Abstract
The bio-wastes (like peels, seeds, etc.) from food industry are rich source of bio-active components, but are poorly managed. In present study, carotenoids were extracted from carrot pomace using ultrasonication and high shear dispersion techniques and flaxseed oil as green solvent (green biorefinery approach). Various combinations of time and temperature were used and final selection was made on the basis of maximum recovery of carotenoids. High shear disperser yielded maximum carotenoids (recovery 94.8 ± 0.08%). The total carotenoid content, antioxidant activity as ABTS, DDPH and FRAP and β-carotene of carotenoid rich extract from carrot pomace (CREP) were 82.66 ± 0.06 μg/g, 1596.04 ± 69.45 μg Trolox eq./ml, 380.21 ± 39.62 μg Trolox eq./ml, 941.20 ± 19.91 μM Trolox eq./ml, 78.37 μg/g, respectively were significantly higher (p < 0.05) when compared with the extracting medium. The L*, a* and b* values of CRE were 18.65 ± 0.037, 19.42 ± 0.21, 27.947 ± 0.65 and were significantly higher than extracting medium. The CRE could be used as a natural source of β-carotene and natural colorant for food applications.
Keywords: Extraction, Carrot pomace, Flaxseed oil, SEM, β-Carotene, HPLC
Introduction
Fruits and vegetables are the richest source of bioactive components and accessory factors such as carotenoids and other phytonutrients, especially, their peels and other solid residues. However, such waste is usually discarded for example, peels of pomegranate, black grapes, tomato, orange etc. Carotenoids (C40), lipophilic isoprenoid molecules, are one of the largest groups of pigments (ranging from yellow to orange to red) that are accumulated in higher levels in the organs and tissues of all photosynthetic plants synthesizing them. The characteristic colour of carotenoids is due to the presence of polyene chain with the number of double bonds that function as chromophores (Klein and Rodriguez-Concepcion 2015). The biosynthesis and accumulation of high levels of carotenoids has a great importance in plant as well as in human health. They act as precursor of Vitamin A, besides possessing the ability of quenching singlet oxygen (good antioxidant properties), and thus have been proposed to be very good chemo preventive agents and lower the risk of cancer (Boeing et al. 2012).
Carrot, an important root vegetable, is a major source of carotene. At cellular level, carotenoids are found in the chromoplasts of fresh carrot. During carrot juice processing, 50% of the raw material remains as pomace and is either used as feed or manure or is disposed off. However, it is a good source of carotenoids and dietary fiber and thus, can potentially be introduced into food chain. But, the problem lies with its utilization as it contains carotenoids bound to pectin and fibers which decrease the micellization of carotenoids, in turn decreasing its bioaccessibility (Saini et al. 2015). Thus, extracting carotenoids using green bio-refinery approach could increase its bioavailability.
Carotenoids, besides possessing health benefits can also be used as a source of natural colourant. However, mostly organic solvents such as acetone, methanol, ethanol, ethyl acetate, isopropyl alcohol, petroleum ether, and hexane etc. are used singly or in combination for the extraction and purification of these colorants which possess toxic effects to the body (Sachindra and Mahendrakar 2005). Halim et al. (2012) has reported extraction using organic solvents. The authors, however, emphasized that organic solvent extraction is slow and uses a large amount of expensive and toxic solvents, requires further treatment like evaporation to concentrate the extract and cost large amount of solvents which results in polluting the environment. The green bio-refinery approach is need of the hour and is based on design of extraction processes that utilizes minimal energy, green solvents and renewable or novel sources from plant origin and eliminate the use of solvents of petrochemical origin so as to ensure a safe and superior quality extract or product (Chemat et al. 2012; Baria et al. 2019). Lipid soluble nature of carotenoids can be taken as an advantage for the extraction of carotenoids in vegetable oils over other solvent extraction methods. Sachindra and Mahendrakar (2005) compared the extraction of carotenoids in different oils from shrimp waste. In the present work, carrot pomace, a bio-waste, was used as a substrate for extraction of carotenoids using flaxseed oil as green solvent by applying modern methods of extraction like high shear dispersion and ultrasonication.
Materials and methods
Materials
The carrots were procured from local market of Karnal, Haryana (India) and stored in cold store. Flaxseed oil, the green solvent used as extracting media, was obtained from local market of Tilak Bazar Chowk, New Delhi. High shear disperser (IKA T18 digital ULTRA-TURRAX Disperser) and Probe Ultrasonicator (Sonics, Vibra-Cell™, USA) were used as extraction technique. The chemicals and enzymes used were procured from either Sigma Aldrich Chemicals Pvt. Ltd. or Merck Specialties Pvt. Ltd.
