Abstract
BACKGROUND
Thiopurine-induced leukopenia (TIL) is a life-threatening toxicity and occurs with a high frequency in the Asian population. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants significantly improve the predictive sensitivity of TIL, more than 50% of cases of this toxicity cannot be predicted by this mutation. The potential use of the 6-thioguanine nucleotide (6TGN) level to predict TIL has been explored, but no decisive conclusion has been reached. Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 R139C genotypes?
AIM
To determine the 6TGN cut-off levels after dividing patients into subgroups according to their NUDT15 R139C genotypes.
METHODS
Patients’ clinical and epidemiological characteristics were collected from medical records from July 2014 to February 2017. NUDT15 R139C, thiopurine S-methyltransferase, and 6TGN concentrations were measured.
RESULTS
A total of 411 Crohn’s disease patients were included. TIL was observed in 72 individuals with a median 6TGN level of 323.4 pmol/8 × 108 red blood cells (RBC), which was not different from that of patients without TIL (P = 0.071). Then, we compared the 6TGN levels based on NUDT15 R139C. For CC (n = 342) and CT (n = 65) genotypes, the median 6TGN level in patients with TIL was significantly higher than that in patients without (474.8 vs 306.0 pmol/8 × 108 RBC, P = 9.4 × 10-5; 291.7 vs 217.6 pmol/8 × 108 RBC, P = 0.039, respectively). The four TT carriers developed TIL, with a median 6TGN concentration of 135.8 pmol/8 × 108 RBC. The 6TGN cut-off levels were 411.5 and 319.2 pmol/8 × 108 RBC for the CC and CT groups, respectively.
CONCLUSION
The predictive sensitivity of TIL based on 6TGN is dramatically increased after subgrouping according to NUDT15 R139C genotypes. Applying 6TGN cut-off levels to adjust thiopurine therapies based on NUDT15 is strongly recommended.
Keywords: Crohn’s disease, Thioguanine nucleotide levels, Nucleoside diphosphate-linked moiety X-type motif 15, Thiopurine-induced leukopenia
Core tip: Thiopurine-induced leukopenia (TIL), a life-threatening toxicity in inflammatory bowel disease, occurs with a high frequency in Asia. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants significantly increase the prediction sensitivity of TIL, more than 50% of cases cannot be predicted by this mutation. The potential use of steady-state thioguanine nucleotide (6TGN) levels to predict TIL has been explored for decades, but no decisive conclusion has been reached. Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 genotypes? Yes! According to our research, applying 6TGN levels to adjust thiopurine therapies based on NUDT15 is strongly recommended.
INTRODUCTION
Thiopurines, including mercaptopurine (MP) and azathioprine (AZA), are immunosuppressive drugs that have been regularly used to maintain remission in patients with Crohn’s disease (CD)[1,2]. Although thiopurine therapy is clinically effective, up to 10%-30% of patients discontinue treatment due to adverse reactions[3,4], among which thiopurine-induced leukopenia (TIL) is the most common and life-threatening toxicity, especially in Asian populations[5,6].
Neither AZA nor MP has an intrinsic activity. They must undergo extensive metabolic transformation into the active metabolites 6-thioguanine nucleotides (6TGN) and 6-methylmercaptopurine ribonucleotides (6MMPR), to exert their pharmacological effect or cytotoxicity[7,8]. The potential use of a steady-state 6TGN level above 450 pmol/8 × 108 red blood cells (RBC) to predict toxicity is still controversial[9-14]. Several studies have reported that 6TGN levels are not significantly correlated with TIL, and no applicable therapeutic range for 6TGN has been determined[15-18]. For example, a prospective study found that only three out of ten Japanese patients with TIL exhibited high 6TGN levels (> 450 pmol/8 × 108 RBC)[17]. In addition, a few groups have reported that extremely elevated 6MMPR concentrations are associated with TIL[12,19]. Therefore, further investigation of the exact relationship between the 6TGN and 6MMPR levels and TIL is warranted, especially in the Asian population, since Asians are more sensitive than Caucasians to thiopurine toxicity[6,20,21].
Nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) has been considered as the key gene which can highly predict thiopurine toxicity in Asian that is comparable to thiopurine S-methyltransferase (TPMT) in Europe[22-24]. TPMT variants with deficient enzyme activity were observed to have a preferential accumulation of 6TGN in TIL, so the guidelines have suggested adjusting the starting doses of thiopurine for variants based on the level of 6TGN[24]. However, NUDT15 variants have been discovered to develop TIL with normal or even lower 6TGN levels, suggesting that NUDT15 has an unfavorable effect on thiopurine metabolism[25,26]. NUDT15 is an enzyme responsible for catalyzing deoxy-6-thioguanine triphosphate (dTGTP) and 6-thioguanine triphosphate (TGTP) into their inactive monophosphates, preventing TGTP and dTGTP from incorporating into DNA[27]. Moriyama et al[28,29] discovered that NUDT15 R139C variants CT and TT were associated with a much higher TGTP/TGMP ratio and DNA-incorporated thioguanine (DNA-TG)/6TGN ratio in children with acute lymphoblastic leukemia (ALL). According to this mechanism, NUDT15 R139C variants are assumed to cause cytotoxicity with TGTP and dTGTP accumulation independent of the total 6TGN levels due to decreases in 6-thioguanine monophosphate (TGMP) and deoxy-6-thioguanine monophosphate (dTGMP). Thus, the guidelines applied to TPMT variants would not be suitable for patients with NUDT15 variants. Can we determine 6TGN cut-off levels thereafter dividing patients into subgroups according to NUDT15 R139C genotypes?
In this study, we investigated the relationship between the levels of 6TGN and 6MMPR and thiopurine-induced TIL based on patients’ NUDT15 R139C genotypes and obtained specific 6TGN cut-off levels to guide thiopurine therapy.
