Abstract
BACKGROUND
Inflammatory bowel diseases (IBD) have been associated with a low quality of life (QoL) and a negative impact on work productivity compared to the general population. Information about disease control, patient-reported outcomes (PROs), treatment patterns and use of healthcare resources is relevant to optimizing IBD management.
AIM
To describe QoL and work productivity and activity impairment (WPAI), treatment patterns and use of healthcare resources among IBD patients in Brazil.
METHODS
A multicenter cross-sectional study included adult outpatients who were previously diagnosed with moderate to severe Crohn’s disease (CD) or ulcerative colitis (UC). At enrolment, active CD and UC were defined as having a Harvey Bradshaw Index ≥ 8 or a CD Activity Index ≥ 220 or calprotectin > 200 µg/g or previous colonoscopy results suggestive of inadequate control (per investigator criteria) and a 9-point partial Mayo score ≥ 5, respectively. The PRO assessment included the QoL questionnaires SF-36 and EQ-5D-5L, the Inflammatory Bowel Disease Questionnaire (IBDQ), and the WPAI questionnaire. Information about healthcare resources and treatment during the previous 3 years was collected from medical records. Chi-square, Fisher’s exact and Student’s t-/Mann-Whitney U tests were used to compare PROs, treatment patterns and the use of healthcare resources by disease activity (α = 0.05).
RESULTS
Of the 407 patients in this study (CD/UC: 64.9%/35.1%, mean age 42.9/45.9 years, 54.2%/56.6% female, 38.3%/37.1% employed), 44.7%/25.2% presented moderate-to-severe CD/UC activity, respectively, at baseline. Expressed in median values for CD/UC, respectively, the SF-36 physical component was 46.6/44.7 and the mental component was 45.2/44.2, the EQ-visual analog scale score was 80.0/70.0, and the IBDQ overall score was 164.0/165.0. Moderate to severe activity, female gender, being unemployed, a lower educational level and lower income were associated with lower QoL (P < 0.05). Median work productivity impairment was 20% and 5% for CD and UC patients, respectively, and activity impairment was 30%, the latter being higher among patients with moderate to severe disease activity compared to patients with mild or no disease activity (75.0% vs 10.0%, P < 0.001). For CD/UC patients, respectively, 25.4%/2.8% had at least one surgery, 38.3%/19.6% were hospitalized, and 70.7%/77.6% changed IBD treatment at least once during the last 3 years. The most common treatments at baseline were biologics (75.3%) and immunosuppressants (70.9%) for CD patients and 5-ASA compounds (77.5%) for UC patients.
CONCLUSION
Moderate to severe IBD activity, especially among CD patients, is associated with a substantial impact on QoL, work productivity impairment and an increased number of IBD surgeries and hospitalizations in Brazil.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Quality of life, Healthcare resources
Core tip: In a large multicenter sample of patients with ulcerative colitis or Crohn’s disease (CD), disease activity, female gender, unemployment, and lower education and income were associated with a poorer quality of life. Approximately one-third of patients had some work and activity impairment, the latter increasing with disease activity. CD patients used more health resources, with 25.4% having at least one surgery and 38.3% being hospitalized in the previous 3 years. Inflammatory bowel disease prevalence is increasing, and health services should be prepared to provide an adequate response, including optimal therapies, to manage the care of such patients.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) with periods of remission and relapse[1,2]. The incidence and prevalence of IBD have been increasing around the world, particularly in developing countries[3-7]. Furthermore, the impact on patients’ quality of life (QoL) can be relevant when considering the onset at usually younger ages and the severity of IBD signs and symptoms, such as abdominal pain, rectal bleeding, diarrhea, and fatigue[1,8,9].
QoL refers to a person’s physical functioning, social and emotional well-being, ability to work and freedom from disease symptoms, and has been reported to be significantly lower in IBD patients compared to the general population[10]. In 2007, a European survey observed that 75.6% of IBD patients reported having symptoms that interfered with their ability to enjoy leisure activities, and almost 70% stated that their symptoms negatively affected work performance[11]. Patients with an active disease usually have poor QoL[2,12], but other studies[13,14] have observed that stress level, anxiety or depression, female gender, and fatigue can also contribute to poor QoL in IBD patients. Several studies[12,15,16] have also shown that IBD impacts work productivity.
Knowing the distribution of IBD features such as disease control, patient-reported outcomes (PRO) and treatment patterns is of paramount relevance when optimizing IBD management and improving QoL[17,18]. Data from Latin American countries are needed, although the few studies available seem to indicate that the IBD burden is relevant[19]. In Brazil, epidemiological information about IBD is also scarce[6,9,12,20,21]. The Real-world Data of Moderate to Severe Inflammatory Bowel Disease in Brazil (RISE BR) study was a noninterventional study designed to evaluate disease control and treatment patterns and to compare the burden of disease and QoL in patients with moderate to severe IBD activity vs those with mild or no activity. In this work, we describe QoL and work and activity impairment experienced by IBD patients enrolled in the RISE BR study. In addition, we characterize treatment patterns and the use of healthcare resources in this setting.
MATERIALS AND METHODS
Study design and participants
The RISE BR study was a multicenter, noninterventional study with a cross-sectional evaluation (baseline) and a 3-year retrospective data collection. In the study reported here, we recorded PROs at baseline and assessed the use of IBDs treatment and healthcare resources in the previous 3 years (i.e., baseline-3 years). The study protocol was reviewed and approved by the Ethics Committees of the participant centers. All study participants provided written informed consent prior to study enrollment (ClinicalTrials.gov Identifier: NCT02822235).
Patients were enrolled consecutively from October 2016 to February 2017, when attending scheduled clinical appointments at one of the 14 reference IBD hospitals distributed across Brazilian geographical regions. Eligible patients were ≥ 18 years old, with a diagnosis, by a gastroenterologist, of moderate-to-severe CD or UC for at least 6 mo. Patients with indeterminate/unclassified colitis, who were hospitalized at baseline or who had participated in an experimental study within the 3 years prior to baseline were excluded.
Study variables
Sociodemographic data (age, gender, educational level and professional status), smoking habits, family history of IBD and extraintestinal manifestations were collected from medical records. Other variables included time since IBD diagnosis, location and severity/behavior of UC and CD (Montreal classification) and steroid behavior (dependent or refractory).
CD activity at baseline was evaluated with the Harvey Bradshaw Index (HBI)[22] and/or the Crohn’s Disease Activity Index (CDAI)[23], according to local clinical practice. Moderately to severely active CD at baseline was considered when patients had an HBI ≥ 8 or a CDAI ≥ 220 or fecal calprotectin > 200 µg/g or previous colonoscopy results suggestive of inadequate control (as per investigator criteria)[22,24,25]. UC activity at baseline was assessed with the 9-point partial Mayo (pMayo) score[26] (moderate-to-severe activity: pMayo ≥ 5), according to local clinical practice[2,26].
QoL was assessed with three self-administered scales validated for the Brazilian population: The 36-item Short-Form Health Survey (SF-36)[27], the 5-dimensional EuroQoL measure (EQ-5D-5L)[28], and the Inflammatory Bowel Disease Questionnaire (IBDQ)[29]. The SF-36 evaluates eight health dimensions: Physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning, and mental health. Two summary scores (Physical Component Score and Mental Component Score) are obtained through a standardization of each dimension score and computation of aggregate scores. The component summary ranges from 0 to 100, with higher scores reflecting more a favorable health status[30-32]. The EQ-5D-5L considers five attributes (mobility, self-care, usual activity, pain/discomfort, anxiety/depression) scored with five possible levels (1-no problems, 2-slight problems, 3-moderate problems, 4-severe problems, 5-extreme problems) and a visual analog scale (VAS) where “100” indicates the best health imagined and a “0” represents the worst health imagined[2,15]. The IBDQ score comprises four domains: Bowel function, Emotional status, Systematic symptoms, and Social function[29,33]. Each IBDQ domain scores from 1 (poorest QoL) to 7 (best QoL), and the overall IBDQ score ranges from 32 to 224.
In addition, the impact of IBD on work productivity and daily activities was assessed with the Brazilian version of the Work Productivity and Activity Impairment (WPAI) questionnaire[34]. The WPAI topics are as follows: (1) If the patient was currently employed; (2) How many hours were missed due to the disease; (3) How many hours were missed for other reasons, (4) How many hours were worked; (5) To what degree did the disease affect productivity while working; and (6) To what degree did the disease affect regular activities. Based on the WPAI questionnaire, total work productivity impairment due to IBD (TWPI) was defined as the subjects’ total percentage of impaired work time that resulted from both absenteeism (work time missed due to IBD) and presenteeism (impairment while working due to IBD)[16]. Total activity impairment (TAI) due to IBD was also estimated.
Utilization of healthcare resources during the previous 3 years was retrieved from medical records regarding imaging and laboratory testing, surgeries, hospitalizations, and consultations with gastroenterologists or other medical specialists. The type and duration of IBD treatment ongoing at baseline and received during the retrospective 3-year period was also collected.
Statistical analysis
The sample size was estimated for the primary objective of the RISE BR study to estimate the prevalence of IBD activity. Hence, a sample size of 400 IBD patients was defined to allow estimates of disease activity at baseline with a 95% confidence interval (CI) and a margin of error less than 5%.
All analyses were presented by IBD type (UC or CD). Descriptive statistics were used to analyze sociodemographic, anthropometric and clinical variables. To compare QoL and WPAI according to disease activity at baseline (moderate to severe vs. mild or no activity), demographic characteristics and the presence of extraintestinal manifestations, the Chi-square or Fisher’s exact tests were used for qualitative variables (i.e., domain items) and the t-test for independent samples, the ANOVA test, the Kruskal-Wallis test or the Mann-Whitney U test were used for quantitative variables (i.e., SF-36 domain scores or component summary scores, EQ-5D VAS, IBDQ domain scores or total score, and WPAI scores). The correlation of IBDQ scores and EQ-5D dimensions/VAS with SF-36 domain scores was determined using Spearman’s correlation coefficient. There was no imputation of missing data in the study. Statistical tests were two-tailed, and significance was set at 5%. Statistical analysis was performed using SAS® (version 9.4, SAS Institute Inc., Cary).
RESULTS
Study participants and IBD characteristics
Of the 421 screened patients, 407 (96.7%) fulfilled the eligibility criteria and were included, 264 (64.9%) presented with CD and 143 (35.1%) had UC. The mean age (± standard-deviation, sd) was 42.9 ± 13.0 and 45.9 ± 13.8 years for CD and UC patients, respectively (Table 1). Most patients were female (54.2% CD and 56.6% UC patients), and over one-third were employed. The median time since the first diagnosis of CD and UC was 11.4 [range: 0.5-45.0] and 10.4 [range: 0.5-31.0] years, respectively. At baseline, CD patients had a median HBI of 2.0 [range: 0.0-37.0], a median CDAI of 137.0 [range: 25.0-495.0], and 44.7% (95%CI: 38.7-50.7%) presented moderate to severe activity. When considering UC patients, the median pMayo score was 1.0 [range: 0.0-9.0], and 25.2% (95%CI: 18.1-32.3%) presented moderate to severe activity.
Table 1.