Methods
Processing and storage of carrots
Carrots were washed with potable water, peeled and juice was extracted. The carrot bio-waste i.e. pomace was blanched so as to inactivate peroxidase enzyme. The blanching of carrots was ascertained by the method of Cemeroglu (1992). Five gram of carrot pomace (after the heat treatment) was taken in a testtube and 5 ml of distilled water was added to it. This was followed by addition of 1 ml each of 0.5% solution of guaiacol and 0.08% solution of hydrogen peroxide. The colour change in testtube was noted till 30 s. The absence of formation of brown ring after the stipulated time indicated the inactivation of peroxidase. The time–temperature combination at which inactivation was completed was selected for blanching carrot pomace used in subsequent steps. Blanched carrot pomace was portioned and packed in laminated pouches of 150 g each and subsequently sealed followed by storing under frozen conditions.
Enzymatic hydrolysis of carrot pomace
The frozen carrot pomace was thawed and a suspension of cellulase from Apergillus niger (~ 0.8 U/mg) and pectinase from Apergillus niger (~ 1.15 U/mg) in water were added into it @ 0.35 and 0.5%, respectively. The enzymatic treatment was given at 45 °C for 3 h. The reaction was terminated by heating suspension at 90 °C for 5 min thereafter cooling to 20 °C (Baria et al. 2019).
Scanning electron microscopy (SEM) of carrot pomace without and with enzymatic treatment
A small quantity of carrot pomace (with and without enzyme treatment) were taken in a petriplate and fixed with 2.5% glutaraldehyde followed by washing with cacodylated buffer (pH 7). The secondary fixation was carried out with 1% osmium tetraoxide and washing with cacodylated buffer followed by dehydration using ethanol with serial dilution of 30% to 100% at an interval of 10 min. The critical drying was done in oven at 50 °C (for 10 h). The dried samples were sprayed on double adhesive tape mounted on aluminium stub and were coated with gold. The samples were finally examined using SEM (HIthachi, 3400 N) at an acceleration voltage of 15 kV under low vacuum of 0.00009 Torr and micrographs were recorded.
Extraction of carotenoid using flaxseed oil from carrot pomace
Enzyme treated carrot pomace and flaxseed oil were mixed in 1:1 ratio (based on the preliminary trials) and carotenoid extraction was carried out by using high shear disperser (Ultra turrax, IKA T18) at 20,000 rpm and ultrasonication at 45% duty cycle with 13 mm probe radius and 750 W power. The carotenoid-rich extract from carrot pomace (CREP) was obtained by subjecting the mixture to centrifugation @ 4000 rpm for 12 min after each treatment. The CREP was analyzed for the parameters mentioned below.
Physico-chemical analysis of CREP
Total carotenoid content (TCC)
The TCC was analysed based on the protocol proposed by Luterotti and Kljak (2010).
Recovery of carotenoids in CREP
The recovery of carotenoids was determined by following formula:
Antioxidant activity
ABTS antioxidant activity
ABTS antioxidant activity was measured by following the method of Awika et al. (2003).
DPPH antioxidant activity
The DPPH antioxidant activity was measured according to the method given by Cuendet et al. (1997) with some modifications. For analysis, 50 µl sample was mixed with 3.95 ml of ethyl acetate and reaction was carried out by adding 1 ml of 0.2 mM DPPH solution in ethyl acetate. The absorbance was measured at 517 nm after an incubation of 30 min in dark at room temperature. Ethyl acetate was used as blank. Standard curve was prepared using Trolox (from 20 to 350 µg/ml) as standard. Results were expressed in terms of TEAC i.e. Trolox equivalent antioxidant capacity (mg/g).
FRAP antioxidant activity
The FRAP antioxidant activity was analyzed according to the method given by Benzie and Strain (1996) with slight modifications. Three ml of FRAP reagent was mixed with 100 µl of sample (the sample was prepared by diluting it with ethyl alcohol to 2% and using diluted sample for analysis). The absorbance was measured at 593 nm after exactly 4 min of mixing sample and FRAP reagent. Standard curve of Trolox was prepared at concentrations of 0, 20, 40, 60, 80 and 100 µM.
Color values
Tristimulus spectrophotometer Hunter Lab Colour Flex was used to measure color of carotenoid extract and results were expressed in terms of L*, a* and b*. Further, chroma, hue and total color difference were measured using following formulae:
Total color difference (ΔE*): The following formula was used to determine total color difference between all three coordinates:
ΔL = L*sample −L*standard = difference in lightness and darkness (+ for lighter, − for darker), Δa = a* sample −a*standard = difference in red and green (+ for redness, − for greener), Δb = b* sample −b* standard = difference in yellow and blue (+ for yellowness, − for blueness).
Peroxide value
Peroxide value was estimated as per the protocol given by ISI (1964).
Conjugated acids
The conjugated acids were reported in terms of % conjugated diene, trienes, tetraenes and pentaenes (AOAC, 1995).