MATERIALS AND METHODS
Patient recruitment
Patients diagnosed with CD according to the criteria of Lennard-Jones and prescribed thiopurines at a steady dose for more than 1 mo were recruited at the Sixth Affiliated Hospital, Sun Yat-sen University from July 1, 2014 to February 1, 2017. The exclusion criteria included blood transfusion or administration of cyclosporine or methotrexate; insufficient function of the heart, liver, or kidney; active infection; and pregnancy.
Weight-based thiopurine dosing was provided in a step-wise approach (the initial dose of AZA was 1.0 mg/kg daily or 0.5 mg/kg daily for MP and gradually increased to the target dose of 2.0 mg/kg or 1.0 mg/kg, respectively). If patients developed adverse effects, such as leukopenia [white blood cell count (WBC) < 3.5 × 109⁄L], gastric intolerance, hepatotoxicity, flu-like symptoms, pancreatitis, rash, or others, the dose was reduced. If the laboratory abnormalities did not subside, the treatment was discontinued.
A total of 2 mL of venous blood samples (EDTA anticoagulation) were obtained for determination of the erythrocyte 6TGN/6MMPR concentrations and NUDT15 R139C and TPMT*3C genotypes at the Institute of Clinical Pharmacology, Sun Yat-sen University[30,31].
NUDT15 R139C and TPMT*3C genotyping
We used allele-specific polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) to test the genotypes of NUDT15 R139C (rs116855232) and TPMT*3C (rs1142345). The sequences of the primers for NUDT15 R139C are: forward, 5-AGCTTACCCAAATAAACACCCT-3 and reverse, 5-TGGGGGATACAT TAAGAGACTGC-3, and those for TPMT*3C are: forward, 5-AAGTGTTGGGATTA CAGGTG-3 and reverse, 5-TCCTCAAAAACATGTCAGTGTG-3. PCR amplification started at 94 °C for 5 min, and continued with 30 cycles of 94 °C for 60 s, 59 °C for 30 s, and 72 °C for 30 s. Final extension was performed at 72 °C for 10 min. The PCR product was digested with restriction enzyme HpyCH4III (New England Biolabs, Hertfordshire, United Kingdom) for NUDT15 R139C testing and the enzyme AccI (New England Biolabs, Hertfordshire, United Kingdom) for TPMT*3C testing.
6TGN and 6MMPR determination
6TGN and 6MMPR concentrations in patients’ erythrocyte lysates were determined using the Dervieux et al’s method[32] by high performance liquid chromatography. First, 6TGN were hydrolyzed (100 °C for 45 min) into their bases (6-thioguanie) and 6MMPR were converted into their derivatives with perchloric acid. Then, they were separated using a C18 column with the mobile phase consisting of methanol-20 mmol/L potassium dihydrogenphosphate-triethylamine (pH adjusted to 3.2 using phosphoric acid) (5:95:0.1) at a flow rate of 1.0 mL/min. Finally, the wavelength for detection was set at 345 nm for 6TGN and at 303 nm for 6MMPR.
The present study was approved by the Ethics Committee of the Sixth Affiliated Hospital of the Sun Yat-sen University.
Statistical analysis
Statistical analyses and calculations were performed with SPSS 21.0 (SPSS, Inc., Chicago, IL, United States) and Prism 6 (GraphPad Software, La Jolla, CA, United States). Quantitative variables are expressed as medians and ranges. Categorical variables are summarized as frequencies. Data such as sex, NUDT15 R139C and TPMT*3C diplotypes, disease location, and co-medication were compared using the X2 method or Fisher’s test. Data such as age and thiopurine dose were compared using the nonparametric Kruskal-Wallis H-test. The nonparametric Kruskal-Wallis H-test was also used to evaluate the relationship between the 6TGN level and TIL. A receiver operating characteristic (ROC) curve was obtained to calculate the sensitivity and specificity of various 6TGN concentrations to predict the development of TIL. Multivariate logistic regression was used to identify the variables associated with TIL. P-values less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics of CD patients
A total of 411 patients with CD were included in this study. Among them, 100 (24.3%) patients had adverse effects during the AZA maintenance treatment, including TIL (n = 72, 17.5%), gastric intolerance (n = 21, 5.1%), flu-like symptoms (n = 6, 1.5%), hepatitis (n = 5, 1.2%), pancreatitis (n = 3, 0.7%), rash (n = 2, 0.5%), and others (n = 3, 0.7%). The characteristics of the patients are summarized in Table 1. The TIL rates were higher in patients carrying the NUDT15 R139C allele T (CT + TT) compared with those carrying the CC genotype (P = 9.04 × 10-20, OR = 8.04, 95%CI: 4.84-13.34). The median dosage was significantly lower for patients with TIL than for those without TIL (1.5 mg/kg per day vs 1.7 mg/kg per day, P = 0.03). Overall, there were no significant differences in gender, age, disease location, or co-medication between individuals with or without TIL (P > 0.05) (Table 1). No significant association of TPMT*3C with TIL was observed. Neither NUDT15 R139C nor TPMT*3C had significant associations with the other adverse effects (P > 0.05).
Table 1.
