Summary
Recent major advances in metagenomics and metabolomics technologies have enabled us to collect more data on the gut microbiome and metabolome to evaluate its influence on host health. In this short opinion article, we have chosen to focus on summarizing the protective mechanisms of bifidobacteria, a highly regarded probiotic, and it's metabolite: acetate; against enteropathogens, specifically in the E. coli O157:H7 mice model. We advocate for using a novel approach metabologenomics, which is an integration of metagenomic and metabolomic information on a systems biology‐wide approach to better understand this interplay between gut microbiome and host metabolism.
Gut microbiota and its metabolites
There are 40 trillions of bacteria residing in the human gut. The gut microbiota greatly influences human physiology, immunology and nutrition (Bäckhed et al., 2005; Kau et al., 2011). Imbalances in the gut environment, also known as dysbiosis, are related to various disorders such as metabolic disorders, inflammatory bowel disease and colon cancer (Frank et al., 2007; Turnbaugh et al., 2008; Wen et al., 2008; Wu et al., 2009). Therefore, in order to maintain good health, controlling gut environment balance may be effective to maintain a good health status in humans. Recent studies have also found that the gut microbiota produces a large variety of metabolites such as short‐chain fatty acids (SCFAs) (Flint et al., 2012; den Besten et al., 2013), trimethylamine (Wang et al., 2011), indole metabolites (Rothhammer et al., 2016; Alexeev et al., 2018), vitamins (LeBlanc et al., 2017), polyamines (Kibe et al., 2014) and secondary bile acids (Wahlström et al., 2016). These molecules play important roles to maintain good health and suppress onset of various diseases in the host. In order to obtain novel knowledge by combining the metabolome and microbiome analysis of the intestinal environment, a multi‐omics approach could be a valuable tool for understanding the entire intestinal ecosystem, including the relationships among microbiota, metabolites and host. Additionally, it is considered that a multi‐omics approach integrating metagenomics and metabolomics, called metabologenomics, can provide new information about the mechanisms underlying the key roles the microbiota plays in the varying conditions of the intestinal environment (Ishii et al., 2018).
Probiotics are live microorganisms that when consumed, exert beneficial effects on their hosts by either restoring imbalanced microbiota or maintaining the healthy microbiota population in the gut. Bifidobacteria and lactobacilli are the most common probiotics, and their biological and bacteriological properties have been studied extensively in order to characterize their beneficial functions (Ventura et al., 2008; Kleerebezem and Vaughan, 2009; Lebeer et al., 2010).
General characteristics of bifidobacteria
In this short opinion, we would be placing our focus on bifidobacteria. Bifidobacteria are anaerobic and belong to the phylum Actinobacteria and the genus Bifidobacterium. They are Gram‐positive and are shaped like a letter Y. Representatives of this genus are present in a wide range of animal hosts and environmental sources but they are also reported to be present in fermented milk products and sewage (Milani et al., 2017). Human‐associated bifidobacteria are among the first colonizers and most abundant bacteria in the gut of infants who have been vaginally delivered and breastfed. Following weaning, the relative abundance decreases whilst their abundance further declines in elderly subjects (Tannock et al., 2016; Duranti et al., 2017). Bifidobacteria are highly regarded probiotics as their presence correlates with various health‐promoting activities and their absence has been reported to be related to health issues, such as obesity and undernutrition, especially with regard to infant gut microbiota (Arboleya et al., 2016). Bifidobacteria constitute part of the colonic gut community, and they contribute significantly to host metabolism via saccharolytic fermentation of glycans that are abundant in the proximal section of the colon (Rivière et al., 2016). The health‐promoting effects in the gut are attributed to the production of many metabolites such as vitamins, antioxidants, polyphenols, conjugated linoleic acids and SCFAs which have a positive impact on epithelial host cells as well as on the gut community (Hamer et al., 2012).