β-Carotene determination using high performance liquid chromatography
Four hundred mg of sample was measured accurately in which 0.2 g of L-ascorbic acid, 15 ml of absolute ethanol and 4 ml of 76% potassium hydroxide were added. It was then incubated at 70 °C for 30 min. Five ml of sodium chloride was added (25 g/l) after cooling and suspension was extracted three times with 15 ml portions of n-hexane and ethyl acetate (85:15 v/v). The organic phase was evaporated to dryness at 40 °C and residue was dissolved in hexane (for flaxseed oil, it was dissolved in 1 ml of hexane and for CREP, it was dissolved in 1.5 ml of hexane). The samples were passed through 0.22 µm filter before injecting for chromatographic analysis.
Twenty μl of sample was injected and elution was performed at a flow-rate of 0.8 mL/min in analytical column of HPLC, Zorbax, 5 μm C18 column, 250*4.6 mm, Agilent, USA having guard column of Delta-Pack, 5 μm C18 column, Agilent Technologies, USA. Column temperature was maintained at 30 °C. The method involved isocratic flow with a ratio of Methanol:Tetrahydrofuran:Water:Triethylamine to be 67:27:5:1. Photo-diode array was used as detector for taking absorbance at 450 nm. Standard curve was plotted using peak area of different concentration of standards of β-carotene. The R2 was 0.9603 with linear equation of y = 136716 × − 281383. Peak-area of sample was used for calculating β-carotene in sample using appropriate dilution factor as mentioned above.
Statistical analysis
The results of the analysis were statistically analyzed using One way ANOVA (Duncan Post Hoc test) and paired t test (SPSS statistical tools) at p = 0.05.
Results and discussion
Blanching of carrot pomace
Blanching is the process of dipping cut fruits or vegetables in water maintained at fixed temperature for a specific period of time so as to inactivate peroxidase, index enzyme for blanching. Blanching of carrot pomace was carried out at 90 °C for 1, 2.5 and 5 min and the enzyme was found to be inactivated at 2.5 and 5 min. Carotenoids are water insoluble pigments. Therefore, these are retained during blanching. However, prolonged treatment may result in leaching of some carotenes into water in turn causing the significant losses of carotenoids. Therefore, 2.5 min treatment at 90 °C was selected for blanching carrot pomace.
Enzymatic treatment of carrot pomace
The TCC of extract from carrot pomace without enzymatic treatment was 53.86 ± 0.084 µg/g, while after enzymatic treatment it increased significantly (p < 0.05) to 73.03 ± 1.182 µg/g. This could be due to the presence of carotenoids in the matrix of pectin, pectin-like substance and cellulosic materials in carrots. Thus, food matrix is required to be disrupted so as to break cell structure and achieve the highest recovery of carotenoids. The major advantage of enzymatic hydrolysis is to decrease the activation energy of chemical reaction and provide milder conditions for the process. These benefits attract researchers to use enzyme aided extraction for carotenoids. Ghosh and Biswas (2016) reported cellulase and pectinase enzymes to be highly efficient for obtaining a good yield of carotenoids from pumpkin tissues.
Effect of enzymatic hydrolysis on micrograph of carrot pomace
Scanning electron microscopy of carrot pomace was done to study the difference in structure of carrot pomace before and after enzymatic treatment at microscopic level (Fig. 1). The samples of carrot pomace without enzymatic treatment showed uniformity in the structure with negligible cavities, indicating intactness of the tissues. On the other hand, alteration in structure vis-à-vis formation of cavities, irregularity and open structure was prominent in enzyme treated sample, which could have eased in extraction of carotenoids. Similar structural changes were observed by Nowacka and Wedzik (2016) who processed carrots using ultrasound treatment. Figure 1 clearly indicates that enzymatic treatment has efficiently assisted in extraction of carotenoids from carrot pomace.
Fig. 1.
×1000 SEM images of carrot pomace with and without enzymatic treatment
Extraction of carotenoids from carrot pomace using different treatments
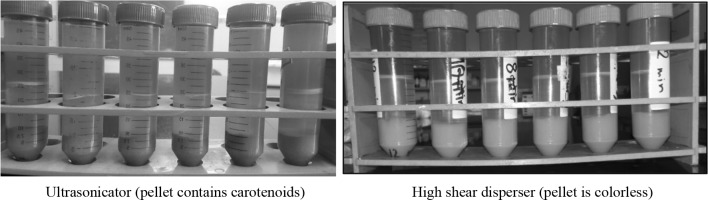
The blanched and enzyme treated carrot pomace was used for extraction of carotenoids using flaxseed oil as extracting medium. The carrot pomace to flaxseed oil ratio of 1:1 was subjected to different extracting techniques i.e. ultrasonication (probe method, UAE) and high shear dispersion (HSD) for different times i.e. 2–12 min. It is evident from Table 1 that as the time increased from 2 to 12 min, extraction of carotenoids increased significantly (p < 0.05) using both the techniques. This could probably be due to high shearing action and contact time which in turn facilitated in rupturing of carrot pomace matrix. In case of HSD technique, it was observed that there was non-significant (p > 0.05) increase in TCC when extraction time was increased from 12 to 14 min. The total carotenoids recovered from HSD technique was significantly higher as compared to that of UAE (Table 1). The results are supported by Fig. 2 as the pellet obtained in case of HSD treated extract was almost colorless, while that obtained after UAE treatment were not colorless indicating retention of carotenoids in pellet. Thus, 12 min using HSD carried out at 20 000 rpm was selected for extraction of carotenoids from carrot bio-waste (i.e. carrot pomace).
