Abstract
C-phycocyanin (C-PC) a blue color phycobiliproteins used as a food colorant, therapeutics, medicines, health food and biomarkers. In the present study, morphological property of encapsulated C-PC and its stability under various conditions like temperature, pH conditions are discussed. Microencapsulated droplets formed by extrusion found to be spherical with average size 1.2 ± 0.1 mm. SEM micrographs of freeze dried encapsulate confirmed the spherical shape. The effect of droplet formation with varying alginate percentage (1.5%, 2.0% and 2.5% w/v) was studied. In the stability test at 70 °C and 80 °C relative concentration (CR %) was found to be 86.89 and 88.19%, respectively. The encapsulated C-PC showed a slow degradation at higher temperature compared to without encapsulated C-PC which was confirmed by UV–visible absorbance. At 45 °C and 55 °C temperatures the stability was studied at various pH conditions (pH 4.5, 5.5, 6.5, and 7.0) and reported. Aggregation of C-PC protein will not change during encapsulation was confirmed by SDS-PAGE. FTIR analysis of encapsulate and the alginate depicted similar characteristics of the compound compared to that of native C-phycocyanin colorant. Microencapsulation improves the stability and increases the shelf life of colorant.
Keywords: Encapsulation, C-phycocyanin, Stability, Microencapsulation, Colour, Alginate
Introduction
C-phycocyanin (C-PC) a one of the major phycobiliproteins pigment present in prokaryotic blue-Green (Cynobactreia) and eukaryotic red algae (Rhodophyta) (Romay et al. 2003; Patil and Raghavarao 2007; Tavanandi et al. 2018). It occurs as a super molecular protein complex with phycoerthrin, allophycocyanin to harvest light to perform photosynthesis in photosystem-II reaction centre. C-phycocyanin composed of two subunits, α and β, which occur in equal numbers, but the exact number of α and β pairs make up the molecule may vary among the species (Tandeau de Marsac 2003). C-PC is commonly used as natural dye in food (Chewing gum, dairy product, ice-cream jellies etc.) and cosmetics (lipstick and eye liners) and replaced the synthetic dyes as it is nontoxic and non-carcinogenic (Eriksen 2008; Fernández-Rojas et al. 2014; Jiang et al. 2017; Kuddus et al. 2013; Martelli et al. 2014). C-PC has a significant antioxidant, anti-inflammatory, hepatoprotective due to radical-scavenging properties (Patil et al. 2006; Romay et al. 1998).
C-PC and other phycobiliproteins have large applications as a convenient markers for gel electrophoresis, isoelectric focusing, and gel exclusion chromatograph (Gupta et al. 2011). C-PC pigment is widely used as natural colorant as food additives due to its deep and intense blue color (Suresh et al. 2013; Chethana et al. 2015). They are also used as a fluorescent reagent in immunological analysis (Suresh et al. 2013). They are been used as a valuable fluorescent probe for analysis of cells and molecules. Another important applications of phycobiliproteins is that it is been used as fluorescent markers for cells and macromolecules in biomedical research (Sekar and Chandramohan 2008).
The application of C-phycocyanin in food, pharmaceutical, beverages and other commercial uses depends up on the aggregation state of the protein. The degradation of protein is influenced by pH, temperature, light and protein concentration (Chaiklahan et al. 2012; Edwards et al. 1997; Jespersen et al. 2005; Sarada et al. 1999; Wu et al. 2016). Microencapsulation is an effective and economical method for protecting and improving bioactivity of any biomolecules against adverse conditions (Dubey et al. 2009). To protect the bioactivity or aggregation of protein structure and reactivity of the side chain against variation of temperature, pH and degradation against light can be controlled by encapsulating in different coating or wall materials (Lee and Mooney 2012; Zhang et al. 2016a, b). To improve the stability of C-phycocyanin lot of effort`s were carried out by various authors for improving a stability of C-phycocyanin to effecting parameter. Such as keeping or preserving in preservatives, by changing characteristics by modifying gene or protecting by coating or entrapping with coating material by various methods (Li et al. 2009; Manconi et al. 2010; Yan et al. 2014). In current study, microencapsulated C-phycocyanin was synthesized by extrusion technology by sodium alginate matrix in order to improve stability of C-phycocyanin and to study its morphological nature of droplet formed.
Materials and methods
Biomolecule
Food grade C-phycocyanin from Spirulina platensis spray/freeze dry powder was gifted from M/s. Delhi Nutraceuticals Private Limited, New Delhi, India. The pure C-phycocyanin was used without further processing for all our experiments.
Chemicals and reagents
Anhydrous calcium chloride (CaCl2) and Sodium Alginate food grade were procured from Sigma-Aldrich. Analytical grade chemicals are used for phosphate buffer (pH 7.4) preparation. All other chemicals used were of analytical grade.
