Abstract
In the present study, peel oils were extracted through hydrodistillation and cold pressing from three Citrus species (Valencia orange, Ponkan and Eureka lemon) to investigate their volatile constituents and antioxidant activities. A total of 47 volatile components were identified by GC–MS, and then grouped by principal component analysis. The extraction methods were found to have an obvious effect on the proportion of terpenes and oxygenated compounds in the six Citrus oils, especially for Eureka lemon oils. The major fractions in the Citrus oils were found to be monoterpenes (78.65–96.57%), with limonene occupying a dominant percentage (51.22–86.65%). Furthermore, γ-terpinene and terpinolene displayed strong DPPH (1,1-diphenyl-2-picrylhydrazyl) scavenging abilities and efficient inhibition of lipid peroxidation, while oxygenated compounds of α-terpineol and terpinen-4-ol showed poor DPPH radical-scavenging abilities. Therefore, hydrodistillated Eureka lemon oil with high levels of α-terpineol (9.11%) and terpinen-4-ol (4.69%) presented low radical scavenging capability. Citral displayed a high pro-oxidant ability against thiobarbituric acid reactive species formation, which might lead to the decreased ability of the Eureka lemon oils in inhibition of lipid peroxidation, since citral was significantly high in Eureka lemon oils. This study facilitated the understanding of volatile constituents and antioxidant activities in different Citrus peel oils.
Keywords: Citrus peel oil, Hydrodistillation, Cold pressing, Individual component, Antioxidant activities
Introduction
Citrus fruits, with a world-wide popularity for their nutritional value and special flavor, are usually consumed as fresh product or processed juice. During the processing of Citrus juice, massive wastes (peel and bagasse), are produced, accounting for 40–50% of the total fruit weight (Silvestre et al. 2016). However, improper disposal of Citrus peel will not only lead to the waste of resources, but also pose harm to the environment, suggesting the importance of comprehensive utilization of Citrus wastes.
Essential oils extracted from Citrus peel are the important by-product of Citrus industry. In nature, essential oils are synthesized by plants as secondary metabolites and contribute to the growth and survival of plants as antimicrobial agents or insecticides (Bakkali et al. 2008). With the wide use of Citrus essential oils in food preservation, pharmaceutical industry, perfumery and cosmetics, the development of essential oils from peel can facilitate the comprehensive utilization of Citrus wastes (Bousbia et al. 2009; Mahato et al. 2019).
Due to their existence in oil sacs or oil glands of fruit peel (Allaf et al. 2013), Citrus essential oils are usually extracted from citrus peel by cold pressing and distillation. Essential oils are a complex mixtures of terpenes, hydrocarbons and oxygenated derivatives such as alcohols, aldehydes, ketones and esters (Bassolé and Juliani 2012). The quantitative determination of essential oils in different Citrus varieties and cultivars has been widely explored in several previous studies (Chutia et al. 2009; González-Mas et al. 2019; Guo et al. 2018). The effects of different extraction processes on oil constituents have also been evaluated by several researchers (Allaf et al. 2013; Boukroufa et al. 2015; Bousbia et al. 2009). Moreover, intensive research has also been performed on the biological actions of Citrus essential oils as fragrance (Graziano et al. 2012; Singh et al. 2010; Tongnuanchan et al. 2012) especially, their antimicrobial activities (Espina et al. 2011; Fisher and Phillips 2008).
The antioxidant activities of Citrus essential oils have also attracted much attention from researchers (Guo et al. 2018; Menichini et al. 2011). However, most of previous studies are mainly focused on the antioxidant activities of whole oils, while the function of individual oil components remains poorly understood. Currently, the information is still incomplete about the antioxidant properties of Citrus essential oils as well as the corresponding individual volatile components. Therefore, this study aims to explore the potential contributions of individual oil components to the antioxidant activities of the entire oil by analyzing the volatile components and antioxidant abilities of the peel oils extracted from three different Citrus varieties by hydrodistillation and cold pressing.
Materials and methods
Materials
Three species of mature Citrus fruits were used in this study. Valencia orange (C. sinensis Osbeck forma Valencia) and Eureka lemon (C. limon Burm. f. cv. Eureka) fruits were harvested respectively in June and December of 2014 in Yichang, Hubei, China. Ponkan (C. reticulata Blanco) fruits were obtained in December of 2014 from Huazhong Agricultural University Wuhan, China. The outermost pigmented layers (flavedo) of the peel were separated by a sharp knife, collected in glass tubes and stored at − 80 °C for further extraction.
Chemicals and reagents
Standards of n-paraffins (C8–C22), α-pinene, β-pinene, myrcene, d-limonene, γ-terpinene, terpinolene, 3-carene, α-terpineol, terpinen-4-ol and citral were purchased from Sigma-Aldrich Chemical Company (Saint Louis, MO, USA). DPPH (1,1-diphenyl-2-picrylhydrazyl), thiobaribituric acid (TBA), malondialdehyde (MDA), 2,2′-azobis (2-amidinopropane) dihydrochloride(AAPH) and sodium dodecyl sulfate (SDS) were obtained from Yuanye Bio-Technology Co., Ltd (Shanghai, China). All other analytical grade chemicals were bought from Sinopharm chemical reagent Co., Ltd (Shanghai, China).
Extraction of essential oils
The essential oils were extracted from Citrus peels using two classical methods: hydrodistillation and cold pressing. The hydrodistillation method was carried out as previously reported with minor modifications (Périno-Issartier et al. 2013). Briefly, 100 g of peel was hydrodistilled using a clevenger-type apparatus, and extracted with 500 mL of distilled water for 300 min. The upper layer was collected and treated with anhydrous sodium sulphate overnight at 4 °C. Finally, the supernatant phase was then collected by centrifugation (10,000 rpm) at 4 °C for 30 min and stored in a sealed vial at − 20 °C for further analysis.
