
Gait Events
Kinematic data were analyzed offline using Vicon Nexus (Vicon Motion Systems Ltd, Oxford, UK) and Matlab (Mathworks, Natick, MA). The gait events of heel strike and toe off were calculated using an automated algorithm (Zeni et al. 2008). To ensure the accuracy of the automated custom-written scripts, gait events were also visually inspected for all trials across participants.
Locomotor Learning Parameters
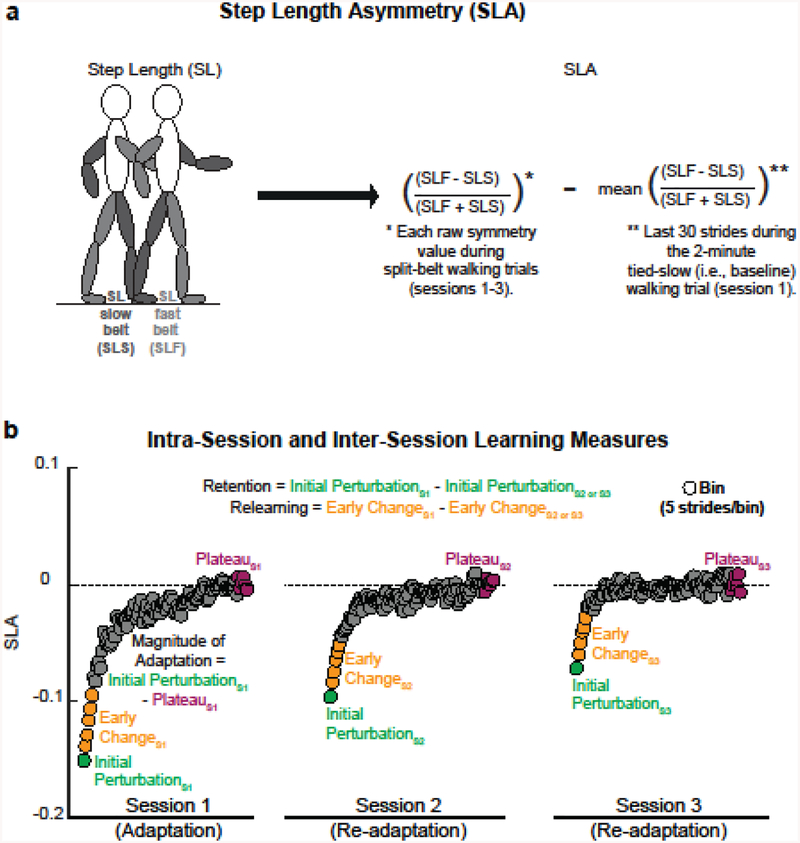
To quantify the intra- and inter-session learning of locomotor patterns, step length asymmetry (SLA: the difference between the step length of the fast and slow legs normalized by the sum of the two step lengths) was first calculated (Fig. 2a). As opposed using other gait-related metrics which may indicate certain components of walking capacity, SLA was used because numerous studies in both healthy participants and stroke survivors have demonstrated that this index is a robust measure of intra-session (adaptation) and inter-session (re-adaptation) learning during walking on the split-belt treadmill (Reisman et al. 2005; Reisman et al. 2007; Reisman et al. 2009; Malone and Bastian 2010; Malone et al. 2011; Vasudevan et al. 2011; Finley et al. 2013; Malone and Bastian 2014; Charalambous et al. 2018a; Day et al. 2018; Leech et al. 2018). To correct for between-participant differences in baseline symmetry, the average baseline symmetry was subtracted from each raw symmetry value during split-belt walking trials (Fig. 2a).
Fig. 2.
Schematic of the calculation of SLA and locomotor learning measures, (a) We first calculated the step length (sagittal distance between ipsilateral and contralateral foot at ipsilateral foot contact) bilaterally and then calculated the SLA, which was normalized to baseline symmetry, (b) Three distinct time periods from the split-belt treadmill walking (initial perturbation: first 5 strides; early change: strides 6–30; plateau: last 30 strides) were calculated during split-belt treadmill walking within each session. Using these measures, one intra-session and two inter-session learning measures were calculated to quantify the amount of adaptation on session 1 and the amount of retention and relearning on session 2 and 3 compared to session 1, respectively. Data are from a representative subject.
