Abstract
Several species of cosmopolitan marine macroalgal genus Ulva (Chlorophyta) are economically important due to high growth, carbohydrate, protein and lipid content. Nevertheless, analysis pertaining these traits of any species has by no means been explicitly investigated. We herein investigated 109 samples of U. rigida from fifteen locations of Indian coast for carbohydrate, protein and lipid content suitable for further development of scaled-up production. The carbohydrate, protein and lipid content ranged from 16.63 ± 1.07 to 65.93 ± 0.49% dry weight, 4.14 ± 0.45 to 26.0 ± 1.43% dry weight and 0.8 ± 0.08 to 3.1 ± 0.04% dry weight respectively. Principal component analysis provides an interpretable overview of main information enclosed in a multidimensional data set satisfactorily explained 72.1% of the total variability in the present data, with principal component 1 accounting for 38.7% and principal component 2 for 33.4% of the total variation. The study confirmed that the strain collected from Gopnath, Gujarat possesses high potential for industrial exploitation due to its high carbohydrate level. Growing this alga on large-scale might pave ways for socio-economic development of coastal populace.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03929-w) contains supplementary material, which is available to authorized users.
Keywords: Carbohydrate, Lipid, Marine macro algae, Principal component analysis, Protein, Ulva
Introduction
Sustainability pertaining to human food is a key challenge that needs to be comprehended with respect to burgeoning global population. Nevertheless, with the era of seemingly inexpensive and plentiful resources coming to an end, harnessing non-conventional biological feedstock is pivotal. Marine macroalgae are diverse assemblage of photosynthetic aquatic plants and represent a relatively under-utilized, renewable resource (Dominguez 2013). As per the estimates from the World Bank, by achieving the target of 14% annual growth of marine algal farming could generate 500 million tons dry weight by 2050, adding about 10% to the world’s present supply of food, generating revenues and improving environmental quality (Yarish et al. 2016). Marine macroalga have been traditionally used as food in China, Korea, and Japan for over 2000 years (Nisizawa 2002). Further, substantial research efforts are being made recently for characterisation of functional food ingredients from this resource.
Green marine macroalgae, especially species of Ulva are potential candidate for developing functional food since they are rich in proteins, essential amino acids, vitamins, dietary fibers and minerals vital for human nutrition (McDermid and Stuercke 2003; Yaich et al. 2015). It has been also reported that they are an excellent source of nutritionally important long-chain polyunsaturated fatty acids (McCauley et al. 2007) The incorporation of Ulva compressa in traditional snack food has reported fivefold increase in iron content [26.4–126 mg/100 g] and fourfold increase in calcium content [30.1–124 mg/100 g] (Mamatha et al. 2007). Herein we present proximate composition profile of 109 samples of Ulva rigida collected from several locations along the Indian coast. The findings presented in this paper have important implications pertaining to utilising Ulva in functional food or food supplement in fast growing snack food industry. The food industry in India is worth around US $ 155 billion which is expected to touch US $ 344 by 2025 with an increment of 4.1% per annum; while the share of snack food alone is US $ 3 billion with impressive annual growth of 15–20% (Mantri and Reddy 2015) To our knowledge, there are no exemplified studies ascertaining proximate composition of this alga with extensive sampling efforts from any part of the world. Objective of the study was to determine the extent of variation in the proximate composition of U. rigida from Indian coast and to identify potential strain for industrial exploration. The study arrived to a judgement that U. rigida can be utilised for commercial farming aimed at edible applications.
