Abstract
Introduction
Pre-eclampsia contributes to maternal and fetal morbidity and mortality all over the world. Endothelial dysfunction is postulated to be the crux of the pathogenesis. Recent meta-analysis of aspirin trials showed aspirin to be effective when started early in pregnancy (at ≤ 16-week gestation). We aimed to study the effect of low-dose prophylactic isosorbide mononitrate (ISMN) 20 mg/day on the incidence of hypertensive diseases in high-risk women receiving standard aspirin prophylaxis.
Methods
Design: Randomized double-blind placebo-controlled parallel-arm superiority trial. Setting: Antenatal clinic of a tertiary teaching hospital, South India. Participants and methods: One hundred women fulfilling NICE guideline criteria for aspirin prophylaxis recruited at 12–16 weeks were randomized to receive either 20 mg/day of ISMN or placebo, in addition to 75 mg/day of oral aspirin from recruitment till delivery. Main outcome measure: Rate of hypertensive disorder of pregnancy (HDP). Sample Size: One hundred women (50 in each arm) to detect a decrease of HDP from 20% in the placebo group to 5% in the ISMN group with a power of 80% and at 0.05.
Results
One hundred women (50 in each arm) participated and completed the trial. Intention to treat analysis of these 100 women showed that the groups were comparable in terms of age, BMI, parity, and vascular indices (such as mean arterial pressure, uterine artery pulsatility index, flow-mediated vasodilatation index, brachial–ankle pulse wave velocity, Ankle–Brachial Index, brachial arterial stiffness index, and ankle arterial stiffness index). The rate of hypertensive disorders (gestational hypertension, pre-eclampsia, or superimposed pre-eclampsia) was not significantly different between the groups (14/50, 28% in ISMN vs. 12/50, 24% in placebo group; p = 0.7). The mean gestational age at diagnosis of hypertensive disease (35.4 vs. 36 weeks, ISMN vs. placebo groups, p = 0.7) or the rate of severe disease (8/50, 16% in ISMN vs. 7/50, 14% in the placebo group; p = 0.9) did not differ significantly between the two groups. Stillbirths (1 vs. 2), NICU admission rates (18 vs. 10%), and neonatal mortality (2 vs. 2) were also similar between the groups.
Conclusion
The results of the randomized controlled trial of nitric oxide in the prevention of pre-eclampsia (NOPE) showed that in high-risk women receiving standard aspirin prophylaxis from less than 16 weeks, there is no significant reduction in the incidence of hypertensive disorders of pregnancy in the ISMN group, to the desired extent. There was no significant effect on the severity of disease, gestational age at diagnosis of disease or maternal–perinatal morbidity due to low-dose isosorbide mononitrate.
Electronic supplementary material
The online version of this article (10.1007/s13224-018-1100-1) contains supplementary material, which is available to authorized users.
Keywords: Pre-eclampsia, Prevention, Prophylaxis, Nitric oxide, Isosorbide mononitrate, Aspirin, Randomised controlled trial, NOPE
Introduction
Pre-eclampsia is a pregnancy-specific multi-system disorder characterized by hypertension and proteinuria, often accompanied by maternal and fetal complications. Pre-eclampsia contributes significantly to maternal and fetal morbidity in both developed and developing countries [1, 2]. Commonly, the disease manifests in the third trimester; however, the pathophysiological processes are thought to be related to the process of placentation that occurs during the first and early second trimester. The exact pathogenesis remains unclear and is a matter of intense research over decades. Several important concepts have evolved from extensive research including multi-factorial causation, an increased sensitivity of the maternal vasculature to endogenous vasopressor agents, and implication of vaso-active agents. Although the CLASP trial [3] concluded that routine prophylactic aspirin was ineffective against pre-eclampsia, recent meta-analyses of aspirin trials have revealed protective effect of early institution of aspirin (at ≤ 16-week gestation). The overall reduction in pre-eclampsia risk with aspirin has been estimated to be 17% [4], whereas reduction in early onset severe pre-eclampsia is about 45–50% when started before 16-weeks gestation [5]. With the advent of first trimester screening models that predict pre-eclampsia with an increasing sensitivity at acceptable false-positive rates [6], it is imperative to develop preventive strategies that can offer women effective prophylaxis against the development of pre-eclampsia and its attendant complications.
