Abstract
Background: Glioblastoma multiforme is the most aggressive brain tumor with poor prognosis and an average survival of 1-2 years. Animal models that simulate the features of human glioma are the key to newer agents or therapeutic strategies. In order to establish such models, the C6 glioma cell line has been mostly used in neuro-oncology research.
Methods: In this narrative review, we systematically reviewed the international literature in order to retrieve and present the most important biological and molecular features of C6 cell line.
Results: Even though many cell lines have been developed, each cell line presents with slight differences from human glioma behavior. C6 cancer cell line is a rat glioma cell line, which can simulate in overall the high growth rate, the high vascularization, and the highly infiltrative character of glioblastoma multiforme.
Conclusions: Most of the C6 glioma research has been focused on testing a wide diversity of agents for their tumoricidal activity. C6 cell line is considered to be a safe and popular glioma model in the literature, providing a good simulation of glioblastoma multiforme. HIPPOKRATIA 2018, 22(3): 105-112.
Keywords: C6, cell line, rat glioma model, glioma, glioblastoma multiforme
Introduction
Gliomas are tumors that derive from glial cells and are the most common tumors of the central nervous system (CNS). The classification of CNS tumors has recently been updated in the 2016 World Health Organization (WHO) classification, which is based on the type of the primary cell along with histological and molecular characteristics1. The most aggressive glioma tumor is glioblastoma multiforme (GBM), which has a poor prognosis with an average survival of 1-2 years depending on the isocitrate dehydrogenase (IDH) status1. Glioblastomas exhibit high growth rate, high vascularization, and are considered to be highly infiltrative. Unfortunately, in the last years, there has been only a few therapeutic advances on gliomas2. Even though researchers have proposed several molecular pathways and a variety of therapeutic targets, there was no success in the clinical trials3,4. This failure points out that as existing molecular knowledge for glioma tumors advances, it becomes clearer that in order to study innovative therapies, appropriate and predictive animal glioma models are of great importance. Animal glioma models are generated from glioma cell lines that are tumorigenic to laboratory animals and can simulate the fundamental biological properties of human gliomas. The key to a successive glioma model is the proper choice of a cancer cell line.
Methods
This review aims to present an update on the C6 glioma cell line and discuss newer therapeutic applications and effects using C6 glioma rat model. Therefore, we searched the Medline (PubMed) for C6 rat glioma model articles in English related to therapeutic applications in terms of tumor growth and proliferation, invasiveness, migration, immunogenicity, angiogenesis, and genetic profile published from 2000 to 2018. The following keywords were used in advanced search: (glioma models OR cell lines) AND rat AND C6 and the results were sorted by the most recent. We proceeded in discussing the genetic, morphologic, and angiogenic profile of C6 cell line, presenting newer aspects of its profile and the most recent therapeutic applications. Finally, we investigated the characteristics of C6 cell line that constitute it as the best glioma model for studying GBM. To answer these topics, a narrative but comprehensive review with systematic intent was conducted and is presented. The flow chart of the recovered and analyzed studies from PubMed is shown in Figure 1.
Figure 1. Flow chart of the recovered and analyzed studies in PubMed regarding articles involving therapeutic applications in terms of tumor growth, proliferation, invasiveness, migration, immunogenicity, angiogenesis, and genetic profile, focusing on literature of the period 2000-2018.
Results
Genetics
C6 cell line was developed in adult Wistar-Furth rats in the late 1960s after the rats were repetitively exposed to N-Nitroso-N-methylurea5. This glioma cell line is composed of pleomorphic cells with variably shaped nuclei. Genetically, the cells are reported to have a wild type of p53 gene, an increase in the expression of the Rb gene and mutant p16/Cdkn2a/Ink4a locus but without the expression of p16 and p19ARF mRNAs6. They also overexpress the same genes that are expressed in human gliomas: the PDGFβ, insulin-like growth factor (IGF)-1, epidermal growth factor receptor (EGFR), and Erb3/Her3 precursor proteins7,8. Furthermore, there is a reduced expression of IGF-2, FGF-9, and FGF-10, while there is no change in the expression of MMP-7 gene. As human gliomas exhibit increased activity of genes of Ras pathway9, C6 cells also exhibit upregulation of Ras pathway. Nevertheless, increased expression of Ras guanine triphosphate activator protein keeps the Ras pathway under control. In addition, increased expression of TGFα precursor was also reported10.
Mutations in genes encoding IDH1 and IDH2 in gliomas have been linked to patient’s prognosis11, but they are not detected in C6 cells12. However, researchers have proved that artificial mutagenesis of IDH2 in C6 cells increases their sensitivity to chemotherapy and promotes cell migration and tumor growth12,13. IDH2 mutated C6 cells could be a new promising proliferation and migration glioma model for the development of new agents14,15. Cell adhesion and signal transduction are essential features of tumors, regulated by cell surface antigens. CD9 is a cell surface antigen that is typically expressed in the myelin sheath of nerves. Its increased expression is found in high-grade gliomas, and it has been proposed as a marker for the degree of glioma malignancy. In C6 glioma cell line, CD9 has a significant increase10. Finally, it should be noted that there is a sub-clone of C6 cell line that expresses β-galactosidase marker protein, which acts as a tumor antigen. Even though this marker protein can help at in vivo immunohistochemical analysis of C6-derived tumors, it must be taken into consideration that immunization of rats against the gene of β-galactosidase can protect against tumor growth16.