Table 1.
Effect of extraction time and technique on the total carotenoid content and its recovery
| Time (min.) | Extraction technique (TCC content µg/g) | |||
|---|---|---|---|---|
| UAE (probe method) | HSD | |||
| TCC | % Recovery | TCC | % Recovery | |
| 2 | 14.34 ± 0.02ax | 1.32 ± 0.02 | 62.98 ± 0.18ay | 66.54 ± 0.24 |
| 4 | 15.28 ± 0.30ax | 2.62 ± 0.41 | 67.64 ± 24by | 74.3 ± 0.32 |
| 6 | 16.41 ± 0.02bx | 4.15 ± 0.02 | 69.32 ± 1.12cy | 76.60 ± 1.53 |
| 8 | 17.83 ± 0.20cx | 6.10 ± 0.27 | 77.08 ± 0.12dy | 87.21 ± 0.16 |
| 10 | 19.40 ± 0.46dx | 8.24 ± 0.63 | 79.12 ± 0.24ey | 90.01 ± 0.32 |
| 12 | 21.67 ± 0.40ex | 11.36 ± 0.54 | 82.66 ± 0.06fy | 94.85 ± 0.08 |
| 14 | – | – | 82.97 ± 0.02f | 95.28 ± 0.02 |
Mean ± SD (n = 3)
Different superscripts (a–f) are significantly different with each other within column (p < 0.05), different superscripts (x–y) are significantly different with each other within row (p < 0.05)
Fig. 2.
Comparison between high shear dispersion and ultrasonication
The significantly higher yield using HSD treatment could mainly be due to mechanical disruption of cell wall and cell membranes, enabling the release of intracellular compounds (Poojary et al. 2016), while ultrasonication uses the principle of acoustic cavitation phenomenon which induces sound energy. This sound energy leads to cavities or bubbles formation, implosion of cavitation bubbles can hit surface of solid matrix and disintegrate the cells causing release of desired components (Knorr et al. 2004). The superior efficiency of extraction for various components using UAE have been reported to be obtained by using organic solvents such as ethyl acetate, ethanol, methanol, n-heptane, n-hexane etc.
Physico-chemical analysis of carotenoid rich extract from carrot pomace (CREP)
The results of CREP analyzed for different parameters were compared and statistically analyzed (paired t-test) for significant differences (Table 2).
Table 2.
Physico-chemical analysis extracting medium and carotenoid rich extract
| Parameter | Flaxseed oil | CREP |
|---|---|---|
| Total carotenoids content (µg/g) | 13.38 ± 0.141a | 82.66 ± 0.06b |
| β-carotene (µg/g) | 6.75 | 78.37 |
| Antioxidant activity | ||
| ABTS (µg Trolox eq./ml) | 930.33 ± 64.61a | 1596.04 ± 69.45b |
| DPPH (µg Trolox eq./ml) | 255.32 ± 9.05a | 380.21 ± 39.62b |
| FRAP (µM Trolox eq./ml) | 521.91 ± 12.65a | 941.20 ± 19.91b |
| Color values | ||
| L* | 4.085 ± 0.12a | 18.65 ± 0.037b |
| a* | 1.502 ± 0.025a | 19.42 ± 0.206b |
| b* | 2.163 ± 0.106a | 27.947 ± 0.65b |
| Chroma | 2.63 ± 0.07a | 34.03 ± 0.41b |
| Hue | 55.19 ± 0.79a | 55.19 ± 0.92a |
| Peroxide value (meqO2/1000 g of sample) | 1.0736 ± 0.104a | 1.0493 ± 0.08a |
| Conjugated acids (%) | ||
| Conjugated dienes | 0.311 ± 0.000a | 0.406 ± 0.000b |
| Conjugated trienes | 0.0423 ± 0.001a | 0.055 ± 0.001b |
| Conjugated tetraenes | 0.0009 ± 0.001a | 0.0046 ± 0.001b |
| Conjugated pentaenes | 0.0001 ± 0.000a | 0.0004 ± 0.000b |
Mean ± SD (n = 3)
CREP carotenoid rich extract from carrot pomace
Different superscripts (a, b) are significantly different with each other within a row (p < 0.05)
Total carotenoid content
The TCC of CREP was significantly higher than flaxseed oil (p < 0.05) and it increased from 13.38 ± 0.141 (µg/g) to 82.66 ± 0.06 (µg/g). The pretreatment given to carrot pomace i.e. enzymatic treatment followed by HSD were found to be a good combination for extracting carotenoids from carrot pomace using green bio refinery concept. HSD is associated with disruption of cell wall by involving principles of turbulence, cavitation, shear stress which helps in rupturing of rigid cell walls and removing the carotenoids from fiber matrix resulting in removal of maximum amount of carotenoids from carrot pomace (Clarke et al. 2010). Also, slight increase in temperature during homogenization was hypothesized to have further assisted in softening of cell walls.