In the search for another clinically useful prophylactic intervention, nitric oxide appears to be a promising candidate. Nitric oxide has been identified as an important participant in the trophoblastic invasion of the decidua and myometrium, regulation of vascular tone of the uteroplacental vessels, and regulation of platelet function [7]. Several studies have explored the usefulness of nitric oxide in the prevention and treatment of pre-eclampsia. A Cochrane review in 2007 concluded that the use of nitric oxide appears to have an “attractive role” in the prophylaxis of pre-eclampsia, but there is a need for reliable evidence to support its routine clinical use [8]. The studies reviewed had used nitric oxide for either too short a duration or too late in the pregnancy to have produced any beneficial prophylactic effect on pre-eclampsia. Therefore, we aimed to study the effect of early administration of a nitric oxide donor, isosorbide mononitrate, on the incidence of pre-eclampsia and on the maternal–perinatal outcome in women at risk of developing hypertensive disorders of pregnancy [9].
Materials and Methods
Study Design
We tested the research hypothesis that addition of isosorbide mononitrate early in the pregnancy (i.e., before 16 weeks) to the standard aspirin prophylaxis decreases the incidence of pre-eclampsia in high-risk women from a pragmatic practice point of view, with a large effect size. Therefore, a superiority, parallel-arm, double-blind, randomized controlled trial was designed. The study was part of a postgraduate dissertation.
Participants and Enrollment
The study was conducted in the Department of Obstetrics and Gynaecology in Women and Children Hospital attached to Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER)—Pondicherry. This is a tertiary-level teaching hospital, handling 11,000–12,000 births annually. The population that is served by the institute is typically South Indian Tamil-speaking people coming from different socio-economic classes and educational statuses. The study included women who present before 16 weeks of gestation with any of the demographic risk factors for pre-eclampsia as outlined by the NICE guidelines on hypertension [9] [box 1]. Eligible and willing women, after signing the informed consent, were randomized to one of the two study arms.
Box 1.
Inclusion and exclusion criteria for enrollment into the NOPE trial
| Inclusion criteria |
| High-risk factors |
| 1. Previous pregnancy with h/o hypertensive disorders (pre-eclampsia, eclampsia, gestational hypertension, chronic hypertension), IUGR, abruption |
| 2. Chronic hypertension |
| 3. Chronic kidney disease |
| 4. Autoimmune diseases such as SLE, antiphospholipid antibody syndrome |
| Moderate-risk factors |
| 1. First pregnancy |
| 2. Pregnancy interval > 10 years |
| 3. Age > 40 years |
| 4. BMI > 35 kg/m2 |
| 5. Family h/o pre-eclampsia |
| Participants were considered eligible if they have at least one high-risk or at least two moderate-risk factors |
| Exclusion criteria |
| 1. Women with history of heart disease (congenital heart disease, valvular heart disease, or ischemic heart disease) |
| 2. Women with hemoglobin level < 8 gm/dL |
| 3. Women with medical diseases such as epilepsy, jaundice, or renal disorders |
| 4. Women with history of allergy to isosorbide mononitrate |
Randomization, Allocation and Intervention
Participants were randomized using computer-generated random blocks maintained by the pharmacology department. Each block consisted of ten codes, five each for the treatment and placebo arms. The study pharmacologist [JM] maintained a sequence of identical opaque envelopes, each of which had a unique pre-determined treatment assignment code inside. Whenever a participant was enrolled into the study, the pharmacologist added either the experimental drug or placebo into the envelope according to the randomization code and handed over the sealed envelope to the principal investigator [GP] who then dispensed it to the participant after explaining the schedule.