Sociodemographic characteristics and clinical features of included patients
| CD patients (n = 264) | UC patients (n = 143) | |
| Age (yr), mean ± SD | 42.9 ± 13.0 | 45.9 ± 13.8 |
| Female | 143 (54.2) | 81 (56.6) |
| Educational level | ||
| Primary school | 55 (26.6) | 38 (38.8) |
| Secondary school | 83 (40.1) | 37 (37.7) |
| Higher education | 69 (33.3) | 23 (23.5) |
| Missing | 57 | 45 |
| Professional situation | ||
| Employed | 101 (44.3) | 53 (42.7) |
| Unemployed | 61 (26.8) | 33 (26.6) |
| Student | 10 (4.4) | 5 (4.0) |
| Retired | 30 (13.2) | 15 (12.1) |
| Other | 26 (11.4) | 18 (14.5) |
| Missing | 36 | 19 |
| Current smokers1 | 24 (9.9) | 3 (2.3) |
| Missing | 22 | 11 |
| Time since IBD diagnosis (yr), median [range] | 10.0 [0.5-45.0] | 10.0 [0.5-31.0] |
| Time since moderate-to-severe diagnosis (yr), median [range] | 6.0 [0.5-30.0] | 5.0 [0.5-25.0] |
| Steroid response2 | ||
| Steroid-dependent | 31 (14.8) | 23 (19.3) |
| Steroid-refractory | 16 (7.7) | 11 (9.2) |
| Not applicable (no previous use) | 87 (41.6) | 36 (30.3) |
| Unknown | 75 (35.9) | 49 (41.2) |
| Missing | 55 | 24 |
| Any extraintestinal manifestations | 54 (37.8) | 30 (38.0) |
| Family IBD history | 33 (12.5) | 15 (10.5) |
| Moderately to severely active disease at baseline3 | 118 (44.7) | 36 (25.2) |
| UC location [Montreal classification] | ||
| E1- distal UC | -- | 43 (30.1) |
| E2- left-sided | -- | 26 (18.2) |
| E3- pancolitis | -- | 74 (51.7) |
| UC severity [Montreal classification] | ||
| S0- asymptomatic | -- | 57 (39.9) |
| S1- mild UC | -- | 32 (22.4) |
| S2- moderate UC | -- | 40 (28.0) |
| S3- severe UC | -- | 14 (9.8) |
| CD location [Montreal classification] | ||
| L1- ileal | 67 (25.4) | -- |
| L2- colonic | 42 (15.9) | -- |
| L3- ileocolonic | 150 (56.8) | -- |
| L4- upper GI tract disease | 17 (6.4) | -- |
| CD behavior [Montreal classification] | ||
| B1- Nonstricturing/nonpenetrating | 58 (22.0) | -- |
| B2- Stricturing | 110 (41.7) | -- |
| B3- Penetrating | 91 (34.5) | -- |
| Perianal disease | 105 (39.8) | -- |
| Ileal surface involved ≥ 1 m [n = 201] | 38 (18.9) | -- |
Data are shown as n (%), except where otherwise mentioned.
Patients were smoking at baseline and has smoked 100 cigarettes in his/her lifetime.
Steroid-dependent disease: patients who either (1) Were unable to reduce steroids below the equivalent of prednisolone 10 mg/d within 3 mo of starting steroids, without recurrent disease, or (2) Had a relapse within 3 mo of stopping glucocorticoids. Steroid-refractory disease: active disease despite prednisolone up to 0.75 mg/kg/d over 4 wk.
Moderate-to-severe activity at baseline was defined as pMayo ≥ 5 (UC) and having HBI ≥ 8 or CDAI ≥ 220 (CD). IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease.
QoL evaluation by IBD type and disease activity
Regarding the SF-36 results (Table 2), the median scores of the physical and mental components were 46.6 [range: 20.6-68.6] and 45.2 [range: 5.8-67.2] for CD patients and 44.7 [range: 23.4-63.8] and 44.2 [range: 7.9-65.0] for UC patients, respectively. CD patients with moderate to severe activity had significantly lower scores in all SF-36 domains except for the vitality domain and had a lower physical component score (median: 44.0 vs 48.6, P < 0.001) and mental component score (median: 42.3 vs. 48.4, P = 0.022) compared to CD patients with mild or no activity. For UC patients, those with moderate to severe activity also presented lower SF-36 scores in all domains, and both the physical component score (median: 40.5 vs 46.2, P = 0.007) and mental component score (median: 35.0 vs 46.6, P < 0.001) were lower than those of UC patients with mild or no activity.
Table 2.
Quality of life according to type of inflammatory bowel disease and disease activity
|
CD |
UC |
|||||||
| Total | Moderate to severe activity | No or mild activity | P value | Total | Moderate to severe activity | No or mild activity | P value | |
| n | 263 | 118 | 145 | 143 | 36 | 107 | ||
| Missing value1 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| SF-36 scores | ||||||||
| Physical component | 46.6 [20.6-68.6] | 44.0 [20.6-62.3] | 48.6 [27.1-68.6] | < 0.001 | 44.7 [23.4-63.8] | 40.5 [23.4-58.8] | 46.2 [23.9-63.8] | 0.007 |
| Physical functioning | 75.0 [10.0-100.0] | 70.0 [10.0-100.0] | 85.0 [15.0-100.0] | < 0.001 | 70.0 [0.0-100.0] | 55.0 [0.0-100.0] | 77.5 [0.0-100.0] | 0.043 |
| Physical role | 68.8 [0.0-100.0] | 50.0 [0.0-100.0] | 75.0 [0.0-100.0] | < 0.001 | 59.4 [0.0-100.0] | 40.6 [0.0-100.0] | 75.0 [0.0-100.0] | < 0.001 |
| Body pain | 52.0 [0.0-100.0] | 51.0 [0.0-100.0] | 62.0 [10.0-100.0] | < 0.001 | 51.0 [0.0-100.0] | 31.0 [0.0-100.0] | 60.5 [0.0-100.0] | 0.001 |
| General health | 52.0 [0.0-100.0] | 46.0 [0.0-100.0] | 57.0 [10.0-100.0] | < 0.001 | 51.0 [5.0-100.0] | 31.0 [5.0-100.0] | 55.0 [5.0-100.0] | < 0.001 |
| Mental component | 45.2 [5.8-67.2] | 42.3 [14.0-63.6] | 48.4 [5.8-67.2] | 0.022 | 44.2 [7.9-65.0] | 35.0 [7.9-64.1] | 46.6 [7.9-65.0] | < 0.001 |
| Vitality | 56.3 [0.0-100.0] | 50.0 [6.3-100.0] | 56.3 [0.0-100.0] | 0.074 | 50.0 [0.0-100.0] | 37.5 [0.0-93.8] | 56.3 [0.0-100.0] | 0.002 |
| Social role functioning | 62.5 [0.0-100.0] | 50.0 [0.0-100.0] | 75.0 [0.0-100.0] | 0.002 | 62.5 [0.0-100.0] | 50.0 [0.0-100.0] | 62.5 [0.0-100.0] | 0.002 |
| Emotional role | 75.0 [0.0-100.0] | 66.7 [0.0-100.0] | 79.2 [0.0-100.0] | 0.012 | 75.0 [0.0-100.0] | 45.8 [0.0-100.0] | 75.0 [0.0-100.0] | 0.002 |
| Mental health | 65.0 [0.0-100.0] | 55.0 [0.0-100.0] | 72.5 [0.0-100.0] | 0.008 | 60.0 [0.0-100.0] | 45.0 [5.0-100.0] | 70.0 [0.0-100.0] | < 0.001 |
| EQ-VAS (cm) | 80.0 [5.0-100.0] | 70.0 [5.0-100.0] | 80.0 [15.0-100.0] | 0.003 | 70.0 [0.0-100.0] | 50.0 [0.0-100.0] | 80.0 [0.0-100.0] | < 0.001 |
| EQ-5D [no problems], n (%) | ||||||||
| Mobility | 193 (73.4) | 80 (67.8) | 113 (77.9) | 0.0802 | 85 (59.9) | 17 (47.2) | 68 (64.2) | 0.0732 |
| Self-care | 225 (85.6) | 95 (80.5) | 130 (89.7) | 0.0522 | 120 (84.5) | 29 (80.6) | 91 (85.8) | 0.4482 |
| Usual activities | 125 (47.5) | 47 (39.8) | 78 (53.8) | 0.1582 | 66 (46.8) | 10 (27.8) | 56 (53.3) | 0.0092 |
| Pain/disco-mfort | 87 (33.1) | 31 (26.3) | 56 (38.6) | 0.0362 | 30 (21.3) | 3 (8.3) | 27 (25.7) | 0.0122 |
| Anxiety/de-pression | 95 (36.1) | 31 (26.3) | 64 (44.1) | 0.0392 | 51 (36.2) | 9 (25.0) | 42 (40.0) | 0.1142 |
| IBDQ score | 164.0 [50.0-224.0] | 153.5 [50.0-222.0] | 178.0 [57.0-224.0] | < 0.001 | 165.0 [47.0-224.0] | 106.0 [48.0-217.0] | 178.5 [47.0-224.0] | < 0.001 |
| Bowel symptoms | 5.7 [1.4-7.0] | 5.3 [1.4-7.0] | 6.0 [1.8-7.0] | < 0.001 | 5.4 [1.3-7.0] | 3.7 [1.3-7.0] | 6.1 [1.5-7.0] | < 0.001 |
| Emotional health | 4.8 [1.2-7.0] | 4.4 [1.2-6.9] | 5.3 [1.5-7.0] | 0.001 | 4.9 [1.0-7.0] | 2.9 [1.2-6.6] | 5.3 [1.0-7.0] | < 0.001 |
| Systemic symptoms | 4.8 [1.0-7.0] | 4.4 [1.2-7.0] | 5.0 [1.0-7.0] | < 0.001 | 4.8 [1.0-7.0] | 3.2 [1.0-7.0] | 5.2 [1.0-7.0] | < 0.001 |
| Social function | 5.4 [1.2-7.0] | 5.0 [1.2-7.0] | 5.8 [1.4-7.0] | < 0.001 | 5.4 [1.2-7.0] | 3.8 [1.2-7.0] | 6.0 [1.2-7.0] | < 0.001 |
| WPAI scores | ||||||||
| % TWPI | 20.0 [0.0-100.0] | 30.0 [0.0-100.0] | 19.7 [0.0-100.0] | 0.053 | 5.0 [0.0-100.0] | 34.8 [0.0-100.0] | 0.0 [0.0-100.0] | 0.082 |
| % work time missed | 0.0 [0.0-100.0] | 4.7 [0.0-100.0] | 0.0 [0.0-64.0] | 0.009 | 0.0 [0.0-100.0] | 5.9 [0.0-100.0] | 0.0 [0.0-100.0] | 0.287 |
| % impairment while working | 10.0 [0.0-100.0] | 10.0 [0.0-80.0] | 10.0 [0.0-100.0] | 0.336 | 0.0 [0.0-80.0] | 20.0 [0.0-80.0] | 0.0 [0.0-80.0] | 0.08 |
| % TAI | 30.0 [0.0-100.0] | 50.0 [0.0-100.0] | 20.0 [0.0-100.0] | < 0.001 | 30.0 [0.0-100.0] | 75.0 [0.0-100.0] | 10.0 [0.0-100.0] | < 0.001 |
The missing data is due to a patient who has not completed SF-36 at Day 1. All p-values from Mann-Whitney U test, except
Chi-square test. Data are shown as median [range], except otherwise mentioned. CD: Crohn’s disease; UC: Ulcerative colitis. SF-36: Short-form 36; EQ-5D: Euro quality of life – 5 dimensions; EQ-VAS: EQ-5D visual analog scale. IBDQ: Inflammatory bowel disease questionnaire. WPAI: Work Productivity and Activity Impairment Questionnaire. TWPI: Total work productivity impairment; TAI: Total activity impairment.
