Abstract
Seaweed is a novel food source that is packed with bioactive compounds but is rarely used in its raw/cooked form. An efficient nutrient delivery medium is required to make the potential health benefits of seaweeds accessible to the general population while maintaining its palatability. In this study, coffee infused with different seaweed concentrations (1%, 3%, 5%) were prepared and their physico-chemical, phenolics, flavonoids, antioxidants, rheology, thermal and spectral characteristics were observed. Increase in seaweed concentration resulted in increased acidity and decreased total soluble solids of the beverage with no distinct color change. Rheological measurements showed flow behavior index in the range of 1.09–1.34 indicating dilatant tendency of the seaweed-coffee infusions which gradually decreased towards a Newtonian nature with increase in seaweed concentration. Higher detection of flavonoids and ferric reducing antioxidant power was possible with increase in seaweed concentration from 1 to 5%. However, no significant changes in total phenols and 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity was observed. Sensory evaluation of the coffee drinks was done using fuzzy logic which showed highest sensory acceptability for the infusion with 1% seaweed concentration. Thermograms showed changes in flavor profile on increasing seaweed concentration which was later confirmed using Fourier transform-infrared spectroscopy. Results of the study highlight that coffee can be successfully used to mask the off-flavor of seaweed while disseminating its health benefits to the general population through an effective and extensively utilized food medium.
Keywords: Seaweed, Antioxidant activity, Coffee, Infrared spectroscopy, Sargassum wightii
Introduction
Seaweed is a marine algae and a miracle source of food that provides various health benefits. It is similar to a medicine that contains many bioactive compounds to reduce the risk of chronic diseases. Sargassum wightii, a brown seaweed contains several bioactive compounds such as fucoidan and fucoxanthin along with substantial quantities of polyphenols (phloroglucinol, phlorotannins, phenolic acids etc.) and flavonoids (flavonols and flavonol glycosides). Fucoidan, is a soluble polysaccharides responsible for many biological activities such as anticoagulant, antithrombotic, immunomodulation, anticancer and antiviral activity. The prominent biological activity of fucoidan is due to the presence of sulfate groups in varying amounts (Wijesinghe and Jeon 2012; Borazjani et al. 2017). Biological activities of fucoxanthin include antioxidant, antiinflammatory, anticancer activity, antiobesity and antidiabetic activity (Padua et al. 2015; Kumar et al. 2016).
Seaweeds are rich in vitamins, minerals and dietary fibres (Dhargalkar 2014). Due to high nutritional properties, seaweeds can be consumed as basic food in our daily diet but its consumption is limited to coastal parts of the world. Brown seaweed, Sargassum spp. are boiled and consumed directly in many coastal countries. In India, Japan and Korea, it is consumed as salad, in soups, as rice dishes, and as a savory food ingredient (Kumar et al. 2015). Seaweeds, however, have a distinct fishy smell due to the presence of amines which restrict its potential for ready consumption. To overcome this hurdle, the incorporation of seaweeds to foods with a strong characteristic aroma can be an effective alternative to increase its sensory acceptability.
Many industries have dedicated themselves to overcome the limitation and challenges existing in seaweeds as a functional food ingredient and to prepare traditional and commercial nutritious food such as nori, kombu, wakame, chocolates, soup, cookies, pakoda, bread, crackers, pickles, jam, wafer, porridge, jelly and pasta (Kaliaperumal 2003; Prabhasankar et al. 2009). However, due to the regionality of some of these food products, the major fraction of world population is deprived of the incredible health benefits of seaweed. Moreover, the authors could not find any seaweed based beverage that could be convenient to consume on the go. The major reason for this limitation is the off-flavor of seaweeds which make it undesirable for ready consumption. Therefore, a medium that can mask this off-flavor while providing wide range product access is critical to the commercialization of seaweed based products.
Coffea arabica and Cofffea canephora (Robusta) are the most consumable coffee brew in the world. Coffee brew contains important compounds like flavonoids, hydroxycinnamic acid, caffeic, ferulic acid, pyrogallic acid, quinolinic acid, tannic acid, nicotinic acid, melanoidins, trigonelline, and caffeine (Esquivel and Jimenez 2012). When roasted, 800 different compounds in coffee beans exhibit maillard reaction to produce the characteristic ‘coffee’ aroma which is sufficiently strong to mask any undesired smell from seaweeds. Seaweed infused coffee can also produce a synergistic effect to reduce various health problems as both these natural sources are rich in bioactive components. Moreover, seaweeds have stable antioxidants as compared to the terrestrial plants (Kumar et al. 2015).
Although there are different kinds of food preparations that contain seaweed, the authors could not come across any work that utilizes seaweed (Sargassum wightii) powder in the formulation of coffee infusions. In the present study, Sargassum wightii powder is utilized in coffee brew to investigate its effects on various physico-chemical characteristics (pH, acidity, color, TSS), phytochemical content, antioxidant activity, rheology, spectroscopic, and thermal analysis. The objective of this study is to utilize seaweeds in processed food products and to enable food industries to commercialize seaweed based functional food products.
