Abstract
MicroRNAs (miRNAs) are key players in the integrated regulation of cellular processes, and shape many of the functional properties that define the “cancer stem cell” (CSC) phenotype. Little is known, however, about miRNAs that regulate such properties in human colorectal carcinoma (CRC). In this study, we compared the expression levels of 754 miRNAs between paired samples of EpCAM+/CD44+ cancer cells (enriched in CSCs) and EpCAM+/CD44neg cancer cells (with CSC depletion) sorted in parallel from human primary CRCs, and identified miR-221 as the miRNA that displayed the highest level of preferential expression in EpCAM+/CD44+ cancer cells. High levels of miR-221 expression were associated with Lgr5+ cells in mouse colon crypts and reduced survival in CRC patients. Constitutive over-expression of miR-221 enhanced organoid-forming capacity of both conventional CRC cell lines and patient-derived xenografts (PDXs) in vitro. Importantly, constitutive downregulation of miR-221 suppressed organoid-forming capacity in vitro and substantially reduced the tumorigenic capacity of CSC populations from PDX lines in vivo. Finally, the most abundant splicing isoform of the human Quaking (QKI) gene, QKI-5, was identified as a functional target of miR-221; overexpression of miR-221 reduced QKI-5 protein levels in human CRC cells. As expected, overexpression of QKI-5 suppressed organoid-forming capacity in vitro and tumorigenic capacity of CRC PDX cells in vivo. Our study reveals a mechanistic link between miR-221 and QKI, and highlights their key role in regulating CSC properties in human colorectal cancer.
Keywords: colorectal cancer, cancer stem cells, miR-221, QKI, tumorigenic capacity
INTRODUCTION
Advanced stage, metastatic colorectal carcinomas (CRCs) are difficult to cure, as they often display limited sensitivity to conventional anti-tumor therapies. Among the key sources of tumor resistance to cytotoxic agents is the heterogeneous cell composition of malignant tissues, which originates not only from the divergent somatic mutations within the transformed population, but also from the capacity to recapitulate the multi-lineage differentiation processes that enable adult stem cell populations to sustain the formation of different cell types (1, 2). The cell sub-populations that, within a specific tumor, retain tumorigenic capacity upon serial transplantation and are able to sustain the formation of tumors that recreate the cellular diversity of the parent lesions, are operationally defined as “cancer stem cells” (CSCs) (3). Despite important progress in the understanding of the molecular identity of CSC populations in human CRCs, the molecular regulation of their tumor-initiation capacity remains only partially understood.
A large body of experimental evidence indicates that, among the key molecular regulators of CSC properties are microRNAs (miRNAs), non-coding RNAs that contribute to the post-transcriptional regulation of messenger RNAs (mRNAs) (3, 4). For example, in epithelial malignancies such as breast and pancreatic cancer the self-renewal ability of malignant cells is negatively regulated by miR-200c, which suppresses the expression of BMI1 (5, 6). Similar inhibitory effects are exerted, in human CRCs, by miR-34a, which suppresses NOTCH1 (7). On the other hand, selected miRNA species can act as positive regulators of tumorigenic capacity, as observed in the case of miR-22, which suppresses TET2 in mammary epithelia (8), and of miR-142, which suppresses APC in breast cancer (9).
In this study, we aimed to identify miRNAs involved in the regulation of CSC properties in human CRCs. Our results identified miR-221 as a positive regulator of tumorigenic capacity in human CRCs and an RNA binding protein QKI, as one of its key functional targets.
MATERIALS AND METHODS
Supplementary Appendix.
A comprehensive and detailed description of all methods used in this study is also provided in the Supplementary Information.
Ethics statements.
Human primary CRCs were obtained from patients admitted to the Division of Gastrointestinal Surgery of Kobe University Hospital. The research was pre-approved by Kobe University’s Institutional Review Board (permission number: 1299) and was conducted in accordance with recognized ethical guidelines (Declaration of Helsinki, CIOMS). All patients included in the study provided written informed consent. Animal experiments were performed with the approval of Kobe University’s Animal Care and Use Committee (permission number: 150802).
Flow cytometry.
Primary tumor specimens, patient-derived xenografts (PDXs) and normal colon epithelia were dissociated and analyzed as previously described (1). Dissociated cells were stained with monoclonal antibodies (mAbs) conjugated to fluorescent dyes. A complete list of all antibodies used in this study is provided in the Supplementary Information.
Analysis of miRNA expression by multiplex semi-quantitative real-time PCR.