The median [range] EQ-VAS scores for CD and UC patients were 80.0 [5.0-100.0] and 70.0 [0.0-100.0], respectively (Table 2). The majority of CD and UC patients had pain/discomfort (66.9% and 78.7%, respectively), anxiety/depression (63.9% and 63.8%), and problems with conducting their usual activities (52.5% and 53.2%). CD patients with moderate to severe activity had lower EQ-VAS scores (median: 70.0 vs 80.0, P = 0.003), and more patients reported problems of pain/discomfort (P = 0.036) and anxiety/depression (P = 0.039) compared to CD patients with mild or no activity. UC patients with moderate to severe activity had lower EQ-VAS scores (median: 50.0 vs 80.0, P < 0.001), and more patients reported problems in usual activities (P = 0.009) and pain/discomfort (P = 0.012) compared to UC patients with mild or no activity.
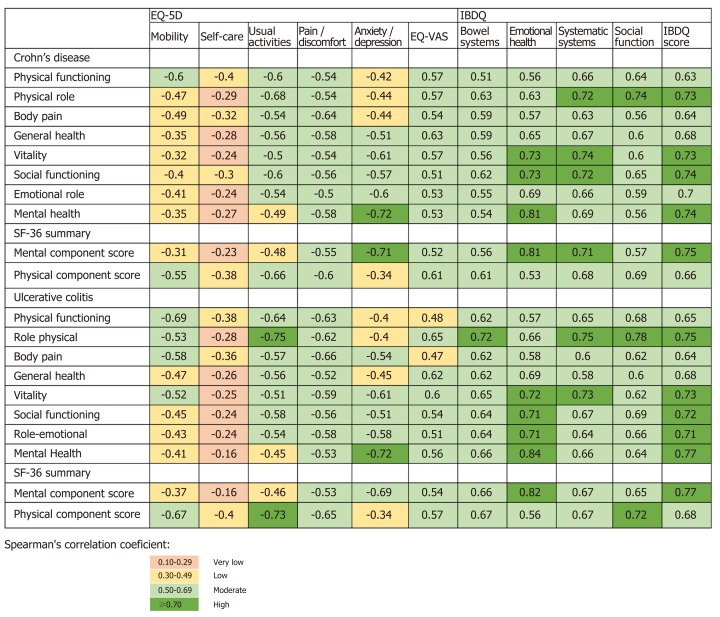
In both IBD types, EQ-VAS scores, as well as the EQ-5D dimensions, were statistically correlated with all SF-36 domains and component summary measures, except for the self-care and mental health dimensions in UC patients (Figure 1). Related dimensions presented larger coefficients, such as physical functioning in SF-36 and mobility in EQ-5D (CD: rs = -0.60 and UC: rs = -0.69) or body pain in SF-36 and pain/discomfort in EQ-5D (CD: rs = -0.64 and UC: rs = -0.66).
Figure 1.
Spearman’s correlation coefficients between domains and summary measures of the different quality of life measures. SF-36: Short-form 36; EQ-5D: Euro quality of life – 5 dimensions; EQ-VAS: EQ-5D visual analog scale; IBDQ: Inflammatory bowel disease questionnaire.
The median overall scores of the IBDQ for CD and UC patients were 164.0 and 165.0, respectively (Table 2). The lowest score was obtained for the systemic symptoms’ domain (median: 4.8 for both CD and UC patients), and the highest score was observed for the bowel symptoms domain (median: 5.7/5.4 for CD/UC patients, respectively). In both IBD types, patients with moderate to severe activity at baseline presented statistically lower median scores in all IBDQ domains and overall score compared to patients with mild or no activity (CD: 153.5 vs 178.0, P < 0.001 and UC: 106.0 vs 178.5, P < 0.001). The IBDQ dimensions and overall score presented a significantly strong correlation with all SF-36 domains and component summary measures (Figure 1). The correlation coefficient of mental health in SF-36 and emotional health in IBDQ was higher than 0.8 (CD: rs = 0.81 and UC: rs = 0.84).
QoL vs. demographic and clinical characteristics
The SF-36 mental component score was statistically higher among CD/UC male and employed subjects, CD patients aged ≥ 60 years and UC patients with higher income (Table 3). The SF-36 physical component score was statistically higher among CD/UC male, employed subjects and those with higher income, and UC patients with higher education. For both IBD types, the EQ-VAS score was statistically higher among men. The EQ-VAS score was significantly different according to the professional situation of CD patients, with employed patients presenting higher scores. The IBDQ overall score was statistically higher in CD patients who were ≥ 60 years, male, and students. Considering UC patients, higher IBDQ scores were observed in males, employed individuals and those with a higher income.
Table 3.
Quality of Life scores by sociodemographic and clinical characteristics and by type of inflammatory bowel disease
|
CD |
UC |
|||||||||||||||
|
SF-36 mental com-pon-ent |
SF-36 phys-ical com-pon-ent |
EQ-VAS |
IBDQ score |
SF-36 mental com-pon-ent |
SF-36 phys-ical com-pon-ent |
EQ-VAS |
IBDQ score |
|||||||||
| mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | |
| Age (years) | 0.018 | 0.895 | 0.514 | 0.002 | 0.1553 | 0.263 | 0.534 | 0.8473 | ||||||||
| 18-39 | 44.8 ± 13.0 | 43.1 ± 10.5 | 72.4 ± 20.3 | 158.9 ± 41.0 | 46.5 ± 10.0 | 46.0 ± 10.0 | 67.7 ± 22.2 | 151.6 ± 43.8 | ||||||||
| 40-59 | 41.9 ± 12.9 | 43.5 ± 14.3 | 73.4 ± 20.5 | 151.0 ± 43.4 | 43.1 ± 10.1 | 45.0 ± 9.8 | 65.5 ± 25.6 | 153.0 ± 54.8 | ||||||||
| ≥60 | 48.8 ± 9.4 | 42.0 ± 16.8 | 76.1 ± 19.8 | 178.7 ± 30.1 | 46.1 ± 9.7 | 48.2 ± 7.9 | 69.9 ± 30.5 | 157.2 ± 46.8 | ||||||||
| Gen-der | < 0.0011 | 0.0271 | 0.0041 | < 0.0011 | 0.0041 | 0.0321 | 0.0421 | < 0.001 | ||||||||
| Male | 48.0 ± 11.3 | 45.9 ± 13.6 | 77.5 ± 18.0 | 169.5 ± 38.0 | 47.5 ± 9.7 | 47.3 ± 8.9 | 72.4 ± 22.0 | 169.7 ± 43.3 | ||||||||
| Fem-ale | 41.2 ± 12.9 | 41.0 ± 13.1 | 69.8 ± 21.4 | 149.9 ± 42.1 | 42.7 ± 9.9 | 44.8 ± 10.0 | 62.8 ± 26.8 | 140.7 ± 50.8 | ||||||||
| Prof-essi-onal situa-tion | 0.002 | 0.030 2 | 0.003 | < 0.001 | < 0.0012 | < 0.001 | 0.130 | 0.001 | ||||||||
| Emp-loyed | 47.5 ± 11.5 | 47.6 ± 11.4 | 78.1 ± 19.4 | 168.1 ± 40.4 | 49.4 ± 8.9 | 49.2 ± 8.0 | 73.2± 23.2 | 175.7 ± 39.3 | ||||||||
| Une-mplo-yed | 40.0 ± 14.0 | 39.2 ± 13.5 | 67.6 ± 21.2 | 145.3 ± 40.6 | 41.2 ± 9.8 | 42.9 ± 9.3 | 64.9± 25.9 | 135.8 ± 51.5 | ||||||||
| Stu-dent | 43.0 ± 16.0 | 44.2 ± 11.2 | 75.5 ± 18.6 | 172.6 ± 42.8 | 42.0 ± 12.6 | 54.2 ± 9.8 | 61.6± 15.2 | 139.6 ± 35.3 | ||||||||
| Reti-red | 46.4 ± 8.2 | 39.3 ± 18.3 | 75.2 ± 15.8 | 165.2 ± 33.5 | 41.7 ± 10.7 | 45.4 ± 7.8 | 55.2± 31.4 | 135.7 ± 53.3 | ||||||||
| Other | 39.0 ± 14.0 | 40.3 ± 12.7 | 64.8 ± 23.4 | 135.5 ± 50.7 | 41.2 ± 8.9 | 39.8 ± 10.2 | 62.2± 24.3 | 138.4 ± 52.2 | ||||||||
| Edu-cati-onal level | 0.802 | 0.176 2 | 0.990 | 0.398 2 | 0.122 2 | 0.0282 | 0.510 | 0.185 | ||||||||
| Pri-mary school | 43.7 ± 11.4 | 43.7 ± 11.4 | 73.2 ± 20.8 | 156.3 ± 40.8 | 44.0 ± 9.3 | 44.0 ± 9.3 | 66.6 ± 27.9 | 143.0 ± 56.8 | ||||||||
| Seco-ndary school | 43.0 ± 13.3 | 43.0 ± 13.3 | 73.1 ± 20.7 | 152.9 ± 44.9 | 45.3 ± 9.9 | 45.3 ± 9.9 | 66.4 ± 25.8 | 160.0 ± 47.6 | ||||||||
| Hig-her educ-ation | 44.1 ± 13.2 | 44.1 ± 13.2 | 72.8 ± 20.1 | 162.2 ± 39.1 | 48.4 ± 8.9 | 48.4 ± 8.9 | 76.0 ± 6.5 | 171.4 ± 42.3 | ||||||||
| Inc-ome | 0.112 | 0.0072 | 0.547 | 0.0872 | < 0.001 | 0.0952 | 0.0702 | 0.0022 | ||||||||
| > 3x MW | 46.2 ± 11.9 | 47.7 ± 9.2 | 74.6 ± 21.4 | 169.2 ± 39.6 | 52.3 ± 9.4 | 49.3 ± 8.0 | 75.3 ± 16.2 | 179.0 ± 39.7 | ||||||||
| > 1x – 3x MW | 43.6 ± 11.8 | 46.1 ± 14.0 | 73.9 ± 20.2 | 157.6 ± 40.2 | 44.1 ± 9.0 | 46.2 ± 9.1 | 69.8 ± 24.6 | 158.7 ± 49.3 | ||||||||
| < 1x MW | 40.4 ± 13.6 | 36.2 ± 13.3 | 70.9 ± 20.8 | 149.4 ± 43.9 | 39.2 ± 10.0 | 45.9 ± 9.8 | 58.8 ± 26.2 | 124.0 ± 50.7 | ||||||||
| Any EIM | 0.4703 | 0.9243 | 0.6871 | 0.750 1 | 0.3433 | 0.2423 | 0.6011 | 0.7011 | ||||||||
| Yes | 44.8 ± 13.0 | 42.0 ± 14.9 | 70.7 ± 19.6 | 158.0 ± 42.2 | 42.6 ± 8.6 | 44.4 ± 7.9 | 69.6 ± 19.0 | 149.6 ± 48.5 | ||||||||
| No | 43.2 ± 11.9 | 42.3 ± 12.9 | 71.3 ± 22.0 | 161.0 ± 40.7 | 44.7 ± 10.4 | 46.2 ± 10.4 | 64.1 ± 27.8 | 152.2 ± 49.8 | ||||||||
All P-values from Kruskal-Wallis test, except
Mann-Whitney U test,
ANOVA test, and
t-test. EIM: Extraintestinal Manifestations; MW: Minimum wage.