Characteristics of the 411 Crohn’s disease patients included in this study
| Characteristic | All patients | With leukopenia | Without leukopenia | P value |
| No. of subjects (%) | 411 | 72 (17.5) | 339 (82.5) | - |
| Gender | ||||
| Male, n (%) | 305 (74.0) | 52 (17.0) | 253 (83.0) | 0.180 |
| Female, n (%) | 106 (26.0) | 20 (18.9) | 86 (81.1) | |
| NUDT15 R139C | ||||
| CC, n (%) | 342 (80.4) | 35 (10.2) | 307 (89.8) | 2.40 × 10-15 |
| CT, n (%) | 65 (17.9) | 33 (50.2) | 32 (49.8) | |
| TT, n (%) | 4 (1.7) | 4 (100) | 0 | |
| TPMT*3C | 0.26 | |||
| AA, n (%) | 398 (96.8) | 68 (17.1) | 330 (82.9) | |
| AG, n (%) | 13 (3.2) | 4 (30.8) | 9 (69.2) | |
| Age in years, median (range) | 28 (12-70) | 26(12-70) | 27(12-60) | 0.734 |
| Medication | ||||
| Azathiopurine | 337 (82.0) | 57 (17.3) | 273 (82.7) | 0.113 |
| Mercaptopurine | 74 (18.0) | 14 (18.9) | 60 (81.1) | |
| Thiopurines dose, mg/kg per day, median (range) | 1.7 (0.2-3.1) | 1.5 (0.5-2.6) | 1.7 (0.2-3.1) | 0.030 |
| CD | ||||
| Ileal L1 | 34 | 6 | 28 | 0.495 |
| Colorectal L2 | 21 | 6 | 15 | |
| Ileocolonic L3 | 292 | 48 | 244 | |
| Upper gastrointestinal L4 | 61 | 12 | 49 | |
| Co-medication | 211 | 45 | 166 | 0.74 |
| Corticosteroids | 79 | 15 | 64 | |
| Thalidomide | 51 | 13 | 38 | |
| Anti-TNF agent | 64 | 13 | 51 | |
| 5-ASA | 4 | 0 | 4 | |
| Allopurinol | 13 | 4 | 9 |
Patients carrying the nucleoside diphosphate-linked moiety X-type motif 155 R135C CT and TT genotypes were more likely to develop thiopurine-induced leukopenia (bP < 0.01). NUDT15: Nucleoside diphosphate-linked moiety X-type motif 15; CD: Crohn’s disease.
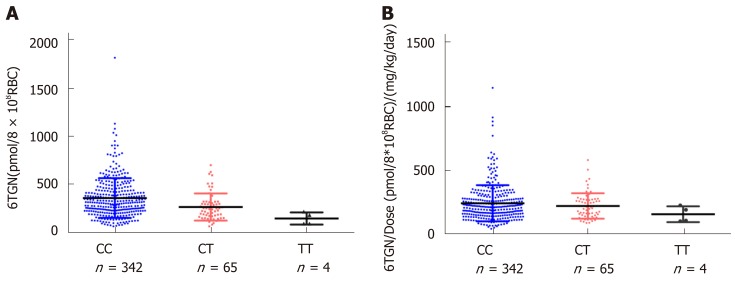
6TGN and 6MMPR levels and their ratios to the thiopurine dose according to NUDT15 R139C and TPMT*3C diplotype
For NUDT15 R139C, the median 6TGN was 312.4 pmol/8 × 108 RBC in the CC group, 240.5 pmol/8 × 108 RBC in the CT group, and 135.8 pmol/8 × 108 RBC in the TT group. The level of 6TGN was much higher in the NUDT CC group than in the CT and TT groups (P = 9.0 × 10-6) (Figure 1), while the median level of 6MMPR was also significantly higher in CC patients (P = 0.030). To assess the levels of 6TGN and 6MMPR with equal doses of thiopurine, the ratios of the levels of 6TGN and 6MMPR to the thiopurine dose were compared across groups. There was no difference in the ratios between these three groups (P= 0.29, P= 0.58) (Figure 1). For TPMT*3C, the patients carrying TPMT*3C variants had excessively higher 6TGN (P = 2.0 × 10-6) and lower 6MMPR levels (P = 3.7 × 10-4), which could not be offset by the thiopurine dose (P = 4.8 × 10-5, P = 7.7 × 10-5) (Supplemental Figure 1).
Figure 1.
6-thioguanine nucleotide level and its ratio to thiopurine dose according to nucleoside diphosphate-linked moiety X-type motif 15 R139C diplotype. A: The level of 6-thioguanine nucleotide (6TGN) was much higher in the nucleoside diphosphate-linked moiety X-type motif 15 CC group than in the CT and TT groups [bP < 0.01, 312.4 pmol/8 × 108 red blood cells (RBC) vs 312.4 pmol/8 × 108 RBC vs 135.8 pmol/8 × 108 RBC]. B: There was no difference in the ratio of the level of 6TGN to the thiopurine dose among these three groups. 6TGN: 6-thioguanine nucleotide; RBC: Red blood cells.
Associations between thiopurine metabolite concentrations and TIL based on NUDT15 R139C genotypes
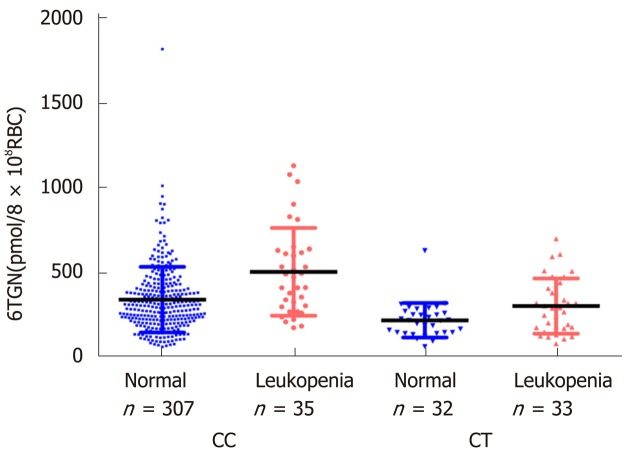
In this study, neither the concentration of 6TGN nor 6MMPR was significantly different between patients with or without TIL (P = 0.071, P = 0.95). According to the NUDT15 R139C genotypes, the samples were divided into three subgroups. In the CC group (n = 342), the median 6TGN concentration was significantly higher in patients who developed TIL than in patients who did not [P = 9.4 × 10-5, 474.8 (174.2-1133.6) pmol/8 × 108 RBC vs 306.0 (62.2-1823.0) pmol/8 × 108 RBC] (Figure 2). In the CT genotype (n = 65), the level of 6TGN was significantly higher in patients who developed TIL [P = 0.039, 291.7 (80.6-701.5) pmol/8 × 108 RBC vs 217.6 (62.9-631.0) pmol/8 × 108 RBC] (Figure 2). All patients with the TT genotype (n = 4) developed TIL, with a median 6TGN concentration of 135.8 (90.0-291.3) pmol/8 × 108 RBC. No correlations between the 6-MMPR concentrations and TIL were found in the subgroups of CC (P = 0.55) and CT (P = 0.30), respectively.