RNA was extracted from 100 cells purified from primary CRCs, and directly collected into TRIzol (Invitrogen). The expression level of 754 miRNAs was measured by multiplex semi-quantitative real-time PCR (TaqMan™ Array Human MicroRNA A+B Cards Set v3.0 with Megaplex™ RT Primers, Human Pool Set v3.0; Thermo Fisher Scientific) as previously described (5). Results were normalized to RNU48 small nuclear RNA (snRNA) and analyzed for statistical significance using the Mann-Whitney U-test.
miRNA-sequence experiments.
Total RNA was isolated from sorted normal murine colon epithelial cells using the NucleoSpin miRNA kit (MACHEREY-NAGEL, Germany). The microRNA-seq profiling was performed on three pairs of biological replicates by LC Sciences (Houston, TX). Results were analyzed using two different mapping and normalization pipelines (LC Sciences, ENCODE) and evaluated for statistical significance using a one-tailed t-test for paired samples.
Bioinformatics analysis of RNA-sequencing (RNA-seq) datasets.
Associations were tested on a dataset downloaded from the Broad Institute Firehose (www.gdac.broadinstitute.org), containing mature miRNA expression data of 293 colon cancer patients from the colon adenocarcinoma (COAD) collection of The Cancer Genome Atlas (TCGA) database. Patients were stratified into two groups based on miR-221 expression levels (miR-221low vs. miR-221high) using the minimum P-value approach. Overall survival and disease-free survival rates were estimated using Kaplan-Meier survival curves and tested for statistical differences using the log-rank test and the Cox proportional hazards model. The presence of linear correlations between the expression levels of miR-221 and QKI was tested in an expanded release of the TCGA-COAD database (n=439, the TCGA public repository, https://cancergenome.nih.gov; March 8, 2016) (10). Correlations were evaluated using Pearson’s correlation coefficients, and tested for statistical significance using a two-tailed t-test (null hypothesis: r=0).
Cell lines.
All cell lines used in this study were obtained from the American Type culture Collection (ATCC; http://www.atcc.org) and include: HCT116 human colon cancer cells (ATCC catalog: CCL-247) and HEK293 human embryonic kidney cells (ATCC catalog: CRL-1573). All cell lines were cultured in RPMI-1640 (Sigma-Aldrich) containing 10% fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 mg/mL; Nacalai, Japan). Early passage cells were used in all experiments. All cell lines were tested to be Mycoplasma free by PCR and authenticated using short tandem repeat profiling (BEX, Japan).
Lentivirus plasmids.
The full-length sequence of miR-221 and the full-length coding region of the QKI-5 mRNA (NM_001301085) were amplified by PCR (Table S1) and cloned into the pEIZ-HIV-ZsGreen lentivirus vector and the pLentiLox3.7-EF1α-mCherry vector, a derivative of pLentiLox3.7 (Addgene: #11795), respectively (5). The lentivirus vectors encoding for the anti-miR-221 construct (miRZip-221) and a non-targeting pre-miRNA (negative control) were purchased from System Biosciences (USA).
Organoid assays.
Cells were infected with either test or control lentivirus constructs, and seeded on Matrigel™ in 96-well plates (3 × 103 cells/well), and cultured at 37°C with 5% CO2, as previously described (11). The number of organoids larger than 100 μm in diameter was counted 10 days after seeding. Results were tested for statistical significance using Student’s t-test (two-tailed) and/or, a two-way ANOVA test.
Xenotransplantation assays.
PDX-KUC1 cells were infected with lentivirus constructs at a multiplicity of infection (MOI) of 20, mixed with Matrigel and injected subcutaneously into NOD/SCID/IL2Rγ−/− (NSG) mice (Charles River) as previously described (12). Results were analyzed for statistical significance using Fisher’s exact test.
Cloning and mutagenesis of the QKI-5 3’UTR.
A 415-bp fragment of the QKI-5 3’UTR (nucleotides 2395–2809 of NM_001301085 (GenBank)) was amplified by PCR (Table S1) and cloned into the pGL3-MC vector, at the 3’-end of the firefly Luciferase gene (5). Mutations in the putative miR-221 target sequence within the QKI-5 3’UTR were introduced using a PrimeSTAR Mutagenesis Basal Kit (Takara Bio) (Table S1).
Luciferase reporter assays.
Cells were co-transfected with: 1) a pGL3-MC luciferase expression construct; 2) the pRL-TK Renilla luciferase vector (Promega); and 3) a pEIZ expression plasmid, containing either miR-221 or an empty backbone, using Lipofectamine 3000 (Invitrogen). Luciferase activity was quantified and normalized to Renilla luciferase activity, using the Dual-Luciferase Reporter Assay System (Promega). Results were analyzed for statistical significance using a two-tailed t-test and a two-way ANOVA test.