The cold pressed essential oil was obtained as previously reported with minor modifications (Peng et al. 2013). In brief, a spiral juicer was used to squeeze 300 g of citrus peel. The cold pressed product was collected and treated with anhydrous sodium sulphate. The oil layer was further separated by centrifugation at 10,000 rpm and 4 °C for 30 min. The supernatant was gently collected and stored in a sealed vial at − 20 °C. Each extraction experiment was carried out in triplicate. The extraction yield was expressed as the weight percentage of the weight of the essential oil to the fresh material (100 g).
Identification of volatile components by GC–MS
The composition of volatile components in Citrus essential oils was analyzed by an Agilent 6890 N gas chromatography (GC) equipped with an Agilent 5973 mass spectrometer as previously described with minor modifications (Ren et al. 2015). A flame ionization detector (FID) was used for quantitative analysis of volatile components. The GC injector and detector were both set at 250 °C. Samples (1 μL) were injected under a split mode at the ratio of 1:50. The column temperature was initially kept at 45 °C for 1 min, increased to 165 °C at a rate of 10 °C/min and held for 2 min, then increased to 200 °C at a rate of 2 °C/min and maintained for 10 min. Volatile components were identified by comparing the obtained mass spectra and retention indices (RI) with those of authentic standards and the National Institute of Standards and Technology (NIST) MS Library, Version 2008 (Lan-Phi et al. 2009; Ren et al. 2015).
DPPH free radical scavenging assay
The DPPH radical scavenging ability of the essential oils was measured by a modified method (Asikin et al. 2012). Briefly, 50 μL of samples at different concentrations (diluted in ethanol) were placed in a 96-well microplate and then supplemented with 150 μL of DPPH (0.1 mM) solution added. After incubation for 30 min in the dark at room temperature, the absorbance was measured at 517 nm using a microplate reader (Thermo Scientific Multiskan GO). BHT (butylated hydroxytoluene) was used as the positive control, and ethanol served as the negative control. All experiments were carried out in triplicate. Radical-scavenging activity was calculated as a percentage of DPPH discoloration using the following equation:
| 1 |
in this equation, As is the absorbance of the reaction mixture containing the sample, and ADPPH is the absorbance of the negative control.
Thiobarbituric acid reactive species (TBARS) assay
A modified TBARS assay was used to detect the potential antioxidant capacity in the lipid-rich media with egg yolk homogenates (Ruberto and Baratta 2000). Briefly, 0.5 mL of 10% (w/v) tissue homogenate was mixed with 0.1 mL of sample solution in a test tube, followed by the addition of 0.05 mL of 2,2′-azobis (2-amidinopropane) dihydrochloride solution (0.07 M) to induce lipid peroxidation. Next, the mixture was supplemented sequentially with 1.5 mL of 20% acetic acid (pH 3.5) and 1.5 mL 0.8% (w/v) of thiobarbituric acid in 1.1% (w/v) sodium dodecyl sulfate solution, followed by vortexing and heating at 95 °C for 60 min. After cooling to room temperature, 5.0 mL of butan-1-ol was added to the mixture, then extensively vortexed and centrifuged at 1000 rpm for 10 min. Finally, the absorbance of the organic upper layer was measured at 532 nm. The antioxidant index (AI %) was calculated based on the following equation:
| 2 |
In this equation, AC is the absorbance value of the fully oxidized control and AS is the absorbance of the test sample.
Statistical analysis
All the experiments were performed in triplicate. Results were presented as mean values ± SD of triplicate independent experiments. One-way ANOVA was applied to test the differences between the means and significant levels were considered at p < 0.05 (Duncan’s post hoc test). All statistical analyses were processed by IBM SPSS Statistics version 20.0. Principal component analysis (PCA) of the data was conducted on the normalized variables by XLSTAT 2010 (Addinsoft, New York, NY).
Results and discussion
Oil yields
After extraction by hydrodistillation and cold pressing, Citrus essential oils were obtained, and their yields and volatile constituents are shown in Table 1. Oil contents in Citrus peels have been reported to be influenced by Citrus genotype, origin, season, environmental factors, extraction and analytical method (Asikin et al. 2012; Hosni et al. 2010; Wu et al. 2013). In this study, Citrus fruits were harvested in their mature conditions, and Valencia orange and Ponkan fruits showed higher oil yields than Eureka lemon. A similar trend was also reported previously and this phenomenon can be attributed to genetic difference (Ferhat et al. 2006; Guimarães et al. 2010). Additionally, the morphology of Citrus peels, especially the thickness and the number of oil glands, can also affect the oil yield. In this study, the detected oil yields ranged from 0.37 to 0.62%, which agreed with previously reported yields (Minh Tu et al. 2003).
Table 1.
