Abstract
We applied RT‐QuIC assay to detect α‐synuclein aggregates in cerebrospinal fluid (CSF) of patients with suspected Creutzfeldt–Jakob disease who had a neuropathological diagnosis of dementia with Lewy bodies (DLB) (n = 7), other neurodegenerative diseases with α‐synuclein mixed pathology (n = 20), or without Lewy‐related pathology (n = 49). The test had a sensitivity of 92.9% and specificity of 95.9% in distinguishing α‐synucleinopathies from non‐α‐synucleinopathies. When performed in the CSF of patients with DLB (n = 36), RT‐QuIC was positive in 17/20 with probable DLB, 0/6 with possible DLB, and 0/10 with Alzheimer disease. These results indicate that RT‐QuIC for α‐synuclein is an accurate test for DLB diagnosis.
Introduction
Dementia with Lewy bodies (DLB) is a progressive dementia characterized by fluctuating cognitive decline, visual hallucinations, parkinsonism, and REM sleep behavior disorder.1 Although the diagnosis of DLB is supported by SPECT dopamine transporter imaging, meta‐iodobenzylguanidine myocardial scintigraphy, and polysomnography,1 the diagnostic accuracy of DLB and other α‐synucleinopathies, including the early diagnosis of Parkinson disease (PD) is still poor and a significant proportion of patients do not receive a correct diagnosis in life.2, 3
Moreover, because clinical signs in DLB patients often overlap those observed in Alzheimer disease (AD), Creutzfeldt–Jakob disease (CJD), or other rapidly progressive dementias (RPD), neurologists need to consider alternative diagnoses.4, 5 There is a demand for specific and sensitive tests that should be based on the detection of α‐synuclein (α‐syn) aggregates in tissues, such as cerebrospinal fluid (CSF), commonly tested in the differential diagnosis of dementia. Misfolded forms of α‐syn are associated with DLB and other α‐synucleinopathies, that is PD and multisystem atrophy (MSA), similar to the pathological prion protein in CJD and other prion diseases.6 Although the expectations would be promising, ELISA assays for the detection of α‐syn in the CSF finally resulted poorly specific and sensitive.7 Recently, the Real‐Time Quaking Induced Conversion (RT‐QuIC) assay,8 originally developed for detecting the pathological prion protein in the CSF of patients with CJD, was successfully tailored for the identification of aggregated α‐syn in patients with α‐synucleinopathies, particularly PD patients.9, 10, 11, 12
The novelty of this study is that we use RT‐QuIC assay for α‐syn on CSF samples from patients who were referred as suspect CJD to the Italian CJD surveillance network but with neuropathological diagnosis of DLB or other α‐synucleinopathies with the aim of validating a test for an early diagnosis of DLB in rapidly dementing patients.
Patients and Methods
Brain tissues
Brain tissue samples for the development of the RT‐QuIC assay for α‐syn included seven cases of α‐synucleinopathies (DLB, parietal and frontal cortices, n = 3; PD, substantia nigra, n = 2; MSA, putamen, n = 2) and 12 frontal cortex specimens of other neurodegenerative diseases [CJD, n = 4; AD, n = 2; progressive supranuclear palsy, n = 3; corticobasal degeneration, n = 1; IgLON5 tauopathy, n = 1; frontotemporal lobar degeneration with TDP‐43, FTLD‐TDP, n = 1] obtained from the Dementia Laboratory of the Department of Pathology and Laboratory Medicine, at Indiana University School of Medicine and the Neuropathology laboratory of the University of Verona. For RT‐QuIC assay, brain areas were selected based on extent of α‐syn pathology observed in the immunostained brain sections.