Morphology and mechanisms of development
C6 cells are spindle-like cells that simulate human GBM when they are injected in the brain of neonatal rats17,18. Glioma models have been developed in Wistar rats18,19 and exhibit the same histological features as human GBM, such as foci of tumor necrosis, nuclear polymorphism and high mitotic index17,20,21. The main histological differences are that C6 does not express glial fibrillary acidic protein (GFAP), whereas vimentin is variably expressed22. The tumor doubling times can be evaluated by experimental volume data after fitting in a modified Gompertz function20. The brain tumor C6 model has been shown to occur as early as 5-7 days post-implantation after magnetic resonance imaging (MRI) tumor detection and growth monitoring with volumetric analysis23-25. Even though immune microenvironment of C6 gliomas resembles that of a human GBM21, the cell line is capable of producing an immune response in Wistar and BDX (inbred rat strain X) rats26, and therefore, it cannot be used for assessing immunotherapy. However, several studies report significant tumor growth ranging from 70 % to 91 % after the implantation of C6 in Wistar rats19,27. Initial assessments on C6 implantation in Long-Evans and Sprague-Dawley rats do not support simulation of a human GBM model. In these animals, C6 formulates in most cases a rounded and well-demarcated brain tumor without evidence of parenchymal invasion resembling more in brain metastasis than GBM28,29. In general, C6 glioma model has been used to study several biological features of brain tumors, such as tumor growth, tumor invasion and migration, angiogenesis, growth factor production and regulation, and blood-brain barrier disruption30-34.
C6 glioma cells invade and migrate in cerebral cortex post-implantation by attaching to the endothelial basement membrane18,35. This route of migration resembles the human xenografted cell lines in a rat brain, and it simulates the migration of malignant gliomas in humans36. Tumor invasion is achieved through the degradation of the basement membrane as well as of the extracellular matrix. Tumors can be developed due to the invasion of a single C6 cell into the surrounding brain tissue, depending mainly on metalloprotease activity, but not on cell proliferation itself37,38. Metalloprotease activity includes factors that comprise a family of endopeptidases that are metal ion-dependent. They are responsible for the degradation process but are also needed for angiogenesis. Orthotopic C6 brain gliomas have been found to exhibit high amounts of matrix metalloprotease (MMP) proMMP2 and its activated form, which is usually found only in tumoral brain tissue39. Activated MMP2 is detected as part of collagenase activity and the basement membrane degradation process40. The matrix metalloprotease activity of C6 cells can transform CNS myelin into a substrate for cell migration41. Myelin degradation enables C6 cells to invade and migrate through white matter; this process is attributed to membrane type 1 MMP (MT1-MMP), found on the cytoplasmic membrane of the C6 cells42. Furthermore, other molecules, which are overexpressed in C6 cells, play an essential role in the invasion and particularly in the adhesion of the C6 cells at the surrounding tissue. These include the intercellular adhesion molecule (ICAM) and the cell surface antigen CD9, which is usually found in the myelin sheaths43-45.
Angiogenesis in C6 derived tumors
The development of the C6 tumor is also associated with the vascular status of the C6-derived gliomas. The lack of oxygen in the center of the tumor is an essential factor of neovascularization. C6 cells secrete several angiogenic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)46,47, that contribute to tumor growth via tyrosine kinase receptors and MMPs, respectively48,49. C6 implanted cells present with several growth phases related to the vascular status of the tumor. Therefore, there is the lag phase, the proliferative phase, and the exponential phase of tumor growth, which is associated with the vascularization process50. The latter includes three stages: the avascular, the early vascular, and the late vascular stage. During the vascularization process, MRI and pathology study of an orthotopic C6 glioma model in Sprague-Dawley rats revealed four patterns of neovascularization. Two of them are found inside the tumor and include: i) splitting angiogenesis, also known as intussusception angiogenesis, in which the extent of the capillary wall inside the lumen splits the single vessel in two, and ii) sprouting angiogenesis, in which activated endothelial cells of the existing vessels release proteases that degrade the basement membrane and allow the endothelial cells to escape their site and proliferate into the surrounding matrix in a tandem way. The third pattern refers to vascular co-option found in the tumor margin, and the last is vascular mimicry, which is recognized in the surrounding necrotic area51.