Volatile aroma components (relative concentration %) of Citrus essential oils
| No. | Component | RIa | Orange-HD | Orange-CP | Ponkan-HD | Ponkan-CP | Lemon-HD | Lemon-CP |
|---|---|---|---|---|---|---|---|---|
| 1 | 3-Thujene | 934 | – | – | 1.24 ± 0.20b | 1.62 ± 0.13a | – | – |
| 2 | α-Pinene | 939 | 0.98 ± 0.13d | 1.59 ± 0.11c | 1.47 ± 0.11c | 1.84 ± 0.14c | 2.70 ± 0.18b | 5.80 ± 0.4a |
| 3 | Camphene | 953 | – | – | – | – | 0.45 ± 0.05a | 0.12 ± 0.03b |
| 4 | Sabenene | 968 | 0.52 ± 0.05c | 0.90 ± 0.04b | 0.81 ± 0.05b | 1.03 ± 0.08a | – | – |
| 5 | β-Pinene | 980 | 0.38 ± 0.05d | 0.58 ± 0.06 cd | 1.35 ± 0.08c | 1.34 ± 0.04c | 9.82 ± 0.14b | 16.91 ± 1.10a |
| 6 | β-Myrcene | 988 | 2.96 ± 0.46b | 4.69 ± 0.58a | 3.26 ± 0.20b | 3.81 ± 0.69ab | – | – |
| 7 | Octanal | 1002 | 0.84 ± 0.13b | – | 1.66 ± 0.24a | 0.41 ± 0.06c | – | – |
| 8 | Limonene | 1030 | 86.65 ± 8.64a | 85.50 ± 6.95a | 68.16 ± 6.03b | 74.93 ± 8.06ab | 51.22 ± 5.78c | 55.47 ± 6.12c |
| 9 | γ-Terpinene | 1071 | 0.34 ± 0.05c | – | 9.72 ± 1.05b | 9.70 ± 1.16b | 9.70 ± 0.15b | 11.78 ± 1.78a |
| 10 | Terpinolene | 1088 | 0.14 ± 0.01c | – | 0.85 ± 0.11b | 0.88 ± 0.13b | 1.65 ± 0.25a | 0.91 ± 0.06b |
| 11 | p-cymenene | 1093 | – | – | – | – | 0.46 ± 0.08 | – |
| 12 | Linalyl acetate | 1098 | 1.80 ± 0.15a | 0.94 ± 0.12b | – | – | – | – |
| 13 | 3-Carene | 1101 | 0.06 ± 0.01c | – | 5.54 ± 0.81a | 1.42 ± 0.20b | 0.85 ± 0.08b | 0.97 ± 0.19b |
| 14 | 1,5,8-p-Menthatriene | 1128 | – | – | – | – | 0.52 ± 0.04 | – |
| 15 | Ocimene | 1144 | – | – | 0.10 ± 0.00b | – | 1.29 ± 0.11a | – |
| 16 | Perilla alcohol | 1158 | 0.10 ± 0.01a | – | 0.04 ± 0.00b | 0.01 ± 0.00c | – | – |
| 17 | Citronellal | 1165 | 0.30 ± 0.02b | 0.30 ± 0.02b | 0.72 ± 0.08a | 0.34 ± 0.05b | – | – |
| 18 | (Z)-Nerol | 1170 | – | – | 0.08 ± 0.00 | – | – | – |
| 19 | Decanal | 1185 | 1.57 ± 0.12a | 1.03 ± 0.03b | 0.54 ± 0.09d | 0.70 ± 0.04c | – | – |
| 20 | Terpinen-4-ol | 1191 | 0.50 ± 0.04b | – | – | – | 4.69 ± 0.33a | – |
| 21 | a-Terpineol | 1198 | – | – | 1.37 ± 0.22b | – | 9.11 ± 1.11a | – |
| 22 | P-menth-1-en-9-al | 1213 | 0.14 ± 0.02 | – | – | – | – | – |
| 23 | Methyl thymol ether | 1229 | – | – | 0.83 ± 0.08a | 0.20 ± 0.02b | – | – |
| 24 | d-Citronellol | 1237 | 0.21 ± 0.03b | 0.05 ± 0.00c | – | – | 0.79 ± 0.08a | – |
| 25 | Geranial | 1244 | 0.28 ± 0.03b | 0.17 ± 0.02c | – | – | 1.30 ± 0.13a | – |
| 26 | Carveol | 1247 | – | – | – | – | – | 0.98 ± 0.07 |
| 27 | D(+)-Carvone | 1269 | 0.23 ± 0.02 | – | – | – | – | – |
| 28 | Neryl alcohol | 1271 | 0.01 ± 0.00d | 0.03 ± 0.00c | 0.13 ± 0.01b | – | 0.35 ± 0.04a | – |
| 29 | Citral | 1272 | 0.28 ± 0.03c | 0.11 ± 0.12d | 0.11 ± 0.01d | – | 1.66 ± 0.11a | 1.10 ± 0.12b |
| 30 | Perilla aldehyde | 1295 | 0.23 ± 0.04c | 0.06 ± 0.00d | 0.40 ± 0.05a | 0.01 ± 0.00d | 0.32 ± 0.03b | – |
| 31 | δ-Elemene | 1339 | – | – | 0.22 ± 0.08a | 0.17 ± 0.02a | – | – |
| 32 | Thymol | 1386 | – | – | 0.12 ± 0.03 | – | – | – |
| 33 | Copaene | 1397 | 0.05 ± 0.00b | 0.24 ± 0.03a | – | 0.03 ± 0.00bc | 0.02 ± 0.00 cd | – |
| 34 | β-Elemene | 1407 | 0.04 ± 0.00b | – | 0.05 ± 0.00b | 0.09 ± 0.01a | – | – |
| 35 | Tridecanal | 1418 | 0.13 ± 0.02b | 0.32 ± 0.03a | 0.07 ± 0.00c | 0.15 ± 0.04b | – | – |
| 36 | Caryophyllene | 1454 | 0.06 ± 0.00c | 0.06 ± 0.00c | – | – | 0.16 ± 0.05b | 0.57 ± 0.08a |
| 37 | g-Elemene | 1463 | – | – | 0.04 ± 0.00a | 0.06 ± 0.01a | – | – |
| 38 | Cubebene | 1469 | 0.07 ± 0.00b | 0.17 ± 0.02a | – | – | – | – |
| 39 | Farnesene | 1475 | 0.04 ± 0.00d | 0.10 ± 0.01c | 0.03 ± 0.00d | 0.05 ± 0.00d | 0.59 ± 0.04b | 1.28 ± 0.07a |
| 40 | alpha-Cedrene | 1482 | – | – | – | – | – | 0.02 ± 0.00 |
| 41 | Germacrene D | 1490 | – | 0.08 ± 0.00b | 0.03 ± 0.00c | 0.13 ± 0.02a | – | – |
| 42 | Valencen | 1491 | 0.33 ± 0.03b | 0.43 ± 0.05a | 0.01 ± 0.00c | – | – | – |
| 43 | β-Bisabolene | 1510 | – | – | 0.03 ± 0.00c | – | 0.86 ± 0.06b | 1.86 ± 0.21a |
| 44 | d-Cadinene | 1524 | 0.06 ± 0.01b | 0.16 ± 0.03a | 0.03 ± 0.00b | 0.04 ± 0.00b | – | – |
| 45 | Alloaromadendren | 1567 | – | – | 0.01 ± 0.00 | – | – | – |
| 46 | a-Sinensal | 1695 | – | 0.07 ± 0.00 | – | – | – | – |
| 47 | Nootkanone | 1811 | – | 0.05 ± 0.00 | – | – | – | – |
| Monoterpenes | 92.03 (8) | 93.43 (6) | 92.51 (10) | 96.57 (9) | 78.65 (10) | 91.95 (7) | ||