Experimental setup
Extrusion of Sodium alginate and C-phycocyanin solution into droplet by using an infusion syringe pump (NE-300) from New Era Pump Systems, Inc., USA. Syringe needle tip used for droplet formation was grinded and polished to uniform size to get uninterrupted droplet formation.
Methods
Phosphate buffer preparation
Phosphate buffer of pH 7.4 was prepared using double distilled water. It used to dissolve droplets after subjecting to stability test to find the quantity and purity of C-phycocyanin.
Microencapsulated of C-phycocyanin droplet formation extrusion dripping technique
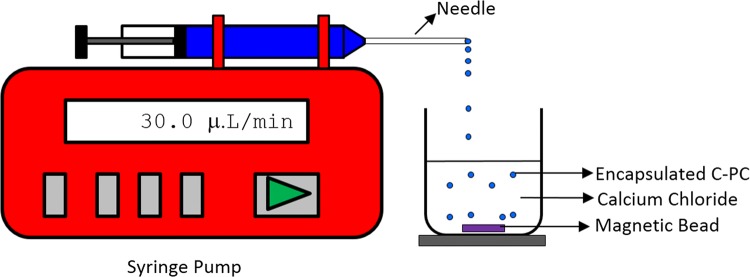
Matrix type of microencapsulated C-phycocyanin (> 1 mm) were prepared by extrusion dripping technique using sodium alginate as a coating material. Sodium alginate solutions were prepared with 1.5%, 2% and 2.5% (w/v) concentration. The solutions were kept in magnetic stirrer for 2 h to get uniform homogeneous solution. C-phycocyanin powder was added to alginate solution according to the ration of 1:1 (w/w) and stirred uniformly for 30 min. The prepared solution was further taken in a syringe of volume 20 ml. The flow rate of the infusion syringe pump was maintained at 30.0 ± 0.1 µl/min. Uniform and constant droplet were extruded by needle into a beaker contain 2% (w/v) calcium chloride solution. The dropped droplets were kept for 24 h in calcium chloride solution to complete ionic gelation process at room temperature. The encapsulated calcium alginate droplets were filtered using Whatman filter paper number 40. Droplets were washed with double distilled water to remove unreacted sodium alginate. The filtered droplets were analyzed and tested for its C-phycocyanin content and stability by subjecting it to various temperature and pH conditions. The schematic representation of the experimental setup for preparation of microencapsulated of C-phycocyanin is depicted in Fig. 1.
Fig. 1.
Schematic diagram of extrusion of C-phycocyanin and sodium alginate mixture into CaCl2 by syringe pump
Stability studies of encapsulated C-phycocyanin
To study the effect of encapsulation on C-phycocyanin, encapsulated droplets were incubated in water bath and maintained at different temperature (40, 50, 60, 70, and 80 °C). To evaluate the effect of pH, encapsulated C-phycocyanin droplets were incubated at different pH levels (4.5, 5.5, 6.5 and 7.0) with respect two different temperatures (i.e., 45 and 55 °C). After stipulated time droplets were removed and dissolved in phosphate buffer to find out remaining concentration of C-phycocyanin.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of encapsulated C-PC
To study the change in nativity of C-PC protein, SDS PAGE gel–electrophoresis (vertical mini gel system, Bio-world, Bangalore) was carried out. SDS-page was composed of 5% stacking gel in pH 6.8 Tris–HCl buffer and 10% SDS and 12% separating gel with buffer of pH 8.8. Native protein, encapsulated protein and standard marker were used for electrophoresis at 150 V for 1 h. Finally bands were visualized by silver staining (Chethana et al. 2015).
Analysis
Spectroscopic measurements
The purity and concentration of C-phycocyanin were measured using a calibrated UV/Visible Spectrometer (Model: T80 + Double Beam UV Spectrophotometer, PG instruments Ltd., United Kingdom) at the wavelengths of 280, 615 and 652 nm.
Purity or grade of C-phycocyanin
The purity or grade of C-phycocyanin is given by the ratio of absorbance at 620 nm to 280 nm i.e., A620/A280, wherein A620 is the maximum absorbance of C-phycocyanin and A280 is the absorbance of total proteins (Patil and Raghavarao 2007).
Concentration of C-phycocyanin
The concentration of C-phycocyanin was determined by measuring the absorbance at 620 nm and 615 nm. Quantitative amount of C-phycocyanin in mg/ml is calculated using the following Eq. (1) as given by (Yan et al. 2014).
| 1 |
Relative concentration of C-phycocyanin (CR)
Relative concentration (CR) of C-phycocyanin is represented by the remaining concentration of C-phycocyanin (C) as percentage of the initial concentration (C0) is calculated (Chaiklahan et al. 2012) as shown in Eq. (2)
| 2 |
where C is the concentration at time t, C0 is the initial concentration.
Microscopic size analysis
The diameter of the encapsulated C-phycocyanin droplet size was visualized and measured under light microscope at 40 × resolution. Using optical microscope (282A Moticam Pro, Motic, Hong Kong, China) consists of digital camera. 50 droplets were collected and size was measured and averaged.
