Abstract
Gastric cancer (GC) represents a leading cause of cancer related morbidity and mortality worldwide accounting for more than 1 million of newly diagnosed cases and thousands of deaths every year. In the last decade, the development of targeted therapies and the optimization of already available chemotherapeutic drugs has expanded the available treatment options for advanced GC and granted better survival expectations to the patients. At the same time, global efforts have been undertaken to investigate in detail the genomic and epigenomic heterogeneity of this disease, resulting in the identification of new specific and sensitive predictive and prognostic biomarkers and in innovative molecular classifications based on gene expression profiling. Nonetheless, several randomized studies aimed at exploring new innovative agents, such as immune checkpoint inhibitors, failed to demonstrate clinically meaningful survival advantages. Therefore, it is essential to further improve the molecular characterization of GC subgroups in order to provide researchers and medical oncologists with new tools for patients’ selection and stratification in future clinical development programs and subsequent trials. The aim of the present manuscript is to provide a global overview of the recent molecular classifications from The Cancer Genome Atlas and the Asian Cancer Research Group and to present key promising developments in the field of immunotherapy and targeted therapies in metastatic GC.
Keywords: Gastric cancer, Personalized medicine, Predictive biomarkers, Molecular diagnostic, The Cancer Genome Atlas, Asian Cancer Research Group
Core tip: Gastric cancer (GC) still represents a leading cause of cancer related morbidity and mortality worldwide accounting for more than 1 million of newly diagnosed cases and thousands of deaths every year. In the last decade, global efforts have been undertaken to investigate in detail the genomic and epigenomic heterogeneity of this disease, resulting in innovative molecular classifications of GC based on gene expression profiling and in the identification of new specific and sensitive predictive and prognostic biomarkers. At the same time, the development of targeted therapies has expanded the treatment scenario for advanced GC. The aim of the present manuscript is to provide a detailed and comprehensive overview of the recent molecular classifications from The Cancer Genome Atlas and the Asian Cancer Research Group and to present key promising developments in the field of immunotherapy and targeted therapies in metastatic GC.
INTRODUCTION
Gastric Cancer (GC) is the third leading cause of cancer death and the fifth most common malignancy worldwide for both sex, accounting for over 1 million new diagnoses and almost 800000 patients deaths in 2018[1]. Over 70% of GCs occur in low/middle income countries with the highest rates in Eastern Asia, Eastern Europe and South America and the lowest rates in North America and Western European countries[2,3].
Since the middle of the 20th century a progressive decline of distal GC incidence has been observed, possibly due to recognition and eradication of certain risk factors such as Helicobacter pylori (H. pylori), the introduction of refrigerators and an increased use of fresh food[4,5]. Conversely, the rate of gastroesophageal junction cancer (GEJC) is increasing in Western countries, which probably reflects the increase of gastroesophageal reflux disease and visceral obesity[6,7]. Considering the rising of worldwide population, the absolute number of new diagnoses per year is increasing, so that GC still remains an important cause of cancer-related mortality and a main global health-care problem.
Surgery is the only potentially curative treatment while neoadjuvant and adjuvant therapies should be integrated with surgery in locally advanced disease. Despite these multimodal treatments the 5 years overall survival is less than 30%, and in metastatic setting the prognosis remains poor with a median overall survival (OS) of 1 year[8].
The last decade has been characterized by a better understanding of molecular mechanisms of pathogenesis and biology of GC with the definition of new genomic classifications and identification of prognostic and predictive biomarkers potentially predictive of response to innovative target agents. Despite this, up to now, few target-directed options have been approved for metastatic GC. The anti-human epidermal growth factor receptor-2 (HER2) drug trastuzumab has been the first target agent approved for HER2 high expressing advanced GCs and GEJCs, while the antiangiogenic drug ramucirumab has received approval in the second-line setting as a monotherapy or in combination with paclitaxel. More recently, anti-PD1 agents such as nivolumab and pembrolizumab have been approved for patient with heavily pretreated advanced GC in some Asian countries and North America, respectively.
More than 90 percent of GCs are adenocarcinomas. According to Lauren’s criteria, gastric adenocarcinomas are divided into intestinal (54%), diffuse (32%), and indeterminate type (15%), which present distinct epidemiology, histological appearance, cell differentiation and molecular pathogenesis[9,10]. Intestinal carcinomas are more likely to be sporadic than inherited and causally related to H. pylori infection and the Correa’s phenotypic multistep cascade (i.e., longstanding gastritis, atrophic gastritis, dysplasia and adenocarcinoma)[11]. Histologically, diffuse-type ade-nocarcinomas are poorly differentiated and composed by discohesive cells usually characterized by with a dysregulation in the expression of E-cadherin, a key cell surface and connection protein[12]. Both Lauren classification and the World Health Organization (WHO) 2010 classification[13], although widely used in the clinical practice, remain insufficient to guide precision treatments for the individual patient and GC histotype is not a parameter considered in the treatment decision process.
GC MOLECULAR CLASSIFICATION
The Cancer Genome Atlas research group
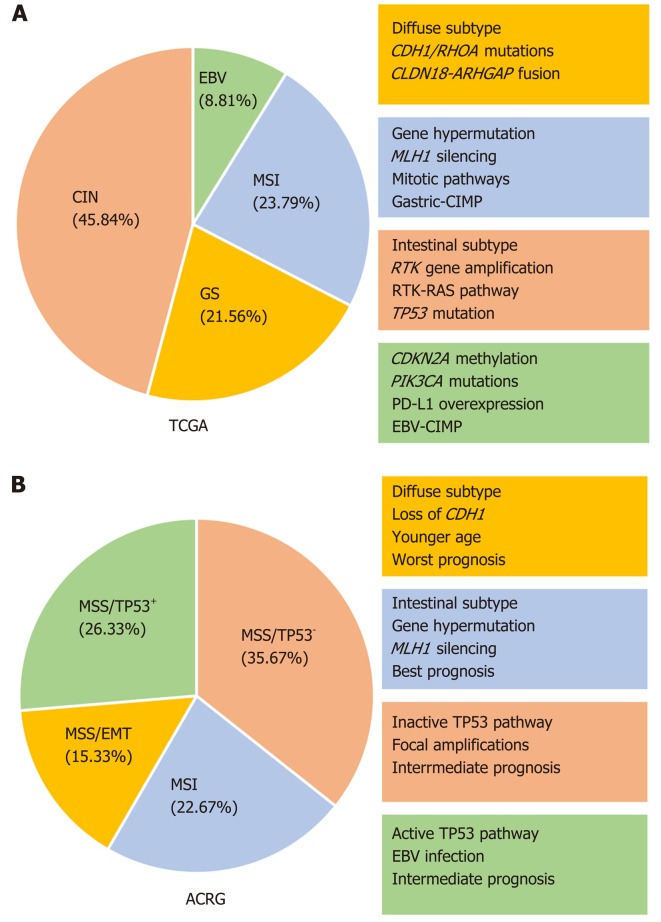
In 2014, the The Cancer Genome Atlas (TCGA) network proposed a comprehensive molecular analyses of 295 primary GC based on array-based somatic copy number analyses, whole exome and genome sequencing, messenger RNA and microRNA sequencing, and reverse-phase protein array profiling. As a result, four GC subgroups were identified: Epstein-Barr (EBV) positive tumors, tumors with microsatellite instability (MSI), genomically stable tumours (GS) and tumors with chromosomal instability (CIN)[14].
EBV activation was found in about 9% of tumor samples. All EBV positive tumours were associated to extreme DNA hypermethylation with high levels of CIMP (i.e., CpG island methylation) of CDKN2A (p16 NK4A) promoter but not of MutL homolog 1 (MLH1), characteristic of MSI associated CIMP. As previously reported, pho-shatidylinositol-4-5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations were detected in 80% of EBV-positive GC[15,16]. Moreover, the finding of an overexpression of programmed death ligands 1 and 2 (PD-L1 and PD-L2) and of a significant immune cell presence supported the rationale to evaluate checkpoint inhibitors in this GC subgroup.