Each participant received an envelope containing either isosorbide mononitrate 20 mg oral tablets or similar-looking placebo tablets. All participants received the standard 75 mg aspirin once daily for pre-eclampsia prophylaxis. The study pharmacologist was the only member of the study team not blinded to the treatment arm and was not involved in assigning patients to treatment arms, assessing patients, or assigning outcomes. None of the clinicians involved in assessing patients’ compliance, recording investigations, follow-up, and outcomes had access to the randomization code breaker.
Each participant was clinically assessed thoroughly with a detailed personal, family, obstetric, and medical history followed by general examination, blood pressure measurement (mercury sphygmomanometer), systemic examination, obstetric examination, and baseline investigations including hemoglobin estimation, blood grouping and Rh typing, infection screening (HIV, HbsAg, VDRL), and urine examination for sugar and protein. All participants underwent baseline measurements of uterine artery Doppler study, and noninvasive endothelial functional assessments such as arterial pulse wave velocity measurements, arterial stiffness indices, and flow-mediated vasodilation test. These vascular parameters were reassessed at 24-weeks gestation to assess the effect of ISMN on the vascular endothelial function. The uterine artery pulsatility index (Ut A PI) was measured according to the protocol described by the Fetal Medicine Foundation [10]. The sagittal section of the uterus was obtained transabdominally and the cervical canal and internal cervical os identified. Subsequently, the transducer was gently tilted from side to side; color flow mapping was used to identify each uterine artery along the side of the cervix and uterus at the level of the internal os. Pulsed wave Doppler was used with the sampling gate of 2 mm at an angle of insonation of less than 30°. When three similar consecutive waveforms were obtained, the pulsatility index was measured. The mean PI of the left and right arteries was calculated.
A volume plethysmographic apparatus (Periscope ™) was used to noninvasively assess endothelial function through various indices such as brachial–ankle pulse wave velocity (ba PWV), Ankle–Brachial Index (ABI), arterial stiffness index (ASI) [11, 12]. The procedure was performed with the patient in supine position at resting state with leads placed over both clavicles and iliac bones. Four BP cuffs were wrapped over both upper arms and above ankles. BP cuffs were connected to a plethysmographic sensor, which determined the pulse volume form and to an oscillometric pressure sensor which determined blood pressure volume waveforms from both brachial and tibial arteries. Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse pressure (PP), heart rate (HR), right and left brachial–ankle pulse wave velocity (ba PWV), Ankle–Brachial Index (ABI), arterial stiffness index (ASI) were measured.
Flow-mediated vasodilatation index was measured according to the technique and guidelines previously published [13, 14]. After a period of rest, the participant assumed a semirecumbent position. Using a linear array high-frequency ultrasound probe (Voluson 730 Pro, GE health care, Austria), a linear image of the brachial artery was obtained 2 cm above the antecubital fossa. Baseline vessel diameter (D1) was obtained by averaging 3 end-diastolic measurements. An appropriately sized cuff was then tied around the forearm and inflated to suprasystolic pressures (50 mmHg above the systolic pressure) for a period of 5 min and then gradually deflated. A second set of measurement of the vessel diameter was then obtained as described above (D2). The flow-mediated vasodilatation index was calculated using the formula FMVDi = (D2 − D1)/D1.
After the baseline data collection, the participants were instructed to take both aspirin and the study tablets once daily, preferably aspirin after a meal in the day, and the test tablet before bed time. The principal investigator [GP] recorded the compliance rate through telephonic follow-up on a weekly basis. The participants were then followed up according to the hospital protocol, i.e., every 4 weeks for the first 28 weeks, every fortnight till 36 weeks, and every week thereafter, if no complications supervened. All women were started on iron and calcium supplementation according to national guidelines, starting from 16 weeks. The women were educated about the warning signs and symptoms of pre-eclampsia and the importance of regular follow-up.