Materials and methods
Material procurement
Sargassum wightii, an edible brown seaweed, was collected from Mandapam, Gulf of Mannar region of the Indian coast, Tamil Nadu, India, in shed-dried form. Coffee powder, sugar and toned milk were procured from the local market of Sonipat, Haryana, India. The procured coffee powder consisted of 20% protein, 80% carbohydrates and 0% fat while toned milk had 3.1% protein, 4.7% carbohydrates and 3.1% fat (as specified by manufacturer).
Preparation of seaweed coffee
Procured seaweed Sargassum wightii was cleaned with tap water to remove epiphytes, sand and debris and then shade dried at room temperature up to a moisture content of 21.53 ± 0.05% (w.b.). The shed-dried seaweed was ground to powder using a mixer-grinder (Sujata, India) and passed through an 850 micron screen. Three different concentrations of seaweed powder (1%, 3%, 5%) along with fixed quantities of coffee powder (1.5 g) and sugar (10 g) were added to 120 mL of boiled toned milk (Kumar and Badgujar 2018). The formulation was covered and allowed to stand for 5 min following which it was strained to produce a particle-free beverage. All samples were prepared in SS 304 grade stainless steel utensils.
Physicochemical properties
Total soluble solids (TSS) of seaweed coffee samples was measured using a digital refractometer (RX 7000i, ATAGO, Japan), pH was measured using a digital pH meter (EUTECH Instruments). Titratable acidity was determined by titrating 5 mL sample against 0.1 N NaOH in the presence of phenolphthalein indicator. Color characteristics were measured using a hand-held colorimeter (CR-400, Konika Minolta, Japan) and the values for L* (lightness or darkness), a* (redness or greenness) and b* (yellowness or blueness) were recorded. Change in color, chroma and hue were calculated using the equations described by Tarafdar et al. (2018). All measurements were carried out in triplicates.
Rheological properties
Rheological measurements of seaweed coffee samples were performed as suggested by Sirohi et al. (2019) using an Anton Paar MCR 52 rheometer equipped with temperature control unit. Concentric cylinder geometry was utilized for rheological measurements. Data analysis was carried out using Rheoplus software. Measurements were performed over a shear rate () of 1–1000 s−1 at 25 °C. A constant sample volume of 4 mL was used for measurement. Power law equation (Eq. 1) was used to describe the stress–strain relationship for the samples. Rheological properties of seaweed coffee samples such as flow behavior index (n) and consistency index (k) were determined from Eq. (1).
| 1 |
where, τ is the shear stress (Pa). Equation (1) was linerarized as follows:
| 2 |
The slope of Eq. (2) represents the flow behavior index and the apparent viscosity can be determined by the exponential of the intercept as defined by Eq. (3).
| 3 |
Thermal properties
Thermal properties were analyzed using a differential scanning calorimeter (DSC 200 F3, Maia, NETZSCH, Germany). Seaweed coffee samples weighing 5–10 mg were taken in a DSC aluminium pan and hermetically sealed using a lid. The sealed pan was loaded into the equipment at room temperature. An empty pan was used as reference. Flow rate of nitrogen was adjusted at 60 and 40 mL/min in purge line 1 and 2 respectively. Heating was linearly ramped from 30 °C to 250 °C at a rate of 10 °C/min.
FTIR analysis
Seaweed coffee samples were analyzed using Fourier transform infrared spectrometer (Alpha Bruker, USA) at the wavenumber range of 4000–600 cm−1. Samples were analyzed by ATR (Attenuated Total Reflectance) technology using a ZnSe crystal. Smoothening of the sample spectra was done using Opus computer software.
Sensory evaluation using Fuzzy logic model
Sensory analysis of seaweed coffee samples was determined over a nine point hedonic scale. Quality attributes selected for sensory evaluation of seaweed coffee samples were aroma, color, taste and overall acceptability. Judges were initially subjected to a preliminary training to familiarize with seaweed coffee samples. Fourteen semi-trained judges (8 male + 6 female) in the age group of 25–37 years were selected including faculty members and research scholars from the Food Science and Technology and Food Engineering department of National Institute of Food Technology Entrepreneurship and Management, Kundli. Sensory score was analyzed using fuzzy logic to determine the overall score of the seaweed coffee samples (Kumar et al. 2019).
Preparation of seaweed coffee infusion extracts
Samples were extracted according to the protocol defined by Sreeramulu and Raghunath (2011) with slight modifications. Briefly, 5 mL of seaweed coffee infused sample was taken and extract was prepared in 20 mL of 70% methanol containing 0.1% HCl. The mixture was shaken vigorously for 4 h at room temperature. The sample suspension was centrifuged at 7800g for 10 min at 10 °C. The supernatant was collected and filtered through Whatman 1 filter paper and resultant filtrate was stored at − 20 °C. Analysis was completed within a week of extraction.
Phytochemical contents of seaweed coffee infusion extracts
Total phenolic content (TPC)
TPC of seaweed coffee infusion extracts was determined as described by Wang et al. (2009) with minor modifications. Briefly, 1 mL aliquot of diluted samples (the extract stock solution further diluted 10 times with absolute methanol) was mixed with 5 mL of Folin-Ciocalteu reagent (10% in distilled water) in a test tube. After 5 min, 4 mL of sodium carbonate (7.5% in distilled water) was added to each test tube. The test tubes were cap screwed and vortexed. The samples were incubated at room temperature for 2 h under dark conditions. Absorbance was measured at 750 nm using a UV–Vis spectrophotometer (Shimadzu, Japan). Gallic acid was used as standard with a concentration range of 0–0.1 mg/mL. All experiments were performed in triplicates. Results were expressed as mg gallic acid equivalent per mL of extract (mg GAE/mL).