Cerebrospinal fluid samples from neuropathologically confirmed and clinical cases
Cerebrospinal fluid samples were obtained from patients with an initial clinical suspect of CJD who finally underwent autopsy for neuropathological examination (n = 77). These included cases of pure DLB (n = 7) and MSA (n = 1); Lewy body dementia (LBD) and AD mixed pathology (LBD/AD, n = 15); LBD with tau mixed pathology (LBD/PART n = 2) and CJD with incidental α‐syn pathology (CJD/LBD, n = 3). Cerebrospinal fluid samples from other non‐α‐syn neurodegenerative (n = 30) or nondegenerative neurological diseases (n = 19) were used as controls (Table 1). Cerebrospinal fluid samples from patients with rapidly progressive cognitive decline mimicking CJD but with final clinical diagnosis of probable (n = 20) or possible (n = 6) DLB, and probable AD (n = 10) were also recruited. Clinical and neuropathological diagnoses were according to established criteria.1, 13, 14, 15, 16, 17, 18, 19 The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all subjects.
Table 1.
α‐Synuclein RT‐QuIC assay in cerebrospinal fluid of patients with suspected CJD.
| Neuropathologically verified cases (n = 77) | ||
|---|---|---|
| Definite Diagnosis (n) | Clinical diagnosis of Probable DLB | α‐syn RT‐QuIC results +/‐ |
| Pure α‐synucleinopathies (8) | ||
| DLB (7) | 6/7 | 7/0 |
| MSA‐C (1) | 1/1 | 1/0 |
| Other neurodegenerative diseases with α‐synuclein co‐pathology (20) | ||
| LBD/AD (15) | 1/15 | 14/1 |
| LBD/PART (2) | 1/2 | 2/0 |
| CJD/LBD (3) | 0/3 | 2/1 |
| Non‐α‐synucleinopathies (49) | ||
| Sporadic CJD (19) | 0/19 | 0/19 |
| Other neurodegenerative diseases (11)a | 1/11 | 1/10 |
| Other neurological diseases (19)b | 0/19 | 1/18 |
| α‐Synucleinopathies vs. non‐α‐Synucleinopathies |
Sensitivity (95% CI) = 92.9% (76.5–99.1) Specificity (95% CI) = 95.9% (86.0–99.5) |
|
| Clinical cases (n = 36) | ||
|---|---|---|
| Final clinical diagnosis | n | α‐syn RT‐QuIC results +/‐ |
| Probable DLB | 20 | 17/3 |
| Possible DLB | 6 | 0/6 |
| Probable AD | 10 | 0/10 |
| Probable DLB vs Possible DLB + Probable AD |
Sensitivity (95% CI) = 85.0% (62.1–96.8) Specificity (95% CI) = 100% (79.4–100) |
|
| Probable DLB + Possible DLB vs. Probable AD |
Sensitivity (95% CI) = 65.4% (44.3–82.8) Specificity (95% CI) = 100% (69.2‐100) |
|
RT‐QuIC, Real‐Time Quaking induced Conversion; DLB, Dementia with Lewy bodies; MSA‐C, Multiple system atrophy, cerebellar dysfunction subtype; LBD, Lewy body dementia; AD, Alzheimer disease; PART, primary age‐related tauopathy; CJD, Creutzfeldt–Jakob disease; CI, Confidence interval.
AD (n = 6); FTLD‐TDP 43 (n = 1); PSP (n = 2); CBD (n = 1); PART (n = 1).
VD (n = 4); encephalitis (n = 7); autoimmune encephalitis (n = 2); brain tumor (n = 2); pontine myelinolysis (n = 1); Wernicke encephalopathy (n = 1); anoxic encephalopathy (n = 2).
Expression and purification of recombinant human α‐synuclein and prion protein
Recombinant human α‐syn and hamster prion protein were expressed in E. coli and purified as previously reported.20, 21
Alpha‐synuclein RT‐QuIC analysis in brain and CSF samples
RT‐QuIC analyses of brain homogenates and CSF samples were performed as described.10 One microliter of serially diluted brain homogenate or 15 µL of CSF were plated and sealed with a plate sealer film (Nalgene Nunc International) and then incubated at 30°C in a BMG FLUOstar Omega plate reader with cycles of 1 min shaking (200 rpm double orbital) and 14 min rest. ThioflavinT (ThT) fluorescence measurements were taken every 45 min. Four replicate reactions were tested for each sample. The test was considered positive when the reaction occurred in two of four wells. A positive response was defined as a relative fluorescence unit (rfu) value of >3 SD above the mean value of all samples between 20 and 30 h. For sensitivity determinations, cutoff time was assessed at 75 h. The final fluorescence value was the mean fluorescence value at 75 h. The lag‐phase was the time for a sample to reach 165.000 rfu.