Discussion
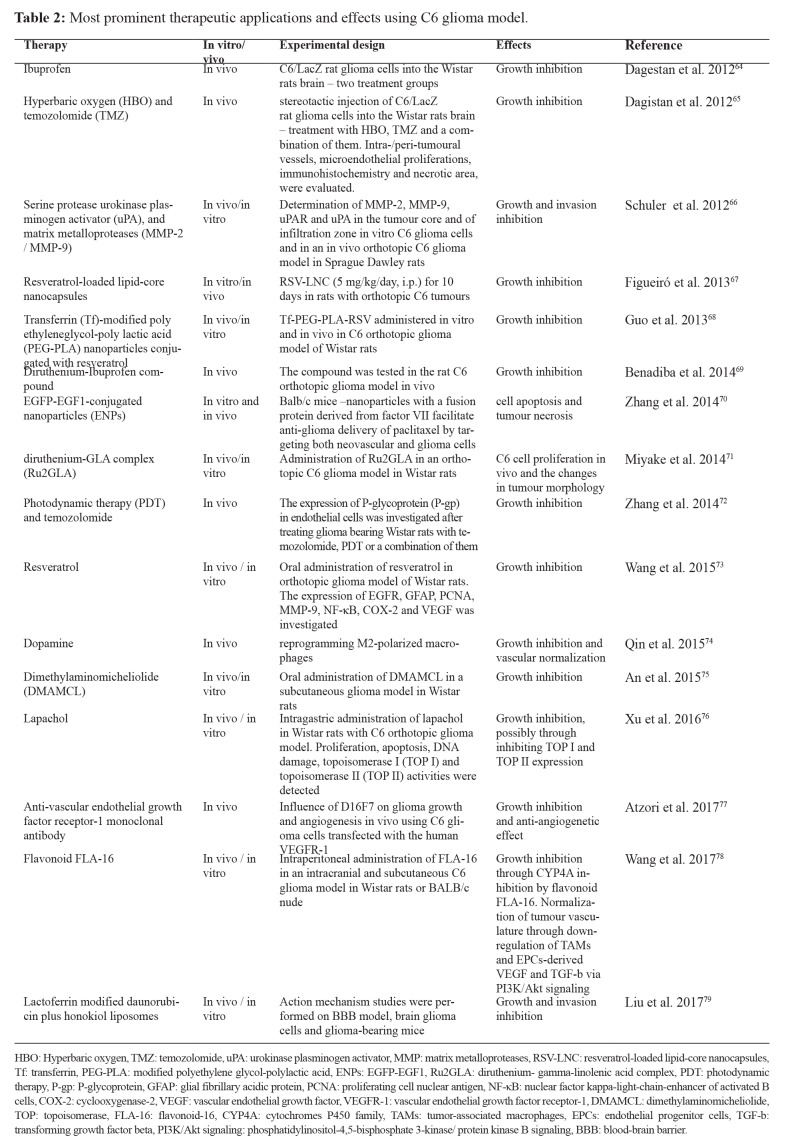
The ideal glioma model should be similar to human GBM in terms of morphological characteristics, its invasive pattern and ability, its vascular behavior, and its immune microenvironment. There is a plethora of cell lines that simulate human GBM and are used in research. The most commonly used include human-derived cell lines such as U251 and U87, murine cell line GL261, and rat cell lines 9L/LacZ, F98, RG2, CNS-1, and C6. U251 and U87 are xenograft models that can be developed only in immunocompromised rodents, whereas the rest cell lines can be developed in immunocompetent syngeneic models. This restriction constitutes human-derived lines inadequate for a tumor-immune microenvironment study. All models exhibit similar morphological characteristics except 9L/LacZ and F98, which resemble gliosarcoma and anaplastic glioma respectively. All models share similar to GBM nuclear pleomorphism and high mitotic index, while F98 and 9L/LacZ present a low percentage of tumor necrotic foci and C6 moderate. The most aggressive and invasive models are RG2, F98, and 9L/LacZ, whereas C6, GL261, and CNS-1 have a moderate invasive ability and poor invasiveness is a feature of human cell lines U87 and U25152. Each glioma model is able to create its own vascular network with vessels of different length and diameter. All models exhibit high neovascularization but either by neovascularization or recruitment of existent vasculature. U87 has been shown to exhibit profuse neovascularization and has been widely used to study GBM angiogenesis53. Half of the cell lines mentioned above can cause an immune response of the host. The most vigorous immune response is reported in 9L/LacZ, whereas C6, U87, U251 cause a moderate response. Non-immunogenic cell lines are considered to be GL261, CNS-1, F98, and RG2. However, when a study of the tumor-immune microenvironment is of need, C6 is well studied and resembles human GBM immune infiltrates21. A series of markers and most common genetic mutations complete the profiling of the cell lines. S100 protein is expressed in all cell lines except U87, F98, and RG2, whereas GFAP is expressed in U251, GL261, CNS-1, and F98. A wild type p53 can be found in C6, U87, F98, and RG2, while EGFR overexpression is a common feature in all cell lines except U87 and RG2. A summary of the most important characteristics of the above cell lines is presented in Table 1.
Table 1. A comparative profile of the most important features of the commonest used in research cell lines.
GFAP: glial fibrillary acidic protein, PTEN: phosphatase and tensin homolog, EGFR: epidermal growth factor receptor, +: presence, -: absence, N/A: not available.
The C6 rat glioma model is one of the commonest experimental models used in neuro-oncology in order to study the growth and the invasion of high-grade gliomas. A recent MRI and magnetic resonance angiography (MRA) in vivo study has reported that C6 resembles human GBM better than other rodent glioma models20,54. The most common host of C6 cell line for an in vivo study is immunocompetent Wistar rats, but other species such as Sprague-Dawley and Long-Evans have also been used. However, Long-Evans rats have not been extensively used in glioma models, and studies using this species are very scarce in the literature55,56. On the contrary, although it has been primarily reported that implantation of C6 in Sprague-Dawley rats does not produce a similar invasion pattern as human GBM28,29, recent studies state otherwise and have used this model to investigate tumor growth or the extent of resection57,58. C6 glioma model has been used to test variable treatment modalities, including newer and more effective drugs against glioma, radiation therapy, photodynamic therapy, or even gene therapy59-62. Despite the fact that it has been extensively used, allogeneic proteins of the major histocompatibility complex (MHC) found in the C6-derived tumors can cause an immune response. These proteins are up-regulated in the C6 glioma model and simulate a therapeutic response63. The immunological reaction is present in both intracranial C6 glioma models and subcutaneous flank C6 models of Wistar rats26. Even though the cell implantation protocol affects tumor formation, allogenicity is the main problem for low rates of tumor intake. Nevertheless, it has been documented that C6 presents a similar composition of the immune infiltrates to human GBM. The invasion and immunosuppression-related genes in C6 gliomas produce similar immune evasion pattern as in human GBM. Therefore, C6 glioma model is considered to be a good model of an immunocompetent host for in vivo studies21 and has been widely used in research studying tumor growth and invasion as well as anti-tumor drug effectiveness (Table 2).
Table 2. Most prominent therapeutic applications and effects using C6 glioma model.