RESULTS AND DISCUSSION
miR-221 is over-expressed in EpCAM+/CD44+ as compared to EpCAM+/CD44neg CRC cells.
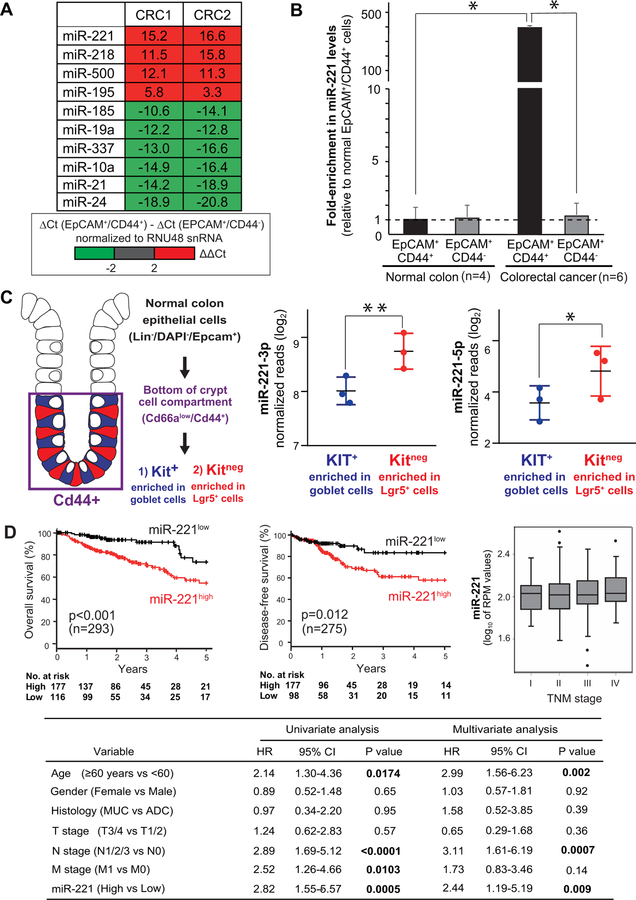
To identify miRNAs involved in the regulation of CSC properties in human CRCs, two autologous pairs of EpCAM+/CD44+ (enriched in cells with CSC properties) and EpCAM+/CD44neg (depleted in cells with CSC properties) cancer cells were isolated by fluorescence activated cell sorting (FACS) and purified in parallel from two independent primary CRC specimens (CRC1, CRC2). A screening of the expression levels of 754 miRNAs identified miR-221 as the miRNA that showed the highest level of preferential expression in EpCAM+/CD44+ as compared to EpCAM+/CD44neg cells (Fig. 1A), a finding subsequently validated on 6 primary CRC tissues (Fig. 1B). In normal mouse colon epithelia, Epcam+/Cd44+ cells contain both Kitneg Lgr5+ stem cells and Kit+ goblet cells (13) (Fig. 1C). A comparison of miR-221 expression levels between Epcam+/Cd66alow/Cd44+/Kitneg cells (enriched in Lgr5+ stem cells) and Epcam+/Cd66alow/Cd44+/Kit+ cells (enriched in goblet cells) confirmed over-expression of both miR-221–3p and miR-221–5p in Kitneg (Lgr5+) stem cells (Fig. 1C, Fig. S1, Fig. S2). These findings were in agreement with our previous studies in human breast cancer, which identified miR-221 among the miRNA species over-expressed in mammary CSC populations (5), and with previous studies in several malignancies, which identified miR-221 as over-expressed in cancerous as compared to normal tissues (14).
Figure 1. In human CRCs, miR-221 is preferentially expressed in EpCAM+/CD44+ cancer cells and associates with reduced survival outcomes.