Morphology studies
The morphology of encapsulated was observed by Scanning electron microscope (SEM). The freeze-dried encapsulated droplets were attached to aluminum stubs using two-sided adhesive carbon tape and coated under vacuum with 200 Å gold before examination using a scanning electron microscope (SEM Model: Tescan Vega 3 LM Octane Pro, Module Info:2.18/1116129, Česká Republika, Europe). The SEM was operated at 10 to 20 kV and magnified to 150 × and 5000 ×.
Fourier-transform infrared spectroscopy (FTIR) analysis
Freeze dried microencapsules, phycocyanin and the alginate were grounded and mixed well with potassium bromide, further the prepared grounded powder were made into thin discs using hydraulic press at 100 bar pressure. The spectra were recorded using FTIR spectrophotometer (Model Spectrum 2, PerkinElmer Spectrum Version 10.4.3) from 4000 to 500 cm−1 wave number at a data acquisition rate of 1 cm per point at ambient temperature.
Results and discussion
Experiments were carried out initially using equal ratio of concentration of 0.5%, 1.0% 1.5%, 2.0%, 2.5% and 3.0% (gm/ml) sodium alginate and was mixed with C-Phycocyain (C-PC) purified powder 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 gm, respectively. The alginate CPC prepared mixture at 1:1 ratio was subjected to extrusion process and dropped into calcium chloride solution to get encapsulated C-PC. The concentration of alginate and viscosity of the solution are another major affecting parameters for droplet formation. At lower concentration of alginate the droplets of irregular shape and less stiffness were formed (Viscosity < 19.6 cP). As the concentration of alginate mixture increase the shape and stiffness is also increased. But with an increase in alginate mixture, the viscosity of the mixture also increases. This increase of viscosity (> 1180 cP) will makes the flow difficult and plugs the needle. Similar problems were reported by Yan et al. (2014) where extrusion of more than 3% concentration of sodium alginate plugs the needle and less concentration forms tailings (Yan et al. 2014). To avoid the plug and free flow of the mixture further experiments were carried out at 1.5%, 2.0% and 2.5% sodium alginate concentrations to have a uniform droplet size.
Morphology studies of microencapsulated C-phycocyanin (C-PC) droplets
The surface morphology is one of the important parameter for release of active ingredients from an encapsulated product. For a high throughput production of the encapsulate the size uniformity of droplets plays a significant role during scale-up of the encapsulation process (Jyothi et al. 2010). In this study, 2.5% concentration of sodium alginate and C-PC (1:1) were used for droplet formation. The native C-PC purity was measured and found to be 1.2. Encapsulated C-PC, the size of encapsulate was measured using optical microscope (Motic B302 Model) consists of digital camera. Around 50 numbers of encapsulated C-PC droplets were collected and its images were taken using the digital microscope. The sizes of encapsulated droplets were averaged and the average size was found 1.2 ± 0.1 mm. Size of droplet depends on orifice of the needle and the droplet size varies as orifice of the needle varies. The frequency of droplet formation depends up on flow rate of the feed (Nayak and Pradeep 2018). During the experiment, a constant flow rate (30 μL/min) was maintained and needle size of 0.55 mm was used for encapsulation process.
Microencapsules containing C-phycocyanin appeared smooth spherical appearance without any surface cracks. C-PC is homogeneous within alginate matrix. This is clearly confirmed by Fig. 2a, b. The Fig. 2a depicts with magnification of 40 × using the optical digital microscope. The images show a clear spherical shape with a sharp dark blue colour encapsulation of C-phycocyanin. Examination of SEM micrographs of freeze dried encapsulated C-PC is shown Fig. 2b. The Fig. 2b confirms the spherical appearance of the encapsulated C-PC even after the freeze dried process. The freeze dried encapsulate size was measured and found to be 0.868 ± 0.15 mm. The surface is found to be rough this can be attributed to the evaporation of water during freeze-drying process. Nayak and Pradeep (2018) and Yan et al. (2014) have reported similar observations and structure in SEM observation for C-phycocyanin pigment (Pradeep and Nayak 2016; Yan et al. 2014). Yan et al. 2014, carried out for C-phycocyanin encapsulation by using a chitosan-alginate matrix by extrusion method. The droplet formed from this matrix for 2.5% alginate was found to be in spherical shape with mean diameter 1.41 ± 0.07 mm before freeze drying. Further, after freeze drying the size was reduced to 1.05 ± 0.11 mm. The size reduction and the rough surface after freeze drying is due to fast water evaporation during the freeze drying process (Yan et al. 2014). Similarly, another literature by Li et al. (2009) reported that C-PC doped silica biomaterial matrix SEM image shows cracks in the surface cluster which is attributed to the evaporation process (Li et al. 2009).
Fig. 2.