| Sesquiterpenes | 0.65 (7) | 1.23 (7) | 0.45 (9) | 0.57 (7) | 1.63 (4) | 3.73 (4) | ||
| Total oxygenated compounds | 6.61 (14) | 3.13 (11) | 6.08 (12) | 1.81 (7) | 18.23 (7) | 2.08 (2) | ||
| Total identified | 99.29 (29) | 97.79 (24) | 99.04 (31) | 98.95 (22) | 98.52 (21) | 97.76 (13) | ||
| Hydrocarbons/oxygenates | 14.02 | 30.24 | 15.29 | 53.67 | 4.40 | 46.00 | ||
| Oil yields | 0.61 ± 0.02a | 0.49 ± 0.02b | 0.62 ± 0.04a | 0.45 ± 0.02bc | 0.54 ± 0.03ab | 0.37 ± 0.01c |
Values are expressed as mean ± SD, n = 3. Values followed by different small letters in the same row are significantly different (p < 0.05)
aRI: Retention indexes relative to C5–C28 n-alkanes calculated on non-polar HP5MS™ capillary
Compared with cold pressing, hydrodistillation resulted in higher oil yields for the three Citrus species (Table 1), and similar results were also reported in previous studies (Asikin et al. 2012; Bousbia et al. 2009). A possible explanation for the higher yields by hydrodistillation is that the repeated exposure to boiling water or steam by the hydrodistillation system might lead to the release of most crude oil from the flavedo peel (Asikin et al. 2012). While in cold pressing extraction, peels and cuticle oils were squeezed mechanically, leading to the emulsification of colloid substances and the liberated Citrus oils, thus decreasing the oil yield (Ferhat et al. 2007). However, cold pressing is usually recommended for Citrus oil extraction because of its contribution to the fresh aroma.3.2 Volatile aroma composition.
A total of 47 compounds were identified, and Table 1 shows their contents and classifications. In the Citrus essential oils, the volatile components were dominated by monoterpenes, accounting for 78.65–96.57% of the total, and the most representative monoterpene was limonene, amounting to 51.22–86.65% of the total, which was in agreement with previous findings (Ferhat et al. 2007; Sawamura et al. 2004). The amounts of hydrocarbons (compounds 9–11) accounted for 1.81–18.23% of the total volatiles, with the highest amount of hydrocarbons observed in the hydrodistillated lemon oil.
Compared with Eureka lemon oil, volatiles in Valencia orange and Ponkan essential oils were much more complicated in volatile aroma composition (Table 1). Specifically, Valencia orange and Ponkan essential oils showed a similar ratio of hydrocarbons to oxygenates in hydrodistillated oils (14.02 vs 15.29, Table 1). However, the distilled lemon oil exhibited a higher amount of oxygenated compounds (18.23%), coupled with the presence of α-terpineol (9.11%), terpinen-4-ol (4.69%) and citral (1.66%). Compared with hydrodistillated oil, the cold-pressed oil exhibited a higher ratio of hydrocarbons to oxygenated compounds (Table 1). The aforementioned results showed that more terpene hydrocarbons were retained in oils separated by cold pressing, in contrast to more oxygenates in oils extracted by hydrodistillation. This phenomenon might be attributed to the gradual diminution of water-soluble oxygenated compounds in oils induced by the vigorous mixing of essential oil with water during cold pressing. (Menichini et al. 2011).
Principal component analysis
Principal component analysis (PCA) was performed to understand the segregation and correlation among these volatile aroma compounds (Ren et al. 2015). Figure 1 shows the correlations between the two dimensions and compound variables. Three principal components were obtained by the PCA analysis, explaining 75.97% of the total variance, with PC1 and PC2 taking up 54.13% and 21.84% respectively. A significant difference was observed in the aroma profiles of essential oils extracted by hydrodistillation and cold pressing, especially for the Eureka lemon oil (Fig. 1a). Lemon-CP (cold pressing) was positively correlated with PC2, whereas lemon-HD (hydrodistillation) was negatively correlated with PC2, indicating the significant effect of extraction methods on the aroma profile in Eureka lemon oil. Additionally, Orange-CP and ponkan-CP were clustered in the first quadrant with no significant difference, while orange-HD and ponkan-HD appeared in the third quadrant, suggesting that the aroma profiles of essential oils from Valencia orange and Ponkan were similar under the same extraction method.
Fig. 1.