(A) List of miRNAs displaying the highest degree of differential expression between paired samples of EpCAM+/CD44+ cancer cells (enriched in CSCs) and EpCAM+/CD44neg cancer cells (depleted in CSCs) isolated in parallel from human primary CRCs. The expression level of 754 miRNAs was measured by real-time qPCR. Numbers represent the difference in threshold-cycle (ΔCt) values. (B) Relative miR-221 expression levels in EpCAM+/CD44+ and EpCAM+/CD44neg cells from mouse normal primary colon epithelia (n=4) and primary human CRCs (n=6; *p<0.05). (C) Comparison of miR-221 expression levels in Epcam+/Cd66alow/Cd44+/Kit+ cells (enriched in Lgr5+ stem cells) and Epcam+/ Cd66alow/Cd44+/Kitneg cells (enriched in goblet cells) sorted in parallel from the normal colon epithelium (n=3; *p<0.05, **p<0.01). (D) Relationship between miR-221 expression levels, 5-year overall survival and disease-free survival rates, and tumor stage (TNM) in CRC patients from the TCGA-COAD dataset. Box-plots display 10th, 25th, 50th, 75th, and 90th percentiles of miR-221 expression levels. The association between miR-221 expression levels and 5-year survival outcomes remained statistically significant in multivariable analysis. HR: hazard ratio; CI: confidence interval; MUC: mucinous adenocarcinoma; ADC: adenocarcinoma.
High levels of miR-221 expression are associated with reduced survival in CRC patients.
In many forms of human cancer, tumors characterized by a gene-expression profile similar to that of phenotypic sub-populations enriched in CSCs are associated with reduced survival outcomes (1, 15, 16). Indeed, analysis of a public miRNA-sequencing database from human colon carcinomas revealed that miR-221high tumors were associated with worse clinical outcomes than miR-221low tumors, with regard to both 5-year overall survival (54.6% vs. 73.6%, n=293; p<0.001) and 5-year disease-free survival rates (57.9% vs. 83.4%, n=275; p=0.012) (Fig. 1D). Importantly, the association between high miR-221 expression levels and worse clinical outcomes did not appear to be confounded by major clinical or pathological variables (Fig. 1D), and remained associated with a statistically significant reduction in overall survival rates in a multivariable analysis based on the Cox proportional hazards method (HR=2.44. 95%CI=1.19–5.19, p=0.009) (Fig. 1D), in agreement with results from an independent cohort (17).
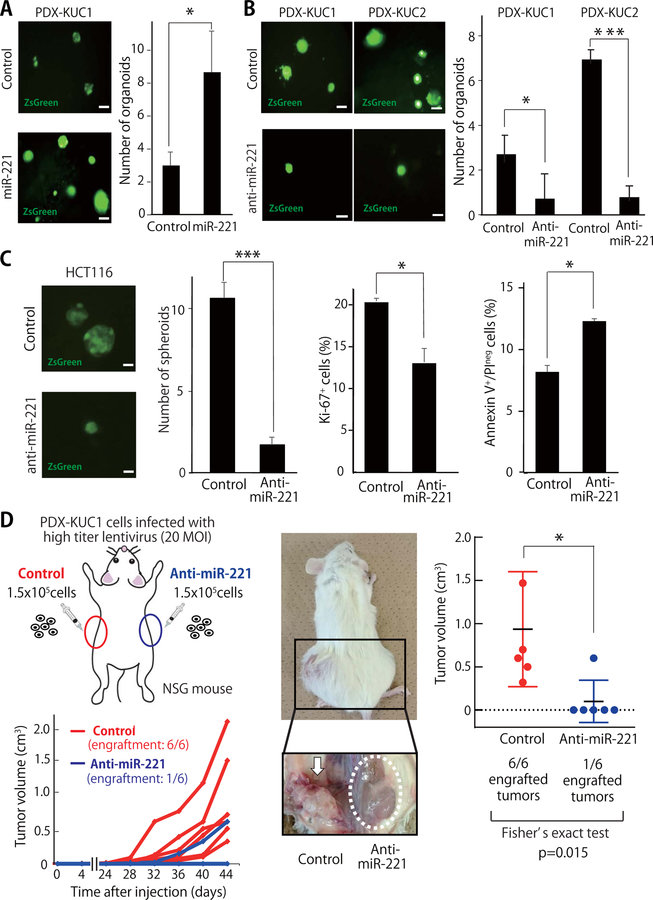
Over-expression of miR-221 enhances the in vitro clonogenicity and three-dimensional (3D) organoid-forming capacity of human CRC cells.