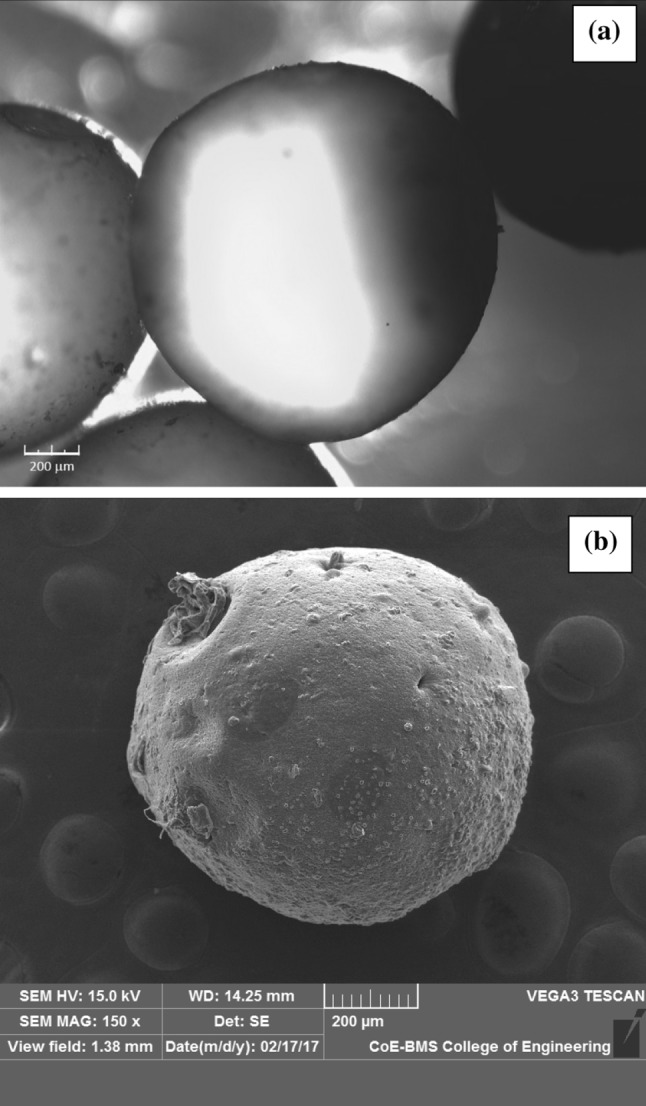
Morphology of encapsulated microsphere of C-PC a with magnification using microscope × 40 and b scanning electron microscope (SEM) images of encapsulated microsphere of C-PC magnification of × 150
Effect of temperature on encapsulated C-phycocyanin (C-PC)
Temperature is one of the important parameter which affects the stability of C-phycocyanin. To study the effect of temperature on encapsulated C-PC 1.5%, 2.0% and 2.5% sodium alginate concentrations were taken. Around fifty numbers of microencapsulated C-phycocyanin was incubated in water bath maintained at 40, 50, 60, 70 and 80 °C temperatures. After incubation at specific time the droplets were dissolved in phosphate buffer solution and its C-PC concentration was analysed. For 2.5% concentration CR value of microencapsulated C-PC after incubating for 30 min at 40 °C, 50 °C and 60 °C were about 95.13%, 94.74% and 92.02%, respectively. This indicates the rate of degradation was slow for 2.5%. This slow rate of degradation may be attributed to the alginate structure and strong matrix formation at 2.5% as well as the C-PC is stable up to 55–60 °C in its native form. While at 70 °C and 80 °C, the rate was observed to be 90.46% and 88.15%, respectively for 30 min of incubation. But after 120 min of incubation the CR values decreased to 88.19 and 86.89%. This change is small at 2.5% alginate at higher temperature. Thus encapsulated C-PC is stable for a longer time (p < 0.05) compared to that of the native C-PC. The similar results are reported by Yan et al. (2014) where C-PC shows greater stability at 40 and 50 °C temperature for a longer duration when it encapsulated with chitosan-sodium alginate matrix (Yan et al. 2014). Martelli et al. (2014) reported the stability with Manuk honey as preservative for C-PC (Martelli et al. 2014). This report stated that the rate of degradation increased at higher degree temperatures when compared to that of lower temperatures. After 90 min of time during their study, it was found that the CR values were not significantly decreased for all the temperatures. This indicates that microencapsulated C-PC can maintain an increased stability at temperatures even at 70 °C and 80 °C.
