Abstract
Enzymes are some of the most efficient catalysts in nature. If small catalytic peptides mimic enzymes, there is potential for broad applications from catalysis for new material synthesis to drug development, due to the ease of molecular design. Recently a hydrogel-based combinatory phage display library was developed and protease-mimicking peptides were identified. Here we advanced the previous discovery to apply one of these catalytic peptides for the synthesis of bimetal oxide nanocrystals through the catalytic ester-elimination pathway. Conventional bimetal oxide crystallization usually requires high temperatures above several hundred °C; however, this catalytic peptide could grow superparamagnetic MnFe2O4 nanocrystals at 4°C. Superconducting quantum interference device (SQUID) analysis revealed that MnFe2O4 nano-crystals grown by the catalytic peptide exhibit superpara-magnetism. This study demonstrates the usefulness of protease-mimicking catalytic peptides in the field of material synthesis.
Keywords: biomineralization, catalytic peptide, ester elimination, MnFe2O4, superparamagnetism
Enzymes in nature are evolved efficiently to catalyze material growths in high yield and high selectivity at low temperature. Up to now, biomineralizing enzymes and peptides that catalyze the growth of metal nanocrystals have been isolated from tissues and cells of animals and microorganisms.[1] Recent comprehensive proteomic studies discovered metal-binding proteins that play critical roles in biomineralization.[2] For instance, the proteins derived from magnetotactic bacteria (e.g., Mms6)[2a] control the morphologies of magnetite crystals in the magnetosome organelles.[3] The biomineralization pathway starts from the uptake of metal precursors on metal-binding peptide sites, and then their catalytic properties nucleate and facilitate crystal growths. The biomineralization process is so efficient that crystals can be grown with low energy consumption (e.g., at low temperature). Thus far, several promising peptides or proteins can be used to catalyze the growth of inorganic nanomaterials.[4]
Recently, protease-mimicking CP4 peptides were used to catalyze ZnO nanocrystal formation through ester elimination.[5] Ester elimination favors the growth of metal oxides from metal acetate precursors through reverse hydrolysis of esters in organic environments, such as alcohols at high temperature.[6] The CP4 peptide was discovered from a combinatorial phage-display peptide library through a hydrogel-based biopanning process where phage viruses possessing protease-like activity can be purified by centrifugation due to product gel formation around peptides displayed at the tail of viruses.[5,7] Previously, this type of phage biopanning was also applied to identify peptides that can target cancer and stem cells.[8] The CP4 peptide is an efficient catalyst for metal oxide formation through the ester elimination pathway, and its amino acid residues (in particular, serine and lysine residues) could play an important role for crystallization.[5] While protease can catalyze the reverse hydrolysis of ester,[9] natural proteases are not ideal catalysts for metallization reactions because of the use of organic solvents. Small-peptide catalysts could have an advantage in catalyzing ZnO formation through ester elimination in organic solvents because organic-solvent-induced denaturing could be less problematic, as the conformation of the catalytic pocket of enzymes is more sensitive to the environment.[5]
Thus, the biomimetic approach is now recognized as a potential route to catalyze novel inorganic nanocrystal syntheses; however, most of these demonstrations were to grow simple noble metal or metal oxide nanocrystals, and the biosynthesis of bimetal oxide materials has not explored extensively.[10] In particular, spinel ferrites, MFe2O4 (M=Mn, Co, Zn, Ni, etc.), attracts high interest due to the great technological importance for electrode materials of Li-ion battery[11] as well as magnetic storage,[12] ferrofluids,[13] catalysts,[14] and biomedical applications.[15] The issue for the synthesis of spinel ferrites is that iron species could readily generate amorphous and low-crystallinity ferrihydrite at low temperature,[16] and thus there are few reports to mineralize the highly crystalline metal iron oxide below room temperature with biomolecular catalytic approaches.[17]
We hypothesize that this catalytic peptide-mediated ester elimination approach can be applicable for the synthesis of bimetal oxide materials at low temperature.[18] In a variety of bimetal oxides, MnFe2O4 is an interesting material for medical imaging and therapeutics in addition to magnetic storage.[19] Recently, MnFe2O4 nanocrystals were grown in a methanol (MeOH)–benzyl alcohol (BA) solvent system (50% volume ratio) with hydrothermal ester elimination.[20] In this study, the catalytic CP4 peptide was examined as to whether it can crystalize MnFe2O4 nanoparticles in the MeOH-BA system through the ester elimination pathway with no external energy inputs at low temperature. It was demonstrated that as-prepared MnFe2O4 nanocrystals consisted of single crystalline domains and these nanoparticles exhibited superparamagnetism, with a blocking temperature comparable with conventional MnFe2O4 nanoparticles synthesized with high-temperature treatments (100–3008C).