To understand whether miR-221 had a direct mechanistic role in supporting the capacity of CSC populations to initiate tumor growth, we tested whether miR-221 was able to affect the 3D organoid-forming capacity of human colon cancer PDX lines (PDX-KUC1, PDX-KUC2) (Fig. 2A–B) (18). Their clinico-pathological characteristics and gene mutation status are summarized in Fig. S3, S4 and Table S2. Infection of PDX-KUC1 cells with a lentivirus encoding for miR-221 significantly enhanced their capacity to grow as 3D organoids (Fig. 2A), while infection with a lentivirus encoding for an anti-miR-221 construct significantly suppressed it (Fig. 2B, Fig. S5A). Similar results were obtained in PDX-KUC2 cells and a human CRC cell line (HCT116) (Fig. 2B–C). In HCT116 cells, forced expression of the anti-miR-221 construct caused a reduction in the percentage of proliferating cells, an increase in the percentage of apoptotic cells, and a reduction of the expression levels of stem cell-related genes, such as LGR5, SOX2 and OCT4 (Fig. 2C, Fig. S5B). These findings were in agreement with previous observations on the oncogenic effects of constitutive miR-221 expression (19), which has a capacity to inhibit tumor suppressor genes involved in the regulation of cell-cycle progression, apoptosis and WNT signaling, such as CDKN1B/p27, CDKN1C/p57, PTEN, DKK2, and AXIN2 (14).
Figure 2. Inhibition of miR-221 reduces both the in vitro three-dimensional (3D) organoid-forming capacity and in vivo tumorigenicity of human CRC cells.
(A-B) Representative appearance and number of organoids formed by colorectal cancer PDX cells following infection with lentivirus vectors encoding either miR-221 (A) or an anti-miR-221 construct (B) (n=5; *p<0.05, ***p<0.001). scale bar: 100 μm. (C) Infection of HCT116 cells with a lentivirus vector driving constitutive miR-221 expression was associated with a reduction in 3D spheroid forming capacity (n=3; ***p<0.001), a reduction in the percentage of Ki67+ cells (n=3; *p<0.05), and an increase of the percentage of Annexin-V+/Propidium Iodideneg (PIneg) cells (n=3; *p<0.05). (D) Schematic illustration of in vivo xenotransplantation experiments and growth curves of tumors originated from PDX-KUC1 cells infected with either a lentivirus vector encoding for the anti-miR-221 construct or an empty vector used as negative control (n=6; 1.5×105 cells/injection). Two months after xenotransplantation, PDX-KUC1 cells infected with the control vector formed tumors in 6 out 6 cases (100%), while those with the anti-miR-221 construct formed tumors in only 1 out 6 cases (17%; *p<0.05). All sub-cutaneous injection sites were dissected and visually inspected.
Inhibition of miR-221 reduces the in vivo tumorigenic capacity of human CRC cells from PDX lines.