Bifidobacteria and its protective mechanisms in the E. coli O157:H7 murine infection model
Enterohaemorrhagic Escherichia coli (EHEC) has been reported to be a cause for many illnesses ranging from mild diarrhoea to more severe diseases like haemolytic uraemic syndrome or haemorrhagic colitis (Tarr et al., 2005). EHEC O157:H7 produces Shiga toxin and is the major EHEC serotype involved in public health problems worldwide. Previous studies have shown previously that Shiga toxins 1 and 2 produced by E. coli O157 are pivotal factors in deadly infections (Eaton et al., 2008) and that if mice are pretreated with certain probiotics, including bifidobacteria, they survive with ideal outcomes. However, there is much to be understood about the protective mechanisms of bifidobacteria. In this report, we will review several articles on the protective mechanisms of bifidobacteria in the E. coli O157 murine infection model (Asahara et al., 2004; Gagnon et al., 2006; Yoshimura et al., 2009; Fukuda et al., 2011, 2012).
In a study by Yoshimura et al., the efficacy of bifidobacteria protection against E. coli O157:H7 infections was investigated using E. coli O157:H7‐infected gnotobiotic mice associated with Bifidobacterium strains (6 species, 9 strains). Survival rate in mice pre‐fed each Bifidobacterium strain for a week was recorded after they were orally infected with E. coli O157:H7. Mice gavaged with Bifidobacterium longum subsp. infantis 157F‐4‐1 and B. longum subsp. longum NCC2705 survived. On the other hand, mice associated with other Bifidobacterium strains, including type strains of B. longum subsp. infantis and B. longum subsp. longum, unfortunately perished. There were no significant differences in the numbers of E. coli O157:H7 in the faeces among the Bifidobacterium‐associated groups. However, in the gnotobiotic mice associated with B. infantis 157F‐4‐1 and B. longum NCC2705, they had significantly lower Shiga toxin concentrations in the caecal contents and sera than those of the other groups were observed. This experimental data the protection against the lethal infections of E. coli O157:H7 by B. longum subsp. longum/infantis could be due to prevention of caecal Shiga toxin production as well as Shiga toxin transfer from the intestinal lumen to the bloodstream (Yoshimura et al., 2009).
In another study using BALB/c mice, the efficacy of Bifidobacterium thermacidophilum RBL 71 as a probiotic against enterohaemorrhagic E. coli O157:H7 infection was studied. The mice were fed the probiotic for 7 days before or after a single challenge with E. coli O157:H7. In B. thermacidophilum‐treated mice, improvements in marked body weight loss and intestinal histopathological changes were observed as compared to the infected group. Pre‐feeding B. thermacidophilum RBL 71 for a week before infection resulted in larger food intake and increase in body weight, lesser extent of intestinal injuries, larger reaction in the lymphoid component of the ileal mucosa and lower faecal levels post E. coli O157:H7 challenge as compared to the infected controls. After infection, the concentrations of anti‐E. coli O157:H7‐specific faecal IgA and sera IgG + IgM were also increased in mice fed bifidobacteria. These results demonstrate that feeding the probiotic can alleviate the severity of E. coli O157:H7 infection (Gagnon et al., 2006).
In another report using the Shiga toxin‐producing E. coli O157:H7 mice model, Asahara et al. investigated the antiinfectious activity of probiotic bifidobacteria. Bifidobacterium breve strain was colonized in intestines of mice administered drinking water containing 5 mg ml−1 streptomycin. The infected controls had marked body weight loss and subsequently died, but this was significantly inhibited in the B. breve‐colonized group. Moreover, Shiga toxin production by intestinal cells was almost undetectable in the B. breve‐colonized group. Several Bifidobacterium strains that were naturally resistant towards streptomycin were compared for anti‐Shiga toxin‐producing activity. In the potent strains such as B. breve strain Yakult and Bifidobacterium pseudocatenulatum DSM 20439, higher concentration of acetic acid of approximately 56 mM and lower intestinal pH were observed as compared to the infected control group (acetic acid concentration, 28 mM; pH, 7.15). Both high concentrations of acetic acid and a low pH due to these bifidobacterial strains could impede Shiga toxin production in vitro and these characteristics contributed to the protective effect against the lethal infection (Asahara et al., 2004).
In our previous studies, we also reported that acetate produced by certain bifidobacterial strains in mice associated with bifidobacteria was protected against enterohaemorrhagic E. coli O157:H7 using a multi‐omics approach. Certain bifidobacterial possess an ATP‐binding‐cassette‐type carbohydrate transporter, thereby increasing the production of acetate and inhibited translocation of E. coli O157:H7 produced Shiga toxin from the gut lumen to the blood. The protective bifidobacteria produced acetate, thereby upregulating intestinal defence mediated by epithelial cells and protected the host against lethal infection (Fukuda et al., 2011, 2012).