HBO: Hyperbaric oxygen, TMZ: temozolomide, uPA: urokinase plasminogen activator, MMP: matrix metalloproteases, RSV-LNC: resveratrol-loaded lipid-core nanocapsules, Tf: transferrin, PEG-PLA: modified polyethylene glycol-polylactic acid, ENPs: EGFP-EGF1, Ru2GLA: diruthenium- gamma-linolenic acid complex, PDT: photodynamic therapy, P-gp: P-glycoprotein, GFAP: glial fibrillary acidic protein, PCNA: proliferating cell nuclear antigen, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, COX-2: cyclooxygenase-2, VEGF: vascular endothelial growth factor, VEGFR-1: vascular endothelial growth factor receptor-1, DMAMCL: dimethylaminomicheliolide, TOP: topoisomerase, FLA-16: flavonoid-16, CYP4A: cytochromes P450 family, TAMs: tumor-associated macrophages, EPCs: endothelial progenitor cells, TGF-b: transforming growth factor beta, PI3K/Akt signaling: phosphatidylinositol-4,5-bisphosphate 3-kinase/ protein kinase B signaling, BBB: blood-brain barrier.
C6 glioma model has been proved to express a diversity of proteins, growth factors and/or their receptors, which constitute targets for tumor research. Among most popular targets are angiogenic factors such as VEGF and bFGF and its receptors vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR) respectively80,81. Other factors targeted are platelet-derived growth factor and its receptors platelet-derived growth factor receptor (PDGFR)82, that regulate cell growth and division, and factors that stimulate cell growth and differentiation such as epidermal growth factor (EGF) and its receptor EGFR83. More complexed targets are IGF and its receptors84 that are responsible for cell proliferation and inhibition of cell death.
Tumor growth inhibition remains the primary target of C6 glioma model research. Several popular drugs such as ibuprofen, dopamine, and aspirin have been tested on such models. The first two have succeeded in that direction64,74, whilst aspirin reduced the glioma invasion85. C6 glioma models have also been a preferred model for experimental therapies with nanoparticles. Transferrin (Tf)-modified polyethylene glycol-polylactic acid (PEG-PLA) nanoparticles conjugated with resveratrol as well resveratrol-loaded lipid-core nanocapsules have been shown to reduce tumor growth67,68. Furthermore, nanoparticles with a fusion protein derived from factor VII facilitated anti-glioma delivery of paclitaxel. In that way, they targeted both neovascular and glioma cells leading to cell apoptosis and tumor necrosis70. Combined therapeutic approaches have also been tested against C6 glioma models. Hyperbaric oxygen, as well as photodynamic therapy, have been used against glioma, in combination with temozolomide, which is an approved by The Food and Drug Administration (FDA) chemotherapeutic agent for glioblastoma65,72. These combinations have shown to augment growth inhibition of gliomas. Among newer therapeutic techniques, clinical research offers gene therapy. C6 glioma cells have been used to explore the limits of gene therapy in glioblastomas. An example of gene therapy was the introduction of INF-γ gene in C6 cells by retroviral delivery, which led to tumor growth inhibition by B- and T-cells activation and by inhibition of angiogenesis86.
Another promising field of research is that of chemokines and their receptors that are involved in proliferation and migration of glial precursor cells. Chemokines are also involved in tumor metastasis, tumor growth and progression87,88. More specifically, tumor research has shown great interest in the chemokine CXCL12 and the axis CXCL12-CXCR789. CXCL12 chemokine is found in necrotic areas, as well as in areas of neo-angiogenesis, and are responsible for the proliferation of glioblastoma progenitor cells90. CXCR7 can be found in capillaries of the neo-angiogenetic tumor tissue of human glioblastoma91. Furthermore, CXCR7 is localized in the adult rat brain and particularly in astrocytes, and Schwann cells92. It is noteworthy that the axis CXCL12-CXCR7 has been proven not only to mediate migration but also to reduce apoptosis induced by the chemotherapeutic agent temozolomide91,93.
Additionally, recent studies have highlighted the potential role of activated macrophages and microglia in glioma development. The endothelial monocyte-activated polypeptide II (EMAPII) is a cytokine expressed by macrophages and microglia. It plays a role against angiogenesis and acts as a pro-inflammatory cytokine94. It is also responsible for the activation and infiltration of macrophages and induces endothelial apoptosis. ED1 is a lysosomal protein found both in macrophages, who underwent phagocytosis and microglia. Another marker which is observed in several CNS pathologies such as ischemia and Wallerian degeneration is CD895,96. The early accumulation of the aforementioned markers was found in C6 rat glioma models97, and this could prove a promising research field for studying either the development of the glioma or test new tumoricidal agents in gliomas.
Conclusions
C6 cell line is quite popular within glioma research. It has been widely used in order to establish a rat glioma model, which simulates human glioblastoma. Even though literature supports low tumor development rates in rats and mice due to C6 allogenicity, literature is also very rich regarding in vivo studies, as this review demonstrated. C6 cell line gives the advantage of a syngeneic model without the need of immunocompromised rodents in comparison to xenograft models. It can produce a highly angiogenic, invasive glioma model with distinct peritumoral environment altering pre-existing vasculature for its needs and with many of the human GBM morphological characteristics. It can be developed as an orthotopic model in rats which offers easier MRI study investigation in comparison to murine models due to the size of the animal. MRI and MRA studies have shown the superiority of C6 cell line in GBM similarity in comparison to other rodent models. Furthermore, its expressed markers facilitate an immunohistochemical investigation as in other rat cell lines, but its genetic profile resembles better human GBM than the rest rat glioma models. Finally, C6 is preferred in tumor-immune microenvironment studies since it employs similar immune infiltrates and evasion strategies as does human GBM. Therefore, C6 cell line offers a wide variety of therapeutic studies including growth and invasive pattern studies, angiogenic and immune models, and a plethora of molecular and genetic targets for newer pharmacological agents. Most of the C6 glioma research has been focused on testing a wide diversity of agents for their tumoricidal activity. Moreover, C6 rat glioma models have also been extensively used to analyze glioma characteristics such as development, invasion, migration, and angiogenesis. In general, the C6 rat glioma model is thought to be a quite safe model, and it has been widely used throughout the timeline. Nevertheless, a researcher should not forget to take into consideration the pros and cons of every cell line, in accordance with the appropriate model to be studied.