Total flavonoid content (TFC)
TFC of seaweed coffee infusion extracts was determined by aluminium chloride colorimetric method following the method proposed by Chan et al. (2014). Briefly, an aliquot of 0.5 mL (extract stock solution) was mixed with 1.5 mL methanol, 0.1 mL aluminium chloride (10%), 0.1 mL of 1 M potassium acetate and 2.8 mL distilled water in a test tube. The samples were incubated at room temperature for 30 min under dark conditions. The absorbance was measured at 415 nm using a UV–Vis spectrophotometer. Standard curve was made with Quercetin (0–0.1 mg/mL). All experiments were performed in triplicates. Results were expressed as mg quercetin equivalent per mL extract (mg QE/mL).
Antioxidant activity of seaweed coffee infusion extracts
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was performed according to the method described by Benzie and Strain (1996). In FRAP assay, antioxidants present in the samples reduces the Fe3+/tripyridyltriazine complex (Fe3+-TPTZ) to Fe2+-TPTZ form (blue colored). This indicates that antioxidant compounds are electron donors and can reduce the oxidized intermediates of the lipid peroxidation process that act as primary and secondary antioxidants (Matanjun et al. 2008). FRAP reagent was freshly prepared by mixing 300 mM acetate buffer at pH 3.6, 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), and 20 mM ferric chloride in the ratio of 10:1:1 (v/v). The mixture was warmed to 37 °C for 30 min before use. The FRAP solution (3 mL) was added to 0.3 mL of seaweed coffee infusion extracts, and the mixture was kept in dark for 30 min. The absorbance was read at 593 nm using a UV–Vis spectrophotometer. A standard curve of Trolox (5–100 μM) was used for estimation of unknown concentrations. All experiments were performed in triplicates. Results were expressed as µM Trolox equivalent/mL extract (µM TE/mL).
2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity (DPPH) assay
The radical scavenging activity (RSA) of seaweed coffee infusion extracts on DPPH radical was determined according to Brand-Williams et al. (1995) with minor modifications. In DPPH assay, antioxidants donate hydrogen, and DPPH, being a stable free radical, accepts an electron or hydrogen radical to become a stable molecule (Chan et al. 2014). An aliquot of 0.5 mL of freshly prepared 0.06 mM methanolic DPPH was added to test tubes with 0.5 mL of seaweed coffee infusion extracts (the extract stock solution further diluted 10 times with absolute methanol). The reaction mixture was mixed thoroughly and incubated in the dark for 30 min at room temperature. The absorbance was measured at 517 nm with UV–Vis spectrophotometer against blank (methanol). An equal amount of methanol and DPPH served as control. All measurements were performed in triplicates. The radical scavenging activity was calculated as follows:
| 4 |
where A0 is the absorbance of the control solution, AB is the absorbance of the DPPH solution in the presence of extracts, and As is the absorbance of the sample extract without DPPH.
Statistical analysis
All measurements were carried out in triplicates and the values were reported as mean ± standard deviation. Data was analyzed using one-way Analysis of variance (ANOVA) and Duncan’s multiple comparison post hoc test using SPSS statistical software v.20 at 5% level of significance.
Results and discussion
Physico-chemical properties
Table 1 shows the physico-chemical characteristics of seaweed-coffee infusions. Control showed highest per centage of soluble solids which gradually declined with increase in seaweed concentration (SWC) from 1% to 5% (p < 0.05). Decrease in TSS was more prominent with SWC > 1%. Presence of soluble polysaccharides such as fucoidan and phenolic compounds in brown seaweed could form a reversible complex with protein via hydrogen bonding leading to a decrease in TSS (Wong and Cheung 2001). Binding of milk protein to soluble components of the infusion could also reduce the total soluble solids.
Table 1.
Physicochemcial characteristics of Sargassum wightii coffee infusions
| Parameters | Control | 1% SWC | 3% SWC | 5% SWC |
|---|---|---|---|---|
| % TSS | 20.31 ± 0.00c | 20.29 ± 0.00c | 19.96 ± 0.00b | 16.90 ± 0.00a |
| pH | 6.73 ± 0.01c | 6.70 ± 0.01b | 6.67 ± 0.01ab | 6.66 ± 0.01a |
| % Acidity | 0.141 ± 0.005a | 0.144 ± 0.009ab | 0.147 ± 0.005ab | 0.156 ± 0.005c |
| L* | 58.83 ± 0.05c | 58.51 ± 0.38c | 57.50 ± 0.72b | 56.50 ± 0.09a |
| a* | 3.75 ± 0.30a | 3.73 ± 0.03a | 3.77 ± 0.04a | 4.47 ± 0.06b |
| b* | 19.26 ± 0.03b | 18.96 ± 0.10a | 19.24 ± 0.13b | 21.16 ± 0.05c |
| ∆E | – | 0.47 ± 0.27a | 1.33 ± 0.67b | 3.08 ± 0.07c |
| Hue | 78.97 ± 0.10b | 78.85 ± 0.12b | 78.90 ± 0.10b | 78.07 ± 0.13a |
| Chroma | 19.62 ± 0.30b | 19.33 ± 0.17a | 19.60 ± 0.14b | 21.63 ± 0.06c |
Values are represented as mean ± SD of n = 3. Mean values bearing different superscript letters (b, c, d) in the same row differ significantly between groups at p < 0.05 in Duncan’s multiple comparison post hoc test
Minor increase in titratable acidity was observed in the samples with increase in SWC. However, sample with SWC of 5% had considerably higher acidity than control (p < 0.05). Increase in acidity was complemented with an observed decrease in recorded pH. Sargassum wightii contains soluble polysaccharide; fucoidan which consists of glucuronic acid residues in its structure (Zvyagintseva et al. 1999) which may be responsible for the decrease in pH and increase in acidity of seaweed coffee samples.
