Summary
Metschnikowia reukaufii is a widespread yeast able to grow in the plants’ floral nectaries, an environment of extreme conditions with sucrose concentrations exceeding 400 g l−1, which led us into the search for enzymatic activities involved in this sugar use/transformation. New oligosaccharides were produced by transglucosylation processes employing M. reukaufii cell extracts in overload‐sucrose reactions. These products were purified and structurally characterized by MS‐ESI and NMR techniques. The reaction mixture included new sugars showing a great variety of glycosidic bonds including α‐(1→1), α‐(1→3) and α‐(1→6) linkages. The main product synthesized was the trisaccharide isomelezitose, whose maximum concentration reached 81 g l−1, the highest amount reported for any unmodified enzyme or microbial extract. In addition, 51 g l−1 of the disaccharide trehalulose was also produced. Both sugars show potential nutraceutical and prebiotic properties. Interestingly, the sugar mixture obtained in the biosynthetic reactions also contained oligosaccharides such as esculose, a rare trisaccharide with no previous NMR structure elucidation, as well as erlose, melezitose and theanderose. All the sugars produced are naturally found in honey. These compounds are of biotechnological interest due to their potential food, cosmeceutical and pharmaceutical applications.
Introduction
Bioactive oligosaccharides are highly demanded products mainly for food and pharmaceutical industries, and their biotechnological production has increased in Asia, Europe and USA markets (Goffin et al., 2011; Sorndech et al., 2018). This sort of carbohydrates include lactulose, lactosucrose, soybean‐oligosaccharides, fructo‐oligosaccharides (FOS), galacto‐oligosaccharides (GOS) and xylo‐oligosaccharides (XOS), as well as gluco‐oligosaccharides (GlcOS) containing α‐(1→6) (isomalto‐oligosaccharides, IMOS), α‐(1→4), α‐(1→2) and α‐(1→3) linkages (Crittenden and Playne, 1996; Kim et al., 2014). In addition to its potential prebiotic effects, which would promote the colonic beneficial bacteria growth/activity (mainly Lactobacilli sp. and Bifidobacteria sp.), several physicochemical and biological properties would make them of interest for food, and biotechnological applications (Remaud‐Simeon et al., 2000; Singla and Chakkaravarthi, 2017; Singh et al., 2017). For instance, the consumption of bioactive oligosaccharides is associated with an increase in calcium and magnesium absorption, the prevention of colon cancer and cardiovascular diseases, or even with the immune system improvement (Patel and Goyal, 2012; Florowska et al., 2016). Besides, oligosaccharides are frequently included in food and beverages as non‐cariogenic ingredients, sugar substitutes or bulking agents (Crittenden and Playne, 1996; Kim et al., 2014). In addition, the gelling properties of certain bioactive carbohydrates allow their use as low‐caloric fat replacement that maintain the spreadable texture of creamy products like yoghurts and sauces, or due to its moisture retention capacity increase foodstuff shelf life (Al‐Sheraji et al., 2013).
Bioactive oligosaccharides can be produced by direct isolation from natural sources (plants, milk and honey), chemical processes and biotechnological procedures employing biocatalysts. The enzymatic methods can include the controlled hydrolysis of polysaccharides or the synthesis of oligomers from mono‐ and di‐saccharides, processes with high substrate specificity, regio‐ and stereo‐ selectivity (Díez‐Municio et al., 2014). Enzymes involved in the bioactive carbohydrate synthesis are glycosidases (EC 3.2) or glycosyltransferases (EC 2.4), that catalyse the hydrolysis of glycosidic bonds of oligo‐ and polysaccharides or the glycosyl group‐transfer from a donor to an acceptor molecule respectively (Plou et al., 2007). It was extensively reported that the hydrolytic activity of glycosidases, such as α/β‐glucosidases or β‐fructofuranosidases, could be shifted towards synthesis (trasnsglycosylating activity) using a high substrate concentration. Thus, the α‐glucosidase from the yeast Xanthophyllomyces dendrorhous produced IMOS (mainly panose) using 200–525 g l−1 maltose (Fernández‐Arrojo et al., 2007; Gutierrez‐Alonso et al., 2016) and β‐fructofuranosidases from Aspergillus awamori or yeast such as Schwanniomyces occidentalis and Saccharomyces cerevisiae synthesized FOS using different sucrose‐rich by‐products from a variety of palm tree dates (the first) or 600 g l−1 sucrose (the last two) (Álvaro‐Benito et al., 2007; Lafraya et al., 2011; Smaali et al., 2012). In fact, sucrose is a relatively cheap and renewable raw material that has already been widely employed in the synthesis of bioactive oligosaccharides (Monsan and Ouarné, 2009), and among them several hetero‐oligosaccharides. As examples, novel hetero‐FOS were obtained using sucrose as fructosyl donor, different sugar acceptors and β‐fructofuranosidases from microorganisms such as X. dendrorhous (Gimeno‐Pérez et al., 2014) or S. occidentalis (Piedrabuena et al., 2016). In addition, the α‐glucosidase from Bacillus licheniformis produced the trisaccharide theanderose by transglucosylation of sucrose, while glucansucrases GtfA and Gtf180 from Lactobacillus reuteri generated different lactose derivatives by transferring the glucose unit of sucrose to lactose (Nimpiboon et al., 2011; Pham et al., 2017).
Metschnikowia reukaufii is a widespread budding yeast (Ascomycota, Saccharomycetales) able to grow in the floral nectaries, an environment of extreme conditions with sucrose concentrations exceeding 400 g l−1. It is the predominant microorganism in nectaries of plants such as Helleborous foetidus (Pozo et al., 2011; Belisle et al., 2012; Herrera, 2014; Dhami et al., 2016). Although the ecological function of this yeast is relatively unknown, several studies relate it to the nectar sugar composition, the synthesis of volatile compounds or even with the nectaries temperature increases, all factors that may have incidence on the pollinators behaviour (Herrera and Pozo, 2010; Canto et al., 2015; Yang et al., 2019).
In this work, we have analysed an enzymatic activity associated to M. reukaufii cell extracts able to hydrolyse sucrose and generate a mixture of oligosaccharides, which were purified and structurally characterized using mass spectrometry (MS‐ESI) and nuclear magnetic resonance (NMR) techniques. The mixture of sugars obtained is found naturally in honey, and includes several molecules of biotechnological interest whose production profiles were also evaluated.
Results and discussion
Analysis of the standard sucrose‐splitting activity in Metschnikowia reukaufii cultures
Initially, the potential hydrolytic activity on sucrose of M. reukaufii was assessed using a solid rich medium for yeast including sucrose and bromothymol blue (BTB), a pH indicator (yellow, greenish and blue in acidic, neutral and basic solutions respectively). Schwanniomyces occidentalis and Pichia pastoris were used as positive and negative controls in this assay due to their proven or absent capacity to hydrolyse sucrose respectively (Álvaro‐Benito et al., 2007; Gimeno‐Pérez et al., 2015). As expected, all yeast species were able to grow on this rich medium. Thus, S. occidentalis turned the colour of YEP(S‐BTB) plates from greenish to yellow due to the acidic compounds formed as a result of sucrose utilization, whereas P. pastoris turned it into dark blue because of the use of peptone and ammonium excretion. As proof of its sucrose‐splitting activity, the medium where M. reukaufii grew was also yellow (Fig. 1A).
Figure 1.