The carotenoids from carrot pomace are rich in β-carotene which possess high pro-vitamin A activity. Therefore, this extract could also be used as dietary supplement, besides providing coloring attributes to food products. Furthermore, ω-3 rich flaxseed oil is known for its health promoting attributes like preventing diabetes, cancer, arthritis, inflammatory diseases, depression, heart disease, memory problems, etc. (Goyal et al. 2014). Thus, CREP could be a good source of both β-carotene and α-linolenic acid.
Estimation of β-carotene using high performance liquid chromatography (HPLC)
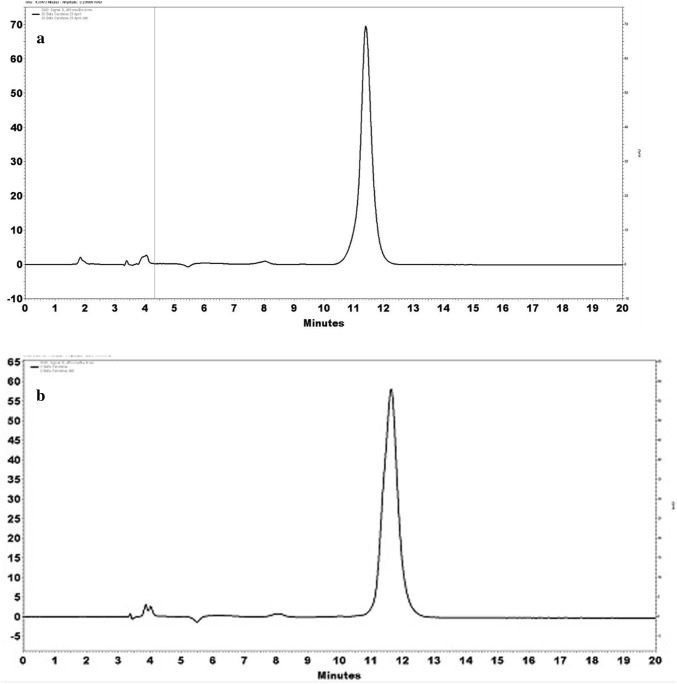
Carotenoids are the major lipophilic antioxidants present in carrot. Among the different varieties of carrots, orange carrots mainly contain appreciable amount of β-carotene over other carotenoids. The typical chromatogram of standard of β-carotene indicated retention time of 11.68 min (Fig. 3). The β-carotene content of flaxseed oil was approximately 6.75 µg/g, while for CREP it was 78.37 µg/g. Koley et al. (2014) reported that amount of β-carotene present in orange variety of carrots is approximately 45 µg/g, while our study showed higher content of β-carotene which may be attributed to varietal differences. Tadesse et al. (2015), on the other hand, reported that fresh carrot contains approximately 71 ppm of β-carotene. Our results are in accordance with the reported value.
Fig. 3.
Peak for β-carotene a standard indicating the retention time of 11.68 min, b peak obtained from carotenoid rich extract from carrot pomace
Antioxidant activity
The antioxidant activity is reported to be dependent on the extracting solvent (type and polarity), procedures adopted for isolation, purity of active compounds and technique of estimation (Meyer et al. 1998). Carotenoids are lipophilic pigments and possess antioxidant potential. The electron-rich conjugated system of polyene is responsible for antioxidant activities of carotenoids, both by quenching singlet oxygen (Conn et al. 1991) and scavenging radicals to terminate chain reactions (Everett et al. 1996). The antioxidant activity of carotenoids are mainly due to reducing nature of conjugated double bond system and ring structure of terminal ends. The antioxidants could have an added advantage of preventing unsaturated fatty acids of flaxseed oil from being oxidized.