Color characteristics
CIE Lab color values of seaweed coffee samples are shown in Table 1. Positive values of a* and b* indicated reddish-yellow color of the control sample. Values of a* and b* ranged from 3.73–4.47 to 18.96–21.16, respectively. Increasing SWC in coffee samples resulted in increased a* and b* values but decreased L* value by up to 3.96% (p < 0.05). Maximum L* value of 58.83 was measured for control and the lowest for sample with SWC of 5% (L* = 56.50). Lower L* values signify gradual darkening of the samples with increase in SWC. Since, fucoxanthin pigment has an inherently greenish-brown color, the infusion of coffee and seaweed was expected to show reducing in lightness values. Although, overall change in color (∆E) varied significantly (p < 0.05) in the seaweed coffee samples, no perceivable color change was detected up to 3% SW incorporation. ΔE of 0.5–1.0 is detectable only by expert observers and ΔE of 1.0–2.0 is considered minor color change that can be detected by the human eye (Witzel et al. 1973). Uribe et al. (2018) reported that drying of brown seaweeds at 40 °C results in dark brownish color whereas increased drying temperature results in light brownish color. Since seaweed coffee infusions were subjected to higher temperatures, a relatively lower ∆E was observed. Hue (h°*) values for all the samples were similar (p > 0.05) except for the sample with 5% SWC. Hue refers to the attribute of visible light by which it can be differentiated from primary colors. Similarity in hue indicated a combination of colors rather than an observable distinctive color in the samples. A constant hue with incorporation of seaweeds would be sensorially desirable in such a case. Increased hue in 5% SW incorporated sample can therefore, be rendered unsuitable. Chroma (C*) values varied significantly (p < 0.05) with increase in concentration of seaweed in coffee samples. C* values indicate the intensity or purity of color. Here, a higher chroma suggested a dark yellow color of the seaweed coffee samples.
Rheological properties
Flow behavior was expressed as a logarithmic plot of shear stress (τ) versus shear rate () for the seaweed coffee samples and control as shown in Fig. 1. Control sample showed shear thickening behavior with highest flow behavior index (n) of 1.385. Increasing the concentration of seaweed in coffee samples decreased the value of n thereby, decreasing the shear thickening (dilatant) tendency of the fluid with increased consistency index (k). The flow behavior index for coffee samples with SWCs of 1%. 3% and 5% was 1.34, 1.11 and 1.09, respectively. The consistency index however, increased from 3.0 × 10−4 for control to 3.6 × 10−4, 1.5 × 10−3 and 1.8 × 10−3 for samples with SWC of 1%, 3% and 5%, respectively. Increase in consistency index could be due to the presence of soluble polysaccharide and phenolic compounds of seaweed which increase the percent total soluble solids in the prepared beverages. These compounds bind with soluble solids in coffee samples that decrease the flow behavior index. Strong positive correlation (r = 0.999) was observed between the consistency index and TSS.
Fig. 1.
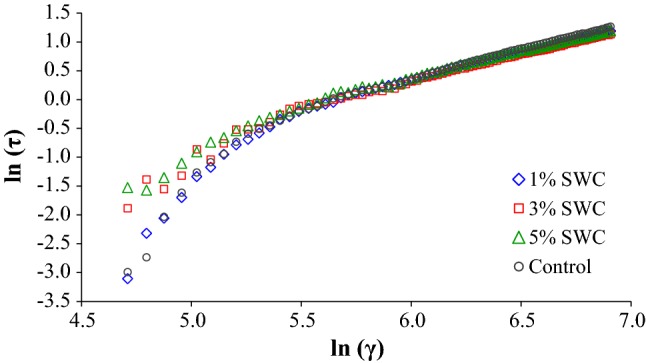
Flow characteristics of Sargassum wightii coffee infusions
DSC analysis
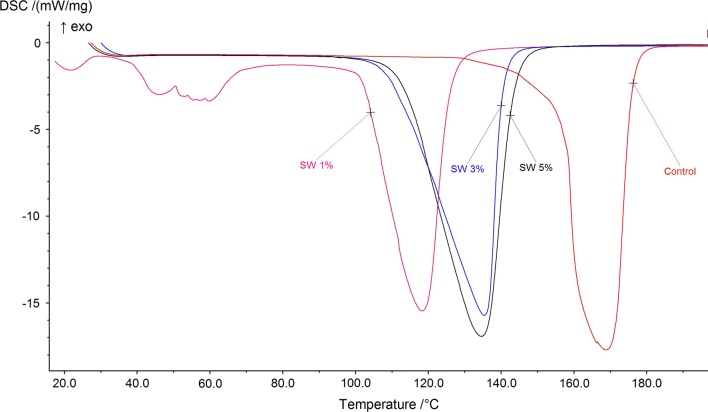
Thermograms of the seaweed infused coffee and control samples showed a characteristic endothermic peak indicating their respective glass transition temperatures (Tg). Tg of control coffee sample was determined as 163 °C. Addition of seaweeds to coffee decreased the Tg of the resulting beverage. Seaweed infused coffee samples with SWC of 1%, 3% and 5% exhibited a Tg of 121.3 °C, 138.1 °C and 139.3 °C, respectively. In control sample, shift in endothermic peak (Fig. 2) was observed which could be due to structural modifications in hydrated caffeine and protein. Rivera et al. (2011) reported that endothermic peak in green coffee beans corresponded to a change in diffraction pattern from a transformation of the a-polymorph of caffeine into a b-crystal phase at 141 °C. A clear shift in Tg of seaweed infused coffee samples bears evidence of flavor change in seaweed infused samples. Changes in sulphated polysaccharides (fucoidan) present in seaweed and denaturation of protein with temperature may be responsible for a shift in Tg (Kumar et al. 2014, 2019). A minor exothermic peak was observed in the sample with 1% SWC possibly due to food protein aggregation caused by hydrophobic interactions (Etemadian et al. 2017) which gradually subsided with increase in SWC.