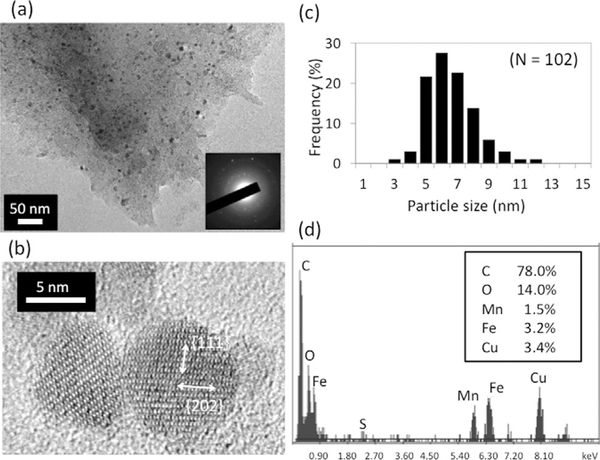
For the proposed catalytic MnFe2O4 nanocrystal growth, first the CP4 peptide (SMESLSKTHHYR, 1 mgmL−1) and metal pre-cursors (50 mm manganese acetate [Mn(OAc)2] and 100 mm iron acetylacetonate [Fe(AcAc)3]) were mixed in BA and MeOH in a 50% volume ratio. The formation of MnFe2O4 nanocrystals was observed, as shown in Figure 1a and 1b. Mn(OAc)2 and Fe(AcAc)3 provide Mn2+ and Fe3+ sources for the formation of manganese ferrite.[18] Selected area electron diffraction (SAED) confirms the formation of MnFe2O4 nanocrystals (inset of Figure 1a and Figure S1 in the Supporting Information). High-resolution TEM (HRTEM) imaging revealed that individual nanoparticles consisted of single crystalline domains and d-spacings of 5.000.71 and 3.17±0.14 Å were measured. These correspond to the {111} and {202} lattice fringes of MnFe2O4 (Figure 1b), further supporting the formation of target MnFe2O4. The as-prepared nanocrystals had a narrow size distribution of 5.9±1.2 nm in diameter (Figure 1c). EDX analysis of nanocrystals also supports the growth of MnFe2O4 with the catalytic peptide (Figure 1-d). In the absence of CP4, no crystal growth was observed (data not shown), thus demonstrating that the CP4 peptide is necessary for catalytic MnFe2O4 crystallization at low temperature.
Figure 1.
Formation of MnFe2O4 nanocrystals with enzyme-mimicking CP4 peptide and Fe and Mn precursors in MeOH/BA at 4°C. (a) TEM image of MnFe2O4 nanocrystals (dark spots). SAED (inset) supports formation of MnFe2O4 (a facet-assigned pattern at higher magnification is shown in the Supporting Information). (b) MnFe2O4 nanocrystals are confirmed to be single crystalline by HRTEM observation. (c) The narrow size distribution of MnFe2O4 nanocrystals. (d) EDX analysis of a nanocrystal detects both manganese and iron.