The methods for estimation of antioxidant activities for natural products can broadly be classified into two categories: (1) those based on evaluation of radical scavenging activity (RSA) of samples i.e., 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay; 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay; oxygen radical absorbance capacity (ORAC) assay; chemiluminescent assays and (2) those based on evaluation of reducing activity of natural samples i.e., ferric reducing/antioxidant power (FRAP) assay; cupric reducing antioxidant capacity (CUPRAC) assay (Laguerre et al. 2007). In the present study, ABTS, DPPH and FRAP methods were used for determination of antioxidant activities of carotenoids extract.
ABTS antioxidant activity
The ABTS antioxidant activity of CREP was 1596.04 ± 69.45 µg Trolox eq./ml, which was significantly higher (p < 0.05) than that of flaxseed oil (930.33 ± 64.61 µg Trolox eq./ml, Table 2). The ABTS antioxidant activity is based on the extent of reduction of ABTS radical cation and is based upon single electron transfer mechanism. The ABTS free radical scavenging activity of carotenoids is mainly due to carboxyl or hydroxyl functional group at terminal ends as well as number of conjugated double bonds in hydrocarbon chain. It is mainly based on ionization potential of carotenoids in non-polar solvents (Miller et al. 1996). Hence, increase in antioxidant activity is mainly contributed by extracted carotenoids with some minor lipid soluble antioxidant components in CREP.
FRAP antioxidant activity
Antioxidant activity (as FRAP) was also found to have increased significantly (p < 0.05) from 521.91 ± 12.65 µM Trolox eq./ml in flaxseed oil to 941.20 ± 19.91 µM Trolox eq./ml in CREP (Table 2). The principle of FRAP antioxidant activity is based on reduction of ferric ion to ferrous ion at low pH (3.6) via electron transfer mechanism with antioxidant resulting in colored ferrous-tripyridltriazine complex formation due to which it leads to increase in absorbance (Benzie and strain 1996).
FRAP and ABTS are, however, based on single electron transfer mechanism, but the values for both the parameters were different. This could probably be due to different reaction conditions adopted to measure both the antioxidant activities, especially in terms of pH value (pH for FRAP being 3.6, while that for ABTS being 7.4) and the oxidizing molecules (ferric di-TPTZ vs. ABTS) leading to different antioxidant activities (Müller et al. 2011).
DPPH antioxidant activity
DPPH is reported to be a chemical compound possessing a proton free radical having characteristic absorption which decreases significantly when exposed to proton radical scavengers (Yamaguchi et al. 1998). The DPPH free radical-scavenging by antioxidants is attributed to its hydrogen donating ability due to which hydroxyl radical is generated (Chen and Ho 1995). DPPH antioxidant activity increased significantly from 255.32 ± 9.05 µg Troloxeq./ml in flaxseed oil to 380.21 ± 39.62 µg Troloxeq./ml in CREP (Table 2). Müller et al. (2011) reported that carotenoids do not scavenge DPPH free radical, hence lower DPPH antioxidant activity was observed as compared to ABTS and FRAP antioxidant activities. The reported rise in antioxidant activity could be due to some other antioxidants present in extract such as α-tocopherol which may act as radical scavenger.
Color values
The colour values of flaxseed oil and CREP are seen in terms of L*, a*, b*, total color difference (ΔE), Chroma (C*) and Hue angle (h*).
L* a* b* values
Color of flaxseed oil and CREP is mainly because of carotenoid pigment. There was significant difference (p < 0.05) in color values between flaxseed oil and CREP for all parameters i.e. L*, a* and b*. This increase could be attributed to extraction of lipophilic carotenoids in flaxseed oil. The L* value increased from 4.085 ± 0.12 to 18.65 ± 0.037, indicating more bright colour of extract as compared to flaxseed oil. a* value increased significantly from 1.502 ± 0.025 to 19.42 ± 0.206, indicating that colour of extract shifted towards red shade and b* value increased from 2.163 ± 0.106 to 27.947 ± 0.65 which revealed that there was significant increase in yellow shade. The increase in red and yellow shade of extract can mainly be attributed to the use of orange (combination of red and yellow primary colours) variety of carrot in the present study.
Total color difference (ΔE)
The concept of measuring color change as modulus of distance vector between initial and actual color ordinates is termed as total color difference (Martins and Silva 2002). The total color difference values of CREP and flaxseed oil was 34.60. This could be due to increase in total carotenoid pigment in carotenoid rich extract.
Chroma (C*)
Chroma (C*) is quantitative attribute of colorfulness and is used to determine degree of difference in hue with respect to grey color with same lightness. Higher intensity of chroma value is associated with higher intensity of color. The chroma value significantly increased (p < 0.05) in CREP which can again be attributed to carotenoid pigment extracted in flaxseed oil (Table 2).