Both intra- and inter-session locomotor learning was quantified using a total of five measures. Fig. 2b depicts the calculation of locomotor learning measures using a dataset from representative subject. During the split-belt treadmill walking within each day, we calculated SLA over three distinct time periods to characterize intra-session learning: the initial perturbation (mean of the first 5 strides), early change (mean of strides 6–30), and final adapted state (i.e., plateau: mean of the last 30 strides) (Roemmich and Bastian 2015; Malone and Bastian 2016). For session 1 only, we also calculated the magnitude of adaptation (difference between initial perturbation and plateau: mean of the first 5 strides - mean of the last 30 strides).
To test the main effect of exercise on long-term locomotor learning, we calculated two inter-session learning measures that reflect retention and relearning of what was remembered from session 1 to session 2 (S1–S2) or session 3 (S1–S3). First, we calculated the difference between initial perturbation; this measure reflects the amount of the sensorimotor pattern retained between days (i.e., RETENTI0NS1–S2, RETENTIONS1–S3) (Musselman et al. 2016; Charalambous et al. 2018a). Second, we calculated the difference between early change, which has been used in previous studies to generally reflect adaptation rate (Roemmich and Bastian 2015; Day et al. 2018). The difference in early change thus reflects the differences in the inter-session learning/relearning rate (i.e., RELEARNINGS1–S2, RELEARNINGS1–S3). Inter-session gains in locomotor learning are indicated by greater values in both retention and relearning.
Statistical Analyses
Given the sample size per group, we ran only non-parametric statistical analyses and calculated the effect sizes using SPSS v25 (IBM Corp, Armonk, NY, USA). All data is presented as either group mean ± 1SD or group median (Fig. 3b & 3c) while the significance level was set at P<0.05. First, a Mann-Whitney U test was used to examine the effect of exercise on the exercise-derived and intra- and inter-session locomotor learning measures in the rest and exercise groups without washout (RESTNO_WASH vs. EXENO_WASH) and the rest and exercise groups with washout (RESTWASH vs. EXEWASH). That analysis showed no difference between the rest and exercise groups within the NO_WASH and WASH groups. For this reason, we combined the RESTNO_WASH and RESTWASH groups and the EXENO_WASH and the EXEWASH groups into REST (13 participants, 10 females, 23.7 ±3.3 years) and EXE groups (13 participants, 10 females, 21.8 ± 0.5 years), respectively. Then, we again ran the Mann-Whitney U test to determine if intra-session and inter-session locomotor learning measures differed between the REST and EXE groups. Therefore, as indicated earlier, only the results from the last analysis are presented in the following section.
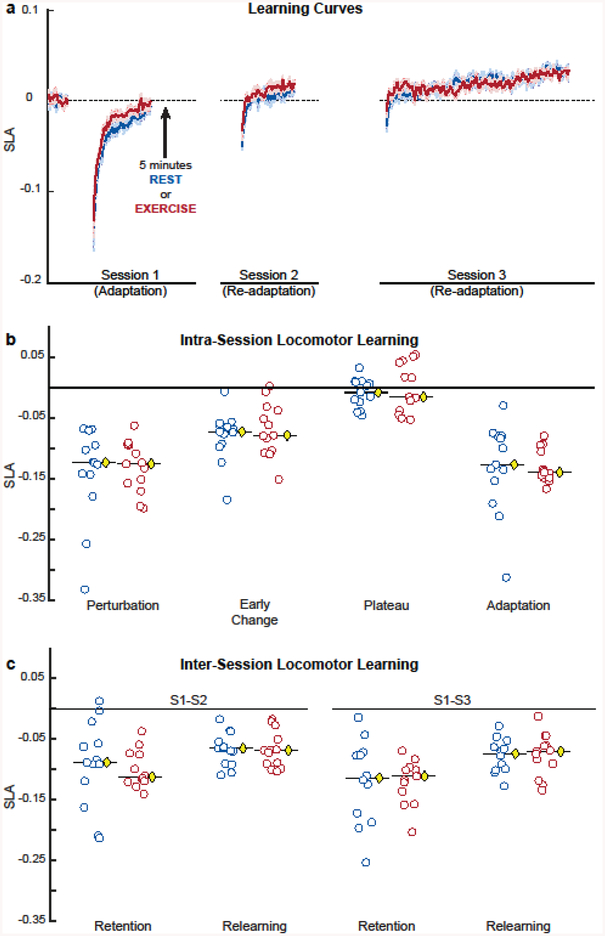
Fig. 3.