Statistical analysis
Statistical comparisons of mean relative ThT fluorescence responses in CSF samples from patients with pure DLB or with other α‐synucleinopathies and between groups with α‐syn co‐pathologies were performed with the t‐test. Sensitivity, specificity, and their relative 95% confidence intervals (C.I.) of the α‐syn RT‐QuIC were calculated.
Prion RT‐QuIC analysis in CSF samples
RT‐QuIC assay was performed using the improved conditions (IQ‐QuIC) as described.20
Results
α‐Syn RT‐QuIC analysis of brain tissues from α‐synucleinopathies
Brain samples from DLB, PD, and MSA cases had positive α‐syn RT‐QuIC reactions as early as 20 h in 10−4 brain dilutions and within 35 h in increasing brain dilutions up to 10−8. α‐Syn RT‐QuIC seeding reactions were still positive at 10−8 DLB brain dilutions while the end point dilution in PD and MSA samples was 10−5. Brain homogenates from other neurodegenerative diseases remained negative after 75 h of reaction (Figure S1) even at the 10−2 dilution.
α‐Syn RT‐QuIC assay of CSF samples from neuropathologically confirmed cases
The sensitivity and specificity of α‐syn RT‐QuIC assay were assessed in blinded CSF samples obtained from 77 patients (Table 1). Twenty‐six of 28 CSF samples from definite α‐synucleinopathies were positive by α‐syn RT‐QuIC. Interestingly, 20 samples were from cases with α‐syn concurrent pathologies, including AD and CJD cases (Table 1). Positive reactions in serially diluted CSF samples of DLB cases were obtained in as little as 0.3 µL of CSF (Figure S2) and seeding reactivity was observed as early as 40 h with a plateau at 75 h (Fig. 1A). The initial increase of RT‐QuIC curves observed up to 10 h was caused by unbound ThT fluorescence (Fig. 1A).
Figure 1.
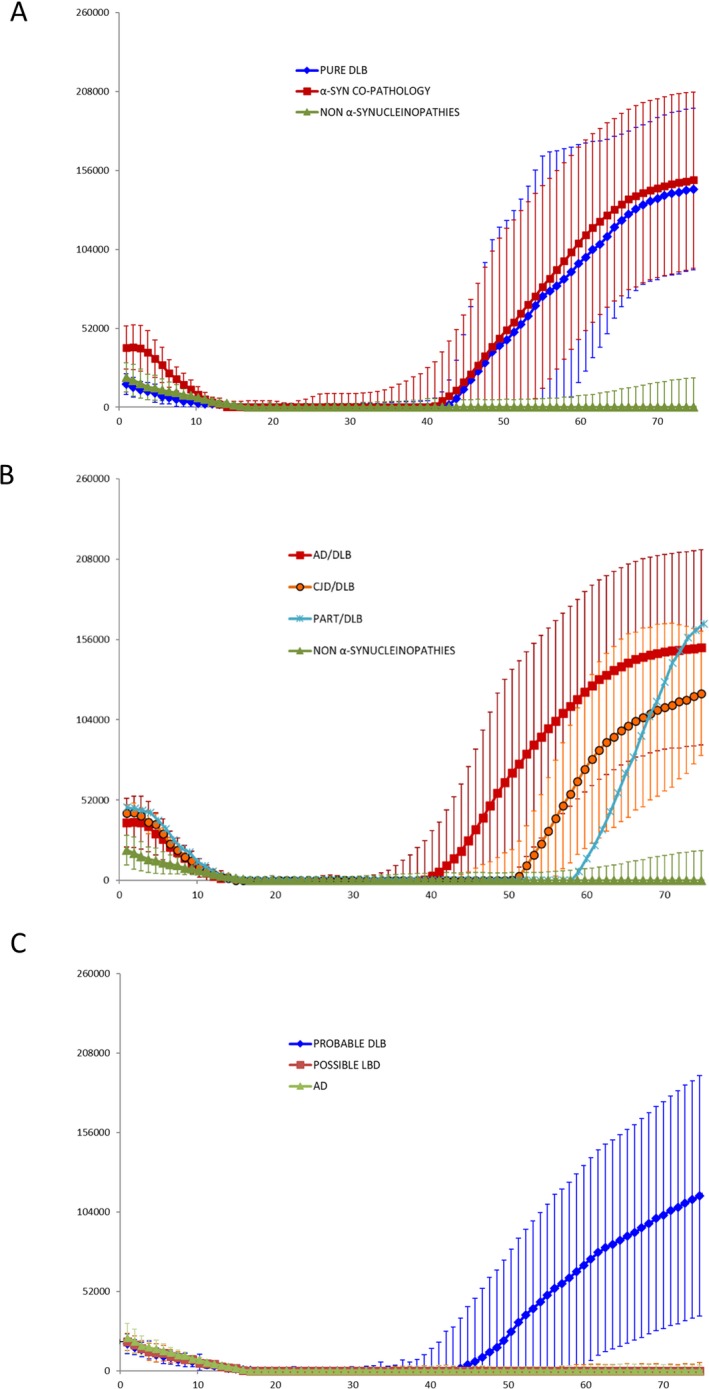