Work productivity impairment due to IBD
Considering employed patients (CD: n = 111, 42.0%; UC: n = 58, 40.8%), the median TWPI was 20.0% (CD) and 5.0% (UC) (Table 2). The median TAI was 30.0% for both CD and UC patients. CD patients with moderate to severe disease activity presented higher absenteeism (median: 4.7% vs 0.0%, P = 0.009) and TAI (median: 50.0% vs 20.0%, P < 0.001) than those with mild or no disease activity. Moderately to severely active UC patients had higher TAI (75.0% vs 10.0%, P < 0.001).
Women presented higher activity impairment due to CD than men (P = 0.014). Activity impairment due to CD was significantly different by age group (p=0.007) and professional situation (P < 0.001) (Table 4). Higher TAI due to UC was observed in women when compared to men (P = 0.043). TAI due to UC presented statistically significant differences by professional situation (P = 0.001) and by income level (P = 0.032) (Table 4).
Table 4.
Work productivity and activity impairment scores by sociodemographic and clinical characteristics and by type of inflammatory bowel diseases
|
CD |
UC |
|||||||||||||||
|
% TWPI |
% work time missed |
% impairment while working |
% TAI |
% TWPI |
% work time missed |
% impairment while working |
% TAI |
|||||||||
| mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | |
| Age (yr) | 0.522 | 0.208 | 0.541 | 0.007 | 0.803 | 0.453 | 0.929 | 0.433 | ||||||||
| 18-39 | 31.8 ± 32.4 | 13.8 ± 23.6 | 23.3 ± 26.4 | 36.3 ± 34.1 | 28.5 ± 34.6 | 13.7 ± 27.1 | 19.6 ± 28.0 | 44.3 ± 36.1 | ||||||||
| 40-59 | 35.2 ± 35.1 | 12.9 ± 25.8 | 27.4 ± 30.8 | 41.2 ± 34.2 | 23.7 ± 33.6 | 11.7 ± 29.3 | 15.9 ± 25.0 | 37.6 ± 37.5 | ||||||||
| ≥ 60 | 19.6 ± 25.8 | 4.2 ± 11.8 | 15.6 ± 21.3 | 21.2 ± 25.4 | 19.6 ± 29.4 | 3.6 ± 6.2 | 18.0 ± 26.8 | 37.4 ± 40.9 | ||||||||
| Gen-der | 0.186 1 | 0.9141 | 0.148 1 | 0.0141 | 0.4651 | 0.6491 | 0.0921 | 0.0431 | ||||||||
| Male | 27.8 ± 30.5 | 13.0 ± 24.4 | 20.0 ± 23.4 | 30.4 ± 32.6 | 22.5 ± 32.0 | 13.4 ± 27.9 | 12.4 ± 21.9 | 31.8 ± 36.2 | ||||||||
| Fem-ale | 37.6 ± 35.5 | 12.2 ± 23.2 | 29.6 ± 32.0 | 40.3 ± 33.6 | 29.5 ± 35.1 | 9.3 ± 25.2 | 24.6 ± 29.8 | 45.9 ± 37.5 | ||||||||
| Prof-essi-onal situa-tion | 0.198 | 0.753 | 0.078 | < 0.001 | - | - | - | 0.001 | ||||||||
| Emp-loyed | 30.1 ± 31.4 | 11.0 ± 20.5 | 23.0 ± 27.8 | 24.2 ± 30.6 | 23.4 ± 31.5 | 9.4 ± 23.8 | 17.7 ± 26.2 | 23.7 ± 30.7 | ||||||||
| Une-mpl-oyed | 54.7 ± 18.0 | 5.4 ± 6.7 | 56.0 ± 16.7 | 49.8 ± 33.3 | - | - | - | 52.4 ± 39.1 | ||||||||
| Stu-dent | - | - | - | 23.0 ± 27.9 | - | - | - | 56.0 ± 24.1 | ||||||||
| Reti-red | 62.5 ± 17.7 | 25.0 ± 35.3 | 33.3 ± 28.9 | 32.3 ± 32.2 | 0.0 | 0.0 | 0.0 | 48.7 ± 43.4 | ||||||||
| Other | 45.0 ± 63.6 | 33.3 ± 47.1 | 35.0 ± 49.5 | 56.2 ± 29.1 | - | - | - | 51.1 ± 36.3 | ||||||||
| Edu-cati-onal level | 0.608 | 0.591 | 0.455 | 0.114 | 0.331 | 0.571 | 0.186 | 0.252 | ||||||||
| Pri-mary school | 37.4 ± 30.8 | 12.3 ± 15.6 | 28.6 ± 29.0 | 39.6 ± 34.9 | 15.7 ± 29.1 | 8.0 ± 17.7 | 8.0 ± 19.3 | 39.2 ± 40.8 | ||||||||
| Sec-onda-ry school | 37.1 ± 33.4 | 9.0 ± 17.7 | 29.1 ± 30.5 | 43.6 ± 36.6 | 26.3 ± 32.5 | 10.1 ± 28.6 | 21.2 ± 26.0 | 42.2 ± 37.5 | ||||||||
| Hig-her educ-ation | 30.0 ± 33.6 | 16.0 ± 26.6 | 20.5 ± 25.6 | 29.1 ± 28.4 | 10.6 ± 19.4 | 1.1 ± 2.7 | 10.0 ± 18.3 | 25.7 ± 31.7 | ||||||||
| Inc-ome | 0.293 | 0.708 | 0.042 | 0.079 | 0.341 | 0.648 | 0.453 | 0.032 | ||||||||
| > 3x MW | 33.0 ± 35.5 | 13.4 ± 25.6 | 25.8 ± 30.2 | 25.9 ± 30.8 | 23.0 ± 33.0 | 9.3 ± 27.5 | 22.0 ± 28.6 | 25.8 ± 36.7 | ||||||||
| > 1x – 3x MW | 28.0 ± 28.5 | 13.9 ± 23.8 | 16.8 ± 20.1 | 38.6 ± 34.2 | 27.6 ± 27.4 | 7.1 ± 16.4 | 16.7 ± 22.7 | 38.9 ± 36.8 | ||||||||
| < 1x MW | 60.0 ± 10.8 | 7.3 ± 8.9 | 50.0 ± 24.5 | 42.3 ± 35.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 56.8 ± 35.8 | ||||||||
| Any EIM | 0.0051 | 0.0041 | 0.199 1 | 0.6561 | 0.3451 | 0.8241 | 0.214 1 | 0.8961 | ||||||||
| Yes | 53.9 ± 33.4 | 23.3 ± 28.2 | 32.9 ± 31.8 | 37.2 ± 32.1 | 28.5 ± 31.2 | 1.8 ± 4.4 | 27.3 ± 31.0 | 37.0 ± 36.4 | ||||||||
| No | 23.5 ± 31.9 | 6.2 ± 15.4 | 21.0 ± 28.4 | 35.1 ± 33.8 | 19.0 ± 31.6 | 9.4 ± 26.6 | 13.7 ± 23.9 | 36.7 ± 38.4 | ||||||||
All P-values from Kruskal-Wallis test, except
Mann-Whitney U test. EIM: Extraintestinal manifestations; MW: Minimum wage; TWPI: Total work productivity impairment; TAI: Total activity impairment.
Healthcare utilization
A total of 108 surgeries were performed in 67 (25.4%) CD patients over the 3-year retrospective period. The median number of surgeries per CD patient was 1.0, and most (20.4%) were anal procedures (fistulectomy) (Table 5). Seven surgeries were performed in 4 (2.8%) UC patients (median 2.0), namely, 2 (28.6%) total colectomies and 2 (28.6%) enterostomy closures, among other interventions (n = 3, 42.9%). No statistically significant differences were observed when comparing patients by disease activity.
Table 5.
Utilization of healthcare resources during the retrospective 3-year period
|
CD |
UC |
|||||||
| Total | Moderate to severe activity | No or mild activity | P value | Total | Moderate to severe activity | No or mild activity | P value | |
| n | 264 | 118 | 146 | 143 | 36 | 107 | ||
| IBD surgeries | ||||||||
| At least one IBD surgery | 67 (25.4) | 32 (27.1) | 35 (24.0) | 0.559 1 | 4 (2.8) | 1 (2.8) | 3 (2.8) | -- |
| Surgeries, n | 108 | 45 | 63 | 7 | 2 | 5 | ||
| Surgeries/pt, median [range] | 1.0 [1-5] | 1.0 [1-4] | 2 [1-5] | 0.074 | 2.0 [1-2] | 2 | 2.0 [1-2] | -- |
| More than one IBD surgery | 28 (41.8) | 10 (31.2) | 18 (51.5) | 3 (75.0) | 1 (100.0) | 2 (66.7) | ||
| Type [frequency ≥ 5%] | ||||||||
| Partial colectomy | 13 (12.0) | 5 (11.1) | 8 (12.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total colectomy | 1 (0.9) | 0 (0.0) | 1 (1.6) | 2 (28.6) | 1 (50.0) | 1 (20.0) | ||
| Drainage of anorectal abscess | 6 (5.6) | 3 (6.7) | 3 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Fistulectomy | 22 (20.4) | 9 (20.0) | 13 (20.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Enterostomy | 13 (12.0) | 6 (13.3) | 7 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Enterostomy closure | 5 (4.6) | 5 (11.1) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 2 (40.0) | ||
| IBD hospitaliza-tions | ||||||||
| At least one IBD hospitaliza-tion | 101 (38.3) | 51 (43.2) | 50 (34.2) | 0.136 1 | 28 (19.6) | 11 (30.6) | 17 (15.9) | 0.055 1 |
| Hospitaliza-tions, n | 168 | 93 | 75 | 43 | 18 | 25 | ||
| Hospitaliza-tions/pt, median [range] | 1.0 [1-5] | 2.0 [1-4] | 1.0 [1-5] | 0.031 | 1.0 [1-5] | 1.0 [1-5] | 1.0 [1-3] | 0.978 |
| More than one hospitalization | 47 (46.5) | 29 (56.8) | 18 (36.0) | 10 (35.7) | 4 (36.4) | 6 (35.3) | ||
| Duration (d), median [range] | 6 [1-98] | 5 [0-76] | 4.5 [0-97] | 4 [1-737] | 2.5 [0-20] | 5 [0-737] | ||
| IBD medical appointments | ||||||||
| At least one IBD consultation | 263 (99.6) | 117 (99.2) | 146 (100.0) | 143 (100.0) | 36 (100.0) | 107 (100.0) | ||
| Consultations, n | 3192 | 1466 | 1726 | 1541 | 423 | 1118 | ||
| Consultations /pt, median [range] | 11.0 [1-45] | 12.0 [1-45] | 11.0 [1-35] | 0.801 | 10.0 [1-39] | 9.5 [1-39] | 10.0 [2-30] | 0.896 |
| More than 20 consultations | 27 (10.3) | 15 (12.8) | 12 (8.2) | 9 (6.3) | 4 (11.1) | 5 (4.7) | ||
| Type | ||||||||
| IBD specialist | 2989 (93.6) | 1356 (92.4) | 1633 (94.6) | 1426 (92.5) | 394 (93.1) | 1032 (92.3) | ||
| Emergency | 43 (1.3) | 25 (1.7) | 18 (1.0) | 34 (2.2) | 20 (4.7) | 14 (1.3) | ||
| Other specialist | 160 (5.0) | 85 (5.8) | 75 (4.3) | 81 (5.3) | 9 (2.1) | 72 (6.4) | ||
| Consultations with registered score | ||||||||
| HBI | 11 (0.3) | 1 (0.1) | 10 (0.6) | -- | -- | -- | ||
| CDAI | 103 (3.2) | 54 (3.7) | 49 (2.8) | -- | -- | -- | ||
| Other CD scores | 30 (0.9) | 21 (1.4) | 9 (0.5) | -- | -- | -- | ||
| pMayo score | -- | -- | -- | 95 (6.2) | 12 (2.8) | 83 (7.4) | ||
| Consultations with change of IBD treatment | 522 (16.4) | 269 (18.3) | 253 (14.7) | 0.005 1 | 353 (22.9) | 86 (20.3) | 267 (23.9) | 0.139 1 |
| IBD imaging and laboratory testing | ||||||||
| At least one IBD test | 260 (98.5) | 116 (98.3) | 144 (98.6) | -- | 141 (98.6) | 36 (100.0) | 105 (98.1) | -- |
| IBD tests, n | 5674 | 2722 | 2952 | 2509 | 643 | 1866 | ||
| Tests/pt, median [range] | 19.0 [1-143] | 20.0 [1-143] | 16.0 [1-94] | 0.372 | 15.0 [1-82] | 14.5 [2-82] | 16 [1-75] | 0.767 |
| More than 20 tests | 118 (45.4) | 56 (48.3) | 62 (43.1) | 57 (40.4) | 15 (41.7) | 42 (40.0) | ||
| Type [frequency ≥ 5%] | ||||||||
| Complete blood cell count | 2317 (40.8) | 1077 (39.6) | 1240 (42.0) | 973 (38.8) | 250 (38.9) | 723 (38.7) | ||
| C-reactive protein | 1773 (31.2) | 837 (30.7) | 936 (31.7) | 748 (29.8) | 214 (33.3) | 534 (28.6) | ||
| Serum albumin | 647 (11.4) | 346 (12.7) | 301 (10.2) | 356 (14.2) | 59 (9.2) | 297 (15.9) | ||
| Colonoscopy | 346 (6.1) | 183 (6.7) | 163 (5.5) | 228 (9.1) | 57 (8.9) | 171 (9.2) | ||
All P-values from Mann-Whitney U test, except
Chi-square test. Data are shown as n (%), except otherwise mentioned. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease; pt: Patient.