We tested whether inhibition of miR-221 was able to suppress tumorigenic capacity in immuno-deficient NSG mice (Fig. 2D). The results showed that infection of PDX-KUC1 cells with the lentivirus vector encoding for the anti-miR-221 construct caused a statistically significant reduction of their in vivo tumorigenic capacity (Fig. 2D). Importantly, lack of tumor growth associated with forced anti-miR-221 expression did not appear to be caused by a delay in growth kinetics, but rather by a lack of tumor engraftment, as revealed by anatomical dissection of the injection sites, which showed lack of even small tumor masses (Fig. 2D). Our data, therefore, suggested that miR-221 acts not simply as a positive modulator, but as necessary element of the molecular machinery that enables in vivo CRC growth.
QKI is a direct molecular target of miR-221.
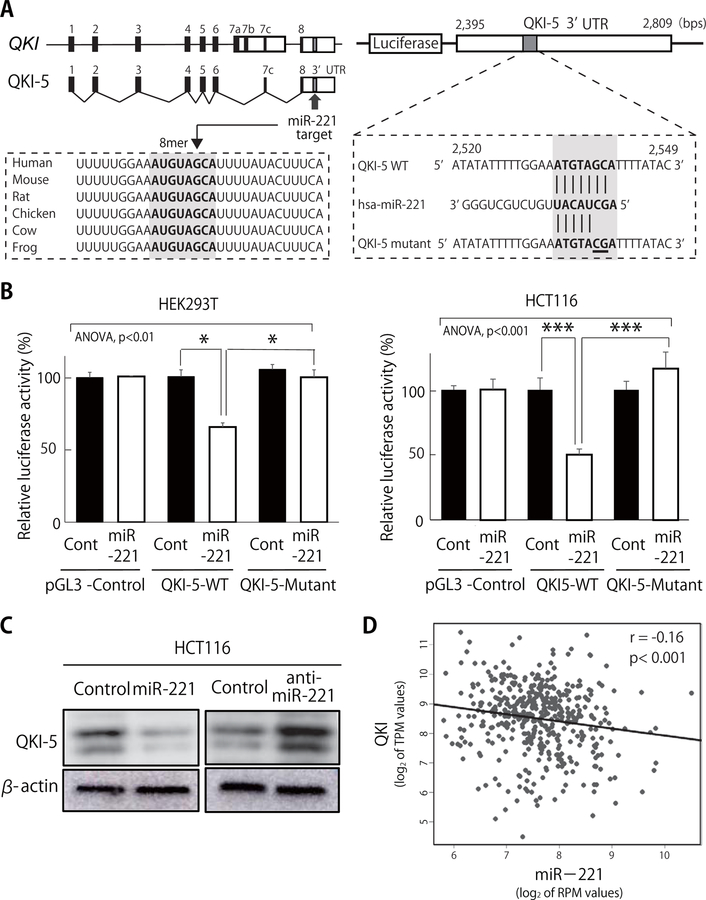
We used the TargetScan 6.2 algorithm (http://www.targetscan.org) to search for putative miR-221 target genes that play important roles in both oncogenesis and the regulation of stem cell functions. The search identified the Quaking (QKI) gene as one of the most promising candidates (20). Importantly, QKI is a transcriptional target of p53, often functions as a tumor-suppressor, and is a marker associated with better patient prognosis (20). In addition, QKI plays important roles in both the normal development and epigenetic regulation of stem cells (20, 21), and regulates epithelial-to-mesenchymal transition (EMT) processes (22), WNT signaling (23), and the expression of the transcription factors SOX2, NANOG and OCT4 (3, 21).
Among the four known splice variants of the human QKI gene (QKI-5, QKI-6, QKI-7, and QKI-7b) (20), QKI-5 is the variant most abundantly expressed in normal human colon tissues, and preferentially down regulated in human CRCs (23, 24). Within its 3’UTR, QKI-5 carries a predicted 8-mer miR-221 target site that is highly conserved across mammalian species (Fig. 3A). Indeed, miR-221 over-expression suppressed the luciferase activity driven by the Luc-QKI-5 (3’UTR) construct, and this effect was completely abrogated by the introduction of mutations restricted to the target sequence itself (Fig. 3A–B). Moreover, forced expression of miR-221 caused a reduction of QKI-5 protein levels in human CRC cells, while inhibition of miR-221 led to their increase (Fig. 3C). As predicted based on our in vitro data, the expression of the two genes was inversely correlated in the TCGA COAD database (n=439) (Fig. 3D) (20).
Figure 3. QKI-5 is a direct molecular target of miR-221.
(A) Schematic representation of the predicted miR-221 target recognition sequence within the 3’UTR of the QKI-5 mRNA, and of the two mutations introduced to functionally disable it. Numbers correspond to nucleotide positions in QKI-5 sequence (GenBank: NM_001301085). (B) Suppression of the luciferase activity of pGL3 constructs encoding the WT version of the QKI-5 3’UTR, but not that encoding mutant QKI-5 3’UTR by miR-221 (n=3; *p<0.05, ***p<0.005). (C) Forced expression of miR-221 down-regulated endogenous QKI-5 protein levels in human HCT116 cells, while forced expression of an anti-miR-221 construct up-regulated them. Expression of β-actin was used as a control. (D) QKI and miR-221 expression levels are inversely correlated (r=−0.16, p<0.001) in the TCGA COAD database of human primary CRCs (n=439).
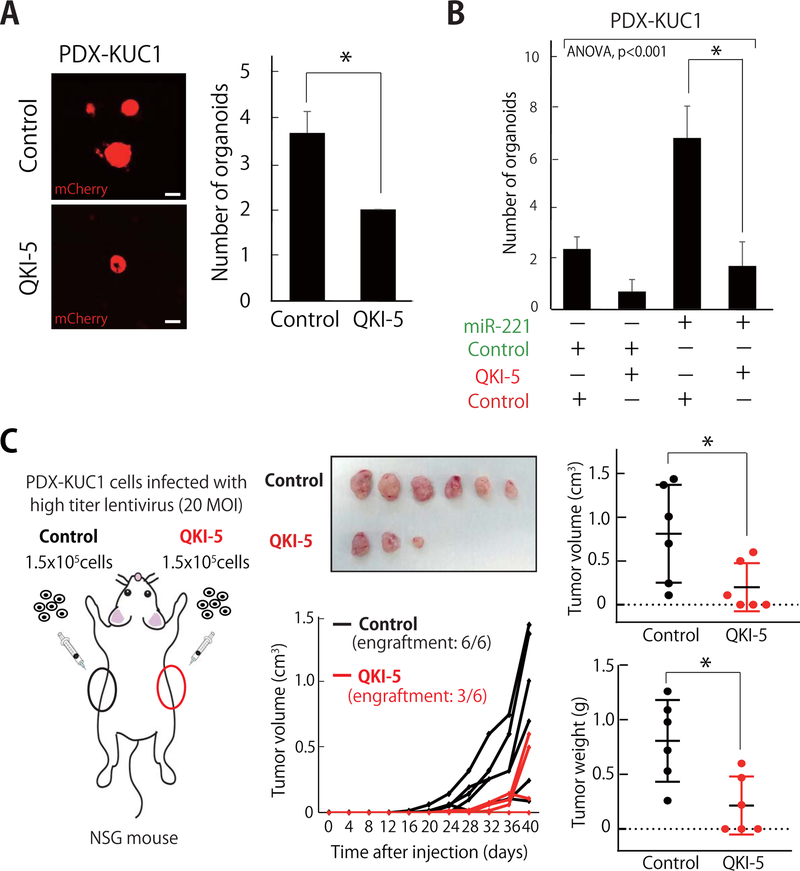
QKI-5 suppresses the in vitro three-dimensional (3D) organoid-forming and tumor formation capacities of human CRC PDX cells.
