Abstract
Our objective is to report longitudinal results of the MG‐ADL, MG‐Composite, MG‐MMT, and MG‐QoL15 in an open‐label trial of therapeutic plasma exchange in myasthenia gravis. Ten MG patients experiencing exacerbation had assessments prior to, immediately following, and at selected time points post‐TPE. Changes from baseline to 2 weeks post‐TPE were: MG‐ADL median −5.0, P < 0.0033, MG‐QoL15 median −13.0, P < 0.001, MG‐MMT median −10.0, P < 0.0001, and MG‐Composite median −10.0, P < 0.005. TPE produced a rapid, clinically significant change in all instruments, indicating these outcome measures are robust endpoints for clinical trials of rapidly efficacious MG therapies.
Introduction
Myasthenia gravis (MG) is a debilitating and potentially fatal autoimmune disease characterized by autoantibodies directed against epitopes of the postsynaptic muscle membrane, including the nicotinic acetylcholine receptor (AChR) and the muscle‐specific tyrosine kinase receptor (MuSK), and complement‐mediated destruction of the postjunctional membrane. Clinical manifestations include fluctuating weakness of ocular, bulbar, respiratory, and limb muscles.1 Current long‐term therapies for MG include thymectomy (THX), cholinesterase inhibitors, and immunosuppressive or immunomodulatory agents. Exacerbations are typically treated with rapidly efficacious therapies such as intravenous or subcutaneous immunoglobulins (IVIg or SCIg) and therapeutic plasma exchange (TPE).
Formal recommendations for clinical research standards identified a need for validated, disease‐specific measures to assess therapeutic responses in MG clinical trials, including patient‐reported functional and quality of life quality of life measures.2 These recommendations led to validation studies of the Quantitative MG score (QMG),3 MG‐Activities of daily living profile (MG‐ADL),4 manual muscle test (MG‐MMT),5 MG‐Composite (MG‐C),6 and Quality of Life 15 (MG‐QoL15).7 These measures provide a consistent methodology for assessing clinical response and include patient‐centered outcomes. These outcome measures, particularly the QMG and MG‐ADL, are being used as primary endpoints in clinical trials for new MG therapies including: eculizumab (NCT00727194 and NCT01997229), ARGX113 (NCT03669588), RA101495 (NCT03315130), CFZ533 (NCT02565576), M281 (NCT03772587), and UCB7665 (NCT03052751). However, data on the performance of these outcome measures in the setting of rapidly efficacious therapies, such as TPE, are sparse. Published data have relied upon clinical muscle strength testing8 and demonstration of reduction in immunoglobulin or autoantibody levels9, 10 during and immediately following TPE, but have not utilized validated, MG‐specific outcome measures.11, 12 Data regarding the responsiveness of MG‐specific outcome measures could be invaluable in planning clinical trials of rapidly efficacious therapies for MG.
Methods
This report utilized data from an open‐label study of MG patients who received TPE for a MG exacerbation at Duke University Medical Center and The University of North Carolina at Chapel Hill Hospital. The primary objective of the trial was to characterize the immunoglobulin and autoantibody response following TPE for treatment of MG exacerbation.13 Secondary objectives were to evaluate changes in MG‐specific clinical outcome measures and correlate with changes in immunoglobulin and autoantibody levels. Details of the study have been reported previously.13 In summary, eligible subjects were ≥18 years old with detectable antibodies to the AChR (AChR+), had a clinical diagnosis of MG, and an indication for use of TPE. Major exclusion criteria included antibodies to MuSK or low‐density lipoprotein receptor‐related protein 4, and history of thymoma, thymectomy, or rituximab infusion in the 6 months prior to enrollment. Enrolled subjects received five to six TPE sessions (1 plasma volume per session) at a frequency of every other day, performed in accordance with institutional practices. Clinical assessments included the MG‐ADL, MG‐QoL15, MG‐MMT, and MGC. Immunoglobulin and autoantibody levels were performed prior to the first and third TPE sessions, after the last TPE session, and at weeks 1, 2, 3, 6, and 12 post‐TPE. Immunologic assays were performed as previously described.13 The primary outcome for this analysis was the change in clinical outcome scores from baseline to 2 weeks post‐TPE as this time point has been used in prior studies of TPE.12 Statistical significance was determined by ANOVA with one‐way repeat measures for the primary endpoints at 2 weeks post‐TPE. A clinically significant change for the purposes of this analysis was defined as a change in score of ≥3 points for the MG‐ADL, MG‐QoL, MG‐MMT, and MGC and reductions in the total score indicated clinical improvement. Linear regression analysis was used to estimate the strength of correlations between immunologic markers and clinical outcome measures. Spearman correlation was performed to calculate strength of the relationship between clinical outcome measures. All data analysis was performed using SAS® version 9.1 (SAS Institute, Cary, NC). Level of statistical significance was set at P < 0.05.