Fig. 2.
Thermograms of Sargassum wightii coffee infusions over a range of 20–200 °C
Narrowing of the peaks was observed with increase in seaweed concentration in coffee samples. The area under these peaks was used to evaluate the enthalpy of the respective samples. Enthalpy of control coffee sample was calculated as 1.401 kJ/g which decreased to 1.264 kJ/g upon 1% incorporation of seaweed. Further addition of seaweeds increased the enthalpy to 1.602 kJ/g and 1.808 kJ/g for 3% and 5% incorporations, respectively. Increased enthalpy indicates more energy required for peptide and glycosidic bond-breakage. Therefore, the net enthalpy demonstrates the cumulative effects of endothermic (breakdown of hydrogen bonds) and exothermic events (aggregation of food proteins due to hydrophobic interactions) (Kumar et al. 2014).
FT-IR analysis
Infrared spectra of seaweed infused coffee samples were investigated from the plot of % absorbance versus wavenumber (ν). Weak absorption peak above 3700 cm−1 in seaweed coffee samples was seen mostly due to O–H stretching vibrations which show unique patterns that can be used to characterize the compositions of hydrated inorganic compounds. In seaweed infused coffee samples, peak shift in the region ν > 3700 cm−1 as compared to control, can be associated with breakdown of hydroxyl group due to incorporation of seaweed powder. The carbonyl region of the spectra (ν = 1800–1680 cm−1) provides a flavor print of coffee which appears to be consistent with the taste and aroma (Lyman et al., 2003). Higher concentration of seaweed powder in coffee samples disrupted the C=C, C=O and N=O bonds (Kannan 2014) which suppressed the coffee flavor and can be characterized by the lack of peak at (ν = 1547, 1659 cm−1). Peaks from ν = 3000–2900 cm−1 to 2900–2700 cm−1 can be attributed to C–H stretching bond (Stuart 2004) indicative of chlorophyll groups present in seaweeds (Kannan 2014). Peak from ν = 1100–1000 cm−1 indicate several modes such as C–H deformation or C–O or C–C stretching, pertaining to carbohydrates and polysaccharides (Kannan 2014). Table 2 shows a comprehensive list of functional groups observed in the samples under consideration.
Table 2.
FT-IR absorption frequencies (cm−1) and functional group of Sargassum wightii coffee infusions
| Wavenumber (cm−1) | Functional groupa | |||
|---|---|---|---|---|
| Control | 1% SWC | 3% SWC | 5% SWC | |
| 3743 | 3740 | 3750 | 3736 | OH-stretching |
| 2927 | 2927 | 2927 | 2924 |
NH-stretching CH3 and CH2 stretching |
| 2858 | 2858 | 2861 | 2861 | C–H symmetric stretching |
| 1748 | 1748 | 1745 | 1748 | C=O stretching |
| 1656 | 1659 | 1656 | 1659 |
C=O stretching, N=O asymmetric stretching (Nitrate) |
| 1537 | 1550 | 1547 | 1547 | C=C stretching |
| 1055 | 1055 | 1058 | 1055 |
C–F stretching Si–O |
aStretching corresponds to change in bond length. In symmetric stretching, all bonds vibrate in and out together. In asymmetric stretching, some bonds get longer while others get shorter
Phytochemical content of seaweed coffee infusion
With increased levels of seaweed incorporation, the phenolic content increased from 2.79 mg GAE/100 mL to 2.96 mg GAE/100 mL of seaweed infused coffee (Table 3). This increase can be due to leaching of phenolic constituents after addition of seaweed; however, increase was not significant (p > 0.05). Prabhasankar et al. (2009) reported higher phenolic content of 0.29 mg GAE/g of gruels collected after cooking of seaweed (Sargassum marginatum) pasta indicating leaching of phenolic compounds during cooking. Similar trend was observed in seaweed infused coffee samples. Sample with 5% SWC showed highest TPC. Polyphenols like phlorotannins have been reported as the major fractions of antioxidants in brown seaweeds. In the presence of methanol, phlorotannins exhibit structural changes upon polymerization due to which different subunits (such as fucols, fuhalols, eckol, and fucophlorethols) are formed which increase the phenolic content. Since, seaweed powder was prepared from methanolic extracts there could be formation of such subunits along with formation of other components like bromophenols, catechins, and tetraprenyltoluquinols (Airanthi et al. 2011) which impact the phenolic composition of seaweeds. Sachindra et al. (2010) also observed higher polyphenols in methanolic extracts of seaweeds among other solvents.
