Abstract
The trace element selenium is an essential micronutrient that plays an important role in maintaining homeostasis of several tissues including the immune system of mammals. The vast majority of the biological functions of selenium are mediated via selenoproteins, proteins which incorporate the selenium containing amino acid selenocysteine. Several bacterial infections of humans and animals are associated with decreased levels of selenium in the blood and an adjunct therapy with selenium often leads to favorable outcomes. Many pathogenic bacteria are also capable of synthesizing selenocysteine suggesting that selenoproteins may have a role in bacterial physiology. Interestingly, the composition of host microbiota is also regulated by dietary selenium levels. Therefore, bacterial pathogens, microbiome, and host immune cells may be competing for a limited supply of selenium. Elucidating how selenium, in particular selenoproteins, may regulate pathogen virulence, microbiome diversity, and host immune response during a bacterial infection is critical for clinical management of infectious diseases.
Keywords: Selenium, Selenoproteins, Bacteria, Pathogen, Immune Response, Microbiota
Introduction
Selenium is an essential micronutrient utilized by organisms across all three domains of life [1–3]. Selenium is co-translationally incorporated into the 21st amino acid selenocysteine [Sec] [4–7]. A vast majority of the biological effects of selenium are mediated by selenoproteins, proteins that contain one or more selenocysteines. These proteins make up an organisms’ selenoproteome which vary from zero selenoproteins in some plants and fungi to more than 30 in some species of fish and algae [8]. In humans and other mammals, selenium has been shown to provide several health benefits such as decreasing cancer incidence, preventing cardiovascular disease, and improving overall immune function [9–11]. Conversely, selenium deficiency has been shown to lead to several disease conditions such as Keshan disease, white muscle disease, and Kashin Beck Disease [12, 13]. Interestingly, numerous chronic infectious diseases such as infections with Human Immunodeficiency virus, Hepatitis C virus, and Mycobacterium tuberculosis have been associated with selenium deficiency [9, 14–16]. Accordingly, nutritional intervention studies have shown a beneficial effect of selenium supplementation in treating patients with these illnesses [17–23].
Similar to eukaryotes, prokaryotes also express a diverse set of selenoproteins [8, 24]. It is estimated that approximately 20% of sequenced prokaryotic genomes encode at least one trait for selenium utilization [2, 3, 25]. Multiple selenium-dependent enzymes have been found in microorganisms such as numerous formate dehydrogenases, some hydrogenases, glycine reductase of Clostridia, and xanthine dehydrogenase [26]. Therefore, it is plausible that prokaryotes which possess selenoproteins may exhibit increased fitness in the presence of selenium similar to the benefits observed in humans and other mammals. This represents a unique challenge for the mammalian host upon infection with bacteria that possess the ability to utilize selenium with both host and parasite competing for the limited selenium resources. However, there is limited information available related to the role of selenium in bacterial physiology and virulence as well as overall bacterial pathogenesis.
Selenium Utilization by Bacteria
Prokaryotic Selenoprotein Synthesis and their Evolution
The mechanism of Sec insertion in prokaryotes has been most thoroughly described in Escherichia coli [27–30]. Unlike eukaryotes, Sec insertion is a one step process in prokaryotes. Briefly, Sec insertion in bacteria requires an in-frame UGA codon and unique tRNASec [SelC] [27–29]. The UGA codon [typically read as a stop codon] is reprogrammed to a Sec insertion codon by the presence of a Sec insertion sequence [SECIS], an mRNA stem-loop structure present in the selenoprotein mRNA immediately downstream of the Sec-encoding UGA codon [1, 28]. Reprogramming of the UGA stop codon also requires a Sec-specific elongation factor [SelB] which binds GTP, the SECIS element, and the tRNASec [SelC] [27, 28]. Conversely, in eukaryotes, the SECIS element is located in the 3’-untranslated region of selenoprotein mRNA and reprogramming of the UGA codon is carried out by two other proteins, EF-Sec and SECIS-binding protein [SBP2] [7]. Here, EF-Sec binds to GTP, tRNASec, and SBP2 while SBP2 binds the SECIS element [1,7].
Sec synthesis is an important step in selenium metabolism and is required prior to the synthesis of selenoproteins. In bacteria, tRNASec is initially aminoacylated with serine via seryl-tRNA synthetase resulting in seryl-tRNASec [29]. Seryl-tRNASec is then converted into selenocysteyl-tRNASec by Sec synthase [SelA] [29]. SelA utilizes selenophosphate as a selenium donor which is provided by selenophosphate synthetase [SelD] [31]. Synthesis of Sec in eukaryotes follows a similar process to that in bacteria with the exception of an O-phosphoseryl-tRNASec intermediate which is generated by O-phosphoseryl-tRNASec kinase [PSTK] phosphorylating the serine of seryl-tRNASec [31,32]. Eukaryotic Sec synthase then converts O-phosphoseryl-tRNASec to selenocysteyl-tRNASec [32, 33]. tRNASec generation in conjunction with the presence of the required Sec insertion machinery ultimately results in selenocysteine being transferred to the nascent polypeptide and the generation of selenoproteins.
In addition to selenocysteine and selenoproteins, some prokaryotes are able to utilize selenium from selenophosphate for the synthesis of a modified tRNA nucleotide, 5-methylaminomethyl-2-selnouridine [SeU] located at the anticodon wobble position of several bacterial tRNAs, including tRNALys, tRNAGlu, and tRNAGln [34–36]. SeU present on such modified tRNAs is believed to improve the translation accuracy and efficiency [34]. However, in order to replace a sulfur atom with selenium in 2-thiouridine within these tRNAs, a 2-selenouridine synthases [YbbB] is required [2]. Selenium has also been shown to be utilized in the form of a co-factor in molybdenum containing hydroxylases such as nicotinic acid hydroxylase and xanthine dehydrogenase [37, 38]. However, much like the sec insertion machinery and tRNASec, SeU and YbbB are not ubiquitously expressed in all prokaryotes.
Numerous studies have investigated the evolution and distribution of these selenium utilization traits in various sequenced bacterial genomes [1–3, 31, 39]. Such studies suggest that the evolution of the Sec insertion system most likely arose once from a common ancestor and has been adjusted over time through speciation and differential gene loss with some contribution of horizontal gene transfer [1]. These studies also indicate a strong correlation between the ability to decode Sec and the presence of formate dehydrogenases which are important for anaerobic metabolism; thus, it is reasonable to believe that selenium may be important for respiration during restricted oxygen supply [1, 3]. Conversely, more recent studies involving larger data sets were unable to identify a trend between oxygen levels and Sec utilization [39]. However, they did note that the majority of selenoprotein rich organisms are anaerobic signifying that low oxygen levels may contribute to the evolution of new selenoprotein genes [39]. This study also demonstrated that bacteria with Sec and selenium-cofactor traits favor host-associated environments while SeU appears to favor more aquatic environments [39]. This may be due to the fact that host associated and aquatic environments may provide a more stable and abundant supply of selenium while terrestrial bacteria may be exposed to more diverse environmental selenium levels [39].
Selenoproteins of Bacteria
Formate Dehydrogenases
Selenium has been identified as a common component of formate dehydrogenases that are often identified in anaerobic organisms [40]. Formate dehydrogenase is an enzyme that supports the growth of multiple facultative and obligate anaerobic bacteria by catalyzing the reversible two electron oxidation of formate [40]. Because formate is produced during the fermentation of sugars, aromatic compounds, L-[+]-tartaric acid and oxalate, there tends to be a greater range of anaerobes which are able to metabolize formate [40].
E. coli is a facultatively anaerobic bacteria which possesses Sec within its formate dehydrogenase O, N, and H [6, 40, 41]. Axley et.al. investigated the role of this Sec residue by replacing the Sec contained in E. coli’s formate dehydrogenase H with a cysteine resulting in a sulfur analog of the selenoprotein [42]. While both enzymes maintained similar overall structural properties and pH dependencies related to activity and stability, the mutant was found to bind formate with greater affinity than that of the Sec-containing wild type enzyme. However, the mutant was found to have a turnover rate (Kcat) two orders of magnitude (300 times) lower than that of the native enzyme due to a diminished reaction rate during the first step of the overall reaction. Thus, selenium containing formate dehydrogenases may provide an evolutionary advantage to facultative anaerobic bacteria allowing them to thrive under diverse conditions [42].
Campylobacter jejuni naturally resides in the intestinal tracts [specifically the cecum] of birds and is a known gastrointestinal pathogen of both humans and animals [43]. Due to its anaerobic location, C. jejuni metabolizes formate for energy which occurs via its formate dehydrogenase A [FdhA] which is known to contain a Sec. The bacterium utilizes its fdhTU genes for the selenium-controlled biosynthesis of formate dehydrogenase and inactivation of these genes results in the absence of FdhA. Likewise, inactivation of C. jejuni’s selA and selB genes results in a lack of Sec insertion and the absence of formate dehydrogenase activity. Thus, selenium is essential for FdhA activity in C. jejuni which directly affects its pathogenic potential [43]. The fdhTU genes have been shown to affect C. jejuni’s motility, chemotactic behavior, and play a role in invasion of host cells [44]. This poses a potential problem for the infected mammalian host which also requires selenium in order to mount an effective immune response to C. jejuni.