Results
Demographics
Ten AChR + MG patients were enrolled. All 10 patients completed the study protocol to the primary endpoint, and one patient discontinued during the observation period after the primary outcome time point due to an unrelated stroke. Most patients were taking concomitant immunomodulatory therapy and had moderate disease severity at the time of enrollment (Table 1). The racial distribution and severity of disease reflect enrollment in recent MG clinical trials where patients tend to be Caucasian and MGFA Severity Class II‐IV, whereas our population was somewhat older, had a relatively short disease duration overall, and was predominantly male.
Table 1.
Baseline demographics of enrolled MG patients (N = 10).
| Median age in years, (range) | 72.9 (20–86) |
| Male N (%) | 6 (60%) |
| Caucasian N (%) | 9 (90%) |
| Median BMI (kg/m2), range | 28.4 (20.2–32.4) |
| Concomitant MG medications | |
| Acetylcholinesterase inhibitors | 80% |
| Corticosteroids | 60% |
| Oral immunomodulators1 | 50% |
| Median duration of MG in years, (range) | 0.8 (0.0–38.0) |
| Baseline MGFA severity class | |
| IIa | 20% |
| IIIa | 30% |
| IIIb | 40% |
| Iva | 10% |
Primarily mycophenolate mofetil and azathioprine.
Clinical outcome measures
The MG‐ADL, MG‐QoL15, MG‐MMT, and MG‐Composite all demonstrated a statistically significant change and a clinically significant improvement at 2 weeks post‐TPE (Table 2 and Fig, 1). The maximal improvement occurred at 6 weeks (MG‐ADL, MG‐QoL) or 12 weeks (MG‐MMT and MG‐Composite) post‐TPE (Table 2). Individual‐level data are presented in Figures S1–S4. Strong correlations were observed in the change in outcome measures at 2 weeks post‐TPE for the MG‐ADL and MG‐Composite (r = 0.82, P < 0.003), MG‐QoL15 and MG‐Composite (r = 0.74, P < 0.014), and MG‐MMT and MG‐Composite (r = 0.67, P < 0.033).
Table 2.
Summary of clinical outcome measures (N = 10).
| Time point | Baseline | End of TPE | 2 weeks post‐TPE | Change at 2 weeks post‐TPE | 6 weeks post‐TPE | Change at 6 weeks post‐TPE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (range) | Mean (SD) | Median (Range) | Mean (SD) | Median (range) | Median (range) | P‐value | Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| MG‐ADL | 8.7 (2.0) | 8.5 (6–12) | 3.9 (1.9) | 3.5 (2–7) | 4.4 (2.7) | 3.5 (1–9) | −5.0 (−10 to 1) | <0.0033 | 3.6 (3.5) | 2.0 (0–12) | −5.3 (4.4) | –5.0 (−12 to 3) |
| MG‐QoL15 | 28.8 (9.2) | 25.0 (18–43) | 20.2 (13.8) | 19.5 (3–41) | 16.5 (13.7) | 13.5 (2–41) | –13.0 (−32 to 5) | <0.001 | 12.8 (14.3) | 5.0 (0–36) | –15.2 (11.9) | –18.0 (−32 to 10) |
| MG‐MMT | 29.5 (21.7) | 22.5 (11–81) | 15.3 (19.5) | 8.0 (3–64) | 15.1 (22.0) | 7.0 (2–75) | –10 (−44 to 0) | <0.0001 | 14.2 (23.2) | 7.0 (1–74) | –14.3 (11.7) | −10.0 (−42 to −4) |
| MG‐Composite | 20.7 (4.5) | 21 (12–27) | 10.2 (5.2) | 9.0 (4–19) | 10.0 (6.2) | 8.0 (3–20) | –10.0 (−22 to −3) | <0.0015 | 10.1 (7.7) | 6.0 (3–24) | –10.7 (7.3) | –14.0 (−25 to 4) |
TPE, therapeutic plasma exchange; SD, standard deviation; MG, myasthenia gravis; ADL, Activities of Daily Living; QoL15, Quality of Life 15; MMT, manual muscle test.