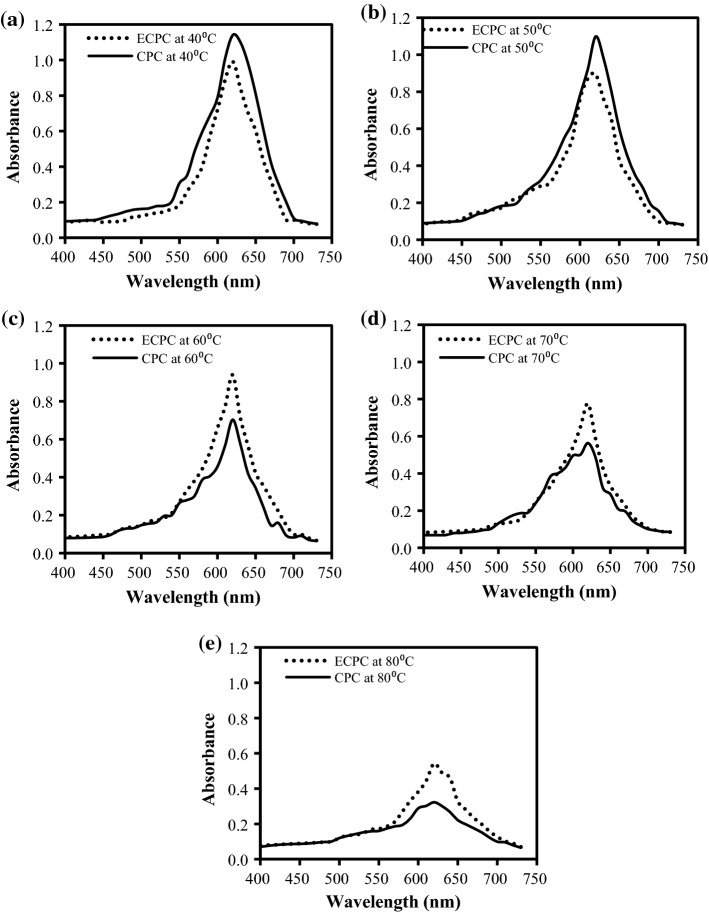
Microencapsulation enhances the stability of the C-phycocyain with respect to temperature. The encapsulated C-PC was compared with the native proteins at various temperatures to confirm the stability. These results were also confirmed by absorbance peak at 620 nm wavelength and shown in Fig. 3a–e. The peak of encapsulated C-phycocyanin shows some sharp absorbance at lower temperature i.e., 40 °C and 50 °C depicting that the biomolecule is not degraded and is in native form as shown in Fig. 3a, b. As temperature increases from 50 to 80 °C absorbance peak decreases Fig. 3c–e. The decrease in the absorbance peak is due to the denaturation of the protein. The change in colour of the protein was observed at 70 °C and 80 °C due to denature of the protein. Similar absorption spectra was reported after exposure of the C-PC/Manuka honey mixture for 30 min at 25, 50, 60, 70 and 80 °C, whereas as the temperature increases spectra gives low C-PC value. Shu and Zhu (2002) studied the release behaviour for encapsulated C-PC coated with sodium alginate and chitosan and reported that at higher concentration of bio-polymer the integrity and strength of encapsulated droplet is high (Shu and Zhu 2002).
Fig. 3.
Absorbance spectra of Native C-PC (CPC) and encapsulated C-PC (ECPC) at various temperatures a 40 °C, b 50 °C, c 60 °C, d 70 °C, and e 80 °C
Effect of pH on encapsulation
C-phycocyanin is highly unstable above 50 °C at its native form. Its property altered and denatured after 50 °C (Sarada et al. 1999). To verify the stability of microencapsulated C-PC was studied at four different pH levels at 4.5, 5.5, 6.5 and 7.0 at two different temperatures (45 °C and 55 °C). Degradation of C-PC was found as relative concentration after incubating at two different temperature i.e., 45 and 55 °C. The results are shown in the Fig. 4a, b. The maximum stability was found to be at pH 6.5 and the lowest stability was at pH 7.0 as well as at pH 4.5. The protein degrades at alkaline or acidic pH conditions which attributes the lower relative concentration. The degradation was found to be minimum for pH-6.5 at both 45 and 55 °C temperatures. It indicates that the encapsulated C-PC was not stable at lower pH and at higher pH value. Similar results are reported by Jiang et al. (2014) showed phycobiliprotein is unstable at strong acidic and alkaline environment, whereas encapsulated C-PC was stable at a large range of pH value (Jiang et al. 2014).
Fig. 4.