Materials and methods
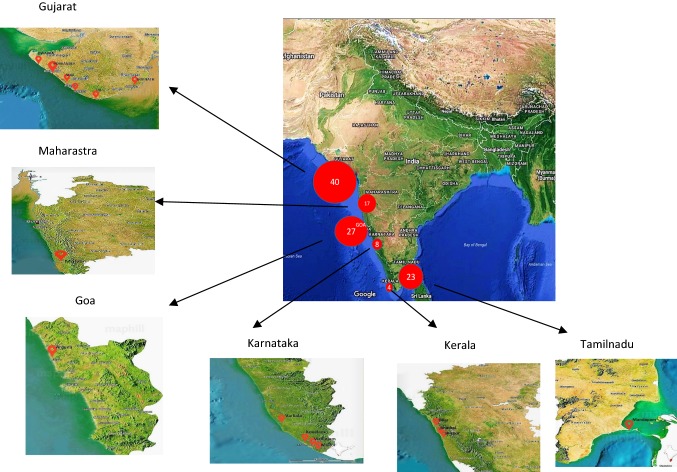
All the samples (109) were collected from intertidal areas of 15 locations includes namely Porbandar, Diu, Veraval, Gopnath, Dwarka, Malvan, Anjuna beach, Ullal, Kovalam, VInzhijam, Muller, Verkala, Shiroor, Bhatkal, Mandapam belonging to six coastal states of India includes Gujarat, Maharashtra, Goa, Kerala, Tamilnadu, Karnataka during November 2014 to August 2016 and given unique code for data handling (Fig. 1). The fronds were transported to the laboratory immediately in plastic zip bags with sufficient seawater from the vicinity in cool packs. The collected samples were subjected taxonomic identification based on the morphological and anatomical features using standard keys provided for Indian taxa (Krishnamurthy 2006). Those which referred to U. rigida only were selected for the further experiments in triplicate (n = 3).
Fig. 1.
Locations of collection sites in India
The carbohydrate extraction was performed using Dubois method targeting effective cell lysis and hydrolysis of carbohydrates (DuBois et al. 1956). The algal tissue (100 mg fresh wt.) was ground to fine powder in liquid nitrogen, to this; 5 ml of 2.5 N HCL was added. The mixture was kept at 100 °C for 150 min in water bath and was allowed to cool at room temperature, to neutralize with solid sodium carbonate powder. The final volume was brought to 50 ml post neutralisation and centrifuged at 3000g for 5 min at room temperature. The supernatant was used for estimation of carbohydrate. About 50 μl sample was taken and working volume was brought to 1 ml using distilled water. To this, 1 ml 5% phenol and 5 ml concentrated H2SO4 were added. The solution was mixed thoroughly and allowed to stand at room temperature to 20 min. Absorbance was measured at 490 nm, and total carbohydrate was calculated based on standard curve of glucose. To determine total protein content, 10 mg of fresh algal sample was mixed with 200 uL of Tris chloroacetic acid and the mixture was incubated at 65 °C for 20 min. After incubation the mixture was diluted using 600 μl distilled water. The mixture was centrifuged at 15,000 g for 20 min at 4 °C. Supernatant was discarded and to the pellet 500 μl, Lowry’s reagent added. The mixture was incubated at 55 °C for 60 min. It was then centrifuged at 15,000 g for 20 min at 4 °C. About 200 μl of supernatant was diluted up to 1 ml using distilled water. To this, 2 ml Lowry’s reagent was added and further incubated at room temperature for 10 min. 200 μl 1 N Folin–Cioacalteu’s reagent was added to this mixture and incubated in the dark at room temperature for 30 min. The absorbance was measured at 660 nm and total protein content was calculated based on a standard curve of bovine serum albumin (Lowry et al. 1951). The lipid extraction was performed using gravimetric analysis (Bligh and Dyer 1959). The algal thallus (100 mg fresh wt.) was first washed with distilled water to remove surface salts and then blotted with paper towel. The sample was grounded to fine powder in liquid nitrogen and extracted with chloroform: methanol (1:2 v/v). The residue obtained was re-extracted three times with small amount of chloroform: methanol (1:1). The extract was filtered and mixed with water for phase separation. The organic phase was collected and evaporated to dryness in vacuum to obtain total lipids. We have found that water content in this alga is ca. 90% (data not shown) and therefore corresponding dry weight of samples was considered while providing the results in % dw.
The data matrix pertaining to carbohydrate, protein and lipid of 109 strains of Ulva were subjected to principal component analysis (PCA) to establish the relationship between and within the different strains collected from various locations along Indian coast using the JMP Pro statistical package version 12.