Follow-Up Observations and Outcome Measures
The study participants were reviewed at 24 weeks and underwent uterine artery Doppler velocimetry and the noninvasive tests of endothelial function as mentioned above. At each antenatal visit participants were enquired about symptoms of pre-eclampsia and underwent blood pressure examination and urine testing for protein using dipstick method. A growth scan was scheduled between 28 and 32 weeks if there was a clinical suspicion or if clinical assessment was unsatisfactory. All women were followed up until delivery and discharge subsequently, and the outcome measures were noted. The classification criteria for the hypertensive disorders of pregnancy followed in the hospital and in this study were those laid down in the report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy, 2000 [15]. Pre-eclampsia was classified as severe or mild according to the ACOG practice guideline [16]. All data were documented in a preformatted case report form manually and counterchecked by two other investigators after transferring to an electronic spreadsheet. The primary outcome was defined as the development of any of the hypertensive disorders of pregnancy; secondary outcome included a composite maternal morbidity index defined as the occurrence of eclampsia, abruptio, HELLP (Hemolysis, Elevated Liver enzymes and Low Platelets syndrome), DIC (disseminated intravascular coagulation), pulmonary edema or CVA (cerebro-vascular accident); requirement of more than one antihypertensive drug; and perinatal events such as stillbirth, neonatal death, and admission to neonatal intensive care unit (NICU). The outcome measures used in the current study are defined in supplemental material S1.
Safety and Ethical Considerations
The trial was scheduled to be stopped if 5% or more of participants experienced symptomatic hypotension. The participants were regularly contacted over telephone by one of the investigators and asked about any adverse events, side effects, and compliance. No separate data safety monitoring board was instituted. The study protocol and informed consent forms were approved by the JIPMER Scientific Advisory Committee (for postgraduate dissertations) and JIPMER Institute Ethics Committee for Human Studies. The Drug Controller General of India approved the use of isosorbide mononitrate for the purpose of this study in the proposed dose and route [CT Drugs/178/2012]. The trial was registered with the Clinical Trials Registry of India: CTRI/2012/12/003251.
Sample Size
The expected incidence of hypertensive disorders in women with high-risk factors such as chronic hypertension who are on aspirin prophylaxis is about 20%; the background risk of hypertensive disorders in low-risk women is about 5%. We regarded a reduction in the risk comparable to the background risk to be clinically relevant in the prophylactic setting. To detect this reduction, 50 participants were required in each arm to be able to reject the null hypothesis with a probability (power) of 0.8. The type I error probability associated with this test of null hypothesis was 0.05.
Data Analysis
Data analysis was done using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Chi-square test/Fisher’s exact test was used to analyze the incidence of hypertensive disorders, mortality/morbidity due to pre-eclampsia, and for perinatal outcome. Unpaired t test was used to analyze the time of onset of pre-eclampsia; Mann–Whitney U test to analyze the severity of pre-eclampsia. The Student’s t test for paired samples was used to study the effect of the drug on the vascular indices in each arm. The analysis was done on an intention to treat basis.
Results
The trial began in December 2012 and recruitment was completed in August 2014. Of the 103 eligible women identified, three declined participation and 100 women underwent randomization (Fig. 1). Optimal compliance, defined as adherence to trial-prescribed medication intake of at least 80%, was observed in 95% of women. An interim analysis of 60 subjects was reported in August 2014 to the postgraduate dissertation committee, and this analysis showed no major adverse effect of the study drug. The final distribution of the baseline characteristics of the women between the two arms is presented in Table 1. The groups were comparable with respect to the risk factors for pre-eclampsia. Of note, 31 women in ISMN arm and 37 women in the placebo arm had a previous pregnancy complicated by a hypertensive disorder of pregnancy. In 28 and 33 women in ISMN and placebo arms, respectively, the uterine artery pulsatility index at enrollment was more than the ninetieth centile for the gestational age. The effect of ISMN on the endothelial function was assessed by the change in the vascular characteristics of the participants between the enrollment readings and at 24 weeks. Table 2 describes the vascular changes between enrollment and 24 weeks in both arms. In both groups, women showed a significant drop in the Ut A PI at 24 weeks compared to entry; in the ISMN group, the FMVD index at 24 weeks was lower than at entry (0.05 vs. 0.09, respectively, p = 0.02). None of the other vascular index showed a significant change between entry and 24 weeks in either arm of the study.
Fig. 1.