Conflicts of interest
Authors declare no conflicts of interest.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 5.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel J, Piontek G, Kersting M, Schuermann M, Kappler R, Scherthan H, et al. The p16/Cdkn2a/Ink4a gene is frequently deleted in nitrosourea-induced rat glial tumors. Pathobiology. 1999;67:202–206. doi: 10.1159/000028073. [DOI] [PubMed] [Google Scholar]
- 7.Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimberger AB, Suki D, Yang D, Shi W, Aldape K. The natural history of EGFR and EGFRvIII in glioblastoma patients. J Transl Med. 2005;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibenaller ZA, Etame AB, Ali MM, Barua M, Braun TA, Casavant TL, et al. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg Focus. 2005;19:1–9. doi: 10.3171/foc.2005.19.4.2. [DOI] [PubMed] [Google Scholar]
- 11.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Huang R, Zheng Y, Zhang Z, Liang A. Glioma-derived mutations in isocitrate dehydrogenase 2 beneficial to traditional chemotherapy. Biochem Biophys Res Commun. 2011;410:218–223. doi: 10.1016/j.bbrc.2011.05.108. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Zheng Y, Li K, Huang R, Zheng S, An N, et al. Mutations in isocitrate dehydrogenase 2 accelerate glioma cell migration via matrix metalloproteinase-2 and 9. Biotechnol Lett. 2012;34:441–446. doi: 10.1007/s10529-011-0800-8. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y, Jiao Y, Zheng S, Liang A, Hu F. Combination of lithium chloride and pEGFP-N1-BmK CT effectively decreases proliferation and migration of C6 glioma cells. Cytotechnology. 2016;68:197–202. doi: 10.1007/s10616-014-9768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Y, Zheng Y, Chan KG, Liang A, Hu F. Lithium chloride decreases proliferation and migration of C6 glioma cells harboring isocitrate dehydrogenase 2 mutant via GSK-3β. Mol Biol Rep. 2014;41:3907–3913. doi: 10.1007/s11033-014-3258-7. [DOI] [PubMed] [Google Scholar]
- 16.Lampson LA, Lampson MA, Dunne AD. Exploiting the lacZ reporter gene for quantitative analysis of disseminated tumor growth within the brain: use of the lacZ gene product as a tumor antigen, for evaluation of antigenic modulation, and to facilitate image analysis of tumor growth in situ. Cancer Research. 1993;53:176–182. [PubMed] [Google Scholar]
- 17.Auer RN, Del Maestro RF, Anderson R. A simple and reproducible experimental in vivo glioma model. Can J Neurol Sci. 1981;8:325–331. doi: 10.1017/s0317167100043468. [DOI] [PubMed] [Google Scholar]
- 18.Nagano N, Sasaki H, Aoyagi M, Hirakawa K. Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol. 1993;86:117–125. doi: 10.1007/BF00334878. [DOI] [PubMed] [Google Scholar]
- 19.Chicoine MR, Silbergeld DL. Invading C6 glioma cells maintaining tumorigenicity. J Neurosurg. 1995;83:665–671. doi: 10.3171/jns.1995.83.4.0665. [DOI] [PubMed] [Google Scholar]
- 20.Doblas S, He T, Saunders D, Pearson J, Hoyle J, Smith N, et al. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. J Magn Reson Imaging. 2010;32:267–275. doi: 10.1002/jmri.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gieryng A, Pszczolkowska D, Bocian K, Dabrowski M, Rajan WD, Kloss M, et al. Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas. Sci Rep. 2017;7:17556. doi: 10.1038/s41598-017-17752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468–1478. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huhndorf M, Moussavi A, Kramann N, Will O, Hattermann K, Stadelmann C, et al. Alterations of the Blood-Brain Barrier and Regional Perfusion in Tumor Development: MRI Insights from a Rat C6 Glioma Model. PLoS One. 2016;11:e0168174. doi: 10.1371/journal.pone.0168174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souza TKF, Nucci MP, Mamani JB, da Silva HR, Fantacini DMC, de Souza LEB, et al. Image and motor behavior for monitoring tumor growth in C6 glioma model. PLoS One. 2018;13:e0201453. doi: 10.1371/journal.pone.0201453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le TNT, Lim H, Hamilton AM, Parkins KM, Chen Y, Scholl TJ, et al. Characterization of an Orthotopic Rat Model of Glioblastoma Using Multiparametric Magnetic Resonance Imaging and Bioluminescence Imaging. Tomography. 2018;4:55–65. doi: 10.18383/j.tom.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsa AT, Chakrabarti I, Hurley PT, Chi JH, Hall JS, Kaiser MG, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. discussion 999-1000. [DOI] [PubMed] [Google Scholar]
- 27.Kondziolka D, Somaza S, Comey C, Lunsford LD, Claassen D, Pandalai S, et al. Radiosurgery and fractionated radiation therapy: comparison of different techniques in an in vivo rat glioma model. J Neurosurg. 1996;84:1033–1038. doi: 10.3171/jns.1996.84.6.1033. [DOI] [PubMed] [Google Scholar]
- 28.Farrell CL, Stewart PA, Maestro RF. A new glioma model in rat: the C6 spheroid implantation technique permeability and vascular characterization. J Neurooncol. 1987;4:403–415. doi: 10.1007/BF00195612. [DOI] [PubMed] [Google Scholar]
- 29.San-Galli F, Vrignaud P, Robert J, Coindre JM, Cohadon F. Assessment of the experimental model of transplanted C6 glioblastoma in Wistar rats. J Neurooncol. 1989;7:299–304. doi: 10.1007/BF00172924. [DOI] [PubMed] [Google Scholar]