The MSI group (22%) was characterized by loss of mismatch repair functions which lead to alterations in length of repetitive regions in DNA known as microsatellites. The hypermethylation of MLH1 promoter region was the most representative mismatch repair defect in patient with MSI sporadic GCs. Alterations of PIK3CA, ERBB3 and ERB22 were found and in contrast to MSI colorectal cancers, BRAF mutations have never been described in MSI-GCs. MSI GCs can be part of the spectrum of inherited malignancies such as Lynch syndrome and nonpolyposis colorectal cancer syndrome which are associated to inherited germline mismatch repair defects[17]. Although colorectal and endometrial cancers are the most common cancer associated to these syndromes, other extracolic tumours including GC, can occur[18]. MSI GCs are mainly associated with intestinal histotype, are localized in the antrum, with less frequent lymph-node involvement, occur mainly in elderly age and have a more favourable prognosis[19,20].
GS tumors (20%) are characterized by low copy number alterations and a low mutation rate. ARID1, RHOA and CDH1 mutations are the principal somatic genomic alterations observed in this class. An interchromosomal translocation between CLDN18 and ARHGAP26, mutually exclusive from RHOA mutations, was found in the 15% of GS GCs. These tumors usually occur in younger patients (median age 59), and are correlated with diffuse histology and distal localization. GS subtype was associated with the worst OS and prognosis among the four TCGA subtypes.
The fourth TCGA group are GCs with CIN (50%) characterized by DNA aneuploidy, highly variable chromosomal copy numbers, and mutations of the tumor suppressor TP53, which is responsible for chromosomal instability. Frequent genomic amplifications of receptor tyrosine kinases (RTKs)/RAS pathway were found, including epidermal growth factor receptor (EGFR), ERBB2, ERBB3, MET proto-oncogene (MET), fibroblast growth factor receptor 2 (FGFR2), vascular endothelial growth factor A (VEGFA), and KRAS. Most of CIN GCs are classified as intestinal-type, frequently located at the gastroephageal junction/cardia[21,22].
The Asian Cancer Research Group
In 2015 the Asian Cancer Research Group (ACRG) proposed a different molecular classification based on gene expression profiling, genome-wide copy number microarrays and targeted gene sequencing of 300 primary tumors with the definition of four molecular subtypes: Microsatellite unstable type, epithelial to mesenchymal-like type (MSS/EMT), MSS/TP53 and MSS/TP53 negative subtypes[23]. Each of these molecular subtypes was correlated to distinct clinical phenotypes and genomic alterations.
MSI GCs occurred preferentially in the antrum, with intestinal histology (more than 60%) and half of them were diagnosed at an early stage disease. MSI tumors were characterized by loss of expression of MLH1 and an elevated DNA methylation signature. The MSI subtype was associated with the presence of hypermutation, with mutations of ARID1A (44.2%), the PI3K-PTEN-mTOR pathway (42%), KRAS (23.3%) and ALK (16.3%).
The MSS/EMT subtype was observed at significantly younger age, with diffuse histology at stage III/IV and showed CDH1 loss of expression. The EMT subtype presented a lower number of mutation events when compared to the other MSS groups. The MSS/EMT had the worst prognosis, while the MSI subtype showed the best prognosis of the four. The authors observed that the MSS/EMT group presented a higher percentage of recurrence vs the MSI group (63% vs 23%). The MSS/EMT GC subtype was associated to a higher frequency of peritoneal metastases compared to all other subtypes, while a higher percentage of liver-limited metastasis in the MSI and MSS/TP53 subtypes was found.
MSS/TP53 positive and MSS/TP53 negative showed an intermediate prognosis and also an intermediate chance of recurrence. EBV infection was more frequently associated to MSS/TP53 positive group. MSS/TP53 negative subtype exhibited the highest prevalence of TP53 mutations (60%) and a low frequency of other mutations, as well as recurrent focal amplification of ERBB2, EGFR, CCNE1, CCND1, MDM2, ROBO2, GATA6 whereas the MSS/TP53 positive subtype showed a relative higher (compared to MSS/TP53 negative) of mutations in APC, ARID1A, KRAS, PIK3CA and SMAD4.
Comparison between the TCGA and ACRG classification
Comparisons between TCGA and ACRG genomic subtypes showed similarities and differences (Figure 1). Both molecular classifications revealed MSI positive tumors and TCGA GS, EBV and CIN subtypes are likely to be approximated to ACRG MSS/EMT, MSS/TP53 positive and MSS/TP53 negative subtypes, respectively. Tumors classified as the GS subtype in the TCGA set were present across all ACRG subtypes in the ACRG data set, while tumors classified as the TCGA CIN subtype were present across all ACRG subtypes in the TCGA data set. A substantially lower percentage of Lauren’s diffuse subtype cases were found in the TCGA cohort compared to ACRG database (24% vs 45% respectively) with the majority (57%) of Lauren’s diffuse-sub-type cases present in the TCGA GS group but only 27% cases present in the ACRG MSS/EMT subtype. Additionally, CDH1 and RHOA mutations, which were mutated in TCGA GS, were infrequent in the ACRG MSS/EMT subtypes. These differences suggest that TCGA GS type is not equivalent to the ACRG MSS/EMT subtype.
Figure 1.
The cancer genome atlas and the Asian cancer research group molecular classification of gastric cancer. EBV: Epstein-Barr; CIN: Chromosomal instability; MSI: Microsatellite instability; GS: Genomically stable tumours; MSS/EMT: Microsatellite unstable type, epithelial to mesenchymal-like type.
Collectively, these findings confirm that the TCGA and ACRG classification systems are related but distinct in terms of demographics, molecular mechanisms, driver genes and prognosis. Although these novel classifications have provided a deeper understanding of GC biology, some limitations can be observed. First, these analyses are based on complex molecular technologies and could not be replied in standard laboratories. Furthermore, a prospective validation on large scale including patients of different age and ethnicity is needed. Finally TCGA and ACRG classifications are the result of comprehensive molecular analysis on malignant GC epithelial cells that don’t consider the impact of peritumoral stromal cells. Of note, novel stromal gene signatures have been analyzed in comparison to the dominant cancer phenotypes identified by TCGA and ACRG, revealing distinct stromal phenotypes[24,25].
CURRENT STANDARD TREATMENTS IN METASTATIC GC
Chemotherapy remains the backbone of therapy in patients with metastatic GC and good performance status. Available data from randomized clinical trials showed a statistically significant benefit of palliative chemotherapy, compared with best supportive care (BSC), in terms of symptom control, improvement of quality of life (QoL) and overall survival (OS).
In the first line setting a variety of cytotoxic drugs, including platinum compounds, fluoropyrimidines, anthracyclines, taxanes, and irinotecan, have shown activity in GC. Currently, a combination including a platinum compound (cisplatin or oxaliplatin) plus a fluoropyrimidine (5-FU,capecitabine, or S-1) agent is one of the most widely used doublet on the basis of a balanced benefit-to risk ratio[26].
Currently, trastuzumab is the only molecularly targeted drug accepted in first-line therapy, in combination with cisplatin and a fluoropyrimidine, for the treatment of patients with metastatic HER2-overexpressing GC or GEJC who have not received anti-cancer treatment for their metastatic disease. As a result, all cases of advanced GC should be characterized for HER2 status. HER2 is a member of the epidermal growth factor receptors (EGFRs) family and is involved in transmembrane signaling, and overexpression/activation leads to increased cell proliferation, growth and survival[27]. HER2 overexpression or/and amplification is found in approximately 20% of metastatic GC, although there are differences depending on tumor subtype, is more common in intestinal GC than diffuse GC, and more common in GEJC than distal GC[28].
The phase III, open-label, randomised controlled ToGA trial (Trastuzumab for GC) compared in a population of 594 previously untreated patients standard chemotherapy (six courses of cisplatin plus either infusional fluorouracil or capecitabine) with and without trastuzumab until disease progression. All end points were improved with the addition of trastuzumab to chemotherapy, including objective response rate (ORR) (47.3% vs 34.5%), PFS (6.7 vs 5.5 mo; HR: 0.71; 95%IC: 0.59-0.85; P < 0.0002), and at a median follow-up of 17 to 19 mo, median OS was significantly better with trastuzumab (13.8 vs 11.1 mo) (HR: 0.74; 95%CI: 0.60-0.91; P = 0.0046). The inclusion criteria of the ToGA trial were a 3+ HER2 immuno-histochemical (IHC) overexpression or the presence of HER2 gene amplification as assessed by fluorescent in situ hybridization (FISH), irrespective of IHC score[29].