Locomotor learning curves (a), and intra-session (b) and inter-session (c) locomotor learning measures of REST (N = 13) and EXE (N = 13) groups, (a) Locomotor learning curves during split-belt treadmill walking across sessions. Group learning curves (smoothed by 5 strides) and shaded 1SD regions for SLA during split-belt treadmill walking across sessions. Value of 0 (dashed line) denotes baseline symmetry while any deviation from 0 denotes step length asymmetry compared to baseline. Blue lines and light blue shaded regions represent the group that simply rested after practice. Red lines and light red shaded regions represent the group that completed high-intensity exercise after split-belt practice on Session 1. For both REST and EXE groups, with initial exposure to 2:1 split-belt ratio, participants were initially perturbed, but they all returned to their baseline symmetry. On the second (session 2) and third (session 3) exposures, both groups were less perturbed initially and readapted back toward baseline, (b) Intra-session learning on session 1. There were no differences between REST and EXE groups on any measure, suggesting that all groups learned the same during session 1. (c) Inter-session learning on session 2 and 3 relative to session 1. In both REST and EXE groups, exercise had no effect on any of the inter-session learning measures. Larger values denote greater retention and relearning. (b) and (c) Dots denote individual data while the yellow diamonds with the black line indicate group medians. Blue dots represent the group that simply rested after practice on Session 1 (REST), whereas the red dots represent the group that completed high intensity exercise after split-belt practice on Session 1 (EXE).
Results
All participants successfully completed the experimental procedures in all three sessions with the exception of one female in REST, who did not return for session 3, but her data from session 1 and 2 were still included in the analysis. In EXE group, all participants successfully completed the high-intensity exercise bout without asking for breaks or any adverse events (e.g., nausea, lightheadedness, pain) with vitals returning to baseline within a few minutes. Participants in both REST and EXE groups successfully completed all walking trials without breaks.
Exercise-Derived Measures
As anticipated, the target high-intensity range (i.e., 77–94 % HRmax) was never reached by REST group but was quickly reached by EXE group, which exercised for the remaining time (EXE: 4.02 ± 0.31 minutes) at relatively high intensities (EXE: 88 ± 3 % HRmax). EXE group had increases in lactate that exceeded 10 mmol/l (EXE: 10.83 ± 2.26 mmol/l). As expected REST group had no changes in lactate that approached 10 mmol/l (REST: −0.19 ± 0.69 mmol/l). The change in lactate was a significantly greater in the EXE group than the REST group (REST vs. EXE: U < 0.001, z = −4.247, P < 0.001, r = −0.89).
Locomotor Learning
As anticipated, both REST and EXE groups adapted similarly in session 1; however, contrary to our hypotheses, both REST and EXE groups retained and relearned similarly in sessions 2 and 3 (Fig. 3a).
Given that either exercise or rest followed the locomotor task on session 1, we expected that participants in both groups would experience the same amount of intra-session locomotor learning on session 1. In session 1, both REST and EXE groups were similarly perturbed at the beginning of split-belt walking (U = 81.000, z = −0.180, P = 0.858, r = −0.04), experienced the same early change (U = 78.500, z = −0.308, P = 0.758, r = −0.06), demonstrated the same plateau at the end of split-belt walking (U = 75.000, z = −0.487, P = 0.626, r = −0.10), and had the same magnitude of adaptation (U = 68.500, z = −0.821, P = 0.411, r = −0.16) (Fig. 3b).
We also expected that participants in EXE group would show greater retention and relearning during session 2 and 3 than participants in REST, respectively. However, in contrast to our hypotheses, there was no significant effect of exercise on inter-session locomotor learning. Relative to session 1, participants in REST group retained and relearned similarly as participants in EXE group in session 2 (RETENTIONSS1–S2: U = 67.500, z = −0.872, P = 0.383, r = −0.17; RELEARNINGS1–S2: U = 82.00, z = −0.128, P = 0.898, r = −0.03) and session 3 (RETENTIONS1–S3: U = 74.000, z = −0.218, P = 0.828, r = −0.04; RELEARNINGS1–S3: U = 72.500, z = −0.299, P = 0.765, r = −0.06) (Fig. 3c).