Figure 2.
Relationship between thiopurine-induced leukopenia and 6-thioguanine nucleotide concentrations in different nucleoside diphosphate-linked moiety X-type motif 15 R139C genotypes. In the CC group (n = 342), the median 6-thioguanine nucleotide (6TGN) concentration in patients who developed leukopenia was significantly higher than that in patients who did not [bP < 0.01, 474.8 (174.2-1133.6) pmol/8 × 108 red blood cells (RBC) vs 306.0 (62.2-1823.0) pmol/8 × 108 RBC]. In the CT group (n = 65), 6TGN level was also significantly higher in patients who developed leukopenia [aP < 0.05, 291.7 (80.6-701.5) vs 217.6 (62.9-631.0) pmol/8 × 108 RBC]. 6TGN: 6-thioguanine nucleotide; RBC: Red blood cells.
6TGN cut-off values to predict TIL
Based on ROC analysis, we defined the cut-off level of 6TGN to be 411.5 pmol/8 × 108 RBC in the CC group and 319.2 pmol/8 × 108 RBC in CT group. After considering the level of 6TGN in different subgroups, the area under the curve (AUC) was increased from 0.57 to 0.65 and 0.70. Moreover, in the CT group, the specificity was as high as 96.9%, with a sensitivity of 42.4%, and in the CC group the specificity was 73.3%, with a high sensitivity of 60.0%, compared with the values in the total samples (Table 2).
Table 2.
Relationship between concentrations of 6-thioguanine nucleotide and thiopurine-induced leukopenia in different nucleoside diphosphate-linked moiety X-type motif 15 R139C genotype groups among 411 patients according to receiver operating characteristic curves
| NUDT15 genotype (No. of patients) | 6TGNs (pmol/8 × 108 RBC) | P value | AUC | 95%CI | Sensitivity (%) | Specificity (%) |
| CT + CC | ≥ 474.7 | 0.071 | 0.57 | (0.49-0.65) | 34.7 | 82.6 |
| CC (342) | < 474.7 | 9.4 × 10-5 | (0.61-0.79) | 60.0 | 73.3 | |
| ≥ 411.5 | 0.70 | |||||
| < 411.5 | ||||||
| CT (65) | ≥ 319.2 | 0.039 | 0.65 | (0.51-0.79) | 42.4 | 96.9 |
| < 319.2 |
In subgroups, the area under the curve was increased from 0.57 to 0.65 and 0.70. Moreover, in the CT group, the specificity was as high as 96.9%, with a sensitivity of 42.4%, and in the CC group, the specificity was 73.3%, with a high sensitivity of 60.0%, compared with the values in the total samples. AUC: Area under the curve; NUDT15: Nucleoside diphosphate-linked moiety X-type motif 15; RBC: Red blood cells.
Multivariable prediction model for TIL
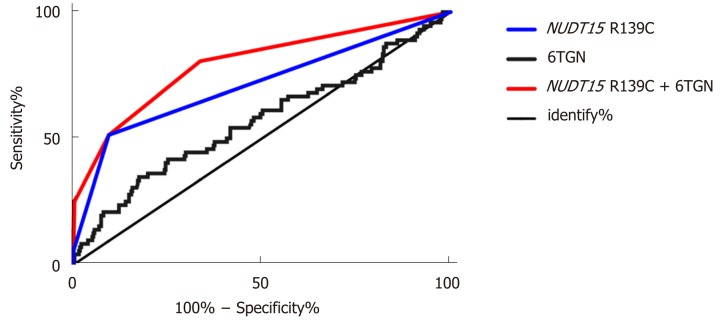
After performing the multivariate regression analysis, we found that the NUDT15 R139C genotypes and 6TGN concentration were relevant determinants for the development of TIL. Table 3 presents the prevalence of TIL and the odds of developing it for each category compared with the reference group of patients with the CC genotype and a 6TGN level below 411.5 pmol/8 × 108 RBC. Patients carrying the NUDT15 TT allele, irrespective of the 6TGN concentration, were at the highest risk, as all of them developed TIL in this study (Table 3). Patients with the CT genotype and a 6TGN concentration above the cut-off value of 319.2 pmol/8 × 108 RBC were at the second highest risk of developing TIL [OR 225.0 (95%CI: 27.6-1836.2)], followed by the CT genotype with a 6TGN concentration below 319.2 pmol/8 × 108 RBC [OR 9.9 (95%CI: 4.5-21.6)] and the CC genotype with a 6TGN concentration above 411.5 pmol/8 × 108 RBC [OR 4.1 (95%CI: 2.0-8.5)] (Table 3). The area under the ROC curve for the obtained predicted probabilities based on NUDT15 and the 6TGN level was 0.79 (95%CI: 0.76-0.92) (Figure 3).
Table 3.