Glycine Reductases
Glycine reductases are composed of three proteins [protein A, B, and C] which catalyze the deamination of glycine and ammonia with simultaneous esterification of orthophosphate which reacts with ADP in order to form ATP [26]. These proteins are known to contain Sec and are expressed in a variety of anaerobes such as Eubacterium acidaminophilum and various Clostridium species [45]. Protein A of Clostridium sticklandii is an acidic, 18 kDa protein that contains a Sec residue and two Cysteine residues [46]. These three residues are highly reactive with oxygen resulting in an oxidized protein that is converted to the active, reduced form [26]. Further investigation of Protein A demonstrated a dependency on the amount of selenium present in the culture media for catalytic activity. However, the production of immunogenic material appears to be independent of the selenium level in vitro. Under selenium deficient conditions, cysteine is incorporated into the protein in the place of Sec resulting in an enzymatically inactive protein [46]. Therefore, selenium is an essential component of Clostridium’s glycine reductase which allows the bacterium to produce ATP for energy. However, the role of selenium in different Clostridium species have not been elucidated.
Xanthine Dehydrogenases
Selenium has been shown to be beneficial in increasing the catalytic efficiency of various enzymes and while most selenoproteins utilize Se via incorporation of selenocysteine, it is also known to occur in enzymes as a cofactor [38, 47]. Enzymes known to require selenium in this form include xanthine dehydrogenases, nicotinic acid hydroxylase, and purine hydroxylase [48]. Each of these enzymes are complex and also contain a molybdopterin cofactor [49]. Xanthine dehydrogenase [XDH] is a flavoprotein which catalyzes the reaction of purines, hypoxanthine, and xanthine into uric acid via a complex mechanism [50].
Enterococcus faecalis is an opportunistic pathogen which produces biofilms during infection of the heart and bladder [49]. E. faecalis is a facultative anaerobe which is capable of utilizing selenium as a cofactor for XDH. Srivastava et.al. demonstrated that the density of biofilm was increased following the addition of uric acid to the culture media of E. faecalis [49]. However, this biofilm increase following the addition of selenium was only observed in the presence of molybdate in the media. Subsequent deletion of selD resulted in decreased biofilm formation following the addition of exogenous selenium. Likewise, disruption of the gene encoding the XDH resulted in diminished biofilm formation. It is known that enhanced biofilm proliferation correlates with increased extracellular production of peroxide following selenite addition. These data demonstrate that selenium dependent XDH is involved in the formation of biofilms presumably via oxidant production [49]. Therefore, selenium supplementation may increase the pathogenicity of E. faecalis which poses a potential risk for the selenium supplemented host during infection with this bacterium.
Selenoproteins in Host Responses to Bacteria
The host’s micronutrient status is increasingly being linked to the response to infectious diseases as several micronutrients including selenium have been shown to play an important role in the immune system [16, 21, 51]. The primary method of selenium intake in humans/animals is via the diet; selenium rich foods include bread, cereals, meat, fish, eggs, as well as dairy products. In animal products, selenium status directly reflects the amount of selenium within the feed and thus that of the soil where the feeds were grown. It is recommended that humans consume a daily allowance of ~20 μg/day of selenium in order to prevent illnesses such as Keshan Disease, a form of cardiomyopathy [52]. However, intake of excess selenium [between 3,200 and 6,700 μg/day] is known to result in selenium toxicity. Thus, it is essential to consume selenium within the target range in order to prevent negative effects from selenium deficiency or over-supplementation. Absorption of selenium primarily occurs within the lower part of the small intestine. Both inorganic and organic selenium are generally absorbed with an efficiency between 70 and 90 percent under homeostatic conditions. Selenite, however, is only absorbed with an efficiency of 60 percent [53]. The selenium obtained via the diet is then utilized to form selenoproteins which are responsible for executing the biological functions of selenium.
The human selenoproteome consists of 25 selenoproteins which have been shown to play a variety of functions important for human health. These proteins are indispensable for the proper functioning of the immune system, male reproductive system, endocrine system, muscular system, etc. [9, 11, 52, 54]. Selenoproteins expressed by the cells of the immune system are responsible for carrying out essential functions such as antioxidant functions, protein folding, play a role in cell signaling events, and other yet-to-be defined functions [55]. Therefore, selenium deficiency may result in a suppressed immune system while supplementation may improve overall immune function and aid in microbial clearance following infection. While more information regarding the influence of selenium on the immune system are reviewed elsewhere [55], the following sections will focus on the influence of selenium on immune responses during specific bacterial infections. A more specific relationship between selenoproteins and bacterial infections are listed in Table 1.
Table 1.
Key findings related to the role of selenium in animal models of bacterial infections.
| Study | Year | Model | Pathogen | Selenium* | Dose & Route** | Major Findings |
|---|---|---|---|---|---|---|
| Gao [116] | 2016 | Mouse | S. aureus | NaSe | 0.03, 0.13, 1.5mg/kg diet | Deficiency lead to higher levels of proinflammatory cytokines, MPO activity, TLR2 signaling and NF-κB activation. |
| Smith [117] | 2011 | Mouse | C. rodentium | NaSe | 0 or 0.2μg/g diet | Deficiency lead to increased cytokine levels, pathology and colonic hyperplasia. Maintenance of diet for 20 weeks verses 5 weeks exacerbated phenotype. |
| Smith [90] | 2011 | Mouse | C. rodentium | NaSe (Vit E) | 0 or 0.2μg/g diet | Vitamin E and Se deficiency lead to increased bacterial burden reduced response and increased pathology. |
| Wang [99] | 2009 | Mouse | L. monocytogenes | Se | 0.005 or 0.2mg/kg diet | Deficiency lead to increased bacterial burden, less infiltrating immune cells in the spleen and NK cells had lower activity. |
| Berg [118] | 2005 | Mouse | E. coli LPS | NaSe | 0.05, 0.15, 2g/kg diet | Deficiency was marked by reduced levels of GPX in a dose dependent manner. |
| Altimira [119] | 2000 | Mouse | L. monocytogenes | Se | 350 or <8μg/g diet | Deficiency leads to greater damage in CNS tissue. |
| Liu [88] | 2016 | Rat | S. aureus | MCS/MSA | 1.5mg/kg Se i.p. | Deficiency leads to increased TRL-2 activation increasing caspase cleavage and apoptosis. |
| Kim [120] | 2012 | Rat | E. coli | Se | 12μg/g water | Se with ciprofloxacin decreased bacterial burden. |
| Boyne [121] | 1986 | Rat | S. typhimurium S. aureus | Se | 0.01 or 0.1 mg/kg | Deficiency had no effect on response to infection. |
| Sjunnesson [122] | 2001 | Guinea Pig | H. pylori | Se (Vit: A,C, E) | 0.15 or 1 mg/kg diet | Dietary antioxidant levels increased with supplementation this protected against type-B gastritis. Data suggest there may be a correlation between bacterial load and gastric scores. |
Forms of Se: sodium selenite (NaSe), unknown or labeled as selenium (Se), selenium nanoparticles (SeNp), methylselenocysteine (MCS), methylseleninic acid (MSA)
Intraperitoneal injection (i.p.)
Mycobacterium tuberculosis infections
M. tuberculosis, the causative agent of Tuberculosis [TB], is a slow-growing, gram-positive, non-selenium utilizing, non-spore-forming bacteria responsible for infecting over 9,000 individuals in the United States in 2017. Today, TB remains one of the world’s deadliest illnesses and is the leading cause of death in individuals who are co-infected with Human Immunodeficiency Virus [HIV] [56]. TB induces a Th1-type immune response characterized by CD4+ T cell production of IFN-γ, a cytokine which has shown to be crucial for the clearance of M. tuberculosis [51]. Due to the reduced CD4+ T cell numbers and overall impaired cell-mediated immune response, individuals with a pre-existing HIV infection are extremely susceptible to TB and pose an increased risk for developing overt tuberculosis resulting in death [21,51]. TB alone often results in decreased apatite, malabsorption of nutrients and micronutrients, and increased metabolic demands which collectively result in poor nutritional status and increased immunodeficiency [57]. It is unknown whether malnutrition leads to increased susceptibility to TB or if TB infection results in malnutrition due to the increased metabolic demands and reduced nutrient intake.
Numerous studies have demonstrated a reduction in serum selenium levels in patients with TB suggesting that selenium supplementation may be a beneficial therapeutic strategy for these individuals [21,58–60]. One such study conducted in Botswana showed that TB and HIV were both associated with decreased levels of micronutrients and supplementation with multivitamins and selenium reduced recurrence of TB as well as TB-related mortality in coinfected participants. Selenium supplementation also reduced the risk of developing TB in patients with a pre-existing HIV [18, 61]. Thus, selenium supplementation should be considered for the prevention of TB in high risk populations such as those with pre-existing HIV infections where TB is endemic. Other studies have demonstrated that micronutrient supplementation [including selenium] also significantly decrease the risk of TB recurrence in all TB patients regardless of HIV co-infection [62]. While it remains unknown how selenium reduces TB recurrence and mortality, it may be attributed to its antioxidant effects which prevent tissue damage and inflammation caused by the production of reactive oxygen species during M. tuberculosis infection [23, 62]. One study attempted to elucidate the molecular mechanism behind multi-micronutrient supplementation [including selenium] and TB. However, they found no significant differences in T cell proliferation following T cell mitogen challenge between the micronutrient supplemented group and control group [62]. While many studies have demonstrated the benefit of selenium supplementation on TB outcome, others have found no significant improvement following micronutrient supplementation [22, 63]. However, these studies did not control for the varied selenium status of subjects. Therefore, additional studies with carefully monitored selenium status of test patients are needed to investigate the mechanism by which selenium alone functions during TB infection are necessary to understand the potential of selenium supplementation during host response to bacterial pathogenesis.