Results and discussion
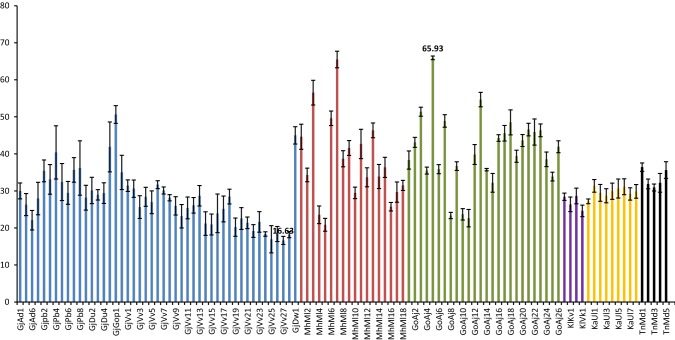
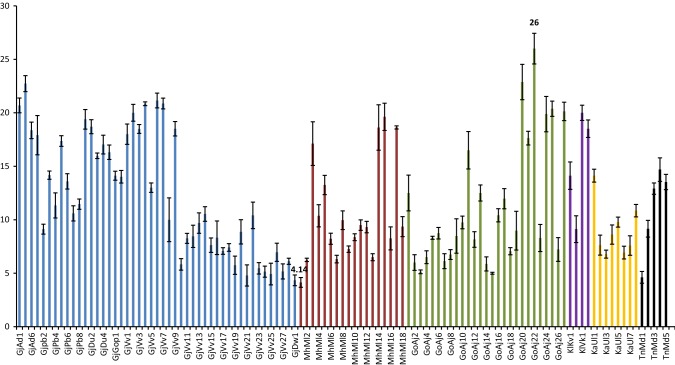
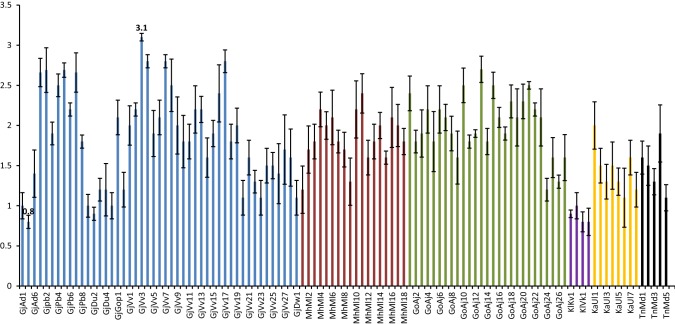
The proximate composition reported in the present study corroborated well with earlier studies (Supplementary table 1). The marine macro algae have been only source of certain high value sulphated polysaccharides like agar, agarose, alginates, ulvan that have been industrially exploited. Protein and lipid especially omega-3 fatty acid plays vital role in the human nutrition (Mantri and Reddy 2015). The carbohydrate content varied from 16.63 ± 1.07 to 65.93 ± 0.49% dw in the samples studied during the present investigation (Fig. 2). The lowest and highest content for the samples collected from Gujarat were represented by strain from Gopnath ‘GjGop1’ (50.63% dw) and Veraval ‘GjVv27’ (16.63% dw) respectively. The same from Maharashtra was from Malvan ‘MhMl7’ (65.49% dw) and ‘MhMl5’ (20.85% dw) respectively. Anjuna ‘GoAj5’ (65.93% dw) reported highest and ‘GoAj11’ (22.64% dw) reported lowest content from Goa. Similarly for collection from Karnataka it was Ullal ‘KaUl1’ (31.3% dw) and ‘KaUl3’ (28.69%) respectively. Muller ‘KlMu1’ reported 28.64% dw carbohydrate which was highest among all the samples from Kerala, while that of Varkala ‘KlVk1’ was lowest (24.61% dw). Tamil Nadu collection from Mandapam ‘TnMd1’ reported highest (36.42% dw) and lowest from ‘TnMd3’ (30.93% dw) respectively. The lower carbohydrate content in U. lactuca was reported in the literature; 4–14% dw (Wong and Cheung 2000) 14.6 – 18.10% dw (Marinho-Soriano et al. 2006) and 5.6% dw (Tabarsa et al. 2012). While, Trivedi et al. (2013) reported 43% dw carbohydrate in U. fasciata. Similarly, higher content was also reported in U. fasciata (46.73% dw), U. reticulata (57.96% dw) and U. rigida (56.07% dw) (Kumar et al. 2011). The carbohydrate content in genus Ulva comes from starch which is the storage material besides several structural polysaccharides namely cellulose, xyloglucan, glucuronan including sulphated heteropolymer called ulvan (Usov and Zelinsky 2013). In the present study we reported quantitative analysis of total carbohydrates. The protein content ranged from 4.14 ± 0.45 to 26.0 ± 1.43% dw among the samples analysed (Fig. 3). The maximum protein content was from Adri ‘GjAd2’ (22.73% dw) while lowest was from Dwarka ‘GjDw1’ (4.34% dw) among the samples collected from Gujarat. The strain from Maharashtra, Malvan ‘MhMll15’ reported highest protein (19.62% dw) while that of Malvan ‘MhMl1’ was lowest (4.14% dw). The highest protein among samples collected from Goa was reported from Anjuna ‘GoAj22’ (26% dw) and lowest was ‘GoAj15’ (5% dw). Sample ‘KaUl1’ (14.12% dw) reported highest protein while lowest was from ‘KaUl3’ (6.79%) of Ullal from Karnataka. Strain from Varkala Kerala ‘KlVk1’ reported 20% dw protein which was highest, while that of Muller ‘KlMu1’ was lowest (9.12% dw). Among the samples from Tamil Nadu sample from Mandapam ‘TnMd4’ (14.69% dw) reported highest protein and lowest was from ‘TnMd1’ (4.61% dw) respectively. The values corroborated well with the previous reports 10–26% dw (Fleurence et al. 1999) and 7.06–19% dw (Wong and Cheung 2000). Nevertheless, lower values of protein (6.4–7.06% dw) were also recorded in U. lactuca (Marinho-Soriano et al. 2006). The total protein extracted includes proteins, endogenous bio-active peptides, oligopeptides and free amino acids as well. However, the methods reported in the literature for extraction and determination of protein were not same and most of the studies estimated protein from field-collected samples. The comparison of protein from different strains enabled us to identify the strain with high protein content that is from Anjuna, Goa. The lipid content in marine macro-algae is generally low, but when compare to land plants they significantly have higher level of polyunsaturated fatty acids (PUFA) such as omega-3 fatty acids (Kumari et al. 2013) We reported lipid content in a range of 0.8 ± 0.08–3.1 ± 0.04% dw in the present investigation (Fig. 4). Among the samples from Gujarat strain collected from Veraval ‘GjVv3’ (3.1% dw) reported maximum lipid while lowest was from Adri ‘GjAd2’ (0.8% dw). The strain ‘MhMll1’ reported highest lipid (2.4% dw) while that of ‘MhMl1’ was lowest (1.2% dw) both collected from Malvan, Maharashtra. The highest lipid among samples collected from Goa was reported from Anjuna ‘GoAj13’ (2.7% dw) and lowest was ‘GoAj24’ (1.2% dw). Highest lipid was reported from Ullal ‘KaUl1’ (2% dw) while lowest from Ullal ‘Kaul5’ (1.1%) of Karnataka. Muller ‘KlMu1’ reported 1% dw lipid which was highest among all the samples from Kerala, while that of Varkala ‘KlVk1’ was lowest (0.8% dw). Tamil Nadu reported high lipid from the sample ‘TnMd4’ (1.9% dw) and lowest from ‘TnMd5’ (1.1% dw) both collected from Mandapam. The lower lipid (0.8% dw) was reported in three samples collected from Adri along Gujarat; Vizhinjam and Varkala from Kerala respectively. Five strains (4.59%) from collected samples showed lipid content less than 1% dw basis, while 61 (55.96%) showed lipid ranged between 1 and 2% dw and 43 (39.45%) reported lipid content over 2% dw. We also reported lipid content of 2.13 ± 0.23% dw from Ulva tubulosa; 2.10 ± 0.10% dw from U. linza; 1.83 ± 0.20% dw from U. fasciata; 2.03 ± 0.20 dw from U. rigida; 2.00 ± 0.20% dw from U. reticulata and 1.27 ± 0.11% dw from U. lactuca (Kumari et al. 2010).
Fig. 2.
Carbohydrate Content of Ulva rigida collected from Indian coast (% dw). Vertical bar represents SD
Fig. 3.
Protein Content of Ulva rigida collected from Indian coast (% dw). Vertical bar represents SD
Fig. 4.
