Abstract
Objective
To study if treatment with triheptanoin, a 7‐carbon triglyceride, improves exercise tolerance in patients with McArdle disease. McArdle patients have a complete block in glycogenolysis and glycogen‐dependent expansion of tricarboxylic acid cycle (TCA), which may restrict fat oxidation. We hypothesized that triheptanoin metabolism generates substrates for the TCA, which potentially boosts fat oxidation and improves exercise tolerance in McArdle disease.
Methods
Double‐blind, placebo‐controlled, crossover study in patients with McArdle disease completing two treatment periods of 14 days each with a triheptanoin or placebo diet (1 g/kg/day). Primary outcome was change in mean heart rate during 20 min submaximal exercise on a cycle ergometer. Secondary outcomes were change in peak workload and oxygen uptake along with changes in blood metabolites and respiratory quotients.
Results
Nineteen of 22 patients completed the trial. Malate levels rose on triheptanoin treatment versus placebo (8.0 ± SD2.3 vs. 5.5 ± SD1.8 µmol/L, P < 0.001), but dropped from rest to exercise (P < 0.001). There was no difference in exercise heart rates between triheptanoin (120 ± SD16 bpm) and placebo (121 ± SD16 bpm) treatments. Compared with placebo, triheptanoin did not change the submaximal respiratory quotient (0.82 ± SD0.05 vs. 0.84 ± SD0.03), peak workload (105 ± SD38 vs. 102 ± SD31 Watts), or peak oxygen uptake (1938 ± SD499 vs. 1977 ± SD380 mL/min).
Interpretation
Despite increased resting plasma malate with triheptanoin, the increase was insufficient to generate a normal TCA turnover during exercise and the treatment has no effect on exercise capacity or oxidative metabolism in patients with McArdle disease.
Introduction
Patients with McArdle disease have an inherited defect of myophosphorylase. Usually, mutations in the responsible gene, PYGM, result in no functional enzyme, causing a complete block in the glycogenolysis in skeletal muscle. This manifests as exercise intolerance, premature muscle fatigue with exercise and a risk of developing muscle pain, contractures and rhabdomyolysis with continued effort.1 During sustained exercise, patients with McArdle disease partially compensate for the lack of energy from the blocked muscle glycogenolysis by increasing the rate of fatty acid oxidation and by increased uptake of extra‐muscular glucose produced by the liver.1 However, these fuels cannot fully cover the energy deficit during exercise in McArdle patients. The inability to further increase fat oxidation in McArdle disease is likely due to blocked production of pyruvate from glycogenolysis.1, 2 In addition to its crucial role in generating acetyl‐CoA, pyruvate can be converted to oxaloacetate, which is an intermediate of the tricarboxylic acid (TCA) cycle that combines with acetyl‐CoA to form citrate. Reduced oxaloacetate may limit acetyl‐CoA metabolism, impair the normal exercise expansion of TCA intermediates, and slow the turnover of the TCA cycle and the aerobic production of ATP, and in this way may also restrict fat oxidation (Fig. 1).3, 4
Figure 1.
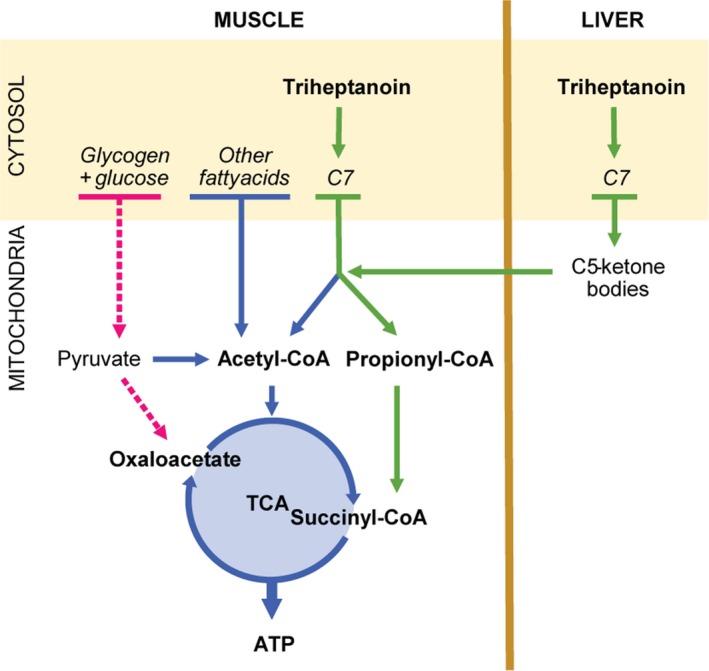
The suggested principles of treatment with triheptanoin in McArdle disease. The pyruvate pool is depleted in patients with McArdle disease due to the block in glycogenolysis. This may reduce levels of oxaloacetate in the tricarboxylic acid cycle (TCA), which slows the turnover rate of the TCA and limits the entry of acetyl‐CoA to the TCA. Almost all naturally occurring fatty acids have an even number of carbons. They are metabolized through beta‐oxidation cleaving off two carbons at a time producing acetyl‐CoA. Triheptanoin is a triglyceride of three 7‐carbon fatty acids (C7). The final step in the metabolism of odd number carbon fatty acids, such as C7, produces both acetyl‐CoA and propionyl‐CoA, which are converted to succinyl‐CoA, an intermediate of the TCA. The supplied succinyl‐CoA can potentially replenish the pool of TCA intermediates, boost the turnover of the TCA and enhance oxidative phosphorylation through increased metabolism of fatty acids and other substrates passing through the cycle. The liver can convert C7 to 5‐carbon (C5) ketone bodies, which are readily exported from the liver to the muscle. The breakdown of C5‐ketone bodies in the muscle produces acetyl‐CoA and propionyl‐CoA and contributes to the delivery of intermediates to the TCA.
Triheptanoin oil has been proposed as a treatment for a range of inherited metabolic diseases as the metabolism of the oil generates both acetyl‐CoA and intermediates of the TCA cycle. Case series and uncontrolled studies report improvements in a variety of symptoms in patients with inborn errors of fat metabolism.5, 6, 7, 8, 9, 10, 11, 12
Triheptanoin consists of glycerol and three 7‐carbon fatty acids (heptanoate). In the mitochondria, fatty acids undergo beta‐oxidation, which shortens the fatty acids two carbons at a time producing acetyl‐CoA. Due to the odd number of carbons in heptanoate, the final step in beta‐oxidation also produces propionyl‐CoA, which is converted to succinyl‐CoA, an intermediate of the TCA cycle.12 Increasing levels of intermediates of the TCA cycle is called anaplerosis, and triheptanoin therefore acts as an anaplerotic substrate.5, 13 A smaller proportion of ingested triheptanoin is converted to 5‐carbon ketone bodies in the liver, which is secreted and metabolized in muscle and other tissues, which also produces succinyl‐CoA (Fig. 1).13, 14, 15
In the present study, we hypothesized that treatment with triheptanoin could enhance fatty acid metabolism by boosting the TCA cycle intermediate levels and turnover rate in patients with McArdle disease, and thereby augment oxidative metabolism in working muscles to improve exercise performance.