Hue angle (h*)
The hue angle (h*) is qualitative attribute of color and is used to define color with respect to grey color with same lightness. Hue angle of 0° or 360° denotes red hue, whereas angles of 90°, 180°, and 270° represent yellow, green and blue hues, respectively (Rentfrow et al. 2004). The h* value of flaxseed oil and CREP were 55.19 ± 0.79 and 55.19 ± 0.92, respectively which is between 0° and 90° (Table 2). The results depict that the hue of both flaxseed oil as well as CREP lie in red-yellow region with more inclination towards yellowness.
Peroxide values
The onset of oxidation was checked by analyzing the development of primary oxidation products (peroxides and hydroperoxides) in flaxseed oil and carotenoid rich extract (reported as peroxide value). This peroxide value is an empirical measure of fat oxidation that is highly useful for samples that are oxidized to relatively lower levels. There was no significant difference (p > 0.05) between the peroxide value of flaxseed oil as well as CREP (Table 2) which was within limits as given by New Zealand Food Regulations (1984) for edible fats and oils i.e. 10 meq peroxides/kg of oil and Codex Alimentarius Commission (1999) i.e. 5 meq peroxides/kg of oil, indicating that both oil and CREP were free from oxidation. Burton and Ingold (1984) reported that carotenoids can prevent vegetable oils from being oxidized as are potent antioxidants.
Conjugated acids
Conjugated dienes and trienes are formed on oxidation of polyunsaturated fatty acid which can be monitored by intense absorption in ultraviolet region at 233 and 269 nm for dienes and trienes, respectively (Abdulkarim et al. 2007). Conjugated dienes and trienes were very less in oil as well as in CREP (Table 2). Abdulkarim et al. (2007) observed effect of frying on conjugated dienes and trienes and reported an increase in these values on frying. The authors reported higher increase in these values in soybean oil (more unsaturation) over palm oil (more saturation). Farmer and Sutton (2002) indicated that increase in absorption due to formation of conjugated dienes and trienes is proportional to uptake of oxygen and formation of peroxides during early stages of oxidation. The low levels of both conjugated dienes and trienes observed in present study indicate the oxidative stability. Tetraenes and pentaenes are formed from dienes and triene and these also showed negligible values.
Conclusion
The study concludes that carotenoids can be very effectively extracted from natural bio-waste using green bio-refinery concept. Blanching at 90 °C for 2.5 min resulted in inactivation of peroxidase enzyme. The enzymatic treatment of carrot pomace caused a significant increase (p < 0.05) in extraction of carotenoids. The carrots and flaxseed oil (as the extracting medium) in the ratio of 1:1 yielded highest carotenoids content by using high shear disperser at 20,000 rpm for 12 min over ultrasonication. The oxidative stability of carotenoid rich extract from carrot pomace was high in terms of peroxide value and conjugated acids, which can also be attributed to increased antioxidant activity in terms of ABTS, DPPH and FRAP. The β-carotene content was found to be high i.e. 78.37 µg/g. Thus, the carrot bio-waste can be diverted to human use as the extracted carotenoids can be used as a source of β-carotene or natural colorant in oil based products or it can be used in the form of emulsion in aqueous type products.
Acknowledgements
The first author acknowledge the financial support received from Indian Council of Agricultural Research in the form of Junior Research Fellowship and Institutional Fellowship. Scanning Electron Microscopy was carried out at Punjab Agricultural University, Ludhiana.
Compliance with ethical standards
Conflict of interest
Authors do not have any conflict of interest.