Lipid Content of Ulva rigida collected from Indian coast (% dw). Vertical bar represents SD
A statistical analysis was performed on the proximate composition including carbohydrate, lipid and protein data matrix of 109 taxa of Ulva in order to select a better taxa for seaweed farming purpose and its further utilization in seaweed food and nutraceutical industry. PCA that provides an interpretable overview of main information enclosed in a multidimensional data set satisfactorily explained 72.1% of the total variability in the present data, with PC1 accounting for 38.7% and PC2 for 33.4% of the total variation (Fig. 5). The discriminant variables along the axis I were lipid and carbohydrate contents and along axis II was the protein content. The lipid and carbohydrate contents being positioned close to each other on the loadings plot indicated that they are closely related. This may be due to both are the part of central carbon metabolism and are positively regulated. It’s a well-known fact that carbohydrate is the first assimilatory product of carbon in the metabolism and excess carbon is mobilized to lipids in any organism, and, thus both are positively correlated. However, the relative position of protein indicated that it was not correlated with lipid and carbohydrate contents in taxa Ulva. Remarkably, although all the taxa collected from different sites, those collected from same collection sites were grouped together as apparent from the bi-plot (with a few exceptions for e.g., MhMl3, MhMl7, MhMl13, GoAj2, GoAj5, GoAj7, GoAj14, GoAj18, GoAj26, GjVv10, GjVv16, GjVv19, GjVv21, GjVv23, KlMu, TnMd1, KaUl3). This may be attributed to genetic differences between different U. rigida strains collected from different geographical locations and physiology. The physico-chemical characters of seawater at study location also varied considerably (Supplementary table 2). Another possibility is phenotypic plasticity between different U. rigida strains collected from same site in this study that despite of sharing similar genetic makeup they respond differently. Twenty-one taxa out of 28 collected from Veraval were clustered together with the taxa from Karnataka due to their lower loadings of carbohydrate and lipid contents with the exception of 6 taxa (GjVv1, GjVv2, GjVv4, GjVv6, GjVv7 and GjVv9) that were located differently due to their higher loadings for protein content and GjVv3 for high lipid content (3.1% dw). Similarly, the taxa collected from Adri and Diu were grouped together due to their higher loadings of protein contents. The taxa collected from Anuja were also clustered together due to their higher carbohydrate and lipid contents with the highest carbohydrate content being reported in the taxa from Anuja GoAj5 (65.93%). The single taxon collected from Gopnath was grouped with them due to its higher carbohydrate content (50.63%). Most of the variations were observed from the taxa collected from Malvan, Maharashtra, with most of the taxa had lower protein content except for MhMl14, MhMl15 and MhMl17, being situated with a few taxa from Anuja and Veraval along PC2, while MhMl3 and MhMl7 had higher carbohydrate and lipid contents. All other taxa were positioned in between them showing intermediate values for their proximate composition. Hereby, it is noteworthy to consider that macroalgae generally exhibit remarkable phenotypic plasticity across large spatial scales (Fowler-Walker et al. 2006). A correlative study of the proximate composition of U. rigida species collected from different geographical regions along with relevant molecular markers may give a better insight of this broad range variability in proximate compositions. The variation in proximate composition could be attributed to the individual genetic make and physiology. This further explained the mechanism of their adaptability across large geographical scales.
Fig. 5.
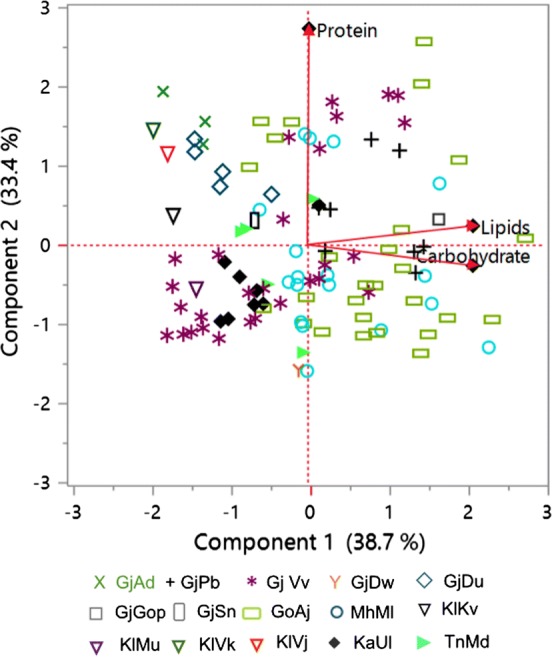
Principal component analysis of proximate composition data matrix of Ulva rigida strains