Conclusions
Lately, there has been overwhelming developments in identifying bifidobacterial structures that play important roles in host colonization and exert health‐promoting effects on the host. In this short opinion article, we have chosen to focus on summarizing the protective effects of bifidobacteria and its metabolite: acetate; against enteropathogens, specifically in the E. coli O157:H7 mice model (Fig. 1). There is much more to be elucidated, and more contributions to the scientific field could be made possible by integrating multi‐omics evaluations using various disease murine models or clinical subjects.
Figure 1.
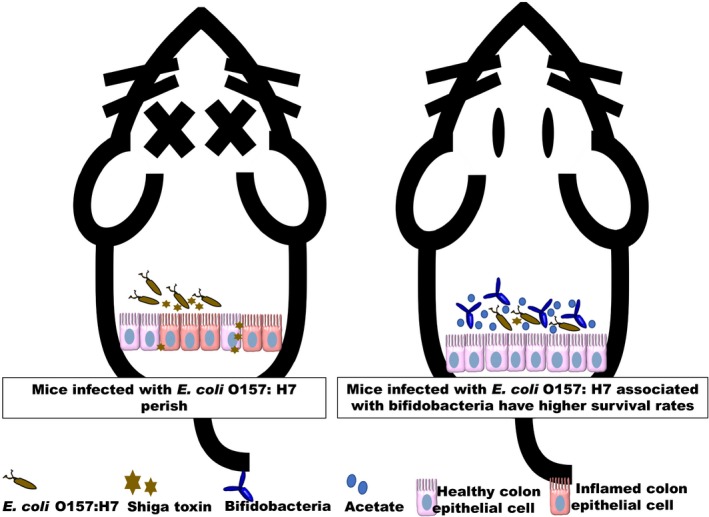
Mice infected with E. coli O157: H7 associated with bifidobacteria have higher survival rates as some bifidobacterial strains possess an ATP‐binding‐cassette‐type carbohydrate transporter, thereby increasing the production of acetate and inhibited translocation of E. coli O157:H7 produced Shiga toxin from the gut lumen to the blood.
Conflicts of interest
The authors declare no conflict of interest.
Microb Biotechnol (2019) 12(6), 1097–1100
Funding Information
No funding information provided.
References
- Alexeev, E.E. , Lanis, J.M. , Kao, D.J. , Campbell, E.L. , Kelly, C.J. , Battista, K.D. , et al (2018) Microbiota‐derived indole metabolites promote human and murine intestinal homeostasis through regulation of Interleukin‐10 Receptor. Am J Pathol 188: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleya, S. , Watkins, C. , Stanton, C. , and Ross, R.P. (2016) Gut bifidobacteria populations in human health and aging. Front Microbiol 7: 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara, T. , Shimizu, K. , Nomoto, K. , Hamabata, T. , Ozawa, A. , and Takeda, Y. (2004) Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin‐producing Escherichia coli O157:H7. Infect Immun 72: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed, F. , Ley, R.E. , Sonnenburg, J.L. , Peterson, D.A. , and Gordon, J.I. (2005) Host‐bacterial mutualism in the human intestine. Science 307: 1915–1920. [DOI] [PubMed] [Google Scholar]
- den Besten, G. , van Eunen, K. , Groen, A.K. , Venema, K. , Reijngoud, D.‐J. , and Bakker, B.M. (2013) The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti, S. , Lugli, G.A. , Mancabelli, L. , Armanini, F. , Turroni, F. , James, K. , et al (2017) Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 5: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, K.A. , Friedman, D.I. , Francis, G.J. , Tyler, J.S. , Young, V.B. , Haeger, J. , et al (2008) Pathogenesis of renal disease due to Enterohemorrhagic Escherichia coli in germ‐free mice. Infect Immun 76: 3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, H.J. , Scott, K.P. , Louis, P. , and Duncan, S.H. (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9: 577. [DOI] [PubMed] [Google Scholar]
- Frank, D.N. , St Amand, A.L. , Feldman, R.A. , Boedeker, E.C. , Harpaz, N. , and Pace, N.R. (2007) Molecular‐phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, S. , Toh, H. , Hase, K. , Oshima, K. , Nakanishi, Y. , Yoshimura, K. , et al (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543. [DOI] [PubMed] [Google Scholar]
- Fukuda, S. , Toh, H. , Taylor, T.D. , Ohno, H. , and Hattori, M. (2012) Acetate‐producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 3: 449–454. [DOI] [PubMed] [Google Scholar]
- Gagnon, M. , Kheadr, E.E. , Dabour, N. , Richard, D. , and Fliss, I. (2006) Effect of Bifidobacterium thermacidophilum probiotic feeding on enterohemorrhagic Escherichia coli O157:H7 infection in BALB/c mice. Int J Food Microbiol 111: 26–33. [DOI] [PubMed] [Google Scholar]