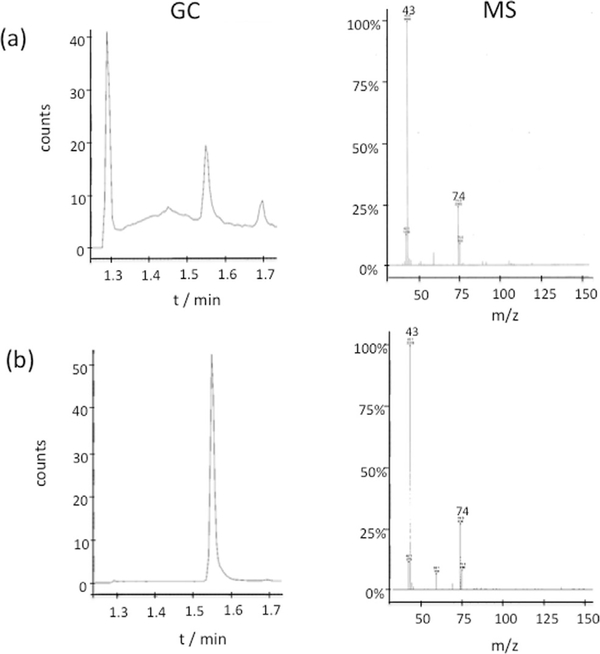
To support the hypothesis that the crystallization of MnFe2O4 goes through the ester-elimination pathway, we looked for a by-product of methyl acetate from the esterification of acetate ion and alcohol.[6a] After the reaction mixtures were stirred with decane to dissolve nonpolar methyl acetate in the decane phase, remaining polar acetate and metal ions in the alcohol phase could be separated. When these by-products in the decane phase are analyzed by gas-chromatography mass-spectroscopy (GC-MS), methyl acetate is detected (Figure 2). This analysis supports the hypothesis that the CP4 peptides crystallize MnFe2O4 nanoparticles by catalyzing the ester-elimination pathway. It should also be noted that 100% BA cannot be used because of low solubility of the Mn(OAc)2 precursor (the detailed GC-MS procedure is described in the Supporting Information).
Figure 2.
GC-MS analysis of the by-product, methyl acetate, in the sample extracted from the supernatant after mixing MnFe2O4 growth precursors and CP4 peptide. (a) The sample from the supernatant, and (b) a standard methyl acetate. Molecular weight for methyl acetate is 74.08.
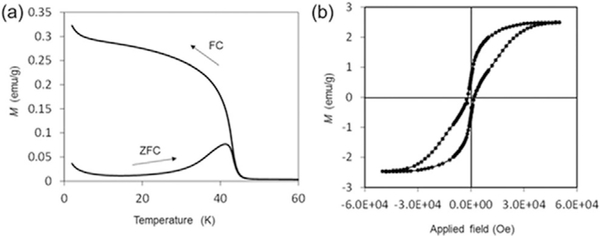
The magnetic properties of resulting MnFe2O4 nanocrystals such as blocking temperature (TB), coercivity and saturation magnetization were studied by using superconducting quantum interference device (SQUID) magnetometry. As shown in Figure 3a, the TB for the MnFe2O4 nanocrystals is 41 K. Zero-field-cooled (ZFC) and field-cooled (FC) curves are superimposed at temperatures above TB, suggesting that MnFe2O4 nanocrystals become superparamagnetic in this temperature range. The blocking temperature in this study is comparable to that of conventional MnFe2O4 nanoparticles prepared at high temperature (100–3008C), which have a similar size distribution.[21] This result indicates that both MnFe2O4 nanoparticles have comparable energy barriers of the magnetic anisotropy. As compared to the ferrimagnetic bulk MnFe2O4, these superparamagnetic nanoparticles are beneficial for many applications such as drug delivery, hyperthermic treatments, and contrast agents for magnetic resonance imaging (MRI).[22] The magnetic field dependence of magnetization is displayed in Figure 3b. At 2 K, that is, a temperature lower than TB, MnFe2O4 nanocrystals displayed ferrimagnetic behavior with a coercivity value of 150 Oe and a saturation moment (Ms) of 2.5 emug-1. The saturation moment is an order of magnitude lower than that reported by other study,[19] which indicates that our sample could contain amorphous domains around crystalline nanoparticle domains.