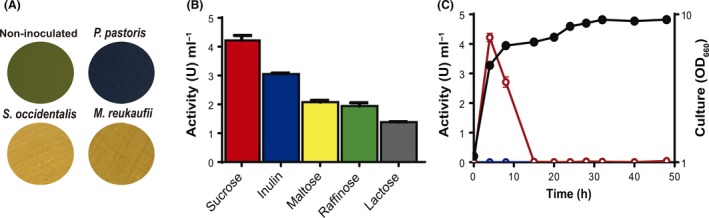
Analysis of the standard hydrolytic activity on sucrose expressed in M. reukaufii cultures. A. Activity assay in YEP(S‐BTB) medium. Plate inoculated with M. reukaufii cells (yellow) is shown. S. occidentalis (yellow) and P. pastoris (dark blue) or non‐inoculated (greenish) plates were used as positive and negative controls respectively. B. Maximum activity levels detected in YEP cultures containing the referred sugars, all 20 g l−1. C. Time‐course of standard activity. Inocula from M. reukaufii were grown (black circles) in 250 ml flasks containing 25 ml of YEP(S) medium and samples were withdrawn at the indicated times. OD660 measurements differing < 1% were obtained for all cultures analysed. The extracellular (blue circles) and cell‐associated (red circles) activity were determined using sucrose as substrate. Each point represents the average of three independent measurements with its standard deviation.
Metschnikowia reukaufii was grown in YEP liquid media supplemented with different carbon sources as described in the Experimental procedures section and the standard sucrose‐splitting activity was evaluated in both, extracellular and cell‐associated fractions. Hydrolytic activity towards sucrose was detected in the cellular fractions of yeast grown in media containing sucrose, inulin, maltose, raffinose and lactose (Fig. 1B), but not in the extracellular fractions in any of the analysed conditions. The maximum activity detected (~ 4.2 U ml−1) was obtained using sucrose‐based culture, the major component in the nectar sugar mixture of Helleborous foetidus (Pozo et al., 2011), and was expressed at the beginning of the microorganism stationary growth phase (OD660 ~ 3.6; ~ 4 h; Fig. 1C). As expected, no activity was detected when using a glucose‐based medium, a very common process known as glucose (catabolic) repression, since it is widely documented that glucose represses the expression of a large number of genes involved among others, in the use of alternative carbon sources and gluconeogenesis (Trumbly, 1992; Kayikci and Nielsen, 2015).
Analysis of the translgycosylating activity in M. reukaufii cell extracts
The search and characterization of new enzymatic activities involved in the production of oligosaccharides with possible bioactive properties is an interesting biotechnological challenge. Therefore, the potential transferase activity of the M. reukaufii cell extracts, which were able to hydrolyse sucrose, was assessed using a high this sugar concentration (500 g l−1) and the composition of the reaction mixtures analysed by HPLC.
A representative HPLC‐ELSD chromatographic profile of the sugar mixture (72 h of reaction) is shown in Fig. 2A. In addition to the expected signals corresponding to sucrose (peak 3) and its hydrolysis products, fructose (peak 1) and glucose (peak 2), new signals (peak 4 to 8) were detected in the chromatograms. Interestingly, the amount of glucose detected was significantly lower than that of fructose in the HPLC‐ELSD chromatograms analysed, suggesting that a potential glucosyltransferase activity was transferring the glucosyl moiety from sucrose (glucosyl donor) to another molecule (glucosyl acceptor). As expected, only the sucrose signal was detected at time 0 reactions (Fig. 2A) and in a control reaction lacking the enzymatic extract (data not shown). Besides, only sucrose was detected when using enzymatic extracts obtained from yeast cells grown in a glucose‐based medium (data not shown), very probably due to the absence of activity caused by the glucose repression effect already mentioned.
Figure 2.
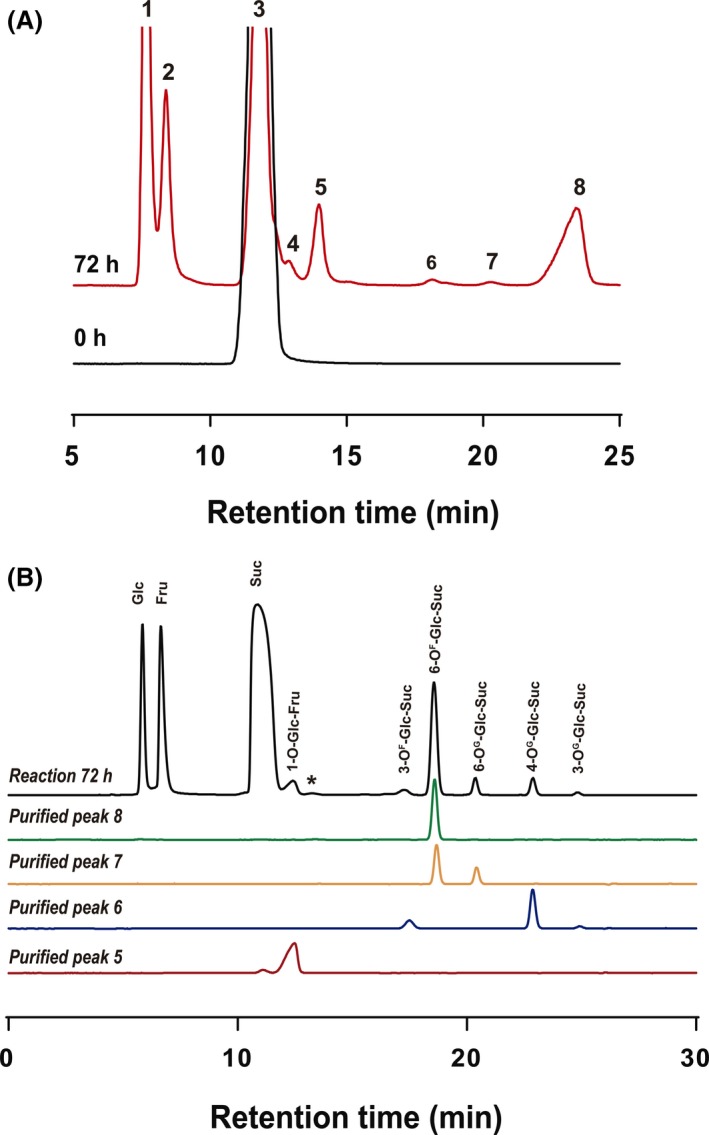
Representative HPLC chromatograms of the reaction mediated by the M. reukaufii extract on sucrose 500 g l−1. A. HPLC‐ELSD analysis. Chromatograms correspond to the sugar mixtures at 0 (black line) and 72 h (red line) of reaction. Similar product profiles were obtained by using longer reaction times. Peaks assignation: (1) fructose, (2) glucose, (3) sucrose, (4–8) new signals, initially unknown compounds. B. HPAEC‐PAD analysis of carbohydrate fractions purified by semi‐preparative HPLC. Chromatographic profiles of the 72 h reaction mixture (black line) and the referenced purified peaks (red, blue, yellow and green lines) are shown. Peaks assignation using the corresponding standards: Glc: glucose; Fru: fructose; Suc: sucrose. New products assignation after MS‐ESI and NMR analysis of the purified peaks are indicated: ( * ) unknown compound.
Identification and structural characterization of the transfer products
The new oligosaccharides synthesized in overload‐sucrose reaction (peaks 5 to 8, Fig. 2A) were purified by semi‐preparative HPLC. Although it was attempted, it was not possible to purify peak 4 due to its proximity to the sucrose signal. The purity of each collected fraction was analysed by HPAEC‐PAD given the higher sensitivity of this system compared to that of HPLC‐ELSD. Figure 2B shows the HPAEC‐PAD chromatographic profile of the reaction mixture including sucrose as single substrate after 72 h and each one of the purified product profiles. Although a high degree of purity was achieved in all the analysed fractions, sugars corresponding to the purified peaks 5 and 7 show traces of sucrose and the compound responsible of the peak 8 in the HPLC‐ELSD chromatogram (Fig. 2A) respectively.