Results of α‐synuclein Real‐Time Quaking‐Induced Conversion (RT‐QuIC) assay of CSF samples from neuropathologically confirmed cases (A), α‐syn co‐pathology (B) and clinical cases (C). Traces represent the average percentage of Thioflavin T (ThT) fluorescence from four replicate reactions (normalized as described in the Methods section) with the means (thick lines) of those average and SDs (thin lines) shown as a function of RT‐QuIC reaction time; (Panel A) Curves representative of α‐syn RT‐QuIC from seven patients with pure DLB (blue trace) and 20 patients with α‐syn co‐pathology (LBD/AD; LBD/PART, and CJD/LBD) (burgundy trace), and from 49 control with other neurodegenerative and neurological diseases (green trace); (Panel B) Curves representative of RT‐QuIC results in different groups with α‐syn co‐pathology; (Panel C) Curve representative of CSF samples positive to α‐syn RT‐QuIC from 17 patients with clinical diagnosis of probable DLB (blue trace) and 19 negative samples (green trace), which include three patients with probable DLB, six with possible DLB and 10 with AD.
Samples from pure α‐synucleinopathies and α‐syn concurrent pathologies had an average final ThT fluorescence (P = 0.809) and lag‐time phase (P = 0.269) not statistically different (Fig. 1A and Table 1). Moreover, we did not observe any significant differences between groups with different α‐syn co‐pathologies (Fig. 1B and Table 1). As shown in Table 1, only 9 of 28 patients with definite α‐synucleinopathy had a clinical diagnosis of probable DLB or MSA with the majority of them in the group of pure α‐synucleinopathy. However, in one patient, who was clinically diagnosed as probable DLB (confirmed LBD/AD), α‐syn RT‐QuIC was negative in the CSF. Only two of 49 CSF samples from patients with non‐α‐synucleinopathies were borderline positive for α‐syn RT‐QuIC assay within 75 h of reaction. The false‐positive sample in the group “other neurodegenerative diseases” belonged to a patient with AD, whereas that in the group “other neurological diseases” belonged to a patient with encephalitis. (Table 1). These data indicate that the α‐syn RT‐QuIC assay is both highly specific (2/49, 95.9%) and sensitive (26/28, 92.9%). Finally, all CJD CSF samples were positive to the prion IQ‐QuIC assay while all other samples were negative (data not shown).