Table 3.
Phytochemical properties of Sargassum wightii coffee infusions
| Sample | TPC (mg GAE/100 mL) | TFC (mg QE/100 mL) | FRAP (µM Trolox/mL) | DPPH (% radical scavenging activity) |
|---|---|---|---|---|
| Control | 2.817 ± 0.001a | 1.405 ± 0.000b | 176.185 ± 20.855a | 74.451 ± 0.382a |
| 1% SWC | 2.791 ± 0.000a | 1.376 ± 0.001a | 194.981 ± 21.570bc | 72.598 ± 0.479a |
| 3% SWC | 2.936 ± 0.000a | 1.501 ± 0.000c | 205.694 ± 6.558bc | 71.834 ± 0.163a |
| 5% SWC | 2.969 ± 0.001a | 1.525 ± 0.000c | 210.861 ± 14.650c | 71.479 ± 1.554a |
Values are represented as mean ± SD of n = 3. Mean values bearing different superscript letters (b, c, d) in the same column differ significantly between groups at p < 0.05 in Duncan’s multiple comparison post hoc test
With increase in SWC, the flavonoid content increased from 1.37 mg QE/100 mL to 1.52 mg QE/100 mL (p < 0.05) of seaweed infused coffee. However, in samples with 1% SWC, a reduction in TFC was observed. This could be due to the binding of SW flavonoids to milk proteins which were later not detected by aluminum chloride colorimetric assay. Arts et al. (2002) also reported similar interactions between tea flavonoids and proteins. Trend in data for TFC was similar to that observed for TPC. The flavonoid concentration in seaweed extracts depends on the polarity of solvents used in the extract preparation. It was found that the highest flavonoid concentration was in the extracts obtained using solvents of high polarity (Stankovic et al. 2011). Less polar flavonoids (isoflavones, flavanones, methylated flavones, and flavonols) are extracted with low and moderate polar solvents while more polar flavonoid such as flavonoid glycosides and aglycones are extracted with high polar solvents like methanol (Chan et al. 2014). This justifies the increase in TPC and TFC with increase in SWC in coffee samples as a polar solvent is likely to leach the phenolic and flavonoid components of seaweed more responsibly.
Antioxidant activity of seaweed coffee infusion
FRAP assay and DPPH assay
Seaweed infused coffee samples exhibited lower free radical scavenging activity, as compared to control. DPPH radical scavenging activity decreased from 72.59% to 71.47% with increase in level of seaweed incorporation (p < 0.05). Prabhasankar et al. (2009) reported that radical scavenging activity of cooked pasta increased from 12.35% to 38.62% with increase in level of seaweed incorporation. Upon cooking, heat degraded compounds from carbohydrate and protein enzyme hydrolysates of Sargassum spp. may be released which could show higher antioxidant activity. But, in seaweed infused coffee samples, no such degradation of carbohydrates and protein on boiling was observed, which was evident from lowering of radical scavenging activity.
In contrast to DPPH scavenging activity, FRAP increased with increase in SWC in coffee. Leaching of seaweed components responsible for reducing power upon boiling can contribute to antioxidant potential (Prabhasankar et al. 2009). In brown seaweeds, other than polyphenols; fucoxanthin (carotenoid) and sterols are also involved in radical scavenging activity (Airanthi et al. 2011).
Correlation between TPC, TFC and antioxidant potential
A strong significant correlation between FRAP and DPPH assays indicated that the antioxidants which scavenge the free radicals may be reducing the ferric ions (Chan et al. 2014). However, the correlation between FRAP and DPPH was negative (r = − 0.996) since the change in radical scavenging activity was constant (p > 0.05) while FRAP increased (p < 0.05) with increase in SWC. Radical scavenging activity of antioxidants including phenolics and flavonoids, depend on their ability to donate electrons, their structural conformation and hydroxyl group arrangement (Loganayaki et al. 2013). Also, not all compounds can react to every kind of free radical. Therefore, there is a possibility that a particular antioxidant assay will produce better scavenging activity for a particular type of substrate than another. The present study portrays a similar situation wherein the antioxidants present in the seaweed coffee beverage are able to react with Fe3+ ions more effectively than DPPH free radical.