Helicobacter pylori infections
Helicobater pylori is a gram-negative, microaerophilic, helix-shaped bacterium which colonizes the gastric mucous layer or adheres to the epithelial lining of the stomach [64, 65]. This bacterium is present within ~50% of the human population worldwide and is responsible for causing 90% of duodenal ulcers and 80% of gastric ulcers [21,65]. H. pylori transmission most often occurs via fecal-oral and oral-oral exposures. Once acquired, those infected with H. pylori have an increased risk of developing gastric cancer and mucosal-associated-lymphoid type lymphoma [64, 65]. Treatment is currently available to those infected with H. pylori and includes a 10 to 14-day course of “triple therapy” which includes proton pump inhibitors, amoxicillin and clarithromycin [64]. Unfortunately, H. pylori has begun to develop resistance to clarithromycin resulting in decreased eradication rates. Thus, alternative treatments have been suggested which include proton pump inhibitors and amoxicillin for the first half of treatment followed by proton pump inhibitors, metronidazole, and clarithromycin for the second half of treatment [66].
Micronutrient homeostasis is often impaired during H. pylori infection, an effect which is restored following eradication of the bacteria [67]. Alteration of host micronutrient status is most likely due to lowered gastric acid secretion, atrophy of the gastric mucosa, and malabsorption [67]. While plasma selenium levels have not been shown to differ between patients with or without H. pylori caused inflammation, it has been shown that there are higher levels of selenium located in the antral mucosa in individuals suffering from H. pylori associated gastritis [68–70]. Moreover, the concentration of gastric tissue selenium tend to be increased with greater inflammation scores of the antral mucosa [68]. This is likely due to the strong relationship between H. pylori tissue damage and the generation of reactive oxygen species [ROS] with concomitant reduction in the levels of various antioxidants. Thus, an increase in the selenium concentration at the infected mucosa may be a protective response where selenium is acting as an antioxidant to prevent further damage caused by ROS [68, 71] or mediating resolution of inflammation. This is further supported by the decrease of gastric tissue selenium observed in patients after successful eradication of H. pylori [68]. It is important to note, that selenium deficiency has been shown to be a risk factor for the conversion of precancerous gastric lesions into carcinomas [68, 70, 72, 73]. This decrease in selenium may be due to long-lasting mucosal inflammation which results in an altered gastric microenvironment leading to gastric carcinogenesis [68]. These findings suggest that selenium supplementation may aid in preventing the onset of gastric carcinogenesis in chronically infected individuals and mortality in those whom already have gastric cancer [74, 75]. Furthermore, one study suggests that selenium status may be correlated to the location of gastric cancer [72]. More research is required to investigate why selenium levels drop prior to carcinogenesis and the mechanism by which this occurs.
Sepsis and Septic Shock
Clinically, sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Such organ dysfunction is identified as an acute change in the total Sequential Organ Failure Assessment [SOFA] score due to the infection. A subset of sepsis, known as septic shock, occurs when underlying circulatory and cellular/metabolic abnormalities are so profound that they significantly increase the likelihood of mortality [76]. Sepsis, while incompletely understood, is a pathophysiologic process comprised of activation and dysregulation of pro-inflammatory/anti-inflammatory responses, complement and coagulation systems, metabolic alterations, hormonal changes, mitochondrial dysfunction, and epithelial and microcirculatory dysfunction. The organ injuries sustained during sepsis are generally caused by the patient’s immune response to the pathogen[s] which results in significant oxidative cellular stress. Thus, early antibiotic intervention, re-establishment of cellular perfusion, and control of the source of sepsis are essential for a positive patient outcome [77]. A vast majority of sepsis is caused by bacteria, which are primarily gram-negative in nature.
Several studies have reported a relationship between selenium status and sepsis/septic shock. Sakr et. al, showed that plasma selenium concentrations were below the standard value 92 percent of the time in critically ill patients admitted to the surgical Intensive Care Unit [ICU]. All critically ill patients demonstrated a consistent decrease in plasma selenium concentrations throughout the course of their ICU stay. Moreover, the plasma selenium concentration was lower upon admission and decreased more significantly throughout the ICU stay in non-survivors compared to survivors. This affect is most likely due to selenium’s role as an antioxidant and involvement with immune system function. Overall, lower plasma selenium levels were correlated with increased tissue damage, organ dysfunction/failure, and increased mortality [78].
In vitro models of sepsis have demonstrated that the selenium environment influences LPS/PepG-induced mitochondrial dysfunction. Mitochondrial dysfunction plays a significant role during the course of sepsis due to a loss of membrane potential and metabolic activity which ultimately results in decreased ATP production [79]. In vivo models have shown that the administration of selenium results in decreased septic alterations within lung tissue and improved glutathione peroxidase activity [specifically GPx3] which plays a role in preventing oxidative damage. Additionally, myeloperoxidase [MPO] activity [a marker for neutrophil accumulation and activity within the lung] is altered during sepsis. Following selenium supplementation, MPO activity significantly decreased indicating that selenium suppresses the severity of sepsis by decreasing MPO activity. This is thought to be due to decreasing neutrophil accumulation resulting in less tissue damage [80]. It should also be noted that few studies have demonstrated that MPO contributes to protection against endotoxemia [81]. Thus, the role of selenium may be more complex as the overall effect may be dependent on tissues, selenium status, causative agents, and immune status of the host.
Studies investigating the role of hepatic selenium metabolism during sepsis demonstrates that selenium metabolism is disturbed during sepsis which results in decreased serum selenium due to decreased synthesis of Selenoprotein P [SelP], the selenoprotein responsible for transporting selenium. These data support the idea that SelP is the main determinant of the altered serum selenium status during sepsis as the expression of other selenoproteins rely on the availability of selenium for Sec biosynthesis within tissue specific locations. Moreover, SelP biosynthesis and selenium status may be under the control of the limiting trans-acting factors which are required for selenoprotein synthesis. This study demonstrated that redox status may control SBP2 trafficking and thus, may alter the rate of selenoprotein biosynthesis. However, how supplementation of selenium may reverse this condition has not been addressed. Thus, more research is required to determine the exact effect selenium exerts on various tissues during acute sepsis/septic shock and the mechanism by which selenium is regulated during these events.
Staphylococcus aureus Infections
Staphylococcus aureus is an opportunistic gram-positive commensal bacterium which may overcome the immune system gaining access to deep tissues. The resulting infections range from mild skin infections to more severe infections such as pyomyositis, necrotizing fasciitis, necrotizing pneumonia, and bacteremia [82]. Internalization of S. aureus by macrophages can occur via non-opsonic uptake [such as internalization via Pattern Recognition Receptors [PRRs]], complement dependent, and complement-independent uptake depending on the structure of the bacterium’s capsule. Activation of PRRs result in the activation of the Nuclear Factor Kappa B [NFκB] signaling pathway which is thought to play a central role in inflammation leading to the activation of genes responsible for various cytokines and chemokines. The Mitogen Activated Protein Kinase [MAPK] signaling pathway has also been shown to be involved during inflammation and is able to induce pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [83]. Following internalization, macrophages produce Reactive Oxygen Species [ROS] as well as Reactive Nitrogen Species [RNS] to induce microbial killing. However, S. aureus produces a variety of virulence factors which protect them against microbicidal agents generated by phagocytosis allowing for their intracellular survival [84]. S. aureus pathogenicity is also related to its ability to produce arginase which can compete with inducible nitric oxide synthase [iNOS] for the shared substrate arginine leading to decreased NO production and reduced microbicidal activity by activated macrophages. Lower NO production results in bacterial expression of NO-inducible-L-lactate dehydrogenase improving S. aureus resistance to oxidative stress [85]. Because selenium is known to be an antioxidant and is required for optimal immune cell functioning, it is plausible that it will aid in the host response to infection of Staphylococcus.
In fact, upon internalization of S. aureus, macrophages supplemented with selenium produced a significantly reduced amount of NO with a concomitant increase in ROS production [specifically H202]. Selenium supplementation also led to a decrease in the bacterial arginase activity, limiting the bacterial tolerance to oxidative stress. Moreover, selenium was shown to enhance phagocytosis of the bacterium and increase bactericidal capacity in a dose-dependent manner. Thus, selenium supplementation could result in an enhanced immune response to S. aureus [85].