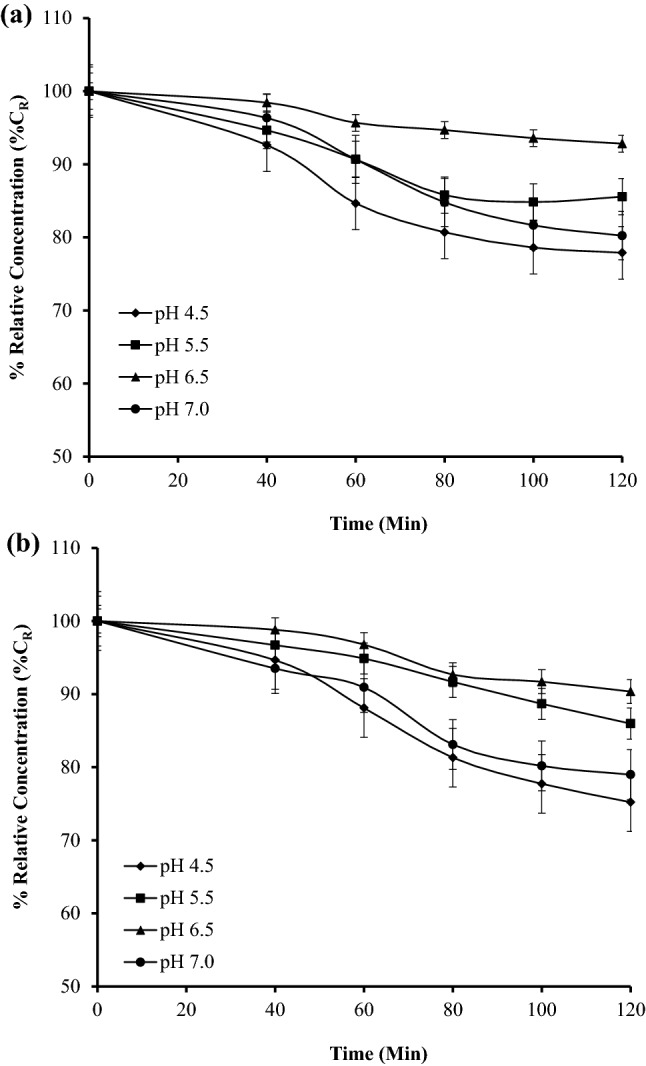
Relative concentration (%CR) of encapsulated C-PC at different pH at a 45 °C and b 55 °C temperatures
Effect of microencapsulation process on C-phycocyanin protein structure stability
Effect of encapsulation process on protein structure stability or integrity of protein was studied. The released C-PC from the encapsulated C-PC was subjected in SDS–PAGE with along with native C-phycocyanin protein. The developed SDS-PAGE is shown in Fig. 5. The protein marker, pure native C-PC and released C-PC from microencapsulate are shown in Lane-1, 2, and 3 respectively in Fig. 5. The bands of the standard protein marker are indicated in the Lane-1 consist of two bands of molecular weight 18.4 kDa and 21.3 kDa respectively. In Lane-2 and Lane-3 also consists of two bands of α and β subunits of C-PC are clearly visible. These molecular weights were consistent with those of earlier literature reports (Chethana et al. 2015). The purity of the released protein was found to be 1.2 ± 0.01 which was not degraded even after encapsulation. Released protein from the encapsulate band height is same height of that of the pure native protein. This confirms that there is no change during encapsulation process as well as later released protein. The retaining of C-PC structure is very important to maintain its properties. This finding suggests or clears that encapsulation process does not change structure of proteins hence it can be used to improve the stability at severe conditions of temperature and pH. Encapsulation of liposome and Human Serum Albumin (HSA) efficiently were entrapped within the alginate microcores. Observation were made that the cross linking of calcium and alginate could significantly affect the integrity of the entrapped protein (Dhoot and Wheatley 2003). Another research article, a protein was entrapped within the alginate matrix to protect from organic solvents, the matrix helped to preserve the structural integrity of the protein (Zhou et al. 2001).
Fig. 5.
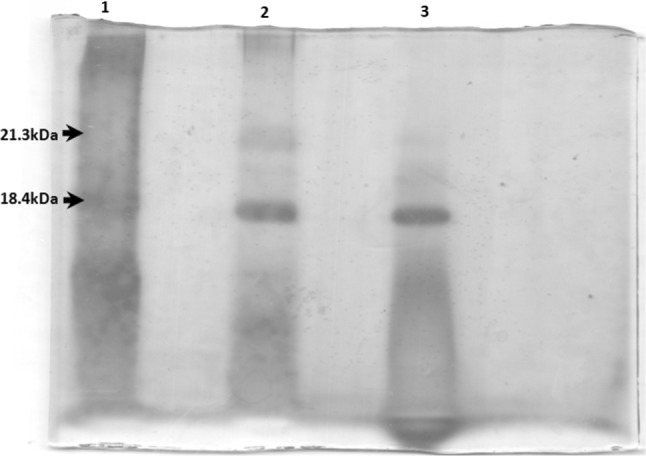
SDS PAGE of silver staining
FTIR analysis of the microencapsules
The FTIR analysis of microencapsulate, C-phycocyanin and the alginate are depicted in Fig. 6. The spectrum of microencapsulated and that of the alginate are similar, but it is very different from that of the C-phycocyain pigment only. This may be attributed to the alginate is well embedded with the pigment. Spectra mainly consists of wave number ranges of 3560–3500 cm−1, 3500–3000 cm−1 and 1750–1735 cm−1. These ranges represents O–H, N–H and C=O bonds respectively for characterization of proteins and sodium alginate (Devi et al. 2012). After encapsulation the wave number range 3500–3000 cm−1 (N–H Secondary amines) starching to higher wave number due to formation of weak electrostatic bond. O–H group peak is shifted from 3352 to 3426 cm−1 wave number is due to breaking of intra molecular hydrogen bond. Wave number of C=O peak also shifted from 1654 to 1628 cm−1 may due to formation of hydrogen bond (Chaiklahan et al. 2012; Kanokpanont et al. 2018). FTIR confirms there are no much chemical changes of CPC during encapsulation. Encapsulates are formed by the electrostatic interactions, this interactions help the stability of the pigments in the matrix and sustained release of protein form alginate matrix easily for its end uses.