Regarding IBD hospitalizations, 101 (38.3%) CD patients had a total of 168 hospitalizations (median frequency: 1.0; median duration: 6 d), and 28 (19.6%) UC patients had 43 hospitalizations (median: 1.0; mean duration: 4 days). CD patients with moderate to severe disease activity at baseline had more hospitalizations (median: 2.0 vs 1.0 hospitalizations/patient, P = 0.031) than those with mild or no disease activity; no statistically significant differences were observed for UC patients by disease activity.
CD and UC patients attended 3192 (median 11.0) and 1541 (median 10.0) medical appointments, respectively. More than 90% of consultations were with IBD specialists for both IBD types. The assessment of disease activity through common scores, such as CDAI and HBI for CD patients and Partial Mayo Score for UC patients, was not performed in most medical appointments. When comparing by disease activity, no statistically significant differences were observed regarding the number or type of consultations for both IBD types. Changes in treatment occurred in 16.4% and 22.9% of CD and UC consultations, respectively, and CD patients with moderate to severe activity had a higher proportion of medical appointments with treatment changes (18.3% vs 14.7%, P = 0.005) than those with mild or low disease activity.
A total of 5674 imaging/laboratory tests were performed by 260 CD patients (median 19.0) and 2509 by 141 UC patients (median 15.0). Hemograms were the most frequent test, followed by quantification of C-reactive protein. Colonoscopies accounted for 6.1% (CD) and 9.1% (UC) of the total tests. No statistically significant differences were observed in IBD activity at baseline.
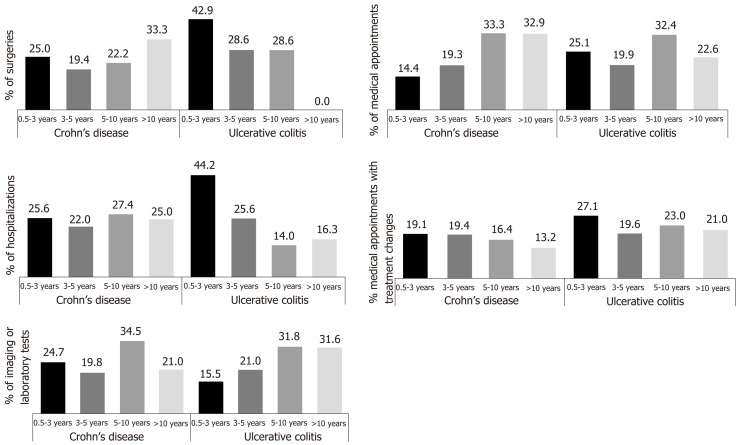
Most surgeries (CD/UC: 44.4%/71.5%) and hospitalizations (CD/UC: 47.6%/69.8%) occurred among IBD patients < 5 years since the first diagnosis of moderate to severe disease compared to patients diagnosed 5 to 10 years or 10 or more years prior (Figure 2). In addition, UC patients diagnosed for less than 5 years had the most medical appointments (45.0%), with 25.1% occurring in patients diagnosed between 6 months and 3 years prior. Medical appointments with treatment changes occurred most frequently (CD/UC: 38.5%/46.7%) among patients diagnosed for less than 5 years, as did imaging/laboratory tests (CD/UC: 44.5%/36.5%), compared to patients diagnosed 5 to 10 years or 10 or more years.
Figure 2.
Healthcare resource utilization by time since the first diagnosis of moderate to severe inflammatory bowel diseases.
IBD treatment at baseline and changes over the previous 3 years
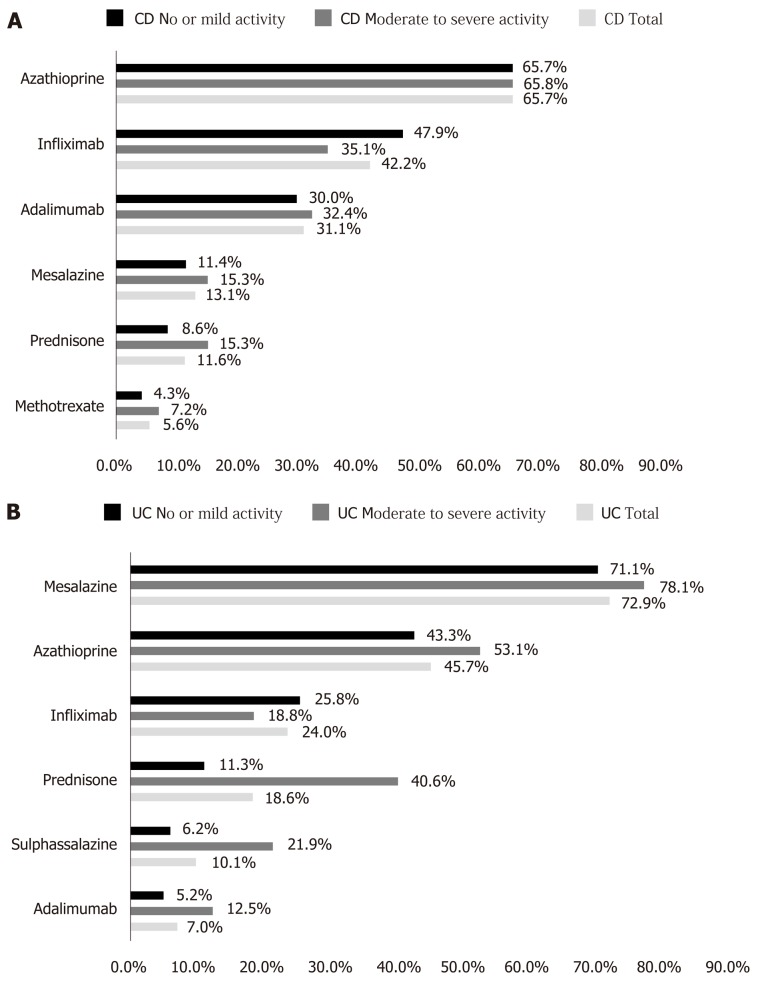
At baseline, the majority of CD (95.1%) and UC (90.2%) patients were on IBD treatment (Table 6). The median number of concurrent medicines at baseline, by patient, was 2.0 for both CD and UC patients. The most common treatments at baseline were biologics (75.3%) and immunosuppressants (70.9%) for CD patients and 5-ASA compounds (77.5%) for UC patients. Considering each IBD medicine (Figure 3), azathioprine was used by 65.7% CD patients, followed by infliximab (42.2%) and adalimumab (31.1%). Among UC patients, 5-ASA (72.9%), azathioprine (45.7%) and infliximab (24.0%) were the three most frequently used treatments.
Table 6.
Treatment for inflammatory bowel diseases at baseline and changes during the previous 3 years
|
CD |
UC |
|||||||
| Total | Moderate to severe activity | No or mild activity | P value | Total | Moderate to severe activity | No or mild activity | P value | |
| n | 264 | 118 | 146 | 143 | 36 | 107 | ||
| IBD treatment at baseline | ||||||||
| Treated patients | 251 (95.1) | 111 (94.1) | 140 (95.9) | 129 (90.2) | 32 (88.9) | 97 (90.7) | ||
| IBD medicines/pt, median [range]2 | 2.0 [1-7]] | 2.0 [1.6] | 2.0 [1-7] | 2.0 [1-6] | 3 [1-6] | 2 [1-5] | ||
| Main IBD therapy3 | ||||||||
| 5-ASA compounds | 39 (15.5) | 20 (18.0) | 19 (13.6) | 100 (77.5) | 27 (84.4) | 73 (75.3) | ||
| Biologic therapy | 189 (75.3) | 79 (71.2) | 110 (78.6) | 41 (31.8) | 11 (34.4) | 30 (30.9) | ||
| Immunosup-pressants | 178 (70.9) | 80 (72.1) | 98 (70.0) | 63 (48.8) | 17 (53.1) | 46 (47.4) | ||
| Any corticosteroid | 30 (12.0) | 17 (15.3) | 14 (10.0) | 26 (20.2) | 13 (40.6) | 13 (13.4) | ||
| Any antibiotic | 14 (5.6) | 11 (9.9) | 3 (2.1) | 7 (5.4) | 5 (15.6) | 2 (2.1) | ||
| Changes to IBD treatment | ||||||||
| Treatment changes /pt, median [range]2 | 1 [0-23] | 1 [0-23] | 1 [0-9] | 0.526 | 2 [0-15] | 1 [0-15] | 2 [0-10] | 0.226 |
| At least one change on IBD treatment2 | 186 (70.7) | 81 (69.2) | 105 (71.9) | 0.6341 | 111 (77.6) | 24 (66.7) | 87 (81.3) | 0.0681 |
| Type of change2 | ||||||||
| Ongoing or beginning | 437 (35.6) | 190 (31.6) | 247 (39.5) | 265 (36.1) | 75 (38.7) | 190 (35.2) | ||
| Dose change | 256 (20.9) | 128 (21.3) | 128 (20.4) | 189 (25.7) | 39 (20.1) | 150 (27.8) | ||
| Discontinued | 534 (43.5) | 283 (47.1) | 251 (40.1) | 280 (38.1) | 80 (41.2) | 200 (37.0) | ||
| Dose change by main IBD therapy | 146 | 71 | 75 | 128 | 31 | 97 | ||
| 5-ASA compounds | 19 (13.0) | 5 (7.0) | 14 (18.7) | 89 (69.5) | 20 (64.5) | 69 (71.1) | ||
| Biologic therapy | 53 (36.3) | 27 (38.0) | 26 (34.7) | 12 (9.4) | 5 (16.1) | 7 (7.2) | ||
| Immunosup-pressants | 74 (50.7) | 39 (54.9) | 35 (46.7) | 27 (21.1) | 6 (19.4) | 21 (21.6) | ||
| Discontinua-tion by main IBD therapy | 226 | 123 | 103 | 128 | 36 | 92 | ||
| 5-ASA compounds | 64 (28.3) | 33 (26.8) | 31 (30.1) | 82 (64.1) | 20 (55.6) | 62 (67.4) | ||
| Biologic therapy | 72 (31.9) | 44 (35.8) | 28 (27.2) | 11 (8.6) | 8 (22.2) | 3 (3.3) | ||
| Immunosup-pressants | 90 (39.8) | 46 (37.4) | 44 (42.7) | 35 (27.3) | 8 (22.2) | 27 (29.3) | ||
| Reason for dose change of biologics [frequency ≥ 5%]4 | ||||||||
| Adverse reaction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 2 (40.0) | 0 (0.0) | ||
| Patient decision/ad-herence | 1 (1.9) | 1 (3.7) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (28.6) | ||
| Poor effectiveness | 22 (41.5) | 12 (44.4) | 10 (38.5) | 3 (25.0) | 1 (20.0) | 2 (28.6) | ||
| Remission | 5 (9.4) | 2 (7.4) | 3 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Serum level of biologic drug | 2 (3.8) | 1 (3.7) | 1 (3.8) | 3 (25.0) | 1 (20.0) | 2 (28.6) | ||
| Reason for discontinua-tion of biol-ogics [frequency ≥ 5%] | ||||||||
| Adverse reaction | 21 (29.2) | 13 (29.5) | 8 (28.6) | 3 (27.5) | 2 (25.0) | 1 (33.3) | ||
| Contraindica-tion | 6 (8.3) | 5 (11.4) | 1 (3.6) | 1 (9.1) | 0 (0.0) | 1 (33.3) | ||
| Patient decision/ad-herence | 5 (6.9) | 4 (9.1) | 1 (3.6) | 2 (18.2) | 1 (12.5) | 1 (33.3) | ||
| Poor effectiveness | 6 (8.3) | 2 (4.5) | 4 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
All P-values from Mann-Whitney U test, except
Chi-square test. Data are shown as n (%), except otherwise mentioned.