The constitutive expression of QKI-5 suppressed the 3D organoid-formation capacity of human CRC cells from both classical cell lines (HCT116) and PDX lines (PDX-KUC1) (Fig. 4A, Fig. S6A). When QKI-5 and miR-221 and were co-expressed together, QKI-5 completely abrogated the capacity of miR-221 to up-regulate organoid-forming ability (Fig. 4B, Fig. S6B).
Figure 4. QKI-5 suppresses the in vitro three-dimensional (3D) organoid-forming and tumor formation capacities of human CRC PDX cells.
(A) The 3D organoid-forming capacity of PDX-KUC1 cells was reduced following infection with a lentivirus vector driving constitutive expression of the QKI-5 cDNA (n=3; *p<0.05). Scale bar: 100 μm. (B) Forced expression of QKI-5 abrogated miR-221’s ability to enhance the 3D organoid forming capacity of PDX-KUC1 cells. Histograms report the number of organoids larger than 100 μm in diameter (n=3; *p<0.05). (C) Schematic illustration of in vivo xenotransplantation experiments and growth curves of tumors originated from PDX-KUC1 cells infected with either a lentivirus vector encoding for QKI-5 or an empty vector used as negative control (n=6; *p<0.05, 1.5×105 cells/injection).
Finally, to understand whether QKI acted as a negative regulator of in vivo tumor engraftment, we infected PDX-KUC1 cells with the lentivirus encoding QKI-5 and evaluated their in vivo tumorigenic capacity (Fig. 4C). We found that over-expression of QKI-5 caused a statistically significant reduction of their growth in vivo (Fig. 4C).
In summary, we identified miR-221 as one of the miRNA species that displays the highest degree of preferential expression in the EpCAM+/CD44+ population of human CRCs. miR-221 is not simply a positive regulator, but rather a necessary component of the molecular machinery involved in sustaining in vivo tumor growth. Furthermore, our study identified QKI-5 as a functional target of miR-221 and a suppressor of in vivo tumor growth. Taken together, our findings suggest that the functional interaction between miR-221 and QKI represents one of the key molecular networks involved in the regulations of CSC biology in human CRCs.
Supplementary Material
Significance.
Findings uncover molecular mechanisms underlying the maintenance of cancer stem cell properties in colon cancer.
ACKNOWLEDGMENTS
We thank Dr. Seetha V. Srinivasan (Herbert Irving Comprehensive Cancer Center, Columbia University) for helpful comments. This work was supported by grants from 1) the Japan Society for the Promotion of Science (JSPS KAKENHI) (17K16555 to J.M.; 15K14381, 18K07231, and Japan-Belgium Research Cooperative Program to Y.S.); 2) the Japan Foundation for Applied Enzymology (to Y.S.); 3) the Itoh-Chubei Foundation (to Y.S.); 4) Cancer Research Institute, Kanazawa University (to Y.S.); 5) Fujita Health University (to Y.S.); 6) the Princess Takamatsu Cancer Research Fund (to Y.S.); 7) the Uehara Memorial Foundation (to J.M.); 8) The Cell Science Research Foundation (to J.M.); 9) the New York State Stem Cell Science (NYSTEM) (to J.M); 10) the Damon Runyon Cancer Research Foundation (DRR-44–16, to P.D.); and 11) the College of Physicians and Surgeons of Columbia University (the 2017 Schaefer Research Scholarship to P.D.).