Despite the demonstrated efficacy of numerous chemotherapy options, only 40% of patients who progressed to first-line chemotherapy are susceptible to a second-line chemotherapy on progression[30]. In this setting, ramucirumab, a fully human monoclonal antibody VEGFR-2 antagonist, is the only molecular-targeted drug with a confirmed, although modest, survival benefit.
The activity of ramucirumab, in second-line treatment of GC was investigated by the phase III REGARD trial (Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma), a randomized, double-blind, placebo-controlled study. In the REGARD trial 355 patients with previously treated advanced GC or GEJC adenocarcinomas were randomized to best supportive care plus either ramucirumab or placebo. Median OS was 5.2 mo in the ramucirumab arm and 3.8 mo in the placebo arm (HR: 0.78; 95%CI: 0.603-0.998; P = 0.047)[31]. However, the RAINBOW trial (Ramucirumab plus paclitaxel vs placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma) was the landmark study that demonstrated the benefit of ramucirumab in second line setting in combination with chemotherapy, which compared weekly paclitaxel (80 mg/m2 on days 1, 8, and 15 of each 28-day cycle) plus ramucirumab (8 mg/kg IV every two wk) or placebo in 665 patients. Median OS and PFS were significantly longer in patients treated with ramicurumab than in the placebo-plus-paclitaxel group (median OS: 9.6 vs 7.4 mo, HR: 0.81, 95%CI: 0.678-0.962, P = 0.017 and median PFS 4.4 vs 2.9 mo, HR: 0.635, 95%CI: 0.539-0.752, P ≤ 0.001, respectively)[32].
Largely based on this trial results, ramucirumab plus paclitaxel is currently the preferred choice for second-line therapy. More recently, the phase III TAGS study (Trifluridine/tipiracil vs placebo in patients with heavily pretreated metastatic GC) proved that trifluridine/tipiracil is an effective treatment option for patients with heavily pretreated metastatic GC. The study demonstrated a 31% reduction in risk of death and a 2.1-month improvement in median OS in treated patients[33].
HER2: PRIMARY AND ACQUIRED RESISTANCE
The anti-HER2 monoclonal antibody trastuzumab plus standard chemotherapy have significantly improved response rate and survival outcomes in primary GC and GEJC displaying HER2 overexpression/amplification. Unfortunately, about 50% of patients did not respond to the combination treatment suggesting the existence of a primary resistance[29]. At same time, acquired resistance usually limits the duration of response to this treatment.
Genomic alterations of the RTK pathway such as EGFR, FGFR2, MET, and KRAS amplification may be responsible for primary resistance to HER2-targeting drugs[14]. Recently, amplifications of cell-cycle–related genes such as CCNE1 and CDK6, PI3K mutations, and amplification of MET have shown to confer resistance to anti-HER2 agents in vitro HER2–amplified cell-line models[34]. Although uncommon, other rare alterations in RTK pathways such as ALK, ROS1, NTRK1/2/3 and RET fusion could be correlated with primary resistance to trastuzumab[35-37]. To confirm these data, a recent study investigated a panel of genomic alterations including mutations in the EGFR / MET / KRAS / PI3K / PTEN genes and amplifications in EGFR / MET / KRAS in 37 patients treated with trastuzumab (17 responders and 20 patients with primary resistance). Interestingly, panel alterations were significantly more frequent in resistant (11 of 20, 55%) as compared with sensitive patients and in HER2 IHC 2+ than 3+ tumors. Patients with no alteration had a significantly longer median PFS and OS[38].
Mechanisms of acquired resistance to anti-HER2 treatment in GC are largely unknown. Pietrantonio et al[39] have analyzed 22 matched tumor samples taken at baseline and post-progression in patients receiving chemotherapy and trastuzumab for advanced HER2 IHC 3+ or 2+ GC. Loss of HER2 was identified as a mechanism of resistance in 32% of samples and the probability of loss of HER2 positivity was significantly higher in patients with baseline IHC score 2+ vs 3+. Similarly, loss of HER2 and frequent secondary alterations in the RTK/RAS/PI3K pathway in HER2 positive adenocarcinoma have been observed in patients treated with trastuzumab[40].
In a recent study evaluating capecitabine and oxaliplatin as first line neoadjuvant therapy in patients with previously untreated, HER2-positive GC, the analysis of circulating free DNA (cfDNA) at disease progression demonstrated the emergences of genomic aberrations such as MYC, EGFR, FGFR2 and MET amplifications[41]. However, none of these biomarkers is evaluated in the current clinical practice.
Other anti-HER2 strategies have failed to demonstrate a clinical benefit in second line HER2 positive GC. In the GATSBY trial (Trastuzumab emtansine vs taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma) the antibody-drug conjugate consisting of the monoclonal antibody Trastuzumab linked to microtubule inhibitor emtansine (T-DM1) compared to taxans, failed to prolong survival in patients with previously treated HER2-positive advanced GC[42]. In the phase III randomized TyTAN trial (Lapatinib plus paclitaxel vs paclitaxel alone in the second-line treatment of HER2-amplified advanced GC in Asian populations) comparing the efficacy and safety of the tyrosine kinase inhibitor of EGFR and HER2 Lapatinib plus paclitaxel with paclitaxel alone, the combination treatment demonstrated activity in the second-line but did not significantly improve OS in the intent-to-treat population (ITT)[43]. Moreover, in a recent trial comparing weekly paclitaxel alone with weekly paclitaxel plus Trastuzumab in patients with HER2-positive advanced GC/GEJC progressing during Trastuzumab-containing therapy, Trastuzumab beyond progression strategy did not improve PFS[44].
Results from ongoing phase III (NCT01774786) and phase II (NCT01522768) clinical trials of Pertuzumab and Afatinib respectively, will hopefully contribute to the unmet need in this setting of patients whose therapeutic options still remain limited (Table 1).
Table 1.
Ongoing phase II/III target trials in advanced gastric cancer
| Study | Line | Control arm | Experimental arm | Target | NCT number |
| JACOB | 1st | Placebo + | Pertuzumab + | HER2 | NCT01774786 |
| Trastuzumab + | Trastuzumab + | ||||
| Chemotherapy | Chemotherapy | ||||
| ID NUMBER:11-166 | 2nd | - | Afatinib + | HER2 | NCT01522768 |
| Paclitaxel | |||||
| NIEGA | 2nd | - | Irinotecan + | EGFR | NCT03400592 |
| Nimotuzumab | |||||
| ENRICH | 2nd | Irinotecan | Irinotecan + | EGFR | NCT01813253 |
| Nimotuzumab | |||||
| CheckMate-649 | 1st | Oxaliplatin + | - Nivolumab + Oxaliplatin + | PD-1, CTLA-4 | NCT02872116 |
| Fluoropyrimidine | |||||
| Fluoropyrimidine | |||||
| - Ipilimumab + Nivolumab | |||||
| ATTRACTION-4 | 1st | Placebo + Oxaliplatin + S-1/Capecitabine | Oxaliplatin + S-1/Capecitabine + Nivolumab | PD-1 | NCT02746796 |
| JAVELIN Gastric 100 | 1st | Maintenance 1st line | Avelumab | PD-L1 | NCT02625610 |
| KEYNOTE-062 | 1st | Platin/fluoropyrimidine | - Pembrolizumab | PD-1 | NCT02494583 |
| - Pembrolizumab + Platin/fluoropyrimidine | |||||
| SPOTLIGHT | 1st | Oxaliplatin + | Zolbetuximab + Oxaliplatin + | CLDN18.2 | NCT03504397 |
| Fluoropyrimidine | Fluoropyrimidine | ||||
| ILUSTRO | 1st/3rd | - | - Zolbetuximab monotherapy, 3rd line | CLDN18.2 | NCT03505320 |
| - Zolbetuximab + FOLFOX, 1st line | |||||
| GLOW | 1st | Oxaliplatin + | Zolbetuximab + Oxaliplatin + | CLDN18.2 | NCT03653507 |
| Capecitabine | Capecitabine | ||||
| ANGEL | 3rd | BSC | Apatinib | VEGFR-2 | NCT03042611 |
| INTEGRATE II | 3rd | Placebo | Regorafenib | VEGFR1-3, | NCT02773524 |
| FGFR, | |||||
| PDGFR-β RAF, RET and KIT |