Methods
Design
We conducted a randomized, double‐blind crossover study of the effect of triheptanoin in patients with McArdle disease. Participants completed a treatment period of ≥14 days followed by a ≥7‐day washout period before a second ≥14 days treatment period. Participants were randomized 1:1 to a treatment sequence of either placebo or active treatment in the first treatment period followed by the alternate treatment in the second period. Patients met at the clinic for a screening visit followed by assessment visits before and on the last day of the treatment periods (visits 1–4) (Fig. 2).
Figure 2.
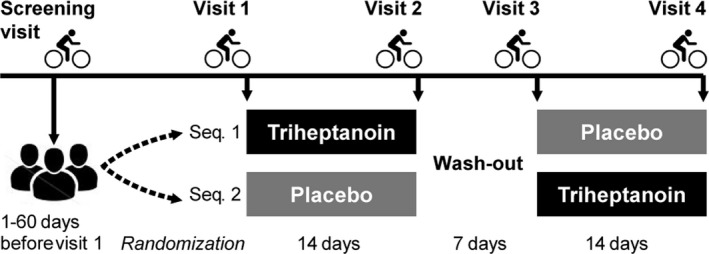
Study design of the randomized double‐blind crossover study comparing triheptanoin to placebo treatment in McArdle disease. Participants completed two treatment periods of 14 days with triheptanoin and placebo in a random sequence (Seq. 1 or Seq. 2) separated by a ≥ 1‐week washout period, and assessments with cycle ergometry exercise were performed on a screening visit and at 4 study visits.
Participants
Inclusion required a diagnosis of McArdle disease verified by two pathogenic mutations in the PYGM gene. Further inclusion criteria were age between 18 and 75 years and a Body Mass Index (BMI) of 18–32. Women of fertile age had to be on contraceptives. Primary exclusion criteria were significant cardiac or pulmonary disease, dysregulated diabetes, pregnancy, or breastfeeding.
The study was conducted at the exercise laboratories and clinics at the Copenhagen Neuromuscular Center, Rigshospitalet, Copenhagen, Denmark, and at the Institut de Myologie and Department of Cardiology at Hôpital Pitié‐Salpêtrière, Paris, France. Participants were patients at the two clinics along with a group of patients from the Centre for Neuromuscular Diseases at the National Hospital for Neurology and Neurosurgery, London, UK, who were referred to the Copenhagen site for trial participation. All participants were recruited at their regular visits to these clinics.
Study treatment
Triheptanoin is a tasteless and odorless oil and placebo treatment was safflower oil with the same characteristics. Both treatments were delivered by Ultragenyx Pharmaceutical Inc. (Novato, CA) and came in 1L round, amber‐colored glass bottles (USP, Ph. Eur. Grade). Participants took an increasing dose of 0.3, 0.5 and 0.7 g × kg−1 × day−1 for the first 7 days of the treatment period followed by 7 days on full dose (1 g × kg‐1 × day−1). The full dose treatment aimed at covering 30–35% of the daily calorie intake, replacing ingestion of other types of fat. The dose was divided and taken with 3–4 meals daily while the participants followed an isocaloric diet by restricting the intake of fat and sugar. The participants received thorough dietary instructions and planned the dose distribution with investigators at the two baseline visits. Participants received the bottles with treatment along with a dietary guideline, dosing tubes, and a treatment diary to register deviations or comments.
Outcome measures
The primary outcome measure was the heart rate during submaximal exercise. Secondary outcome measures were peak workload (W peak), peak oxygen uptake (VO2peak), rate of perceived exertion, respiratory quotient during submaximal exercise assessed with cycle ergometry exercise tests, self‐rated severity of fatigue symptoms on a Fatigue Severity Scale (FSS), urine concentrations of organic acids, and blood concentrations of metabolites. Plasma malate was analyzed as a measure of changes in TCA intermediate availability as this is the immediate precursor for oxaloacetate and as it is exclusively produced from the preceding steps of the TCA cycle.
Screening maximal exercise test
At screening, participants performed a maximal exercise test on an upright cycle ergometer (Copenhagen: Excalibur Sport 925900, Lode BV, Groningen, Netherlands: Paris: ERG 900s, GE Medical Systems, Freiburg, Germany) to approximate the exercise capacity of the participant and to identify a workload to be used for the exercise test on study visits 1–4. Breath‐by‐breath exchange rate of O2 (VO2) and CO2 (VO2) were measured with a metabolic cart (Copenhagen: CPET, Cosmed, Rome, Italy and Paris: Vyntus CPX, CareFusion, Hoechberg, Germany).
Study visits 1–4
Submaximal exercise test with ramp
After an overnight fast, participants met at the laboratory in the morning. They delivered a urine sample for the measurement of urine organic acids (visits 2 and 4) and received a standardized breakfast, described below. A peripheral venous catheter was inserted in an antecubital vein for sampling of blood. Participants then performed an exercise test of 20 min submaximal exercise at an intensity that matched 65% of the participants’ VO2peak, followed by an increasing load until maximal exercise. Participants rated their exertion every 2 min on a Borg scale.16 Blood was drawn at rest, every 10 min during exercise, and at exhaustion.
Standardized breakfast
A maximum of 1 h before the exercise test, participants were served a meal of low fat, sugar‐free yogurt (Cheasy® 1%, Arla Foods amba, Viby, Denmark or Activia Nature 0%, Danone, Paris France <6 g/100 g carbohydrates, <1 g/100 g fat) along with a small amount of oat granola (Øko Müesli, Kornkammeret, Lantmännen Cerealia A/S, Vejle, Denmark or Creamy Superfast Oats, Mornflake, Cheshire, England (≤62 g/100 g carbohydrates, incl. ≤13 g/100 g sugars, and <6 g/100 g fats). Each participant took their own amount of yogurt on the first visit and was served the same amount on all following visits. On visits 2 and 4, a portion of 0.25× the daily dose of study oil was added and mixed with the yogurt.
Blood and urine samples
Blood drawn on heparinized syringes was immediately analyzed for glucose and lactate on a blood gas analyzer (ABL90 Flex, Radiometer, Copenhagen, Denmark). Samples collected on tubes coated with lithium heparin were immediately spun at 3000 rpm for 10 min. Plasma was transferred to Eppendorf tubes and stored at −80°C until analysis.
Plasma‐free fatty acids were analyzed with spectrophotometry (NEFA‐HR(2)‐kit, Fujifilm, Wako Chemicals GmbH, Neuss Germany and a Multiskan GO with SkanIt™ Software; ThermoFisher Scientific Inc., Waltham, MA).