The simultaneous increase in TPC and TFC was supported by a strong positive correlation (r = 0.998). Correlation between DPPH with TPC (r = − 0.746) and, DPPH with TFC (r = − 0.717) was observed to be weak and insignificant. Weak correlation between DPPH and phenolics/flavonoids has been reported earlier for various plant based extracts. Airanthi et al. (2011) reported that there was no significant correlation between TPC and DPPH radical scavenging activity for brown seaweeds. Saeed et al. (2012) also observed weak correlation between total flavonoids and DPPH scavenging activity (EC50) of Torilis Leptophylla L. plant extract. Correlations between FRAP with TPC and TFC were r = 0.799 and 0.772, respectively. The study shows that both antioxidant assays have insignificant correlations with TPC and TFC of Indian edible brown seaweeds.
Sensory evaluation using fuzzy logic
For sensory analysis samples were coded as S1 (1% SWC), S2 (3% SWC), S3 (5% SWC) and S4 (control). Fuzzy logic analysis was performed to establish the order of ranking of seaweed coffee samples as compared with control. All seaweed coffee samples were found to be satisfactory with 1% seaweed infused coffee sample showing highest acceptability among S1, S2 and S3. Control and S1 sample showed ‘very good’ score, however, control scored slightly higher than S1 (Table 4). Final sample ranking was obtained as S4 (0.7325) > S1 (0.7118) > S2 (0.6042) > S3 (0.6507). Studies on toxicity (if any) arising from the interaction of flavor compounds in food systems is warranted for identifying safe levels of SW incorporation (Ravichandran et al. 2018). Flavor profiling is also recommended to weed out undesirable aromatic compounds in seaweeds during processing to utilize seaweeds more generously for food preparations.
Table 4.
Fuzzy logic based similarity values for commercial coffee and seaweed coffee samples
| Scale factor | Similarity value of control (S4) and seaweed coffee samples (S1, S2, S3) | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Not Satisfactory, F1 | 0 | 0 | 0 | 0 |
| Fair, F2 | 0 | 0.0340 | 0.1752 | 0 |
| Satisfactory, F3 | 0.0356 | 0.4989 | 0.6507a | 0 |
| Good, F4 | 0.5066 | 0.6042a | 0.2673 | 0.2548 |
| Very good, F5 | 0.7118a | 0.0650 | 0.0059 | 0.7325a |
| Excellent, F6 | 0.1563 | 0 | 0 | 0.4963 |
aValues represent maximum in each column. Ranking was done giving preference to highest scale factor followed by highest numerical value. For same scale factor, higher value was given preference
Conclusion
Different concentrations of Sargassum wightii seaweed powder were infused in coffee beverage and a sensorially acceptable beverage was developed with 1% seaweed incorporation. Increase in phytochemical and ferric reducing antioxidant power was observed (p < 0.05) with seaweed incorporation; however, no noticeable variation in DPPH scavenging activity was seen. Physico-chemical analysis showed minor increase in the acidity with insignificant color change. The developed beverage can be safely processed up to 120 °C as seen from DSC thermograms without any major phase change phenomenon. Rheological measurements showed dilatant tendency of the beverage at 1% SWC. Studies on flavor profile of the beverage to improve its sensory quality and to increase SW incorporation, is recommended.
Acknowledgements
Authors express their sincere gratitude to Dr. M. Ganesan, Senior Scientist, CSIR Central Salt and Marine Chemicals Research Institute, Mandapam campus, Tamil Nadu for the kind help extended in procuring Sargassum wightii seaweed. The authors also thank Mr. Jai Shankar Prasad, Lab technician, APT Lab, NIFTEM for his constant support for the successful completion of this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Airanthi MKW-A, Hosokawa M, Miyashita K. Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci. 2011;76:C104–C111. doi: 10.1111/j.1750-3841.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- Arts MJ, Haenen GR, Wilms LC, Beetstra SA, Heijnen CG, Voss HP, Bast A. Interactions between flavonoids and proteins: effect on the total antioxidant capacity. J Agric Food Chem. 2002;50(5):1184–1187. doi: 10.1021/jf010855a. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Borazjani NJ, Tabarsa M, You SG, Rezaei M. Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. J Food Sci Technol. 2017;54(12):4016–4025. doi: 10.1007/s13197-017-2867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-williams W, Cuvelier ME, Berset C. Use of a free redical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chan PT, Matanjun P, Yasir SM, Tan TS. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J Appl Phycol. 2014;27(6):2377–2386. doi: 10.1007/s10811-014-0493-1. [DOI] [Google Scholar]
- Dhargalkar VK. Uses of seaweeds in the Indian diet for sustenance and well-being. Sci Cult. 2014;80(7–8):192–202. [Google Scholar]
- Esquivel P, Jimenez VM. Functional properties of coffee and coffee by products. Food Res Int. 2012;46(2):488–495. doi: 10.1016/j.foodres.2011.05.028. [DOI] [Google Scholar]
- Etemadian Y, Shabanpour B, Ramzanpour Z, Shaviklo AR, Kordjazi M. Studies on the functional properties of water extracts of the brown seaweeds Sirophysalis trinodis and Polycladia myrica. J Appl Phycol. 2017;30(3):1989–1999. doi: 10.1007/s10811-017-1361-6. [DOI] [Google Scholar]