- 30.Karmakar S, Olive MF, Banik NL, Ray SK. Intracranial stereotaxic cannulation for development of orthotopic glioblastoma allograft in Sprague-Dawley rats and histoimmunopathological characterization of the brain tumor. Neurochem Res. 2007;32:2235–2242. doi: 10.1007/s11064-007-9450-6. [DOI] [PubMed] [Google Scholar]
- 31.Assadian S, Aliaga A, Del Maestro RF, Evans AC, Bedell BJ. FDG-PET imaging for the evaluation of antiglioma agents in a rat model. Neuro Oncol. 2008;10:292–299. doi: 10.1215/15228517-2008-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valable S, Lemasson B, Farion R, Beaumont M, Segebarth C, Remy C, et al. Assessment of blood volume, vessel size, and the expression of angiogenic factors in two rat glioma models: a longitudinal in vivo and ex vivo study. NMR Biomed. 2008;21:1043–1056. doi: 10.1002/nbm.1278. [DOI] [PubMed] [Google Scholar]
- 33.Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, et al. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2008;40:973–983. doi: 10.1016/j.neuroimage.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Shen G, Shen F, Shi Z, Liu W, Hu W, Zheng X, et al. Identification of cancer stem-like cells in the C6 glioma cell line and the limitation of current identification methods. In Vitro Cell Dev Biol Anim. 2008;44:280–289. doi: 10.1007/s11626-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 35.Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 1996;56:1440–1444. [PubMed] [Google Scholar]
- 36.Bernstein JJ, Goldberg WJ, Laws ER Jr, Conger D, Morreale V, Wood LR. C6 glioma cell invasion and migration of rat brain after neural homografting: ultrastructure. Neurosurgery. 1990;26:622–628. doi: 10.1097/00006123-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein JJ, Laws ER Jr, Levine KV, Wood LR, Tadvalkar G, Goldberg WJ. C6 glioma-astrocytoma cell and fetal astrocyte migration into artificial basement membrane: a permissive substrate for neural tumors but not fetal astrocytes. Neurosurgery. 1991;28:652–658. doi: 10.1097/00006123-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Tamaki M, McDonald W, Amberger VR, Moore E, Del Maestro RF. Implantation of C6 astrocytoma spheroid into collagen type I gels: invasive, proliferative, and enzymatic characterizations. J Neurosurg. 1997;87:602–609. doi: 10.3171/jns.1997.87.4.0602. [DOI] [PubMed] [Google Scholar]
- 39.Kodera T, Nakagawa T, Kubota T, Kabuto M, Sato K, Kobayashi H. The expression and activation of matrix metalloproteinase-2 in rat brain after implantation of C6 rat glioma cells. J Neurooncol. 2000;46:105–114. doi: 10.1023/a:1006387600909. [DOI] [PubMed] [Google Scholar]
- 40.Tamaki M, McDonald W, Del Maestro RF. Release of collagen type IV degrading activity from C6 astrocytoma cells and cell density. J Neurosurg. 1996;84:1013–1019. doi: 10.3171/jns.1996.84.6.1013. [DOI] [PubMed] [Google Scholar]
- 41.Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58:149–158. [PubMed] [Google Scholar]
- 42.Beliën AT, Paganetti PA, Schwab ME. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol. 1999;144:373–384. doi: 10.1083/jcb.144.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamaki M, Aoyagi M, Morita I, Hirakawa K, Murota S. Cell adhesion molecules acting between C6 glioma and endothelial cells. J Neurooncol. 1995;24:181–188. doi: 10.1007/BF01078488. [DOI] [PubMed] [Google Scholar]
- 44.Dietrich JB, Zaepfel M, Kuchler-Bopp S. Expression of intercellular adhesion molecule-1 in C6 glioma cells is up-regulated by thyroid hormone. Neuroreport. 2000;11:2855–2860. doi: 10.1097/00001756-200009110-00006. [DOI] [PubMed] [Google Scholar]
- 45.Kawashima M, Doh-ura K, Mekada E, Fukui M, Iwaki T. CD9 expression in solid non-neuroepithelial tumors and infiltrative astrocytic tumors. J Histochem Cytochem. 2002;50:1195–1203. doi: 10.1177/002215540205000906. [DOI] [PubMed] [Google Scholar]
- 46.Gross JL, Morrison RS, Eidsvoog K, Herblin WF, Kornblith PL, Dexter DL. Basic fibroblast growth factor: a potential autocrine regulator of human glioma cell growth. J Neurosci Res. 1990;27:689–696. doi: 10.1002/jnr.490270429. [DOI] [PubMed] [Google Scholar]
- 47.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 48.Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 49.Tamaki M, McDonald W, Del Maestro RF. The importance of cell density in the interpretation of growth factor effects on collagenase IV activity release and extracellular matrix production from C6 astrocytoma cells. J Neurooncol. 1998;39:205–216. doi: 10.1023/a:1005997919704. [DOI] [PubMed] [Google Scholar]
- 50.Vajkoczy P, Schilling L, Ullrich A, Schmiedek P, Menger MD. Characterization of angiogenesis and microcirculation of high-grade glioma: an intravital multifluorescence microscopic approach in the athymic nude mouse. J Cereb Blood Flow Metab. 1998;18:510–520. doi: 10.1097/00004647-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Xuesong D, Wei X, Heng L, Xiao C, Shunan W, Yu G, et al. Evaluation of neovascularization patterns in an orthotopic rat glioma model with dynamic contrast-enhanced MRI. Acta Radiol. 2017;58:1138–1146. doi: 10.1177/0284185116681038. [DOI] [PubMed] [Google Scholar]