Ammonia and creatine kinase were analyzed at the central hospital laboratories at Rigshospitalet (on a Cobas® 8000, Roche, Basel, Switzerland) at both sites. Malate and C5‐ketones were measured at rest on test days 2 and 4 by gas chromatography–mass spectrometry (Scion TQ, Bruker Daltonics, Billerica, MA, USA) in selected ion monitoring mode following organic extraction and derivatization with N,O‐Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane (BSTFA + TMCS, T6381, Sigma‐Aldrich, St. Louis, MO) according to standard procedures.17 Specific internal standard for quantification included 13C4‐malic acid and 3‐ketopentanoic‐3,4,5‐13C3 acid sodium salt (Eurisotop, Saint‐Aubin, France and Cambridge Isotope Labs. Inc. Tewksbury). Non‐labeled standards for external calibration were used for 3‐hydroxypentanoic acid and 3‐ketopentanoic acid (Epsilon Chimie, Brest, France) and for malic acid (TraCERT 09172, Sigma‐Aldrich). Acylcarnitines were measured by direct injection and mass spectrometry (Xevo‐TQD, Waters, Milford, MA) according to routine laboratory method.18
Statistics
We calculated a sample size of 14 participants to detect a minimal relevant change in the primary outcome measure, heart rate, during submaximal exercise of ≥±5 bpm, and the secondary outcome measure VO2peak of ≥±3.0 mL × kg‐1 × min−1 between active and placebo treatments. This would give 80% power and two‐sided 95% confidence intervals around assumed mean values for each parameter. We assumed a standard deviation (SD) of ±5 bpm for the submaximal heart rate and ±4.0 mL × kg−1 × min−1 for the VO2peak based on previous studies in the same population.19, 20 Because of the risk of dropouts or errors in measurement, we included 21 participants. We considered a P‐value of 0.05 to be significant with two‐tailed testing. Mean values are presented ±SD. We controlled for a period effect using the Grizzle method comparing the active‐placebo group to the placebo‐active group with the nonparametric Mann–Whitney U test due to the small number of participants in the two groups. As we did not find a period effect for any of the outcome measures, the comparison of active to the placebo treatment was evaluated on the pooled data from the two groups using a paired t‐test two‐tailed testing provided that a normality test showed a normal distribution of data. Statistical analyses were performed using SPSS Statistics software for Apple Macintosh version 25.0.0. Differences between active and placebo treatment were considered the primary measure of efficacy and differences between active treatment and baseline were considered secondarily.
Blinding and randomization
An unblinded pharmacist at the Copenhagen Neuromuscular Center created a kit assignment list with 600 unique, random 4‐digit numbers using www.random.org. An unblinded representative at Ultragenyx Pharmaceutical Inc. assigned each number to a treatment bottle. Ultragenyx Pharmaceutical Inc. handled the labeling and shipped a bulk of treatment to each study site upon request. Upon inclusion of a new participant, the local unblinded pharmacist assigned treatment to the patient, informing the local investigator which bottles to hand out in the two treatment periods and informed the unblinded pharmacists at Ultragenyx Pharmaceutical Inc. and the other site of the bottle numbers used. The unblinded pharmacist at Copenhagen Neuromuscular Center drew lots from an envelope with treatment sequences 1:1 in blocks of four.
After the last participant had completed the last visit, the unblinded pharmacist shared the treatment assignment list with the investigators and the investigators informed the participants of the individual treatment sequences. Analyses of urine organic acids, plasma FFA, malate, C3‐acylcarnitines and C5‐ketones were performed when all patients had completed all study visits.
Approvals and registration
The local authorities at each study site approved the study. In Denmark, the scientific ethics committee of the Capital Region of Denmark (H‐8‐2014‐006) and the Danish Health and Medicines Agency approved the study (EudraCT number 2014‐003644‐12) and the study was monitored by the GCP unit at Copenhagen University (Frederiksberg, Denmark). In France, the study was approved by CPP 123‐15 (EudraCT: 2015‐005174‐38). The study plan was published on www.clinicaltrials.gov with identifier NCT02432768 (4 May, 2014) before the inclusion of participants. All participants gave written consent prior to any study procedures.
Data availability
The data of this study are available on request from the corresponding author, KM. The data are not publicly available as they contain information that could compromise the privacy of research participants. The corresponding author had full access to the study data and had the final responsibility for the decision to submit for publication.
Results
Participants and dropouts
Sixteen participants were included in Copenhagen, and six were included in Paris between May 2015 (Table 1) and April 2018, when the last patient had completed all study visits. One patient withdrew after the first treatment period. She failed to perform visit 2 assessments due to nausea and discomfort from the treatment (triheptanoin) (Fig. 3).
Table 1.
Demographics and baseline data from 22 patients with McArdle disease.