- Kaliaperumal N (2003) Products from seaweeds. SDMRI Research Publication, No. 3, pp 33–42
- Kannan S. FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae) Int J Curr Microbiol Appl Sci. 2014;3(4):341–351. [Google Scholar]
- Kumar Y, Badgujar PC. Sensory evaluation of seaweed-coffee infusions using fuzzy logic. Pharm Innov. 2018;7(11):567–572. [Google Scholar]
- Kumar KS, Ganesan K, Selvaraj K, Rao PVS. Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) Doty: an edible seaweed. Food Chem. 2014;153:353–360. doi: 10.1016/j.foodchem.2013.12.058. [DOI] [PubMed] [Google Scholar]
- Kumar S, Sahoo D, Levine I. Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res. 2015;9:117–125. doi: 10.1016/j.algal.2015.02.024. [DOI] [Google Scholar]
- Kumar SR, Narayan B, Kizawa Y, Hosokawa M, Miyashita K. Does squalene alter the antioxidant potential of astaxanthin and fucoxanthinol? In vitro evidence in RAW 264.7 cells, a murine macrophage. J Food Sci Technol. 2016;53(4):2139–2143. doi: 10.1007/s13197-015-2077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Tarafdar A, Kumar Y, Badgujar PC. Intelligent modelling and detailed analysis of drying, hydration, thermal and spectral characteristics for convective drying of chicken breast slices. J Food Process Eng. 2019 doi: 10.1111/jfpe.13087. [DOI] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol. 2013;50(4):687–695. doi: 10.1007/s13197-011-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman DJ, Benck R, Dell S, Merle S, Murray-Wijelath J. FTIR-ATR analysis of brewed coffee: effect of roasting conditions. J Agric Food Chem. 2003;51:3268–3272. doi: 10.1021/jf0209793. [DOI] [PubMed] [Google Scholar]
- Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol. 2008;20:367–373. doi: 10.1007/s10811-007-9264-6. [DOI] [Google Scholar]
- Padua D, Rocha E, Gargiulo D, Ramos AA. Bioactive compounds from brown seaweeds: phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem Lett. 2015;14:91–98. doi: 10.1016/j.phytol.2015.09.007. [DOI] [Google Scholar]
- Prabhasankar P, Ganesan P, Bhaskar N. Influence of Indian brown seaweed (Sargassum marginatum) as an ingredient on quality, biofunctional and microstructure characteristics of pasta. Food Sci Technol Int. 2009;15(5):471–479. doi: 10.1177/1082013209350267. [DOI] [Google Scholar]
- Ravichandran C, Badgujar PC, Gundev P, Upadhyay A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem Toxicol. 2018;120:668–680. doi: 10.1016/j.fct.2018.07.052. [DOI] [PubMed] [Google Scholar]
- Riveraa W, Velascoa X, Galveza C, Rincona C, Rosalesb A, Arangob P. Effect of the roasting process on glass transition and phase transition of Colombian Arabic coffee beans. Proc Food Sci. 2011;1:385–390. doi: 10.1016/j.profoo.2011.09.059. [DOI] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47(1):94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221–232. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi R, Pandey JP. Dilute acid hydrolysis of spoiled wheat grains: analysis of chemical, rheological and spectral characteristics. Bioresour Technol. 2019;283:53–58. doi: 10.1016/j.biortech.2019.03.068. [DOI] [PubMed] [Google Scholar]
- Sreeramulu D, Raghunath M. Antioxidant and phenolic content of nuts, oil seeds, milk and milk products commonly consumed in India. Food Nutr Sci. 2011;2:422–427. [Google Scholar]
- Stankovic MS, Niciforovic N, Topuzovic M, Solujic S. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium Montanum L Var. Montanum, F. Supinum (L.) Reichenb. Biotechnol Biotech Eq. 2011;25:2222–2227. doi: 10.5504/BBEQ.2011.0020. [DOI] [Google Scholar]
- Tarafdar A, Shahi NC, Singh A. Color assessment of freeze-dried mushrooms using photoshop and optimization with genetic algorithm. J Food Process Eng. 2018;55:e12920. doi: 10.1111/jfpe.12920. [DOI] [Google Scholar]
- Uribe E, Galvez AV, Vargas N, Pasten A, Guez KR, Ah-Hen KS. Phytochemical components and amino acid profile of brown seaweed Durvillaea antarctica as affected by air drying temperature. J Food Sci Technol. 2018;55(12):4792–4801. doi: 10.1007/s13197-018-3412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jonsdottir R, Olafsdottir G. Total phenolic compounds, radical scavenging and metallic chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116:240–248. doi: 10.1016/j.foodchem.2009.02.041. [DOI] [Google Scholar]
- Wijesinghe WAJP, Jeon YJ. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohyd Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- Witzel RF, Burnham RW, Onley JW. Threshold and suprathreshold perceptual color differences. J Opt Soc Am. 1973;63:615–625. doi: 10.1364/JOSA.63.000615. [DOI] [PubMed] [Google Scholar]
- Wong K, Cheung PC. Influence of drying treatment on three Sargassum species 2. Protein extractability, in vitro protein digestibility and amino acid profile of protein concentrates. J Appl Phycol. 2001;13:51–58. doi: 10.1023/A:1008188830177. [DOI] [Google Scholar]
- Zvyagintseva N, Shevchenko NM, Popivnich IB, Isakov VV, Scobun AS, Sundukova EV, Elyakova LA. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds Tatiana. Carbohyd Res. 1999;322(1–2):32–39. doi: 10.1016/S0008-6215(99)00206-2. [DOI] [Google Scholar]