The significant differences in the proximate composition between strains of U. rigida opens immense possibility of isolating a desirable strain of promising potential for genetic improvement programmes to selectively breed for desirable traits (such as lipid productivity or carbohydrate or protein contents). In addition, the broad distribution of U. rigida across multiple locations across India makes it an ideal species for aquaculture farming and genetic breeding programmes for bioprospection perspectives. In our study, the PCA data revealed that there is a huge difference in the proximate composition of Ulva species collected from Veraval, Anuja and Malvan and helped to select the species having higher protein, lipid or carbohydrate contents irrespective of their sites of collection. The strain collected from Anuja and Malvan, GoAj5, MhMl7, MhMl3 and GoAj containing 65.93%, 65.49%, 56.54% and 54.66% carbohydrate contents respectively possess high potential for industrial exploitation for their high carbohydrates, taxa collected from Adri for protein GjV3 collected from Veraval form lipid utilization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Analytical Division and Centralized Instrument Facility for support. This work is carried out under Indo-UK collaborative project entitled “Transnational approaches to resolving biological bottlenecks in macroalgal biofuel production (SuBBSea).” The financial support by Department of Biotechnology, Ministry of Science and Technology, New Delhi is gratefully acknowledged [BT/IN/Indo-UK/SuBB/22/AML/2013]. We would like to thank the anonymous reviewers and handling editor for constructive comments on the manuscript. We thanks Dr. CRK Reddy, Retired Scientist and Principal Investigator of the project for facilitating the work and constructive suggestions to improve the original draft of the manuscript. Dr. M. Ganesan, Dr. Monica Kawale, Mr. Ashok Bhagat and Ms. Richa Sharma are acknowledged for enhancing sampling efforts. Authors would like to thank Dr. Amitava Das, Director, CSIR, CSMCRI for facilities and encouragement. This has CSIR-CSMCRI PRIS Registration Number PRIS 010/2017 dt. 18.01.2017.
Abbreviations
- °C
°Celsius
- mg
Milligram
- µl
Microliter
- g
Gravity (relative centrifugal force)
- dw
Dry weight
- PUFA
Poly unsaturated fatty acid
- PCA
Principal component analysis
- PC1
Principal component 1
- PC2
Principal component 2
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bindu MS. Empowerment of coastal communities in cultivation and processing of Kappaphycus alvarezii—a case study at Vizhinjam village, Kerala, India. J Appl Phycol. 2011;23:157–163. doi: 10.1007/s10811-010-9597-4. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Dhargalkar VK, Shaikh N. Primary productivity of marine macrophytes in the coral reef lagoon of the Kadmat Island, Lakshadweep. Current Sci. 2000;79:1101–1104. [Google Scholar]
- Dominguez H, editor. Functional ingredients from algae for foods and nutraceuticals. Series in Food Science, Technology and Nutrition. Cambridge: Woodhead Publishing; 2013. [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Eswaran K, Ghosh PK, Mairh OP. Experimental field cultivation of Kappaphycus alvarezii (Doty) Doty ex. P. Silva at Mandapam region. Seaweed Res Util. 2002;24:67–72. [Google Scholar]
- Fleurence J, Chenard E, Luccon M. Determination of the nutritional value of proteins obtained from Ulva armoricana. J Appl Phycol. 1999;11:231–239. doi: 10.1023/A:1008067308100. [DOI] [Google Scholar]
- Fowler-Walker MJ, Wernberg T, Connell SD. Differences in kelp morphology between wave sheltered and exposed localities: morphologically plastic or fixed traits? Mar Biol. 2006;148:755–767. doi: 10.1007/s00227-005-0125-z. [DOI] [Google Scholar]
- Gore PS, Singbal SYS. Ecology and production in two sandy beaches of Goa. Part (A) Distribution of bacteria in Colva and Siridaon in relation to certain environmental parameters. Fish Technol. 1973;10:55–60. [Google Scholar]
- Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- Krishnakumar PK, Bhat GS. Seasonal and interannual variations of oceanographic conditions off Mangalore coast (Karnataka, India) in the Malabar upwelling system during 1995–2004 and their influences on the pelagic fishery. Fish Ocean. 2008;17:45–60. doi: 10.1111/j.1365-2419.2007.00455.x. [DOI] [Google Scholar]