JACOB: A Study of Pertuzumab in Combination With Trastuzumab and Chemotherapy in Participants With Human Epidermal Growth Factor Receptor 2 Positive Metastatic Gastroesophageal Junction or Gastric Cancer; ID NUMBER:11-1669: Afatinib and Paclitaxel in Patients With Advanced HER2-Positive Trastuzumab-Refractory Advanced Esophagogastric Cancer; NIEGA: Study of Nimotuzumab and Irinotecan as Second Line With Recurrent or Metastatic Gastric Adenocarcinoma; ENRICH: Study of Nimotuzumab and Irinotecan as Second Line With Advanced or Recurrect Gastric and Gastroesophageal Junction Cancer; CheckMate649: Efficacy Study of Nivolumab Plus Ipilimumab or Nivolumab Plus Chemotherapy Against Chemotherapy in Stomach Cancer or Stomach/Esophagus Junction Cancer; ATTRACTION-4: Study of ONO-4538 in Gastric Cancer; JAVELIN: Gastric 100Avelumab in First-Line Maintenance Gastric Cancer; KEYNOTE-062: Study of Pembrolizumab as First-Line Monotherapy and Combination Therapy for Treatment of Advanced Gastric or Gastroesophageal Junction Adenocarcinoma; SPOTLIGHT: A Phase 3 Efficacy, Safety and Tolerability Study of Zolbetuximab Plus mFOLFOX6 Chemotherapy Compared to Placebo Plus mFOLFOX6 as Treatment for Gastric and Gastroesophageal Junction Cancer; ILUSTRO: A Study to Assess the Antitumor Activity, Safety, Pharmacokinetics and Biomarkers of Zolbetuximab in Participants With Claudin 18.2 Positive, Metastatic or Advanced Unresectable Gastric and Gastroesophageal Junction Adenocarcinoma; GLOW: A Study of Zolbetuximab Plus CAPOX Compared With Placebo Plus CAPOX as First-line Treatment of Subjects With Claudin 18.2-Positive, HER2-Negative, Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma; ANGEL: Efficacy and Safety Trial of Apatinib Plus Best Supportive Care Compared to Placebo Plus Best Supportive Care in Patients With Gastric Cancer; INTEGRATEII: A Study of Regorafenib in Refractory Advanced Gastro-Oesophageal Cancer, Best supportive care; HER2: Human epidermal growth factor receptor 2; EGFR: Epidermal growth factor receptor; PD-L1: Programmed death ligand 1; PD-1: Programmed cell death protein 1; CTLA-4: Cytotoxic T-lymphocyte-associated antigen 4; CLDN18.2: Claudine-18.2; VEGFR1-3: Vascular endothelial growth factor receptor 1-3; FGFR: Fibroblastic growth factor receptor; PDGFR-β: Platelet-derived growth factor receptor beta; RAF: Serine/threonine-specific protein kinases RAF; RET: Rearranged during transfection; KIT: Tyrosine-protein kinase Kit.
RTK/RAS - TARGET THERAPIES
CIN tumors are frequently characterized by the presence of activation of the RTK/RAS pathway and EGFR, HER2, HER3, JAK2, MET, FGFR2, PIK3CA and KRAS/NRAS amplification[14]. Other works have reported that at least one third of GC patients present alterations of the RTK/RAS pathway and may be potentially treatable by directed therapies[45]. Unfortunately, most of phase II and III trials evaluating RTK/RAS target therapies failed to demonstrate activity in metastatic GC.
The EGFR gene is amplified in the 5% and EGFR is overexpressed in more than 30% of GC[14,46]. Both anti-EGFR drug cetuximab and panitumumab have been tested in two phase III trial. In the EXPAND trial (Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced GC), the addition of the chimeric monoclonal antibody cetuximab to capecitabine-cisplatin provided no additional benefit in terms of PFS to chemotherapy alone in the first-line treatment of advanced GC (HR: 1.09; 95%CI: 0.92-1.29; P = 0.32)[47]. The REAL 3 trial (Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer), with 553 patients randomized to receive epirubicin, oxaliplatin, and capecitabine (EOC) plus the human monoclonal antibody panitumumab or EOC alone, failed to show a benefit in OS of the combination therapy compared with the only chemotherapy (HR: 1.37; 95%CI: 1.07-1.76; P = 0.013)[48]. However, none of these studies have selected patients on the basis of EGFR overexpression/amplification. In metastatic colorectal cancer, RAS mutations are a negative predictive biomarkers of response to anti-EGFR therapy but can be detected only in about 3% of GC and GEJC.
Several works have reported that EGFR expression, EGFR gene copy number, or expression of other EGFR ligands such as epiregulin and amphiregulin, might be potential markers for efficacy of anti-EGFR target therapies[49-51]. However, in the EXPAND trial, no substantial differences between the treatment groups for PFS or OS according to EGFR immunohistochemistry score was noted[47]. Results from a phase III trial comparing the efficacy of nimotuzumab, a recombinant humanized anti-EGFR antibody, and irinotecan on irinotecan alone in patients with EGFR overexpressed advanced GC/GEJC are expected (ENRICH study, NCT01813253, Table 1).
The tyrosine kinase receptor c-MET and its own ligand, hepatocyte growth factor (HGF), have been investigated as potential target in advanced GC. In GC, alteration of the MET/HGF pathway is related to a more aggressive disease and poor prognosis, with MET activation stimulating tumor invasiveness[52,53]. Onartuzumab, a monovalent monoclonal antibody binding with the extracellular of MET, has been tested in a phase III trial of 562 patients randomized to receive onartuzumab plus FOLFOX6 vs placebo plus mFOLFOX6 in patients with metastatic HER2-negative and MET-positive GEC. However, the addition of onartuzumab to mFOLFOX6 did not attain significant differences in OS or PFS compared with placebo plus mFOLFOX6 in ITT (OS HR: 0.82; 95%CI: 0.59-1.15; P = 0 .24; PFS HR: 0.90; 95%CI: 0.71-1.16; P = 0.43) or MET 2+/3+ populations (OS HR: 0.64; 95%CI: 0.40-1.03; P = 0 .06; PFS HR: 0.79; 95%CI: 0.54-1.15; P = 0.22)[54]. The RILOMET phase III trial (Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer), evaluating the fully human monoclonal antibody anti-MET Rilotumumab plus epirubicin, cisplatin, and capecitabine or placebo plus epirubicin, cisplatin, and capecitabine as first line in advanced GC, was ceased subsequently the finding by an independent data monitoring committee of a higher number of deaths in the rilotumumab group compared with the placebo group[55].
Approximately 5%-10% of GCs present an fibroblast growth factor receptor-2 (FGFR2) gene amplification, which appears to confer poor prognosis[56-58]. The selective FGFR-1, 2, 3 tyrosine kinase inhibitor AZD4547 showed potent activity in preclinical models[59]. The randomized phase II SHINE study (Efficacy and Safety of AZD4547 vs Paclitaxel in Patients With Advanced Gastric or Gastro-oesophageal Cancer) comparing AZD4547 vs paclitaxel as second-line treatment in patients with advanced GC displaying FGFR2 polysomy or gene amplification did not demonstrated a PFS improvement in the experimental arm (1.8 mo with AZD4547 vs 3.5 mo with paclitaxel; HR: 1.57; 80%CI: 1.12-2.21; P = 0.9581)[60].
IMMUNOTHERAPY
GCs/GEJCs are associated with immune system evasion and overexpression of immune checkpoint proteins including the programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) expressed on the surface of tumor and immune cells. An high expression of PD-L1 has been reported in both Western and Asian GC/GEJC cohorts and has been associated with an elevated tumor mutational burden (TMB) and specific subtypes of adenocarcinomas[61,62]. The binding of PD1, a protein of CD28 family expressed on T cells functioning as a negative costimulatory receptor, and its ligands-PD-L1 and PD-L2, can inhibit cytotoxic T-cell responses, allowing tumor cells to evade immune surveillance. Checkpoint inhibitors such as antibodies anti PD1 (pembrolizumab, nivolumab) and PD-L1 (atezolizumab, avelumab, durvalumab) and inhibitors of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4, like ipilimumab) have demonstrated to antagonize this immune tolerance, which results in an enhanced antitumor effect. In the last years, checkpoint inhibitors have shown activity in several solid tumors and have received approval for a number of clinical indications including advanced melanoma, renal cell carcinoma and non-small lung cancer (NSCLC)[63].