Discussion
Our previous study of the effect of a short bout of high intensity exercise on sensorimotor adaptation-based locomotor learning in neurologically intact participants (Helm et al, 2017) did not find the positive effects on retention that have been shown in upper extremity skill-based learning tasks (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016b; Lundbye-Jensen et al. 2017; Nepveu et al. 2017; Stavrinos and Coxon 2017; Thomas et al. 2017; Dal Maso et al. 2018). These conflicting findings have been suggested to be the result of a differing effect of exercise on locomotor sensorimotor adaptation and/or due to the non-optimization of exercise parameters and time points of retention testing (e.g., 1 day vs. 7 days)(Helm et al. 2017; Charalambous et al. 2018a). In the present study, therefore, we refined the exercise and retention testing parameters to investigate whether this would result in a positive effect of exercise on locomotor learning. In contrast to our hypotheses, in session 2 and 3 both groups similarly retained and relearned the locomotor patterns learned on session 1. This result suggests that exercise may not have the same positive effect on sensorimotor adaptation-based locomotor learning as it does on upper extremity skill-based learning tasks. In the following sections, we will present and discuss potential factors that may explain the present findings, acknowledge methodological considerations that may influence our results, and suggest future directions that may advance our knowledge on the potential approaches to probe different types of locomotor learning.
Exercise parameters.
Cumulative evidence points out that the exercise effect on motor learning in skill-based upper extremity learning is maximized when exercise is administered immediately after motor practice at high intensity (i.e., post-exercise lactate level > 10 mmol/l). Regarding the first exercise parameter (i.e., timing), it has been speculated that exercise may have a time-dependent effect on motor learning (Roig et al. 2016). Exercise prior to motor practice may improve skill acquisition (i.e., motor performance during task) (Statton et al. 2015), but exercise may increase memory formation when it is performed after the practice of the task to be learned therefore improving retention (Roig et al. 2012; Thomas et al. 2016a). Because of the latter factor, both exercise groups in this study exercised immediately after motor practice (i.e., during the early phase of consolidation at which memory is transformed from short-term to a long-term form and is less susceptible to disruption) (Brashers-Krug et al. 1996; McGaugh 2000; Dudai 2012). Intensity is the other exercise parameter that was reported to be crucial for maximizing motor learning gains. All exercise priming studies that reported a positive exercise effect on motor learning aimed for post-exercise lactate levels greater than 10 mmol/l. In addition to being a proxy of exercise intensity, lactate was suggested to be a potential linkage between exercise and motor learning (Skriver et al. 2014). Therefore, to optimize the potential effect of exercise on locomotor learning, we ensured that participants in both exercise groups had large changes in lactate, even if exercise duration was much shorter than the previous work (5 minutes vs. 12–28 minutes).
Despite employing the exercise parameters that have been previously suggested to potentially maximize motor learning gains on upper-extremity task, we did not observe a significant effect of a short high-intensity exercise bout on locomotor learning in healthy participants. This unanticipated finding suggests that factors other than the parameters of the exercise priming may have contributed to the lack of exercise effect in this study.
Type of motor learning and motor task.
Past studies demonstrating a significant effect of exercise on motor learning have all used a similar type of motor task and form of learning (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b; Lundbye-Jensen et al. 2017; Nepveu et al. 2017; Thomas et al. 2017; Dal Maso et al. 2018). The motor task employed in those studies was either an upper extremity visuomotor tracking task (procedural skill) or time-on-target motor task (motor sequence), both of which required single-joint upper extremity movements. Furthermore, explicit instructions regarding how to perform the task, online feedback about task performance, and reward-based on performance were provided to the participants during the learning. Thus, the form of learning employed in those studies is likely a combination of explicit, strategy-based learning (Taylor and Ivry 2012) and reinforcement learning (i.e., reward-based) (Doya 2000). On the other hand, the dominant form of learning during the split-belt treadmill walking is thought to be implicit sensorimotor adaptation (Bastian 2008; Day et al. 2018). Recent studies using the split-belt paradigm demonstrated that explicit instructions may not affect within and across days locomotor learning (Malone and Bastian 2010; Roemmich et al. 2016), thus conscious motor strategies may not be involved in split-belt locomotor learning as much as in upper extremity learning (Day et al. 2018). Therefore, both the types of motor tasks and the forms of learning in the previous studies, which reported a significant exercise effect on motor learning (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b; Lundbye-Jensen et al. 2017; Nepveu et al. 2017; Stavrinos and Coxon 2017; Thomas et al. 2017; Dal Maso et al. 2018), are likely quite different than the split-belt treadmill walking studies.