Multivariable prediction model for thiouprine-induced leukopenia, including the nucleoside diphosphate-linked moiety X-type motif 1 R139C genotypes and 6-thioguanine nucleotide cut off levels
| Category |
Leukopenia |
% of total | OR (95%CI) | P value | |
| Yes | No | ||||
| NUDT15 R139C TT | 4 | 0 | 4/4 (100%) | ↑↑↑ | - |
| NUDT15 R139C CT + 6TGN > 319.2 | 14 | 1 | 14/15 (93.3%) | 225.0 (27.6-1836.2) | 1.5 × 10-14 |
| NUDT15 R139C CT + 6TGN < 319.2 | 19 | 31 | 19/50 (38.0%) | 9.9 (4.5-21.6) | 8.1 × 10-11 |
| NUDT15 R139C CC + 6TGN > 411.5 | 21 | 82 | 21/103 (20.4%) | 4.1 (2.0-8.5) | 4.8 × 10-5 |
| NUDT15 R139C CC + 6TGN < 411.5 | 14 | 225 | 14/239 (5.9%) | Reference | - |
| Total | 72 | 339 | 72/411 (17.5%) | ||
Patients carrying the TT allele, irrespective of the concentration of 6-thioguanine nucleotide (6TGN), were at the highest risk, as all of them developed leukopenia in this study. Patients with the CT genotype and a 6TGN concentration above the cut-off value of 319.2 pmol/8 × 108 red blood cells (RBC) were at the second highest risk of developing leukopenia [OR 225.0 (95%CI: 27.6-1836.2)], followed by those carrying the CT genotype with a 6TGN concentration below 319.2 pmol/8 × 108 RBC [OR = 9.9 (95%CI: 4.5-21.6)] and those carrying the CC genotype with a 6TGN concentration above 411.5 pmol/8 × 108 RBC [OR 4.1 (95%CI: 2.0-8.5)]. NUDT15: Nucleoside diphosphate-linked moiety X-type motif 15; 6TGN: 6-thioguanine nucleotide.
Figure 3.
Receiver operating characteristic curves for the concentration of 6-thioguanine nucleotide with the threshold parameters and nucleoside diphosphate-linked moiety X-type motif 15 R139C genotypes. The area under the receiver operating characteristic curve for the obtained predicted probabilities based on nucleotide concentrations in different nucleoside diphosphate-linked moiety X-type motif 15 R139C genotypes and the level of 6-thioguanine nucleotide was 0.79 (95%CI: 0.76-0.92). 6TGN: 6-thioguanine nucleotide; NUDT15: Nucleoside diphosphate-linked moiety X-type motif 15.
DISCUSSION
In this retrospective study, for the first time, we found that the level of 6TGN level was significantly associated with thiopurine-induced TIL in different NUDT15 R139C genotypes. After combing NUDT15 R139C genotypes and different 6TGN cut-off levels, 80% of TIL cases could be explained by an ROCAUC of 0.79.
The incidence of thiopurine-induced TIL is more common in Asians than in Caucasians, even at the lower standard dose[6,33]. The discovery of NUDT15 R139C as a major genetic determinant predicting TIL in the Asian population was a milestone[22,23,34]. In our study, there was a strong relationship between NUDT15 R139C genotypes and TIL (P = 9.04 × 10-20, OR = 8.04, 95%CI: 4.84-13.34) (Table 1). However, in our study, no significant association of TPMT*3C with TIL was observed (Table 1), with only four (30.7%, 4/13) variants experiencing TIL, which is similar to our previous report[33]. Thus, in the Asian population, NUDT15 R139C might be a better biomarker to predict thiopurine toxicity.
NUDT15 is a gene that encodes the purine-specific Nudix hydrolase, which dephosphorylates the active metabolites TGTP and dTGTP into their inactive monophosphates, thus preventing TGTP from binding to Rac1 and dTGTP from incorporating into DNA[27,28]. When the function of NUDT15 is decreased, the excessive accumulation of TGTP and dTGTP may induce TIL, with decreased levels of TGMP and dTGMP[28]. Thus, the total 6TGN remains apparently unchanged. In our study, there were lower median levels of 6TGN in the CT and TT groups compared with the CC group (P = 9.0 × 10-6) (Figure 1). A study on Korean children with ALL also found that the level of 6TGN was negatively correlated with the number of NUDT15 risk alleles (P = 5.3 × 10-6), and the association remained significant after adjusting for the MP dosage (P = 1.7 × 10-6)[26]. However, in our study, this difference was counteracted by the thiopurine dosage (P = 0.29) (Figure 1), suggesting lower 6TGN and 6MMPR levels in patients carrying the T allele due to lower thiopurine administration. Contrary to the function of NUDT15, the patients carrying TPMT variants had excessively higher 6TGN and lower 6MMP levels, which could not be offset by the thiopurine dose (Supplemental Figure 1). These data suggested different mechanisms of TPMT and NUDT15, which made us believe that considering the 6TGN level alone may overlook NUDT15-deficient Asian patients who are prone to TIL[14,26,27]. In our study, we found no difference in the 6TGN concentration between patients with and without TIL in the total samples (P = 0.071). Thus, we subgrouped the entire sample based on patients’ NUDT15 R139C genotypes.
Few studies have analyzed the relationship of the level of 6TGN with TIL according to patients’ NUDT15 diplotypes[31,26]. Based on our previous research, we found that the concentration of 6TGN was significantly correlated with TIL in patients with the CC genotype, while no correlation was found in patients with the CT genotype due to a small sample size (24 patients). However, in this study, which had a larger sample size (n = 65), the level of 6TGN was significantly associated with TIL in the CC and CT groups. In the CC group (n = 342), the median 6TGN concentration was significantly higher in patients who developed TIL than in those who did not [P = 9.4 × 10-5, 474.8 (174.2-1133.6) pmol/8 × 108 RBC vs 306.0 (62.2-1823.0) pmol/8 × 108 RBC] (Figure 2). Similarly, in the CT genotype (n = 65), the level of 6TGN was higher in patients who developed TIL than in patients who did not [P = 0.039, 291.7 (80.6-701.5) pmol/8 × 108 RBC vs 217.6 (62.9-631.0) pmol/8 × 108 RBC] (Figure 2). According to ROC analysis, we defined the cut-off level of 6TGN to be 319.2 pmol/8 × 108 RBC in the CT group, which was significantly lower than that in the CC group (411.5 pmol/8 × 108 RBC). Moriyama et al[28,29] reported that all patients with deficient NUDT15 diplotypes had a higher DNA-TG/dosage ratio, even for the lower level of 6TGN. Thus, in our cohort, CT individuals might have a higher percentage of active dTGTP incorporated into their DNA (DNA-TG), even with a lower TGN level compared with that of CC genotype individuals, which needs to be further investigated.