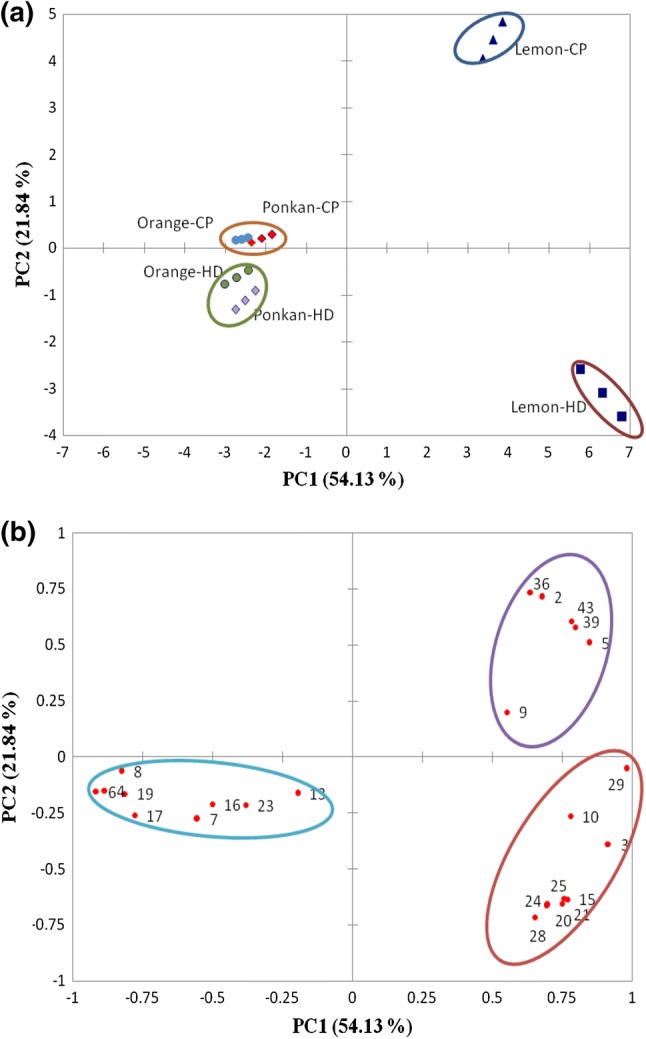
Principal component analysis (PCA) of volatile compounds in six essential oils. a Score plot of PC2 against PC1. b Variable plot of PC2 against PC1. Variables are those of identified volatile compounds shown in Table 1
The variable plot of PC2 against PC1 was further analyzed. As shown in Fig. 1b, the volatile aroma compounds were scattered in three quadrants. Notably, most of the oxygenated compounds (octanal, perilla alcohol, citronellal, decanal, methyl thymol ether) and other terpenes (β-myrcene, limonene) were negatively correlated with both PC1 and PC2. These compounds contributed greatly to the volatile contents of the essential oils, with a high accumulation in Valencia orange and Ponkan oils. Meanwhile, the volatile aroma compounds in the second quadrant (α-pinene, β-pinene, γ-terpinene, caryophyllene, farnesene and β-bisabolene) were positively correlated with both PC1 and PC2, serving as a major contributor to the lemon-CP. Comparatively, volatiles (camphene, terpinolene, ocimene, terpinen-4-ol, a-terpineol, d-citronellol, geranial, neryl alcohol and citral) were closer to lemon-HD. These results indicated that Eureka lemon was significantly different from the other two species in the composition of volatiles, and these volatile compounds varied greatly with the extraction methods.
DPPH free radical scavenging activity
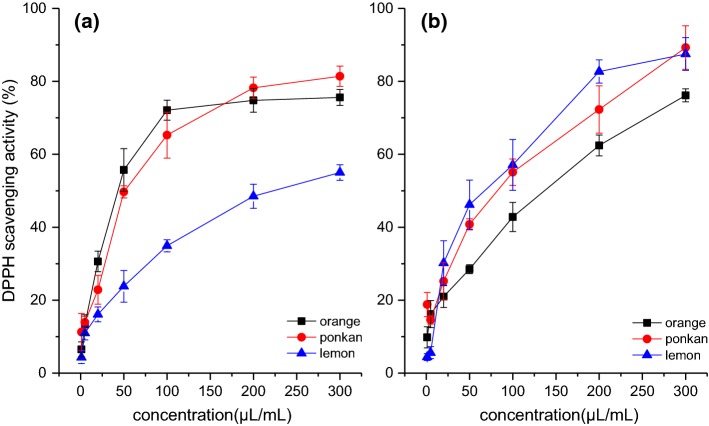
The antioxidant activities of the entire oils as well as ten individual components were measured to explore the possible contribution of each component to DPPH free radical scavenging activity. As shown in Fig. 2, all the three Citrus essential oils exhibited DPPH free radical scavenging activity in a concentration-dependent manner. In Fig. 2a, the DPPH scavenging activities were shown to increase dramatically with the increasing concentration of hydrodistillated oils. Comparatively, the antioxidant activities were higher in the hydrodistillated oils of Valencia orange and Ponkan than Eureka lemon, with the highest DPPH scavenging activity being only 54.5% for Eureka lemon versus 81.7% and 77.8% for Ponkan and Valencia orange, respectively. In the case of cold-pressed oils, the Eureka lemon oil showed higher DPPH scavenging activity than the Valencia orange oil (Fig. 2b). Table 2 shows the radical scavenging activity (EC50) of different oils. Compared with cold pressing, hydrodistillation obviously decreased the EC50 values of Valencia orange and Ponkan oils, but significantly increased the EC50 value of the Eureka lemon oil.
Fig. 2.
DPPH free radical scavenging activities of Citrus essential oils. a Hydrodistillated oils; b cold pressed oils
Table 2.