Bi et.al., demonstrated that RAW264.7 macrophages supplemented with selenium during S. aureus infection resulted in decreased inflammatory cytokine gene expression and protein levels (TNF-α, IL-1β, and IL-6]. Additionally, selenium supplementation appeared to inhibit the activation of both NF-κB and MAPK signaling pathways. This occurred via inhibition of the phosphorylation of IκBα and p65 as well as Erk, Jnk, and p38 resulting in the suppression of NF-κB and MAPK signaling, respectively. Together, the inhibition of these pathways by selenium led to the attenuation of the overall inflammatory pathways [83].
S. aureus infection is also the primary cause of mastitis (mammary inflammation disease) in both humans and animals which results in inflammation injury to the mammary tissue [86]. Selenium has been shown to perform an immunoregulatory function on inflammation in mammary epithelial cells and glandular tissue, indicating it may play a role during S. aureus-induced mastitis [87]. Bacterial infection in selenium deficient mice resulted in mammary alveolus damage due to damaged and apoptotic epithelial cells while selenium supplementation improved the observed histological changes. Selenium deficiency was also shown to increase the levels of pro-inflammatory cytokines during infection with S. aureus [NO, IL-17, IL-8, IFN-γ]. Moreover, IL-10 expression was highest in the high selenium infection group indicating that selenium deficiency promotes pro-inflammatory cytokines while selenium supplementation promotes antiinflammatory cytokines. This study also demonstrated inhibition of NF-κB under selenium supplemented condition [86]. Additional work has demonstrated that selenium also plays a role inhibiting S. aureus infection of the uterus and reduces the activation of Toll Like Receptor-2 [TLR2] inflammatory signaling reducing Caspase activity [88]. Overall, selenium appears to benefit the host by limiting the inflammatory damage caused by S. aureus.
Escherichia coli infections
Escherichia coli is a versatile bacterium which is typically a part of the normal intestinal microflora of humans and various other mammals. While the commensal strains of E. coli are rarely involved in causing diseases in the healthy human host, there are numerous adapted E. coli strains which possess virulence attributes that allow them to cause disease. Infection with pathogenic E. coli are able to produce a variety of infections such as enteric/diarrheal disease, urinary tract infections, and sepsis/meningitis [89].
Citrobacter rodentium is a murine pathogen which mainly infects the colon and closely resembles enteropathogenic E. coli [EPEC] and enterohemorrhagic E. coli [EHEC] in humans. C. rodentium infections in mice have been shown to induce lesions similar to those caused by EPEC and EHEC making it an excellent model to study these human diseases. Furthermore, C. rodentium has been shown to alter gut homeostasis by causing crypt hyperplasia, epithelial cell proliferation, crypt dilation, mucosal thickening, and apical enterocyte surfaces. Mice fed diets deficient in both Vitamin E and selenium resulted in increased pathology, higher bacterial burdens, and elevated bacterial translocation to the spleen following C. rodentium infection. Moreover, the deficiency of Vitamin E enhanced the effects of selenium deficiency indicating that combined deficiency may exacerbate pathology. This is most likely due to a greater pro-inflammatory response to infection as demonstrated by increased chemokine and cytokine expression [90]. Thus, deficiency of Vitamin E and selenium may result in increased oxidative stress leading to greater proinflammatory signaling which ultimately results in greater tissue damage during gastrointestinal tract disease.
Selenium-enriched probiotics have also been investigated as useful means to protect against pathogenic E. coli within the gut. Selenium-enriched probiotics demonstrated superior ability to increase the serum selenium levels over sodium selenite presumably due to the enhanced absorption of organic selenium compounds over that of inorganic selenium compounds. Four varieties of probiotic bacteria [Candida utilis, Lactobacillus acidophilus, Lactobacillus rhamnosus GG, and Streptococcus thermophilus] were grown in the presence of selenium and were fed to mice. These bacteria adhered well to the intestine and resulted in the inability of other pathogenic bacteria, such as E. coli, to interact with potential binding sites. This demonstrates the enhanced ability of selenium-enriched probiotics to support the internal environment of the gut by increasing antioxidant performance, preventing pathogenic bacterial colonization, increasing immunity and preventing enteric illness [91].
Similar effects of selenium have been observed in an E. coli model of chronic bacterial prostatitis [CBP]. Currently, the primary method to treat this disease is the use of antibiotics. However, this requires small molecular weight antibiotics which are fat soluble in order for the antibiotic to diffuse on the prostate epithelial membrane. Kim et. al. demonstrated that selenium in addition to ciprofloxacin resulted in the most significant reduction of E. coli. Moreover, treatment with selenium lead to a significant reduction in inflammatory cell infiltration of the prostate. This suggests that selenium may play a role in the resolution of CBP especially when used in conjunction with antibiotics [92]. Overall, selenium supplementation may aid the host immune response ultimately preventing illnesses caused by pathogenic E. coli.
Clostridium Infections
Clostridium perfringens is a gram-positive, spore-forming bacteria which often colonizes the gastrointestinal tracts of humans and animals. This bacterium can be found in diverse environments such as soil, sewage, and food such as raw meat [93]. C. perfringens is often associated with severe systemic and enteric disease, food poisoning, and enterocolitis which is believed to be caused by the secretion of >20 identified toxins or enzymes [94].
In poultry, C. perfringens is known to colonize the intestines of chickens where it causes necrotic enteritis resulting in high morbidity and mortality; this disease results in an economic loss greater than 2 billion dollars. Broiler chickens which were supplemented with selenium and subjected to colonization of C. perfringens, demonstrated increased body weight gain, fewer intestinal lesions, improved antibody production to Net-B toxin, and upregulated transcript levels of IL-1β, IL-6, IL-8, iNOS, LITAF [Lipopolysaccharide induced TNF Factor], and TNSF15 [Tumor Necrosis Factor Superfamily, Member 15] compared to those which were not supplemented during infection. The upregulation of pro-inflammatory cytokines represents the initiation of the innate immune response which is important for protection against pathogens during the early phase of infection. The upregulation of these pro-inflammatory cytokines following selenium supplementation suggests that they are essential during the host immune response to necrotizing enteritis [95]. These results support previous findings which demonstrated a beneficial effect of injected selenium into the amniotic cavity of developing eggs followed by exposure to C. perfringens in post-hatched chickens [96]. Further research is required to elucidate the direct mechanism by which selenium exerts its effects during necrotizing enteritis.
Vibrio cholerae Infections
Vibrio cholerae is a pathogenic bacterium which causes toxin-mediated diarrhea in humans. This waterborne pathogen leads to extreme dehydration which may result in death in untreated patients [97]. In order to cause infection within its host, V. cholerae relies on its motility, intestinal colonization, and the production of cholerae toxin [98]. Bhattaram et. al., investigated the effect of a sub-inhibitory concentration of selenium [400 μg] on V. cholera’s pathogenicity. They observed that selenium supplementation resulted in reduced motility, an important step in its pathogenesis which allows the bacterium to traverse the host’s intestine. While they failed to elucidate the mechanism by which this occurs, prior literature focusing on other bacterial species has suggested that it may be due to an alteration of membrane integrity which affects flagellar structure [98]. Furthermore, Bhattaram and collogues demonstrated that selenium reduced intestinal cell-bacterial attachment and was effective at reducing the production of cholera toxin which causes profuse diarrhea by 95%. This effect was not the result of an alteration to the host but an alteration to bacterial virulence factors [98]. This case demonstrates an instance where selenium supplementation may benefit the host by increasing their immune response while concomitantly decreasing the virulence of the bacterial pathogen.
Other Bacterial Infections
Various additional studies have demonstrated a range of benefits from selenium supplementation during infection. Listeria monocytogenes is a gram-positive, food-borne bacterium that causes severe infections in immunocompromised patients. A study demonstrated that mice with a reduced resistance to infection were associated with reduced antioxidant activity and an overall decreased innate immune response. Moreover, the persistence of bacteria was greater in selenium deficient groups compared to those of the selenium adequate group. Selenium deficient mice also demonstrated reduced antioxidant activity compared to those in the selenium adequate group. Additionally, selenium deficient mice had a decreased natural killer cell response to infection. Thus, selenium deficiency results in a compromised response following infection with L. monocytogenes [99]. Such alterations of the immune response caused by selenium deficiency has also been demonstrated in ruminants with foot rot caused by Dichelobacter nodosus. While selenium did not prevent diseases in these animals, selenium supplementation was able to restore immune functions [100]. New studies are beginning to investigate the role of selenium during leprosy which have demonstrated that low selenium status occurs in patients with high bacterial load [101, 102]. While many studies indicate a benefit for selenium supplementation during infection by bacterial pathogens, there are studies which suggest there is no benefit of supplementation [103]. Thus, there is a need for more studies investigating the outcome of selenium supplementation following bacterial infections.
Selenium and Gut Microbiota
There is a growing body of evidence that links alterations of the host gut microbiome to various diseases such as colon cancer, Crohn’s disease, inflammatory bowel disease, and obesity [104–112]. One strategy that may be valuable in preventing such alterations of the microbiome may involve modulating the diet specifically through alterations in micronutrient intake. Selenium supplementation in mice has shown to affect the composition of the existing microbiota as well as the establishment of the microflora. Significant changes were seen in the microbiota upon selenium supplementation as some groups increased in diversity while fewer groups exhibited decreases in diversity. The most prominent effect was seen in the decline of the genus Parabacterioides as well as several alterations in phylotypes of Firmicutes including Clostridia. Overall, selenium appeared to increase the diversity of the microbiome which is most likely due to the fact that selenium is able to be utilized in some bacteria while it remains toxic to others. Thus, the microbiome may sequester selenium for incorporation into bacterial selenoproteins. This may result in the microbiota competing with the host for dietary selenium [113]. A similar study investigating the effects of selenium nanoparticles in poultry also demonstrated positive effects in improving gut health by altering the microbiome’s diversity [114].