- 52.Radaelli E, Ceruti R, Patton V, Russo M, Degrassi A, Croci V, et al. Immunohistopathological and neuroimaging characterization of murine orthotopic xenograft models of glioblastoma multiforme recapitulating the most salient features of human disease. Histol Histopathol. 2009;24:879–891. doi: 10.14670/HH-24.879. [DOI] [PubMed] [Google Scholar]
- 53.Candolfi M, Curtin JF, Nichols WS, Muhammad AG, King GD, Pluhar GE, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doblas S, He T, Saunders D, Hoyle J, Smith N, Pye Q, et al. In vivo characterization of several rodent glioma models by 1H MRS. NMR Biomed. 2012;25:685–694. doi: 10.1002/nbm.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bing G, Notter MF, Hansen JT, Kellogg C, Kordower JH, Gash DM. Cografts of adrenal medulla with C6 glioma cells in rats with 6-hydroxydopamine-induced lesions. Neuroscience. 1990;34:687–697. doi: 10.1016/0306-4522(90)90175-4. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi DK, Dinday MT, Barbaro NM, Baraban SC. Abnormal cortical cells and astrocytomas in the Eker rat model of tuberous sclerosis complex. Epilepsia. 2004;45:1525–1530. doi: 10.1111/j.0013-9580.2004.23004.x. [DOI] [PubMed] [Google Scholar]
- 57.Chang KT, Lin YY, Lin YY, Lin YL, Cheng H, Chang Y, et al. In Vivo Real-Time Discrimination Among Glioma, Infiltration Zone, and Normal Brain Tissue via Autofluorescence Technology. World Neurosurg. 2019;122:e773–e782. doi: 10.1016/j.wneu.2018.10.144. [DOI] [PubMed] [Google Scholar]
- 58.Löhr M, Linsenmann T, Jawork A, Kessler AF, Timmermann N, Homola GA, et al. Implanting Glioblastoma Spheroids into Rat Brains and Monitoring Tumor Growth by MRI Volumetry. Methods Mol Biol. 2017;1622:149–159. doi: 10.1007/978-1-4939-7108-4_12. [DOI] [PubMed] [Google Scholar]
- 59.Zhao S, Zhang X, Zhang J, Zhang J, Zou H, Liu Y, et al. Intravenous administration of arsenic trioxide encapsulated in liposomes inhibits the growth of C6 gliomas in rat brains. J Chemother. 2008;20:253–262. doi: 10.1179/joc.2008.20.2.253. [DOI] [PubMed] [Google Scholar]
- 60.Sheehan J, Ionescu A, Pouratian N, Hamilton DK, Schlesinger D, Oskouian RJ Jr, et al. Use of trans sodium crocetinate for sensitizing glioblastoma multiforme to radiation. J Neurosurg. 2008;108:972–978. doi: 10.3171/JNS/2008/108/5/0972. [DOI] [PubMed] [Google Scholar]
- 61.Yang WQ, Lun X, Palmer CA, Wilcox ME, Muzik H, Shi ZQ, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 62.Tanriover N, Ulu MO, Sanus GZ, Bilir A, Canbeyli R, Oz B, et al. The effects of systemic and intratumoral interleukin-12 treatment in C6 rat glioma model. Neurol Res. 2008;30:511–517. doi: 10.1179/174313208X289516. [DOI] [PubMed] [Google Scholar]
- 63.Beutler AS, Banck MS, Wedekind D, Hedrich HJ. Tumor gene therapy made easy: allogeneic major histocompatibility complex in the C6 rat glioma model. Hum Gene Ther. 1999;10:95–101. doi: 10.1089/10430349950019228. [DOI] [PubMed] [Google Scholar]
- 64.Dagestan Y, Karaca I, Bozkurt ER, Bilir A. Effects of Ibuprofen on orthotopic glioma model in rats. J Coll Physicians Surg Pak. 2012;22:690–693. [PubMed] [Google Scholar]
- 65.Dagistan Y, Karaca I, Bozkurt ER, Ozar E, Yagmurlu K, Toklu A, et al. Combination hyperbaric oxygen and temozolomide therapy in C6 rat glioma model. Acta Cir Bras. 2012;27:383–387. doi: 10.1590/s0102-86502012000600005. [DOI] [PubMed] [Google Scholar]
- 66.Schuler PJ, Bendszus M, Kuehnel S, Wagner S, Hoffmann TK, Goldbrunner R, et al. Urokinase plasminogen activator, uPAR, MMP-2, and MMP-9 in the C6-glioblastoma rat model. In Vivo. 2012;26:571–576. [PubMed] [Google Scholar]
- 67.Figueiró F, Bernardi A, Frozza RL, Terroso T, Zanotto-Filho A, Jandrey EH, et al. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J Biomed Nanotechnol. 2013;9:516–526. doi: 10.1166/jbn.2013.1547. [DOI] [PubMed] [Google Scholar]
- 68.Guo W, Li A, Jia Z, Yuan Y, Dai H, Li H. Transferrin modified PEG-PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. Eur J Pharmacol. 2013;718:41–47. doi: 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 69.Benadiba M, de M Costa I, Santos RL, Serachi FO, de Oliveira Silva D, Colquhoun A. Growth inhibitory effects of the Diruthenium-Ibuprofen compound, [Ru2Cl(Ibp) 4], in human glioma cells in vitro and in the rat C6 orthotopic glioma in vivo. J Biol Inorg Chem. 2014;19:1025–1035. doi: 10.1007/s00775-014-1143-4. [DOI] [PubMed] [Google Scholar]
- 70.Zhang B, Wang H, Liao Z, Wang Y, Hu Y, Yang J, et al. EGFP-EGF1-conjugated nanoparticles for targeting both neovascular and glioma cells in therapy of brain glioma. Biomaterials. 2014;35:4133–4145. doi: 10.1016/j.biomaterials.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 71.Miyake JA, Benadiba M, Ribeiro G, DE Oliveira Silva D, Colquhoun A. Novel ruthenium - gamma-linolenic acid complex inhibits C6 rat glioma cell proliferation in vitro and in the orthotopic C6 model in vivo after osmotic pump infusion. Anticancer Res. 2014;34:1901–1911. [PubMed] [Google Scholar]