| Study site/residence | Mutation | Sex | Age | Age at diagnosis | BMI | W peak | W submax | VO2submax/VO2peak | Physical activity | |
|---|---|---|---|---|---|---|---|---|---|---|
| M:F | Years | Years | kg/m2 | Watt | Watt | % | Hours/week | |||
| Sequence 1 | ||||||||||
| DK/DK | c.148C>T/ | c.148C>T | F | 21 | 18 | 23 | 60 | 30 | 57 | 2 |
| DK/DK | c.148C>T/ | c.2262delA | M | 65 | 54 | 24 | 105 | 50 | 62 | 1 |
| DK/GB | c.148C>T/ | c.148C>T | F | 48 | 38 | 25 | 91 | 55 | 79 | 2 |
| DK/DK | c.482G>A/ | c.482G>A | M | 40 | 40 | 24 | 67 | 35 | 70 | 1 |
| *1FR/FR | c.148C>T/ | c.148C>T | M | 43 | 27 | 31 | 120 | 40 | 53 | 3 |
| *2FR/FR | c.148C>T/ | c.2392T>C | F | 45 | 32 | 25 | 65 | 25 | N/A | 4 |
| FR/FR | c.507G>T/ | c.507G>T | M | 36 | 34 | 19 | 70 | 36 | 68 | 7 |
| FR/FR | c.148C>T/ | p625N | F | 36 | 26 | 20 | 75 | 45 | 62 | 2 |
| DK/GB | c.965C>T/ | c.1430G>A | M | 23 | 11 | 36 | 48 | 18 | 59 | 0 |
| DK/GB | c.148C>T/ | c.148C>T | M | 54 | 51 | 28 | 155 | 65 | 53 | 10 |
| Group mean (n = 10): | 6:4 | 41 ± 13 | 36 ± 12 | 27 ± 4 | 89 ± 36 | 40 ± 16 | 62 ± 10 | 3 ± 3 | ||
| Sequence 2 | ||||||||||
| DK/DK | c.148C>T/ | c.2392T>C | F | 47 | 32 | 31 | 100 | 50 | 67 | 6 |
| DK/SE | c.148C>T/ | IVS5‐60G>A | F | 49 | 39 | 23 | 110 | 55 | 55 | 2 |
| *3DK/DK | c.148C>T/ | c.2392T>C | M | 62 | 45 | 35 | 110 | 55 | 69 | 0 |
| DK/DK | c.148C>T/ | c.2392T>C | M | 48 | 34 | 32 | 65 | 20 | 63 | 4 |
| DK/DK | c.148C>T/ | c.1948C>T | M | 61 | 62 | 20 | 105 | 40 | 51 | 3 |
| DK/NL | c.148C>T/ | c.1760T>C | M | 30 | 23 | 24 | 125 | 40 | 48 | 6 |
| DK/DK | c.148C>T/ | c.148C>T | M | 67 | 53 | 26 | 103 | 55 | 61 | 6 |
| FR/FR | c.148C>T/ | c.148C>T | M | 26 | 22 | 19 | 81 | 31 | 72 | 1 |
| DK/DK | c.148C>T/ | c.2262delA | M | 25 | 25 | 23 | 134 | 50 | 55 | 3 |
| FR/FR | c.148C>T/ | c.2262delA | M | 45 | 12 | 26 | 200 | 80 | 50 | 6 |
| DK/GB | c.965C>T/ | c.965C>T | M | 50 | 42 | 27 | 138 | 60 | 86 | 3 |
| DK/GB | c.965C>T/ | c.1430G>A | M | 27 | 16 | 29 | 105 | 45 | 59 | 2 |
| Group mean (n = 12): | 10:2 | 45 ± 15 | 35 ± 15 | 25 ± 4 | 115 ± 34 | 48 ± 16 | 61 ± 11 | 3 ± 2 | ||
| All included (n = 22) | 16:6 | 43 ± 14 | 35 ± 13 | 26 ± 5 | 101 ± 36 | 45 ± 15 | 62 ± 10 | 3 ± 2 | ||
| Qualified for analysis (n = 19) | 14:5 | 43 ± 14 | 36 ± 14 | 26 ± 4 | 104 ± 37 | 44 ± 16 | 61 ± 10 | 3 ± 2 | ||
Demographics and baseline data for participants in a randomized double‐blind crossover trial in patients with McArdle disease receiving 14 days of treatment with triheptanoin followed by placebo (Sequence 1) or placebo followed by triheptanoin (Sequence 2). Study sites and countries of residence are Denmark (DK), Great Britain (GB), France (FR), Sweden (SE), and the Netherlands (NL). *1) excluded from analysis due to dysregulated diabetes, *2) withdrew due to side effects, *3) excluded from analysis due to incompliance. M, Male; F, female; BMI, body mass index; W peak, exercise workload; W submax, submaximal exercise workload; VO2submax, submaximal exercise oxygen uptake, VO2peak, peak oxygen uptake; wk, week.
Figure 3.
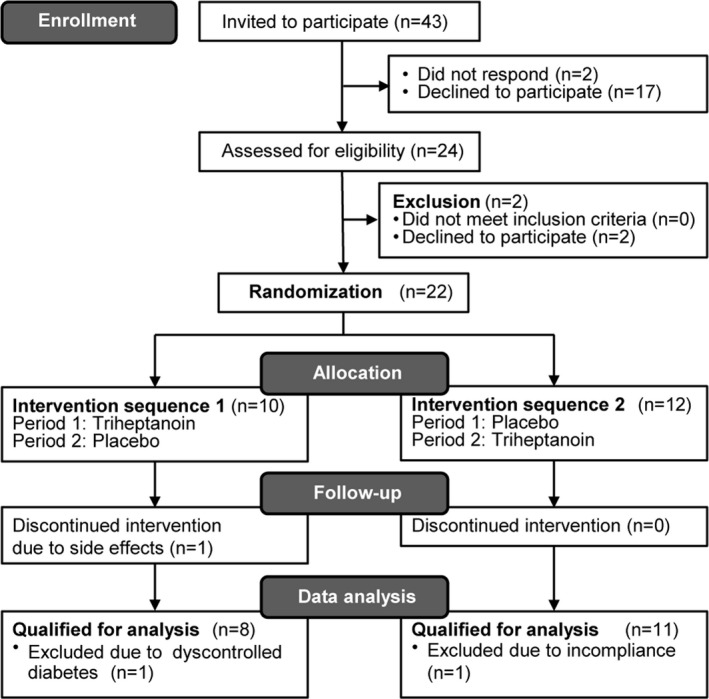
Participant enrollment, allocation, and completion in a randomized double‐blind crossover study where the included participants with McArdle disease were allocated 1:1 to two 14‐days periods of treatment with triheptanoin and placebo in a random sequence separated by a >1‐week washout period.
All participants had genetically confirmed McArdle disease (Table 1) and had experienced symptoms of exercise intolerance with muscle pain (n = 20/22), contractures (n = 17/22), premature fatigue (21/22) or having experienced rhabdomyolysis (15/22) or myoglobinuria (14/22) triggered by physical effort.
Randomization to treatment sequence was balanced with regards to demographics and exercise capacity (Table 1).
One participant was excluded from data analysis because of dysregulated diabetes with resting blood glucose varying from 5.3 to 19.7 mmol/L between visits. Another participant was not compliant enough to be included in the analysis. He had taken less than half of the oil according to his own reports, his treatment diary and measurement of the returned study oil, and he stopped taking the oil 2 days prior to the last visit (Fig. 3 and Table 1). All participants completed a minimum of 14 days of treatment in both periods.
Standardized meal
Participants took on average 192 ± 65 mL of yogurt and 55 ± 46 g of oat granola and on test days 2 and 4, and added the study oil. The study oil constituted 41 ± 16% of the total consumed calories (446 ± 185 kcal) at the breakfast. The breakfast took place on average 44 ± 22 min before the submaximal exercise test with ramp and this interval varied among tests with 4 min on average (CI: −5.4 to 13.4 min).
Submaximal exercise
There was no change in the primary outcome measure, submaximal exercise heart rate, between placebo and triheptanoin treatments, P = 0.69 or between triheptanoin and baseline, P = 0.09 (Figs. 4 and 5). The submaximal heart rate in each patient varied between tests with −1.5 bpm (CI: −5.4 to 2.4) from test to test, but without correlation to treatment. There was no difference in the response between the two crossover groups (P = 0.40), and data from the two groups are therefore pooled and presented together.
Figure 4.