Selenium supplementation has also shown to play a role in the intestinal barrier functions due to its effects on the microbiota. Selenium supplemented mice subjected to Dextran Sulfate Sodium [DSSj-induced colitis demonstrated enhanced survival, fewer symptoms of colitis, and decreased gut permeability. Moreover, fecal transplantation from selenium supplemented mice into deficient mice was able to alleviate colitis. This is thought to be attributed to the altered microbiota of supplemented mice which showed decreases in Dorea and increases in microbes with protective effects [115].
Conclusions
It is also important to recognize that many infectious diseases are associated with a reduced serum selenium level in the host. However, it is not clear if the low-selenium hosts are more prone to infectious diseases or infected hosts deplete selenium at a higher rate due to inflammation. It is also important to determine the levels of selenium in specific tissues during infections as the levels may vary between serum and infected organs. Lastly, more reliable biological markers that indicate the selenium status of hosts are required.
It is clear from the limited studies which have investigated the effect of selenium on bacterial infections that some bacterial species are able to benefit from the presence of selenium in their surrounding environment. Thus, when such bacteria are able to establish an infection in a mammalian host, there is a complex interaction that occurs between the host immune response, microbial pathogen, microbiota, and host selenium status. Many of the enzymes which utilize selenium allow the bacterium to survive in anaerobic conditions such as the mammalian intestine. When these infections occur, the bacterium may ultimately benefit from the selenium-supplemented host by leeching selenium in order to increase its virulence and pathogenicity. At the same time, the host may also benefit from the improved immune functions due to the beneficial effects of selenium on host immune responses. Host microbiota may also differ in the presence of selenium that may prevent infection with selenium-dependent bacteria either by competing for selenium or by producing toxic metabolites that may be detrimental to the pathogenic bacteria. Conversely, it is known that selenium deficiency in the host may place them in an immunocompromised state where bacteria which do not require selenium may establish an infection and cause pathology. Microbiota in the absence of selenium may promote the establishment of infection as well. Because of this complex relationship, more research is required investigating the effects of selenium utilization among pathogenic bacteria, microbiota and hosts at various selenium status. Advances in next generation sequencing, availability of germ-free mice and specific immune deficient mice, in addition to the ability to grow and transplant monocultures of microbiota will allow better elucidation to the exact role of selenium in bacterial pathogenesis.
Figure 1. Schematic representing the close interactions of a pathogen, microbiome and immune cells in the context of requirement for selenium.
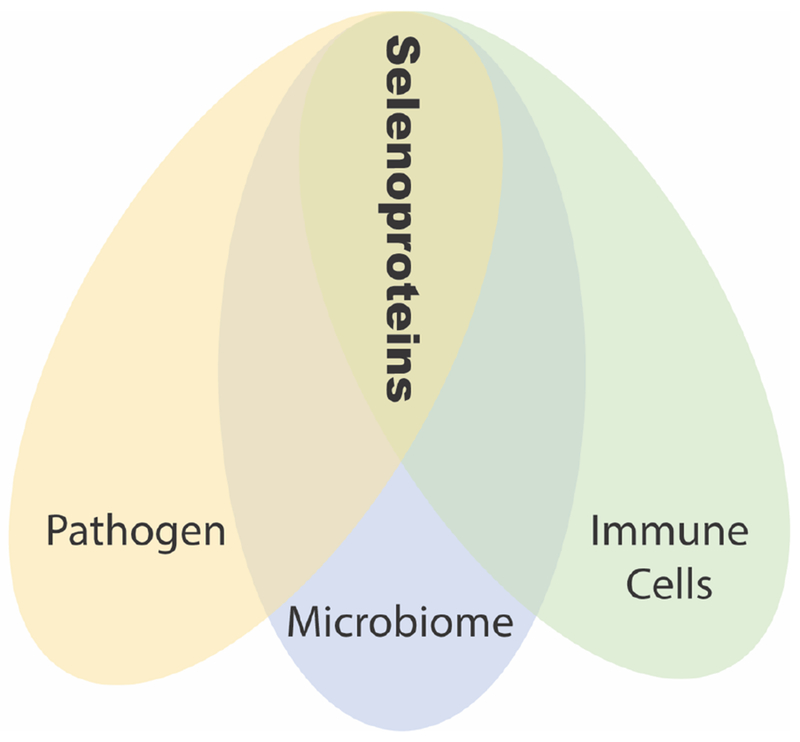
Several bacterial pathogens require selenium for adapting to host environment, particularly to the anaerobic environment. Host microbiome also requires selenium as selenium depletion leads to dysbiosis of microbiota. Host immune cells also require selenium for their optimum functions.
Figure 2. Involvement of selenium and selenoproteins in host-bacteria interactions. Several immune functions are dependent or modulated by selenoproteins.
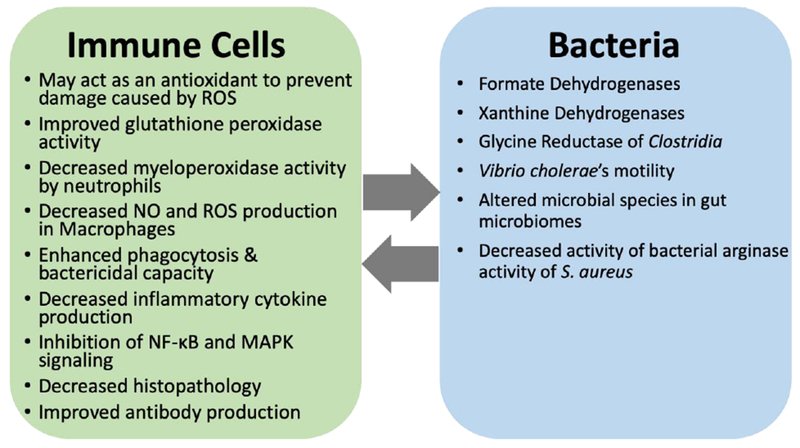
Similarly, selenoproteins also contribute to the pathogen fitness in host environment. The dynamic interaction and consequent pathogenesis is thus regulated in part by the limited selenium availability.
Table 2.
Key findings related to the role of selenium in human clinical trials.
| Study | Year | Patients | Selenium | Dose | Levels of Se/GPX | Mortality |
|---|---|---|---|---|---|---|
| Angstwurm [123] | 1999 | 42 | Selenite | 535 μg/24hr/3d to 285 μg/24hr/3d to 155 μg/24hr/3d then maintained as control group on 35 μg/24hr/d | Increased | Total p=0.13 Post hoc p=0.0278 |
| Berger [124] | 2006 | 41 | Selenite | copper 2.5-3.1 mg/d selenium 315-380μg/d zinc 26.2 - 31.4 mg/d | Increased | p=0.57 |
| Angstwurm [125] | 2007 | 249 (238, 189) | Selenite | 1,000 μg/bolus followed by 1,000 μg/24hr/14d or placebo | Increased | p=.109 p=0.049 |
| Forceville [126] | 2007 | 60 | Selenite | 4,000 μg/24hr/1d to 1000 μg/24hr/9d or placebo | N/A* | p=0.691 |
| Mishra [127] | 2007 | 40 | Selenite | 474 μg/24hr/3d to 316 μg/24hr/3d to 158 μg/24hr/3d then maintained as control group on 31.6 μg/24hr/d | Increased | p=0.94 |
| Andrews [128] | 2011 | 502 | Selenite | Parenteral glutamine 20.2 g/24hr/7d or selenium 500 μg/24hr/7d | N/A* | p=0.54 |
| Manzanares [129] | 2011 | 35 | Selenite | 1,000 μg/bolus followed by 1,000 μg/24hr/14d or placebo | Increased | p=0.55 p=0.95 |
| Valenta [130] | 2011 | 150 | Selenite | 1000μg/24hr/1d to 500μg/24hr/13d then maintained as controls group on 75μg/24hr/14d | Increased | p=0.367 |
| Janka [131] | 2013 | 72 | Selenite | 750 μg/24 h/6d or placebo | Increased | p=0.159 |
N/A signifies information that is not available.