Figure 3.
SQUID studies for the MnFe2O4 nanocrystals prepared with the CP4 peptide in MeOH/BA. (a) ZFC and FC magnetizations of MnFe2O4 nanocrystals measured in applied field of 100 Oe. (b) Hysteresis loop measurement of MnFe2O4 nanocrystals at 2 K.
In conclusion, MnFe2O4 crystallization was achieved at extremely low temperature through the ester elimination pathway catalyzed by the CP4 dodeca-peptide. It was demonstrated that as-prepared nanocrystals exhibited superparamagnetic properties with SQUID analysis. The current study shows the potential to develop enzyme-mimicking oligo-peptide catalysts through environmental friendly synthesis for technologically important metal oxide materials.
Experimental Section
Catalytic-peptide, esterification-mediated MnFe2O4 growth
Manganese acetate [Mn(OAc)2, 50 mm=17.3 mgmL−1] and [Fe(AcAc)3, 100 mm=70.6 mgmL−1] were dissolved in a mixture of MeOH and benzyl alcohol (1:1 volume ratio) by sonication. The mixture was centrifuged (18000×g, 30 min), and the supernatant was used as precursor solution. CP4 peptide (SMESLSKTHHYR, 0.5 mg) was suspended in the precursor solution (500 μL), sonicated and stirred on a shaker for 3 days at 4°C. The resulting suspension was transferred to a new tube and centrifuged (18000×g, 30 min). The collected pellet was washed with methanol (500 μL) and water (500 μL) under sonication and centrifuged again. The collected pellet was suspended in water, and used for further experiments.
TEM and EDX analysis
For the TEM observation, a drop of the MnFe2O4 sample (4 μL) was applied on a carbon-coated copper TEM grid (Electron Microscopy Sciences, PA, USA) and dried. The sample was examined by TEM (JEOL 2100) at 200 kV and EDX. The obtained digital images were analyzed using ImageJ program.
SQUID measurement
The magnetic properties such as blocking temperature (TB), coercivity, and saturation magnetization were studied by using superconducting quantum interference device (SQUID) magnetometry (Quantum Design, MPMS). The MnFe2O4 sample (0.7 mg) was used for measurement. The temperature sweep from 2 to 60 K was performed in applied field of 100 Oe under both zero-field-cooled (ZFC) and field-cooled (FC; on cooling) conditions. The value of TB was determined by the peak position in the ZFC curve.
Supplementary Material
Acknowledgements
All works were supported by the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health (NIH) (MD007599). Y.M. and H.M. thank Dr. Natasha A. Chernova (Binghamton University, SUNY) for SQUID measurements. Y.M. thanks Japan Society for the Promotion of Science, and the International Training Program provided through Tokyo University of Agriculture and Technology. E.T. thanks David Warren (Weill Cornell Medical College) for assisting with GC-MS.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/cnma.201500181.
This manuscript is part of a Special Issue on Nanobiointerfaces. A link to the Table of Contents will appear here once the Special Issue is assembled.