EC50 values (μL/mL) for the six Citrus essential oils and two representative violate components
| EC50-DPPH | EC50-TBARS | |
|---|---|---|
| Lemon-HD | 215.45 ± 16.25a | 5.75 ± 0.28b |
| Orange-HD | 41.68 ± 3.86d | 225.95 ± 17.54a |
| Ponkan-HD | 51.44 ± 1.25cd | 25.68 ± 1.89b |
| Lemon-CP | 65.89 ± 2.33cd | 10.26 ± 0.91b |
| Orange-CP | 133.57 ± 10.88b | 7.48 ± 0.67b |
| Ponkan-CP | 77.22 ± 4.56c | 14.34 ± 1.04b |
| γ-Terpinene | 54.51 ± 1.75cd | < 5 |
| Terpinolene | 65.77 ± 4.98cd | < 5 |
Values are expressed as mean ± SD, n = 3. Values followed by different small letters in the same row are significantly different (p < 0.05)
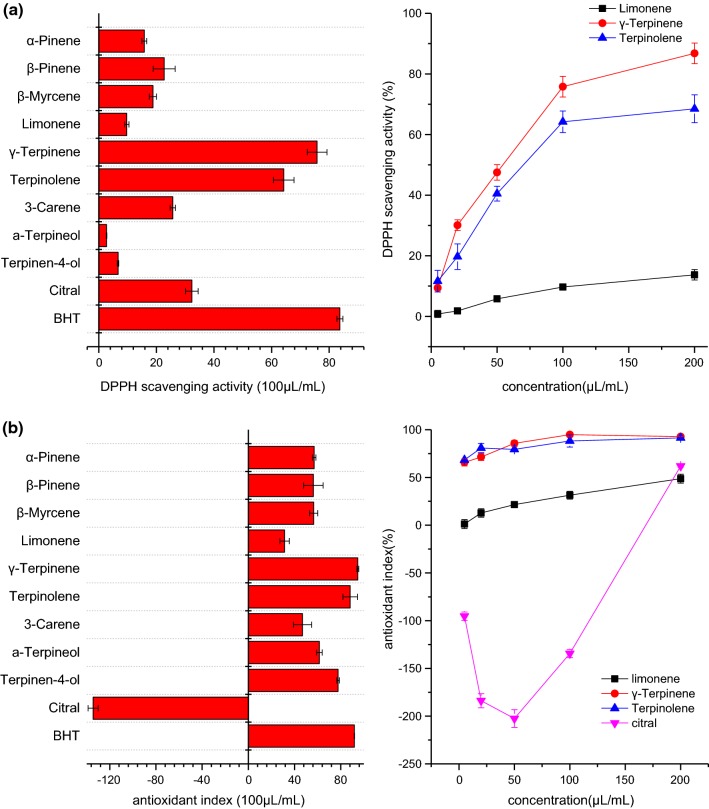
Figure 4a shows the DPPH free radical scavenging activity of ten major oil components at the concentration of 100 μL/mL. The γ-terpinene and terpinolene scavenged 75.8% and 64.2% DPPH radicals respectively (Choi et al. 2000), and these values were comparable to the activity of BHT (100 µg/mL). γ-Terpinene has been reported to have higher DPPH radical-scavenging activity than the other components of flavedo peel extracts (Asikin et al. 2012). Compared with other tested monoterpenes, limonene exhibited low DPPH radical-scavenging ability (less than 20%), indicating that limonene may not playa principal role in DPPH radical-scavenging ability. A previous study also mentioned that the higher antioxidant efficiency of essential oils might be related to the higher concentration of terpenes with the exception of limonene and myrcene (Choi et al. 2000).
Fig. 4.
The antioxidant abilities of ten individual authentic components (100 μL/mL) and BHT (100 µg/mL). a DPPH free radical scavenging activity; b lipid peroxidation inhibition abilities
The oxygenated compounds of α-terpineol and terpinen-4-ol showed low DPPH radical-scavenging abilities (less than 8%). PCA analysis indicated that the hydrodistillated Eureka lemon oil contained high levels of oxygenated compounds, especially α-terpineol and terpinen-4-ol, which might be responsible for the poor performance of the hydrodistillated Eureka lemon oil in scavenging radicals (Fig. 2). A previous study also indicated that the EC50 values of the cold-pressed oils from unripe Shiikuwasha (Citrus depressa Hayata) fruits were much lower than those of the distillation oils in DPPH radical scavenging activity (Asikin et al. 2012). Since PCA analysis showed similar volatile profiles for orange-HD, orange-CP, ponkan-HD and ponkan-CP, the increased radical scavenging ability of orange-HD and ponkan-HD might be attributed to other hydrodistillation-enhanced heat-resistant antioxidant compounds increased by hydrodistillation, such as flavonoids (Salem et al. 2013).
Inhibition of lipid peroxidation (TBARS)
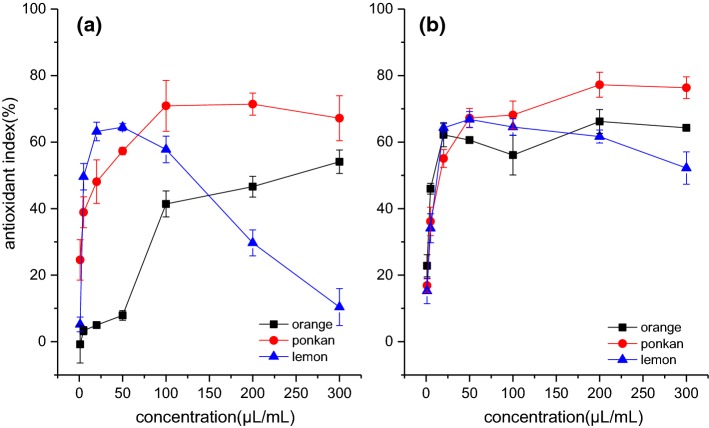
The TBARS method was used to evaluate the inhibition ability of Citrus essential oils on lipid peroxidation. Figure 3 shows the dose-related antioxidant indexes of Citrus essential oils. For hydrodistillated oils (Fig. 3a), the antioxidant index of Ponkan oil increased with its increasing concentration, with the maximal value of 70.9% being obtained at the concentration of 100 μL/mL. A similar trend was also found in the Valencia orange oil, with the highest antioxidant index reaching 54.1%. The hydrodistillated Eureka lemon oil was found to protect 64.5% lipid from oxidation at the concentration of 50 μL/mL, and a further concentration increase led to a decrease in the antioxidant effect. A similar trend was also observed in the cold-pressed Eureka lemon oil. Compared with the cold pressing method, the hydrodistillation decreased the EC50 values of Eureka lemon oil, but increased the EC50 value of Valencia orange and Ponkan oils (Table 2).