Since their introduction in the treatment scenario, lots of efforts have been undertaken to establish predictive biomarkers of response to these novel agents. Combined data from disease-specific trials with the humanized IgG4 monoclonal antibody pembrolizumab, demonstrated that tumors with a large number of somatic mutations due to high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) are susceptible and can benefit to immune checkpoint blockade. On these findings, in 2017 FDA decided to accelerate the approval to Pembrolizumab for patients with unresectable or metastatic solid tumours with positive dMMR or MSI-H biomarkers[64]. Other studies have shown that PD-L1 expression on the membranes of tumor cells or tumor-infiltrating immune lymphocytes (TILs) was associated with a better OS in certain types of tumours treated with checkpoint inhibitors. However, there is currently no consensus on the role of PD-L1 expression as prognostic and predictive biomarker in advanced GC[65].
In GC, checkpoint inhibitors have been firstly assessed in the salvage setting showing a rather wide range of response rate (11.6%-22%)[66,67]. In the phase III Asian ATTRACTION-2 trial (Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens) 493 patients with refractory GC to two or previous chemotherapy regimens were randomized to receive nivolumab (n = 330) or placebo (n = 163) resulting in a median OS of 5.26 mo (95%CI: 4.60-6.37) in the nivolumab group and 4.14 mo (3.42-4.86) in the placebo group (HR: 0.63; 95%CI: 0.51-0.78; P < 0.0001). The OS rates of nivolumab and placebo were 27.3% and 11.6% at 12 mo, and 10.6% and 3.2% at 24 mo, respectively. Based on these results, nivolumab was granted accelerated approval in Japan, South Korea and Taiwan for the treatment of advanced GC progressed to standard chemotherapy[68].
Moreover, Pembrolizumab has recently received accelerated approval by FDA considering the promising results of the KEYNOTE-059 trial (Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer). In this phase II, single arm study, 259 patients with advanced GC or GEJC whose disease progressed to two or more lines of therapy, received pembrolizumab every 3 wk achieving an objective response rate (ORR) of 11,6 % (95%CI: 8.0%-16.1%; n = 30/259) with complete response in 2.3% (95%CI: 0.9%-5%; n = 6/259) and manageable safety. Interestingly, patients with PD-L1 positive tumors (PD-L1 combined positive score ≥ 1) had an ORR of 22.7% (95%CI: 13.8-33.8) and patients PD-L1-negative had an ORR of only 8.6% (95%CI: 2.9-19.0). Excluding MSI-H tumors (ORR of 57%, 4 of 7 patients) from that group, the percentage of response to pembrolizumab decreased to 13.3% in PD-L1 positive microsatellite stable (MSS) (or MSI status not available) patients, and 9% (15 of 167) of MSS patients independently of PD-L1 status responded, confirming the importance of the microsatellite status as marker of response to checkpoint inhibitors[66].
Despite the initial enthusiasm, some randomized phase III trial reported negative outcomes with checkpoint inhibitors when compared to chemotherapy. The KEYNOTE-061 phase III trial (Pembrolizumab vs paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer) comparing pembrolizumab vs chemotherapy with weekly paclitaxel as second line treatment in patients with GC or GEJC with PD-L1 positivity in at least 1 % of tumor cells, failed to improve OS and PFS[69]. Similarly, the randomized, phase III Javelin Gastric 300 trial comparing the anti-PD-L1 IgG1 monoclonal antibody Avelumab vs chemotherapy as third line therapy in 379 patients with advanced GC/GEJC, did not meet its primary endpoint of improving OS or the secondary end points of PFS[70].
In the TCGA study an high level of intra- or peritumoral immune cell infiltration and frequent amplification of the CD274 gene (which encodes PD-L1) and the PDCD1LG2 gene (which encodes PD-L2) in the EBV-positive subgroup GC was found[14]. Furthermore, subsequent studies confirmed remarkable PD-L1 expression both in cancer and immune cells in EBV positive GCs[71]. Consistent with these findings, a prospective phase II Korean clinical trial of pembrolizumab with whole exome and RNA sequencing in pre and post biopsy specimens was performed to better define those patient who may benefit from pembrolizumab. Among 61 patients with advanced GC that received pembrolizumab as a second or greater line of treatment, those with MSI-H and EBV positive tumours, which are mutually exclusive, showed dramatic responses to pembrolizumab with ORR of 85.7% (6/7) in the MSI-H group and of 100% (6/6) EBV positive GCs. In addition for the 55 patients for whom PD-L1 combined positive score positivity (cut off value ≥ 1) was available, ORR was significantly higher for PD-L1 positive (n = 28) tumors when compared to PD-L1 negative (n = 27) GCs (50.0% vs 0.0%, P < 0.001)[72]. Although this study have provided the first clinical evidence of high activity of pembrolizumab EBV positive GCs, the percentage of EBV positive or MSI-H GCs was higher in this patient cohort compared to Western cohorts. This can be explained at least in part with the different regional risk of acquiring EBV associated GCs that ranges from 1.3%-30.9% (average of 10% worldwide) with the highest risk in Far East Asia, which also presents the highest incidence of GCs[73].
In order to optimize treatment strategies with checkpoint inhibitors, a number of ongoing trials are evaluating these agents in the first line setting (NCT02872116, NCT02746796, NCT02625610, NCT02494583, Table 1). Novel predictive biomarker are needed to select patient subgroups most likely to benefit from checkpoint inhibitors. Recently, Sundar et al. reported that epigenomic promoter alterations occur in a substantial proportion of metastatic GCs and cause reduced expression immunogenic peptides, which allow immune evasion and remarkable resistance to anti-PD1 immune checkpoint inhibition[74].
CLAUDIN 18.2
Claudins are main components of tight junctions in epithelial cellular sheets. Distinct claudins isoforms have been identified in different organs and their altered function has been discovered to be associated to the cancerogenesis of respective tissues[75,76]. Claudin 1-5, 7-12,16 and 18 proteins are expressed in healthy gastric tissue[77]. In particular the isoform 2 of the tight junction molecule Claudine-18 (CLDN18.2) is strictly confined to differentiated gastric epithelial cells where controls the paracellular permeability to Na+ and H+. In a significant percentage of primary GCs and metastases, the cell polarity perturbations lead to exposure of CLDN18.2 on the surface of GC cells so that it is available for monoclonal antibody binding[78]. CLDN18.2 is not exclusive of GC and is broadly expressed in various cancer types including biliary duct, pancreatic, ovarian cancer and NSCLC. A recent work have analyzed 286 GC/GEJC tissue samples from North America, Asia and Europe, demonstrating that 30% of samples (n = 86/286) presented high expression CLDN18.2 (moderate-to-strong CLDN18.2 membrane staining in ≥ 75% of tumor cells) with limited overlap with HER2[79]. These biological findings suggested that CLDN18.2 could be targetable and led to the further development of monoclonal antibodies against this protein. Zolbetuximab (IMAB362) is an anti-CLDN18.2 antibody that binds GC cell lines with high relative affinity and selectivity, then mediates a lysis of CLDN18.2-positive cells through antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In the phase II FAST trial a total of 161 patients were randomized to receive zolbetuximab plus epirubicine and oxaliplatin (EOX) or EOX alone. Median PFS was significantly higher with zolbetuximab + EOX (7.5 mo) vs EOX alone (5.3 mo; P < 0.0005; HR: 0.44, 95%CI: 0.29-0.67) and median OS (13 vs 8.4 mo; P = 0.0008; HR: 0.56, 95%CI: 0.40-0.79) and ORR (39 vs 25%; P = 0.022) were also demonstrated to be longer with zolbetuximab + EOX vs EOX alone with an increased efficacy in patients with high CLDN18.2 expression[80]. Consistent with these results, several trials are investigating zolbetuximab in different setting (NCT03504397, NCT03504397, NCT03653507, Table 1).