The molecular weight of the purified products was determined by MS‐ESI using a hybrid QTOF analyser. The mass spectra of the purified compound responsible for peak 5 showed a major signal in positive mode at m/z 365.11 corresponding to a disaccharide‐[Na]+ form (Fig. S1), while those for peaks 6, 7 and 8 at m/z 527.16 to trisaccharides‐[Na]+ (Figs S2, S3 and S4). A standard NMR analysis permitted to deduce the structure of these new oligosaccharides. The compound in the purified fraction‐peak 5 was identified as trehalulose: [α‐D‐Glc‐(1→1)‐β‐D‐Fru]. The results from the 2D 1H‐13C spectra (Fig. 3A) allowed the identification of two signal sets corresponding to the most abundant tautomeric forms, in which the β‐fructosyl ring exists as a pyranosyl (major form) and furanosyl (minor form) mixture: α‐D‐Glcp‐(1→1)‐β‐D‐Frup and α‐D‐Glcp‐(1→1)‐β‐D‐Fruf respectively. Although the α‐fructosyl tautomers of trehalulose were not detected, they should be present in the α/β equilibrium as described by Lichtenthaler and Rönninger (1990).
Figure 3.
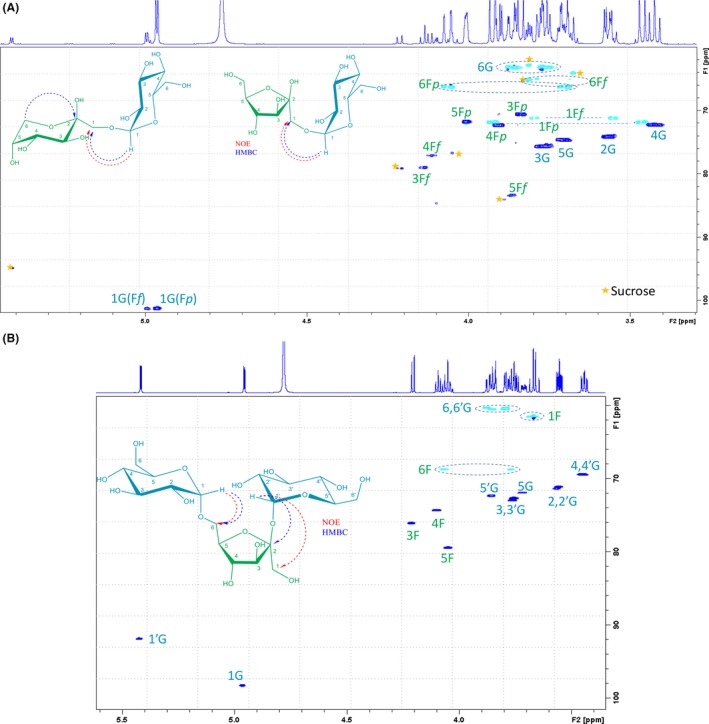
NMR spectra (600 MHz, D2O, 300 K) for the compounds separated as peaks 5 and 8. A. 1H‐13C HSQC‐edited spectra with signal assignation for the compound present in peak 5 (trehalulose). Signals corresponding to sucrose are depicted with an orange star ( ). B. 1H‐13C HSQC‐edited spectra with signal assignation for the compound present in peak 8 (isomelezitose). The key NOE or HMBC correlations that confirmed the different linkages are indicated for each molecule.
). B. 1H‐13C HSQC‐edited spectra with signal assignation for the compound present in peak 8 (isomelezitose). The key NOE or HMBC correlations that confirmed the different linkages are indicated for each molecule.
In the purified fraction corresponding to peak 6, three different trisaccharides were characterized from the combination of the signals from COSY, TOCSY, NOESY, HSQC and HMBC experiments. The major components were identified as erlose [α‐D‐Glcp‐(1→4)‐α‐D‐Glcp‐(1→2)‐β‐D‐Fruf] (Fig. S5A) and melezitose [α‐D‐Glcp‐(1→3)‐β‐D‐Fruf‐(2→1)‐α‐D‐Glcp] (Fig. S5B). The data obtained for these compounds were reasonably consistent with those previously reported by Wei et al. (1997) and Anteunis et al. (1975) respectively. Despite its low concentration, the third component of the mixture was also analysed. The presence of two α‐Glc moieties (G1 and G2) was deduced from the measurement of the JH1H2 couplings (4 Hz). The analysis of the standard COSY, HSQC (Fig. 4A), Heteronuclear Multiple Bond Correlation (HMBC) and HSQC‐TOCSY (Fig. 4B) experiments showed the existence of [α‐Glc‐(1→2)‐β‐Fru] and [α‐Glc‐(1→x)‐Fru/Glc] for G1 and G2 respectively. Moreover, the analysis of the NOESY experiments (Fig. 4C, left) permitted to deduce the existence of a G1‐H1/F‐H1 cross peak highlighting the spatial proximity of these residues, strongly suggesting the existence of an α‐G1‐(1→2)‐β‐Fru fragment. One glycosylated carbon was detected at 80 ppm (Fig. 4B, red). This carbon was part of the G1 residue, as deduced from HSQC‐TOCSY experiment (Fig. 4B, black). Both anomeric G1‐H1 and G2‐H1 protons showed correlation in the HMBC spectra with this carbon (Fig. 4B, blue), thus demonstrating that G2 was linked to G1. This G2‐G1 connectivity led to different trisaccharide molecules. The α‐G2‐(1→2)‐α‐G1‐(1→2)‐β‐Fru trisaccharide has been previously described (Fischer et al., 2006), but no matching with the reported NMR data was found (Table S1). The α‐G2‐(1→3)‐α‐G1‐(1→2)‐β‐Fru trisaccharide has also been described (Chiba et al., 1984), but no NMR data were published. Our NMR data for the major product fit well with those described for the α‐G2‐(1→4)‐α‐G1‐(1→2)‐β‐Fru (Erlose) (Wei et al., 1997). Finally, the NMR data reported for the α‐G2‐(1→6)‐α‐G1‐(1→2)‐β‐Fru trisaccharide (Theanderose) do not match with those deduced for our molecule (Ruiz‐Aceituno et al., 2017). Therefore, the acquired evidences, after elimination of the previously described products, suggest that the third component of peak 6 of the HPLC‐ELSD chromatograms corresponds to esculose, α‐G2‐(1→3)‐α‐G1‐(1→2)‐β‐Fru. Although few signals are unassigned due to the large overlapping with those belonging to major component erlose (the Fru residue), the assigned signals and the previously described data provide solid evidences for this interpretation. As far as we know, this is the first time that the structural characterization of this trisaccharide is described in a detailed way.
Figure 4.