A question that arises, then, is whether the difference in exercise effects on motor learning between studies are due to the type of motor task, form of learning, or both. Ferrer-Uris et al. (Ferrer-Uris et al. 2017) investigated the exercise effect on the learning of an upper extremity visuomotor adaptation task, which may have both explicit and implicit learning components (Taylor et al. 2014), in healthy participants, who learned to adapt to a 60° visual rotation of a cursor during a reaching task. As in our previous work (Helm et al. 2017; Charalambous et al. 2018a) and the present study, Ferrer-Uris et al. also observed no significant exercise effect on the long-term retention of learning, which measured one day and seven days post-practice. Taken together these findings, a short high-intensity exercise bout may not positively influence sensorimotor adaptation-based learning, regardless of whether the task involves locomotion or the upper extremity.
This differential effect could be related to the different neural bases of different forms of learning. Sensorimotor adaptation is thought to be heavily cerebellar-dependent (Morton and Bastian 2006) while visuomotor tracking tasks are thought to depend on cortical substrates (Sanes 2003). Consolidation of learning in both tasks likely relies heavily on primary motor cortex (Muellbacher et al. 2002; Luft et al. 2004); however, recent work suggested that a complex brain network involving not just the primary motor cortex, but also the cerebellum, is involved in the consolidation of sensorimotor adaptation based learning (Della-Maggiore et al. 2017). This is not the case for consolidation of a visuomotor tracking task, which relies heavily on the premotor cortex along with the primary motor cortex (Boyd and Linsdell 2009; Kantak et al. 2010; Meehan et al. 2013). Thus, a short high-intensity exercise bout may differentially influence the different neural pathways involved in consolidation of different forms of motor learning. Recent work, which quantified brain activity and connectivity using electroencephalography, demonstrated that sensorimotor areas were involved during the consolidation of skill-based arm task after an acute high-intensity exercise bout (Dal Maso et al. 2018). Future work should employ similar techniques to systematically examine the brain networks responsible for locomotor memory consolidation and the effect of acute high-intensity exercise bout on those networks.
Methodological considerations.
We acknowledge that the exercise duration used in this study was much shorter (5 minutes vs. 12–28 minutes) than in the previous studies of high intensity exercise and upper extremity motor learning (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b; Nepveu et al. 2017; Stavrinos and Coxon 2017; Dal Maso et al. 2018). Nevertheless, this specific short duration was selected because it has been found to be feasible in neurologically impaired participants with motor learning deficits (Charalambous et al. 2018a), which is critical for future application to neurorehabilitation. Given that high intensity and high lactate levels could be reached within five minutes, we anticipate that this short exercise duration may not have affected our findings; however, it is possible that to have a positive influence on motor learning, it is not only the intensity and timing of the exercise that are important, as previously suggested (Thomas et al. 2016a; Thomas et al. 2016b), but also the duration of the high intensity exercise. Future work should further investigate the effect and interaction of exercise duration with exercise intensity and timing on the subsequent gains in motor learning. This would be important to ascertain because if long durations (i.e., > 10 minutes) of high intensity exercise is necessary to observe the effects on motor learning previously found in healthy participants (Roig et al. 2012; Skriver et al. 2014; Thomas et al. 2016a; Thomas et al. 2016b), this will limit the feasibility of implementing exercise priming in a rehabilitation setting (Charalambous et al. 2018a), especially for patients with severe impairment. Lastly, the sample size in this study is similar to ones used in the previous priming studies (12–16 participants per group), and along with the small effects sizes (see r values in results section) observed suggest that sample size may not be the reason for the lack of significant findings.
Future directions.
The majority of previous studies focused on exercise parameters, while the form of motor learning and types of motor tasks have received less investigation. Therefore, given that our previous and present work demonstrated no effect of exercise on sensorimotor-based locomotor learning, future work should examine whether these findings persist in other types of locomotor learning (e.g., strategic learning using visual distorted feedback)(French et al. 2018).
Conclusion
Though our results did not support our hypotheses, our findings, together with previous work, provide important insights about high intensity exercise as a “primer” of motor learning. Specifically, the effects of high intensity exercise on motor learning appear to differ based on the form of learning and type of motor task. Our results, along with previous studies, suggest that sensorimotor adaptation-based locomotor learning does not appear to be influenced by a short high-intensity exercise bout, even when the parameters of exercise (i.e., timing and intensity) and retention testing (i.e., one day and seven days post-practice) are similar with the ones used in the previous work that reported positive effect of exercise on skill-based arm motor task.