ANGIOGENIC AND STROMAL INHIBITORS
Based on the positive results of the REGARD and RAINBOW trial, other agents were assessed for angiogenic inhibition in GC. The VEGFR-2 tyrosine kinase inhibitor apatinib was tested in a phase II trial of patients with advanced GC refractory to two or more lines of prior chemotherapy, showing compared to placebo, prolonged OS (6.5 mo; 95%CI: 4.8 -7.6 vs 4.7 mo; 95%CI: 3.6-5.4; P = 0.0149; HR: 0.709; 95%CI: 0.537-0.937; P = 0.0156) and PFS (2.6 mo; 95%CI: 2.0-2.9 vs 1.8 mo; 95%CI: 1.4-1.9; P < 0.001; HR: 0.444; 95%CI: 0.331-0.595; P < 0.001)[81]. The ongoing ANGEL phase III trial (Efficacy and Safety Trial of Apatinib Plus Best Supportive Care Compared to Placebo Plus Best Supportive Care in Patients With Gastric Cancer) is evaluating the clinical benefit and safety of apatinib plus Best Supportive Care (BSC) in comparison to placebo plus BSC in patients who failed to at least two prior lines of standard chemotherapies (NCT03042611, Table 1). Other phase III trials are assessing the efficacy of apatinib as maintenance treatment after first line induction therapy (NCT03598348, NCT02510469, NCT02509806). Regorafenib is an oral multi-kinase inhibitor which targets angiogenic (VEGFR-1 and -2, tie-2), stromal (PDGF-β) and oncogenic RTK, largely used in metastatic colorectal cancer and GIST. In the INTEGRATE phase II study (Regorafenib for the treatment of advanced GC) patients with previously treated GC had statistically significantly improved PFS when treated with regorafenib compared to placebo [2.6 vs 0.9 mo (HR: 0.40; 95%CI: 0.28-0.59; stratified log-rank: P < 0.001)][82]. Consistent with these results, regorafenib is currently evaluated in the INTEGRATE II phase III trial (NCT02773524, Table 1). Bevacizumab is a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting VEGF-A. The AVAGAST and AVATAR trials, comparing the VEGF-antibody bevacizumab plus cisplatin/capecitabine to chemotherapy alone in different populations, failed to show significant benefit in OS[83,84]. Subgroup analysis of the AVAGAST trial showed that non-Asian patients were more likely to benefit from an anti-angiogenic therapy than Asian patients, although in the overall study population, this effect was not observed. Despite the encouraging results in the second line setting, in the recent phase III trial RAINFALL (Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma) that randomized patients to receive ramucirumab plus fluoropyrimidine and cisplatin or placebo plus fluoropyrimidine and cisplatin as first-line treatment, the addiction of ramucirumab to chemotherapy did not demonstrated a statistical significant advantage in PFS (HR: 0.961, 95%CI: 0.768-1.203, P = 0.74) and OS [HR: 0.962, 95%CI: 0.801-1.156, P = 0.6757; median OS 11.2 mo (9.9-11.9) in the ramucirumab group vs 10.7 mo (9.5-11.9) in the placebo group][85]. Other studies have investigated innovative approach to target the tumor microenvironment. A phase I/Ib study found that the addition of andecaliximab, a monoclonal antibody that inhibits matrix metalloproteinase 9, to FOLFOX showed activity in GC and GEJC. Unfortunately the phase III GAMMA-1 trial (A phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of andecaliximab combined with mFOLFOX6 as first-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma) comparing FOLFOX6 plus andecaliximab or mFOLFOX6 plus placebo showed a median OS of 12.5 vs 11.8 mo in the andecaliximab vs placebo treatment groups, respectively (HR: 0.93, two-sided: P = 0.56) and a median PFS of 7.5 mo in the andecaliximab group vs 7.1 mo in the placebo group (HR: 0.94, two-sided: P = 0.10)[86].
CONCLUSION
Recent high-throughput molecular profiling studies have provided a deeper understanding of the multiple genomic and epigenetic landscape of this complex and heterogeneous disease. New gene mutations, chromosomal aberrations, transcriptional and epigenetic alterations have been described with potentially implications for the development of personalized treatment options. However, at present, few target therapies are still available for metastatic GC.
Researches are focusing on the comprehension of primary and acquired mechanisms of resistance to anti-HER2 drugs. Moreover the targeting of other RTKs such as EGFR, MET or FGFR by TKIs or monoclonal antibodies failed to demonstrate a clinical benefit in GC. However, an appropriate molecular selection have not been conducted in many target driven clinical trials and retrospective analyses of these studies have provided a potential benefit from RTK-inhibitors in molecularly selected subgroups.
It has to be noted that an excessive GC tumor heterogeneity and evolution complicates the efficacy of target strategies. Recent studies showed a significant discrepancy in genomic alterations within the primary tumor and between the primary tumor and disseminated disease and the potential use of plasma-based circulating-tumor DNA (cfDNA) to enhance selection of therapy in GC[41,87].
Based on the promising results of clinical trials of patients with pretreated advanced GC, pembrolizumab and nivolumab were granted accelerated approval in in the United States and in some Asian countries respectively. In contrast, none of the current checkpoint inhibitors have been approved by the European Medicines Agency (EMA). As demonstrated in other solid tumors, GC with MSI-H or dMMR is more likely to respond to checkpoint inhibitors. EBV positive GCs seem to benefit significantly from these drugs, while the role of PD-L1 expression as prognostic and predictive biomarker of response to checkpoint inhibitors has not confirmed in all the studies. In addition, epigenomic promoter alterations have been recently described as a novel potential mechanism of resistance to checkpoint inhibitors in a substantial proportion of GC. The anti-CLDN18.2 antibody zolbetuximab has shown promising results and it is currently investigated in different ongoing trials. As regard angiogenesis, in addition to ramucirumab, other antiangiogenic agents including apatinib and regorafenib are currently under investigation.
In conclusion, remarkable advances in the molecular characterization of GC have expanded our knowledge and paved the way to novel treatment options that will hopefully improve the survival outcomes of patients with metastatic GC.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Fotios Loupakis had roles as consultant or advisor for Roche, Bayer, Amgen and Genentech. Sara Lonardi had roles as consultant or advisor for Amgen, Bayer, Merck Serono, Lilly; she received research funding from Amgen, Merck Serono and she is part of speakers bureau of Lilly, BMS. Vittorina Zagonel received honoraria and had roles as consultant or advisor for Bristol-Mayers Squibb, Bayer, Roche, Pfizer, Janssen, Novartis, Astellas, Servier; he had roles as consultant or advisor for Celgene, Merck. Matteo Fassan received research funding from Astellas Pharma. All the others authors declare no conflict of interest regarding the publication of this article.
Peer-review started: July 16, 2019
First decision: August 2, 2019
Article in press: September 27, 2019
P-Reviewer: Cao ZF, Cheng H, Chen Z, Li Y, Wu SD, Wang YG S-Editor: Wang J L-Editor: A E-Editor: Zhang YL
Contributor Information
Antonio Pellino, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy; Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua 35100, Italy.
Erika Riello, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy; Surgical Pathology & Cytopathology Unit, Department of Medicine, University of Padua, Padua 35100, Italy.
Floriana Nappo, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy; Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua 35100, Italy.
Stefano Brignola, Surgical Pathology & Cytopathology Unit, Department of Medicine, University of Padua, Padua 35100, Italy.
Sabina Murgioni, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy.
Selma Ahcene Djaballah, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy.
Sara Lonardi, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy.
Vittorina Zagonel, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy.
Massimo Rugge, Surgical Pathology & Cytopathology Unit, Department of Medicine, University of Padua, Padua 35100, Italy; Veneto Cancer Registry, Padua 35100, Italy.
Fotios Loupakis, Department of Oncology, Veneto Institute of Oncology IOV-IRCCS, Padua 35100, Italy.