Although NUDT15 genotype screening can inform the initial drug dosage of thiopurine, therapeutic drug monitoring of its metabolites still serves as a valuable tool for further fine-tuning of the treatment, especially to reduce the incidence of TIL. Dubinsky et al[9] reported that excessive 6TGN levels (> 450 pmol/8 × 108 RBC) were associated with a higher risk of TIL in Caucasians. However, in our study, the sensitivity of 450 pmol/8 × 108 RBC to predict TIL was 34.7% (25/72), as 79% of patients in the NUDT15 CT group and all patients in the TT group developed TIL with a 6TGN level far below 450 pmol/8 × 108 RBC, which indicated that NUDT15 deficient Asian patients were more sensitive to thiopurines. Feng et al[20] reported that the cut-off level for 6TGN in Chinese IBD was from 180-355 pmol/8 × 108 RBC, but patients’ with NUDT15 genotypes were not incorporated and the sample size of TIL was small (n = 12), indicating that the result was not conclusive. Based on our study including 411 patients, the sensitivity of the 6TGN cut-off level to TIL was increased to 42.4% in the CT group and 60.0% in the CC group compared with the threshold values in the studies of Dubinsky and Feng. Thus, different 6TGN cut-off levels should be considered in different NUDT15 genotypes in CD patients.
Finally, multivariable regression analysis showed that both the NUDT15 genotypes and 6TGN level were associated with TIL. Based on the combinations of these two parameters, patients can be categorized to assess their degree of risk for the development of TIL. Patients with the TT genotype, irrespective of the 6TGN level, developed TIL. Patients with the CT genotype and a 6TGN level above 319.2 pmol/8 × 108 RBC exhibited the highest risk of TIL [OR = 225.0 (95%CI: 27.6-1836.2)], followed by patients carrying either the CT genotype (< 319.2 pmol/8 × 108 RBC) or the CC genotype (> 411.5 pmol/8 × 108 RBC). In a Japanese report, the AUC of the model based on NUDT15 to predict TIL was 0.71[23]. Cao et al[33] reported a similar AUC value of 0.69 in a Chinese multicenter study. However, in our study, the AUC of the combination model to predict TIL was increased to 0.79, and 80% of TIL cases could be predicted. Based on this model, we can distinguish the thiopurine refractoriness if the patient has the steady-state 6TGN levels above the cut-off values without efficacy, which suggests that the thiopurine dose should not be increased and the alternative therapeutic agent should be chose to avoid the incidence of TIL.
There were some limitations in our research. The concentration of DNA-TG was not detected in our study. DNA-TG, the final active metabolite of thiopurines, has been reported to be a new biomarker to predict relapse and is correlated with the concentration of 6TGN in childhood acute lymphoblastic TIL based on a prospective study[35]. Curffari et al[36] also discovered that the levels of DNA-TG metabolites were correlated with the erythrocyte 6TGN level, but not the total leukocyte level. However, a recent study performed by Coulthard et al[37] showed contradictory results, indicating that the exact function of DNA-TG still requires further validation in IBD. On the other hand, we performed this retrospective research to determine the cut-off levels of 6TGN for predicting TIL, which need to be validated in further prospective studies.
In conclusion, this study found that the level of 6TGN was significantly correlated with thiopurine-induced TIL in Chinese CD patients with different NUDT15 genotypes, and specific cut-off values were determined. It is strongly recommended that these specific 6TGN cut-off levels be applied to adjust thiopurine therapy to ensure the therapeutic effects and decrease the incidence of TIL.
ARTICLE HIGHLIGHTS
Research background
Thiopurine-induced leukopenia (TIL) is life-threatening and occurs with a high frequency in Asia. Although nucleoside diphosphate-linked moiety X-type motif 15 (NUDT15) variants improve the predictive sensitivity of TIL, more than 50% of TIL cannot be predicted by this mutation. The potential use of the 6-thioguanine nucleotide (6TGN) level to predict TIL is controversial.
Research motivation
Can we increase the predictive sensitivity based on 6TGN by subgrouping patients according to their NUDT15 R139C genotypes?
Research objectives
To obtain a better model with combination of 6TGN levels and NUDT15 R139C genotypes to predict thiopurine-induced TIL and improve the safety for the thiopurine treatment.
Research methods
A total of 411 patients diagnosed with Crohn’s disease at the Sixth Affiliated Hospital of the Sun Yat-sen University were included in this study. Peripheral blood from patients was collected to detect the NUDT15 R139C/TPMT*3C genotypes and 6TGN concentrations at School of Pharmaceutical Sciences, Sun Yat-sen University. The X2 method or Fisher’s exact test was used to check the association of TIL with NUDT15 R139C/TPMT*3C diplotypes. A receiver operating characteristic (ROC) curve was used to obtain 6TGN cut-off levels to predict the development of TIL.
Research results
TIL was observed in 72 individuals with a median 6TGN level of 323.4 pmol/8 × 108 red blood cells (RBC), which was not different from that of patients without TIL (P = 0.071). After comparing the 6TGN levels based on NUDT15 R139C, for CC (n = 342) and CT (n = 65), the median 6TGN level in patients with TIL was significantly higher than that in patients without (P = 9.4 × 10-5, 474.8 pmol/8 × 108 RBC vs 306.0 pmol/8 × 108 RBC and P = 0.039, 291.7 pmol/8 × 108 RBC vs 217.6 pmol/8 × 108 RBC, respectively). The four TT carriers developed TIL, with a median 6TGN concentration of 135.8 pmol/8 × 108 RBC. The 6TGN cut-off levels were 411.5 and 319.2 pmol/8 × 108 RBC for the CC and CT groups, respectively. The area under the ROC curve for the obtained predicted probabilities based on NUDT15 R139C and the 6TGN level was 0.79 (95%CI: 0.76-0.92).