Abbreviations:
- CSC
cancer stem cell
- CRC
colorectal carcinoma
- FACS
fluorescence activated cell sorting
- mAb
monoclonal antibody
- MOI
multiplicity of infection
- miRNA
microRNA
- mRNA
messenger RNA
- NSG
NOD/SCID/IL2Rγ−/−
- PDX
patient-derived xenograft
- PI
propidium iodide
- QKI
quaking
- RNA-seq
RNA-sequencing
- snRNA
small nuclear RNA
Footnotes
Conflicts of interest statement:
Y.S. and P.D. are co-inventors on patents and patent applications filed by Stanford University and licensed to Quanticel Pharmaceuticals Inc. (US-9329170, US-9850483, US-20110021607). P.D. is a co-inventor on patents and patent applications filed by the University of Michigan and licensed to Oncomed Pharmaceuticals Inc. (US-7723112, US-20140030786). J.M., T.I., Q.H., T.H., T.W., M.M., H.Y., X.Q., K.Y., H.M., K.M., D.S., Y.K., A.S. declare no competing interests.
REFERENCES
- 1.Dalerba P, Kalisky T, Sahoo D, Rajendran PS, Rothenberg ME, Leyrat AA, et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol. 2011;29(12):1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukohyama J, Shimono Y, Minami H, Kakeji Y, Suzuki A. Roles of microRNAs and RNA-Binding Proteins in the Regulation of Colorectal Cancer Stem Cells. Cancers. 2017;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimono Y, Mukohyama J, Nakamura S, Minami H. MicroRNA Regulation of Human Breast Cancer Stem Cells. Journal of clinical medicine. 2015;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–95. [DOI] [PubMed] [Google Scholar]
- 7.Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell. 2013;12(5):602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154(2):311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isobe T, Hisamori S, Hogan DJ, Zabala M, Hendrickson DG, Dalerba P, et al. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. eLife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu A, Robertson G, Brooks D, Mungall AJ, Birol I, Coope R, et al. Large-scale profiling of microRNAs for The Cancer Genome Atlas. Nucleic Acids Res. 2016;44(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimono Y, Mukohyama J, Isobe T, Johnston DM, Dalerba P, Suzuki A. Organoid Culture of Human Cancer Stem Cells. Methods in molecular biology (Clifton, NJ). 2016. [DOI] [PubMed] [Google Scholar]
- 12.Mukohyama J, Shimono Y, Yamashita K, Sumi Y, Mukohara T, Minami H, et al. Effect of Xenotransplantation Site on MicroRNA Expression of Human Colon Cancer Stem Cells. Anticancer Res. 2016;36(7):3679–86. [PubMed] [Google Scholar]
- 13.Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F, et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142(5):1195–205 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki J, Suzuki H. Role of MicroRNAs-221/222 in Digestive Systems. Journal of clinical medicine. 2015;4(8):1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med. 2016;374(3):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–26. [DOI] [PubMed] [Google Scholar]
- 17.Cai K, Shen F, Cui JH, Yu Y, Pan HQ. Expression of miR-221 in colon cancer correlates with prognosis. Int J Clin Exp Med. 2015;8(2):2794–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Mukohyama J, Iwakiri D, Zen Y, Mukohara T, Minami H, Kakeji Y, et al. Evaluation of the risk of lymphomagenesis in xenografts by the PCR-based detection of EBV BamHI W region in patient cancer specimens. Oncotarget. 2016;7(31):50150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbelli L, Richard S. Emerging functions of the Quaking RNA-binding proteins and link to human diseases. Wiley interdisciplinary reviews RNA. 2016;7(3):399–412. [DOI] [PubMed] [Google Scholar]
- 21.Shingu T, Ho AL, Yuan L, Zhou X, Dai C, Zheng S, et al. Qki deficiency maintains stemness of glioma stem cells in suboptimal environment by downregulating endolysosomal degradation. Nat Genet. 2017;49(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillman KA, Phillips CA, Roslan S, Toubia J, Dredge BK, Bert AG, et al. miR-200/375 control epithelial plasticity-associated alternative splicing by repressing the RNA-binding protein Quaking. The EMBO journal. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji S, Ye G, Zhang J, Wang L, Wang T, Wang Z, et al. miR-574–5p negatively regulates Qki6/7 to impact beta-catenin/Wnt signalling and the development of colorectal cancer. Gut. 2013;62(5):716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, Fu H, Zhang J, Lu X, Yu F, Jin L, et al. RNA-binding protein quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology. 2010;138(1):231–40 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.