- Krishnamurthy V (2006) Key to taxonomic identification of green and brown marine algae of India. In: Tewari A (ed) Recent advances on applied aspect of Indian marine algae with reference to global scenarion, 1st ed. Central Salt and Marine Chemicals Research Institute, India publication, Bhavnagar, pp 1–45
- Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, Reddy CRK, Jha B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol. 2011;23:797–810. doi: 10.1007/s10811-010-9578-7. [DOI] [Google Scholar]
- Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010;120:749–757. doi: 10.1016/j.foodchem.2009.11.006. [DOI] [Google Scholar]
- Kumari P, Bijo A, Mantri V, Reddy CRK, Jha B. Fatty acid profiling of tropical marine macroalgae: an analysis from chemotaxonomic and nutritional perspectives. Phytochem. 2013;86:44–56. doi: 10.1016/j.phytochem.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mamatha BS, Namitha KK, Senthil A, Smitha J, Ravishankar GA. Studies on use of Enteromorpha in snack food. Food Chem. 2007;101:1707–1713. doi: 10.1016/j.foodchem.2006.04.032. [DOI] [Google Scholar]
- Mantri VA, Reddy CRK. Marine algae, compounds for health, nutrition. Ingredients South Asia. 2015;60:16–31. [Google Scholar]
- Marinho-Soriano E, Fonseca P, Carneiro M. Seasonal variation in the chemical composition of two tropical seaweeds. Biores Technol. 2006;97:2402–2406. doi: 10.1016/j.biortech.2005.10.014. [DOI] [PubMed] [Google Scholar]
- McCauley J, Meyer B, Winberg P, Skropeta D. Parameters affecting the analytical profile of fatty acids in the macroalgal genus Ulva. Food Chem. 2007;209:332–340. doi: 10.1016/j.foodchem.2016.04.039. [DOI] [PubMed] [Google Scholar]
- McDermid KJ, Stuercke B. Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol. 2003;15:513–524. doi: 10.1023/B:JAPH.0000004345.31686.7f. [DOI] [Google Scholar]
- Nisizawa K (2002) Seaweeds Kaiso: bountiful harvest from the seas. Japan Seaweed Association
- Sridhar R, Thangaradjou T, Kumar SS, Kannan L. Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India. J Environ Biol. 2006;27:561–566. [PubMed] [Google Scholar]
- Subba Rao PV, Suresh Kumar K, Ganesan K, Thakur MC. Feasibility of cultivation of Kappaphycus alvarezii (Doty) Doty at different localities on the Northwest coast of India. Aqua Res. 2008;39:1107–1114. doi: 10.1111/j.1365-2109.2008.01976.x. [DOI] [Google Scholar]
- Tabarsa M, Rezaei M, Ramezanpour Z, Waaland JR. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J Sci Food Agric. 2012;92:2500–2506. doi: 10.1002/jsfa.5659. [DOI] [PubMed] [Google Scholar]
- Trivedi N, Gupta V, Reddy CRK, Jha B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Biores Technol. 2013;150:106–112. doi: 10.1016/j.biortech.2013.09.103. [DOI] [PubMed] [Google Scholar]
- Usov AI, Zelinsky ND. Chemical structures of algal polysaccharides. In: Domínguez H, editor. Functional ingredients from algae for foods and nutraceuticals. Oxford: WoodheadpublishingLimited; 2013. pp. 23–86. [Google Scholar]
- Varshney PK, Nair VR, Abidi SAH (1983) Primary productivity in nearshore waters of Thal, Maharashtra coast
- Wong K, Cheung P. Nutritional evaluation of some subtropical red and green seaweeds: part I—proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000;17:475–482. doi: 10.1016/S0308-8146(00)00175-8. [DOI] [Google Scholar]
- Yaich H, Garna H, Bchir B, Besbes S, Paquot M. Chemical composition and functional properties of dietary fibre extracted by Englyst and Prosky methods from the alga Ulva lactuca collected in Tunisia. Algal Res. 2015;9:65–73. doi: 10.1016/j.algal.2015.02.017. [DOI] [Google Scholar]
- Yarish C, Rummett RE, Hansen S, Bjerregaard R, Valderrama D, Sims N, Radulovich R, Diana Capron J, Forster J, Goudey C, Hopkins K, Rust M, McKinnie C (2016) Seaweed aquaculture for food security, income generation and environmental health in tropical developing countries. Report number 107147, World Bank Group 16
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.