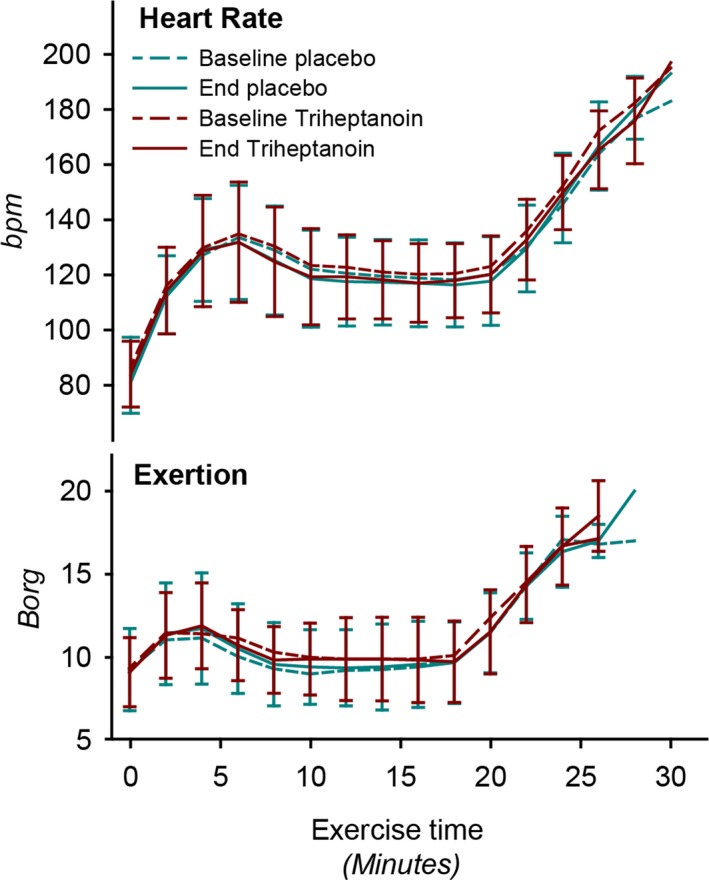
Heart rate and self‐reported exertion during submaximal (until 20 min) to peak exercise in 19 McArdle patients at baseline and after treatment with triheptanoin and placebo for 14 days in a double‐blind crossover study. Values are means with error bars of standard deviations.
Figure 5.
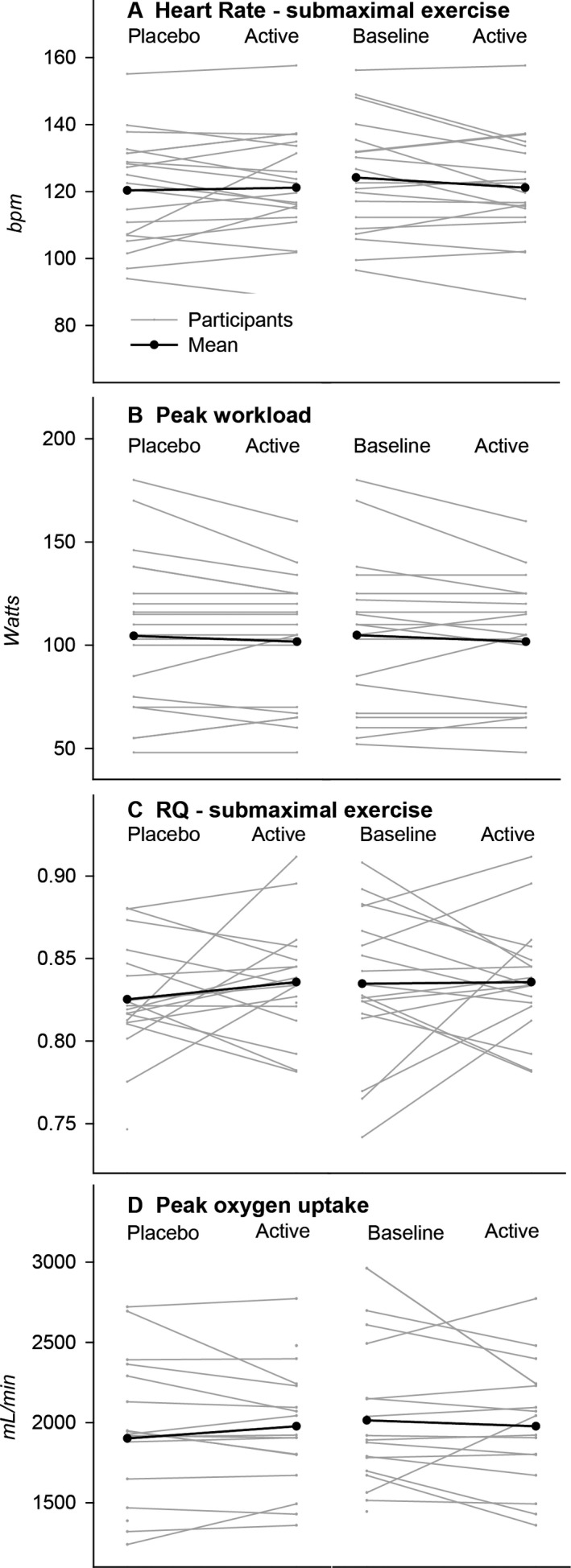
Individual and mean changes from placebo to active treatment and from baseline to active treatment in (A) submaximal exercise heart rate, (B) submaximal exercise respiratory quotient (RQ), (C) peak exercise workload and (D) peak oxygen uptake in 19 patients with McArdle disease in a double‐blind crossover study investigating the effect of 14 days treatment with triheptanoin.
Secondary outcome measures
Exercise performance
Self‐reported exertion during exercise was not different on triheptanoin versus placebo treatment (P = 0.51) or from baseline (P = 0.97). There was no change in the exercise duration (P = 0.39 vs. placebo and P = 0.13 vs. baseline) (Fig. 4 and Table 2). The peak workloads and peak oxygen uptakes achieved on triheptanoin were not different compared to placebo (P = 0.29 and P = 0.37 respectively) or to baseline (P = 0.23 and P = 0.30) (Fig. 5 and Table 2). The respiratory quotient during submaximal exercise did not differ between placebo and active treatments (P = 0.14) or between active treatment and baseline (P = 0.81) (Fig. 6).
Table 2.
Plasma metabolites and exercise test measures.
| Placebo ± SD | Triheptanoin ± SD | ||
|---|---|---|---|
| Metabolites before standardized meal | |||
| Creatine kinase | U/L | 2061 ± 1417 | 2972 ± 2538 |
| 3‐Keto pentanoic acid | µmol/L | 0.5 ± 0.2 | 28.7 ± 15.9* |
| 3‐OH‐pentanoic | µmol/L | 0.6 ± 0.3 | 37.8 ± 27.8* |
| C3‐acylcarnitines | µmol/L | 0.87 ± 0.62 | 1.23 ± 0.63* |
| Exercise test measures | |||
| Fatigue Severity Score | 0–40 | 30 ± 16 | 32 ± 15 |
| Body weight | kg | 81 ± 15 | 82 ± 15 |
| Exercise duration | min:sec | 26:47 ± 1:35 | 26:31 ± 1:13 |
| Glucoserest | mmol/L | 5.9 ± 1.1 | 5.9 ± 1.1 |
| Glucosesubmax | mmol/L | 4.9 ± 0.6 | 4.6 ± 0.8 |
Metabolites and exercise test measures in 19 patients with McArdle disease with 14 days of triheptanoin treatment and 14 days of placebo treatment in a randomized double‐blind crossover study.