Acknowledegements:
The authors thank all participants and undergraduate student volunteers for their assistance during data collections. This material is the result of work supported in part by the National Institute of Health 1R01HD078330-01A1 and S10RR028114-01A1.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Balady GJ, Chaitman B, Driscoll D, et al. (1998) Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Circulation 97:2283–2293 [DOI] [PubMed] [Google Scholar]
- Barros LF (2013) Metabolic signaling by lactate in the brain. Trends Neurosci 36:396–404 doi: 10.1016/j.tins.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Bastian AJ (2008) Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21:628–633 doi: 10.1097/WCO.0b013e328315a293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA (2009) Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci 10:72doi: 10.1186/1471-2202-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382:252–255 doi: 10.1038/382252a0 [DOI] [PubMed] [Google Scholar]
- Charalambous CC, Alcantara CC, French MA, et al. (2018a) A single exercise bout and locomotor learning after stroke: physiological, behavioural, and computational outcomes. J Physiol 596:1999–2016 doi: 10.1113/JP275881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalambous CC, Helm EE, Lau KA, Morton SM, Reisman DS (2018b) The feasibility of an acute high-intensity exercise bout to promote locomotor learning after stroke. Top Stroke Rehabil 25:83–89 doi: 10.1080/10749357.2017.1399527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JT, Bastian AJ (2007) Adaptation reveals independent control networks for human walking. Nat Neurosci 10:1055–1062 doi: 10.1038/nn1930 [DOI] [PubMed] [Google Scholar]
- Christina RW, Shea JB (1993) More on assessing the retention of motor learning based on restricted information. Res Q Exerc Sport 64:217–222 doi: 10.1080/02701367.1993.10608800 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472 doi: 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Dal Maso F, Desormeau B, Boudrias MH, Roig M (2018) Acute cardiovascular exercise promotes functional changes in cortico-motor networks during the early stages of motor memory consolidation. Neuroimage 174:380–392 doi: 10.1016/j.neuroimage.2018.03.029 [DOI] [PubMed] [Google Scholar]
- Day KA, Leech KA, Roemmich RT, Bastian AJ (2018) Accelerating locomotor savings in learning: compressing four training days to one. J Neurophysiol 119:2100–2113 doi: 10.1152/jn.00903.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Villalta JI, Kovacevic N, McIntosh AR (2017) Functional Evidence for Memory Stabilization in Sensorimotor Adaptation: A 24-h Resting-State fMRI Study. Cereb Cortex 27:1748–1757 doi: 10.1093/cercor/bhv289 [DOI] [PubMed] [Google Scholar]
- Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10:732–739 [DOI] [PubMed] [Google Scholar]
- Dudai Y (2012) The restless engram: consolidations never end. Annu Rev Neurosci 35:227–247 doi: 10.1146/annurev-neuro-062111-150500 [DOI] [PubMed] [Google Scholar]
- El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ (2018) Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 0:1073858418771538doi: 10.1177/1073858418771538 [DOI] [PubMed] [Google Scholar]
- Ferrer-Uris B, Busquets A, Angulo-Barroso R (2018) Adaptation and Retention of a Perceptual-Motor Task in Children: Effects of a Single Bout of Intense Endurance Exercise. J Sport Exerc Psychol 40:1–9 doi: 10.1123/jsep.2017-0044 [DOI] [PubMed] [Google Scholar]
- Ferrer-Uris B, Busquets A, Lopez-Alonso V, Fernandez-Del-Olmo M, Angulo-Barroso R (2017) Enhancing consolidation of a rotational visuomotor adaptation task through acute exercise. PLoS One 12:e0175296doi: 10.1371/journal.pone.0175296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JM, Bastian AJ, Gottschall JS (2013) Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol 591:1081–1095 doi: 10.1113/jphysiol.2012.245506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MA, Morton SM, Charalambous CC, Reisman DS (2018) A locomotor learning paradigm using distorted visual feedback elicits strategic learning. J Neurophysiol 120:1923–1931 doi: 10.1152/jn.00252.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MM, Rajasekaran S, Thomsen TW, Peterson AR (2016) Lactate: Friend or Foe. PM R 8:S8–S15 doi: 10.1016/j.pmrj.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Hart S, Drevets K, Alford M, Salacinski A, Hunt BE (2013) A method-comparison study regarding the validity and reliability of the Lactate Plus analyzer. BMJ open 3:e001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm EE, Matt KS, Kirschner KF, Pohlig RT, Kohl D, Reisman DS (2017) The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. Neurobiol Learn Mem 144:77–85 doi: 10.1016/j.nlm.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm EE, Tyrell CM, Pohlig RT, Brady LD, Reisman DS (2016) The presence of a single-nucleotide polymorphism in the BDNF gene affects the rate of locomotor adaptation after stroke. Exp Brain Res 234:341–351 doi: 10.1007/s00221-015-4465-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ (2010) Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci 13:923–925 doi: 10.1038/nn.2596 [DOI] [PubMed] [Google Scholar]