Figure 1.
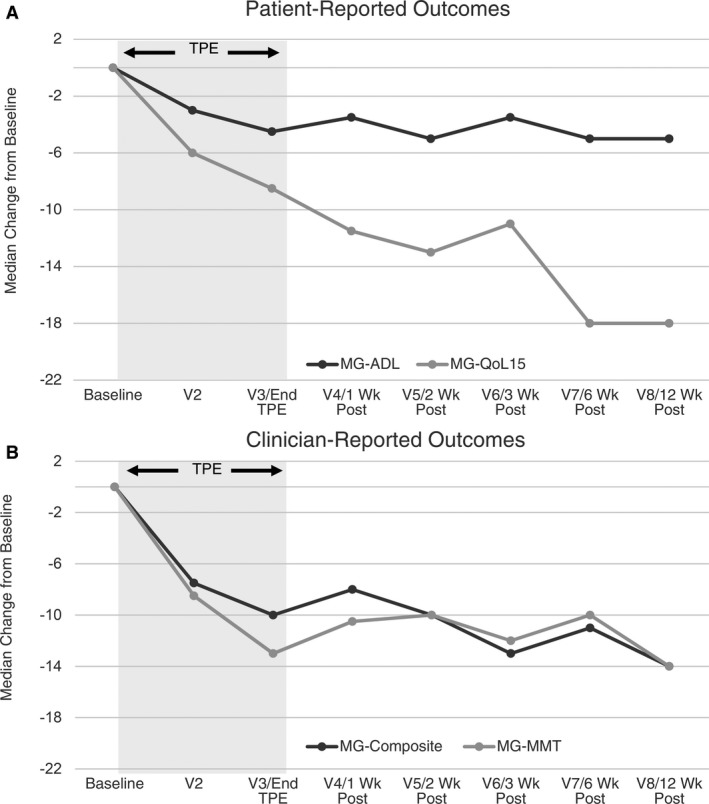
Change in outcome measures relative to baseline. A negative change indicates improvement in patient‐reported (A) and clinician‐reported (B) outcome measures. Interpretation of clinical changes beyond 6 weeks post‐TPE is limited by the confounding effect of changes in concomitant medications that were permitted by the study design starting at 4 weeks post‐TPE.
Immunologic correlations
There was poor correlation between total IgG levels and MG‐Composite at baseline (R 2 = 0.018) and at 2 weeks post‐TPE (R 2 = 0.042). Correlation between AChR antibody titer and MG‐Composite score at baseline (R 2 = 0.022) and at 2 weeks post‐TPE (R 2 = 0.093) and between AChR antibody titer and MG‐ADL at baseline (R 2 < 0.0001) and at 2 weeks post‐TPE (R 2 = 0.198) was also poor.
Discussion
Prior clinical studies of rapidly efficacious therapies such as TPE and IVIG have focused on the QMG score10, 11, 12, 14 which was the primary efficacy endpoint accepted by the U.S. Food and Drug Administration at the time. Recent trials have used the MG‐ADL as the primary or key secondary efficacy endpoint15 but not the MG‐QoL15. Published data from clinical trials for recently validated outcome measures, particularly the MG‐Composite, are limited and are rarely reported together. Outcome measure data from our TPE study, which included the MG‐ADL, MG‐Composite, MG‐MMT, and MG‐QoL15, will aid in the planning of future clinical trials with rapidly efficacious drugs by providing insights into sample size determination and timing of outcome measure assessment.
We found that TPE produces a rapid and statistically significant change from baseline in all outcome measures assessed 2 weeks post‐TPE. Our findings are similar to those of Barnett and colleagues16 who assessed MG‐QoL15 following administration of IVIG and TPE in patients with myasthenia gravis. Their evaluator‐masked trial found a mean change of −7 points in the MG‐QoL15 score at 2 weeks post‐TPE (n = 30), whereas our trial found a mean change of −12 points at 2 weeks post‐TPE. The observed differences in the magnitude of the MG‐QoL15 score between Barnett’s study and ours may be attributable to our inclusion of a more severe disease population (70% MGFA Class III vs. 43% in Barnett), a smaller sample size (N = 10 vs. N = 30), and the unblinded nature of our study. We were not able to make any generalizations relating to age or demographics due to the small numbers, but these relationships would be worth exploring in larger studies. Our study also demonstrated that patient‐reported outcome scores continued to improve across all measures with a nadir at 6 weeks post‐TPE for the MG‐ADL and MG‐QoL15, suggesting a continued clinical effect after completion of treatment and maximal reduction of autoantibodies levels. Some of the continued reduction in patient‐reported outcomes after the completion of TPE treatment may reflect a delay in returning to a more normal lifestyle after intensive therapy and in some cases, hospitalization. This factor should be considered while planning future clinical trials that use these outcome measures as a key endpoint.