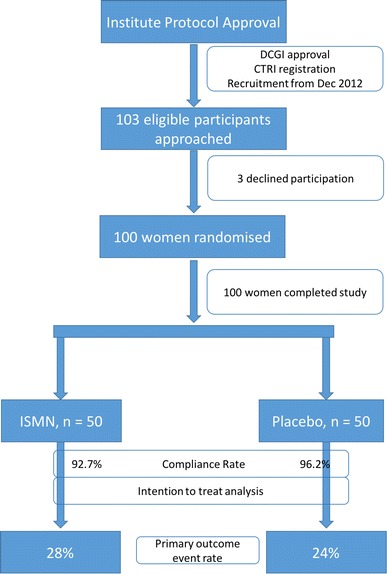
CONSORT diagram—NOPE trial
Table 1.
Baseline characteristics of the participants
| Characteristic | ISMN, n = 50 | Placebo, n = 50 | p value |
|---|---|---|---|
| Age in years, mean (SD) | 27.2 (4.8) | 26.4 (3.9) | 0.4 |
| BMI, mean (SD) | 25.5 (6.8) | 24.9 (3.9) | 0.6 |
| Gestational age at entry in weeks, mean (SD) | 14.6 (1.3) | 14.4 (1.3) | 0.6 |
| Primigravidae, n (%) | 4 (8%) | 6 (12%) | 0.7 |
| Mean arterial pressure in mmHg, mean (SD) | 97.1 (13.8) | 93.2 (9) | 0.1 |
| Mean uterine artery PI, mean (SD) | 1.6 (0.6) | 1.7 (0.6) | 0.3 |
| Risk factors at entry | |||
| Previous PIH spectrum, n (%) | 31 (62%) | 37 (74%) | 0.1 |
| Chronic hypertension, n | 18 (36%) | 16 (32%) | 0.8 |
| Uterine artery PI > 90th centile, n | 28 | 33 | 0.3 |
| Chronic kidney disease | 1 | 2 | |
| SLE | 2 | 2 | |
| Age > 40 years, n | 1 | ||
| BMI > 35 kg/m2, n | 4 | 1 | 0.3 |
Table 2.
Vascular changes over time
| Vascular characteristic | ISMN | Placebo | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | Mean difference | p value | Baseline | 24 weeks | Mean difference | p value | |
| Mean arterial pressure | 96.4 (13.0) | 91.1 (10.3) | 5.3 | < 0.001 | 93.5 (8.8) | 92.0 (8.2) | 1.5 | 0.3 |
| Uterine artery PI | 1.6 (0.6) | 0.98 (0.5) | 0.6 | < 0.001 | 1.7 (0.6) | 0.92 (0.49) | 0.8 | < 0.001 |
| Ba PWV (cm/s) | 1135.6 (158.6) | 1048.6 (398.9) | 87 | 0.24 | 1072.1 (288.9) | 1108.0 (211.8) | − 35.9 | 0.5 |
| Ankle–Brachial Index | 0.9 (0.15) | 1.0 (0.1) | − 0.05 | 0.08 | 0.95 (0.15) | 0.96 (0.12) | − 0.01 | 0.5 |
| Brachial ASI | 22.9 (3.2) | 21.3 (4.0) | 1.5 | 0.12 | 20.7 (4.2) | 22.2 (5.6) | − 1.5 | 0.2 |
| Ankle ASI | 25.2 (8.4) | 27.1 (6.6) | − 1.8 | 0.3 | 27.1 (6.0) | 27.6 (6.2) | − 0.5 | 0.6 |
| FMVD Index | 0.09 (0.09) | 0.05 (0.07) | 0.04 | 0.02 | 0.1 (0.08) | 0.08 (0.09) | 0.01 | 0.3 |
In all, there were 26 women who developed gestational hypertension or pre-eclampsia in the study (26%). The event rate was not different between the two arms (14 vs. 12, RR 1.1 95% CI 0.7–1.8, ISMN vs. placebo group). Severe pre-eclampsia occurred in 15 women (15%) and placental abruption in one woman. There were no maternal deaths in the study participants. Six women reported headaches and three reported myalgia after enrollment; however, these effects were not significantly different between the two arms. There were no statistically significant differences between the two arms in terms of gestational age at disease onset or delivery; mean birthweight; stillbirths, neonatal deaths, or admission to NICU (Table 3).