- Leech KA, Roemmich RT, Bastian AJ (2018) Creating flexible motor memories in human walking. Sci Rep 8:94doi: 10.1038/s41598-017-18538-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB (2004) Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci 24:6515–6520 doi: 10.1523/JNEUROSCI.1034-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbye-Jensen J, Skriver K, Nielsen JB, Roig M (2017) Acute Exercise Improves Motor Memory Consolidation in Preadolescent Children. Front Hum Neurosci 11:182doi: 10.3389/fnhum.2017.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ (2010) Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103:1954–1962 doi: 10.1152/jn.00832.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ (2014) Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil Neural Repair 28:230–240 doi: 10.1177/1545968313505912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ (2016) Age-related forgetting in locomotor adaptation. Neurobiol Learn Mem 128:1–6 doi: 10.1016/j.nlm.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Vasudevan EV, Bastian AJ (2011) Motor adaptation training for faster relearning. J Neurosci 31:15136–15143 doi: 10.1523/JNEUROSCI.1367-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Campbell KL, Ross CJ, Boyd LA (2013) Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther 93:1707–1716 doi: 10.2522/ptj.20130053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJD, Boyd LA (2014) A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. Journal of Applied Physiology 117:1325–1336 doi: 10.1152/japplphysiol.00498.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Wadden KP, Campbell KL, Boyd LA (2016) High-Intensity Aerobic Exercise Enhances Motor Memory Retrieval. Med Sci Sports Exerc 48:2477–2486 doi: 10.1249/MSS.0000000000001040 [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2000) Memory--a century of consolidation. Science 287:248–251 [DOI] [PubMed] [Google Scholar]
- Meehan SK, Zabukovec JR, Dao E, Cheung KL, Linsdell MA, Boyd LA (2013) One hertz repetitive transcranial magnetic stimulation over dorsal premotor cortex enhances offline motor memory consolidation for sequence-specific implicit learning. Eur J Neurosci 38:3071–3079 doi: 10.1111/ejn.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26:9107–9116 doi: 10.1523/JNEUROSCI.2622-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, et al. (2002) Early consolidation in human primary motor cortex. Nature 415:640–644 doi: 10.1038/nature712 [DOI] [PubMed] [Google Scholar]
- Musselman KE, Roemmich RT, Garrett B, Bastian AJ (2016) Motor learning in childhood reveals distinct mechanisms for memory retention and re-learning. Learn Mem 23:229–237 doi: 10.1101/lm.041004.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu JF, Thiel A, Tang A, Fung J, Lundbye-Jensen J, Boyd LA, Roig M (2017) A Single Bout of High-Intensity Interval Training Improves Motor Skill Retention in Individuals With Stroke. Neurorehabil Neural Repair 31:726–735 doi: 10.1177/1545968317718269 [DOI] [PubMed] [Google Scholar]
- Neva JL, Ma JA, Orsholits D, Boisgontier MP, Boyd LA (2019) The effects of acute exercise on visuomotor adaptation, learning, and inter-limb transfer. Exp Brain Res 237:1109–1127 doi: 10.1007/s00221-019-05491-5 [DOI] [PubMed] [Google Scholar]
- Ostadan F, Centeno C, Daloze JF, Frenn M, Lundbye-Jensen J, Roig M (2016) Changes in corticospinal excitability during consolidation predict acute exercise-induced off-line gains in procedural memory. Neurobiol Learn Mem 136:196–203 doi: 10.1016/j.nlm.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Pescatello LS (2014) ACSM’s Guidelines for Exercise Testing and Prescription. Wolters Kluwer Health, Philadelphia, PA: [DOI] [PubMed] [Google Scholar]