Matteo Fassan, Surgical Pathology & Cytopathology Unit, Department of Medicine, University of Padua, Padua 35100, Italy. fotios.loupakis@iov.veneto.it.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 5.Anderson WF, Camargo MC, Fraumeni JF, Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol. 2010;22:669–678. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 8.Marano L, Polom K, Patriti A, Roviello G, Falco G, Stracqualursi A, De Luca R, Petrioli R, Martinotti M, Generali D, Marrelli D, Di Martino N, Roviello F. Surgical management of advanced gastric cancer: An evolving issue. Eur J Surg Oncol. 2016;42:18–27. doi: 10.1016/j.ejso.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano K, Graham DY. Gastric Cancer as Preventable Disease. Clin Gastroenterol Hepatol. 2017;15:1833–1843. doi: 10.1016/j.cgh.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol. 2003;14:1705–1713. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- 13.Bosman F, Carneiro F, Hruban R, Theise N. WHO classification of tumours of the digestive system. 2010. IARC-Press. [Google Scholar]
- 14.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, van Hummelen P, Go C, Palescandolo E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L, Kim KM. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS One. 2012;7:e38892. doi: 10.1371/journal.pone.0038892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M, Matsuno T, Hasegawa T, Hirata K, Imai K, Shinomura Y. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012;18:6577–6586. doi: 10.3748/wjg.v18.i45.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gylling A, Abdel-Rahman WM, Juhola M, Nuorva K, Hautala E, Järvinen HJ, Mecklin JP, Aarnio M, Peltomäki P. Is gastric cancer part of the tumour spectrum of hereditary non-polyposis colorectal cancer? A molecular genetic study. Gut. 2007;56:926–933. doi: 10.1136/gut.2006.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinicrope FA. Lynch Syndrome-Associated Colorectal Cancer. N Engl J Med. 2018;379:764–773. doi: 10.1056/NEJMcp1714533. [DOI] [PubMed] [Google Scholar]
- 19.Velho S, Fernandes MS, Leite M, Figueiredo C, Seruca R. Causes and consequences of microsatellite instability in gastric carcinogenesis. World J Gastroenterol. 2014;20:16433–16442. doi: 10.3748/wjg.v20.i44.16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedrazzani C, Corso G, Velho S, Leite M, Pascale V, Bettarini F, Marrelli D, Seruca R, Roviello F. Evidence of tumor microsatellite instability in gastric cancer with familial aggregation. Fam Cancer. 2009;8:215–220. doi: 10.1007/s10689-008-9231-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Johnson A, Ali SM, Klempner SJ, Bekaii-Saab T, Vacirca JL, Khaira D, Yelensky R, Chmielecki J, Elvin JA, Lipson D, Miller VA, Stephens PJ, Ross JS. Comprehensive Genomic Profiling of Advanced Esophageal Squamous Cell Carcinomas and Esophageal Adenocarcinomas Reveals Similarities and Differences. Oncologist. 2015;20:1132–1139. doi: 10.1634/theoncologist.2015-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, Cheong JH, Jeong W, Cho JY, Kim J, Chae J, Lee J, Kang WK, Kim S, Noh SH, Ajani JA, Lee JS. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 24.Dunne PD, McArt DG, Bradley CA, O'Reilly PG, Barrett HL, Cummins R, O'Grady T, Arthur K, Loughrey MB, Allen WL, McDade SS, Waugh DJ, Hamilton PW, Longley DB, Kay EW, Johnston PG, Lawler M, Salto-Tellez M, Van Schaeybroeck S. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin Cancer Res. 2016;22:4095–4104. doi: 10.1158/1078-0432.CCR-16-0032. [DOI] [PubMed] [Google Scholar]
- 25.Uhlik MT, Liu J, Falcon BL, Iyer S, Stewart J, Celikkaya H, O'Mahony M, Sevinsky C, Lowes C, Douglass L, Jeffries C, Bodenmiller D, Chintharlapalli S, Fischl A, Gerald D, Xue Q, Lee JY, Santamaria-Pang A, Al-Kofahi Y, Sui Y, Desai K, Doman T, Aggarwal A, Carter JH, Pytowski B, Jaminet SC, Ginty F, Nasir A, Nagy JA, Dvorak HF, Benjamin LE. Stromal-Based Signatures for the Classification of Gastric Cancer. Cancer Res. 2016;76:2573–2586. doi: 10.1158/0008-5472.CAN-16-0022. [DOI] [PubMed] [Google Scholar]
- 26.Lordick F, Lorenzen S, Yamada Y, Ilson D. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer. 2014;17:213–225. doi: 10.1007/s10120-013-0297-z. [DOI] [PubMed] [Google Scholar]
- 27.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 30.Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol. 2011;29:4709–4714. doi: 10.1200/JCO.2011.36.7599. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 33.J Tabernero, K Shitara, M Dvorkin, W Mansoor H, T Arkenau A, Prokharau et al. LBA-002 Overall survival results from a phase III trial of trifluridine/tipiracil versus placebo in patients with metastatic gastric cancer refractory to standard therapies (TAGS) Annals of Oncology. 2018;29:suppl 5. [Google Scholar]
- 34.Kim J, Fox C, Peng S, Pusung M, Pectasides E, Matthee E, Hong YS, Do IG, Jang J, Thorner AR, Van Hummelen P, Rustgi AK, Wong KK, Zhou Z, Tang P, Kim KM, Lee J, Bass AJ. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest. 2014;124:5145–5158. doi: 10.1172/JCI75200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R, Jiang W, Li X, Zhang W, Song L, Chang Z, Cao W, Cao X, Zong H. Anaplastic lymphoma kinase (ALK) gene alteration in gastric signet ring cell carcinoma. Cancer Biomark. 2016;16:569–574. doi: 10.3233/CBM-160599. [DOI] [PubMed] [Google Scholar]
- 36.Chon HJ, Kim HR, Shin E, Kim C, Heo SJ, Lee CK, Park JK, Noh SH, Chung HC, Rha SY. The Clinicopathologic Features and Prognostic Impact of ALK Positivity in Patients with Resected Gastric Cancer. Ann Surg Oncol. 2015;22:3938–3945. doi: 10.1245/s10434-015-4376-8. [DOI] [PubMed] [Google Scholar]
- 37.Kamiya A, Inokuchi M, Otsuki S, Sugita H, Kato K, Uetake H, Sugihara K, Takagi Y, Kojima K. Prognostic value of tropomyosin-related kinases A, B, and C in gastric cancer. Clin Transl Oncol. 2016;18:599–607. doi: 10.1007/s12094-015-1407-7. [DOI] [PubMed] [Google Scholar]
- 38.Pietrantonio F, Fucà G, Morano F, Gloghini A, Corso S, Aprile G, Perrone F, De Vita F, Tamborini E, Tomasello G, Gualeni AV, Ongaro E, Busico A, Giommoni E, Volpi CC, Laterza MM, Corallo S, Prisciandaro M, Antista M, Pellegrinelli A, Castagnoli L, Pupa SM, Pruneri G, de Braud F, Giordano S, Cremolini C, Di Bartolomeo M. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: the AMNESIA Case-Control Study. Clin Cancer Res. 2018;24:1082–1089. doi: 10.1158/1078-0432.CCR-17-2781. [DOI] [PubMed] [Google Scholar]
- 39.Pietrantonio F, Caporale M, Morano F, Scartozzi M, Gloghini A, De Vita F, Giommoni E, Fornaro L, Aprile G, Melisi D, Berenato R, Mennitto A, Volpi CC, Laterza MM, Pusceddu V, Antonuzzo L, Vasile E, Ongaro E, Simionato F, de Braud F, Torri V, Di Bartolomeo M. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer. 2016;139:2859–2864. doi: 10.1002/ijc.30408. [DOI] [PubMed] [Google Scholar]
- 40.Janjigian YY, Sanchez-Vega F, Tuvy Y, Bouvier N, Cathleen Riches J, Margolis M, Millang BM, Scott SN, Kundra R, Castel P, Ku GY, Hechtman JF, Kelsen DP, Ilson DH, Vakiani E, Scaltriti M, Solit DB, Taylor BS, Berger MF, Schultz N. Emergence of RTK/RAS/PIK3 pathway alterations in trastuzumab-refractory HER-2 positive esophagogastric (EG) tumours. J Clin Oncol. 2016;34:15_suppl 11608. [Google Scholar]
- 41.Kim ST, Banks KC, Pectasides E, Kim SY, Kim K, Lanman RB, Talasaz A, An J, Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, Park SH, Park JO, Park YS, Lim HY, Kim NKD, Park W, Lee H, Bass AJ, Kim K, Kang WK, Lee J. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29:1037–1048. doi: 10.1093/annonc/mdy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G, Shitara K, Phillips GDL, van der Horst T, Harle-Yge ML, Althaus BL, Kang YK. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640–653. doi: 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 43.Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, Miwa H, Qin SK, Chung IJ, Yeh KH, Feng JF, Mukaiyama A, Kobayashi M, Ohtsu A, Bang YJ. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 44.Makiyama A, Sagara k, Kawada J, Kashiwada T, Hosokawa A, Horie Y. A randomized phase II study of weekly paclitaxel ± trastuzumab in patients with HER2-positive advanced gastric or gastro-esophageal junction cancer refractory to trastuzumab combined with fluoropyrimidine and platinum: WJOG7112G (T-ACT) J Clin Oncol. 2018;36:15_suppl 4011. [Google Scholar]