Including antibiotics and corticosteroids.
Patients with at least one of the main IBD drug classes, excluding antibiotics, corticosteroids and other medications.
More than one possible reason for dose change. CD: Crohn’s disease; UC: Ulcerative colitis; IBD: Inflammatory bowel disease; pt: Patient.
Figure 3.
Most frequently used drugs for inflammatory bowel diseases (overall frequency ≥ 5%). A: Drugs used by patients with Crohn’s disease (total and by disease activity); B: Drugs used by patients with ulcerative colitis (total and by disease activity). Note: % refers to patients using at least one medicine. CD: Crohn’s disease; UC: Ulcerative colitis.
Most CD patients with moderate to severe disease activity were receiving an immunosuppressant (72.1%) and/or biologic (71.2%). Among CD patients with mild or no disease activity, most were receiving a biologic (78.6%) and/or im-munosuppressant (70.0%) as well. With regard to UC patients, the majority were receiving at least one 5-ASA compound (84.4% vs 75.3%, in moderate to severe vs. mild or no disease activity, respectively) (Table 6).
The majority of CD (70.7%) and UC (77.6%) patients changed treatment at least once during the previous 3 years, including changes in antibiotics and corticosteroids. CD patients had fewer treatment changes than UC patients (median: 1.0 vs 2.0; P = 0.036), and changes were not statistically associated with disease activity in both IBD types. Most of the treatment changes were discontinuations (CD/UC: 43.5%/38.1%). When considering only biologics, immunosuppressants and 5-ASA compounds, most dose changes and discontinuations occurred in immunosuppressants (50.7% and 39.8%) for CD patients and 5-ASA compounds (69.5% and 64.1%) for UC patients. With regard to biologic therapy, dose changes were due to poor effectiveness according to physician criteria in 22 (41.5%) CD patients and poor effectiveness and serum level of the biologic drug in 3 (each) UC patients. Discontinuations of biologics were mainly due to adverse reactions (CD/UC: 29.2%/27.5%).
DISCUSSION
The RISE BR study is the first study with a real-world characterization of the burden of moderate to severe IBD in Brazil, both in the patient and payer perspectives. Overall, 407 (143 UC and 264 CD) patients diagnosed with moderate to severe disease were included, and at enrolment, 25% UC and 45% CD patients had moderately to severely active disease.
QoL was assessed with both generic and disease-specific questionnaires. Irrespective of IBD type, the SF-36 summary scores were low (i.e., scores less than 50, in a range from 1-100). Other Brazilian single-center studies have observed no low SF-36 scores[29] or that SF-36 was low regarding physical limitations and emotional aspects domains[35,36]. Our results may reflect that patients with a previous diagnosis of moderate to severe IBD, even though treated, still perceive their general health as poor and that IBD physically and emotionally impacts their life. These findings are supported by the EQ-5D results, with the most compromised dimensions being pain/discomfort and anxiety/depression and better results in the mobility and self-care dimensions. Not surprisingly, a correlation was observed between these two general questionnaires, especially when considering the EQ-VAS and the SF-36 summary scores and comparing between related dimensions (e.g., SF-36 physical functioning vs. EQ-5D mobility).
The IBDQ results are in line with those of the general QoL questionnaires, and a significant correlation was observed between SF-36 and IBDQ, as reported by other Brazilian studies[29,36]. Considering that the overall IBDQ score ranged between 32 and 224, the observed scores were slightly above the midpoint value, with poorer results for the emotional health and systemic symptoms domains, irrespective of IBD type. Pontes et al. observed a higher range of IBDQ overall scores (min-max: 114-222), probably due to the inclusion of mostly patients with no active IBD[29].
Notably, patients with moderate to severe active disease had lower QoL scores in SF-36 domains and summary measures, EQ-VAS and IBDQ dimensions and overall score versus those with mild or no disease activity. In fact, patients with moderate to severe disease activity had a median IBDQ greater than 170 and scored more than 16 points of difference when compared to patients with mild or no disease activity[37]. Other clinically significant differences were also observed in the SF-36 summary measures (more than 2 points of difference) and EQ-VAS scores (more than 8 points)[37,38]. This pattern has been described in several studies across different world regions[38,39]. For instance, one recent French survey reported that the risk of low QoL was significantly increased with greater disease activity[40]. A Polish study showed that CD patients in remission had lower QoL and work productivity impairment compared to patients with active disease[41]. The same trend was observed in one local single-site study conducted in Mato Grosso, Brazil[36]. Parra et al. also showed that during maintenance treatment with infliximab, adequate serum levels are associated with higher rates of clinical remission, mucosal healing and QoL[12]. In line with other studies[13,39,40,42,43], female gender, being unemployed, lower educational level and lower income were associated with poor QoL in almost all domains and summary measures of the different QoL scales. Pain and the intensity of other symptoms during relapses are disruptive of daily life and are particularly relevant for younger and more socially active patients. However, disease activity is not the only determinant of QoL, as other sociodemographic characteristics play a role in the way patients perceive their disease. To provide the best care to IBD patients, subgroups of patients at higher risk of poor QoL should be identified and offered additional coping strategies and social support.
IBD has a relevant impact on work productivity and daily activities. In our study, patients had approximately 30% impaired worktime, with approximately 12% absenteeism and 18%-24% presenteeism, and approximately 36%-40% TAI. Of note, unemployment frequency in IBD patients (23%) was higher than that of the general population of Brazil (12.6%), in 2017[44]. In addition, TAI was higher among IBD patients with moderate to severe disease activity but also among women, middle-aged patients (40-59 years old, CD only) and patients with lower income (UC only). Other studies have reported the same association, with a TAI of approximately 30%[15,40]. Froes et al. have described that IBD in Brazil leads to frequent and prolonged disability periods and contributes to early retirement, especially among CD patients[45]. A European survey conducted in 2010-2011 with 4670 IBD patients showed that, during previous year, only 25% had not been absent from work due to IBD and that 25% had been absent for more than 25 d[46].
To the best of our knowledge, this is the first study to report the use of healthcare resources for IBD patients in Brazil, even though studies from other countries have observed the high economic burden of these conditions[2,47-51]. Approximately one-quarter of CD patients had at least one surgery during the last 3 years. The proportion of UC patients with previous surgeries was considerably smaller (3%), but approximately 30% underwent a high-cost colectomy. Almost half of CD patients and approximately 35% of UC patients had at least one previous hospitalization, with a median duration of 6 and 4 days, respectively. Gibson et al. observed a higher frequency (43.5%) of hospitalization in UC patients in Australia, which may reflect differences in the access of hospital care[2]. On the other hand, the large majority of the medical appointments in our study were with IBD specialists, while in other countries, IBD follow-up is also performed by general practitioners[2,52-54]. Interestingly, CD patients with moderate to severe disease activity at baseline and those with less time since the first diagnosis of moderate to severe disease had more previous hospitalizations and medical appointments with treatment changes, which may reflect the difficulty of maintaining or achieving remission.
In our study, almost all patients had at least one imaging or laboratory test, with almost half having more than 20 tests in the previous 3 years. However, colonoscopy only accounted for approximately 6%-9% of the procedures, and other IBD-specific tests had an even smaller frequency. This finding, aligned with the rare use of clinical scores during medical appointments, make us speculate that other information (such as specific symptoms or patient-reported disease activity) may be more relevant for IBD specialists in Brazil when deciding about treatment[55].
Almost all IBD patients were on some form of treatment at baseline, mainly with immunosuppressants and biologics among CD patients and with 5-ASA compounds in UC patients. In fact, the treatment pattern at baseline is in line with other studies from Latin America[19]. International guidelines recommend the use of 5-ASA compounds (especially in proctitis and left-sided UC) and/or corticosteroids (preferred in CD patients) for the induction phase and, in more severe or refractory cases, azathioprine and biological agents, while salicylates, thiopurines and biologics are usually recommended for the maintenance period[56-58].
Some study limitations should be addressed. The retrospective data collection may bias the estimates of treatment changes and healthcare use due to missing data on patient’s medical records. For instance, patients could have more than one IBD specialist, with medical appointments that are not registered, thus underestimating healthcare use. On the other hand, the cross-sectional evaluation does not enable us to conclude if patients with higher disease activity at baseline should have received a more effective treatment, especially when considering the relapsing and remission nature of IBD. We cannot exclude that the inclusion of patients with a previous diagnosis of moderate to severe disease, although deliberate, may have contributed to a higher rate of active disease at baseline, thus affecting the generalization of results to all IBD patients. Moreover, selection bias was minimized by the consecutive enrolment at scheduled clinical appointments, but patients with an active disease have a higher chance of having a medical appointment during the recruitment period. Finally, the smaller number of UC patients may have compromised the power of statistical analysis by subgroup.
This was the first multicenter real-world study in Brazil that assessed several PROs, providing insight about the patient and payer perspectives that can contribute to optimizing IBD treatment. Based on clinical and patient-reported assessments, we conclude that moderate-to-severe disease activity, especially among CD patients, is associated with a substantial impact on QoL, work productivity impairment and consumption of healthcare resources (namely, IBD hospitalizations and surgeries) in Brazil. The large sample size selected from reference centers of the most populated regions of Brazil allows a good comprehension of IBD burden and management in the Brazilian context. However, the low use of clinical scores and laboratory examinations of recognized biomarkers (such as fecal calprotectin) should be addressed through specific medical education programs. The frequency of IBD is increasing, and health services should be prepared to provide an adequate response, including by addressing unmet medical needs regarding the access and use of more effective therapies, to help patients with IBD.