Ethical approval
The study did not involve any animal study. Therefore, ethical approval was not required for the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007;105(4):1382–1389. doi: 10.1016/j.foodchem.2007.05.013. [DOI] [Google Scholar]
- AOAC (1995) Official method of analysis. Association of Official Agricultural Chemists, Oils and Fats, Maryland
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51(23):6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Baria B, Upadhyay N, Singh AK, Malhotra RK. Optimization of ‘green’ extraction of carotenoids from mango pulp using split plot design and its characterization. LWT. 2019;104:186–194. doi: 10.1016/j.lwt.2019.01.044. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Stehle P. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51(6):637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GW, Ingold KU. Beta-carotene: an unusual type of lipid antioxidant. Science. 1984;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Cemeroglu B (1992) Meyve Sebze I´sleme Endustrisinde Temel Analiz Metodlar (Analysis methods in fruit and vegetable processing industry, in Turkish), Biltav, Ankara
- Chemat F, Vian MA, Cravotto G. Green extraction of natural products: concept and principles. Int J Mol Sci. 2012;13(7):8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black teas. J Food Lipids. 1995;2(1):35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- Clarke A, Prescott T, Khan A, Olabi AG. Causes of breakage and disruption in a homogeniser. Appl Energy. 2010;87(12):3680–3690. doi: 10.1016/j.apenergy.2010.05.007. [DOI] [Google Scholar]
- Codex Alimentarius Commission (1999). www.fao.org/input/download/report/314/Al99_17e.pdf/ Assessed 22nd June 2018
- Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991;11(1):41–47. doi: 10.1016/1011-1344(91)80266-K. [DOI] [PubMed] [Google Scholar]
- Cuendet M, Hostettmann K, Potterat O, Dyatmiko W. Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta. 1997;80(4):1144–1152. doi: 10.1002/hlca.19970800411. [DOI] [Google Scholar]
- Everett SA, Dennis MF, Patel KB, Maddix S, Kundu SC, Willson RL. Scavenging of nitrogen dioxide, thiyl, and sulfonyl free radicals by the nutritional antioxidant-carotene. J Biol Chem. 1996;271(8):3988–3994. doi: 10.1074/jbc.271.8.3988. [DOI] [PubMed] [Google Scholar]
- Farmer EH, Sutton DA. Peroxidation in relation to oleifenic structure. In: Akoh CC, Min DB, editors. Food lipids: chemistry, nutrition, and biotechnology. New York: Marcel Dekker; 2002. p. 470. [Google Scholar]
- Ghosh D, Biswas PK. Enzyme-aided extraction of carotenoids from pumpkin tissues. Indian Chem Eng. 2016;58(1):1–11. doi: 10.1080/00194506.2015.1046697. [DOI] [Google Scholar]
- Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine and modern functional food. J Food Sci Technol. 2014;51(9):1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim R, Danquah MK, Webley PA. Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv. 2012;30(3):709–732. doi: 10.1016/j.biotechadv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- ISI . IS: 548 determination of peroxide value: methods of sampling and test for oils and fats. Manak Bhavan, New Delhi: Bureau of Indian Standards; 1964. pp. 63–64. [Google Scholar]
- Klein CS, Rodriguez-Concepcion M. Carotenoids in carrot. In: Chen C, editor. Pigments in fruits and vegetables. New York: Springer; 2015. pp. 217–228. [Google Scholar]
- Knorr D, Zenker M, Heinz V, Lee DU. Applications and potential of ultrasonics in food processing. Trends Food Sci Technol. 2004;15(5):261–266. doi: 10.1016/j.tifs.2003.12.001. [DOI] [Google Scholar]
- Koley TK, Singh S, Khemariya P, Sarkar A, Kaur C, Chaurasia SNS, Naik PS. Evaluation of bioactive properties of Indian carrot (Daucus carota L.): a chemometric approach. Food Res Int. 2014;60:76–85. doi: 10.1016/j.foodres.2013.12.006. [DOI] [Google Scholar]
- Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: existing methods, new trends and challenges. Prog Lipid Res. 2007;46(5):244–282. doi: 10.1016/j.plipres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Luterotti S, Kljak K. Spectrophotometric estimation of total carotenoids in cereal grain products. Acta Chim Slov. 2010;57(4):781–787. [PubMed] [Google Scholar]
- Martins RC, Silva CLM. Modelling colour and chlorophyll losses of frozen green beans (Phaseolus vulgaris L.) Int J Refrig. 2002;25(7):966–974. doi: 10.1016/S0140-7007(01)00050-0. [DOI] [Google Scholar]
- Meyer AS, Heinonen M, Frankel EN. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998;61(1–2):71–75. doi: 10.1016/S0308-8146(97)00100-3. [DOI] [Google Scholar]
- Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996;384(3):240–242. doi: 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129(1):139–148. doi: 10.1016/j.foodchem.2011.04.045. [DOI] [Google Scholar]
- New Zealand Food Regulation . General standard for edible fats and edible oils. In: Chen C, editor. A consolidation of the food regulations 1984 incorporating amendments 1 to 7. New Zealand: GP Publications Ltd.; 1984. [Google Scholar]
- Nowacka M, Wedzik M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl Acoust. 2016;103:163–171. doi: 10.1016/j.apacoust.2015.06.011. [DOI] [Google Scholar]
- Poojary MM, Barba FJ, Aliakbarian B, Donsì F, Pataro G, Dias DA, Juliano P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar Drugs. 2016;14(11):214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentfrow G, Linville ML, Stahl CA, Olson KC, Berg EP. The effects of the antioxidant lipoic acid on beef longissimus bloom time. J Anim Sci. 2004;82(10):3034–3037. doi: 10.2527/2004.82103034x. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Mahendrakar NS. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour Technol. 2005;96(10):1195–1200. doi: 10.1016/j.biortech.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Saini RK, Nile SH, Park SW. Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int. 2015;76(3):735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Tadesse TF, Abera S, Worku S. Nutritional and sensory properties of solar-dried carrot slices as affected by blanching and osmotic pre-treatments. Int J Food Sci Nutr Eng. 2015;5(1):24–32. [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62(6):1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]