References
- [1].Sarikaya M, Tamerler C, Jen AK, Schulten K, Baneyx F, Nat. Mater. 2003, 2, 577–585. [DOI] [PubMed] [Google Scholar]
- [2].a) Arakaki A, Webb J, Matsunaga T, J. Biol. Chem. 2003, 278, 8745–8750; [DOI] [PubMed] [Google Scholar]; b) Kroger N, Deutzmann R, Sumper M, Science 1999, 286, 1129–1132; [DOI] [PubMed] [Google Scholar]; c) Matsunaga T, Nemoto M, Arakaki A, Tanaka M, Proteomics 2009, 9, 3341–3352; [DOI] [PubMed] [Google Scholar]; d) Nemoto M, Maeda Y, Muto M, Tanaka M, Yoshino T, Mayama S, Tanaka T, Mar. Genom. 2014, 16, 39–44; [DOI] [PubMed] [Google Scholar]; e) Nemoto M, Wang Q, Li D, Pan S, Matsunaga T, Kisailus D, Proteomics 2012, 12, 2890–2894; [DOI] [PubMed] [Google Scholar]; f) Shimizu K, Cha J, Stucky GD, Morse DE, Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Tanaka M, Okamura Y, Arakaki A, Tanaka T, Takeyama H, Matsunaga T, Proteomics 2006, 6, 5234–5247. [DOI] [PubMed] [Google Scholar]
- [3].a) Arakaki A, Yamagishi A, Fukuyo A, Tanaka M, Matsunaga T, Mol. Microbiol 2014, 93, 554–567; [DOI] [PubMed] [Google Scholar]; b) Tanaka M, Mazuyama E, Arakaki A, Matsunaga T, J. Biol. Chem 2011, 286, 6386–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) de La Rica R, Matsui H, Chem. Soc. Rev 2010, 39, 3499–3509; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wei Z, Maeda Y, Matsui H, Angew. Chem. Int. Ed. 2011, 50, 10585–10588; Angew. Chem. 2011, 123, 10773–10776; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang F, Mao CB, Chem. Commun 2009, 1222–1224; [DOI] [PubMed] [Google Scholar]; d) Nanda N, Koder RL, Nat. Chem 2010, 2, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maeda Y, Wei Z, Ikezoe Y, Matsui H, ChemNanoMat, 2015, 1, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Du H, Yuan F, Huang S, Li J, Zhu Y, Chem. Lett 2004, 33, 770–771; [Google Scholar]; b) Niederberger M, Garnweitner G, Pinna N, Neri G, Prog. Solid State Chem 2005, 33, 59–70. [Google Scholar]
- [7].Maeda Y, Javid N, Duncan K, Birchall L, Gibson KF, Cannon D, Kanetsuki Y, Knapp C, Tuttle T, Ulijn RV, Matsui H, J. Am. Chem. Soc. 2014, 136, 15893–15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Ma K, Wang D-D, Lin Y, Wang J, Petrenko V, Mao CB, Adv. Funct. Mater 2013, 23, 1172–1181; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Abbineni G, Modall S, Safiejko-Mroczka B, Petrenko V, Mao CB, Mol. Pharm 2010, 7, 1629–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koeller KM, Wong CH, Nature 2001, 409, 232–240. [DOI] [PubMed] [Google Scholar]
- [10].a) Iwahori K, Yoshizawa K, Muraoka M, Yamashita I, Inorg. Chem. 2005, 44, 6393–6400; [DOI] [PubMed] [Google Scholar]; b) Nuraje N, Su K, Haboosheh A, Samson, Manning EP, Yang N, Matsui H, Adv. Mater 2006, 18, 807–811; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Reiss BD, Mao C, Solis DJ, Ryan KS, Thomson T, Belcher AM, Nano Lett. 2004, 4, 1127–1132; [Google Scholar]; d) Yamashita I, Hayashi J, Hara M, Chem. Lett 2004, 33, 1158–1159. [Google Scholar]