Acknowledgments
Funding Sources:
This work was supported by AI123521 to GSK and T32 GM108563 to SES.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Romero H, Zhang Y, Gladyshev VN, Salinas G (2005) Evolution of selenium utilization traits. Genome Biol 6:R66 10.1186/gb-2005-6-8-r66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Turanov AA, Hatfield DL, Gladyshev VN (2008) In silico identification of genes involved in selenium metabolism: evidence for a third selenium utilization trait. BMC Genomics 9:251 10.1186/1471-2164-9-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Romero H, Salinas G, Gladyshev VN (2006) Dynamic evolution of selenocysteine utilization in bacteria: a balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome Biol 7:R94 10.1186/gb-2006-7-10-r94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci 39:112–120. 10.1016/j.tibs.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stadtman TC (1996) Selenocysteine. Annu Rev Biochem 65:83–100. 10.1146/annurev.bi.65.070196.000503 [DOI] [PubMed] [Google Scholar]
- 6.Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and Selenium in Microbial Metabolism. Annu Rev Microbiol 60:107–130. 10.1146/annurev.micro.60.080805.142053 [DOI] [PubMed] [Google Scholar]
- 7.Hatfield DL, Gladyshev VN (2002) How selenium has altered our understanding of the genetic code. Mol Cell Biol 22:3565–76. 10.1128/mcb.22.11.3565-3576.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladyshev VN (2016) Eukaryotic Selenoproteomes In: Selenium. Springer International Publishing, Cham, pp 127–139 [Google Scholar]
- 9.Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241. 10.1016/S0140-6736(00)02490-9 [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Rose AH, Hoffmann PR (2012) The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal 16:705–743. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: Molecular Pathways and Physiological Roles. Physiol Rev 94:739–777. 10.1152/physrev.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668. 10.1002/mus.10304 [DOI] [PubMed] [Google Scholar]
- 13.ISHIHARA H, KANDA F, MATSUSHITA T, et al. (1999) White muscle disease in humans: myopathy caused by selenium deficiency in anorexia nervosa under long term total parenteral nutrition. J Neurol Neurosurg Psychiatry 67:829–830. 10.1136/jnnp.67.6.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan HB, Benetucci J, Muzzio E, et al. (2012) High rates of serum selenium deficiency among HIV- and HCV-infected and uninfected drug users in Buenos Aires, Argentina. Public Health Nutr 15:538–545. 10.1017/S1368980011001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Bella S, Grilli E, Cataldo MA, Petrosillo N (2010) Selenium deficiency and HIV infection. Infect Dis Rep 2:18 10.4081/idr.2010.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi R, Kim H-T, Lim Y, et al. (2015) Serum Concentrations of Trace Elements in Patients with Tuberculosis and Its Association with Treatment Outcome. Nutrients 7:5969–5981. 10.3390/nu7075263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph M, Kroll F, Beery M, et al. (2013) A Pilot Study Assessing the Impact of a Fortified Supplementary Food on the Health and Well-Being of Creche Children and Adult TB Patients in South Africa. PLoS One 8:e55544 10.1371/journal.pone.0055544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum MK, Campa A, Lai S, et al. (2013) Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. JAMA 310:2154–63. 10.1001/jama.2013.280923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenbaek K, Friis H, Hansen M, et al. (2006) The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: a randomized trial among chronic hepatitis C virus-infected patients. Eur J Gastroenterol Hepatol 18:985–989. 10.1097/01.meg.0000231746.76136.4a [DOI] [PubMed] [Google Scholar]
- 20.Khan MS, Dilawar S, Ali I, Rauf N (2012) The possible role of selenium concentration in hepatitis B and C patients. Saudi J Gastroenterol 18:106–10. 10.4103/1319-3767.93811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinbrenner H, Al-Quraishy S, Dkhil MA, et al. (2015) Dietary Selenium in Adjuvant Therapy of Viral and Bacterial Infections. Adv Nutr 6:73–82. 10.3945/an.114.007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villamor E, Mugusi F, Urassa W, et al. (2008) A Trial of the Effect of Micronutrient Supplementation on Treatment Outcome, T Cell Counts, Morbidity, and Mortality in Adults with Pulmonary Tuberculosis. J Infect Dis 197:1499–1505. 10.1086/587846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SEYEDREZAZADEH E, OSTADRAHIMI A, MAH BOOB S, et al. (2008) Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology 13:294–298. 10.1111/j.1440-1843.2007.01200.x [DOI] [PubMed] [Google Scholar]
- 24.Kryukov G V, Gladyshev VN (2004) The prokaryotic selenoproteome. EMBO Rep 5:538–543. 10.1038/sj.embor.7400126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santesmasses D, Mariotti M, Guigo R (2017) Computational identification of the selenocysteine tRNA (tRNASec) in genomes. PLOS Comput Biol 13:e1005383 10.1371/journal.pcbi.1005383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axley MJ, Stadtman TC (1989) Selenium metabolism and selenium-dependent enzymes in microorganisms. Annu Rev Nutr 9:127–37. 10.1146/annurev.nu.09.070189.001015 [DOI] [PubMed] [Google Scholar]
- 27.Böck A, Forchhammer K, Heider J, et al. (1991) Selenocysteine: the 21st amino acid. Mol Microbiol 5:515–520. 10.1111/j.1365-2958.1991.tb00722.x [DOI] [PubMed] [Google Scholar]
- 28.Böck A (2001) Selenium metabolism in bacteria In: Selenium. Springer US, Boston, MA, pp 7–22 [Google Scholar]
- 29.Böck A (2000) Biosynthesis of selenoproteins--an overview. Biofactors 11:77–8 [DOI] [PubMed] [Google Scholar]
- 30.Thanbichler M, Böck A (2002) Selenoprotein Biosynthesis: Purification and Assay of Components Involved in Selenocysteine Biosynthesis and Insertion in Escherichia coli. In: Methods in enzymology. pp 3–16 [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Peng T, Jiang L, et al. (2015) Comparative Genomics Reveals New Candidate Genes Involved in Selenium Metabolism in Prokaryotes. Genome Biol Evol 7:664–676. 10.1093/gbe/ew022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulteau A-L, Chavatte L (2015) Update on selenoprotein biosynthesis. Antioxid Redox Signal 23:775–94. 10.1089/ars.2015.6391 [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Peng T, Jiang L, et al. (2015) Comparative Genomics Reveals New Candidate Genes Involved in Selenium Metabolism in Prokaryotes. Genome Biol Evol 7:664–676. https://doi.org/10.1093/gbe/ew022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caton-Williams J, Huang Z (2008) Biochemistry of Selenium-Derivatized Naturally Occurring and Unnatural Nucleic Acids. Chem Biodivers 5:396–407. 10.1002/cbdv.200890040 [DOI] [PubMed] [Google Scholar]
- 35.Ching WM, AIzner-DeWeerd B, Stadtman TC (1985) A selenium-containing nucleoside at the first position of the anticodon in seleno-tRNAGIu from Clostridium sticklandii. Proc Natl Acad Sci 82:347–350. 10.1073/pnas.82.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ching W-M (1986) Characterization of selenium-containing tRNAGIu from Clostridium sticklandii. Arch Biochem Biophys 244:137–146. 10.1016/0003-9861(86)90102-5 [DOI] [PubMed] [Google Scholar]
- 37.Gladyshev VN, KhangulovS V., Stadtman TC (1996) Properties of the Selenium- and Molybdenum-Containing Nicotinic Acid Hydroxylase from Clostridium barkeri. Biochemistry 35:212–223. 10.1021/bi951793i [DOI] [PubMed] [Google Scholar]
- 38.Self WT (2002) Regulation of Purine Hydroxylase and Xanthine Dehydrogenase from Clostridium purinolyticum in Response to Purines, Selenium, and Molybdenum. J Bacteriol 184:2039–2044. 10.1128/JB.184.7.2039-2044.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng T, Lin J, Xu Y-Z, Zhang Y (2016) Comparative genomics reveals new evolutionary and ecological patterns of selenium utilization in bacteria. ISME J 10:2048–2059. 10.1038/ismej.2015.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferry JG (1990) Formate dehydrogenase. FEMS Microbiol Lett 87:377–382. 10.1111/j.1574-6968.1990.tb04940.x [DOI] [PubMed] [Google Scholar]
- 41.Bettenbrock K, Bai H, Ederer M, et al. (2014) Towards a Systems Level Understanding of the Oxygen Response of Escherichia coli. In: Advances in microbial physiology, pp 65–114 [DOI] [PubMed] [Google Scholar]
- 42.Axley MJ, Bock A, Stadtman TC (1991) Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc Natl Acad Sci 88:8450–8454. 10.1073/pnas.88.19.8450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw FL, Mulholland F, Le Gall G, et al. (2012) Selenium-Dependent Biogenesis of Formate Dehydrogenase in Campylobacter jejuni Is Controlled by the fdhTU Accessory Genes. J Bacteriol 194:3814–3823. 10.1128/JB.06586-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tareen AM, Dasti Jl, Zautner AE, et al. (2010) Campylobacter jejuni proteins Cj0952c and Cj0951c affect chemotactic behaviour towards formic acid and are important for invasion of host cells. Microbiology 156:3123–3135. 10.1099/mic.0.039438-0 [DOI] [PubMed] [Google Scholar]