- Proia P, Di Liegro CM, Schiera G, Fricano A, Di Liegro I (2016) Lactate as a Metabolite and a Regulator in the Central Nervous System. Int J Mol Sci 17 doi: 10.3390/ijms17091450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ (2005) Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94:2403–2415 doi: 10.1152/jn.00089.2005 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ (2007) Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130:1861–1872 doi: 10.1093/brain/awm035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ (2009) Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair 23:735–744 doi: 10.1177/1545968309332880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Bastian AJ (2015) Two ways to save a newly learned motor pattern. J Neurophysiol 113:3519–3530 doi: 10.1152/jn.00965.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich RT, Long AW, Bastian AJ (2016) Seeing the Errors You Feel Enhances Locomotor Performance but Not Learning. Curr Biol 26:2707–2716 doi: 10.1016/j.cub.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye-Jensen J, Kiens B, Nielsen JB (2012) A single bout of exercise improves motor memory. PLoS One 7:e44594doi: 10.1371/journal.pone.0044594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig M, Thomas R, Mang CS, Snow NJ, Ostadan F, Boyd LA, Lundbye-Jensen J (2016) Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc Sport Sci Rev 44:81–88 doi: 10.1249/JES.0000000000000078 [DOI] [PubMed] [Google Scholar]
- Sanes JN (2003) Neocortical mechanisms in motor learning. Curr Opin Neurobiol 13:225–231 [DOI] [PubMed] [Google Scholar]
- Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB (2014) Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem 116:46–58 doi: 10.1016/j.nlm.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Snow NJ, Mang CS, Roig M, McDonnell MN, Campbell KL, Boyd LA (2016) The Effect of an Acute Bout of Moderate-Intensity Aerobic Exercise on Motor Learning of a Continuous Tracking Task. PLoS One 11:e0150039doi: 10.1371/journal.pone.0150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statton MA, Encarnacion M, Celnik P, Bastian AJ (2015) A Single Bout of Moderate Aerobic Exercise Improves Motor Skill Acquisition. PLoS One 10:e0141393doi: 10.1371/journal.pone.0141393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrinos EL, Coxon JP (2017) High-intensity Interval Exercise Promotes Motor Cortex Disinhibition and Early Motor Skill Consolidation. J Cogn Neurosci 29:593–604 doi: 10.1162/jocn_a_01078 [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Gao V, Alberini CM (2016) The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front Integr Neurosci 10:10doi: 10.3389/fnint.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykov ME, Corcos DM, Madhavan S (2017) Movement-Based Priming: Clinical Applications and Neural Mechanisms. J Mot Behav 49:88–97 doi: 10.1080/00222895.2016.1250716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykov ME, Madhavan S (2015) Motor priming in neurorehabilitation. J Neurol Phys Ther 39:33–42 doi: 10.1097/NPT.0000000000000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB (2012) The role of strategies in motor learning. Ann N Y Acad Sci 1251:1–12 doi: 10.1111/j.1749-6632.2011.06430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB (2014) Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34:3023–3032 doi: 10.1523/jneurosci.3619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Beck MM, Lind RR, et al. (2016a) Acute Exercise and Motor Memory Consolidation: The Role of Exercise Timing. Neural Plast 2016:6205452doi: 10.1155/2016/6205452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Flindtgaard M, Skriver K, et al. (2017) Acute exercise and motor memory consolidation: Does exercise type play a role? Scand J Med Sci Sports 27:1523–1532 doi: 10.1111/sms.12791 [DOI] [PubMed] [Google Scholar]
- Thomas R, Johnsen LK, Geertsen SS, Christiansen L, Ritz C, Roig M, Lundbye-Jensen J (2016b) Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS One 11:e0159589doi: 10.1371/journal.pone.0159589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ (2010) Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J Neurosci 30:17015–17022 doi: 10.1523/JNEUROSCI.4205-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ (2011) Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci 31:3055–3065 doi: 10.1523/jneurosci.5781-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, et al. (2007) High impact running improves learning. Neurobiol Learn Mem 87:597–609 doi: 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Zeni JA Jr., Richards JG, Higginson JS (2008) Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27:710–714 doi: 10.1016/j.gaitpost.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]