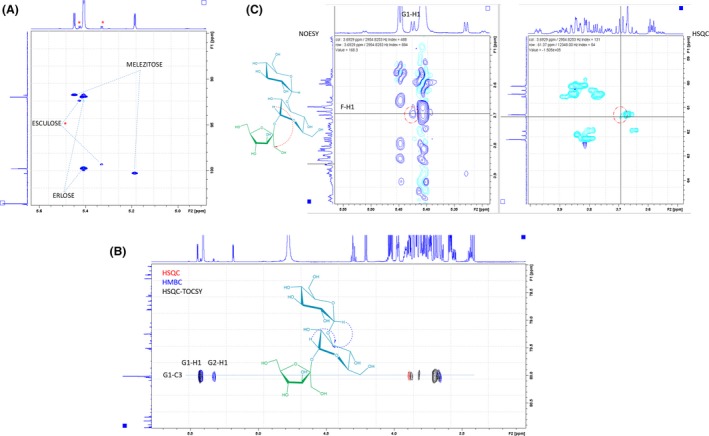
NMR spectra (600 MHz, D2O, 300 K) for the minor component from the purified peak 6. A. Key region of the HSQC experiment. The minor product signals are marked with a red dot and show the existence of [α‐Glc‐(1→2)‐β‐Fru] and [α‐Glc‐(1→x)‐Fru/Glc] type residues for G1 and G2. B. Superimposition of partial HSQC (red), HMBC (blue) and HSQC‐TOCSY (black) spectra, for the 13C region of 80 ppm, demonstrating the G2‐G1 connectivity in a non‐ambiguous manner. C. The key NOE that evidences the spatial proximity of the G1‐H1/F‐H1 pair.
Two trisaccharides were identified in the purified fraction 7, isomelezitose [α‐D‐Glcp‐(1→6)‐β‐D‐Fruf‐(2→1)‐α‐D‐Glcp] (Fig. 3B) and theanderose [α‐D‐Glcp‐(1→6)‐α‐D‐Glcp‐(1→2)‐β‐D‐Fruf] (Fig. S6). The signal pattern obtained for theanderose and isomelezitose spectra were coincident to those described by Ruiz‐Aceituno et al. (2017) and Shi et al. (2016) respectively. Moreover, isomelezitose was the only compound detected in the purified fraction from the peak 8 of the HPLC‐ELSD chromatograms (Figs 2B and 3B).
The structural characterization of the synthesized sugars indicates that the transglycosylating activity of the M. reukaufii cell extracts was able to transfer the glucosyl moiety of sucrose principally to the 6‐OH of the β‐fructofuranosyl residue of sucrose and to the 1‐OH position of the free fructose (released in the reaction), yielding isomelezitose and trehalulose respectively. In addition, the hydroxyls 3‐OH of the β‐fructofuranosyl residue, and the 3‐OH, 4‐OH and 6‐OH of the α‐glucopyranosyl unit of sucrose were also glucosylated, but to a lesser extent, producing melezitose, esculose, erlose and theanderose respectively. This biosynthetic behaviour had been previously described for some glycosidases, specifically α‐glucosidases, which were not only capable of transferring the glucosyl moiety to the more reactive primary hydroxyl groups of acceptor sugars, but also to the secondary ones of several acceptor molecules (Plou et al., 2007). In fact, the synthesis of isomelezitose was first described using a partially purified α‐glucosidase from S. cerevisiae, but this was the only trisaccharide detected in the reactions when using sucrose as substrate (Chiba et al., 1979). Afterwards, three sugars, theanderose (6G‐α‐D‐Glucosyl‐sucrose), isomelezitose (6F‐α‐D‐Glucosyl‐sucrose) and 4F‐α‐D‐Glucosyl‐sucrose were also produced using the Bacillus sp. SAM1606 α‐glucosidase in similar conditions (Inohara‐Ochiai et al., 2000). However, and differing from what has been previously reported, a mixture of oligosaccharides containing a great diversity of glycosidic bonds was obtained, with isomelezitose as the major product, in the transfer reactions mediated by the enzymatic extract of M. reukaufii. Although it seems plausible that an α‐glucosidase activity produces all these compounds, given the varied type of molecules formed, the possibility that not a single enzyme could be implicated in the transglucosylation of sucrose cannot be eliminated by now, which requires further protein characterization that is in progress. In any case, a schematic view of the reactions catalysed by the M. reukaufii cell extract and 500 g l−1 sucrose is represented in Fig. 5.
Figure 5.
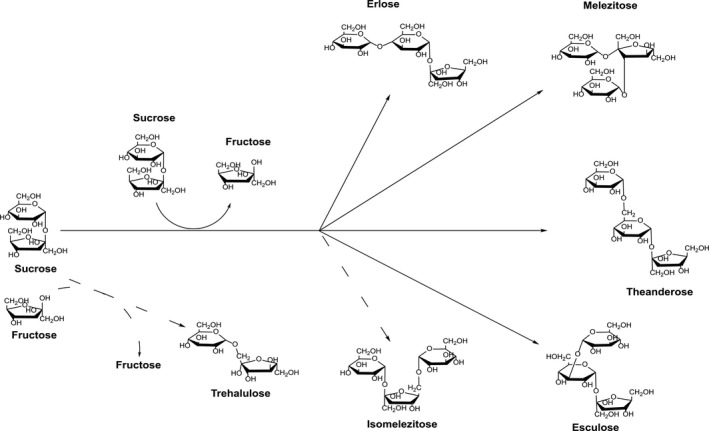
Scheme of the transglucosylation process involving sucrose and M. reukaufii enzymatic extract. The formation of the main transglucosylation products is indicated with broken arrows.
Effect of sucrose concentration on the transfer products’ level
The transglucosylating activity of the M. reukaufii extract was evaluated using different sucrose concentrations and the main transfer products in the reaction mixtures, isomelezitose and trehalulose (Fig. 5), were quantified after 72 h (Fig. 6A). As expected, the concentration of these two compounds increased as the sucrose did from 100 to 500 g l−1. This behaviour has been previously described for many microbial glycosidases, where the substrate concentration and the transglycosylating/hydrolysing ratio increased in parallel. As examples, the transfer activity of the β‐fructofuranosidase from X. dendrorhous improved by 1.5‐fold when the sucrose concentration increased from 420 to 600 g l−1 (Linde et al., 2012), and that of the α‐glucosidase from Aspergillus niger did in 1.8‐fold when the maltose concentration went from 100 to 300 g l−1 (Duan et al., 1995). By contrast, when using the M. reukaufii extract and the initial sucrose concentration increased from 500 to 700 g l−1, the amounts of the majority transfer products were either reduced significantly (~ 50%, from 50 to 24 g l−1) or slightly increased (~ 16%, from 54 to 63 g l−1) for trehalulose and isomelezitose respectively. Thus, the highest total amount of the major transfer products was achieved at 500 g l−1 sucrose (Fig. 6A). Accordingly, this sucrose concentration was used to analyse the evolution of sugars in the reaction mixture for a total of 120 h. Figure 6B shows the production profiles of trehalulose and isomelezitose. The final carbohydrate mixture contained 81 and 51 g l−1 of isomelezitose and trehalulose, which represented 16.1% and 10% (w/w) of total sugars in the mixture respectively. At this point, 120 g l−1 of sucrose (24% of total sugars) was detected. Besides, the concentration of erlose plus melezitose and theanderose reached 8.6 and 5.6 g l−1, which represented 1.7% and 1.1% of total sugars in the reaction mixture respectively (data not shown).
Figure 6.