- 45.Andreesen JR, Wagner M, Sonntag D, et al. (1999) Various functions of selenols and thiols in anaerobic Gram-positive, amino acids-utilizing bacteria. BioFactors 10:263–270. 10.1002/biof.5520100226 [DOI] [PubMed] [Google Scholar]
- 46.Garcia GE, Stadtman TC (1992) Clostridium sticklandii glycine reductase selenoprotein A gene: cloning, sequencing, and expression in Escherichia coli. J Bacteriol 174:7080–7089. 10.1128/jb.174.22.7080-7089.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrader T, Rienhofer A, Andreesen JR (1999) Selenium-containing xanthine dehydrogenase from Eubacterium barkeri. Eur J Biochem 264:862–871. 10.1046/j.1432-1327.1999.00678.x [DOI] [PubMed] [Google Scholar]
- 48.Self WT, Stadtman TC (2000) Selenium-dependent metabolism of purines: A selenium-dependent purine hydroxylase and xanthine dehydrogenase were purified from Clostridium purinolyticum and characterized. Proc Natl Acad Sci 97:7208–7213. 10.1073/pnas.97.13.7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava M, Mallard C, Barke T, et al. (2011) A Selenium-Dependent Xanthine Dehydrogenase Triggers Biofilm Proliferation in Enterococcus faecalis through Oxidant Production. J Bacteriol 193:1643–1652. 10.1128/JB.01063-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rucker RB, Fascetti AJ, Keen CL (2008) Trace Minerals In: Clinical Biochemistry of Domestic Animals. Elsevier, pp 663–693 [Google Scholar]
- 51.van Crevel R, Ottenhoff THM, van der Meer JWM (2002) Innate Immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 15:294–309. 10.1128/CMR.15.2.294-309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54. 10.1039/C3MT00185G [DOI] [PubMed] [Google Scholar]
- 53.Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54. 10.1039/C3MT00185G [DOI] [PubMed] [Google Scholar]
- 54.Arthur JR, McKenzie RC, Beckett GJ (2003) Selenium in the immune system. J Nutr 133:1457S–9S. 10.1093/jn/133.5.1457S [DOI] [PubMed] [Google Scholar]
- 55.Huang Z, Rose AH, Hoffmann PR (2012) The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal 16:705–743. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (2012) CDC | TB | Data and Statistics. In: 2017 [Google Scholar]
- 57.Lazzari TK, Forte GC, Silva DR (2018) Nutrition Status Among HIV-Positive and HIV-Negative Inpatients with Pulmonary Tuberculosis. Nutr Clin Pract 33:858–864. 10.1002/ncp.10006 [DOI] [PubMed] [Google Scholar]
- 58.Muzembo BA, Mbendi NC, Ngatu NR, et al. (2018) Serum selenium levels in tuberculosis patients: A systematic review and meta-analysis. J Trace Elem Med Biol 50:257–262. 10.1016/j.jtemb.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 59.Kassu A, Yabutani T, Mahmud ZH, et al. (2006) Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr 60:580–586. 10.1038/sj.ejcn.1602352 [DOI] [PubMed] [Google Scholar]
- 60.Moraes ML de, Ramalho DM de P, Delogo KN, et al. (2014) Association between serum selenium level and conversion of bacteriological tests during antituberculosis treatment. J Bras Pneumol 40:269–278. 10.1590/S1806-37132014000300010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campa A, Baum M, Bussmann H, et al. (2017) The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr Diet Suppl Volume 9:37–45. 10.2147/NDS.S123545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.KAWAI K, MEYDANI SN, URASSA W, et al. (2014) Micronutrient supplementation and T cell-mediated immune responses in patients with tuberculosis in Tanzania. Epidemiol Infect 142:1505–1509. 10.1017/S0950268813002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grobler L, Nagpal S, Sudarsanam TD, Sinclair D (2016) Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 10.1002/14651858.CD006086.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamboj AK, Cotter TG, Oxentenko AS (2017) Helicobacter pylori: The Past, Present, and Future in Management. Mayo Clin Proc 92:599–604. 10.1016/j.mayocp.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 65.CDC (1998) Helicobacter pylori outer membrane protein (Omp22); used in a recombinant vaccine for therapy or prevention of H. pylori infection Mogam Biotechnol. Res. Inst. Kyonggi World 9728 264; 7 August 1997. Vaccine 16:436 10.1016/S0264-410X(97)80923-1 [DOI] [Google Scholar]
- 66.Kamboj AK, Cotter TG, Oxentenko AS (2017) Helicobacter pylori: The Past, Present, and Future in Management. Mayo Clin Proc 92:599–604. 10.1016/j.mayocp.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 67.Lahner E, Persechino S, Annibale B (2012) Micronutrients (Other than iron) and Helicobacter pylori Infection: A Systematic Review. Helicobacter 17:1–15. 10.1111/j.1523-5378.2011.00892.x [DOI] [PubMed] [Google Scholar]
- 68.Ustiindag Y, Boyacioglu S, Haberal A, et al. (2001) Plasma and gastric tissue selenium levels in patients with Helicobacter pylori infection. J Clin Gastroenterol 32:405–8. 10.1097/00004836-200105000-00009 [DOI] [PubMed] [Google Scholar]
- 69.Hu A, Li L, Hu C, et al. (2018) Serum Concentrations of 15 Elements Among Helicobacter Pylori-Infected Residents from Lujiang County with High Gastric Cancer Risk in Eastern China. Biol Trace Elem Res 186:21–30. 10.1007/s12011-018-1283-4 [DOI] [PubMed] [Google Scholar]
- 70.Camargo MC, Burk RF, Bravo LE, et al. (2008) Plasma Selenium Measurements in Subjects from Areas with Contrasting Gastric Cancer Risks in Colombia. Arch Med Res 39:443–451. 10.1016/j.arcmed.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deshmukh P, Unni S, Krishnappa G, Padmanabhan B (2017) The Keap1-Nrf2 pathway: promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys Rev 9:41–56. 10.1007/s12551-016-0244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji JH, Shin DG, Kwon Y, et al. (2012) Clinical Correlation between Gastric Cancer Type and Serum Selenium and Zinc Levels. J Gastric Cancer 12:217 10.5230/jgc.2012.12.4.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kneller RW, Guo W De, Hsing AW, et al. (1992) Risk factors for stomach cancer in sixty-five Chinese counties. Cancer Epidemiol Biomarkers Prev 1:113–8 [PubMed] [Google Scholar]
- 74.Cai X, Wang C, Yu W, et al. (2016) Selenium Exposure and Cancer Risk: an Updated Metaanalysis and Meta-regression. Sci Rep 6:19213 10.1038/srep19213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong H-Y, He J-G, Li B-S (2016) Meta-analysis of the association between selenium and gastric cancer risk. Oncotarget 7:15600–5. 10.18632/oncotarget.7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singer M, Deutschman CS, Seymour CW, et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong BA, Betzold RD, May AK (2017) Sepsis and Septic Shock Strategies. Surg Clin North Am 97:1339–1379. 10.1016/j.suc.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 78.Sakr Y, Reinhart K, Bloos F, et al. (2007) Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth 98:775–784. 10.1093/bja/aem091 [DOI] [PubMed] [Google Scholar]
- 79.Mertens K, Lowes DA, Webster NR, et al. (2015) Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br J Anaesth 114:990–999. 10.1093/bja/aev073 [DOI] [PubMed] [Google Scholar]
- 80.Zolali E, Hamishehkar H, Maleki-Dizaji N, et al. (2014) Selenium effect on oxidative stress factors in septic rats. Adv Pharm Bull 4:289–93. 10.5681/apb.2014.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reber LL, Gillis CM, Starkl P, et al. (2017) Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J Exp Med 214:1249–1258. 10.1084/jem.20161238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balasubramanian D, Harper L, Shopsin B, Torres VJ (2017) Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis 75:. 10.1093/femspd/ftx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bi C-L, Wang H, Wang Y-J, et al. (2016) Selenium inhibits Staphylococcus aureus-induced inflammation by suppressing the activation of the NF-κB and MAPK signalling pathways in RAW264.7 macrophages. Eur J Pharmacol 780:159–165. 10.1016/j.ejphar.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 84.Cole J, Aberdein J, Jubrail J, Dockrell DH (2014) The Role of Macrophages in the Innate Immune Response to Streptococcus pneumoniae and Staphylococcus aureus. In: Advances in Microbial Physiology, pp 125–202 [DOI] [PubMed] [Google Scholar]
- 85.Aribi M, Meziane W, Habi S, et al. (2015) Macrophage Bactericidal Activities against Staphylococcus aureus Are Enhanced In Vivo by Selenium Supplementation in a Dose-Dependent Manner. PLoS One 10:e0135515 10.1371/journal.pone.0135515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao X, Zhang Z, Li Y, et al. (2016) Selenium Deficiency Deteriorate the Inflammation of S. aureus Infection via Regulating NF-κB and PPAR-γ in Mammary Gland of Mice. Biol Trace Elem Res 172:140–147. 10.1007/s12011-015-0563-5 [DOI] [PubMed] [Google Scholar]