P < 0.001 versus placebo and versus baseline.
Figure 6.
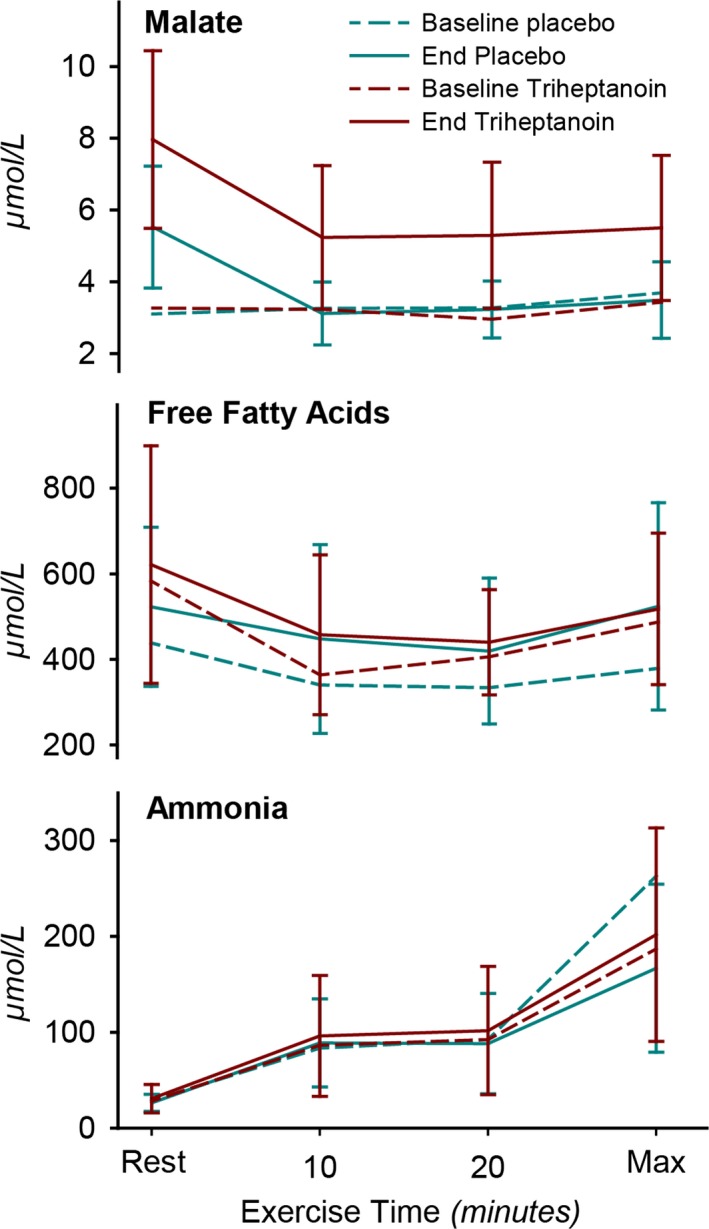
Plasma metabolite concentrations during submaximal (20 min) to peak exercise in 19 McArdle patients at baseline and after treatment with triheptanoin and with placebo for each 14 days in a double‐blind crossover study. *P < 0.001 versus placebo and baseline. Values are means with error bars of standard deviations.
Metabolites
At rest, plasma malate concentration was significantly higher on triheptanoin compared to placebo (P < 0.001). It dropped with the onset of exercise but remained higher during exercise compared to placebo (P < 0.001) and to baseline (P < 0.001) (Fig. 6). Plasma concentrations of C5‐ketone bodies β‐ketopentanoate and (R)‐β‐hydroxypentanoate were 10‐fold higher on triheptanoin compared to placebo (P < 0.001) and C3‐acylcarnitines increased significantly with triheptanoin (Table 2).
There was no difference in the concentration of ammonia and free fatty acids during exercise (Fig. 6). Plasma glucose was not different at rest on triheptanoin and placebo treatments and dropped with submaximal exercise in the same manner on both treatments.
Side effects and compliance
Participants kept a stable body weight with a mean change of −0.4 kg (CI: −0.08 to 0.90, P = 0.10) in the placebo period and −0.3 kg (CI: −0.0665 to 0.645, P = 0.11) in the triheptanoin period (Table 2).
No serious adverse events were reported. None of the participants experienced myoglobinuria after any of the exercise tests or at any other time during the study period. Seven of the 22 randomized participants reported nausea, four had episodes of diarrhea, and three reported stomachache on triheptanoin treatment and one reported stomachache on the placebo treatment. They were all advised to take the oil exclusively with large meals, which solved the issues in all but five participants. Guided by the investigators, they reduced the dose by 30%, which solved the problem for three participants. One withdrew when nausea made her unable to perform the study tests at visit 2, and another reduced the dose to less than 50% and eventually stopped taking the oil due to discomfort. This patient was excluded from data analysis (Fig. 2).
Compliance was evaluated by the amount of oil returned by the participants. Nearly all 19 participants included in the final analysis had taken 90–100% of the assigned total dose, except three participants in the placebo period and two in the triheptanoin period, who had taken 72–89%. This amount corresponded with the recordings in the dosing diaries from each patient.
Discussion
For nearly 20 years, a range of observations, cases and open‐label studies have reported positive effects of triheptanoin for various diseases where energy deficiency contributes to the pathogenesis.5, 6, 7, 8, 9, 10, 11, 12, 21, 22, 23, 24, 25 These uncontrolled studies report improvements in muscle strength, frequency of rhabdomyolysis and hypoglycemia in patients with long‐chain fatty acid oxidation disorders (LC‐FAOD) and reduced symptom severity in diseases of the central nervous system including a 90% reduction in paroxysmal events in patients with GLUT1 deficiency.12, 21, 24, 26, 27 Yet, very few controlled studies have been performed to date. Two showed no effect of triheptanoin on gait, balance or spasticity in adult polyglucosan body disease or on frequency of paroxysmal events in alternating hemiplegia of childhood, while one reports increased left ventricular ejection fraction in patients with LC‐FAODs on triheptanoin treatment.28, 29, 30
The extensive interest in triheptanoin for the treatment of LC‐FAODs assumes that these patients have an exaggerated loss of TCA intermediates from damaged muscle cells through the membrane defect indicated by elevated creatine kinase levels. However, levels of TCA intermediates were not measured in the studied patients, and in fact, there is little evidence that TCA intermediates are low in LC‐FAODs.5, 28 Patients with LC‐FAODs have intact glycolysis and glycogenolysis producing oxaloacetate, which feeds the TCA. This ensures the entry of acetyl‐CoA to the TCA as long as they avoid fasting. The importance of glycolysis is underlined by a study demonstrating that an LC‐FAOD mouse has a faster depletion of TCA intermediates associated with hypoglycemia in response to fasting compared to wild‐type mice.31
Unlike patients with LC‐FAODs, it is well documented that McArdle patients have very low TCA substrate levels during exercise.3 In healthy persons, the muscle and plasma levels of TCA intermediates increase many fold with the onset of exercise, but this response is absent in McArdle patients suggesting that impaired TCA expansion is a key mechanism of impaired oxidative metabolism in these patients.3, 4 In McArdle patients, the rate of fat oxidation during exercise reaches a plateau despite an increased availability of fatty acids and intact beta‐oxidation, and this ceiling effect of fat oxidation is thought to be caused by reduced levels of TCA intermediates, namely succinate, fumarate, malate and ultimately oxaloacetate to support optimal rates of metabolism of acetyl‐CoA derived from beta‐oxidation.1, 2 Therefore, McArdle disease should be one of the most suitable targets for an anaplerotic treatment.