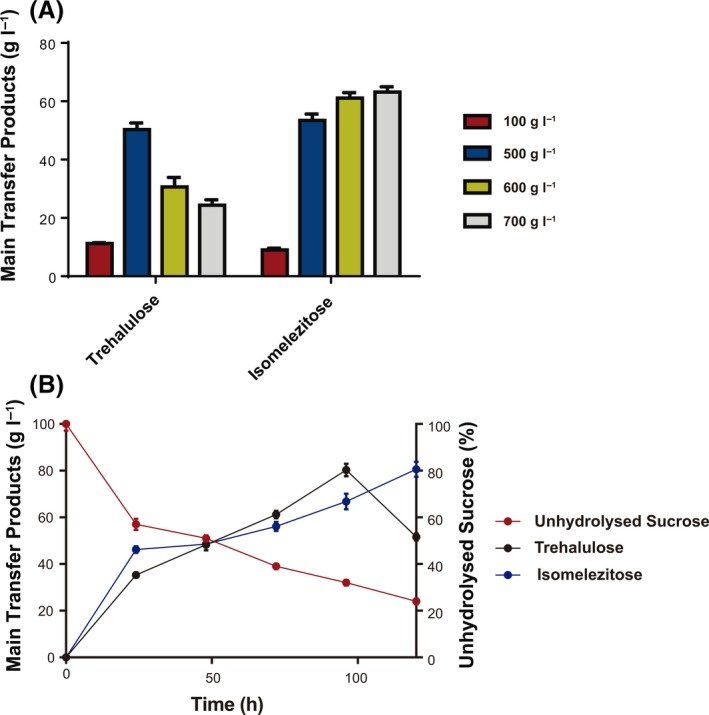
Analysis of the main transglucosylation products synthesized by the M. reukaufii enzymatic extract. A. Trehalulose and isomelezitose concentrations measured by HPLC‐ELSD after 72 h of reaction using the referred initial sucrose amounts. Standard deviation values are shown. B. Time‐course reaction profile of the main transfer products using 500 g l−1 sucrose. The evolution of unhydroylsed sucrose (red line), trehalulose (black line) and isomelezitose (blue line) are shown at the indicated reaction times with the corresponding standard deviations.
The α‐glucosidases mentioned above, responsible for the synthesis of oligosaccharides such as isomelezitose or theanderose, were all structurally included in the family 13 of the glycosyl hydrolases (GH13; carbohydrate‐active enzymes database: CAZy) (Lombard et al., 2014). Therefore, it seems plausible that if more than one protein were responsible for the transglucosylating activity detected in this work they would also be included in this family.
As previously mentioned, several microbial α‐glucosidases has been already used to produce isomelezitose, a rare trisaccharide found in honey, which shows potential nutraceutical and prebiotic properties (Görl et al., 2012). However, and as far as we know, the obtained yields when using native enzymes or cellular extracts were lower than those reported in this work. Thus, employing the partially purified α‐glucosidase from S. cerevisiae and 100 g l−1 sucrose, the isomelezitose production did not exceed 0.4% of total sugars in the reaction mixture (Chiba et al., 1979), and using a Bacillus sp. α‐glucosidase and 600 g l−1 sucrose reached about 8% of total sugars (Inohara‐Ochiai et al., 2000). Interestingly, the proportion of isomelezitose, referred to total sugars produced by transglucosylation in the reaction mixture, increased by site‐directed mutagenesis of this enzyme, but its transferase activity was also reduced and finally isomelezitose constituted only about 3% of total sugars (Okada et al., 2002). In this context, Munir and Vogel (1999) patented the synthesis of isomelezitose using immobilized cells of Protaminobacter and 50ºC with yields close to 11% of total sugars. Besides, Görl et al. (2012) transformed a sucrose isomerase into an isomelezitose synthase, obtaining a protein variant by mutation that yielded 70% of isomelezitose using 100 g l−1 sucrose, but this amount was calculated based on sucrose consumed (~ 32%) instead of the total sucrose added to the reaction mixture, which means to produce about 22.4 g l−1 of this trisaccharide. More recently, Côté et al. (2017) obtained an engineered glucansucrase from the bacteria Leuconostoc mesenteroides that produced isomelezitose very efficiently using ~ 800 g l−1 sucrose. Compared to the wild type enzyme (DsrI), which only synthesized traces of isomelezitose, the mutated variant (Dsr1‐L441E) converted 57% of sucrose in this trisaccharide. Regardless of this excellent result, to our knowledge, the yield of isomelezitose obtained with the M. reukaufii extract (81 g l−1, 16.1% of total sugars in the mixture) is the highest reported for any microbial wild type enzyme, enzymatic extract or cellular system.
In conclusion, this study presents an enzymatic activity from M. reukaufii cell extracts producing a mixture of oligosaccharides with very different glycosidic bonds and biotechnological interest. Among them, isomelezitose, a potential nutraceutical and prebiotic sugar (Görl et al., 2012) and trehalulose, a disaccharide with approximately 60% of the sucrose sweetness and low rate of monosaccharide release into blood, an interestingly property for the development of new food products for diabetics (Wei et al., 2013). In addition, erlose and theanderose were produced, two non‐cariogenic sugars with low‐caloric value that also could be used as alternative to sucrose sweetener (Daudé et al., 2012) or as bifidogenic substrate (Ruiz‐Aceituno et al., 2017) respectively. Because all sugars produced by the M. reukaufii extract are found naturally in honey (Daudé et al., 2012), possibility that this microorganism could have some role in the final composition of this natural product could be considered. The relevance of our results makes further analysis necessary to characterize and tries to improve the biotechnological potential of the activity here analysed, as well as to address its functional improvement using molecular bioengineering.
Experimental procedures
Microorganisms and culture conditions
Metschnikowia reukaufii 1LL10 was isolated from Helleborous foetidus nectaries (Sierra de Cazorla, southeastern Spain) by Dr. Carlos M. Herrera [Estación Biológica de Doñana (CSIC), Sevilla, Spain]. Yeast was maintained at 4ºC on YEP(D) solid media [10 g l−1 yeast extract (Laboratorios Conda, S.A., Madrid, Spain), 20 g l−1 peptone (Laboratorios Conda), 20 g l−1 glucose (Merck, Darmstadt, Germany), 20 g l−1 agar (Laboratorios Conda)]. YEP liquid media including 20 g l−1 of glucose (D), sucrose (S), maltose (M), lactose (L), raffinose (R) or inulin (I) were used to analyse the hydrolytic activity profile on sucrose (also sucrose‐splitting activity). Yeast was cultured at 30ºC with orbital shaking (200 rpm) and growth was monitored spectrophotometrically at a wavelength of 660 nm (OD660). Sucrose, maltose, lactose and raffinose were from Sigma‐Aldrich (St. Louis, MO, USA) and inulin from Beneo Ibérica S.L. (Barcelona, Spain). The YEP(S‐BTB) medium, used to analyse the hydrolytic activity on plates, was the same as YEP(D) but including 50 g l−1 sucrose instead of glucose, supplemented with bromothymol blue 0.25 g l−1 (BTB) and then adjusted to pH 6.5. In this assay, yeast Schwanniomyces occidentalis ATCC2322 and Pichia pastoris GS115 were used as positive and negative yeast controls respectively.
Obtention of enzymatic extracts and sucrose‐splitting activity assay
M. reukaufii was cultivate in YEP(S) liquid medium during 4–8 h at 30ºC, cells were harvested by centrifugation (6000 g for 15 min) and the soluble enzymatic extracts were obtained using YeastBusterTM protein extraction reagent (Novagen, San Diego, CA, USA) according to the manufacturer’s specifications.
Hydrolytic standard activity on sucrose was determined by measuring the amount of reducing sugars (glucose or fructose) released from 60 g l−1 sucrose in 50 mM sodium phosphate pH 6.0 using the 3,5‐dinitrosalicylic acid (DNS) method adapted to a 96‐well microplate scale (Ghazi et al., 2005). Unless otherwise indicated, reactions mixtures (50 µl) including 5 µl of enzymatic extracts were incubated at 30ºC. Glucose in the 0–3 mg ml−1 range was used as calibration curve. Reactions without enzymatic extracts or sucrose were used as negative controls. One unit of standard hydrolytic activity (U) was defined as that catalysing the formation of 1 μmol of reducing sugar per minute under the above described conditions. Each reaction was performed at least in triplicate.