- 87.Wei Z, Yao M, Li Y, et al. (2014) Dietary Selenium Deficiency Exacerbates Lipopolysaccharide-Induced Inflammatory Response in Mouse Mastitis Models. Inflammation 37:1925–1931. 10.1007/s10753-014-9925-y [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Qiu C, Li W, et al. (2016) Selenium Plays a Protective Role in Staphylococcus aureus-Induced Endometritis in the Uterine Tissue of Rats. Biol Trace Elem Res 173:345–353. 10.1007/si2011-016-0659-6 [DOI] [PubMed] [Google Scholar]
- 89.Kaper JB, Nataro JP, Mobley HLT (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 90.Smith AD, Botero S, Shea-Donohue T, Urban JF (2011) The Pathogenicity of an Enteric Citrobacter rodentium Infection Is Enhanced by Deficiencies in the Antioxidants Selenium and Vitamin E. Infect Immun 79:1471–1478. 10.1128/IAI.01017-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Huang K, Qin S, et al. (2009) Antibacterial Action of Selenium-Enriched Probiotics Against Pathogenic Escherichia coli. Dig Dis Sci 54:246–254. 10.1007/s10620-008-0361-4 [DOI] [PubMed] [Google Scholar]
- 92.Kim HW, Ha U-S, Woo JC, et al. (2012) Preventive effect of selenium on chronic bacterial prostatitis. J Infect Chemother 18:30–34. 10.1007/s10156-011-0276-4 [DOI] [PubMed] [Google Scholar]
- 93.Li J, Uzal F, McClane B (2016) Clostridium perfringens Sialidases: Potential Contributors to Intestinal Pathogenesis and Therapeutic Targets. Toxins (Basel) 8:341 10.3390/toxins8110341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiu R, Hall LJ (2018) An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect 7:1–15. 10.1038/s41426-018-0144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu S, Lee S-H, Lillehoj HS, et al. (2015) Effects of dietary selenium on host response to necrotic enteritis in young broilers. Res Vet Sci 98:66–73. 10.1016/j.rvsc.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 96.Lee SH, Lillehoj HS, Jang SI, et al. (2014) Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickensl. Poult Sci 93:1113–1121. 10.3382/ps.2013-03770 [DOI] [PubMed] [Google Scholar]
- 97.CDC (2014) General Information | Cholera | CDC. In: CDC [Google Scholar]
- 98.Bhattaram V, Upadhyay A, Yin H-B, et al. (2017) Effect of Dietary Minerals on Virulence Attributes of Vibrio cholerae. Front Microbiol 8:. 10.3389/fmicb.2017.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C, Wang H, Luo J, et al. (2009) Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol 10:55 10.1186/1471-2172-10-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hall JA, Vorachek WR, Stewart WC, et al. (2013) Selenium Supplementation Restores Innate and Humoral Immune Responses in Footrot-Affected Sheep. PLoS One 8:e82572 10.1371/journal.pone.0082572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Partogi D, Dalimunthe DiA, Hazlianda CP (2018) A Study of Selenium in Leprosy. Open Access Maced J Med Sci 6:485–487. 10.3889/oamjms.2018.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foster R, Sanchez A, Foulkes J, Cameron LJ (1991) Profile of blood elements in leprosy patients. Indian J Lepr 63:12–33 [PubMed] [Google Scholar]
- 103.Kong Z, Wang F, Ji S, et al. (2013) Selenium supplementation for sepsis: a meta-analysis of randomized controlled trials. Am J Emerg Med 31:1170–1175. 10.1016/j.ajem.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 104.Hartstra AV, Bouter KEC, Backhed F, Nieuwdorp M (2015) Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 38:159–165. 10.2337/dc14-0769 [DOI] [PubMed] [Google Scholar]
- 105.Wolf KJ, Lorenz RG (2012) Gut Microbiota and Obesity. CurrObes Rep 1:1–8. 10.1007/si3679-011-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manichanh C (2006) Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 55:205–211. 10.1136/gut.2005.073817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mcllroy J, laniro G, Mukhopadhya I, et al. (2018) Review article: the gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol Ther 47:26–42. 10.1111/apt.14384 [DOI] [PubMed] [Google Scholar]
- 108.Borren NZ, Conway G, Garber JJ, et al. (2018) Differences in Clinical Course, Genetics, and the Microbiome Between Familial and Sporadic Inflammatory Bowel Diseases. J Crohn’s Colitis 12:525–531. 10.1093/ecco-jcc/jjx154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Assa A, Butcher J, Li J, et al. (2016) Mucosa-Associated Ileal Microbiota in New-Onset Pediatric Crohn’s Disease. Inflamm Bowel Dis 22:1533–9. 10.1097/MIB.0000000000000776 [DOI] [PubMed] [Google Scholar]
- 110.Zackular JP, Baxter NT, Iverson KD, et al. (2013) The Gut Microbiome Modulates Colon Tumorigenesis. MBio 4:. 10.1128/mBio.00692-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daniel SG, Ball CL, Besselsen DG, et al. (2017) Functional Changes in the Gut Microbiome Contribute to Transforming Growth Factor β-Deficient Colon Cancer. mSystems 2:. 10.1128/mSystems.00065-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scieszka M, Danch A, Machalski M, Drozdz M (1997) Plasma selenium concentration in patients with stomach and colon cancer in the Upper Silesia. Neoplasma 44:395–7. 10.1515/angl.2010.034 [DOI] [PubMed] [Google Scholar]
- 113.Kasaikina ΜV, Kravtsova MA, Lee BC, et al. (2011) Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J 25:2492–2499. 10.1096/fj.11-181990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gangadoo S, Dinev I, Chapman J, et al. (2018) Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biotechnol 102:1455–1466. 10.1007/s00253-017-8688-4 [DOI] [PubMed] [Google Scholar]
- 115.Zhai Q, Cen S, Li P, et al. (2018) Effects of Dietary Selenium Supplementation on Intestinal Barrier and Immune Responses Associated with Its Modulation of Gut Microbiota. Environ Sci Technol Lett 5:724–730 [Google Scholar]
- 116.Gao X, Zhang Z, Li Y, et al. (2016) Selenium Deficiency Facilitates Inflammation Following S. aureus Infection by Regulating TLR2-Related Pathways in the Mouse Mammary Gland. Biol Trace Elem Res 172:449–457. 10.1007/s12011-015-0614-y [DOI] [PubMed] [Google Scholar]
- 117.Smith AD, Cheung L, Botero S (2011) Long-term Selenium Deficiency Increases the Pathogenicity of a Citrobacter rodentium Infection in Mice. Biol Trace Elem Res 144:965–982. 10.1007/si2011-011-9071-4 [DOI] [PubMed] [Google Scholar]
- 118.Berg BM, Godbout JP, Chen J, et al. (2005) alpha-Tocopherol and selenium facilitate recovery from lipopolysaccharide-induced sickness in aged mice. J Nutr 135:1157–63. 10.1093/jn/135.5.1157 [DOI] [PubMed] [Google Scholar]
- 119.Altimira J, Prats N, Lopez S, et al. (2000) Effect of Selenium Deficiency on the Development of Central Nervous System Lesions in Murine Listeriosis. J Comp Pathol 123:104–109. 10.1053/jcpa.2000.0399 [DOI] [PubMed] [Google Scholar]
- 120.Kim SH, Ha U-S, Sohn DW, et al. (2012) Preventive effect of ginsenoid on chronic bacterial prostatitis. J Infect Chemother 18:709–714. 10.1007/s10156-012-0406-7 [DOI] [PubMed] [Google Scholar]
- 121.Boyne R, Arthur JR, Wilson AB (1986) An in vivo and in vitro study of selenium deficiency and infection in rats. J Comp Pathol 96:379–86 [DOI] [PubMed] [Google Scholar]
- 122.Sjunnesson H, Sturegard E, Willen R, Wadstrom T (2001) High intake of selenium, beta-carotene, and vitamins A, C, and E reduces growth of Helicobacter pylori in the guinea pig. Comp Med 51:418–23 [PubMed] [Google Scholar]
- 123.Angstwurm MWA, Schottdorf J, Schopohl J, Gaertner R (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27:1807–1813. 10.1097/00003246-199909000-00017 [DOI] [PubMed] [Google Scholar]
- 124.Berger MM, Eggimann P, Heyland DK, et al. (2006) Reduction of nosocomial pneumonia after major burns by trace element supplementation: aggregation of two randomised trials. Crit Care 10:R153 10.1186/cc5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Angstwurm MWA, Engelmann L, Zimmermann T, et al. (2007) Selenium in Intensive Care (SIC): Results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock*. Crit Care Med 35:118–126. 10.1097/01.CCM.0000251124.83436.0E [DOI] [PubMed] [Google Scholar]
- 126.Forceville X, Laviolle B, Annane D, et al. (2007) Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care 11 :R73 10.1186/cc5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mishra V, Baines M, Elizabeth Perry S, et al. (2007) Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 26:41–50. 10.1016/j.clnu.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 128.Andrews PJD, Avenell A, Noble DW, et al. (2011) Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 342:d1542–d1542. 10.1136/bmj.d1542 [DOI] [PubMed] [Google Scholar]
- 129.Manzanares W, Biestro A, Torre MH, et al. (2011) High-dose selenium reduces ventilator-associated pneumonia and illness severity in critically ill patients with systemic inflammation. Intensive Care Med 37:1120–1127. 10.1007/s00134-011-2212-6 [DOI] [PubMed] [Google Scholar]
- 130.Valenta J, Brodska H, Drabek T, et al. (2011) High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med 37:808–815. 10.1007/S00134-011-2153-0 [DOI] [PubMed] [Google Scholar]
- 131.Janka V, Ladislav K, Jozef F, Ladislav V (2013) Restoration of antioxidant enzymes in the therapeutic use of selenium in septic patients. Wien Klin Wochenschr 125:316–325. 10.1007/S00508-013-0371-x [DOI] [PubMed] [Google Scholar]