RT‐QuIC testing of CSF in patients showing initial clinical features resembling DLB or CJD
Cerebrospinal fluid samples were obtained from 36 patients with rapidly progressive cognitive decline, visual disturbances, and extrapyramidal signs. The RT‐QuIC assay for prions in the CSF was negative (IQ‐QuIC) and brain MRI did not show the characteristic lesions of hyper‐intense signals in the cortical ribbon or in the basal ganglia in FLAIR or DWI sequences (data not shown). Cerebrospinal fluid was tested by the α‐syn RT‐QuIC assay and 17 samples were positive (Fig. 1C). Patients with positive α‐syn RT‐QuIC assay had a final diagnosis in life (without considering the results of the α‐syn RT‐QuIC assay) of probable DLB. Three patients with final clinical diagnosis of probable DLB and all patients with a final clinical diagnosis of possible DLB (n = 6) or probable AD (n = 10) were α‐syn RT‐QuIC negative in the CSF (Table 2). The sensitivity of the test ranged between 85 and 65% and specificity between 65 and 100% depending on the exclusion or inclusion of possible DLB cases as affected by α‐synucleinopathies (Table 2).
Table 2.
Positive α‐syn RT‐QuIC assay, lag‐phase, and final fluorescence values in CSF from subjects with pure α‐synucleinopathies (Pure DLB, MSA‐C), other neurodegenerative diseases with α‐synuclein co‐pathology (LBD/AD, CJD/LBD, LBD/PART), non‐α‐synucleinopathies, and patients with clinical diagnosis of probable DLB.
|
Positive RT‐QuIC In each subject group (n/total) |
Lag‐phase in positive RT‐QuIC (h) |
Final fluorescence in positive RT‐QuIC (rfu) |
|---|---|---|
| Pure DLB (7/7)a | 57 ± 10.6 | 235,665 ± 31,985 |
| LBD/AD (14/15)a | 56 ± 7.2 | 219,985 ± 36,831 |
| CJD/LBD (2/3) | 59; 61 | 203,233; 205,196 |
| LBD/PART (2/2) | 62; 65 | 235,715; 251,844 |
| MSA‐C (1/1) | 46 | 238,320 |
| Non‐α‐synucleinopathies (2/49)a | 57; 42 | 203,945; 202,690 |
| Probable DLB (17/20)a | 56 ± 10.1 | 236,045 ± 31,629 |
rfu, relative fluorescence units
reported values of lag‐phase and rfu are referred to mean ± standard deviations. Statistical analysis: Lag‐phase: P = 0.066 (Pure DLB vs. LBD/AD); P = 0.992 (Pure DLB vs. Probable DLB). Final fluorescence value: P = 0.858 (Pure DLB vs. LBD/AD); P = 0.823 (Pure DLB vs. Probable DLB).