The hydrolytic on sucrose activity was detected in solid media including sucrose and a pH indicator (BTB), which shows greenish colour at pH 6.5. The use of sucrose would cause the acidification of the medium and the consequent change of the YEP(S‐BTB) colour from greenish to yellow by the BTB protoning (pH < 6.5).
Transglycosylation reactions based on sucrose
The transglycosylation reactions were performed using 500 g l−1 sucrose in 50 mM sodium phosphate pH 6.0, 10 U of standard hydrolytic activity, at 30ºC and 400 rpm in a Vortem 56 shaker (Labnet International, Woodbridge, NJ, USA). The final reaction volume was 1.5 ml. Samples of 100 μl were withdrawn at different reaction times (0–120 h), maintained at 100ºC for 10 min to inactivate the enzyme, and store at −20°C. Sucrose in the range of 100–700 g l−1 and 72 h of reaction time was used to analyse the effect of this sugar concentration on the transfer products level. For HPLC analysis, samples were conveniently diluted (1:5 or 1:10) in distilled water and filtered through 0.45 μm pore size nylon filters (Scharlau, S.L.; Sentmenat, Spain). Each reaction was performed in duplicate or triplicate.
HPLC‐ELSD analysis
Sugars in the reaction mixtures were analysed and quantified by HPLC using a quaternary pump (Delta 600; Waters, Milford, MA, USA) coupled to a Kromasil‐NH2 column (250 × 4.6 mm, 5 μm) from Análisis Vínicos S.L. (Tomelloso, Spain). Detection was performed using an evaporative light scattering detector (ELSD, mod. 1000; Polymer Laboratories, Ltd., Church Stretton, UK) equilibrated at 90°C. Column temperature was kept constant at 30°C. Analytes were eluted with a mixture acetonitrile/water 80:20, degassed with helium, at flow rate of 1.0 ml min−1 for 30 min. The peaks corresponding to the transfer reaction products were purified and identified by MS‐ESI and NMR techniques, as described in the following sections. All compounds were quantified on the base of peak areas using the most closely related commercial standard. Glucose, fructose, sucrose and melezitose monohydrate (all from Sigma) were used as standards. Data obtained were analysed using the Millennium 32 Software (Waters).
Purification of the transglycosylation products
Compounds formed during the transglycosylation reaction with 500 g l−1 sucrose were purified by semi‐preparative HPLC using a system equipped with a quaternary pump (Delta 600; Waters) coupled to a Kromasil‐NH2 column (250 × 10 mm, 5 μm) from Análisis Vínicos S.L. (Tomelloso, Spain). A three‐way flow splitter (Accurate, LC Packings) and the referred ELSD system equilibrated at 90°C was used and acetonitrile/water 90:10 (v:v), degassed with helium, was employed as mobile phase at 5.5 ml min−1 for 55 min. Then, the percentage of acetonitrile was reduced to 80% and kept for 15 min. Finally, it was raised to 90% and maintained for five additional minutes. The column temperature was kept at 30ºC. After collecting the different transglycosylation products, the mobile phase was eliminated by rotary evaporation in a R‐210 rotavapor (Buchi, Essen, Germany).
The purity of the compounds collected in the semi‐preparative HPLC was analysed by high‐performance anion‐exchange chromatography with pulsed amperometric detection (HPAEC‐PAD) on a Dionex ICS3000 system and a CarboPack PA‐1 column (4 × 250 mm) connected to a PA‐1 guard column. An electrochemical detector with a gold working electrode and Ag/AgCl as reference electrode was used. The initial mobile phase was 100 mM NaOH for 8 min. Then, a gradient from 100 to 88% 100 mM NaOH and from 0 to 12% 100 mM NaOH/600 mM sodium acetate was performed in 22 min. This last mobile phase composition was kept for six more minutes and then changed to 50% 100 mM NaOH and 50% 100 mM NaOH/600 mM sodium acetate. The flow rate was maintained at 1 ml min−1 during the analysis. Eluents were degassed by flushing with helium, and peaks were analysed using Chromeleon software.
Characterization of the transglycosylation products
The molecular weight of the purified oligosaccharides was assessed using a mass spectrometer with hybrid QTOF analyser (model QSTAR, Pulsar i, AB Sciex). The samples were analysed by direct infusion and ionized by electrospray (with methanol containing 1% of sodium iodide as ionizing phase) in positive reflector mode.
The structure of the synthesized oligosaccharides was determined using a combination of 1D (1H, 13C{1H}, 1D‐selective TOCSY, NOESY and/or ROESY) and 2D (COSY, HSQC, HSQC‐TOCSY, HMBC, NOESY) NMR techniques. The spectra of the samples, dissolved in D2O (ca. 7 mM), were recorded on a Bruker IVDr 600 spectrometer equipped with a BBI probe with gradients in the Z‐axis, at a temperature of 300 K. Chemical shifts were expressed in parts per million (ppm). TSP‐d4 signal was used as internal reference (0 ppm). Standard Bruker pulse sequences were employed. For the HSQC and HMBC experiments, values of 5 ppm and 1 K points, for the 1H dimension, and 70 ppm and 512 points for the 13C dimension, were used. For the homonuclear COSY and NOESY experiments, 3 ppm windows were used with a 1 K × 384–512 point matrix. For the NOESY the mixing time was set to 500 ms.
Conflict of interests
None declared.
Supporting information
Fig. S1. MS‐ESI Spectrum of purified peak 5.
Fig. S2. MS‐ESI Spectrum of purified peak 6.
Fig. S3. MS‐ESI Spectrum of purified peak 8.
Fig. S4. NMR Spectra (600 Hz, D2O, 300 K) for the compounds separated as peak 6. 1H‐13C HSQC‐edited spectra with signal assignation for erlose (A) and melezitose (B).
Fig. S5. NMR Spectra (600 Hz, D2O, 300 K) for the minor compound of purified peak 7 (theanderose). Signal corresponding to isomelezitose are depicted with a red star (*).
Table S1. NMR data deduced for the minor component of the purified peak 6 (esculose) and those previously described for α‐G2‐(1→2)‐α‐G1 fragment.
Acknowledgements
This work was supported by the Projects BIO2016‐76601‐C3‐2‐R/‐1‐R from the Spanish Ministry of Economy and Competitiveness and Fundación Ramón Areces (XIX Call of Research Grants in Life and Material Sciences). Martin Garcia‐Gonzalez fellowship FPU16/02925 was supported by the Spanish Ministry of Education, Culture and Sport. We thank Fundación Ramón Areces for an institutional grant to the Center of Molecular Biology Severo Ochoa. We also thank Mrs. Asunción Martín‐Redondo for the technical support.
Microbial Biotechnology (2019) 12(6), 1274–1285
Funding Information
This work was supported by the Projects BIO2016‐76601‐C3‐2‐R/‐1‐R from the Spanish Ministry of Economy and Competitiveness and Fundación Ramón Areces (XIX Call of Research Grants in Life and Material Sciences). Martin Garcia‐Gonzalez fellowship FPU16/02925 was supported by the Spanish Ministry of Education, Culture and Sport.