- [11].a) Chu YQ, Fu ZW, Qin QZ, Electrochim. Acta 2004, 49, 4915–4921; [Google Scholar]; b) Zhang D, Zhang X, Ni X, Song JM, Zheng H, Chem. Phys. Lett 2006, 426, 120–123. [Google Scholar]
- [12].Lee DK, Kim YH, Kang YS, Stroeve P, J. Phys. Chem. B 2005, 109, 14939–14944. [DOI] [PubMed] [Google Scholar]
- [13].a) Auzans E, Zins D, Blums E, Massart R, J. Mater. Sci 1999, 34, 1253– 1260; [Google Scholar]; b) Tourinho FA, Franck R, Massart R, J. Mater. Sci 1990, 25, 3249–3254. [Google Scholar]
- [14].Kanazawa A, Kanaoka S, Yagita N, Oaki Y, Imai H, Oda M, Arakaki A, Matsunaga T, Aoshima S, Chem. Commun. 2012, 48, 10904–10906. [DOI] [PubMed] [Google Scholar]
- [15].Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J, Nat. Med. 2007, 13, 95–99. [DOI] [PubMed] [Google Scholar]
- [16].a) Hashimoto H, Yokoyama S, Asaoka H, Kusano Y, Ikeda Y, Seno M, Takada J, Fujii T, Nakanishi M, Murakami R, J. Magn. Magn. Mater 2007, 310, 2405–2407; [Google Scholar]; b) Sakai T, Miyazaki Y, Murakami A, Sakamoto N, Ema T, Hashimoto H, Furutani M, Nakanishi M, Fujii T, Takada J, Org. Biomol. Chem 2010, 8, 336–338. [DOI] [PubMed] [Google Scholar]
- [17].a) Staniland S, Williams W, Telling N, Van Der Laan G, Harrison A, Ward B, Nat. Nanotechnol 2008, 3, 158–162; [DOI] [PubMed] [Google Scholar]; b) Tanaka M, Brown R, Hondow NS, Arakaki A, Matsunaga T, Staniland SS, J. Mater. Chem. 2012, 22, 11919–11921. [Google Scholar]
- [18].Bilecka I, Djerddj I, Niederberger M, Chem. Commun. 2008, 886–888. [DOI] [PubMed] [Google Scholar]
- [19].a) Vestal CR, Zhang ZJ, J. Am. Chem. Soc. 2003, 125, 9828–9833; [DOI] [PubMed] [Google Scholar]; b) Yang H, Zhang C, Shi X, Hu H, Du X, Fang Y, Ma Y, Wu H, Yang S, Biomaterials 2010, 31, 3667–3673; [DOI] [PubMed] [Google Scholar]; c) Peng Y, Wang Z, Liu W, Zhang H, Zuo W, Tang H, Chen F, Wang B, Dalton Trans. 2015, 44, 12871–12877. [DOI] [PubMed] [Google Scholar]
- [20].a) Clavel G, Willinger MG, Zitoun D, Pinna N, Adv. Funct. Mater. 2007, 17, 3159–3169; [Google Scholar]; b) Tonto P, Mekasuwandumrong O, Phatanasri S, Pavarajarn V, Praserthdam P, Ceram. Int. 2008, 34, 57–62; [Google Scholar]; c) Pinna N, Niederberger M, Angew. Chem. Int. Ed 2008, 47, 5292–5304; [DOI] [PubMed] [Google Scholar]
- [21].Vestal CR, Song Q, Zhang ZJ, J. Phys. Chem. B 2004, 108, 18222–18227. [Google Scholar]
- [22].a) Corr SA, Byrne SJ, Tekoriute R, Meledandri CJ, Brougham DF, Lynch M, Kerskens C, O’Dwyer L, Gun’ko YK, J. Am. Chem. Soc. 2008, 130, 4214–4215; [DOI] [PubMed] [Google Scholar]; b) Kim KS, Park J-K, Lab Chip 2005, 5, 657–664; [DOI] [PubMed] [Google Scholar]; c) Laurent S, Dutz S, Hfeli UO, Mahmoudi M, Adv. Colloid Interface Sci. 2011, 166, 8–23; [DOI] [PubMed] [Google Scholar]; d) Motte L, Benyettou F, de Beaucorps C, Lecouvey M, Milesovic I, Lalatonne Y, Faraday Discuss. 2011, 149, 211–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.