Discussion
This study shows that the α‐syn RT‐QuIC assay performed on small amounts of CSF samples is a valuable tool for confirming the diagnosis of DLB and for the identification of other neurodegenerative diseases with α‐syn mixed pathology.22 These and previously published data9 indicate that RT‐QuIC is not influenced by the concomitant presence of other protein aggregates confirming the high specificity of the assay. RT‐QuIC assays performed on CSF or other easily obtainable relevant tissues, such as the olfactory mucosa,23 are therefore a powerful technology for distinguishing different diseases presenting with rapidly evolving cognitive disturbances. Making a correct early diagnosis would improve the prognostic evaluation of patients with dementia.22 Moreover, α‐syn RT‐QuIC assay would be useful for recruiting patients for clinical trials with disease‐specific target drugs24 considering that the co‐occurrence of multiple pathologies might interfere with the efficacy of treatments and a correct molecular diagnosis is likely to enhance the design and possibly the outcome of future trials.25
In the clinical contest, the α‐syn RT‐QuIC assay was positive only in CSF samples from patients with final clinical diagnosis of probable DLB whereas all samples from possible DLB or AD patients were negative confirming the high specificity of the test. The three probable DLB with negative α‐syn RT‐QuIC in the CSF deserves a precautionary interpretation because it might either show the failure of the test to recognize 15% of DLB patients or the inaccuracy of the diagnostic criteria of probable DLB as recently reported.2, 26 Follow‐up studies on a larger group of patients with DLB or other α‐synucleinopathies who are finally neuropathologically confirmed are needed, but our results and those of previous studies9, 10, 11, 12 suggest that the α‐syn RT‐QuIC assay is a much more reliable assay for the detection of α‐syn aggregates in the CSF than conventional ELISA tests.27 It is of note that the negative LBD/AD sample (Table 1) resulted positive when re‐tested after breaking the blinded codes while the two false‐positive samples in the non‐α‐synucleinopathies group resulted negative. Although there are still weaknesses that need to be implemented as shown by the initially wrong evaluation of the three samples in the blinded run, we strongly suggest to introduce α‐syn RT‐QuIC test in the diagnostic criteria of DLB and other α‐synucleinopathies. More importantly, this test should be also routinely performed in patients with clinical signs of progressive dementia for the prompt identification of α‐syn co‐pathologies.
Author Contributions
M.B., A.L., M.P., and G.Z. contributed to the conception and design of the study; M.B., D.P., M.F., and A.P. performed RT‐QuIC experiment and contributed to acquisition and analysis of the data; S.K., S.B., A.C., T.C., F.J., M.T., B.G., S.M., G.B.K., and P.P. provided brain tissues and CSF samples from clinical definite cases; S.C. and G.L. provided recombinant α‐syn; all the authors contributed to drafting the text.
Conflict of Interest
All authors declare no conflict of interests.
Supporting information
Figure S1. α‐Syn RT‐QuIC end‐point dilution analysis of BH samples from DLB (Panel a), MSA‐C (Panel B) and PD (Panel C) patients.
Figure S2. α‐Syn RT‐QuIC end‐point dilution analysis of CSF samples from patients with pure DLB.
Acknowledgments
We thank Drs Dorina Tiple, Luana Vaianella, Elisa Colaizzo of the Italian Registry of CJD and related disorders (Istituto Superiore di Sanità, Rome, Italy) for collecting clinical information of patients; Michele Equestre for valuable technical assistance; Cinzia Gasparrini and Alessandra Garozzo for administrative support.
Funding information
This work was partially supported by the Ministero della Salute, Italy, for the national surveillance of Creutzfeldt‐Jakob disease and by Ministero della Salute RF‐2013‐02354884 and GR‐2013‐02355724 “Development of an assay detecting prions in animals and humans affected with prion disorders in a preclinical and clinical stage” to MP and GZ; Veneto Region Finalized Research 2014. RP‐2014‐00000400 “Experimental Study of a Clinical Network for Diagnosing Rapidly Progressive Dementias” to AC and SM; Dr. Ghetti was supported by the Department of Pathology and Laboratory Medicine and by the grant PHS NIA P30 AG010133.
Funding Statement
This work was funded by Ministero della Salute, Italy grant RF‐2013‐02354884 and GR-2013-02355724; Veneto Region grant RP‐2014‐00000400 ; Department of Pathology and Laboratory Medicine grant PHS NIA P30 AG010133.
Contributor Information
Maurizio Pocchiari, Email: maurizio.pocchiari@iss.it.
Gianluigi Zanusso, Email: gianluigi.zanusso@univr.it.