Research conclusions
The predictive sensitivity of TIL based on 6TGN is dramatically increased after subgrouping patients according to NUDT15 R139C genotypes.
Research perspectives
Applying these specific 6TGN cut-off levels to adjust thiopurine therapies based on NUDT15 R139C is strongly recommended during the thiopurine treatment.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors declare no competing financial interests related to this study.
Data sharing statement: No additional data are available.
Peer-review started: July 24, 2019
First decision: August 17, 2019
Article in press: September 11, 2019
P-Reviewer: Kee BP, Mattar MC S-Editor: Ma RY L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Xia Zhu, Institute of Clinical Pharmacology, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Kang Chao, Department of Gastroenterology, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Miao Li, Department of Gastroenterology, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Wen Xie, Center for Pharmacogenetics and Department of Pharmaceutical Sciences, University of Pittsburgh, Pittsburgh, PA 15261, United States.
Hong Zheng, Institute of Clinical Pharmacology, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Jin-Xin Zhang, School of Public Health, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Pin-Jin Hu, Department of Gastroenterology, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Min Huang, Institute of Clinical Pharmacology, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Xiang Gao, Department of Gastroenterology, the Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China.
Xue-Ding Wang, Institute of Clinical Pharmacology, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China. wangxd@mail.sysu.edu.cn.
References
- 1.Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 2.Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnar T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F European Crohn´s and Colitis Organisation [ECCO] Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 3.Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, Gomollón F, García-Planella E, Merino O, Gutiérrez A, Esteve M, Márquez L, Garcia-Sepulcre M, Hinojosa J, Vera I, Muñoz F, Mendoza JL, Cabriada JL, Montoro MA, Barreiro-de Acosta M, Ceña G, Saro C, Aldeguer X, Barrio J, Maté J, Gisbert JP. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–1410. doi: 10.1097/MIB.0b013e318281f28f. [DOI] [PubMed] [Google Scholar]
- 4.Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549. doi: 10.1002/ibd.21221. [DOI] [PubMed] [Google Scholar]
- 5.Shi HY, Chan FK, Leung WK, Li MK, Leung CM, Sze SF, Ching JY, Lo FH, Tsang SW, Shan EH, Mak LY, Lam BC, Hui AJ, Chow WH, Wong MT, Hung IF, Hui YT, Chan YK, Chan KH, Loo CK, Ng CK, Lao WC, Harbord M, Wu JC, Sung JJ, Ng SC. Low-dose azathioprine is effective in maintaining remission in steroid-dependent ulcerative colitis: results from a territory-wide Chinese population-based IBD registry. Therap Adv Gastroenterol. 2016;9:449–456. doi: 10.1177/1756283X16643509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KM, Kim YS, Seo GS, Kim TO, Yang SK IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID) Intest Res. 2015;13:193–207. doi: 10.5217/ir.2015.13.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon W, Loftus EV., Jr Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43:863–883. doi: 10.1111/apt.13559. [DOI] [PubMed] [Google Scholar]
- 8.de Boer NKH, Peyrin-Biroulet L, Jharap B, Sanderson JD, Meijer B, Atreya I, Barclay ML, Colombel JF, Lopez A, Beaugerie L, Marinaki AM, van Bodegraven AA, Neurath MF. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J Crohns Colitis. 2018;12:610–620. doi: 10.1093/ecco-jcc/jjx181. [DOI] [PubMed] [Google Scholar]
- 9.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Gokhale R, Kirschner BS. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:450–454. doi: 10.1097/00005176-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong L, Sharif JA, Galloway P, McGrogan P, Bishop J, Russell RK. Evaluating the use of metabolite measurement in children receiving treatment with a thiopurine. Aliment Pharmacol Ther. 2011;34:1106–1114. doi: 10.1111/j.1365-2036.2011.04848.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong DR, Coenen MJ, Vermeulen SH, Derijks LJ, van Marrewijk CJ, Klungel OH, Scheffer H, Franke B, Guchelaar HJ, de Jong DJ, Engels LG, Verbeek AL, Hooymans PM TOPIC recruitment team. Early Assessment of Thiopurine Metabolites Identifies Patients at Risk of Thiopurine-induced Leukopenia in Inflammatory Bowel Disease. J Crohns Colitis. 2017;11:175–184. doi: 10.1093/ecco-jcc/jjw130. [DOI] [PubMed] [Google Scholar]
- 13.Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047–1053. doi: 10.1053/j.gastro.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Lim SZ, Chua EW. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front Pharmacol. 2018;9:1107. doi: 10.3389/fphar.2018.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi CY, Bohane TD, Lee D, Naidoo D, Day AS. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:941–947. doi: 10.1111/j.1365-2036.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanai H, Iida T, Takeuchi K, Arai O, Watanabe F, Abe J, Maruyama Y, Oohata A, Ikeya K, Kageoka M, Miwa I, Yoshirou S, Hosoda Y, Kubota T. Thiopurine maintenance therapy for ulcerative colitis: the clinical significance of monitoring 6-thioguanine nucleotide. Inflamm Bowel Dis. 2010;16:1376–1381. doi: 10.1002/ibd.21190. [DOI] [PubMed] [Google Scholar]