References
- Al‐Sheraji, S.H. , Ismail, A. , Manap, M.Y. , Mustafa, S. , Yusof, R.M. , and Hassan, F.A. (2013) Prebiotics as functional foods: a review. J Funct Foods 5: 1542–1553. [Google Scholar]
- Álvaro‐Benito, M. , de Abreu, M. , Fernández‐Arrojo, L. , Plou, F.J. , Jiménez‐Barbero, J. , Ballesteros, A. , et al. (2007) Characterization of a β‐fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6‐kestose. J Biotechnol 132: 75–81. [DOI] [PubMed] [Google Scholar]
- Anteunis, M. , de Bruyn, A. , and Verhegge, G. (1975) The 300‐MHz N.M.R. spectra of melezitose and raffinose in deuterium oxide. Carbohydr Res 44: 101–105. [Google Scholar]
- Belisle, M. , Peay, K.G. , and Fukami, T. (2012) Flowers as islands: spatial distribution of nectar‐inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird‐pollinated shrub. Microb Ecol 63: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto, A. , Herrera, C.M. , García, I.M. , García, M. , and Bazaga, P. (2015) Comparative effects of two species of floricolous Metschnikowia yeast on nectar. An Del Jardín Botánico de Madrid 72: 19. [Google Scholar]
- Chiba, S. , Murata, M. , Matsusaka, K. , and Shimomura, T. (1979) A new trisaccharide, 6F‐α‐D‐glucosyl‐sucrose, synthesized by transglucosylation reaction of brewer’s yeast α‐glucosidase. Agric Biol Chem 43: 775–779. [Google Scholar]
- Chiba, S. , Asada‐Komatsu, Y. , Kimura, A. , and Kawashima, K. (1984) A new trisaccharide, 3G‐α‐D‐glucosyl‐sucrose, synthesized by the transglucosidase action of immobilized buckwheat α‐glucosidase. Agric Biol Chem 48: 1173–1178. [Google Scholar]
- Côté, G. L. , Dunlap, C. A. , Vermillion, K. E. , and Skory, C. D. (2017) Production of isomelezitose from sucrose by engineered glucansucrases. Amylase 1: 82–93. [Google Scholar]
- Crittenden, R.G. , and Playne, M.J. (1996) Production, properties and applications of food‐grade oligosaccharides. Trends Food Sci Technol 7: 353–361. [Google Scholar]
- Daudé, D. , Remaud‐Siméon, M. , and André, I. (2012) Sucrose analogs: an attractive (bio) source for glycodiversification. Nat Prod Rep 29: 945–960. [DOI] [PubMed] [Google Scholar]
- Dhami, M.K. , Hartwig, T. , and Fukami, T. (2016) Genetic basis of priority effects: insights from nectar yeast. Proc R Soc B Biol Sci 283: 20161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez‐Municio, M. , Herrero, M. , Olano, A. , and Moreno, F.J. (2014) Synthesis of novel bioactive lactose‐derived oligosaccharides by microbial glycoside hydrolases. Microb Biotechnol 7: 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, K. , Sheu, D. , and Lin, C. (1995) Transglucosylation of a fungal α‐Glucosidase. Ann NY Acad Sci 750: 325–328. [DOI] [PubMed] [Google Scholar]
- Fernández‐Arrojo, L. , Marín, D. , Gómez De Segura, A. , Linde, D. , Alcalde, M. , Gutiérrez‐Alonso, P. , et al. (2007) Transformation of maltose into prebiotic isomaltooligosaccharides by a novel α‐glucosidase from Xantophyllomyces dendrorhous . Process Biochem 42: 1530–1536. [Google Scholar]
- Fischer, D. , Geyer, A. , and Loos, E. (2006) Occurrence of glucosylsucrose [α‐D‐glucopyranosyl‐(1→2)‐α‐ D‐glucopyranosyl‐(1→2)‐β‐D‐fructofuranoside] and glucosylated homologues in cyanobacteria: structural properties, cellular contents and possible function as thermoprotectants. FEBS J 273: 137–149. [DOI] [PubMed] [Google Scholar]
- Florowska, A. , Krygier, K. , Florowski, T. , and Dłuzewska, E. (2016) Prebiotics as functional food ingredients preventing diet‐related diseases. Food Funct 7: 2147–2155. [DOI] [PubMed] [Google Scholar]
- Ghazi, I. , Gómez De Segura, A. , Fernández‐Arrojo, L. , Alcalde, M. , Yates, M. , Rojas‐Cervantes, M.L. , et al. (2005) Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy‐activated Sepabeads EC for the synthesis of fructo‐oligosaccharides. J Mol Catal B: Enzym 35: 19–27. [Google Scholar]
- Gimeno‐Pérez, M. , Santos‐Moriano, P. , Fernandez‐Arrojo, L. , Poveda, A. , Jimenez‐Barbero, J. , Ballesteros, A. O. , et al. (2014) Regioselective synthesis of neo‐erlose by the β‐fructofuranosidase from Xanthophyllomyces dendrorhous . Process Biochem 49: 423–429. [Google Scholar]
- Gimeno‐Pérez, M. , Linde, D. , Fernández‐Arrojo, L. , Plou, F.J. , and Fernández‐Lobato, M. (2015) Heterologous overproduction of β‐fructofuranosidase from yeast Xanthophyllomyces dendrorhous, an enzyme producing prebiotic sugars. Appl Microbiol Biotechnol 99: 3459–3467. [DOI] [PubMed] [Google Scholar]
- Goffin, D. , Delzenne, N. , Blecker, C. , Hanon, E. , Deroanne, C. , and Paquot, M. (2011) Will isomalto‐oligosaccharides, a well‐established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit Rev Food Sci Nutr 51: 394–409. [DOI] [PubMed] [Google Scholar]
- Görl, J. , Timm, M. , and Seibel, J. (2012) Mechanism‐oriented redesign of an isomaltulose synthase to an isomelezitose synthase by site‐directed mutagenesis. ChemBioChem 13: 149–156. [DOI] [PubMed] [Google Scholar]
- Gutiérrez‐Alonso, P. , Gimeno‐Pérez, M. , Ramírez‐Escudero, M. , Plou, F.J. , Sanz‐Aparicio, J. , and Fernández‐Lobato, M. (2016) Molecular characterization and heterologous expression of a Xanthophyllomyces dendrorhous α‐glucosidase with potential for prebiotics production. Appl Microbiol Biotechnol 100: 3125–3135. [DOI] [PubMed] [Google Scholar]
- Herrera, C. M. (2014) Population growth of the floricolous yeast Metschnikowia reukaufii: effects of nectar host, yeast genotype, and host × genotype interaction. FEMS Microbiol Ecol 88: 250–257. [DOI] [PubMed] [Google Scholar]
- Herrera, C.M. , and Pozo, M.I. (2010) Nectar yeast warm the flowers of a winter‐blooming plant. Proc R Soc B Biol Sci 277: 1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara‐Ochiai, M. , Okada, M. , Nakayama, T. , Hemmi, H. , Ueda, T. , Iwashita, T. , et al. (2000) An active‐site mutation causes enhanced reactivity and altered regiospecificity of transglucosylation catalyzed by the Bacillus sp. SAM 1606 α‐glucosidase. J Biosci Bioeng 89: 431–437. [DOI] [PubMed] [Google Scholar]
- Kayikci, Ö. , and Nielsen, J. (2015) Glucose repression in Saccharomyces cerevisiae . FEMS Yeast Res 15: fov068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.M. , Kang, H.K. , Moon, Y.H. , Nguyen, T.T.H. , Day, D.F. , and Kim, D. (2014) Production and bioactivity of glucooligosaccharides and glucosides synthesized using glucansucrases In Food Oligosaccharides: Production, Analysis and Bioactivity. Moreno F.J., and Sanz M.L. (eds). Chicago, IL, USA: Willey Online Library, pp. 168–183. [Google Scholar]
- Lafraya, Á. , Sanz‐Aparicio, J. , Polaina, J. , and Marín‐Navarro, J. (2011) Fructo‐oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl Environ Microbiol 77: 6148–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, F.W. , and Rönninger, S. (1990) α‐D‐Glucopyranosyl‐D‐fructoses: distribution of furanoid and pyranoid tautomers in water, dimethyl sulphoxide, and pyridine. Studies on ketoses. Part 4. J Chem Soc Perkin Trans 2: 1489–1497. [Google Scholar]
- Linde, D. , Rodríguez‐Colinas, B. , Estévez, M. , Poveda, A. , Plou, F.J. , and Fernández Lobato, M. (2012) Analysis of neofructooligosaccharides production mediated by the extracellular β‐fructofuranosidase from Xanthophyllomyces dendrorhous . Bioresour Technol 109: 123–130. [DOI] [PubMed] [Google Scholar]
- Lombard, V. , Golaconda Ramulu, H. , Drula, E. , Coutinho, P.M. , and Henrissat, B. (2014) The carbohydrate‐active enzymes database (CAZy) in 2013. Nucleic Acids Res 42: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsan, P.F. , and Ouarne, F. (2009) Oligosaccharides derived from sucrose In Prebiotics and Probiotics Science and Technology. Charalampopoulos D., and Rastall R.A. (eds). New York, NY, USA: Springer, pp. 293–336. [Google Scholar]
- Munir, M. , and Vogel, M. (1999) Method for process for producing isomelezitose and isomelezitose‐containing sweeteners. German Patent DE19747642B4.