In this double‐blind, crossover study, we found no improvement of 14 days of triheptanoin treatment on the primary outcome measure, lowering of heart rate during submaximal exercise, in participants with McArdle disease. Neither were there any changes in respiratory exchange rate as a measure of fatty acid oxidation and no change in peak workload, peak oxygen uptake or ammonia production (Figs. 5 and 6).32
We saw significantly higher concentrations of C5‐ketones and C3‐acylcarnitines in plasma on triheptanoin compared to placebo (Table 2) indicating proper metabolism of triheptanoin. As a medium‐chain fatty acid, heptanoate can pass freely into mitochondria independently of membrane transporters.
Triheptanoin succeeded in increasing intermediates of the TCA as indicated by significantly higher blood concentrations of the TCA substrate, malate, at rest and during exercise compared to placebo (Fig. 6). Plasma malate concentrations do not directly reflect the intramuscular levels, which could explain the lack of treatment response in the McArdle patients. However, the change in plasma malate likely indicates the same directional change in muscle as it has been shown that malate concentrations have a parallel response to exercise in plasma and muscle.3, 4 Secondly, the increase in plasma malate on triheptanoin treatment must reflect that triheptanoin has undergone intracellular metabolism. However, malate concentrations did not increase from rest to exercise in the McArdle patients with triheptanoin in contrast to the many fold increase in healthy individuals and in patients with mitochondrial myopathy.3 Thus, the TCA intermediate levels achieved with triheptanoin treatment in McArdle patients appear insufficient to augment oxidative metabolism during exercise, as indicated by the unchanged heart rate and perceived exertion during submaximal exercise (Fig. 4). Meanwhile, it is well established that the same measures of exercise tolerance and capacity can be improved with carbohydrate supplements prior to exercise and with aerobic and resistance training, which McArdle patients can safely perform after a warm‐up.19, 20, 33, 34
We can also speculate if the reason for the reduced TCA cycle turnover is an imbalance in the NADH/NAD+ ratio. The conversion of pyruvate to lactate, serves as an extra source of NAD+ when NAD+ supply from oxidative phosphorylation is maximal. McArdle patients do not produce pyruvate and do not have this alternative source of NAD+. This limits the energy production from the TCA cycle and exercise capacity at high/maximal exercise intensities. In that case, triheptanoin has no chance of improving TCA turnover as its metabolism does not generate NAD+.
However, during submaximal exercise as applied in this study, there is still capacity to increase the NAD+ supply from respiratory chain. Therefore, TCA cycle turnover in McArdle patients is most likely caused by a lack of intermediates rather than a lack of NAD+ supply.
In the present study, participants took a higher dose of triheptanoin compared to patients studied with fatty acid oxidation defects (FAOD)28 and the same dose as the dose given to other adults with FAODs.8, 22 The treatment period of 14 days in our study was chosen as the effect of triheptanoin in other metabolic defects has been reported to start within days.6, 8, 11 This study was however not designed, to evaluate the possible long‐term benefit of triheptanoin treatment in patients with McArdle disease, which would have to be addressed in a long‐term study. Implementing a higher dose of the oil in the diet would be difficult since it constituted about a third of the total caloric intake, and as our participants already had mild but rather frequent side effects to the oil at the dose studied.
The lack of TCA intermediates during exercise may be more profound in phosphofructokinase deficiency where distal glycolysis is impaired thus blocking the utilization of both muscle glycogen and blood glucose. There may therefore be a potential role for triheptanoin in this condition, which is currently under investigation (NCT03642860).35
This study showed no improvement of exercise performance or tolerance in patients with McArdle disease on triheptanoin treatment. The likely explanation is that while triheptanoin increased blood levels of TCA intermediates at rest, it did not evoke a sufficient increase in TCA intermediates to generate a normal rate of TCA turnover during exercise.
Author Contributions
KLM, PL, RGH, FM, and JV conceptualized and designed the study. KLM collected and analyzed the data, and drafted the manuscript and figures. AEB, MGS, CO, SNH, DRP, NSP, MA, MPL, AC, and FM played a major role in acquisition of data, critically revised and approved the final manuscript draft. RGH and PL critically revised and approved the final manuscript draft. RQ played a major role in acquisition of data, data collection critical revision and approved the final manuscript draft. FM. JV collected and analyzed the data, critically revised and approved the final manuscript draft.
Conflict of Interest
KLM, PL, AEB, MGS, CO, SNH, DRP, NSP, MA, MPL, AC, JV and RGH have nothing to report. RQ has been paid honoraria for lecturing and consultancy work, none of which relates to this disease, intervention or study, from Ultragenyx, Genzyme, Santhera, Sarepta and PTC bio. FM has received consulting fees from and conducted other investigator‐sponsored studies supported by Ultragenyx pharmaceuticals, unrelated to this study.
Acknowledgments
We thank the participants and research staff, especially François Renard and Nouella Akay for their extraordinary organizational skills and study coordination. We thank Mina Ghasemilee and Pia Hynne for number generation and randomization and Claude Jardel, Randa Bittar and Jesper Helbo Storgaard for the biochemical analyses. Ultragenyx Pharmaceutical Inc. provided the study treatment and financed the study procedures and patient travel and accommodation expenses. This was a purely investigator‐driven study, designed, conducted, and reported independently from Ultragenyx Pharmaceutical Inc. The company has read the manuscript before publication.
Funding Information
This was a purely investigator‐driven study, designed, conducted, and reported independently from Ultragenyx Pharmaceutical Inc.
Funding Statement
This work was funded by Ultragenyx Pharmaceutical grant .