Fig. 6.
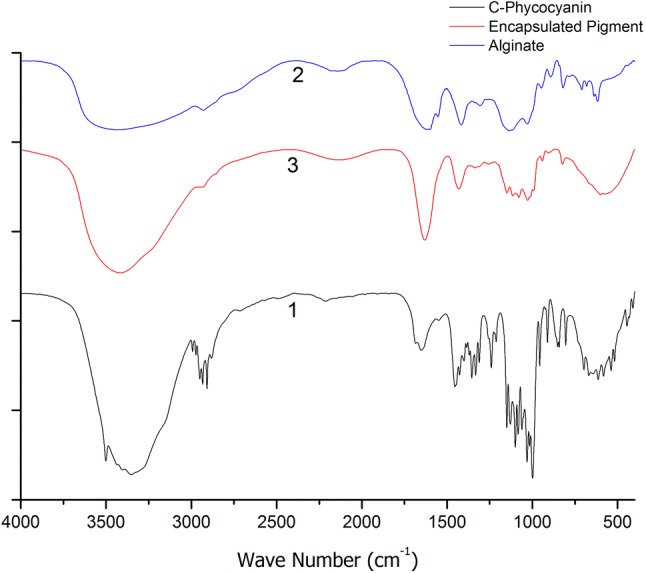
Comparison analysis of Fourier transform infrared spectroscopy graphs for (1) phycocyanin, (2) alginate (3) phycocyanin microcapsule coated by alginate
Conclusion
C-phycocyanin (C-PC) successfully was encapsulated within calcium alginate. The morphology study confirmed the spherical shape of encapsulate and was found to be 1.2 ± 0.1 mm. The stability of encapsulated C-PC was studied for various temperatures (40–80 °C). It was found that the degradation of 2.5% alginate encapsulated C-PC of was slow till 60 °C and relative concentration was found to be 90.28% for 120 min. Whereas, the encapsulated C-PC at higher temperatures 70 °C and 80 °C the degradation was found 88.19% and 86.89%, respectively. The encapsulated C-PC was stable even at high temperature. The protein structural stability of the protein was verified using SDS-PAGE. FTIR confirmed that the nativity of the C-PC was not degraded during encapsulation process. The encapsulation process protects the nativity of the protein as well as the structure of the protein at high temperature. It can be concluded that the controlled extrusion encapsulation process can be utilized to enhance the stability of the C-PC. This method provides high throughput encapsulate for valuable and sensitive components.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chaiklahan R, Chirasuwan N, Bunnag B. Stability of phycocyanin extracted from Spirulina sp.: influence of temperature, pH and preservatives. Process Biochem. 2012;47(4):659–664. [Google Scholar]
- Chethana S, Nayak CA, Madhusudhan M, et al. Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. J Food Sci Technol. 2015;52(4):2415–2421. doi: 10.1007/s13197-014-1287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi N, Hazarika D, Deka C, et al. Study of complex coacervation of gelatin A and sodium alginate for microencapsulation of olive oil. J Macromol Sci Part A. 2012;49(11):936–945. [Google Scholar]
- Dhoot NO, Wheatley MA. Microencapsulated liposomes in controlled drug delivery: strategies to modulate drug release and eliminate the burst effect. J Pharm Sci. 2003;92(3):679–689. doi: 10.1002/jps.19104. [DOI] [PubMed] [Google Scholar]
- Dubey R, Shami TC, Rao KUB. Microencapsulation technology and applications. Def Sci J. 2009;59(1):82–95. [Google Scholar]
- Edwards MR, Hauer C, Stack RF, et al. Thermophilic C-phycocyanin: effect of temperature, monomer stability, and structure. Biochim Biophys Acta (BBA) Bioenerg. 1997;1321(2):157–164. [Google Scholar]
- Eriksen NT. Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol. 2008;80(1):1–14. doi: 10.1007/s00253-008-1542-y. [DOI] [PubMed] [Google Scholar]
- Fernández-Rojas B, Hernández-Juárez J, Pedraza-Chaverri J. Nutraceutical properties of phycocyanin. J Funct Foods. 2014;11:375–392. [Google Scholar]
- Gupta M, Dwivedi UN, Khandelwal S. C-phycocyanin: an effective protective agent against thymic atrophy by tributyltin. Toxicol Lett. 2011;204(1):2–11. doi: 10.1016/j.toxlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Jespersen L, Strømdahl LD, Olsen K, et al. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur Food Res Technol. 2005;220(3):261–266. [Google Scholar]
- Jiang Z, Xuemin T, Kunhuang G, et al. Preparation and characterization of phycobiliprotein mcirocapsule. Adv J Food Sci Technol. 2014;6(1):76–80. [Google Scholar]
- Jiang L, Wang Y, Yin Q, et al. Phycocyanin: a potential drug for cancer treatment. J Cancer. 2017;8(17):3416–3429. doi: 10.7150/jca.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi NVN, Prasanna PM, Sakarkar SN, et al. Microencapsulation techniques, factors influencing encapsulation efficiency. J Microencapsul. 2010;27(3):187–197. doi: 10.3109/02652040903131301. [DOI] [PubMed] [Google Scholar]
- Kanokpanont S, Yamdech R, Aramwit P. Stability enhancement of mulberry-extracted anthocyanin using alginate/chitosan microencapsulation for food supplement application. Artif Cells Nanomed Biotechnol. 2018;46(4):773–782. doi: 10.1080/21691401.2017.1339050. [DOI] [PubMed] [Google Scholar]