- Hamer, H.M. , De Preter, V. , Windey, K. , and Verbeke, K. (2012) Functional analysis of colonic bacterial metabolism: relevant to health?. Am J Physiol Gastrointest Liver Physiol 302: G1–G9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, C. , Nakanishi, Y. , Murakami, S. , Nozu, R. , Ueno, M. , Hioki, K. , et al (2018) A metabologenomic approach reveals changes in the intestinal environment of mice fed on American diet. Int J Mol Sci 19: 4079 10.3390/ijms19124079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau, A.L. , Ahern, P.P. , Griffin, N.W. , Goodman, A.L. , and Gordon, J.I. (2011) Human nutrition, the gut microbiome and the immune system. Nature 474: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibe, R. , Kurihara, S. , Sakai, Y. , Suzuki, H. , Ooga, T. , Sawaki, E. , et al (2014) Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep 4: 4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem, M. , and Vaughan, E.E. (2009) Probiotic and gut Lactobacilli and Bifidobacteria: molecular approaches to study diversity and activity. Annu Rev Microbiol 63: 269–290. [DOI] [PubMed] [Google Scholar]
- Lebeer, S. , Vanderleyden, J. , and De Keersmaecker, S.C.J. (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8: 171. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J.G. , Chain, F. , Martín, R. , Bermúdez‐Humarán, L.G. , Courau, S. , and Langella, P. (2017) Beneficial effects on host energy metabolism of short‐chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 16: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani, C. , Mangifesta, M. , Mancabelli, L. , Lugli, G.A. , James, K. , Duranti, S. , et al (2017) Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life. The ISME journal 11: 2834–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière, A. , Selak, M. , Lantin, D. , Leroy, F. , and De Vuyst, L. (2016) Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer, V. , Mascanfroni, I.D. , Bunse, L. , Takenaka, M.C. , Kenison, J.E. , Mayo, L. , et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22: 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock, G.W. , Lee, P.S. , Wong, K.H. , and Lawley, B. (2016) Why don't all infants have bifidobacteria in their stool? Front Microbiol 7: 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr, P.I. , Gordon, C.A. , and Chandler, W.L. (2005) Shiga‐toxin‐producing Escherichia coli and haemolytic uraemic syndrome. The Lancet 365: 1073–1086. [DOI] [PubMed] [Google Scholar]
- Turnbaugh, P.J. , Hamady, M. , Yatsunenko, T. , Cantarel, B.L. , Duncan, A. , Ley, R.E. , et al (2008) A core gut microbiome in obese and lean twins. Nature 457: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura, M. , O'Flaherty, S. , Claesson, M.J. , Turroni, F. , Klaenhammer, T.R. , van Sinderen, D. , and O'Toole, P.W. (2008) Genome‐scale analyses of health‐promoting bacteria: probiogenomics. Nat Rev Microbiol 7: 61. [DOI] [PubMed] [Google Scholar]
- Wahlström, A. , Sayin, Sama.I. , Marschall, H.‐U. , and Bäckhed, F. (2016) Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24: 41–50. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Klipfell, E. , Bennett, B.J. , Koeth, R. , Levison, B.S. , Dugar, B. , et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, L. , Ley, R.E. , Volchkov, P.Y. , Stranges, P.B. , Avanesyan, L. , Stonebraker, A.C. , et al (2008) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Rhee, K.J. , Albesiano, E. , Rabizadeh, S. , Wu, X. , Yen, H.R. , et al (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15: 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, K. , Matsui, T. , and Itoh, K. (2009) Prevention of Escherichia coli O157:H7 infection in gnotobiotic mice associated with Bifidobacterium strains. Antonie Van Leeuwenhoek 97: 107. [DOI] [PubMed] [Google Scholar]