References
- 1. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rizzo G, Arcuti S, Copetti M, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry 2018;89:358–366. [DOI] [PubMed] [Google Scholar]
- 3. Berg D, Adler CH, Bloem BR, et al. Movement disorder society criteria for clinically established early Parkinson's disease. Mov Disord. 2018;33:1643–1646. [DOI] [PubMed] [Google Scholar]
- 4. Vergouw LJM, Marler LP, van de Berg WDJ, et al. Dementia with lewy bodies: a clinicopathologic series of false‐positive cases. Alzheimer Dis Assoc Disord 2019;. 10.1097/WAD.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 5. Tschampa HJ, Neumann M, Zerr I, et al. Patients with Alzheimer's disease and dementia with Lewy bodies mistaken for Creutzfeldt‐Jakob disease. J Neurol Neurosurg Psychiatry. 2001;71:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol 2013;9:13–24. [DOI] [PubMed] [Google Scholar]
- 7. Gao L, Tang H, Nie K, et al. Cerebrospinal fluid alpha‐synuclein as a biomarker for Parkinson's disease diagnosis: a systematic review and meta‐analysis. Int J Neurosci. 2015;125:645–654. [DOI] [PubMed] [Google Scholar]
- 8. Zanusso G, Monaco S, Pocchiari M, Caughey B. Advanced tests for early and accurate diagnosis of Creutzfeldt‐Jakob disease. Nat Rev Neurol 2016;12:325–333. [DOI] [PubMed] [Google Scholar]
- 9. Fairfoul G, McGuire LI, Pal S, et al. Alpha‐synuclein RT‐QuIC in the CSF of patients with alpha‐synucleinopathies. Ann Clin Transl Neurol 2016;3:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groveman BR, Orrù CD, Hughson AG, et al. Rapid and ultra‐sensitive quantitation of disease‐associated α‐synuclein seeds in brain and cerebrospinal fluid by α‐Syn RT‐QuIC. Acta Neuropathol Commun 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Rumund A, Green AJE, Fairfoul G, et al. α‐Synuclein real‐time quaking‐induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol 2019;85:777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahnawaz M, Tokuda T, Waragai M, et al. Development of a biochemical diagnosis of Parkinson Disease by detection of α‐synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74:163–172. [DOI] [PubMed] [Google Scholar]
- 13. Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt‐Jakob disease. Brain 2009;132:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budka H, Aguzzi A, Brown P, et al. Neuropathological diagnostic criteria for Creutzfeldt‐Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol 1995;5:459–466. [DOI] [PubMed] [Google Scholar]
- 15. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer’s Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging‐Alzheimer's Association guidelines for the neuropathological assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's Disease. Mov Disord 2015;30:1591–1600. [DOI] [PubMed] [Google Scholar]
- 18. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age‐related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orrú CD, Groveman BR, Hughson AG, et al. Rapid and sensitive RT‐QuIC detection of human Creutzfeldt‐Jakob disease using cerebrospinal fluid. MBio 2015;20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plotegher N, Kumar D, Tessari I, et al. The chaperone‐like protein 14‐3‐3η interacts with human α‐synuclein aggregation intermediates rerouting the amyloidogenic pathway and reducing α‐synuclein cellular toxicity. Hum Mol Genet 2014;23:5615–5629. [DOI] [PubMed] [Google Scholar]
- 22. Roudil J, Deramecourt V, Dufournet B, et al. Influence of lewy pathology on Alzheimer's disease phenotype: a retrospective clinico‐pathological study. J Alzheimers Dis 2018;63:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orrú CD, Bongianni M, Tonoli G, et al. A test for Creutzfeldt‐Jakob disease using nasal brushings. N Engl J Med 2014;371:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dehay B, Decressac M, Bourdenx M, et al. Targeting α‐synuclein: therapeutic options. Mov Disord 2016;31:882–888. [DOI] [PubMed] [Google Scholar]
- 25. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti‐α‐synuclein monoclonal antibody, in patients with parkinson disease: a randomized clinical trial. JAMA Neurol 2018;75:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 2015;85:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mollenhauer B, Locascio JJ, Schulz‐Schaeffer W, et al. α‐Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 2011;10:230–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. α‐Syn RT‐QuIC end‐point dilution analysis of BH samples from DLB (Panel a), MSA‐C (Panel B) and PD (Panel C) patients.
Figure S2. α‐Syn RT‐QuIC end‐point dilution analysis of CSF samples from patients with pure DLB.