- Nimpiboon, P. , Nakapong, S. , Pichyangkura, R. , Ito, K. , and Pongsawasdi, P. (2011) Synthesis of a novel prebiotic trisaccharide by a type I α‐glucosidase from B. licheniformis strain TH4‐2. Process Biochem 46: 448–457. [Google Scholar]
- Okada, M. , Nakayama, T. , Noguchi, A. , Yano, M. , Hemmi, H. , Nishino, T. , and Ueda, T. (2002) Site‐specific mutagenesis at positions 272 and 273 of the Bacillus sp. SAM1606 α‐glucosidase to screen mutants with altered specificity for oligosaccharide production by transglucosylation. J Mol Catal ‐ B Enzym 16: 265–274. [Google Scholar]
- Patel, S. , and Goyal, A. (2012) The current trends and future perspectives of prebiotics research: a review. 3 Biotech 2: 115–125. [Google Scholar]
- Pham, H.T.T. , Dijkhuizen, L. , and van Leeuwen, S.S. (2017) Structural characterization of glucosylated GOS derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydr Res 470: 57–63. [DOI] [PubMed] [Google Scholar]
- Piedrabuena, D. , Míguez, N. , Poveda, A. , Plou, F.J. , and Fernández‐Lobato, M. (2016) Exploring the transferase activity of Ffase from Schwanniomyces occidentalis, a β‐fructofuranosidase showing high fructosyl‐acceptor promiscuity. Appl Microbiol Biotechnol 100: 8769–8778. [DOI] [PubMed] [Google Scholar]
- Plou, F.J. , de Segura, A.G. , and Ballesteros, A. (2007) Application of glycosidases and transglycosidases in the synthesis of oligosaccharides In Industrial Enzymes: Structure, Function and Applications. Polaina J., and MacCabe A. P. (eds). Dordrecht: Springer, pp. 141–157. [Google Scholar]
- Pozo, M.I. , Herrera, C.M. , and Bazaga, P. (2011) Species richness of yeast communities in floral nectar of southern Spanish plants. Microb Ecol 61: 82–91. [DOI] [PubMed] [Google Scholar]
- Remaud‐Simeon, M. , Willemot, R.M. , Sarçabal, P. , Potocki De Montalk, G. , and Monsan, P. (2000) Glucansucrases: Molecular engineering and oligosaccharide synthesis. J Mol Catal‐B: Enzym 10: 117–128. [Google Scholar]
- Ruiz‐Aceituno, L. , Sanz, M.L. , De Las Rivas, B. , Muñoz, R. , Kolida, S. , Jimeno, M.L. , and Moreno, F.J. (2017) Enzymatic synthesis and structural characterization of theanderose through transfructosylation reaction catalyzed by levansucrase from Bacillus subtilis CECT 39. J Agric Food Chem 65: 10505–10513. [DOI] [PubMed] [Google Scholar]
- Shi, Q. , Juvonen, M. , Hou, Y. , Kajala, I. , Nyyssölä, A. , Maina, N.H. , et al. (2016) Lactose‐ and cellobiose‐derived branched trisaccharides and a sucrose‐containing trisaccharide produced by acceptor reactions of Weissella confusa dextransucrase. Food Chem 190: 226–236. [DOI] [PubMed] [Google Scholar]
- Singh, S.P. , Jadaun, J.S. , Narnoliya, L.K. , and Pandey, A. (2017) Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl Biochem Biotechnol 183: 613–635. [DOI] [PubMed] [Google Scholar]
- Singla, V. , and Chakkaravarthi, S. (2017) Applications of prebiotics in food industry: a review. Food Sci Technol Int 23: 649–667. [DOI] [PubMed] [Google Scholar]
- Smaali, I. , Jazzar, S. , Soussi, A. , Muzard, M. , Aubry, N. , and Nejib Marzouki, M. (2012) Enzymatic synthesis of fructooligosaccharides from date by‐products using an immobilized crude enzyme preparation of β‐D‐fructofuranosidase from Aspergillus awamori NBRC 4033. Biotechnol Bioprocess Eng 17: 385–392. [Google Scholar]
- Sorndech, W. , Nakorn, K.N. , Tongta, S. , and Blennow, A. (2018) Isomalto‐oligosaccharides: recent insights in production technology and their use for food and medical applications. LWT 95: 135–142. [Google Scholar]
- Trumbly, R. J. (1992) Glucose repression in the yeast Saccharomyces cerevisiae . Mol Microbiol 6: 15–21. [DOI] [PubMed] [Google Scholar]
- Wei, Y.A. , Hendrix, D.L. , and Nieman, R. (1997) Diglucomelezitose, a novel pentasaccharide in silverleaf whitefly honeydew. J Agric Food Chem 45: 3481–3486. [Google Scholar]
- Wei, Y. , Liang, J. , Huang, Y. , Lei, P. , Du, L. , and Huang, R. (2013) Simple, fast, and efficient process for producing and purifying trehalulose. Food Chem 138: 1183–1188. [DOI] [PubMed] [Google Scholar]
- Yang, M. , Deng, G.C. , Gong, Y.B. , and Huang, S.Q. (2019) Nectar yeast enhance the interaction between Clematis akebioides and its bumblebee pollinator. Plant Biol 21: 732–737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. MS‐ESI Spectrum of purified peak 5.
Fig. S2. MS‐ESI Spectrum of purified peak 6.
Fig. S3. MS‐ESI Spectrum of purified peak 8.
Fig. S4. NMR Spectra (600 Hz, D2O, 300 K) for the compounds separated as peak 6. 1H‐13C HSQC‐edited spectra with signal assignation for erlose (A) and melezitose (B).
Fig. S5. NMR Spectra (600 Hz, D2O, 300 K) for the minor compound of purified peak 7 (theanderose). Signal corresponding to isomelezitose are depicted with a red star (*).
Table S1. NMR data deduced for the minor component of the purified peak 6 (esculose) and those previously described for α‐G2‐(1→2)‐α‐G1 fragment.