While Barnett and colleagues demonstrated correlations between the QMG score and individual items of the QoL15, our study utilized the MG‐MMT, making direct comparisons of the clinician‐reported instruments difficult. Correlation between the change in MG‐QoL15 and MG‐Composite scores was very strong, suggesting the MG‐Composite might be a reasonable benchmark for future studies to establish the yet undetermined clinically meaningful improvement in the MG‐QoL15.17 Data from our pilot study show a strong correlation between the MG‐ADL and MG‐Composite, which is not surprising given that the patient‐reported aspects of the MG‐Composite are derived from the MG‐ADL.
Our study13 demonstrated that TPE depletes immunoglobulin and AChR antibodies, but the correlation between these levels and clinical outcome measures, particularly the MG‐Composite and MG‐ADL, were not robust despite a statistically significant improvement in all outcome measures (Fig. 1). This finding may reflect variability in immunoglobulin and AChR antibody titers among individuals within a small overall sample size. However, our data are consistent with prior reports that similarly found that antibody titers correlate only weakly with clinical improvement.18, 19, 20 Such weak correlation between autoantibody and immunoglobulin titers and clinical response indicates that validated, MG‐specific clinical outcome measures should continue to be the primary means of assessing response to existing and novel rapidly efficacious therapies in clinical trials for the foreseeable future.
Author Contributions
SMR performed data analysis and produced the manuscript. JFH and JTG participated in the conception, design, and conduct of the study, data analysis, and provided critical appraisal/editing of the manuscript. VCJ, JMM, and MC participated in the design and conduct of the study and provided critical appraisal/editing of the manuscript.
Conflict of Interest
VCJ receives research support from PCORI and Alexion Pharmaceuticals. MC received research support from Alexion Pharmaceuticals. JH reported research support from Alexion Pharmaceuticals, the Centers for Disease Control and Prevention (Atlanta, GA) and the Muscular Dystrophy Association; grants from Alexion Pharmaceuticals, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke, and the National Institute of Arthritis and Musculoskeletal and Skin Disease), PCORI and UCB Pharma; honoraria from Alexion Pharmaceuticals and nonfinancial support from Alexion Pharmaceuticals, Argenx, Ra Pharmaceuticals and Toleranzia. JTG is supported by K23NS085049; full conflict of interest disclosures are available at: http://www.dcri.duke.edu/research/coi.
Supporting information
Figure S1. Individual‐level data and Group Mean for MG – QoL15.
Figure S2. Individual‐level data and Group Mean for MG – ADL.
Figure S3. Individual‐level data and Group Mean for MG – MMT.
Figure S4. Individual‐level data and Group Mean for MG – Composite.
Acknowledgments
This study was supported by a research grant from UCB Biosciences.
Funding information
This study was supported by a research grant from UCB Biosciences. VCJ receives research support from PCORI and Alexion Pharmaceuticals. MC receives research support from Alexion Pharmaceuticals. JH reported research support from Alexion Pharmaceuticals, the Centers for Disease Control and Prevention (Atlanta, GA) and the Muscular Dystrophy Association; grants from Alexion Pharmaceuticals, the National Institutes of Health (including the National Institute of Neurological Disorders and Stroke, and the National Institute of Arthritis and Musculoskeletal and Skin Disease), PCORI and UCB Pharma; honoraria from Alexion Pharmaceuticals and nonfinancial support from Alexion Pharmaceuticals, Argenx, Ra Pharmaceuticals and Toleranzia. JTG is supported by K23NS085049.
Funding Statement
This work was funded by UCB Biosciences grant ; Centers for Disease Control and Prevention grant ; Muscular Dystrophy Association grant ; National Institutes of Health grant K23NS085049; National Institute of Neurological Disorders and Stroke grant ; UCB grant ; Alexion Pharmaceuticals grant .