- 17.Odahara S, Uchiyama K, Kubota T, Ito Z, Takami S, Kobayashi H, Saito K, Koido S, Ohkusa T. A Prospective Study Evaluating Metabolic Capacity of Thiopurine and Associated Adverse Reactions in Japanese Patients with Inflammatory Bowel Disease (IBD) PLoS One. 2015;10:e0137798. doi: 10.1371/journal.pone.0137798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gennep S, Konté K, Meijer B, Heymans MW, D'Haens GR, Löwenberg M, de Boer NKH. Systematic review with meta-analysis: risk factors for thiopurine-induced leukopenia in IBD. Aliment Pharmacol Ther. 2019;50:484–506. doi: 10.1111/apt.15403. [DOI] [PubMed] [Google Scholar]
- 19.Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423–1431. doi: 10.1136/gut.2005.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng R, Guo J, Zhang SH, Qiu Y, Chen BL, He Y, Zeng ZR, Ben-Horin S, Chen MH, Mao R. Low 6-thioguanine nucleotide level: Effective in maintaining remission in Chinese patients with Crohn's disease. J Gastroenterol Hepatol. 2019;34:679–685. doi: 10.1111/jgh.14465. [DOI] [PubMed] [Google Scholar]
- 21.Komiyama T, Yajima T, Kubota R, Iwao Y, Sakuraba A, Funakoshi S, Negishi K, Minami I, Tanaka Y, Mae H, Hibi T. Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J Crohns Colitis. 2008;2:315–321. doi: 10.1016/j.crohns.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, Haritunians T, Ye BD, Kim KJ, Park SH, Park SK, Yang DH, Dubinsky M, Lee I, McGovern DP, Liu J, Song K. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–1020. doi: 10.1038/ng.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, Nishida A, Nakagawa S, Miura M, Toyonaga T, Onodera K, Shinozaki M, Ishiguro Y, Mizuno S, Takahara M, Yanai S, Hokari R, Nakagawa T, Araki H, Motoya S, Naito T, Moroi R, Shiga H, Endo K, Kobayashi T, Naganuma M, Hiraoka S, Matsumoto T, Nakamura S, Nakase H, Hisamatsu T, Sasaki M, Hanai H, Andoh A, Nagasaki M, Kinouchi Y, Shimosegawa T, Masamune A, Suzuki Y MENDEL study group. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065–1078. doi: 10.1007/s00535-018-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, Moyer AM, Evans WE, Klein TE, Antillon-Klussmann FG, Caudle KE, Kato M, Yeoh AEJ, Schmiegelow K, Yang JJ. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 2019;105:1095–1105. doi: 10.1002/cpt.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, Sung KW, Lee SY, Koo HH. NUDT15 Variants Cause Hematopoietic Toxicity with Low 6-TGN Levels in Children with Acute Lymphoblastic Leukemia. Cancer Res Treat. 2018;50:872–882. doi: 10.4143/crt.2017.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22–29. doi: 10.1007/s00535-015-1142-4. [DOI] [PubMed] [Google Scholar]
- 27.Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, Bevc L, Herr P, Homan E, Sheppard NG, Stenmark P, Jemth AS, Helleday T. NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine. Cancer Res. 2016;76:5501–5511. doi: 10.1158/0008-5472.CAN-16-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, Lin TN, Hoshitsuki K, Nersting J, Kihira K, Hofmann U, Komada Y, Kato M, McCorkle R, Li L, Koh K, Najera CR, Kham SK, Isobe T, Chen Z, Chiew EK, Bhojwani D, Jeffries C, Lu Y, Schwab M, Inaba H, Pui CH, Relling MV, Manabe A, Hori H, Schmiegelow K, Yeoh AE, Evans WE, Yang JJ. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–373. doi: 10.1038/ng.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N, Kato M, Koh K, Inaba H, Manabe A, Schmiegelow K, Yang JJ, Hori H. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017;27:236–239. doi: 10.1097/FPC.0000000000000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding L, Zhang FB, Liu H, Gao X, Bi HC, Wang XD, Chen BL, Zhang Y, Zhao LZ, Zhong GP, Hu PJ, Chen MH, Huang M. Hypoxanthine guanine phosphoribosyltransferase activity is related to 6-thioguanine nucleotide concentrations and thiopurine-induced leukopenia in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:63–73. doi: 10.1002/ibd.21676. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Wang XD, Chao K, Zhi M, Zheng H, Ruan HL, Xin S, Ding N, Hu PJ, Huang M, Gao X. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn's disease. Aliment Pharmacol Ther. 2016;44:967–975. doi: 10.1111/apt.13796. [DOI] [PubMed] [Google Scholar]
- 32.Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44:551–555. [PubMed] [Google Scholar]
- 33.Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, Yang H, Liang J, Lin L, Huang Z, Zhang Y, Huang Y, Sun Y, Xue X, Huang M, Hu P, Lan P, Gao X. Combined Detection of NUDT15 Variants Could Highly Predict Thiopurine-induced Leukopenia in Chinese Patients with Inflammatory Bowel Disease: A Multicenter Analysis. Inflamm Bowel Dis. 2017;23:1592–1599. doi: 10.1097/MIB.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 34.Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, Pei D, Chen Y, Crews KR, Kornegay N, Wong FL, Evans WE, Pui CH, Bhatia S, Relling MV. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–1242. doi: 10.1200/JCO.2014.59.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen SN, Grell K, Nersting J, Abrahamsson J, Lund B, Kanerva J, Jónsson ÓG, Vaitkeviciene G, Pruunsild K, Hjalgrim LL, Schmiegelow K. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol. 2017;18:515–524. doi: 10.1016/S1470-2045(17)30154-7. [DOI] [PubMed] [Google Scholar]
- 36.Cuffari C, Li DY, Mahoney J, Barnes Y, Bayless TM. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn's disease on AZA therapy. Dig Dis Sci. 2004;49:133–137. doi: 10.1023/b:ddas.0000011614.88494.ee. [DOI] [PubMed] [Google Scholar]
- 37.Coulthard SA, Berry P, McGarrity S, McLaughlin S, Ansari A, Redfern CPF. Azathioprine with Allopurinol: Lower Deoxythioguanosine in DNA and Transcriptome Changes Indicate Mechanistic Differences to Azathioprine Alone. Inflamm Bowel Dis. 2017;23:946–955. doi: 10.1097/MIB.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]