Fig. 3.
Lipid peroxidation inhibition abilities of Citrus essential oils. a Hydrodistillated oils; b cold pressed oils
Figure 4b shows the inhibition abilities of ten major oil components on lipid peroxidation. Two monoterpenes, γ-terpinene and terpinolene inhibited over 80% of lipid peroxidation, which is comparable to the standard antioxidant of BHT. This result was in accordance with a previous study (Ruberto and Baratta 2000). The highly activated methylene groups in these molecules were considered as the very reason for their excellent antioxidant behavior (Sharififar et al. 2010). Limonene showed low activity in protecting lipid from oxidation, with an antioxidant index below 50%, and similar results were also reported previously (Ruberto and Baratta 2000; Takahashi et al. 2003). Different from other components, citral displayed a strong pro-oxidant effect in the concentration range of 0–150 μL/mL. The significantly high concentration of citral in Eureka lemon might be responsible for the decreased antioxidant index of Eureka lemon oil (Fig. 3). The extraction methods (cold pressing and hydrodistillation) caused no significant difference in the EC50 values between Ponkan and Eureka lemon oils. The orange-HD showed an obvious increase over the orange-CP in the EC50 value, probably due to other unknown non-volatile compounds extracted by the cold pressing method. Considering the complexity in the antioxidant mechanism of essential oils, other bioactive compounds, including phenolics, carotenoids and fatty acids, need to be further investigated for a better understanding of their antioxidant mechanism.
These two different antioxidant assays provided a more comprehensive understanding of the antioxidant properties of Citrus essential oils. The ratio of hydrocarbons/oxygenated was not found related to the oil’s antioxidant ability of the oils. The high antioxidant activities of γ-terpinene and terpinolene were confirmed by DPPH and TBARS. A possible synergistic effect of other oil compounds on the antioxidant activity can be suggested to further understand the antioxidant mechanism of the entire oils.
Conclusion
In this study, we analyzed the yields, volatile components and antioxidant abilities of six Citrus peel oils prepared by two extraction methods. The hydrodistillation method delivered higher oil yields and more oxygenated chemicals, while the cold pressing method retained more terpene hydrocarbons. PCA analysis revealed a significant difference between Eureka lemon and the other two species in volatile compounds as well as a remarkable effect of the extraction methods on the volatile profile in Eureka lemon oil. The monoterpenes of γ-terpinene and terpinolene displayed antioxidant abilities as strong as BHT based on DPPH and TBARS, while citral showed a strong pro-oxidant ability on TBARS formation. The higher citral concentration in Eureka lemon oils might be responsible for their decreased inhibition ability of lipid peroxidation. Limonene was abundantly present in the oils, but showed poor antioxidant performance. These results suggest that the extraction of Citrus essential oils should focus on the accumulation of γ-terpinene and terpinolene while minimizing the citral content. This study provided valuable information on the volatile components and antioxidant abilities of Citrus essential oils, which would facilitate the development of natural antioxidant products from Citrus essential oil.
Acknowledgements
The authors are grateful to the financial support by the Special Fund for Agro-scientific Research in the Public Interest (201303076-04) and the Scientists Project in the National Modern Agricultural Technology System for Citrus Industry (CARS-27-05B).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qi Lu, Email: luqihzau@126.com.
Nana Huang, Email: 1228973809@qq.com.
Ying Peng, Email: pengkangying@sina.com.
Chunhua Zhu, Email: zchua910@gmail.com.
Siyi Pan, Phone: +86-13554029828, Email: siyipanfood@126.com.
References
- Allaf T, Tomao V, Besombes C, Chemat F. Thermal and mechanical intensification of essential oil extraction from orange peel via instant autovaporization. Chem Eng Process. 2013;72(Supplement C):24–30. doi: 10.1016/j.cep.2013.06.005. [DOI] [Google Scholar]
- Asikin Y, Taira I, Inafuku S, Sumi H, Sawamura M, Takara K, et al. Volatile aroma components and antioxidant activities of the flavedo peel extract of unripe Shiikuwasha (Citrus depressa Hayata) J Food Sci. 2012;77(4):C469–C475. doi: 10.1111/j.1750-3841.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bassolé IHN, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17(4):3989–4006. doi: 10.3390/molecules17043989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukroufa M, Boutekedjiret C, Petigny L, Rakotomanomana N, Chemat F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason Sonochem. 2015;24(Supplement C):72–79. doi: 10.1016/j.ultsonch.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Bousbia N, Vian MA, Ferhat MA, Meklati BY, Chemat F. A new process for extraction of essential oil from Citrus peels: microwave hydrodiffusion and gravity. J Food Eng. 2009;90(3):409–413. doi: 10.1016/j.jfoodeng.2008.06.034. [DOI] [Google Scholar]
- Choi H-S, Song HS, Ukeda H, Sawamura M. Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. J Agric Food Chem. 2000;48(9):4156–4161. doi: 10.1021/jf000227d. [DOI] [PubMed] [Google Scholar]
- Chutia M, Deka Bhuyan P, Pathak MG, Sarma TC, Boruah P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT Food Sci Technol. 2009;42(3):777–780. doi: 10.1016/j.lwt.2008.09.015. [DOI] [Google Scholar]