Table 3.
Outcome
| Outcome | Component | ISMN, n = 50 | Placebo, n = 50 | p |
|---|---|---|---|---|
| Primary outcome | GHT/PE/SIPE, n (%) | 14 (28%) | 12 (24%) | 0.7 |
| Secondary outcome | ||||
| Maternal | Gestational age at disease onset in weeks, mean (SD) | 35.4 (4.6) | 36 (2.0) | 0.7 |
| Gestational age at delivery | 36.7 (6.0) | 37.8 (1.1) | 0.2 | |
| Severe pre-eclampsia, n (%) | 8 (16%) | 7 (14%) | 0.9 | |
| Antihypertensive usage (2 or more), n (%) | 4 (8%) | 3 (6%) | 0.8 | |
| Drug side effect, n headache, myalgia | 3, 2 | 3, 1 | 0.8 | |
| Composite morbidity index, n | 1 | |||
| Perinatal | Stillbirths, n | 1 | 2 | 0.5 |
| Birthweight in grams, mean (SD) | 2730 (456) | 2817 (530) | 0.6 | |
| NICU admission, n (%) | 9 (18%) | 5 (10%) | 0.3 | |
| Neonatal death, n | 2 (4%) | 2 (4%) | ||
| Compliance | Compliance rate | 92.7% | 96.2% | 0.07 |
Discussion
The NOPE trial was powered to detect a decrease in the pre-eclampsia risk from 20 to 5% in ISMN-treated women and has shown no significant effect of ISMN on the incidence of pre-eclampsia in high-risk women. Women in both arms showed a significant decline in the uterine artery PI with advancing gestational age; however, women in the ISMN group, paradoxically, showed a decrease in the flow-mediated vasodilatation index.
Recent meta-analyses of aspirin trials have conclusively shown a reduction in the incidence of pre-eclampsia when aspirin is started before 16 weeks; however, the risk reduction is not uniform across the studies and across the severity of the disease spectrum. In the past, prediction of pre-eclampsia was not always accurate or timely. Tests such as “roll-over” or “isometric hand grip” tests based on the increased vascular sensitivity to vasopressors were too insensitive and too late in the clinical course to be of any use in preventing pre-eclampsia. Second trimester uterine artery Doppler velocimetry improved the lead time in prediction somewhat but still was not enough to institute effective prophylaxis. Currently, studies have demonstrated the high detection rate of combined model for pre-eclampsia prediction—using maternal history, first trimester mean arterial pressure, uterine artery Doppler, PAPP-A, and placental growth factor—in the order of 70–80% for early severe pre-eclampsia for a false-positive rate of 10% [17]. Therefore, there is a need to identify another agent that would substantially supplement the effect of aspirin in reducing pre-eclampsia risk. According to the 2007 Cochrane review [8], nitric oxide appears to be a promising candidate in this respect.
Nitrates are commonly utilized drugs in cardiovascular medicine, especially in ischemic heart disease. Glyceryl trinitrate, isosorbide dinitrate, isosorbide mononitrate are three of the most common organic nitrates in clinical use. Isosorbide mononitrate (ISMN) is an orally active nitric oxide donor that is used routinely in cardiac patients for the treatment of angina pectoris. It is stable in room temperature and relatively cheap than other preparations of nitrates. However, a small percentage of patients experience significant headaches, especially in the first few days after starting therapy. Tolerance is not a particular problem with ISMN when dosing is asymmetric [18]. ISMN has been used in pregnancy as a ripening agent before induction of labor with studies reporting inconsistent results [19]. ISMN has several advantages over the other commonly available nitrates such as orally active form, high bioavailability, once daily dosing, and low frequency of tolerance. All nitrates share similar side effects related to vasodilatation, especially with the first few doses such as headaches, flushing, dizziness, and postural hypotension. Although ISMN appears to be the agent of choice among the organic nitrates, a recent review on the prophylactic role of nitrates has highlighted the potential therapeutic advantages of another, uncommon organic nitrate, namely penta erythrityl tetra nitrate (PETN). The authors have pointed out that in addition to enhanced endothelial action, PETN also has long-term antioxidant properties [20]. However, in keeping with the basic ideals of a prophylactic strategy, we have chosen ISMN over PETN on the grounds of easy availability and affordability in our part of the world. The current study has shown a high rate of compliance for ISMN intake during pregnancy.