- 72.Zhang X, Guo M, Shen L, Hu S. Combination of photodynamic therapy and temozolomide on glioma in a rat C6 glioma model. Photodiagnosis Photodyn Ther. 2014;11:603–612. doi: 10.1016/j.pdpdt.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang Q, et al. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. 2015;46:1739–1747. doi: 10.3892/ijo.2015.2863. [DOI] [PubMed] [Google Scholar]
- 74.Qin T, Wang C, Chen X, Duan C, Zhang X, Zhang J, et al. Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicol Appl Pharmacol. 2015;286:112–123. doi: 10.1016/j.taap.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 75.An Y, Guo W, Li L, Xu C, Yang D, Wang S, et al. Micheliolide derivative DMAMCL inhibits glioma cell growth in vitro and in vivo. PLoS One. 2015;10:e0116202. doi: 10.1371/journal.pone.0116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu H, Chen Q, Wang H, Xu P, Yuan R, Li X, et al. Inhibitory effects of lapachol on rat C6 glioma in vitro and in vivo by targeting DNA topoisomerase I and topoisomerase II. J Exp Clin Cancer Res. 2016;35:178. doi: 10.1186/s13046-016-0455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atzori MG, Tentori L, Ruffini F, Ceci C, Bonanno E, Scimeca M, et al. The Anti-Vascular Endothelial Growth Factor Receptor-1 Monoclonal Antibody D16F7 Inhibits Glioma Growth and Angiogenesis in Vivo. J Pharmacol Exp Ther. 2018;364:77–86. doi: 10.1124/jpet.117.244434. [DOI] [PubMed] [Google Scholar]
- 78.Wang C, Li Y, Chen H, Zhang J, Zhang J, Qin T, et al. Inhibition of CYP4A by a novel flavonoid FLA-16 prolongs survival and normalizes tumor vasculature in glioma. Cancer Lett. 2017;402:131–141. doi: 10.1016/j.canlet.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 79.Liu S, Zhang SM, Ju RJ, Xiao Y, Wang X, Song XL, et al. Antitumor efficacy of Lf modified daunorubicin plus honokiol liposomes in treatment of brain glioma. Eur J Pharm Sci. 2017;106:185–197. doi: 10.1016/j.ejps.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Auguste P, Gürsel DB, Lemière S, Reimers D, Cuevas P, Carceller F, et al. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001;61:1717–1726. [PubMed] [Google Scholar]
- 81.Kirsch M, Strasser J, Allende R, Bello L, Zhang J, Black PM. Angiostatin suppresses malignant glioma growth in vivo. Cancer Res. 1998;58:4654–4659. [PubMed] [Google Scholar]
- 82.Strawn LM, Mann E, Elliger SS, Chu LM, Germain LL, Niederfellner G, et al. Inhibition of glioma cell growth by a truncated platelet-derived growth factor-beta receptor. J Biol Chem. 1994;269:21215–21222. [PubMed] [Google Scholar]
- 83.Fenstermaker RA, Capala J, Barth RF, Hujer A, Kung HJ, Kaetzel DM Jr. The effect of epidermal growth factor receptor (EGFR) expression on in vivo growth of rat C6 glioma cells. Leukemia. 1995;9 Suppl 1:S106–S112. [PubMed] [Google Scholar]
- 84.Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 85.Qin LJ, Jia YS, Zhang YB, Wang YH. Cyclooxygenase inhibitor induces the upregulation of connexin-43 expression in C6 glioma cells. Biomed Rep. 2016;4:444–448. doi: 10.3892/br.2016.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saleh M, Jonas NK, Wiegmans A, Stylli SS. The treatment of established intracranial tumors by in situ retroviral IFN-gamma transfer. Gene Ther. 2000;7:1715–1724. doi: 10.1038/sj.gt.3301273. [DOI] [PubMed] [Google Scholar]
- 87.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 88.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 89.Hattermann K, Mentlein R, Held-Feindt J. CXCL12 mediates apoptosis resistance in rat C6 glioma cells. Oncol Rep. 2012;27:1348–1352. doi: 10.3892/or.2012.1674. [DOI] [PubMed] [Google Scholar]
- 90.Ehtesham M, Mapara KY, Stevenson CB, Thompson RC. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009;274:305–312. doi: 10.1016/j.canlet.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hattermann K, Held-Feindt J, Lucius R, Müerköster SS, Penfold ME, Schall TJ, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70:3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 92.Göttle P, Kremer D, Jander S, Odemis V, Engele J, Hartung HP, et al. Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann Neurol. 2010;68:915–924. doi: 10.1002/ana.22214. [DOI] [PubMed] [Google Scholar]
- 93.Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, et al. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 94.Mueller CA, Schluesener HJ, Conrad S, Meyermann R, Schwab JM. Lesional expression of a proinflammatory and antiangiogenic cytokine EMAP II confined to endothelium and microglia/macrophages during secondary damage following experimental traumatic brain injury. J Neuroimmunol. 2003;135:1–9. doi: 10.1016/s0165-5728(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 95.Gupta S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol. 2014;54:47–52. doi: 10.1016/j.exger.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Popovich PG, van Rooijen N, Hickey WF, Preidis G, McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp Neurol. 2003;182:275–287. doi: 10.1016/s0014-4886(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 97.Zhang ZM, Yang Z, Zhang Z. Distribution and characterization of tumor-associated macrophages/microglia in rat C6 glioma. Oncol Lett. 2015;10:2442–2446. doi: 10.3892/ol.2015.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]