- 45.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH, Lei Z, Goh G, Lim QY, Tan AL, Sin Poh DY, Riahi S, Bell S, Shi MM, Linnartz R, Zhu F, Yeoh KG, Toh HC, Yong WP, Cheong HC, Rha SY, Boussioutas A, Grabsch H, Rozen S, Tan P. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–684. doi: 10.1136/gutjnl-2011-301839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Tang H, Lin J, Hu Y, Luo G, Luo Z, Cheng C, Wang P. Clinicopathologic and prognostic significance of human epidermal growth factor receptor in patients with gastric cancer: An updated meta-analysis. Oncotarget. 2017;8:17202–17215. doi: 10.18632/oncotarget.15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 48.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, Kubicka S, Geissler M, Schwarz S, Galle PR, Kanzler S German Arbeitsgemeinschaft Internistische Onkologie. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol. 2011;22:1358–1366. doi: 10.1093/annonc/mdq591. [DOI] [PubMed] [Google Scholar]
- 50.Han SW, Oh DY, Im SA, Park SR, Lee KW, Song HS, Lee NS, Lee KH, Choi IS, Lee MH, Kim MA, Kim WH, Bang YJ, Kim TY. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298–304. doi: 10.1038/sj.bjc.6604861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luber B, Deplazes J, Keller G, Walch A, Rauser S, Eichmann M, Langer R, Höfler H, Hegewisch-Becker S, Folprecht G, Wöll E, Decker T, Endlicher E, Lorenzen S, Fend F, Peschel C, Lordick F. Biomarker analysis of cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric and oesophago-gastric junction cancer: results from a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO) BMC Cancer. 2011;11:509. doi: 10.1186/1471-2407-11-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 53.Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, Nash GM, Gimbel M, Yamaguchi Y, Culliford AT 4th, D'Alessio M, Barany F, Paty PB. c-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS, Phan S, Cunningham D. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017;3:620–627. doi: 10.1001/jamaoncol.2016.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I, Tejani MA, Udrea AA, Tehfe M, De Vita F, Turkington C, Tang R, Ang A, Zhang Y, Hoang T, Sidhu R, Cunningham D. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467–1482. doi: 10.1016/S1470-2045(17)30566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su X, Zhan P, Gavine PR, Morgan S, Womack C, Ni X, Shen D, Bang YJ, Im SA, Ho Kim W, Jung EJ, Grabsch HI, Kilgour E. FGFR2 amplification has prognostic significance in gastric cancer: results from a large international multicentre study. Br J Cancer. 2014;110:967–975. doi: 10.1038/bjc.2013.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung EJ, Jung EJ, Min SY, Kim MA, Kim WH. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum Pathol. 2012;43:1559–1566. doi: 10.1016/j.humpath.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, Arao T, Hamaguchi T, Shimada Y, Kato K, Oda I, Taniguchi H, Koizumi F, Yanagihara K, Sasaki H, Nishio K, Yamada Y. FGFR2 gene amplification and clinicopathological features in gastric cancer. Br J Cancer. 2012;106:727–732. doi: 10.1038/bjc.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, Xu Y, Gao Z, Liu K, Zhou M, Gao B, Shen D, Zhang L, Ji J, Gavine PR, Zhang J, Kilgour E, Zhang X, Ji Q. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res. 2013;19:2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 60.Van Cutsem E, Bang YJ, Mansoor W, Petty RD, Chao Y, Cunningham D, Ferry DR, Smith NR, Frewer P, Ratnayake J, Stockman PK, Kilgour E, Landers D. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28:1316–1324. doi: 10.1093/annonc/mdx107. [DOI] [PubMed] [Google Scholar]
- 61.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 62.Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269–24283. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med. 2015;373:1490–1492. doi: 10.1056/NEJMp1510079. [DOI] [PubMed] [Google Scholar]
- 64.Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Zhang Q, Ni S, Tan C, Cai X, Huang D, Sheng W. Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status. Cancer Med. 2018;7:2612–2620. doi: 10.1002/cam4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 68.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 69.Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 70.Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, Rabkin CS, Bass AJ. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925–32932. doi: 10.18632/oncotarget.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 73.Bae JM, Kim EH. Epstein-Barr Virus and Gastric Cancer Risk: A Meta-analysis With Meta-regression of Case-control Studies. J Prev Med Public Health. 2016;49:97–107. doi: 10.3961/jpmph.15.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sundar R, Huang KK, Qamra A, Kim KM, Kim ST, Kang WK, Tan ALK, Lee J, Tan P. Epigenomic promoter alterations predict for benefit from immune checkpoint inhibition in metastatic gastric cancer. Ann Oncol. 2019;30:424–430. doi: 10.1093/annonc/mdy550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 77.Caron TJ, Scott KE, Fox JG, Hagen SJ. Tight junction disruption: Helicobacter pylori and dysregulation of the gastric mucosal barrier. World J Gastroenterol. 2015;21:11411–11427. doi: 10.3748/wjg.v21.i40.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L, Sun X, Meng X. Differences in the expression profiles of claudin proteins in human gastric carcinoma compared with non‑neoplastic mucosa. Mol Med Rep. 2018;18:1271–1278. doi: 10.3892/mmr.2018.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moran D, Maurus D, Rohde C, Arozullah A. Prevalence of CLDN18.2, HER2 and PD-L1 in gastric cancer samples. Annals of Oncology. 2018;29:suppl 8. [Google Scholar]
- 80.Sahin U, Tureci O, Manikhas GM, Lordick F, Rusyn A, Vynnychenko I et al. Zolbetuximab combined with EOX as first-line therapy in advanced CLDN18.2+ gastric (G) and gastroesophageal junction (GEJ) adenocarcinoma: Updated results from the FAST trial. J Clin Oncol. 2019;Feb; 37:4_suppl 16. [Google Scholar]
- 81.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 82.Pavlakis N, Sjoquist KM, Martin AJ, Tsobanis E, Yip S, Kang YK, Bang YJ, Alcindor T, O'Callaghan CJ, Burnell MJ, Tebbutt NC, Rha SY, Lee J, Cho JY, Lipton LR, Wong M, Strickland A, Kim JW, Zalcberg JR, Simes J, Goldstein D. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol. 2016;34:2728–2735. doi: 10.1200/JCO.2015.65.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 84.Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y, Xu R, Chen L, Liu Y, Yu S, Bu L, Piao Y. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study) Gastric Cancer. 2015;18:168–176. doi: 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, Ilson DH, Alsina M, Chau I, Lacy J, Ducreux M, Mendez GA, Alavez AM, Takahari D, Mansoor W, Enzinger PC, Gorbounova V, Wainberg ZA, Hegewisch-Becker S, Ferry D, Lin J, Carlesi R, Das M, Shah MA RAINFALL Study Group. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:420–435. doi: 10.1016/S1470-2045(18)30791-5. [DOI] [PubMed] [Google Scholar]
- 86.Shah MA, Yanez Ruiz EP, Bodoky G, Starodub A, Cunningham D. A phase III, randomized,double-blind, placebo- controlled study to evaluate the efficacy and safety of andecaliximab combined with mFOLFOX6 as first-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2019;37:4_suppl 4. [Google Scholar]
- 87.Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, Alpert L, Kwak H, Kindler H, Polite B, Sharma MR, Allen K, O'Day E, Lomnicki S, Maranto M, Kanteti R, Fitzpatrick C, Weber C, Setia N, Xiao SY, Hart J, Nagy RJ, Kim KM, Choi MG, Min BH, Nason KS, O'Keefe L, Watanabe M, Baba H, Lanman R, Agoston AT, Oh DJ, Dunford A, Thorner AR, Ducar MD, Wollison BM, Coleman HA, Ji Y, Posner MC, Roggin K, Turaga K, Chang P, Hogarth K, Siddiqui U, Gelrud A, Ha G, Freeman SS, Rhoades J, Reed S, Gydush G, Rotem D, Davison J, Imamura Y, Adalsteinsson V, Lee J, Bass AJ, Catenacci DV. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov. 2018;8:37–48. doi: 10.1158/2159-8290.CD-17-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]