ARTICLE HIGHLIGHTS
Research background
Inflammatory bowel diseases (IBD) have been associated with a low quality of life (QoL) and a negative impact on work productivity compared to the general population, across several world regions.
Research motivation
Information about the impact of IBD on QoL and work productivity in Latin American countries is scarce and, in Brazil, emerges mostly from single-center studies. It is important to describe IBD control, patient-reported outcomes (PROs), treatment patterns and use of healthcare resources, so that clinicians and health services can optimize IBD management.
Research objectives
To describe QoL and work productivity and activity impairment (WPAI), treatment patterns and use of healthcare resources among IBD patients in Brazil. The association of disease activity with these outcomes was also evaluated.
Research methods
We conducted a multicenter cross-sectional study in several Brazilian IBD centers, with adult IBD outpatients, with clinical evaluation of disease activity at enrolment, chart review of the previous 3-years for collection of treatment and healthcare use, and an extensive collection of PROs, namely: General measures of QoL such as short-form 36 and EQ-5D-5L questionnaires, the Inflammatory and Bowel Disease Questionnaire (IBDQ, a disease-specific QoL measure) and the WPAI questionnaire.
Research results
In a large sample (n = 407) of patients with ulcerative colitis (n = 143) or Crohn’s disease (n = 264), almost half of CD patients (44.7%) and about a quarter of UC patients (25.2%) presented active disease at baseline. Irrespective of IBD type, QoL scores (SF-36, EQ-5D and IBDQ) were low. Disease activity, female gender, unemployment, and lower education and income were associated with a poorer QoL. IBD patients with active disease had a median IBDQ score 16 points higher (i.e., power QoL) than patients with mild or no disease activity, as well as in SF-36 summary measures (more than 2 points of difference) and EQ-VAS scores (more than 8 points). In our study, patients had approximately 30% impaired worktime, with approximately 12% absenteeism and 18%-24% presenteeism, and approximately 36%-40% total activity impairment. Patients with active IBD showed higher total activity impairment. With regard to use of healthcare resources, approximately one-quarter of CD patients had at least one surgery during the last 3 years. The proportion of UC patients with previous surgeries was considerably smaller (3%), but approximately 30% underwent a high-cost colectomy. Almost half of CD patients and approximately 35% of UC patients had at least one previous hospitalization. Almost all IBD patients were on some form of treatment at baseline, mainly with immunosuppressants and biologics among CD patients and with 5-ASA compounds in UC patients.
Research conclusions
Active IBD, especially among CD patients, is associated with a substantial impact on QoL, work productivity impairment and an increased number of IBD surgeries and hospitalizations in Brazil. This is particularly relevant due to the large proportion of IBD patients with active disease at enrolment and to the increase of IBD prevalence, suggesting the need to improve treatment but also the social support and follow-up of IBD patients in Brazil.
Research perspectives
Future research should address the evolution of PROs and its association with treatment changes and control of IBD activity, in a cohort of newly diagnosed patients in Brazil.
ACKNOWLEDGEMENTS
The authors would like to thank the study participants and site staff who collaborated in the study. In addition, the authors acknowledge Eurotrials Scientific Consultants, now a part of CTI Clinical Trial & Consulting Services, for study monitoring, statistical analysis and medical writing assistance.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was reviewed and approved by the Ethics Committees of the participant centers.
Informed consent statement: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest statement: Parra RS has received fees for serving as a speaker and/or an advisory board member for AbbVie, Ferring Pharmaceuticals, Janssen, UCB Pharma and Takeda. Saad-Hossne R has received fees for serving as a speaker for AbbVie, Janssen, Pfizer and Takeda. Miszputen S has received fees for serving as a speaker and/or a consultant for Farmoquimica, Janssen and Marjan. He has received research funding from Ache, Roche and Takeda. Fernandes M is an employee of Eurotrials, now part of CTI, a CRO that provides services for pharmaceutical laboratories. Catapani WR has received fees for serving as a speaker and/or an advisory board member for Janssen and Takeda. Sassaki LY has received fees for serving as a speaker for AbbVie and Takeda. Gomes TNF has received research funding from Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) and Takeda. She has received fees for serving as a speaker for Janssen. Chebli JMF has received fees for serving as a speaker for AbbVie, Janssen, UCB Pharma and Takeda. Senra JT and Caratin RF are employees of Takeda Pharmaceuticals Brazil. Nones RB has received fees for serving as a speaker for AbbVie, Ferring Pharmaceuticals, Janssen, Nestle, Novartis, Pfizer, UCB Pharma and Takeda. Parente JML has received fees for serving as a speaker for Takeda. Ferrari MLA has received fees for serving as a speaker and/or advisory board member for AbbVie, Ferring Pharmaceuticals, Janssen, UCB Pharma, and Takeda. Santana GO has received fees for serving as a speaker for Takeda, AbbVie, Janssen, and UCB Pharma. She has received research funding from Celgene and Roche. She has received fees for serving as an advisory board member for Janssen. Rocha JJR has received fees for serving as a speaker for Nestle. Feitosa MR has received fees for serving as a speaker for AbbVie and Janssen. Scotton AS has received fees for serving as a speaker for Janssen, Novartis, AbbVie, MSD, and EMS. He has received research funding from Janssen, Novartis, AbbVie, Roche, Pfizer, Bristol, Lilly, Novo Nordisk, Anthera, AstraZeneca, GSK, UCB, Sanofi, Takeda, Parexel, IQVIA, PPD, PRA, ICON, INP Research, Covance, and In Trials. Flores C has received fees for serving as a speaker for Janssen, Takeda, and AbbVie. She has received fees for serving as an advisory board member for Janssen. Zaltman C has received fees for serving as a speaker for UCB, Janssen, Takeda, and AbbVie. She has received research funding from AbbVie, Takeda, and Janssen. Bafutto M has received fees for serving as a speaker for Takeda, AbbVie, Janssen, UCB and Farmoquimica. He has received fees for serving as an advisory board member for AbbVie and Janssen. No conflict-of-interest: Omar Feres, Murilo Moura Lima, Roberto Luiz Kaiser Junior, Carolina Dias Gonçalves, Stella Cristina Silva de Souza, Anderson Antonio de Faria, Isabella de Miranda Guimaraes, Heda Maria Barska dos Santos Amarante, Mikaell Alexandre Gouvea Faria, Odery Ramos Junior.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: June 12, 2019
First decision: July 22, 2019
Article in press: September 13, 2019
P-Reviewer: Can G, Filik L, Yang MS, Zhang L S-Editor: Gong ZM L-Editor: A E-Editor: Zhang YL
Contributor Information
Rogerio S Parra, Department of Surgery and Anatomy, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, SP 14049-900, Brazil. rsparra@hcrp.usp.br.
Julio M F Chebli, Inflammatory Bowel Disease Center, Federal University of Juiz de Fora, Juiz de Fora, MG 36036-247, Brazil.
Heda M B S Amarante, Hospital de Clinicas da Universidade Federal do Parana, Curitiba, PR 80060-900, Brazil.
Cristina Flores, Hospital de Clinicas de Porto Alegre, Porto Alegre – RS 90035-007, Brazil.
Jose M L Parente, Universidade Federal do Piaui, Teresina, PI 64073-500, Brazil.
Odery Ramos, Hospital de Clínicas da Universidade Federal do Parana, Curitiba, PR 80060-900, Brazil.
Milene Fernandes, CTI Clinical Trial & Consulting Services, Lisbon 1070-274, Portugal.
Jose J R Rocha, Department of Surgery and Anatomy, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, SP 14049-900, Brazil.
Marley R Feitosa, Department of Surgery and Anatomy, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, SP 14049-900, Brazil.
Omar Feres, Department of Surgery and Anatomy, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, SP 14049-900, Brazil.
Antonio S Scotton, CMIP Centro Mineiro de Pesquisa, Juiz de Fora, MG 36010-570, Brazil.
Rodrigo B Nones, Hospital Nossa Senhora das Gracas, Curitiba, PR 80810-040, Brazil.
Murilo M Lima, Hospital Universitario da Universidade Federal do Piaui, Teresina, PI 64049-550, Brazil.
Cyrla Zaltman, Carolina D Gonçalves, Isabella M Guimaraes, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ 21941-913, Brazil.
Genoile O Santana, Universidade do Estado da Bahia, - Salvador, BA 41150-000, Brazil.
Ligia Y Sassaki, Department of Internal Medicine, Botucatu Medical School at Sao Paulo State University (UNESP), Botucatu, SP 18618-687, Brazil.
Rogerio S Hossne, Department of Internal Medicine, Botucatu Medical School at Sao Paulo State University (UNESP), Botucatu, SP 18618-687, Brazil.
Mauro Bafutto, Instituto Goiano de Gastroenterologia e Endoscopia Digestiva Ltda, Goiania, GO 74535-170, Brazil.
Roberto L K Junior, Kaiser Clinica, Sao Jose do Rio Preto, SP 15015-110, Brazil.
Mikaell A G Faria, Kaiser Clinica, Sao Jose do Rio Preto, SP 15015-110, Brazil.
Sender J Miszputen, Escola Paulista de Medicina, Sao Paulo, SP 04023-900, Brazil.
Tarcia N F Gomes, UNIFESP, Disciplina de Gastroenterologia, Sao Paulo, SP 04040-002, Brazil.
Wilson R Catapani, Faculdade de Medicina do ABC, Santo Andre, SP 09060-870, Brazil.
Anderson A Faria, Faculdade de Medicina UFMG, Belo Horizonte, MG, 30130-100, Brazil.
Stella C S Souza, Faculdade de Medicina UFMG, Belo Horizonte, MG, 30130-100, Brazil.
Rosana F Caratin, Takeda Pharmaceuticals Brazil, Sao Paulo, SP 04709-011, Brazil.
Juliana T Senra, Takeda Pharmaceuticals Brazil, Sao Paulo, SP 04709-011, Brazil.
Maria L A Ferrari, Faculdade de Medicina UFMG, Belo Horizonte, MG, 30130-100, Brazil.