- Kuddus M, Singh P, Thomas G, et al. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res Int. 2013;2013:9. doi: 10.1155/2013/742859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang H, Cao FM. Effect of ultraviolet irradiation on photostability of C-phycocyanin in a silica matrix. Res Chem Intermed. 2009;35(5):607–613. [Google Scholar]
- Manconi M, Mura S, Manca ML, et al. Chitosomes as drug delivery systems for C-phycocyanin: preparation and characterization. Int J Pharm. 2010;392(1):92–100. doi: 10.1016/j.ijpharm.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Martelli G, Folli C, Visai L, et al. Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 2014;49(1):154–159. [Google Scholar]
- Nayak CA, Pradeep HN, et al. Sensing using microfluidic platform. In: Bhattacharya S, Agarwal AK, Chanda N, et al., editors. Environmental, chemical and medical sensors. Springer Singapore: Singapore; 2018. pp. 115–136. [Google Scholar]
- Patil G, Raghavarao KSMS. Aqueous two phase extraction for purification of C-phycocyanin. Biochem Eng J. 2007;34(2):156–164. [Google Scholar]
- Patil G, Chethana S, Sridevi AS, et al. Method to obtain C-phycocyanin of high purity. J Chromatogr A. 2006;1127(1):76–81. doi: 10.1016/j.chroma.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Pradeep HN, Nayak CA. Microencapsulation of C-phycocyanin by microfludics. In: Regupathi I, Shetty KV, Thanabalan M, editors. Recent advances in chemical engineering: select proceedings of ICACE 2015. Singapore: Springer; 2016. pp. 89–95. [Google Scholar]
- Romay C, Armesto J, Remirez D, et al. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47(1):36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- Romay C, Gonzalez R, Ledon N, et al. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4(3):207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- Sarada R, Pillai MG, Ravishankar GA. Phycocyanin from Spirulina sp: influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999;34(8):795–801. [Google Scholar]
- Sekar S, Chandramohan M. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol. 2008;20(2):113–136. [Google Scholar]
- Shu XZ, Zhu KJ. Controlled drug release properties of ionically cross-linked chitosan beads: the influence of anion structure. Int J Pharm. 2002;233(1):217–225. doi: 10.1016/s0378-5173(01)00943-7. [DOI] [PubMed] [Google Scholar]
- Suresh PK, Rajendra BG, Rimal BP, et al. Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. J Appl Pharm Sci. 2013;3(08):149–153. [Google Scholar]
- Tandeau de Marsac N. Phycobiliproteins and phycobilisomes: the early observations. Photosynth Res. 2003;76(1):193–205. doi: 10.1023/A:1024954911473. [DOI] [PubMed] [Google Scholar]
- Tavanandi HA, Mittal R, Chandrasekhar J, et al. Simple and efficient method for extraction of C-phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018;31:239–251. [Google Scholar]
- Wu H-L, Wang G-H, Xiang W-Z, et al. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int J Food Prop. 2016;19(10):2349–2362. [Google Scholar]
- Yan M, Liu B, Jiao X, et al. Preparation of phycocyanin microcapsules and its properties. Food Bioprod Process. 2014;92(1):89–97. [Google Scholar]
- Zhang Z, Zhang R, McClements DJ. Encapsulation of β-carotene in alginate-based hydrogel beads: impact on physicochemical stability and bioaccessibility. Food Hydrocoll. 2016;61:1–10. [Google Scholar]
- Zhang Z, Zhang R, Zou L, et al. Protein encapsulation in alginate hydrogel beads: effect of pH on microgel stability, protein retention and protein release. Food Hydrocoll. 2016;58:308–315. [Google Scholar]
- Zhou S, Deng X, Li X. Investigation on a novel core-coated microspheres protein delivery system. J Control Release. 2001;75(1):27–36. doi: 10.1016/s0168-3659(01)00379-0. [DOI] [PubMed] [Google Scholar]