References
- 1. Sanders DB, Guptill JT. Myasthenia gravis and Lambert‐Eaton myasthenic syndrome. Continuum (Minneap Minn). 2014;20:1413–1425. [DOI] [PubMed] [Google Scholar]
- 2. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000;55:16–23. [DOI] [PubMed] [Google Scholar]
- 3. Barohn RJ, McINTIRE D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann NY Acad Sci 1998;841:769–772. [DOI] [PubMed] [Google Scholar]
- 4. Muppidi S, Wolfe GI, Conaway M, Burns TM. MG‐ADL: still a relevant outcome measure. Muscle Nerve 2011;44:727–731. [DOI] [PubMed] [Google Scholar]
- 5. Sanders DB, Tucker‐Lipscomb B, Massey JM. A simple manual muscle test for myasthenia gravis ‐ validation and comparison with the QMG score. Ann NY Acad Sci 2003;998:440–444. [DOI] [PubMed] [Google Scholar]
- 6. Burns TM, Conaway M, Sanders DB; Composite MG, Group M‐QS . The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology 2010;74:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mullins L. L, Carpentier M. Y, Paul R. H; Muscle Study G . Disease‐specific measure of quality of life for myasthenia gravis. Muscle Nerve 2008;38:947–956. [DOI] [PubMed] [Google Scholar]
- 8. Gajdos P, Chevret S, Clair B, et al. Clinical trial of plasma exchange and high‐dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann Neurol 1997;41:789–796. [DOI] [PubMed] [Google Scholar]
- 9. Volkin RL, Starz TW, Winkelstein A, et al. Changes in coagulation factors, complement, immunoglobulins, and immune complex concentrations with plasma exchange. Transfusion 1982;22:54–58. [DOI] [PubMed] [Google Scholar]
- 10. Liu JF, Wang WX, Xue J, et al. Comparing the autoantibody levels and clinical efficacy of double filtration plasmapheresis, immunoadsorption, and intravenous immunoglobulin for the treatment of late‐onset myasthenia gravis. Ther Apher Dial 2010;14:153–160. [DOI] [PubMed] [Google Scholar]
- 11. Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: a randomized controlled trial. Neurology 2007;68:837–841. [DOI] [PubMed] [Google Scholar]
- 12. Barth D, Nabavi Nouri M, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011;76:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guptill JT, Juel VC, Massey JM, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity 2016;49:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolfe GI, Barohn RJ, Foster BM, et al. Randomized, controlled trial of intravenous immunoglobulin in myasthenia gravis. Muscle Nerve 2002;26:549–552. [DOI] [PubMed] [Google Scholar]
- 15. Howard JF Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti‐acetylcholine receptor antibody‐positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double‐blind, placebo‐controlled, multicentre study. Lancet Neurol 2017;16:976–986. [DOI] [PubMed] [Google Scholar]
- 16. Barnett C, Wilson G, Barth D, et al. Changes in quality of life scores with intravenous immunoglobulin or plasmapheresis in patients with myasthenia gravis. J Neurol Neurosurg Psychiatry 2013;84:94–97. [DOI] [PubMed] [Google Scholar]
- 17. Burns TM, Grouse CK, Conaway MR, et al;Composite and MG QoL15 Study Group . Construct and concurrent validation of the MG‐QOL15 in the practice setting. Muscle Nerve 2010;41:219–226. [DOI] [PubMed] [Google Scholar]
- 18. Sanders DB, Burns TM, Cutter GR, et al. Does change in acetylcholine receptor antibody level correlate with clinical change in myasthenia gravis? Muscle Nerve 2014;49:483–486. [DOI] [PubMed] [Google Scholar]
- 19. Roses AD, Olanow CW, McAdams MW, Lane RJ. No direct correlation between serum antiacetylcholine receptor antibody levels and clinical state of individual patients with myasthenia gravis. Neurology 1981;31:220–220. [DOI] [PubMed] [Google Scholar]
- 20. Olanow CW, Wechsler AS, Roses AD. A prospective study of thymectomy and serum acetylcholine receptor antibodies in myasthenia gravis. Ann Surg 1982;196:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Individual‐level data and Group Mean for MG – QoL15.
Figure S2. Individual‐level data and Group Mean for MG – ADL.
Figure S3. Individual‐level data and Group Mean for MG – MMT.
Figure S4. Individual‐level data and Group Mean for MG – Composite.