The 2007 Cochrane review [8] on the role of nitrates in pre-eclampsia has systematically reviewed six trials using nitric oxide donors for preventing pre-eclampsia and its complications. The overall data were insufficient to delineate the effect of NO on pre-eclampsia. The authors concluded that although nitric oxide appeared to have an “attractive role” in the prophylaxis of pre-eclampsia, there is still paucity of reliable evidence to support its routine clinical use. Kalidindi et al. [20] reviewed the role of nitric oxide donors in the prevention of pre-eclampsia. They identified four interventional studies in which nitric oxide donors were employed prophylactically for a period of more than 7 days. In these trials, the intervention resulted in no significant change in the pre-eclampsia risk. The current study examined the role of nitric oxide donor, ISMN, as a supplement to aspirin use in the prevention of pre-eclampsia among high-risk mothers. In this setting, supplemental NO did not reduce the incidence of pre-eclampsia to the desired level.
Investigators have studied the effect of different nitric oxide donors on the uterine and fetoplacental blood flows [21, 22]. Isosorbide dinitrate and glyceryl trinitrate transdermally have shown to improve uterine artery pulsatility index although no clinically meaningful conclusions could be drawn from these experimental studies. In our study, there was a decline in the uterine artery PI in both groups, with advancing gestational age, as expected from the physiological changes in the uteroplacental circulation. We also tested the hypothesis whether NO supplementation would improve the endothelial function without evident clinical effect on pre-eclampsia. However, none of the indices of endothelial function used in this study showed any significant improvement in the ISMN group compared to the placebo group. These findings may point to the overlapping effect of nitric oxide and aspirin in the endothelial function or may indicate alternative vaso-active pathways operative during pregnancy that are NO independent. Further research in this direction may describe the role of NO in the vascular physiology during pregnancy.
Conclusions
Within the limitations of the study, we conclude that in high-risk women on standard aspirin prophylaxis, the risk of hypertensive disorders of pregnancy is not reduced to background level by the addition of isosorbide mononitrate. A larger randomized study is required to assess whether a clinically relevant but a smaller effect size exists.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr Diwakar Mohan, MD DrPH, Johns Hopkins School of Public Health, Baltimore, USA, for his contribution in designing the study and sample size calculation.
Dr. G. Ponmozhi
is currently pursuing her 3-year fellowship in Fetal Medicine at the prestigious Mediscan Systems under Dr. S. Suresh. She won the Gold Medal in Obstetrics and Gynaecology during her Residency in Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry. The present work is her postgraduate dissertation under the guidance of Dr. K. Manikandan. Dr. Ponmozhi has keen interest in clinical research and is known for her meticulousness in her work. She hails from Orathanadu, a small town near Tanjore, Tamil nadu.
Author Contributions
MK and GP conceived and designed the study, while AK and MJ contributed to the design. MK, GP, and AK performed the experiments; MK and GP performed the data analysis, and AK reviewed the results; MJ provided randomization and allocation masking; all authors contributed to the writing of the manuscript final version. MK is the guarantor of the paper.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflicts of interest.
Ethics Approval
The study protocol and informed consent forms were approved by the JIPMER Scientific Advisory Committee (for postgraduate dissertations) and JIPMER Institute Ethics Committee for Human Studies, No IEC/SC/2012/4/148; Federal Wide Assurance registration number of the ethics committee is FWA00019293. The Drug Controller General of India approved the use of isosorbide mononitrate for the purpose of this study in the proposed dose and route [CT Drugs/178/2012].