References
- 1. Orngreen MC, Jeppesen TD, Andersen ST, et al. Fat metabolism during exercise in patients with McArdle disease. Neurology 2009;72:718–724. [DOI] [PubMed] [Google Scholar]
- 2. Andersen ST, Jeppesen TD, Taivassalo T, et al. Effect of changes in fat availability on exercise capacity in McArdle disease. Arch Neurol 2009;66:762–766. [DOI] [PubMed] [Google Scholar]
- 3. Delaney NF, Sharma R, Tadvalkar L, et al. Metabolic profiles of exercise in patients with McArdle disease or mitochondrial myopathy. Proc Natl Acad Sci USA 2017;114:8402–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahlin K, Jorfeldt L, Henriksson K‐G, et al. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle’s disease. Clin Sci 1995;88:687–693. [DOI] [PubMed] [Google Scholar]
- 5. Roe CR, Sweetman L, Roe DS, et al. Treatment of cardiomyopathy and rhabdomyolysis in long‐chain fat oxidation disorders using an anaplerotic odd‐chain triglyceride. J Clin Invest 2002;110:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roe CR, Mochel F. Anaplerotic diet therapy in inherited metabolic disease: therapeutic potential. J Inherit Metab Dis 2006;29:332–340. [DOI] [PubMed] [Google Scholar]
- 7. Roe CR, Yang BZ, Brunengraber H, et al. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology 2008;71:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roe CR, Brunengraber H. Anaplerotic treatment of long‐chain fat oxidation disorders with triheptanoin: review of 15 years experience. Mol Genet Metab 2015;116:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahapatra S, Ananth A, Baugh N, et al. Triheptanoin: a rescue therapy for cardiogenic shock in carnitine‐acylcarnitine translocase deficiency. JIMD Rep 2017;39:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vockley J, Charrow J, Ganesh J, et al. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain‐fatty acid oxidation disorders. Mol Genet Metab 2016;119:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mochel F, DeLonlay P, Touati G, et al. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab 2005;84:305–312. [DOI] [PubMed] [Google Scholar]
- 12. Mochel F. Triheptanoin for the treatment of brain energy deficit: a 14‐year experience. J Neurosci Res 2017;95:2236–2243. [DOI] [PubMed] [Google Scholar]
- 13. Gu L, Zhang G‐F, Kombu RS, et al. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. II. Effects on lipolysis, glucose production, and liver acyl‐CoA profile. Am J Physiol ‐ Endocrinol Metab 2010;298:E362–E371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng S, Zhang G‐F, Kasumov T, et al. Interrelations between C4 ketogenesis, C5 ketogenesis, and anaplerosis in the perfused rat liver. J Biol Chem 2009;284:27799–27807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marin‐Valencia I, Good LB, Ma Q, et al. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I‐deficient (G1D) brain. J Cereb Blood Flow Metab 2013;33:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381. [PubMed] [Google Scholar]
- 17. Chalmers R, Lawson AM. Organic acids in man: analytical chemistry, biochemistry and diagnosis of the organic Acidurias [Internet]. Springer, Netherlands; 1982. [cited 2019 Feb 19] Available from: https://www.springer.com/us/book/9789400957800. [Google Scholar]
- 18. Vreken P, van Lint AE, Bootsma AH, et al. Quantitative plasma acylcarnitine analysis using electrospray tandem mass spectrometry for the diagnosis of organic acidaemias and fatty acid oxidation defects. J Inherit Metab Dis 1999;22:302–306. [DOI] [PubMed] [Google Scholar]
- 19. Haller RG, Wyrick P, Taivassalo T, Vissing J. Aerobic conditioning: an effective therapy in McArdle’s disease. Ann Neurol 2006;59:922–928. [DOI] [PubMed] [Google Scholar]
- 20. Vissing J, Haller RG. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N Engl J Med 2003;349:2503–2509. [DOI] [PubMed] [Google Scholar]
- 21. Roe CR, Bottiglieri T, Wallace M, et al. Adult polyglucosan body disease (APBD): anaplerotic diet therapy (Triheptanoin) and demonstration of defective methylation pathways. Mol Genet Metab 2010;101:246–252. [DOI] [PubMed] [Google Scholar]
- 22. Vockley J, Burton B, Berry GT, et al. UX007 for the treatment of long chain‐fatty acid oxidation disorders: safety and efficacy in children and adults following 24 weeks of treatment. Mol Genet Metab 2017;120:370–377. [DOI] [PubMed] [Google Scholar]
- 23. Vockley J, Marsden D, McCracken E, et al. Long‐term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment—a retrospective chart review. Mol Genet Metab 2015;116:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mochel F, Duteil S, Marelli C, et al. Dietary anaplerotic therapy improves peripheral tissue energy metabolism in patients with Huntington’s disease. Eur J Hum Genet 2010;18:1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis 2006;29:327–331. [DOI] [PubMed] [Google Scholar]
- 26. Calvert S, Barwick K, Par M, et al. A pilot study of add‐on oral triheptanoin treatment for children with medically refractory epilepsy. Eur J Paediatr Neurol 2018;22:1074–1080. [DOI] [PubMed] [Google Scholar]
- 27. Mochel F, Hainque E, Gras D, et al. Triheptanoin dramatically reduces paroxysmal motor disorder in patients with GLUT1 deficiency. J Neurol Neurosurg Psychiatry 2016;87:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillingham MB, Heitner SB, Martin J, et al. Triheptanoin versus trioctanoin for long‐chain fatty acid oxidation disorders: a double blinded, randomized controlled trial. J Inherit Metab Dis 2017;40:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schiffmann R, Wallace ME, Rinaldi D, et al. A double‐blind, placebo‐controlled trial of triheptanoin in adult polyglucosan body disease and open‐label, long‐term outcome. J Inherit Metab Dis 2018;41:877–883. [DOI] [PubMed] [Google Scholar]
- 30. Hainque E, Caillet S, Leroy S, et al. A randomized, controlled, double‐blind, crossover trial of triheptanoin in alternating hemiplegia of childhood. Orphanet J Rare Dis 2017;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bakermans AJ, Dodd MS, Nicolay K, et al. Myocardial energy shortage and unmet anaplerotic needs in the fasted long‐chain acyl‐CoA dehydrogenase knockout mouse. Cardiovasc Res 2013;100:441–449. [DOI] [PubMed] [Google Scholar]
- 32. Sahlin K, Areskog NH, Haller RG, et al. Impaired oxidative metabolism increases adenine nucleotide breakdown in McArdle’s disease. J Appl Physiol 1990;69:1231–1235. [DOI] [PubMed] [Google Scholar]
- 33. Maté‐Muñoz JL, Moran M, Pérez M, et al. Favorable responses to acute and chronic exercise in McArdle patients. Clin J Sport Med 2007;17:297–303. [DOI] [PubMed] [Google Scholar]
- 34. Santalla A, Munguía‐Izquierdo D, Brea‐Alejo L, et al. Feasibility of resistance training in adult McArdle patients: clinical outcomes and muscle strength and mass benefits. Front Aging Neurosci 2014;6:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haller RG, Vissing J. No spontaneous second wind in muscle phosphofructokinase deficiency. Neurology 2004;62:82–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available on request from the corresponding author, KM. The data are not publicly available as they contain information that could compromise the privacy of research participants. The corresponding author had full access to the study data and had the final responsibility for the decision to submit for publication.