- Espina L, Somolinos M, Lorán S, Conchello P, García D, Pagán R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22(6):896–902. doi: 10.1016/j.foodcont.2010.11.021. [DOI] [Google Scholar]
- Ferhat MA, Meklati BY, Smadja J, Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J Chromatogr A. 2006;1112(1):121–126. doi: 10.1016/j.chroma.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Ferhat MA, Meklati BY, Chemat F. Comparison of different isolation methods of essential oil from Citrus fruits: cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Fragr J. 2007;22(6):494–504. doi: 10.1002/ffj.1829. [DOI] [Google Scholar]
- Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Technol. 2008;19(3):156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- González-Mas MC, Rambla JL, López-Gresa MP, Blázquez MA, Granell A. Volatile compounds in citrus essential oils: a comprehensive review. Front Plant Sci. 2019;10:12. doi: 10.3389/fpls.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano ACE, Cardile V, Crascì L, Caggia S, Dugo P, Bonina F, et al. Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-gamma and histamine exposed human keratinocytes. Life Sci. 2012;90(25):968–974. doi: 10.1016/j.lfs.2012.04.043. [DOI] [PubMed] [Google Scholar]
- Guimarães R, Barros L, Barreira JCM, Sousa MJ, Carvalho AM, Ferreira ICFR. Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem Toxicol. 2010;48(1):99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Guo J-j, Gao Z-p, Xia J-l, Ritenour MA, Li G-y, Shan Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT Food Sci Technol. 2018;97:825–839. doi: 10.1016/j.lwt.2018.07.060. [DOI] [Google Scholar]
- Hosni K, Zahed N, Chrif R, Abid I, Medfei W, Kallel M, et al. Composition of peel essential oils from four selected Tunisian Citrus species: evidence for the genotypic influence. Food Chem. 2010;123(4):1098–1104. doi: 10.1016/j.foodchem.2010.05.068. [DOI] [Google Scholar]
- Lan-Phi NT, Shimamura T, Ukeda H, Sawamura M. Chemical and aroma profiles of yuzu (Citrus junos) peel oils of different cultivars. Food Chem. 2009;115(3):1042–1047. doi: 10.1016/j.foodchem.2008.12.024. [DOI] [Google Scholar]
- Mahato N, Sharma K, Koteswararao R, Sinha M, Baral E, Cho MH. Citrus essential oils: extraction, authentication and application in food preservation. Crit Rev Food Sci Nutr. 2019;59(4):611–625. doi: 10.1080/10408398.2017.1384716. [DOI] [PubMed] [Google Scholar]
- Menichini F, Tundis R, Bonesi M, De Cindio B, Loizzo MR, Conforti F, et al. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat Prod Res. 2011;25(8):789–799. doi: 10.1080/14786410902900085. [DOI] [PubMed] [Google Scholar]
- Minh TuN, Onishi Y, Son U, Ogawa E, Ukeda H, Sawamura M. Characteristic odour components of Citrus inflata Hort. ex Tanaka (Mochiyu) cold-pressed peel oil. Flavour Fragr J. 2003;18(5):454–459. doi: 10.1002/ffj.1252. [DOI] [Google Scholar]
- Peng L-W, Sheu M-J, Lin L-Y, Wu C-T, Chiang H-M, Lin W-H, et al. Effect of heat treatments on the essential oils of kumquat (Fortunella margarita Swingle) Food Chem. 2013;136(2):532–537. doi: 10.1016/j.foodchem.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Périno-Issartier S, Ginies C, Cravotto G, Chemat F. A comparison of essential oils obtained from lavandin via different extraction processes: ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J Chromatogr A. 2013;1305:41–47. doi: 10.1016/j.chroma.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Ren J-N, Tai Y-N, Dong M, Shao J-H, Yang S-Z, Pan S-Y, et al. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015;185:25–32. doi: 10.1016/j.foodchem.2015.03.142. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167–174. doi: 10.1016/S0308-8146(99)00247-2. [DOI] [Google Scholar]
- Salem MZM, Ali HM, El-Shanhorey NA, Abdel-Megeed A. Evaluation of extracts and essential oil from Callistemon viminalis leaves: antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac J Trop Med. 2013;6(10):785–791. doi: 10.1016/S1995-7645(13)60139-X. [DOI] [PubMed] [Google Scholar]
- Sawamura M, Thi Minh TuN, Onishi Y, Ogawa E, Choi H-S. Characteristic odor components of Citrus reticulata Blanco (Ponkan) cold-pressed oil. Biosci Biotechnol Biochem. 2004;68(8):1690–1697. doi: 10.1271/bbb.68.1690. [DOI] [PubMed] [Google Scholar]
- Sharififar F, Yassa N, Mozaffarian V. Bioactivity of major components from the seeds of Bunium persicum (Boiss.) Fedtch. Pak J Pharm Sci. 2010;23(3):300–304. [PubMed] [Google Scholar]
- Silvestre WP, Agostini F, Muniz LAR, Pauletti GF. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J Food Eng. 2016;178:90–94. doi: 10.1016/j.jfoodeng.2016.01.011. [DOI] [Google Scholar]
- Singh P, Shukla R, Prakash B, Kumar A, Singh S, Mishra PK, et al. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, dl-limonene. Food Chem Toxicol. 2010;48(6):1734–1740. doi: 10.1016/j.fct.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Inaba N, Kuwahara S, Kuki W. Antioxidative effect of citrus essential oil components on human low-density lipoprotein in vitro. Biosci Biotechnol Biochem. 2003;67(1):195–197. doi: 10.1271/bbb.67.195. [DOI] [PubMed] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012;134(3):1571–1579. doi: 10.1016/j.foodchem.2012.03.094. [DOI] [PubMed] [Google Scholar]
- Wu Z, Li H, Yang Y, Zhan Y, Tu D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind Crops Prod. 2013;46(Supplement C):311–316. doi: 10.1016/j.indcrop.2013.02.015. [DOI] [Google Scholar]