Footnotes
Dr. G. Ponmozhi is currently pursuing her 3-year post-doctoral fellowship in Fetal Medicine at the prestigious Mediscan Systems under Dr. S. Suresh.
References
- 1.Wilkinson H, on behalf of the Trustees and Medical Advisers Saving mothers’ lives. Reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG. 2011;118(11):1402–1403. doi: 10.1111/j.1471-0528.2011.03097.x. [DOI] [PubMed] [Google Scholar]
- 2.Prakash J, Pandey LK, Singh AK, et al. Hypertension in pregnancy: hospital based study. J Assoc Physicians India. 2006;54:273–278. [PubMed] [Google Scholar]
- 3.CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. CLASP (Collaborative Low-dose Aspirin Study in Pregnancy) Collaborative Group. Lancet Lond Engl. 1994;343(8898):619–29. [PubMed]
- 4.Duley L, Henderson-Smart DJ, Knight M, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2004;(1):CD004659. [DOI] [PubMed]
- 5.Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–146. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 6.Anderson UD, Gram M, Åkerström B, et al. First trimester prediction of preeclampsia. Curr Hypertens Rep. 2015;17(9):584. doi: 10.1007/s11906-015-0584-7. [DOI] [PubMed] [Google Scholar]
- 7.Elzbieta Poniedzialek-Czajkowska BM. Nitric Oxide in Normal and Preeclamptic Pregnancy [Internet]. http://www.eurekaselect.com. [cited 2016 Nov 28]. Available from: http://www.eurekaselect.com/73857/article. [DOI] [PubMed]
- 8.Meher S, Duley L. Nitric oxide for preventing pre-eclampsia and its complications. In: Cochrane database of systematic reviews [Internet]. Wiley, 2007 [cited 2016 Nov 28]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006490/abstract. [DOI] [PMC free article] [PubMed]
- 9.Visintin C, Mugglestone MA, Almerie MQ, et al. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;25(341):c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 10.Martin AM, Bindra R, Curcio P, et al. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18(6):583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 11.Woodman RJ, Kingwell BA, Beilin LJ, et al. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18(2 Pt 1):249–260. doi: 10.1016/j.amjhyper.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Naidu MUR, Reddy BM, Yashmaina S, et al. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng OnLine. 2005;23(4):49. doi: 10.1186/1475-925X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RA, Nishiyama SK, Wray DW, et al. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thijssen DHJ, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Report of the National High Blood Pressure Education Program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. doi: 10.1067/mob.2000.107928. [DOI] [PubMed] [Google Scholar]
- 16.Hypertension in Pregnancy—ACOG [Internet]. [cited 2016 Nov 28]. Available from: http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy.
- 17.Akolekar R, Syngelaki A, Poon L, et al. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 18.Abshagen UW. Pharmacokinetics of isosorbide mononitrate. Am J Cardiol. 1992;70(17):61G–66G. doi: 10.1016/0002-9149(92)90028-W. [DOI] [PubMed] [Google Scholar]
- 19.Kelly AJ, Munson C, Minden L. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2011;(6):CD006901. [DOI] [PubMed]
- 20.Kalidindi M, Velauthar L, Khan K, et al. The role of nitrates in the prevention of preeclampsia: an update. Curr Opin Obstet Gynecol. 2012;24(6):361–367. doi: 10.1097/GCO.0b013e32835a31de. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsuka M, Tada K, Kimura Y, et al. Clinical experience of long-term transdermal treatment with nitric oxide donor for women with preeclampsia. Gynecol Obstet Invest. 1999;47(1):13–19. doi: 10.1159/000010055. [DOI] [PubMed] [Google Scholar]
- 22.Cacciatore B, Halmesmäki E, Kaaja R, et al. Effects of transdermal nitroglycerin on impedance to flow in the uterine, umbilical, and fetal middle cerebral arteries in pregnancies complicated by preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1998;179(1):140–145. doi: 10.1016/S0002-9378(98)70264-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