References
- 1.Moreau J, Mas E. Drug resistance in inflammatory bowel diseases. Curr Opin Pharmacol. 2015;25:56–61. doi: 10.1016/j.coph.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Gibson PR, Vaizey C, Black CM, Nicholls R, Weston AR, Bampton P, Sparrow M, Lawrance IC, Selby WS, Andrews JM, Walsh AJ, Hetzel DJ, Macrae FA, Moore GT, Weltman MD, Leong RW, Fan T. Relationship between disease severity and quality of life and assessment of health care utilization and cost for ulcerative colitis in Australia: a cross-sectional, observational study. J Crohns Colitis. 2014;8:598–606. doi: 10.1016/j.crohns.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 5.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol. 2018;18:87. doi: 10.1186/s12876-018-0822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 8.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 9.Vilela EG, Torres HO, Martins FP, Ferrari Mde L, Andrade MM, Cunha AS. Evaluation of inflammatory activity in Crohn's disease and ulcerative colitis. World J Gastroenterol. 2012;18:872–881. doi: 10.3748/wjg.v18.i9.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeBlanc K, Mosli MH, Parker CE, MacDonald JK. The impact of biological interventions for ulcerative colitis on health-related quality of life. Cochrane Database Syst Rev. 2015;(9):CD008655. doi: 10.1002/14651858.CD008655.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Parra RS, Feitosa MR, Ribeiro LCH, Castro LA, Rocha JJR, Féres O. Infliximab Trough Levels and Quality of Life in Patients with Inflammatory Bowel Disease in Maintenance Therapy. Gastroenterol Res Pract. 2018;2018:1952086. doi: 10.1155/2018/1952086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Have M, van der Aalst KS, Kaptein AA, Leenders M, Siersema PD, Oldenburg B, Fidder HH. Determinants of health-related quality of life in Crohn's disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:93–106. doi: 10.1016/j.crohns.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Magalhães J, Castro FD, Carvalho PB, Moreira MJ, Cotter J. Quality of life in patients with inflammatory bowel disease: importance of clinical, demographic and psychosocial factors. Arq Gastroenterol. 2014;51:192–197. doi: 10.1590/s0004-28032014000300005. [DOI] [PubMed] [Google Scholar]
- 15.Van Assche G, Peyrin-Biroulet L, Sturm A, Gisbert JP, Gaya DR, Bokemeyer B, Mantzaris GJ, Armuzzi A, Sebastian S, Lara N, Lynam M, Rojas-Farreras S, Fan T, Ding Q, Black CM, Kachroo S. Burden of disease and patient-reported outcomes in patients with moderate to severe ulcerative colitis in the last 12 months - Multicenter European cohort study. Dig Liver Dis. 2016;48:592–600. doi: 10.1016/j.dld.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Reilly MC, Gooch KL, Wong RL, Kupper H, van der Heijde D. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford) 2010;49:812–819. doi: 10.1093/rheumatology/kep457. [DOI] [PubMed] [Google Scholar]
- 17.Brazilian Study Group of Inflammatory Bowel Diseases. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. 2010;47:313–325. doi: 10.1590/s0004-28032010000300019. [DOI] [PubMed] [Google Scholar]
- 18.Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246–1256.e6. doi: 10.1016/j.cgh.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Calderón M, Minckas N, Nuñez S, Ciapponi A. Inflammatory Bowel Disease in Latin America: A Systematic Review. Value Health Reg Issues. 2018;17:126–134. doi: 10.1016/j.vhri.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20–25. doi: 10.1590/s0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- 21.Parente JM, Coy CS, Campelo V, Parente MP, Costa LA, da Silva RM, Stephan C, Zeitune JM. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol. 2015;21:1197–1206. doi: 10.3748/wjg.v21.i4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 23.Winship DH, Summers RW, Singleton JW, Best WR, Becktel JM, Lenk LF, Kern F., Jr National Cooperative Crohn's Disease Study: study design and conduct of the study. Gastroenterology. 1979;77:829–842. [PubMed] [Google Scholar]
- 24.Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304–310. doi: 10.1097/01.MIB.0000215091.77492.2a. [DOI] [PubMed] [Google Scholar]
- 25.Lahiff C, Safaie P, Awais A, Akbari M, Gashin L, Sheth S, Lembo A, Leffler D, Moss AC, Cheifetz AS. The Crohn's disease activity index (CDAI) is similarly elevated in patients with Crohn's disease and in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37:786–794. doi: 10.1111/apt.12262. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciconelli RM. Tradução para o português e validação do questionário genérico de avaliação de qualidade de vida “Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)”. Thesis, Escola Paulista de Medicina, Universidade Federal de São Paulo. São Paulo, 1997. Available from: http://repositorio.unifesp.br/handle/11600/15360. [Google Scholar]
- 28.Viegas Andrade M, Noronha K, Kind P, Maia AC, Miranda de Menezes R, De Barros Reis C, Nepomuceno Souza M, Martins D, Gomes L, Nichele D, Calazans J, Mascarenhas T, Carvalho L, Lins C. Societal Preferences for EQ-5D Health States from a Brazilian Population Survey. Value Health Reg Issues. 2013;2:405–412. doi: 10.1016/j.vhri.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Pontes RM, Miszputen SJ, Ferreira-Filho OF, Miranda C, Ferraz MB. [Quality of life in patients with inflammatory bowel diseases: translation to Portuguese language and validation of the "Inflammatory Bowel Disease Questionnaire" (IBDQ)] Arq Gastroenterol. 2004;41:137–143. doi: 10.1590/s0004-28032004000200014. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405–413; discussion 415-420. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- 32.Knowles SR, Keefer L, Wilding H, Hewitt C, Graff LA, Mikocka-Walus A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part II. Inflamm Bowel Dis. 2018;24:966–976. doi: 10.1093/ibd/izy015. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810. [PubMed] [Google Scholar]
- 34.Ciconelli RM, Soárez PC, Kowalski CC, Ferraz MB. The Brazilian Portuguese version of the Work Productivity and Activity Impairment: General Health (WPAI-GH) Questionnaire. Sao Paulo Med J. 2006;124:325–332. doi: 10.1590/S1516-31802006000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calixto RP, Flores C, Francesconi CF. Inflammatory Bowel Disease: Impact on Scores of Quality of Life, Depression and Anxiety in Patients Attending a Tertiary Care Center in Brazil. Arq Gastroenterol. 2018;55:202–207. doi: 10.1590/S0004-2803.201800000-54. [DOI] [PubMed] [Google Scholar]
- 36.de Souza MMH, Barbosa DA, Espinosa MM, Belasco AGS. Qualidade de vida de pacientes portadores de doença inflamatória intestinal. Acta Paul Enferm. 2011;24:479–484. [Google Scholar]
- 37.Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101–109. doi: 10.2147/ceg.s4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coteur G, Feagan B, Keininger DL, Kosinski M. Evaluation of the meaningfulness of health-related quality of life improvements as assessed by the SF-36 and the EQ-5D VAS in patients with active Crohn's disease. Aliment Pharmacol Ther. 2009;29:1032–1041. doi: 10.1111/j.1365-2036.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 39.Høivik ML, Bernklev T, Solberg IC, Cvancarova M, Lygren I, Jahnsen J, Moum B IBSEN Study Group. Patients with Crohn's disease experience reduced general health and vitality in the chronic stage: ten-year results from the IBSEN study. J Crohns Colitis. 2012;6:441–453. doi: 10.1016/j.crohns.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Williet N, Sarter H, Gower-Rousseau C, Adrianjafy C, Olympie A, Buisson A, Beaugerie L, Peyrin-Biroulet L. Patient-reported Outcomes in a French Nationwide Survey of Inflammatory Bowel Disease Patients. J Crohns Colitis. 2017;11:165–174. doi: 10.1093/ecco-jcc/jjw145. [DOI] [PubMed] [Google Scholar]
- 41.Holko P, Kawalec P, Mossakowska M, Pilc A. Health-Related Quality of Life Impairment and Indirect Cost of Crohn's Disease: A Self-Report Study in Poland. PLoS One. 2016;11:e0168586. doi: 10.1371/journal.pone.0168586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huppertz-Hauss G, Lie Høivik M, Jelsness-Jørgensen LP, Henriksen M, Høie O, Jahnsen J, Hoff G, Moum B, Bernklev T. Health-related Quality of Life in Patients with Inflammatory Bowel Disease 20 Years After Diagnosis: Results from the IBSEN Study. Inflamm Bowel Dis. 2016;22:1679–1687. doi: 10.1097/MIB.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 43.Slonim-Nevo V, Sarid O, Friger M, Schwartz D, Chernin E, Shahar I, Sergienko R, Vardi H, Rosenthal A, Mushkalo A, Dizengof V, Ben-Yakov G, Abu-Freha N, Munteanu D, Gaspar N, Eidelman L, Segal A, Fich A, Greenberg D, Odes S Israeli IBD Research Nucleus (IIRN) Effect of psychosocial stressors on patients with Crohn's disease: threatening life experiences and family relations. Eur J Gastroenterol Hepatol. 2016;28:1073–1081. doi: 10.1097/MEG.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 44.OECD. Employment Outlook 2017: How does Brazil compare? OECD Publishing. 2017 [Google Scholar]
- 45.de S B Fróes R, Carvalho ATP, de V Carneiro AJ, de Barros Moreira AMH, Moreira JPL, Luiz RR, de Souza HS. The socio-economic impact of work disability due to inflammatory bowel disease in Brazil. Eur J Health Econ. 2018;19:463–470. doi: 10.1007/s10198-017-0896-4. [DOI] [PubMed] [Google Scholar]
- 46.Lönnfors S, Vermeire S, Greco M, Hommes D, Bell C, Avedano L. IBD and health-related quality of life -- discovering the true impact. J Crohns Colitis. 2014;8:1281–1286. doi: 10.1016/j.crohns.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein CN, Longobardi T, Finlayson G, Blanchard JF. Direct medical cost of managing IBD patients: a Canadian population-based study. Inflamm Bowel Dis. 2012;18:1498–1508. doi: 10.1002/ibd.21878. [DOI] [PubMed] [Google Scholar]
- 48.Bähler C, Vavricka SR, Schoepfer AM, Brüngger B, Reich O. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: a claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol. 2017;17:138. doi: 10.1186/s12876-017-0681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Lofland JH. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–3091. doi: 10.1007/s10620-012-2289-y. [DOI] [PubMed] [Google Scholar]
- 50.Niewiadomski O, Studd C, Hair C, Wilson J, McNeill J, Knight R, Prewett E, Dabkowski P, Dowling D, Alexander S, Allen B, Tacey M, Connell W, Desmond P, Bell S. Health Care Cost Analysis in a Population-based Inception Cohort of Inflammatory Bowel Disease Patients in the First Year of Diagnosis. J Crohns Colitis. 2015;9:988–996. doi: 10.1093/ecco-jcc/jjv117. [DOI] [PubMed] [Google Scholar]
- 51.van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, de Jong DJ, Pierik M, van der Woude CJ, Romberg-Camps MJ, Clemens CH, Jansen JM, Mahmmod N, van de Meeberg PC, van der Meulen-de Jong AE, Ponsioen CY, Bolwerk CJ, Vermeijden JR, Siersema PD, van Oijen MG, Oldenburg B COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79. doi: 10.1136/gutjnl-2012-303376. [DOI] [PubMed] [Google Scholar]
- 52.Bennett AL, Munkholm P, Andrews JM. Tools for primary care management of inflammatory bowel disease: do they exist? World J Gastroenterol. 2015;21:4457–4465. doi: 10.3748/wjg.v21.i15.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubin GP, Hungin AP, Kelly PJ, Ling J. Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther. 2000;14:1553–1559. doi: 10.1046/j.1365-2036.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- 54.Hilsden RJ, Verhoef MJ, Best A, Pocobelli G. A national survey on the patterns of treatment of inflammatory bowel disease in Canada. BMC Gastroenterol. 2003;3:10. doi: 10.1186/1471-230X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derwa Y, Williams CJM, Sood R, Mumtaz S, Bholah MH, Selinger CP, Hamlin PJ, Ford AC, Gracie DJ. Factors affecting clinical decision-making in inflammatory bowel disease and the role of point-of-care calprotectin. Therap Adv Gastroenterol. 2018;11:1756283X17744739. doi: 10.1177/1756283X17744739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira FV, Hossne RS, Kotze PG, Denadai R, Miszputen SJ. Biological therapy in the treatment of moderate-to-severe ulcerative colitis patients: can colectomy be prevented? J Coloproctol (Rio de Janeiro) 2011;31:325–329. [Google Scholar]
- 57.Alencar SSS de, Corrêa R da S, Bezerra C de F, Menezes ESC de, Nascimento AL do, Costa DAA da, Alencar MJC. The surgical treatment of patients with ulcerative colitis from an university hospital at Natal, Brazil. J Coloproctol (Rio de Janeiro) 2012;32:265–270. [Google Scholar]
- 58.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]